Abstract
Objectives:
Acute mesenteric ischemia (AMI) is a life-threatening condition associated with dismal outcomes. This study sought to evaluate the evolution of presentation, treatment, and outcomes of AMI over the past two decades.
Methods:
AMI patients presenting at a single institution were reviewed (1993–2016). Venous thrombosis patients were excluded. Primary outcome was 30-day mortality. Patients were stratified by etiology and diagnosis date (before 2004 versus 2004 and later). Ordered logistic regression was performed for longitudinal temporal analysis.
Results:
303 patients were identified. AMI mechanisms included: embolic (49%), thrombotic (29%), and non-occlusive (NOMI) (22%). The majority were women (55%), 50% had atrial fibrillation, and 23% were on anticoagulation (AC) therapy. Mean age was 72±13 years. 345 procedures were performed in 242 patients: 321 open and 24 hybrid/endovascular. Among the 189 embolic/thrombotic patients who were managed operatively, 45% (n=85) underwent mesenteric revascularization while 39 (21%) had findings of non-survivable bowel necrosis (NSBN). Among the 104 patients who did not undergo revascularization, 64 (62%) died within 30-days compared to 36 out of 85 (42%) patients who were revascularized (p=.01). 30-day mortality was 61% and stable over time (p=.91); when stratified by AMI etiology, the thrombotic cohort had worse survival than embolic and NOMI patients (p=.04). Since 2000, there was a significant decrease in the percentage of embolic AMI events (p=.04). The percentage of patients who underwent operative management decreased also over time (p=.01, 81% → 61%), which was correlated with an increasing number of patients being made comfort measures only (CMO) prior to surgical intervention (50% → 70%, p=.02). The majority of patients (55%) were ultimately made CMO during their hospitalization. Predictors of 30-day mortality included a preoperative white blood cell count (WBC) ≥ 25 K/ μL. (OR 3.0, p=.002) and lactate ≥ 2.3 mmol/L (OR 2.8, p=.045). NSBN predictors included WBC ≥ 24 K/ μL. (OR 3.4 p=.03) and lactate ≥ 3.8 mmol/L (OR 3.6, p=.04).
Conclusions:
Despite advances in critical care over the past 25 years, AMI continues to be associated with poor prognosis. The survival benefit observed in patients who undergo revascularization supports an aggressive approach towards early vascular intervention, although this requires further study. The importance of early diagnosis, prognostication and advanced directives is highlighted given the high morbidity, mortality and use of comfort measures associated with AMI.
INTRODUCTION
Acute mesenteric ischemia (AMI) is a life-threatening condition associated with poor prognosis. Despite advances in diagnostic and treatment modalities, correct diagnosis, management and detection of AMI remain a challenge.1 Over the past several decades, little progress has been made to decrease the high short-term mortality associated with AMI, with rates approaching an astounding 60% to 90%.2 As the prevalence of AMI rises with the aging population, it is increasingly important to understand the evolving patient characteristics and risk factors to improve diagnosis and management.3, 4
While recent trends suggest that incidence of AMI due to embolic events is decreasing due to increased anticoagulation compliance,5 a more granular understanding the contemporary composition of the AMI population in the setting of evolving endovascular and open interventions is important not only for diagnosis, but also for treatment and prognosis.6, 7 We describe the presentation, treatment, and outcomes of AMI over the past two decades. We further characterize the effect of management strategies, including the role of advanced directives, on outcomes, as well as identify early predictors of mortality and non-survivable bowel necrosis.
METHODS
Study Population
All patients who presented to the Massachusetts General Hospital (MGH) with a diagnosis of acute mesenteric ischemia (ICD-9 557.0, ICD-10 K55.0) between January 1993 and March 2016 were identified through our institutional database. 3,628 patients diagnosed with AMI underwent chart review conducted by a single screener (Chou) and those with acute mesenteric venous thrombosis, ischemic colitis, mesenteric ischemia due to mechanical obstruction or volvulus were excluded (2,965). The remaining 303 patients with physician-adjudicated AMI were characterized by etiology: embolic, thrombotic and non-occlusive mesenteric ischemia (NOMI). This protocol was approved by the Institutional Review Board of the MGH and direct informed consent waived.
Clinical Definitions
Demographic and clinical data were collected from review of electronic medical records. Hypertension (HTN), congestive heart failure (CHF), peripheral vascular disease (PVD), chronic obstructive pulmonary disease (COPD), diabetes (DM), and hyperlipidemia were determined by chart documentation. Coronary artery disease (CAD) was defined as a history of angina and/or a history of myocardial infarction, regardless of revascularization status. Baseline chronic kidney disease (CKD) was established by chart review and glomerular filtration rate (GFR); CKD stages based on GFR have been previously defined.8 Causes of AMI were determined by chart review, operative reports and imaging studies. Arterial embolism included emboli from cardiac and other proximal sources compared to the mesenteric vessel affected. Embolic occlusions were defined based on the presence of thrombus in the artery and a relative lack of surrounding calcific disease. Arterial thrombosis included patients with thrombosis superimposed upon preexisting significant atherosclerotic disease. NOMI was defined as nonocclusive ischemia consistent with mesenteric vasospasm in the distribution of the superior mesenteric artery, as previously described.9
Management and Outcome End Points
Procedural data and outcomes were assessed by review of all inpatient and outpatient encounters through July 2019. Additional survival data was obtained from the Social Security Death Index. 30-day complications occurred within 30 days of the diagnosis date and included: urinary tract infection (UTI), pneumonia (PNA), myocardial infarction (MI) and Clostridium difficile colitis. Major adverse events occurring within 30-days was defined as any of the following occurring: UTI, PNA, MI, Clostridium difficile colitis, and/or death. Determination of Comfort Measures Only status (CMO) was determined from chart review; CMO refers to the withdrawal of medical treatment while assuring maximum comfort.
All procedure types and intraoperative findings were obtained by detailed review of operative reports. Patients categorized into the non-survivable bowel necrosis (NSBN) category were found to have intestinal death incompatible with life upon entering the abdomen via laparotomy, prior to any attempt at revascularization. Procedures were categorized into open, endovascular, and hybrid. All endovascular and hybrid interventions involved mesenteric angiography +/− intervention. All hybrid procedures involved exploratory laparotomy to assess for bowel viability and either a diagnostic or interventional endovascular component. Open procedures did not necessarily involve mesenteric revascularization and included: exploratory laparotomy, mesenteric thrombectomy, aorto-mesenteric bypass, bowel resection, or a combination of open procedures. (Figure 1)
Figure 1.
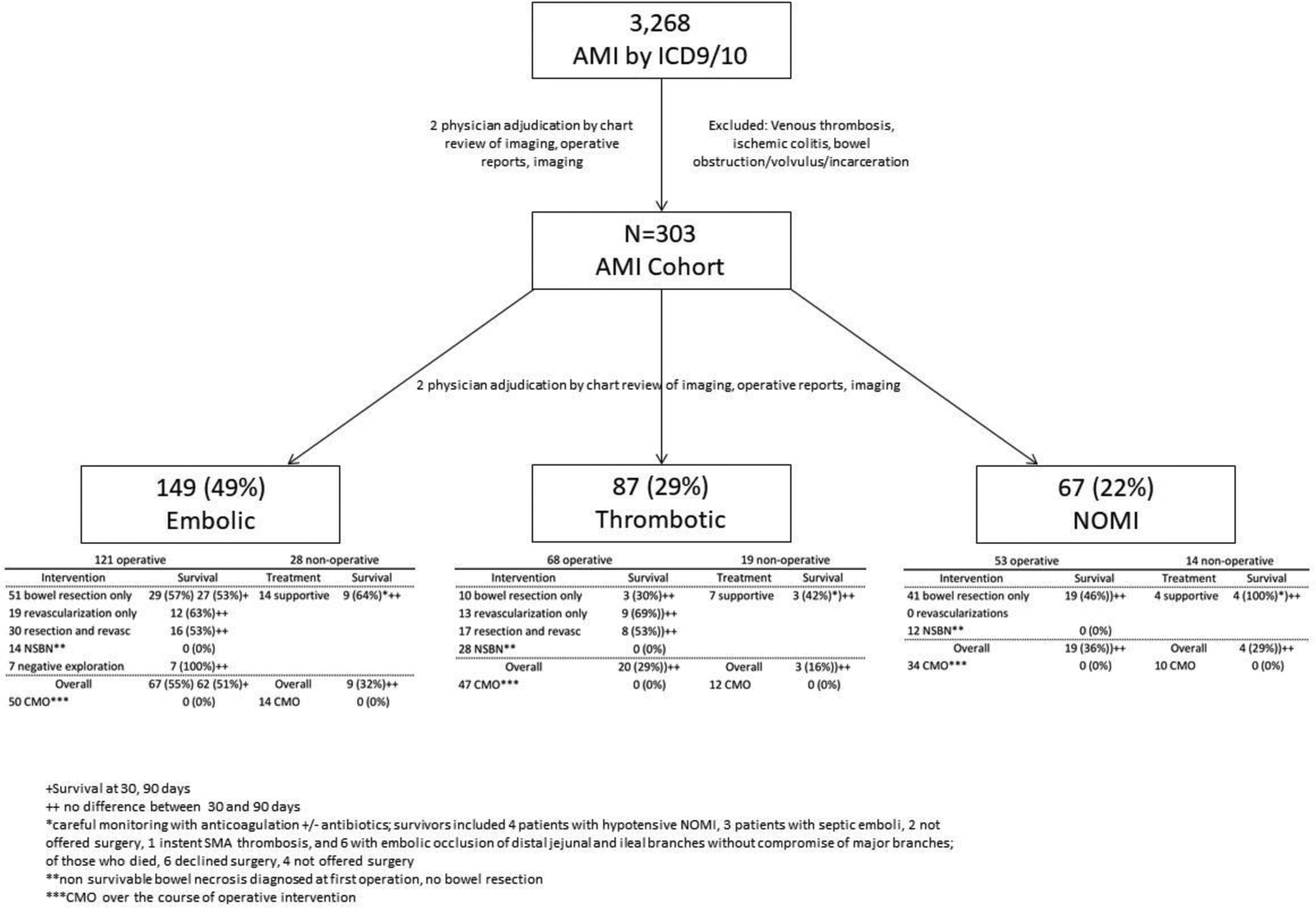
Cohort flowchart and management of patients presenting with acute mesenteric ischemia, stratified by etiology.
Among patients with follow-up, long-term gastrointestinal issues were identified by chart review and included chronic abdominal pain, colitis, nausea, vomiting, and/or diarrhea, not related to acute isolated events.
Statistical Analysis
All statistical analyses were performed using Stata/SE 13 software (StataCorp LLC, College Station, TX). Dichotomous variables are presented as a percentage of the cohort. Continuous, normally distributed variables are expressed as mean ± standard deviation while non-normally distributed variables are expressed as median and interquartile range (IQR). Univariate analysis was performed to compare baseline clinical and demographic features and postoperative complications between the two temporal cohorts. Temporal cohorts were stratified based on diagnosis date: prior to 2004 versus those presenting on January 1, 2004 or later. This date marks the two-year anniversary of the first endovascular treatment options offered at our institution. Two additional years were added to account for implementation and experience. Additional analysis was performed by accounting for AMI etiology. For each comparison: chi-squared test for categorical data, two-sided Student’s t-test for continuous, normally distributed variables, and Kruskal-Wallis test for continuous, non-normally distributed variables were conducted. Temporal trends were assessed and graphed from 2000 and beyond using ordered logistic regression. Time series line plot graphics were constructed using a uniformly weighted moving average of the expression with 2 lagged terms, current observation inclusion, and 2 lead terms. Multivariate logistic regression analysis was performed with implementation of dose-response curves for continuous variables to assess for predictors of mortality and NSBN. Survival was calculated using Kaplan-Meier life tables. A p-value of <.05 was considered significant.
RESULTS
Demographics and Comorbidities
Over the 23-year study period, a total of 3,269 patients were diagnosed with acute mesenteric ischemia. An overwhelming majority were assigned inaccurate ICD codes (1,919), with mechanical obstruction as the most common misdiagnosis or erroneous code. A total of 303 patients were defined to have acute mesenteric ischemia by physician adjudication. 159 (52%) presented prior to 2004 while 144 (48%) presented in 2004 or later. Average age was 72 (± 13) years (range 30 to 95). A majority of patients were female (55%) and Caucasian (86%) while a third were transferred from an outside facility. There were no differences between the temporal cohorts with respect to demographics. Seventy-four percent had hypertension, 50% had atrial fibrillation and 51% were former or active smokers. Patients presenting ≥2004 were more likely to have HTN (80%, vs 69%, p=.03), CKD (33%, vs 23%, p=.04), COPD (46%, vs 27%, p=.001), and smoking history (59%, vs 43%, p=.01). The contemporary cohort was more likely to be on beta blockade (67%, vs 36%, p<.001), statin (51%, vs 16%, p<.001), or antiplatelet therapy (56%, vs 35%, p<.001). (Table 1)
Table 1.
Demographic and clinical information of patients presenting with acute mesenteric ischemia, stratified by diagnosis date. CKD = chronic kidney disease; CHF = congestive heart failure; PVD = peripheral vascular disease; COPD = chronic obstructive pulmonary disease; CAD = coronary artery disease.
| Demographics | Total Cohort n=303 (100%) | Year < 2004 n=159 (52%) | Year ≥ 2004 n=144 (48%) | P |
|---|---|---|---|---|
| Age, years | 72.2 ± 13.3 | 71.6 ± 12.7 | 72.9 ± 13.9 | .40 |
| Sex, female | 168 (55%) | 89 (56%) | 79 (55%) | .85 |
| Caucasian race | 257 (86%) | 136 (87%) | 121 (85%) | .60 |
| Transfer, yes | 101 (33%) | 46 (29%) | 55 (38%) | .09 |
| Comorbidities | ||||
| Hypertension | 225 (74%) | 110 (69%) | 115 (80%) | .03 |
| CKD | 84 (28%) | 36 (23%) | 48 (33%) | .04 |
| CHF | 132 (44%) | 62 (39%) | 70 (49%) | .09 |
| PVD | 142 (47%) | 72 (45%) | 70 (49%) | .56 |
| COPD | 109 (36%) | 43 (27%) | 66 (46%) | .001 |
| Diabetes | 77 (25%) | 33 (21%) | 44 (31%) | .05 |
| CAD | 170 (56%) | 82 (52%) | 88 (61%) | .10 |
| Atrial fibrillation | 152 (50%) | 76 (48%) | 76 (53%) | .39 |
| Hyperlipidemia | 119 (39%) | 35 (22%) | 84 (58%) | <.001 |
| Any smoking history | 154 (51%) | 69 (43%) | 85 (59%) | .01 |
| Current smoker | 53 (17%) | 18 (11%) | 35 (24%) | .003 |
| Medications | ||||
| Beta blocker | 153 (51%) | 57 (36%) | 96 (67%) | <.001 |
| Anticoagulation | 70 (23%) | 32 (20%) | 38 (26%) | .20 |
| Statin | 98 (32%) | 25 (16%) | 73 (51%) | <.001 |
| Antiplatelet therapy, any | 136 (45%) | 56 (35%) | 80 (56%) | <.001 |
| Aspirin | 127 (42%) | 51 (32%) | 76 (53%) | <.001 |
Clinical Presentation and Management
Of the patients with AMI, 149 patients (49%) had an embolic etiology, while 87 (29%) presented with mesenteric thrombosis, and 67 (22%) had NOMI. The majority (n=242, 80%) underwent operative management. (Figure 1) Of those who did not undergo operative management 36 (60%) were CMO and the remainder elected for supportive treatment which consisted of anticoagulation, antibiotics and non-interventional supportive care. (Figure 1) Patients who presented in 2004 or later were less likely to undergo a procedure (74%, vs 86%, p=.004). More than half of patients (n=167, 55%) were made CMO during their hospitalization. Among patients who underwent non-operative management, the majority were made CMO and expired. When reviewing history of interventions, 41% (n=124) had a procedure within 30 days prior to AMI presentation. Open cardiac surgery was the most common intervention preceding AMI. (Table 2) Among the 87 patients in the thrombotic cohort, 27 (31%) had documented chronic abdominal pain and/or known imaging findings consistent with mesenteric vascular disease.
Table 2.
Presentation and management strategies of acute mesenteric ischemia, stratified by diagnosis date. NOMI = non-occlusive mesenteric ischemia; CMO = comfort measures only; PCI = percutaneous coronary intervention.
| Presentation and Management | Total Cohort n=303 (100%) | Year < 2004 n=159 (52%) | Year ≥ 2004 n=144 (48%) | P |
|---|---|---|---|---|
| Cause | .15 | |||
| Embolus | 149 (49%) | 83 (52%) | 66 (46%) | |
| Thrombus | 87 (29%) | 38 (24%) | 49 (34%) | |
| NOMI | 67 (22%) | 38 (24%) | 29 (20%) | |
| CMO | 167 (55%) | 87 (55%) | 80 (56%) | .88 |
| Operative management | 242 (80%) | 137 (86%) | 105 (74%) | .004 |
| Prior surgery/intervention within 30 days | 124 (41%) | 71 (45%) | 53 (37%) | .17 |
| Type* | .12 | |||
| Cardiac, open | 43 (35%) | 21 (30%) | 22 (42%) | |
| PCI | 23 (19%) | 14 (20%) | 9 (17%) | |
| Orthopedic | 8 (6%) | 2 (3%) | 6 (11%) | |
| Vascular | 37 (30%) | 26 (37%) | 11(21%) | |
| Other | 13 (10%) | 8 (11%) | 5 (9%) |
N = 124, < 2004 n = 71, ≥ 2004 n = 53. Percentage calculations based on patients who had a documented procedure within 30 days of diagnosis.
Operative Management
A total of 345 procedures were performed in 242 patients. One patient died on the operating room table prior to incision. Among patients who underwent operative intervention (n=242, 80%), over half (n=131, 54%) were ultimately made CMO and comprised of patients who had NSBN and those whose clinical course after intervention deteriorated to futility or family decided to withdraw care. (Figure 1) Characteristics of intervention included: 18 (5%) endovascular, 321 (93%) open and 6 (2%) hybrid. Revascularizations performed included 54 embolectomies, 7 patch angioplasties, 10 antegrade bypasses, 4 retrograde bypasses, 8 lytic procedures and 11 angioplasties with stenting. Patients in the later ≥2004 cohort were more likely to undergo endovascular and hybrid interventions compared to the <2004 cohort (p=.001). Of those who underwent endovascular-only procedures, lysis was employed in 6 cases and mechanical thrombectomy in 5 cases, with 2 cases of simultaneous stenting. Just under a third of patients (n=76) who underwent initial operative management also underwent a second interval procedure. The majority of those who underwent a secondary procedure (n=49/76, 64%) required bowel resection and/or revascularization. (Table 3) Amongst those who underwent a revascularization during the second procedure, adequate perfusion was evaluated by palpation and doppler signal evaluation at the mesenteric and anti-mesenteric portions of the bowel during the first exploration and revascularization was determined to be not indicated at the time. Twenty-seven patients underwent a third interval operative intervention.
Table 3.
Operative characteristics of patients presenting with acute mesenteric ischemia that underwent a procedure, stratified by diagnosis date.
| Characteristics of Patients Undergoing Operative Management | Total Cohort n=242 (100%) | Year < 2004 n=137 (57%) | Year ≥ 2004 n=105 (43%) | P |
|---|---|---|---|---|
| CMO | 131 (54%) | 77 (56%) | 54 (51%) | .46 |
| Surgical approach | .001 | |||
| Endovascular | 14 (6%) | 3 (2%) | 11 (10%) | |
| Open | 223 (92%) | 134 (98%) | 89 (85%) | |
| Hybrid | 5 (2%) | 0 (0%) | 5 (5%) | |
| Non-survivable bowel necrosis* | 51 (22%) | 33 (25%) | 18 (19%) | .33 |
| Second procedure performed | 76 (31%) | 35 (26%) | 41 (39%) | .03 |
| Needed intervention** | 49 (64%) | 22 (63%) | 27 (64%) | .77 |
n = 228, T<2004 n = 134, T≥2004 n = 94. Only applicable to patients undergoing hybrid or open procedures.
n = 77, T < 2004 n = 35, T ≥ 2004 n = 42. Only applicable to patients undergoing a second procedure.
189 patients who had AMI of embolic/thrombotic etiology were managed operatively: 39 (21%) had initial findings of NSBN; 85 (45%) underwent mesenteric revascularization. 7 patients who had AMI diagnosed by clinical symptoms and CTA showing embolus had no sign of ischemic bowel on exploration. (Figure 1) The remainder underwent exploratory laparotomy with resection of nonviable bowel. Median time from symptom onset to intervention (in days) was: embolic 1 (1,2), thrombotic 1.5 (1,3), and NOMI 1 (1,4), p=.076. As expected, none of the 53 NOMI patients who underwent operative management had attempted mesenteric revascularization.
Among the 228 patients who underwent initial hybrid/open intervention, 134 (59%) had bowel resected during the first procedure. A total of 51 (22%) had NSBN upon entering the abdomen. Percentage of exploratory laparotomies with NSBN findings, stratified by cause was: embolic (12%), thrombotic (40%), and NOMI (22%).
Mortality and Outcomes
Overall 30-day mortality from day of diagnosis was 61%, without significant change between temporal cohorts (p=0.42). (Table 4) Average length of stay (LOS) was 10 days (IQR 4, 21), one-year survival was 28%, and overall survival at 3 and 5 years was 22% and 17%, respectively, without significant differences between temporal cohorts. Among the 130 patients who did not die within 30-days, 21% (n=27) were readmitted to the hospital within 30 days of discharge. Five were admitted for vascular occlusive complications while 11 were admitted for GI-related issues. By one year, 213 patients had died and 13 were lost to follow-up. Among the remaining 77 patients, 49 were readmitted within a year. When patients were stratified by AMI cause, 30-day survival was 34%, 20%, and 24% among embolic, thrombotic, and NOMI, respectively (p=.04). (Figure 2)
Table 4.
30-day complications of patients presenting with acute mesenteric ischemia, stratified by diagnosis date.
| 30-Day Complications | Total Cohort n=303(100%) | Year < 2004 n=159 (52%) | Year ≥ 2004 n=144 (48%) | P |
|---|---|---|---|---|
| Urinary tract infection | 44 (15%) | 24 (15%) | 20 (14%) | .77 |
| Pneumonia | 80 (26%) | 44 (28%) | 36 (25%) | .60 |
| Myocardial infarction | 41 (14%) | 28 (18%) | 13 (9%) | .03 |
| Clostridium difficile colitis | 19 (6%) | 11 (7%) | 8 (6%) | .63 |
| Mortality | 185 (61%) | 102 (64%) | 83 (58%) | .25 |
| Major adverse event, any | 191 (63%) | 100 (62%) | 91 (63%) | .96 |
Figure 2.
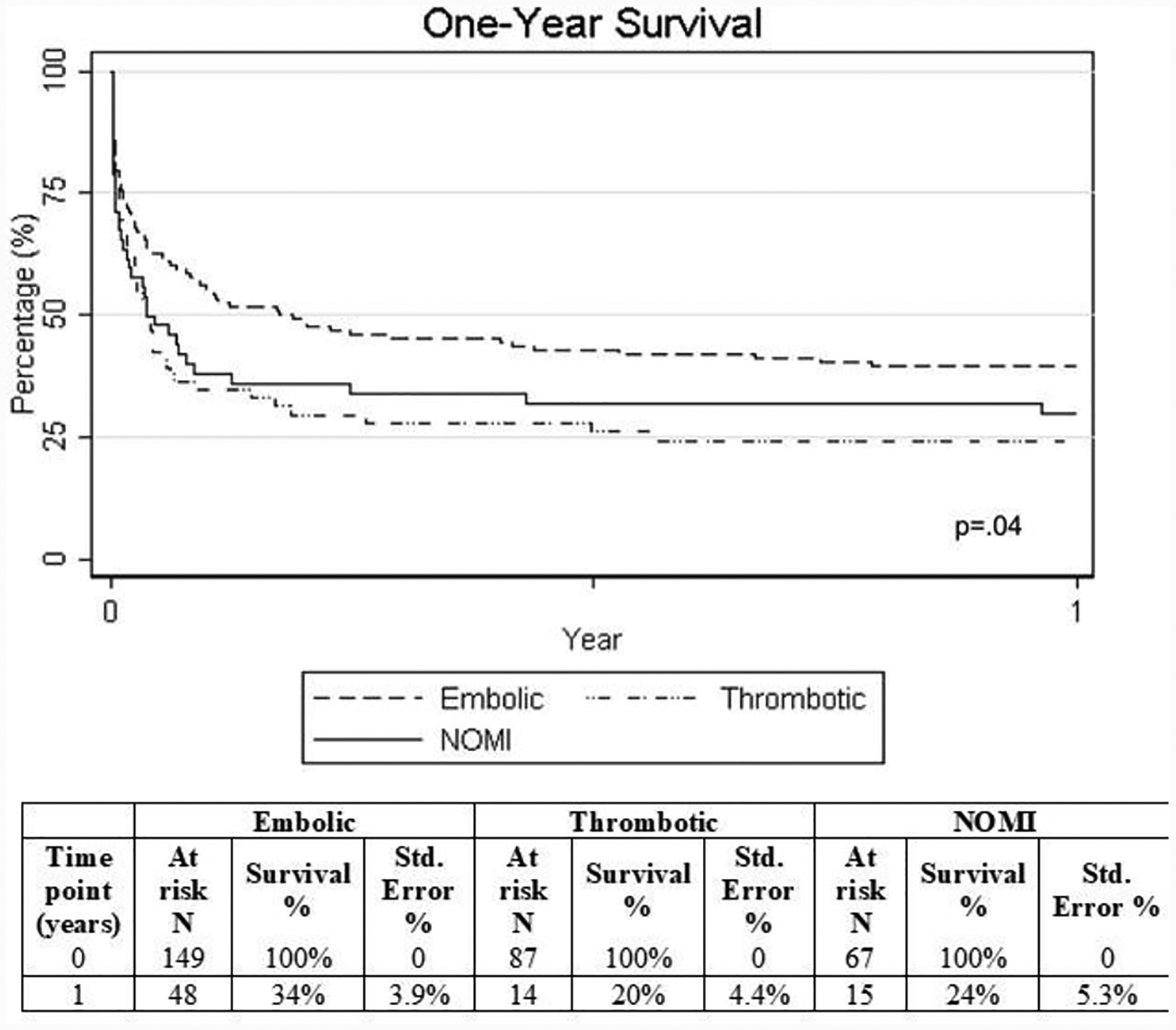
Kaplan-Meier curves and life tables for 1-year survival in patients presenting with acute mesenteric ischemia, stratified by pathophysiology.
In the embolic and thrombotic cohorts, there was a significant difference in 30-day mortality between patients who underwent revascularization versus those who did not. The difference is attributed findings of NSBN on exploration. Amongst those who underwent revascularization, 36 out of 85 (42%) expired. Among the thrombotic population, the 30-day mortality decreased from 82% to 47% in those who were revascularized.
There was a trend towards increased 30-day survival as the time from symptoms to operative intervention increased, however no statistically significant dose response curve was observed for this trend nor was the time variable significant when included categorically in logistic regression analysis. Among the 58 patients who survived beyond three years and were not lost to follow-up, 17 (29%) had chronic GI issues, such as chronic abdominal pain, recurrent colitis, nausea, vomiting, and/or diarrhea, unrelated to acute isolated events.
Temporal Trends
Using ordered logistic regression to assess temporal trends, 30-day mortality remained stable over time (67% → 57%, p=.91) as did the number of patients made CMO (62% → 56%, p=.07). The number of patients who underwent initial exploratory laparotomy and had findings of NSBN also remained stable over time (22% → 19%, p=.75). The percentage of patients who underwent operative management decreased over time (81% → 61%, p=.01) while the number of patients who underwent interval second procedures/interventions increased (24% → 56%, p=.02). The decrease in initial operative management correlated to increased CMO status prior to surgical intervention (50% → 70%, p=.02). The percentage of embolic events as primary cause for AMI decreased over time (62% → 50%, p=.04) while there was a simultaneous trend towards increased pre-diagnosis anti-coagulation use (14% → 28%, p=.056). (Figure 3) Incidence of thrombotic AMI increased over time (15% → 31%, p=.03).
Figure 3.
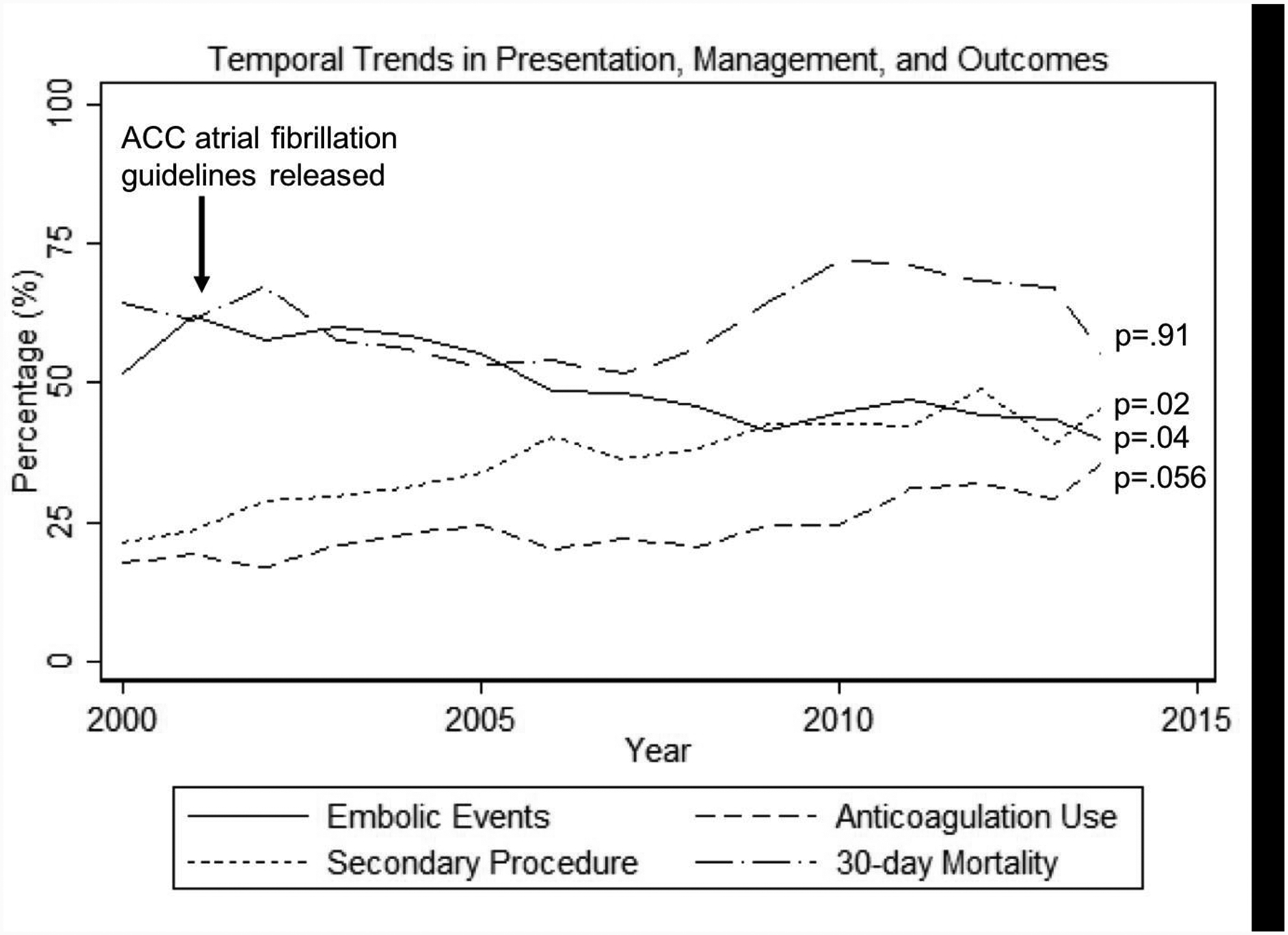
Temporal trends in presentation, management, and outcomes of patients with acute mesenteric ischemia.
Predictors
Significant factors on univariate analysis were included in the logistic regression models and significant predictors of 30-day mortality were: age ≥ 60 years (odds ratio (OR) 5.5, 95% confidence interval (95% CI) 1.6 – 19.5, p=.01), prior surgery/intervention with 30-days of diagnosis (OR 3.4, 95% CI 1.3 – 8.8, p=.01), lactate ≥ 2.3 mmol/L (OR 2.8, 95% CI 1.02 – 7.7, p=.045), and white blood count ≥ 25 K/ μL (OR 3.0, 95% CI 1.1 – 8.2, p=.002). (Table 5) Predictors of intraoperative findings of NSBN included thrombotic cause (OR 3.8, 95% CI 1.03 – 13.6, p=.04), lactate ≥ 3.8 mmol/L (OR 3.6, 95% CI 1.1 – 12.1, p=.04), and white blood count ≥ 24 K/ μL (OR 3.4, 95% CI 1.1 – 10.5, p=.03). (Table 6)
Table 5.
Predictors of 30-day mortality. WBC = white blood count. Lactate in mmol/L. WBC in K/ μL.
| Risk Factor | OR | 95% CI | P |
|---|---|---|---|
| Age ≥ 60 | 5.5 | 1.6 – 19.5 | .01 |
| Previous surgery* | 3.4 | 1.3 – 8.8 | .01 |
| Lactate ≥ 2.3 | 2.8 | 1.02 – 7.7 | .045 |
| WBC ≥ 25 | 3.0 | 1.1 – 8.2 | .002 |
Surgery within 30 days of presentation/diagnosis of AMI.
Table 6.
Predictors of non-survivable bowel necrosis. WBC = white blood count. Lactate in mmol/L. WBC in K/μL.
| Risk Factor | OR | 95% CI | P |
|---|---|---|---|
| Thrombosis | 3.8 | 1.03 – 13.6 | .04 |
| Diabetes | 0.2 | 0.03 – 0.99 | .048 |
| Lactate ≥ 3.8 | 3.6 | 1.1 – 12.1 | .04 |
| WBC ≥ 24 | 3.4 | 1.1 – 10.5 | .03 |
DISCUSSION
This study evaluated 3,268 patients diagnosed with AMI over 23 years and ultimately 303 patients who presented with physician-adjudicated AMI. Over two-thirds of patients were misdiagnosed with AMI. Of those with true AMI, nearly half presented with embolic phenomenon as the primary cause. The percentage of embolic phenomenon, however, appeared to decrease with the initiation of American College of Cardiology (ACC) recommendations on anticoagulation for atrial fibrillation. (Figure 2) Revascularization was associated with survival benefit. Although a large proportion of patients continue to undergo futile open exploration with findings of NSBN, the percentage of patients offered initial operative management has decreased – a finding which appears to correlate with increased CMO status shortly after presentation and diagnosis. Finally, despite advancements in care, 30-day mortality rates remain high at 61%, without significant differences between temporal cohorts.
In this cohort, patients presenting with acute thrombosis were found to have decreased survival compared to those with NOMI or embolus, consistent with prior studies and meta-analyses.7 This is further supported by anatomic autopsy studies where thrombotic occlusions were more likely to be located more proximally than embolic lesions and be associated with prior remote infarcts, aortic wall thrombosis and thus confer more extensive intestinal infarction.10 These findings support that of our cohort, as 40% of exploratory laparotomies with NSBN were due to thrombosis compared with 12% and 22% of embolic and NOMI patients, respectively (p<.001). While not statistically significant, median time from symptom onset intervention was approximately 12 hours longer in the thrombotic cohort. Additionally, nearly a third of thrombotic patients carried a diagnosis of chronic abdominal pain and/or mesenteric vascular disease. These observations suggest that delays in the diagnosis and treatment of patients with acute thrombosis may play a role in the outcomes observed, however further study is warranted.
The recognition and definition of acute mesenteric ischemia is difficult, as evidenced by the number of misdiagnoses in this cohort and the relatively few true AMI events. Incidence of AMI, however, continues to increase with the aging population4 and is more common than appendicitis in the elderly.3, 6 A thorough clinical examination and computer tomography angiography (CTA) allows diagnosis of primary AMI with sensitivity and specificity of 96% and 94%, respectively. Diminished or absent bowel wall enhancement, arterial filling defects and mesenteric haziness have all been described in assisting with the diagnosis of hypoperfusion associated with AMI.11–13 Fundamentally, however, evaluation for AMI depends on an ill-described level of clinical suspicion for AMI, prompting imaging evaluation. There is increasing focus on the development of biomarkers to aid front-line physicians in diagnosing AMI, similar to the use of troponins for myocardial infarction. Consistent with our findings, elevated serum lactate and high WBC, while predictive of intestinal necrosis, are a late finding in AMI, and predictive of NSBN and mortality.2, 14, 15 Biomarkers with improved specificity to reflect bowel-related ischemia such as intestinal fatty acid-binding protein (I-FABP), ischemia modified albumin (IMA) and smooth muscle protein 22kDa (SM22) show promise in small trials.16
To address poor outcomes associated with late recognition, diagnosis and incomplete treatment of acute mesenteric ischemia,17, 18 centers of excellence with dedicated intestinal stroke centers have highlighted the effectiveness of a multimodal approach focusing on: 1) removal of nonviable segments of ischemic bowel 2) preservation of non-necrotic intestine with revascularization 3) medical treatment to prevent progression to multi-organ failure. Utilizing this approach, Corcos et al reported a 95% 30-day survival in a small single-center pilot study of 18 patients presenting with occlusive AMI.19 Consistent with our data, the importance of revascularization, is highlighted.
Options for revascularization include both open and endovascular approaches. Several papers suggest reduced complications and favorable outcomes with endovascular therapy as a primary modality for AMI. In a single-center study of 70 AMI patients, Arthurs et al describe a patient mortality of 36% in those undergoing endovascular repair compared to 50% in those who underwent open repair (p<.05).20 Using the National Inpatient Sample database, Beaulieu et al analyzed 4665 patients who underwent vascular intervention for AMI and report an increase in the proportion of endovascular procedures over the 2005 to 2009 study period, as well as a mortality benefit in those undergoing endovascular (25%) versus open (39%) repair (p=.01).21
While these outcomes reported are more favorable than in our cohort, these studies and similar contemporary investigations rely on procedural coding rather than diagnosis coding to identify patients with AMI and may incompletely capture the entire AMI population.21–23 As demonstrated in our study, a primary challenge for investigating AMI lies in its frequent misdiagnosis or erroneous coding, making population studies inherently difficult and inaccurate. Relying on procedural and billing codes may have the benefit of accuracy, but may not fully characterize the full patient population.
An often overlooked, but important, consideration in the management of AMI is careful evaluation of prognosis and advanced directives. Over time, we observed a decrease in the percentage of patients who underwent operative management of AMI. This was correlated with an increase in the number of documented goals of care discussions and increased rates of CMO status prior to surgical intervention (50% → 70%, p=.02). In a similar trend, official work groups from the American College of Surgeons and World Society of Emergency Surgery created guidelines to prioritize and highlight patient-oriented surgical decision-making with an emphasis on end-of-life goals of care discussions, with specific focus on acute mesenteric ischemia and associated prognosis and advanced directives to determine whether comfort carries the best treatment.24, 25 Determining which patients may benefit from intervention versus those who should undergo comfort measures only remains very difficult. Most of the current literature investigate outcomes after intervention only, due to ease of identification from procedure codes. The resulting lack of information on use of comfort measures or palliation in the AMI patient population remains a largely unexplored area of AMI. Our study provides a unique perspective on the high proportion of patients that eventually undergo CMO during their treatment course. Unfortunately, our cohort remains limited in formal recommendations on which patients should undergo intervention versus palliation due to limited sample size. Further studies are needed to parameter to better establish future clinical practice guidelines for AMI that include intervention and palliation.
There are several limitations to this study. Although this study is one of the largest studies on AMI, it is retrospective, from a single institutional and subject to bias. The high early mortality in this population make assessments of mid- and long-term outcomes associated with AMI challenging. Furthermore, due to lack of granularity on exact onset of symptoms and presentation, a precise analysis correlating timing of intervention with outcomes is not possible. It is also not always feasible to differentiate between thrombotic and embolic causes of occlusion in AMI, especially in patients with existing significant calcific mesenteric arterial disease. While this ambiguity represents a source of bias, a single reviewer (Chou) determined the etiologies with the adjudication of additional reviewer (Wang) for questionable cases, thus limiting inter-reviewer variability. Due to medical record constraints over two decades patient data chart review, some details may not have been fully captured, such as nuanced details in clinical presentation of embolic versus thrombotic events or in the type of vasopressors used or resuscitation efforts employed within the intensive care units. There are also several sources of potential bias that may influence this study. The observed trends of decreased operative intervention and increased CMO status may reflect a surgeon selection bias for survivability and not necessarily a reflection of the effectiveness of an operative intervention. It is possible that the true number of CMO patients with AMI is even higher than suggested in this cohort due to lack of confirmatory surgery or imaging for diagnosis due to critical illness.
There may also be a yet to be elucidated relationship between surgical specialty and frequency of second look operations and endovascular therapies. In our cohort both general surgeons and vascular surgeons conduct open procedures while endovascular interventions are nearly exclusively performed by the vascular surgeons or interventionalists. Coordinating these multidisciplinary efforts may affect patient outcomes and warrant further study. Finally, this study also reveals the gross misdiagnosis and erroneous coding of AMI, making retrospective or even prospective use of large datasets using ICD coding infeasible. Efforts to reconcile these inaccuracies are needed accurately investigate and improve outcomes of this mortal but infrequent disease.
CONCLUSIONS
In conclusion, AMI continues to be associated with poor prognosis with high 30-day mortality rates. There is a survival benefit in patients who undergo revascularization, which support early vascular intervention, but this finding warrants further investigation. The importance of early diagnosis, prognostication and advanced directives is highlighted given the high morbidity, mortality and use of comfort measures associated with AMI.
HIGHLIGHTS:
Acute mesenteric ischemia continues to confer high rate of mortality and morbidity
Etiology of acute mesenteric ischemia affects outcomes, with the incidence of embolic etiology decreasing over time
There is a survival benefit to timely revascularization
ACKNOWLEDGEMENTS
ELC is supported by National Institutes of Health, Grant/Award Number: T32HL007208.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 46th Annual Meeting of the New England Society for Vascular Surgery, Providence, RI, September 13–15, 2019
DECLARATIONS OF INTEREST: none
REFERENCES
- 1.Kassahun WT, Schulz T, Richter O, Hauss J. Unchanged high mortality rates from acute occlusive intestinal ischemia: Six year review. Langenbecks Arch Surg. 2008;393:163–171 [DOI] [PubMed] [Google Scholar]
- 2.Nuzzo A, Maggiori L, Ronot M, Becq A, Plessier A, Gault N, et al. Predictive factors of intestinal necrosis in acute mesenteric ischemia: Prospective study from an intestinal stroke center. Am J Gastroenterol. 2017;112:597–605 [DOI] [PubMed] [Google Scholar]
- 3.Karkkainen JM, Lehtimaki TT, Manninen H, Paajanen H. Acute mesenteric ischemia is a more common cause than expected of acute abdomen in the elderly. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2015;19:1407–1414 [DOI] [PubMed] [Google Scholar]
- 4.Stoney RJ, Cunningham CG. Acute mesenteric ischemia. Surgery. 1993;114:489–490 [PubMed] [Google Scholar]
- 5.Fuster V, Ryden LE, Asinger RW, Cannom DS, Crijns HJ, Frye RL, et al. Acc/aha/esc guidelines for the management of patients with atrial fibrillation: Executive summary a report of the american college of cardiology/american heart association task force on practice guidelines and the european society of cardiology committee for practice guidelines and policy conferences (committee to develop guidelines for the management of patients with atrial fibrillation) developed in collaboration with the north american society of pacing and electrophysiology. Circulation. 2001;104:2118–2150 [PubMed] [Google Scholar]
- 6.Mastoraki A, Mastoraki S, Tziava E, Touloumi S, Krinos N, Danias N, et al. Mesenteric ischemia: Pathogenesis and challenging diagnostic and therapeutic modalities. World J Gastrointest Pathophysiol. 2016;7:125–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schoots IG, Koffeman GI, Legemate DA, Levi M, van Gulik TM. Systematic review of survival after acute mesenteric ischaemia according to disease aetiology. The British journal of surgery. 2004;91:17–27 [DOI] [PubMed] [Google Scholar]
- 8.Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379:165–180 [DOI] [PubMed] [Google Scholar]
- 9.Rutherford RB. Vascular surgery. Philadelphia: Saunders; 2005. [Google Scholar]
- 10.Acosta S, Ogren M, Sternby NH, Bergqvist D, Bjorck M. Clinical implications for the management of acute thromboembolic occlusion of the superior mesenteric artery: Autopsy findings in 213 patients. Annals of surgery. 2005;241:516–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furukawa A, Kanasaki S, Kono N, Wakamiya M, Tanaka T, Takahashi M, et al. Ct diagnosis of acute mesenteric ischemia from various causes. AJR. American journal of roentgenology. 2009;192:408–416 [DOI] [PubMed] [Google Scholar]
- 12.Kirkpatrick ID, Kroeker MA, Greenberg HM. Biphasic ct with mesenteric ct angiography in the evaluation of acute mesenteric ischemia: Initial experience. Radiology. 2003;229:91–98 [DOI] [PubMed] [Google Scholar]
- 13.Menke J Diagnostic accuracy of multidetector ct in acute mesenteric ischemia: Systematic review and meta-analysis. Radiology. 2010;256:93–101 [DOI] [PubMed] [Google Scholar]
- 14.Paladino NC, Inviati A, Di Paola V, Busuito G, Amodio E, Bonventre S, et al. Predictive factors of mortality in patients with acute mesenteric ischemia. A retrospective study. Ann Ital Chir. 2014;85:265–270 [PubMed] [Google Scholar]
- 15.Kougias P, Lau D, El Sayed HF, Zhou W, Huynh TT, Lin PH. Determinants of mortality and treatment outcome following surgical interventions for acute mesenteric ischemia. Journal of vascular surgery. 2007;46:467–474 [DOI] [PubMed] [Google Scholar]
- 16.Acosta S, Nilsson T. Current status on plasma biomarkers for acute mesenteric ischemia. J Thromb Thrombolysis. 2012;33:355–361 [DOI] [PubMed] [Google Scholar]
- 17.Brandt LJ, Boley SJ. Aga technical review on intestinal ischemia. American gastrointestinal association. Gastroenterology. 2000;118:954–968 [DOI] [PubMed] [Google Scholar]
- 18.Gupta PK, Natarajan B, Gupta H, Fang X, Fitzgibbons RJ Jr. Morbidity and mortality after bowel resection for acute mesenteric ischemia. Surgery. 2011;150:779–787 [DOI] [PubMed] [Google Scholar]
- 19.Corcos O, Castier Y, Sibert A, Gaujoux S, Ronot M, Joly F, et al. Effects of a multimodal management strategy for acute mesenteric ischemia on survival and intestinal failure. Clin Gastroenterol Hepatol. 2013;11:158–165 e152 [DOI] [PubMed] [Google Scholar]
- 20.Arthurs ZM, Titus J, Bannazadeh M, Eagleton MJ, Srivastava S, Sarac TP, et al. A comparison of endovascular revascularization with traditional therapy for the treatment of acute mesenteric ischemia. Journal of vascular surgery. 2011;53:698–704; discussion 704–695 [DOI] [PubMed] [Google Scholar]
- 21.Beaulieu RJ, Arnaoutakis KD, Abularrage CJ, Efron DT, Schneider E, Black JH 3rd. Comparison of open and endovascular treatment of acute mesenteric ischemia. Journal of vascular surgery. 2014;59:159–164 [DOI] [PubMed] [Google Scholar]
- 22.Schermerhorn ML, Giles KA, Hamdan AD, Wyers MC, Pomposelli FB. Mesenteric revascularization: Management and outcomes in the united states, 1988–2006. Journal of vascular surgery. 2009;50:341–348 e341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swerdlow NJ, Varkevisser RRB, Soden PA, Zettervall SL, McCallum JC, Li C, et al. Thirty-day outcomes after open revascularization for acute mesenteric ischemia from the american college of surgeons national surgical quality improvement program. Annals of vascular surgery. 2019;61:148–155 [DOI] [PubMed] [Google Scholar]
- 24.Bala M, Kashuk J, Moore EE, Kluger Y, Biffl W, Gomes CA, et al. Acute mesenteric ischemia: Guidelines of the world society of emergency surgery. World journal of emergency surgery : WJES. 2017;12:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Surgeons Palliative Care W. Office of promoting excellence in end-of-life care: Surgeon’s palliative care workgroup report from the field. Journal of the American College of Surgeons. 2003;197:661–686 [DOI] [PubMed] [Google Scholar]


