Abstract
Background:
People post-stroke often walk with a spatiotemporally asymmetric gait, due in part to sensorimotor impairments in the paretic lower extremity. Although reducing asymmetry is a common objective of rehabilitation, the effects of improving symmetry on balance have yet to be determined.
Objective:
We established the concurrent validity of whole-body angular momentum as a measure of balance and we determined if reducing step length asymmetry would improve balance by decreasing whole-body angular momentum.
Methods:
We performed clinical balance assessments and measured whole-body angular momentum during walking using a full-body marker set in a sample of 36 people with chronic stroke. We then used a biofeedback-based approach to modify step length asymmetry in a subset of 15 of these individuals and we measured the resulting changes in whole-body angular momentum.
Results:
When participants walked without biofeedback, whole-body angular momentum in the sagittal and frontal plane was negatively correlated with scores on the Berg Balance Scale and Functional Gait Assessment supporting the validity of whole-body angular momentum as an objective measure of dynamic balance. We also observed that when participants walked more symmetrically, their whole-body angular momentum in the sagittal plane increased rather than decreased.
Conclusions:
Voluntary reductions of step length asymmetry in people post-stroke resulted in reduced measures of dynamic balance. This is consistent with the idea that after stroke, individuals might have an implicit preference not to deviate from their natural asymmetry while walking because it could compromise their balance. Clinical Trials Number: NCT03916562
Introduction
Neuromotor impairments associated with stroke often result in asymmetric walking patterns, and reducing step length asymmetry is a common objective of rehabilitation 1–3. However, the effects of reducing asymmetry on the energy cost of walking post-stroke are heterogeneous 4,5 and depend on individual differences in the level of impairment 6. These findings demonstrate that reducing step length asymmetry in does not always lead to reductions in energetic cost. One alternative advantage of reducing step length asymmetry is that it may improve balance and reduce fall risk, which is critically important post-stroke 7,8. One recent study found that people with lower scores on the Berg Balance Scale (BBS) and greater step widths had more asymmetric step lengths and swing times during walking 9. However, the BBS and measures of step width provide a limited and indirect assessment of dynamic balance during walking. Therefore, it is unclear if step length asymmetry is associated with objective measures of dynamic balance during walking or if reducing asymmetry would improve dynamic balance in both sagittal and frontal planes.
One way to characterize dynamic balance during walking is by measuring the rotational dynamics of the body using measures of whole-body angular momentum 10–14. Whole-body angular momentum captures the combined effects of the rotational dynamics of the body and the relative rotations of the limbs about an axis of rotation that is commonly considered to project through the body’s center of mass (CoM). People without disabilities regulate their balance, in part, by maintaining the peak-to-peak range of angular momentum during walking within a small range 10,11. In addition, angular momentum in the frontal plane has been validated as a measure of mediolateral balance in people post-stroke as higher momentum is associated with lower scores on clinical balance assessments 13,14.
People post-stroke have a higher peak-to-peak range of whole-body angular momentum in both the frontal plane 13 and the sagittal plane 12 compared to age- and speed-matched controls. Increased frontal plane momentum may result from compensatory strategies such as lateral trunk lean, hip hiking, and circumduction 13,15,16. In the sagittal plane, regulation of angular momentum is achieved by the cancellation of limb angular momenta between sides (Herr and Popovic, 2008). Cancellation of angular momenta in the sagittal plane occurs when contralateral limb segments move in anti-phase. This corresponds to a kinematic pattern where the moment of peak flexion for segments on one side of the body happens at the same time as the moment of peak extension for contralateral segments and vice-versa. However, if the contralateral limb segments do not move in anti-phase, the degree of segmental angular momenta cancellation decreases, and whole-body angular momentum increases 17. Angular momentum in the frontal plane is primarily regulated by anti-phase movement of trunk and swing leg during mediolateral weight shift 11. Therefore, one might expect to observe greater angular momentum during walking in stroke survivors in part because their gait asymmetries may reduce the sagittal plane momentum cancellation between the left and right limbs. Although asymmetries may increase angular momentum in the sagittal plane, it has yet to be determined if restoring symmetry could reduce angular momentum.
The strategy that people post-stroke use to reduce step length asymmetry may vary widely due to heterogeneity in the level of motor impairment and in the direction and magnitude of step length asymmetry. Asymmetries in step length and step timing can result from difficulty advancing the paretic leg 18,19, reduced propulsion with the paretic leg 20,21, or both. Depending on the source of impairment, people post-stroke may walk with asymmetries characterized by either longer step lengths with the paretic or non-paretic leg (Finley et al., 2015; Roerdink and Beek, 2011; Sánchez and Finley, 2018). People post-stroke retain the capacity to reduce this step length asymmetry, but this capacity depends on the magnitude and direction of asymmetry 6. Therefore, the strategies used to reduce step length asymmetry are not consistent across the post-stroke population. For example, individuals who have longer step lengths on the paretic side may need to advance the non-paretic limb and increase paretic stance time 24 to reduce step length asymmetry. In contrast, individuals with longer non-paretic steps may rely on strategies such as increasing mediolateral trunk lean 25, hip hiking, or circumduction 15,16 to advance the paretic limb to compensate for weakness of the paretic hip and knee flexors (Balasubramanian et al., 2007; Hsu et al., 2003; Olney and Richards, 1996; Roerdink and Beek, 2011) inadequate ankle plantar flexor power generation 29, or spasticity in the quadriceps or hamstrings 30. What remains to be seen is if reducing step length asymmetry alters dynamic balance and whether those effects depend on the direction of each individual’s asymmetry.
The primary goal of this work is to determine whether reducing step length asymmetry improves dynamic balance by decreasing whole-body angular momentum. Before addressing this goal, we first established the concurrent validity of whole-body angular momentum as a measure of balance for people post-stroke. If angular momentum is a valid measure of balance during walking, the baseline angular momentum in both the sagittal and frontal planes would be inversely correlated with clinical assessments of balance and balance confidence such that a higher angular momentum would be associated with lower balance measures. We also tested the hypothesis that participants would reduce angular momentum during each stride as step length asymmetry decreases. This would be consistent with the notion that reductions in asymmetry would improve dynamic balance. We expected that participants who walked with longer non-paretic step lengths would show smaller reductions in angular momentum compared to participants who walked with longer paretic step lengths given that they would rely more on compensatory strategies to advance the paretic limb when reducing asymmetry. In the frontal plane, we hypothesized that participants would increase mediolateral peak angular momentum as step length asymmetry decreases as participants might rely on compensatory strategies such as circumduction to increase step length.
Methods
Participants
A total of 36 individuals post-stroke (92 ± 84.5 months post-stroke) (Table 1) participated in this study. We computed a necessary sample size of 20 from a set of pilot data where we observed a mean difference in the peak angular momentum in the sagittal plane of 0.004 during asymmetric versus symmetric walking with a standard deviation of 0.006. Inclusion criteria for this study were 1) a history of unilateral stroke more than six months before the study, 2) paresis confined to one side, 3) ability to provide informed consent and communicate with the investigators, 4) ability to walk more than five minutes without the assistance of another individual or walking aids (e.g., a cane or walker). The use of an ankle-foot orthosis was permitted during the experiment. All experimental procedures were approved by the University of Southern California Institutional Review Board, and each participant provided written, informed consent before the experiment began. All aspects of the study conformed to the principles described in the Declaration of Helsinki.
Table 1.
Participants demographics
| Subject | Sex | Age | Months | Paretic side | ABC scale | Fall Efficacy | Berg Balance | FGA | FM-LE | Overground Speed (m/s) | Direction SLA | Fall History | Treadmill speed (m/s) | Baseline Step length asymmetry |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 66 | 394 | R | 90 | 20 | 53 | 19 | 22 | 0.83 | LNP | No | 0.4 | 0.02 |
| 2 | M | 60 | 127 | L | 87.5 | 18 | 45 | 14 | 15 | 0.94 | LP | Yes | 0.55 | −0.12 |
| 3 | F | 57 | 114 | R | 77 | 22 | 50 | 20 | 30 | 0.66 | LP | No | 0.4 | −0.21 |
| 4 | M | 50 | 86 | R | 75 | 26 | 53 | 24 | 29 | 0.915 | LNP | No | 0.62 | 0.00 |
| 5 | M | 75 | 173 | L | 75.7 | 41 | 54 | 23 | 28 | 0.63 | LNP | Yes | 0.65 | 0.02 |
| 6 | F | 59 | 74 | R | 66 | 24 | 49 | 17 | 26 | 0.9 | LNP | Yes | 0.55 | 0.01 |
| 7 | M | 78 | 103 | R | 89 | 17 | 53 | 23 | 34 | 1.3 | LNP | Yes | 0.8 | 0.01 |
| 8 | M | 63 | 189 | R | 83 | 23 | 49 | 16 | 29 | 0.79 | LP | No | 0.38 | −0.05 |
| 9 | M | 35 | 65 | R | 80 | 27 | 55 | 29 | 30 | 0.97 | LNP | Yes | 0.9 | 0.04 |
| 10 | M | 63 | 80 | R | 66 | 23 | 47 | 29 | 30 | 1.01 | LP | No | 0.65 | −0.08 |
| 11 | M | 71 | 123 | L | 56 | 36 | 40 | 11 | 29 | 0.44 | LNP | No | 0.3 | 0.38 |
| 12 | M | 73 | 55 | L | 73 | 28 | 54 | 20 | 28 | 0.9 | LP | No | 0.6 | −0.07 |
| 13 | M | 63 | 127 | L | 65 | 26 | 44 | 15 | 20 | 0.53 | LNP | yes | 0.45 | 0.01 |
| 14 | M | 54 | 16 | L | 53 | 42 | 48 | 22 | 24 | 0.7 | LP | Yes | 0.5 | −0.18 |
| 15 | M | 60 | 16 | R | 64 | 37 | 53 | 22 | 24 | 0.95 | LP | No | 0.5 | −0.10 |
| 16 | M | 66 | 88 | R | 62 | 42 | 50 | 25 | 28 | 0.93 | LNP | Yes | 0.6 | 0.10 |
| 17 | F | 62 | 63 | L | 95 | 24 | 55 | 28 | 31 | 1.15 | LNP | No | 0.95 | 0.03 |
| 18 | M | 49 | 89 | R | 100 | 16 | 53 | 26 | 25 | 0.92 | LNP | No | 0.85 | 0.05 |
| 19 | F | 62 | 24 | L | 82 | 61 | 53 | 16 | 28 | 0.61 | LP | Yes | 0.35 | −0.19 |
| 20 | M | 68 | 24 | R | 54 | 32 | 27 | 6 | 27 | 0.22 | LNP | No | 0.25 | 0.45 |
| 21 | F | 64 | 56 | R | 83.75 | 23 | 54 | 25 | 28 | 0.91 | LNP | yes | 0.55 | 0.02 |
| 22 | M | 77 | 67 | L | 73 | 22 | 54 | 28 | 28 | 1.09 | LNP | no | 0.7 | 0.03 |
| 23 | F | 32 | 136 | R | 80.5 | 22 | 55 | 28 | 31 | 0.96 | LP | No | 0.6 | −0.06 |
| 24 | M | 55 | 28 | R | 86 | 53 | 55 | 29 | 33 | 1.24 | LP | No | 0.75 | −0.07 |
| 25 | M | 42 | 25 | R | 52.5 | 38 | 53 | 23 | 25 | 0.91 | LP | yes | 0.55 | −0.01 |
| 26 | F | 50 | 58 | R | 55.6 | 64 | 47 | 21 | 27 | 0.84 | LP | yes | 0.6 | −0.07 |
| 27 | F | 62 | 270 | R | 78 | 24 | 52 | 18 | 20 | 0.58 | LNP | yes | 0.22 | 0.02 |
| 28 | M | 66 | 31 | L | 90 | 18 | 41 | 13 | 16 | 0.28 | LNP | no | 0.3 | 0.05 |
| 29 | M | 57 | 55 | L | 75 | 30 | 54 | 25 | 26 | 1.06 | LP | yes | 0.72 | −0.01 |
| 30 | M | 62 | 14 | R | 79 | 28 | 49 | 14 | 18 | 0.55 | LNP | yes | 0.35 | 0.08 |
| 31 | F | 76 | 67 | L | 82.5 | 35 | 49 | 24 | 33 | 1.2 | LNP | no | 0.76 | 0.01 |
| 32 | F | 52 | 95 | L | 93 | 20 | 54 | 26 | 31 | 0.98 | LP | no | 0.65 | −0.09 |
| 33 | F | 49 | 10 | R | 68.7 | 30 | 55 | 18 | 18 | 0.64 | LNP | no | 0.28 | 0.06 |
| 34 | F | 46 | 19 | L | 73.8 | 33 | 55 | 26 | 30 | 0.9 | LP | yes | 0.75 | −0.01 |
| 35 | M | 52 | 43 | R | 91 | 19 | 54 | 24 | 20 | 1.05 | LNP | no | 0.85 | 0.06 |
| 36 | M | 63 | 96 | L | 98 | 17 | 51 | 19 | 22 | 0.71 | LP | yes | 0.45 | −0.15 |
| 13 F | 58 ± 11 | 92 ± 84.5 | 15 L | 77 ± 13.2 | 29 ± 11.5 | 50 ± 5.5 | 21 ± 5.6 | 26 ± 4.8 | 0.82 ± 0.26 | 16 LP | 17 yes | 0.55 ± 0.20 |
ABC Scale: Activities-Specific Balance Confidence Scale, FGA: Functional Gait Assessment, FM-LE: Lower extremity portion of the Fugl-Meyer, SLA: Step length asymmetry, LNP: Longer non-paretic step length, LP: Longer paretic step length, Months: Months post-stroke
Experimental protocol
All participants visited the lab once and performed a 10 m walk test before the experiment to determine their self-selected walking speed. We performed the following assessments: 1) the Berg Balance Scale (BBS) 31,32 to assess static balance, 2) the Functional Gait Assessment (FGA) to assess dynamic balance 32,33, 3) the Activity-specific Balance Confidence (ABC) test to evaluate balance self-efficacy 34, 4) the Fall Efficacy scale to assess fear of falling 35, and 5) the lower extremity motor domain of the Fugl-Meyer (FM) assessment to evaluate level of motor impairment 36,37.
After the clinical assessments, we determined the participants’ self-selected walking speed on the treadmill. We started the treadmill at 70% of a participant’s speed measured during the 10 m walk test, and progressively increased or decreased the speed in increments of 0.05 m/s until the participant felt that the speed was comfortable. The experiment began with three minutes of walking at their self-selected treadmill speed to determine a participant’s natural step length asymmetry (Baseline) (Figure 1). Participants then walked with visual feedback of actual and desired step lengths for three different trials 6,38. Visual feedback displayed on a screen in front of the participant showed the position of reflective markers placed on the ankle during the swing phase and a vertical bar on each side, which represented the target step length.
Figure 1.
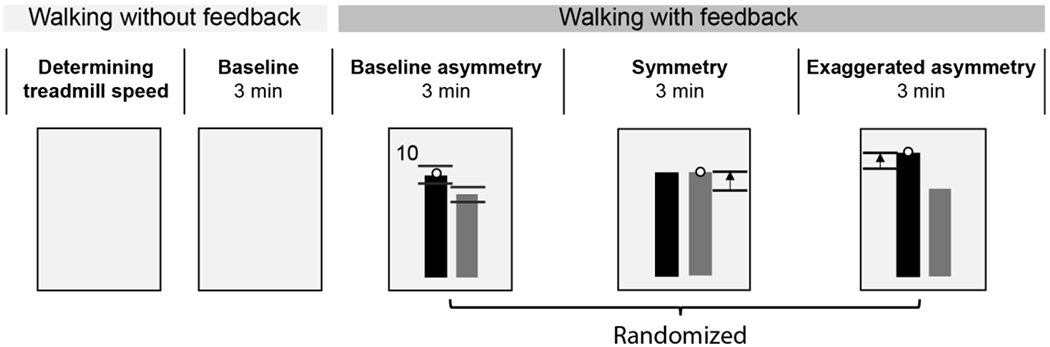
Experimental protocol. We provided online visual feedback to show the position of the ankle markers during swing phase as black dots and a vertical bar on each side, which represented the target step length. Black horizontal lines near the top of each bar in the Baseline asymmetry trial represent the target range which was equal to the standard deviation of the step length measured during baseline. Scores were provided on the top left or right corner of the display to encourage participants to achieve the desired step lengths.
The order of the three feedback trials, baseline asymmetry (Baseline FB), symmetry (Sym),and exaggerated asymmetry (ExggAsym) (Figure 1), was randomized. Each trial lasted three minutes. During Baseline FB, participants walked with visual feedback of their natural step length asymmetry as this allowed us to assess the effects of walking with visual feedback on their angular momentum. During Sym, participants were instructed to walk such that the ankle marker reached the target step length at each foot strike, and we set the target length of the shorter step equal to the length of the longer step. During ExggAsym, we lengthened the target length of the longer step by adding the original difference in step lengths so that their step length asymmetry was doubled. We provided participants with scores to motivate them to achieve the target asymmetry level during each condition. The scores were calculated at every step based on the difference between the actual and target step lengths as described previously 6. If the difference between the actual and target step length was less than 10 mm, the participant would receive a score of 10. The scores were shown adjacent to the top of the bars at every foot-strike. All participants wore a safety harness, and no handrail support was provided during the experiment. Participants also received rest breaks of approximately five minutes between each trial to minimize fatigue.
Data acquisition
Segmental kinematics were recorded using a full-body marker set placing retroreflective markers on bony landmarks 39,40. In addition, sets of cluster markers were placed over the upper arms, lower arms, thighs, shanks, and heels. Marker kinematics were recorded using a 10-camera Qualisys Oqus motion capture system (Qualisys AB, Goteborg, Sweden). We first collected five seconds of a static trial and removed all markers on bony landmarks during the walking trials.
Data processing
Markers were labeled using Qualisys Track Manager and then exported to Visual 3D (C-Motion, Rockville, MD, USA) to construct a full-body model. 3D marker positions were low-pass filtered with a cutoff frequency of 6 Hz. We then created a full-body model in Visual 3D, which included 12 segments: Head, Trunk/Pelvis, left and right Upper arms, Forearms, Thighs, Shanks, Feet. Since participants wore a safety harness over their pelvis, we removed the reflective markers from the pelvis. As this prevented us from tracking the pelvis, we assumed that the pelvis and trunk were rigidly connected in our model. We validated this 12-segment model by comparing the peak-to-peak angular momentum to a 13-segment model in five participants without disabilities. The root-mean-square error for the peak positive and negative whole-body angular momentum during a gait cycle in the 12-segment model compared to the 13-segment model was 2.1 ± 1.5 % and 0.95 ± 0.70 % in the sagittal plane, respectively. Thus, the 12-segment model used in this study introduced relatively small errors in the whole-body angular momentum compared to 13-segment model.
Step length asymmetry (SLA) was computed as the normalized difference in step lengths based on Equation 1.
| (1) |
Here, SLnon-paretic is the fore-aft distance between the heel markers at non-paretic limb foot-strike and SLparetic is the fore-aft distance between heel markers at paretic limb foot-strike. A positive SLA indicates that the participant takes longer non-paretic steps, while a negative SLA indicates a longer paretic step.
Marker data were also used to compute whole-body angular momentum about the CoM in the sagittal and frontal plane as measures of dynamic balance. Whole-body angular momentum (L) is the sum of the angular momenta of individual limb segments with respect to the whole-body CoM 11 (Equation 2).
| (2) |
Whole-body angular momentum was normalized by body mass (M), the CoM height (H), and self-selected walking speed (V)12. The angular momentum of the individual limb segments in the sagittal and frontal plane was calculated as the sum of the angular momentum of each segment about a mediolateral and fore-aft axis, respectively, projecting through the body’s CoM (ri: position of the segmental CoM, mi: segment mass, vi: linear velocity of the segment relative to body’s CoM) and the angular momentum of each segment about its own CoM (Ii: segmental moment of inertia, ωi: segmental angular velocity).
Data Analysis
We calculated whole-body angular momentum (L) during each stride in the sagittal and frontal planes. In the sagittal plane, negative values of angular momentum represented forward rotation, while positive values represented backward rotation. In the frontal plane, we specified rotation toward the paretic side as positive and rotation toward the non-paretic side as negative. A stride was defined as starting with paretic foot-strike and ending with the subsequent paretic foot-strike. Since angular momentum captures the contribution of inertia and velocity, greater angular momentum in any direction would require more effort to restore the body toward its equilibrium position. Thus, we calculated peak negative and positive angular momentum during each stride in the sagittal and frontal planes as our measures of dynamic balance.
Statistical Analysis
All statistical analyses were performed in Matlab version R2018a (Mathworks, Natick, MA, USA). We first tested for correlations between Baseline peak angular momentum and each clinical balance assessment (BBS, ABC, and FGA) with the whole dataset to determine the validity of angular momentum as a measure of dynamic balance. We then used linear regression models to determine if Baseline angular momentum in the sagittal and frontal plane was associated with Baseline step length asymmetry and the direction of asymmetry.
We used mixed-effects regression models to determine if changes in step length asymmetry influenced measures of angular momentum in the sagittal or frontal plane. We used a step length asymmetry of 0.06 as the minimum asymmetry necessary to be included in this analysis based on previous studies 26,41, and we included 15 participants who had a step length asymmetry greater than the cutoff in the regression model. We restricted our analysis to this subset because we were only interested in how changes in asymmetry influenced angular momentum. The independent variables in this model included 1) changes in step length asymmetry magnitude (Δ|SLA|) from Baseline, 2) whether participants took longer paretic or longer non-paretic steps (SLAdirection), and 3) level of impairment measured by over-ground walking speed (OGW) 42. We also included a random intercept for each participant to account for differences in angular momentum across participants. We used the mean values of all variables for each participant during each trial in the regression models. We fit a range of models with main effects and interactions between the independent variables. The most complex potential model included Δ|SLA| SLAdirection, and OGW as main effects and interactions between Δ|SLA| and SLAdirection and between Δ|SLA| and OGW (Equation 3).
| (3) |
The interaction between Δ|SLA| and SLAdirection captures how differences in SLA direction influence the association between changes in SLA magnitude and angular momentum. The interaction between Δ|SLA| and OGW represents how different levels of impairment influence the association between changes in SLA magnitude and angular momentum. We selected the model with the lowest Akaike Information Criterion (AIC) 43 as the best-fit model 44,45, and we conducted post-hoc comparisons using the Bonferroni correction for multiple comparisons. Significance was set at the p < 0.05 level for all analyses.
Results
Peak angular momentum in the sagittal and frontal plane occurred during foot-strike or mid-swing
The magnitude and direction of angular momentum changed systematically during each stride cycle. Whole-body angular momentum in the sagittal plane was most negative during the transition from swing to stance, which corresponded to the moment of peak forward momentum (Figure 2A). Participants then increased angular momentum until it became positive during the swing phase and then fell forward again at foot-strike. The peak forward angular momentum in the sagittal plane occurred between ∼40∼60% of the gait cycle, indicating that participants fell forward most rapidly immediately before non-paretic foot-strike. Furthermore, the two peaks of backward angular momentum occurred within 25 ∼ 35 % and 75 ∼ 85 % of the gait cycle, indicating that the largest backward body rotation occurred during mid-swing of both the paretic and non-paretic sides. In the frontal plane, positive angular momentum peaked at paretic foot-strike and negative angular momentum peaked at non-paretic foot-strike (Figure 2B).
Figure 2.
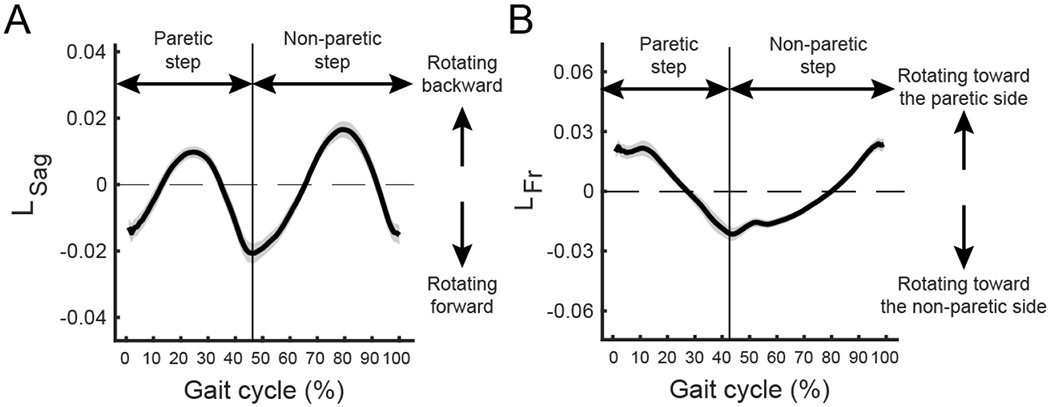
Angular momentum varies with the level of step length asymmetry. (a) Angular momentum in the sagittal plane across the gait cycle. Participants rotated forward most around non-paretic foot-strike (40 ∼ 60% of a gait cycle) and rotated backward most around non-paretic mid swing (75 ∼ 85 % of a gait cycle). The line represents the average across participants and the shaded area represents the standard deviation. The gait cycle starts with foot-strike on the paretic side. (b) Angular momentum in the frontal plane over the gait cycle. Participants rotated toward the non-paretic side near non-paretic foot-strike (40 ∼ 60% of a gait cycle) and rotated toward the paretic side most near paretic foot-strike (0 ∼ 15 % of a gait cycle).
Associations between baseline angular momentum and clinical assessments of balance
We first assessed the concurrent validity of measures of whole-body angular momentum by determining if Baseline angular momentum was associated with clinical balance assessments. We found significant correlations between measures of peak angular momentum and participants’ scores on clinical assessments of balance. For the sagittal plane, was positively correlated with FGA (r = 0.53, p < 0.001) and was negatively correlated with BBS (r = −0.43, p = 0.009) and FGA (r = −0.68, p < 0.001) (Figure 3A, B, and C). This indicates that participants who scored better on clinical assessments of balance had less forward and backward angular momentum in the sagittal plane. In the frontal plane, was positively correlated with FGA (r = 0.40, p < 0.001, Figure 3D). This indicates that individuals who performed better on clinical assessments of dynamic balance during gait tended to rotate more slowly toward the non-paretic side while walking. We did not observe associations between ABC and (r = 0.004, p = 0.98), (r = −0.23, p = 0.17), (r = 0.12, p = 0.48), or (r = 0.01, p = 0.95). Overall, these results support the use of peak angular momentum as a measure of dynamic balance in people post-stroke.
Figure 3.
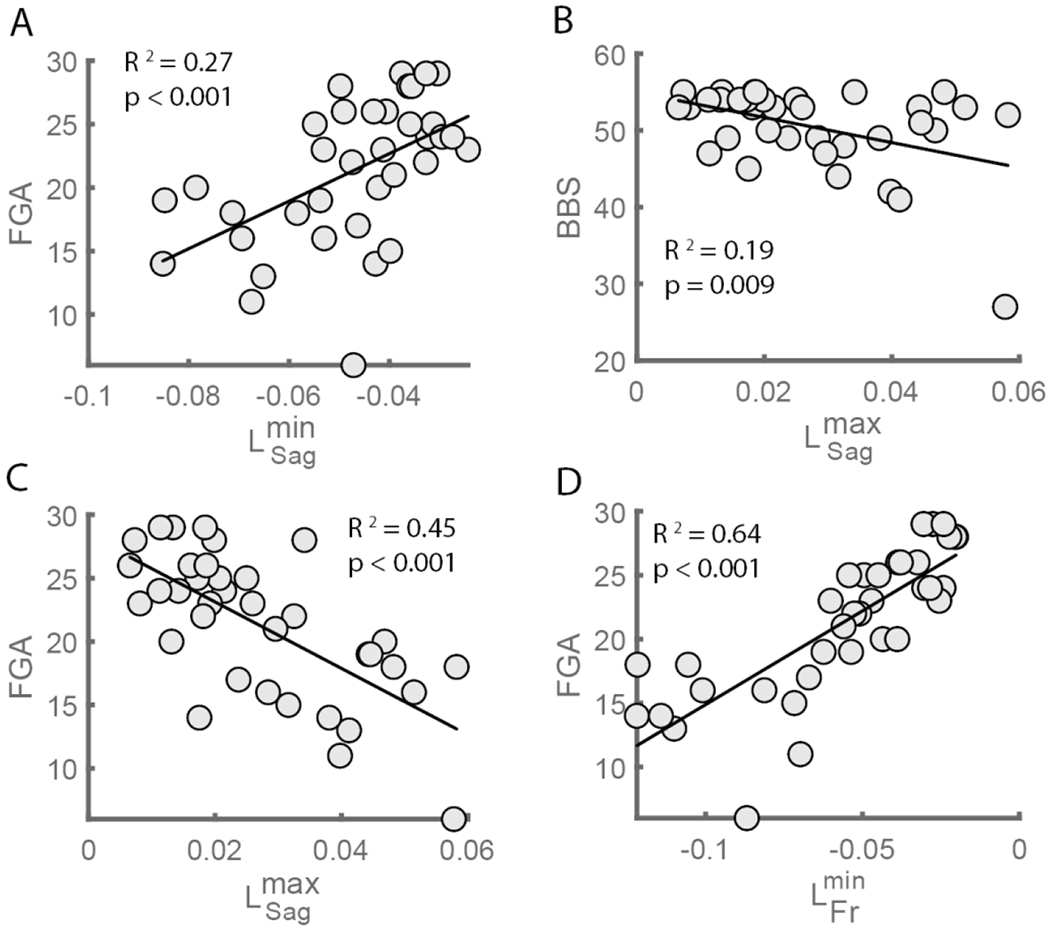
Associations between the peak forward and backward angular momentum in the sagittal (L_Sag^min and L_Sag^max) and frontal plane (L_Fr^min) and clinical balance assessments during Baseline. L_Sag^min was positively associated with (a) the Functional Gait Assessment and L_Sag^min was negatively associated with (b) Berg Balance Scale and (c) the Functional Gait Assessment. L_Fr^min was positively associated with (d) Functional Gait Assessment. FGA: Functional Gait Assessment, BBS: Berg Balance Scale
Baseline backward angular momentum was associated with step length asymmetry magnitude
There was a significant effect of step length asymmetry magnitude on backward angular momentum during Baseline (F1,32 = 6.641, p = 0.015) (Figure 4), but there was no effect of SLAdirection (F1,32 = 1.748, p = 0.195) or interaction between |SLA| and SLAdirection (F1,32 = 1.522, p = 0.226). This association between and |SLA| remained significant after removing two participants who had asymmetry magnitude greater than 0.3 (p = 0.04). In contrast, forward angular momentum during Baseline was not associated with |SLA| (F1,32 = 2.663, p = 0.112) or SLAdirection (F1,32 = 1.307, p = 0.262), nor was there an interaction between |SLA| and SLAdirection (F1,32 = 0.946, p = 0.338).
Figure 4.
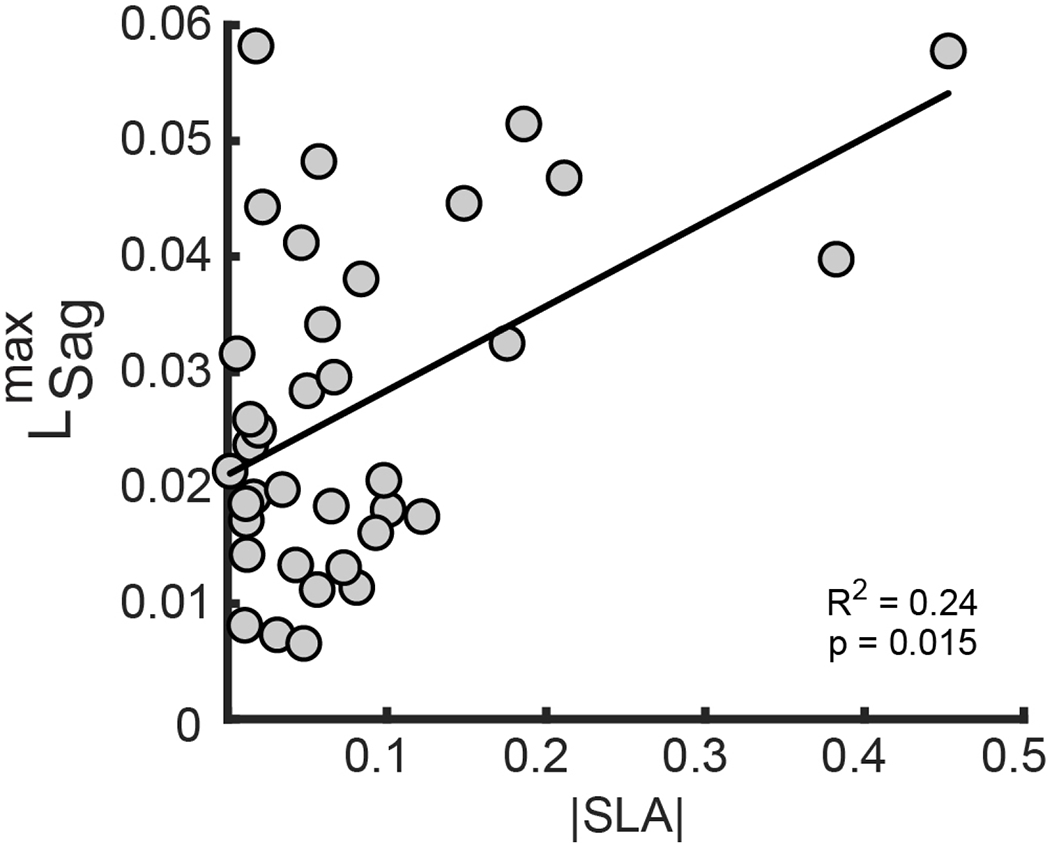
Association between step length asymmetry and baseline peak angular momentum in the sagittal plane. Participant’s natural SLA magnitude was positively associated with L_Sag^max magnitude as measured during the Baseline trial. This indicates that larger SLA magnitude is associated with larger peak backward angular momentum in the sagittal plane.
Baseline angular momentum in the frontal plane was not associated with asymmetry magnitude or the direction of asymmetry. Angular momentum toward the non-paretic side during Baseline was not associated with |SLA| (F1,32 = 0.441, p = 0.511) or SLAdirection (F1,32 = 0.131, p = 0.720), nor was there an interaction between |SLA| and SLAdirection (F1,32 = 0.003, p = 0.955). Similarly, angular momentum toward the non-paretic side during Baseline was not associated with |SLA| (F1,32 = 0.010, p = 0.921) or SLAdirection (F1,32 = 0.043, p = 0.836), nor was there an interaction between |SLA| and SLAdirection (F1,32 = 0.019, p = 0.89).
Participants modified step length asymmetry using visual feedback
Participants successfully modified step length asymmetry using visual feedback. Performance of one representative participant with longer non-paretic steps demonstrates that during Baseline FB, step length asymmetry was maintained at the same level as Baseline since the target asymmetry during Baseline FB was the mean of their natural asymmetry (Figure 5A). The participant reduced step length asymmetry during Sym when walking with feedback to take steps of equal length. Lastly, step length asymmetry increased during ExggAsym compared to Sym since the participant walked with feedback of exaggerated asymmetry. Similarly, a representative participant with longer paretic steps maintained a consistent level of asymmetry during Baseline, Baseline FB, but modified their asymmetry in the Sym and ExggAsym conditions (Figure 5B). At the group level, participants reduced SLA magnitude during Sym (0.08 ± 0.06) compared to Baseline FB (p = 0.004), and increased SLA during ExggAsym (0.18 ± 0.09) relative to Baseline FB (p = 0.02) (Figure 5C).
Figure 5.
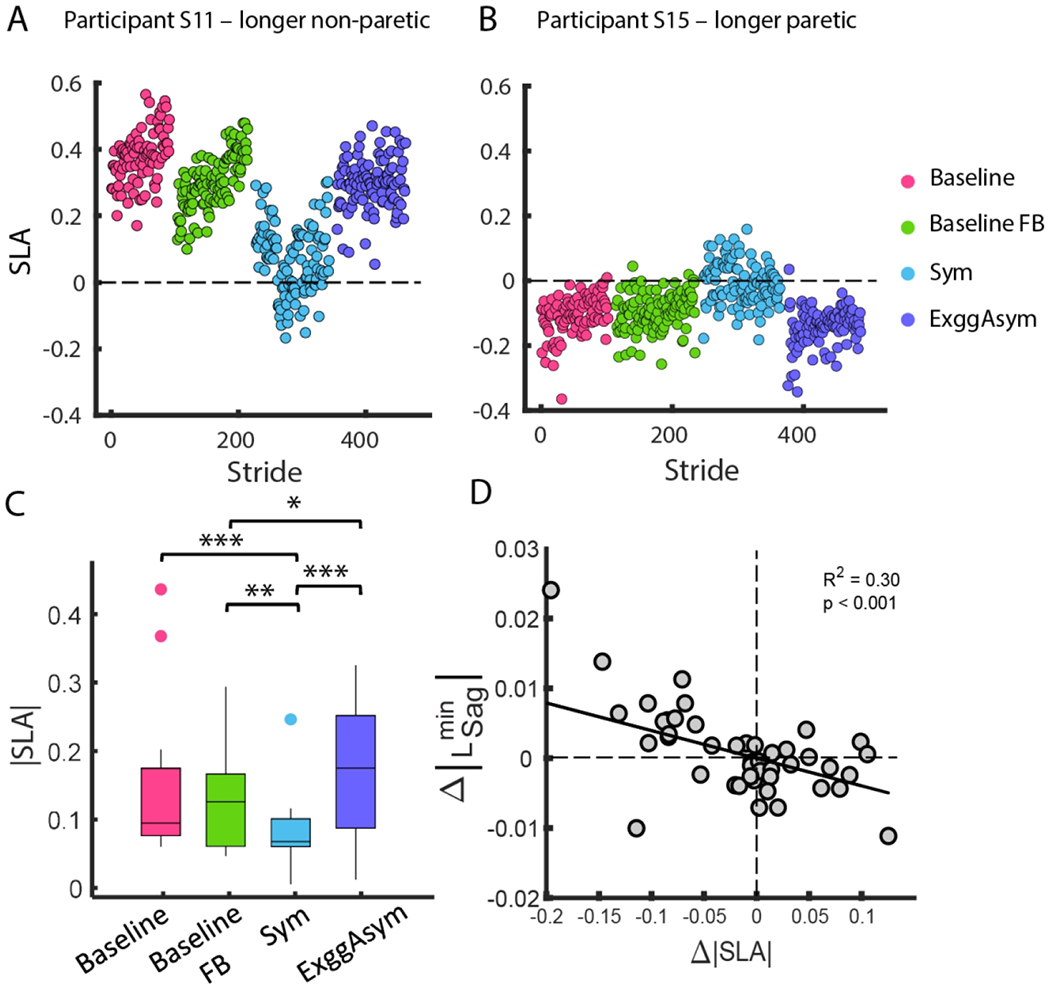
Variation in step length asymmetry (SLA) across trials (a) A representative participant with longer non-paretic step lengths had positive SLA during Baseline, Baseline FB, and ExggAsym. During Sym, the participant reduced step length asymmetry. (b) A representative participant with longer paretic step lengths had negative SLA during Baseline, Baseline FB, and ExggAsym. During Sym, the participant reduced step length asymmetry. (c) Step length asymmetry magnitude for all participants during Baseline, Baseline FB, Sym, and ExggAsym. *p < 0.05, **p < 0.01, and ***p < 0.001 (d) Changes in the magnitude of L_Sag^min in the sagittal plane were negatively correlated with changes in SLA magnitude relative to Baseline indicating that reductions in asymmetry led to increases in angular momentum. *p<0.05
Peak angular momentum in the sagittal plane varied with changes in SLA magnitude
Reductions in step length asymmetry were associated with increases in forward angular momentum in the sagittal plane. The model with the best fit (lowest AIC) for predicting changes in magnitude only included SLA magnitude as a significant predictor. Changes in magnitude were negatively correlated with Δ|SLA| (β = −0.040, p < 0.001), indicating that reductions in asymmetry were associated with an increase in the magnitude of forward angular momentum (Figure 5D). However, no measures of asymmetry or impairment were associated with changes in the magnitude of the peak backward angular momentum in the sagittal plane and angular momentum in the frontal plane.
Discussion
We sought to determine how manipulation of step length asymmetry magnitude and the direction of asymmetry influence changes in angular momentum during walking in people post-stroke. We used online visual feedback to reduce or exaggerate step length asymmetry during walking. We first validated the use of whole-body angular momentum as a measure of dynamic balance by demonstrating that measures of angular momentum were correlated with scores on the clinical balance assessments. Then, we demonstrated that as participants reduced step length asymmetry during the feedback trials, they increased the magnitude of the peak forward angular momentum in the sagittal plane. We also found that the peak backward angular momentum was greater in individuals who were more impaired and took longer non-paretic step lengths.
Associations between clinical balance assessments and baseline angular momentum
Participants’ peak forward angular momentum in the sagittal plane at Baseline was associated with clinical assessments of balance. We expected that participants with smaller peaks in angular momentum would score higher on clinical assessments as this would be consistent with the idea that people who are at a lower risk of falls would have less body rotation during walking. Consistent with this prediction, we found that participants with less angular momentum in the sagittal plane generally had higher scores on BBS and FGA. In the frontal plane, we found that FGA was positively correlated with the peak negative angular momentum indicating that people who had less mediolateral angular momentum at non-paretic foot-strike tended to score better on a clinical assessment of their balance control while walking. This extends previous observations demonstrating that people with a lower rate of change of angular momentum in the frontal plane also scored better on clinical assessments of balance 13. Since exaggerated mediolateral sway in people post-stroke often results from compensatory movements such as hip hiking and circumduction 15,16,25, these compensatory strategies may be reflected by measures of frontal plane angular momentum. Overall, our findings suggest that whole-body angular momentum in the sagittal and frontal planes can be used as a valid measure of balance control during walking in people post-stroke.
The degree of association between BBS and FGA and angular momentum was fair and we did not find a significant association between balance confidence and angular momentum. This should be expected since there are other factors that influence these clinical assessments that are not captured by angular momentum. For example, the BBS evaluates an individual’s ability to transition between chairs, retrieve an object from the floor, and perform alternating steps on a stool. We would not expect that the ability to perform these tasks would be captured by measures of angular momentum during normal walking. Similarly, we did not find significant associations between balance confidence measured by ABC and whole-body angular momentum. This is likely because angular momentum captures people’s actual balance capability rather than their perceived ability. Previous work has established that there was discordance between the actual balance capacity and the perceived balance in people post-stroke who showed low BBS and high ABC and vice versa 46.
Effects of manipulating step length asymmetry on angular momentum
Contrary to our hypothesis, the peak forward angular momentum in the sagittal plane increased when people walked more symmetrically. We expected that angular momentum would reduce as step length asymmetry decreased since walking with more symmetric step lengths would result in more symmetrical, anti-phase limb rotation and better cancellation of the contralateral limb angular momenta. However, we found that participants increased their forward angular momentum as they reduced step length asymmetry, indicating that improving asymmetry did not reduce whole-body rotational behavior during walking. Although changes in asymmetry led to changes in angular momentum in the sagittal plane, peak angular momentum in the frontal plane did not change when walking more symmetrically. This suggests that changes in step length asymmetry in the sagittal plane do not systematically influence frontal plane momentum. However, it is possible that changes in asymmetry could have caused changes in both frontal and sagittal plane balance that we could not detect. For example, our study was not designed to capture changes in the reactive control of balance 38 and this, among other balance-related measures, could be sensitive to changes in asymmetry. Since manipulating asymmetry in our study was not a type of perturbation requiring reactive balance control, the changes in angular momentum in the frontal plane may have not been significant.
Eight of 15 participants increased forward angular momentum near the time of foot-strike on the side where they increased step lengths. Since we manipulated asymmetry by increasing the shorter step length, this may have been associated with an increased excursion of the center of mass (CoM) relative to the trailing leg and higher CoM velocity when lengthening the short step. This increase in velocity may have contributed to increases in momentum that we observed in these individuals. Seven participants increased forward angular momentum near the time of foot-strike on the side contralateral to shorter step length. These individuals may have relied on increasing propulsion before lift-off of the limb on the side where they attempted to increase step lengths. This attempt to generate more swing leg momentum may have caused increased forward angular momentum near the time of foot-strike on the side contralateral to the shorter step length.
Another potential reason why participants did not reduce angular momentum is that reductions in step length asymmetry may not have produced meaningful changes in momentum cancellation between limbs. A recent study found that people post-stroke continue to walk with asymmetric interlimb coordination even when reducing step length asymmetry 5. This suggests that their legs may not move in perfect anti-phase relative to the CoM as they reduce step length asymmetry. Overall, our results suggest that balance-related responses to reductions in step length asymmetry in people post-stroke may vary based on the strategy they use to change their step lengths.
The observed increases in angular momentum during more symmetric walking may reflect impairments in dynamic balance. In our study, the increase in the peak forward angular momentum during foot-strike was about 0.008 (∼16%) as step length asymmetry decreased by 0.2 using the estimated coefficient (β = −0.04) in the linear model relating Δ|SLA| and The size of the changes in angular momentum when walking more symmetrically was smaller than the angular momentum changes when exposed to a perturbation large enough to cause a fall during walking 47. When participants without disabilities experienced fore-aft perturbations characterized by abrupt increases in treadmill speed of approximately 0.6 m/s, they increased peak angular momentum in the sagittal plane by ∼0.03 (∼50%). Thus, the changes in momentum we observed in the current study were approximately equal to the changes that one might expect in individuals without disabilities responding to an unexpected 0.2 m/s increase in treadmill speed. Although the change in angular momentum that we observed is unlikely to be as large as one might expect in a near fall, such changes could be perceived as sizable for people post-stroke depending on their level of sensorimotor function.
However, it remains to be seen how longer-term practice or interventions leading to reductions in step length asymmetry affects the regulation of momentum during walking. Longer-term improvements in the symmetry could be achieved through methods such as repeated practice on a split-belt treadmill 2, using auditory feedback of stance time asymmetry 48, or gait training with functional electrical stimulation 49. Although participants in these studies retained improvements in symmetry after practice, it remains to be seen if these improvements in symmetry impact dynamic balance. Overall, our findings may inform future research efforts that seek to understand how novel interventions affect quantitative measures of balance control during walking post-stroke.
Conclusion
We found that whole-body angular momentum in the sagittal and frontal plane during walking is a valid measure of dynamic balance in people post-stroke. Although we expected that improving symmetry would improve balance post-stroke, we found that the reductions in asymmetry impair dynamic balance by increasing whole-body angular momentum. This may imply, more generally, that people post-stroke have an implicit preference not to deviate from their natural asymmetry because it may perturb their balance. However, additional investigation is necessary to determine the relative contributions of other factors such as energetic optimization, discomfort, and aesthetics in the selection of self-selected walking patterns in people post-stroke. Future studies should determine if the findings observed here generalize to over-ground walking where participants’ walking speed is less constrained. This is critical from a clinical perspective as existing clinical approaches to increase symmetry may rely on providing instructive guidance during overground walking. An additional clinical implication of our results is that if clinicians choose to focus on guiding their patients to improve symmetry, they may also need to consider how to reduce heightened destabilization resulting from these improvements or increase their capacity to withstand the instability.
Acknowledgment
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R01HD091184 and SC CTSI (NIH/NCATS) through Grant UL1TR001855.
References
- 1.Patterson KK, Mansfield A, Biasin L, Brunton K, Inness EL, McIlroy WE. Longitudinal changes in poststroke spatiotemporal gait asymmetry over inpatient rehabilitation. Neurorehabil Neural Repair. 2015;29(2):153–162. doi: 10.1177/1545968314533614 [DOI] [PubMed] [Google Scholar]
- 2.Reisman DS, McLean H, Keller J, Danks KA, Bastian AJ. Repeated split-belt treadmill training improves poststroke step length asymmetry. Neurorehabil Neural Repair. 2013;27(5):460–468. doi: 10.1177/1545968312474118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silver KH, Macko RF, Forrester LW, Goldberg AP, Smith GV. Effects of aerobic treadmill training on gait velocity, cadence, and gait symmetry in chronic hemiparetic stroke: a preliminary report. Neurorehabil Neural Repair. 2000;14(1):65–71. doi: 10.1177/154596830001400108 [DOI] [PubMed] [Google Scholar]
- 4.Nguyen TM, Jackson RW, Aucie Y, Kam D de, Collins SH, Torres-Oviedo G. Self-selected step length asymmetry is not explained by energy cost minimization in individuals with chronic stroke. | medRxiv. Published June 2019. Accessed April 3, 2020. https://www.medrxiv.org/content/10.1101/19013854v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Padmanabhan P, Rao KS, Gulhar S, Cherry-Allen KM, Leech KA, Roemmich RT. Persons post-stroke restore step length symmetry by walking asymmetrically. bioRxiv. Published online October 10, 2019:799775. doi: 10.1101/799775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sánchez N, Finley JM. Individual differences in locomotor function predict the capacity to reduce asymmetry and modify the energetic cost of walking poststroke. Neurorehabil Neural Repair. 2018;32(8):701–713. doi: 10.1177/1545968318787913 [DOI] [PubMed] [Google Scholar]
- 7.Harris JE, Eng JJ, Marigold DS, Tokuno CD, Louis CL. Relationship of balance and mobility to fall incidence in people with chronic stroke. Phys Ther. 2005;85(2):150–158. doi: 10.1093/ptj/85.2.150 [DOI] [PubMed] [Google Scholar]
- 8.Weerdesteyn V, de Niet M, van Duijnhoven HJR, Geurts ACH. Falls in individuals with stroke. J Rehabil Res Dev. 2008;45(8):1195–1213. [PubMed] [Google Scholar]
- 9.Lewek MD, Bradley CE, Wutzke CJ, Zinder SM. The relationship between spatiotemporal gait asymmetry and balance in individuals with chronic stroke. J Appl Biomech. 2014;30(1):31–36. doi: 10.1123/jab.2012-0208 [DOI] [PubMed] [Google Scholar]
- 10.Elftman H. The Function of the Arms in Walking. Hum Biol. 1939;11(4). Accessed October 11, 2016. http://search.proquest.com/docview/1301825789/citation/EB1A37DA804B4DE1PQ/1 [Google Scholar]
- 11.Herr H, Popovic M. Angular momentum in human walking. J Exp Biol. 2008;211(Pt 4):467–481. doi: 10.1242/jeb.008573 [DOI] [PubMed] [Google Scholar]
- 12.Honda K, Sekiguchi Y, Muraki T, Izumi S-I. The differences in sagittal plane whole-body angular momentum during gait between patients with hemiparesis and healthy people. J Biomech. 2019;86:204–209. doi: 10.1016/j.jbiomech.2019.02.012 [DOI] [PubMed] [Google Scholar]
- 13.Nott CR, Neptune RR, Kautz SA. Relationships between frontal-plane angular momentum and clinical balance measures during post-stroke hemiparetic walking. Gait Posture. 2014;39(1):129–134. doi: 10.1016/j.gaitpost.2013.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vistamehr A, Kautz SA, Bowden MG, Neptune RR. Correlations between measures of dynamic balance in individuals with post-stroke hemiparesis. J Biomech. 2016;49(3):396–400. doi: 10.1016/j.jbiomech.2015.12.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cruz TH, Lewek MD, Dhaher YY. Biomechanical impairments and gait adaptations post-stroke: Multi-factorial associations. J Biomech. 2009;42(11):1673–1677. doi: 10.1016/j.jbiomech.2009.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanhope VA, Knarr BA, Reisman DS, Higginson JS. Frontal plane compensatory strategies associated with self-selected walking speed in individuals post-stroke. Clin Biomech. 2014;29(5):518–522. doi: 10.1016/j.clinbiomech.2014.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Popovic M, Hofmann A, Herr H. Angular momentum regulation during human walking: biomechanics and control. In: IEEE International Conference on Robotics and Automation, 2004. Proceedings. ICRA ’04. 2004. Vol 3. ; 2004:2405–2411 Vol.3. doi: 10.1109/R0B0T.2004.1307421 [DOI] [Google Scholar]
- 18.Patten C, Lexell J, Brown HE. Weakness and strength training in persons with poststroke hemiplegia: rationale, method, and efficacy. J Rehabil Res Dev. 2004;41(3A):293–312. doi: 10.1682/jrrd.2004.03.0293 [DOI] [PubMed] [Google Scholar]
- 19.Rybar MM, Walker ER, Kuhnen HR, Ouellette DR, Hunter SK, Hyngstrom AS. The stroke-related effects of hip flexion fatigue on over ground walking. Gait Posture. 2014;39(4):1103–1108. doi: 10.1016/j.gaitpost.2014.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allen JL, Kautz SA, Neptune RR. Forward propulsion asymmetry is indicative of changes in plantarflexor coordination during walking in individuals with post-stroke hemiparesis. Clin Biomech Bristol Avon. 2014;29(7):780–786. doi: 10.1016/j.clinbiomech.2014.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lauzière S, Miéville C, Betschart M, Aissaoui R, Nadeau S. Plantarflexor weakness is a determinant of kinetic asymmetry during gait in post-stroke individuals walking with high levels of effort. Clin Biomech Bristol Avon. 2015;30(9):946–952. doi: 10.1016/j.clinbiomech.2015.07.004 [DOI] [PubMed] [Google Scholar]
- 22.Finley JM, Long A, Bastian AJ, Torres-Oviedo G. Spatial and temporal control contribute to step length asymmetry during split-belt adaptation and hemiparetic gait. Neurorehabil Neural Repair. 2015;29(8):786–795. doi: 10.1177/1545968314567149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roerdink M, Beek PJ. Understanding inconsistent step-length asymmetries across hemiplegic stroke patients: impairments and compensatory gait. Neurorehabil Neural Repair. 2011;25(3):253–258. doi: 10.1177/1545968310380687 [DOI] [PubMed] [Google Scholar]
- 24.Varraine E, Bonnard M, Pailhous J. Intentional on-line adaptation of stride length in human walking. Exp Brain Res. 2000;130(2):248–257. doi: 10.1007/s002219900234 [DOI] [PubMed] [Google Scholar]
- 25.Titus AW, Hillier S, Louw QA, Inglis-Jassiem G. An analysis of trunk kinematics and gait parameters in people with stroke. Afr J Disabil. 2018;7:310. doi: 10.4102/ajod.v7i0.310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balasubramanian CK, Bowden MG, Neptune RR, Kautz SA. Relationship between step Length asymmetry and walking performance in subjects with chronic hemiparesis. Arch Phys Med Rehabil. 2007;88(1):43–49. doi: 10.1016/j.apmr.2006.10.004 [DOI] [PubMed] [Google Scholar]
- 27.Hsu A-L, Tang P-F, Jan M-H. Analysis of impairments influencing gait velocity and asymmetry of hemiplegic patients after mild to moderate stroke1. Arch Phys Med Rehabil. 2003;84(8):1185–1193. doi: 10.1016/S0003-9993(03)00030-3 [DOI] [PubMed] [Google Scholar]
- 28.Olney SJ, Richards C. Hemiparetic gait following stroke. Part I: Characteristics. Gait Posture. 1996;4(2):136–148. doi: 10.1016/0966-6362(96)01063-6 [DOI] [Google Scholar]
- 29.Merlo A, Campanini I. Impact of instrumental analysis of stiff knee gait on treatment appropriateness and associated costs in stroke patients. Gait Posture. 2019;72:195–201. doi: 10.1016/j.gaitpost.2019.06.009 [DOI] [PubMed] [Google Scholar]
- 30.Kerrigan DC, Gronley J, Perry J. Stiff-legged gait in spastic paresis. A study of quadriceps and hamstrings muscle activity. Am J Phys Med Rehabil. 1991;70(6):294–300. [PubMed] [Google Scholar]
- 31.Blum L, Korner-Bitensky N. Usefulness of the Berg Balance Scale in stroke rehabilitation: a systematic review. Phys Ther. 2008;88(5):559–566. doi: 10.2522/ptj.20070205 [DOI] [PubMed] [Google Scholar]
- 32.Langley F, Mackintosh S. Functional balance assessment of older community dwelling adults: A systematic review of the literature. Internet J Allied Health Sci Pract. 2007;5(4). https://nsuworks.nova.edu/ijahsp/vol5/iss4/13 [Google Scholar]
- 33.Leddy AL, Crowner BE, Earhart GM. Functional gait assessment and balance evaluation system test: Reliability, validity, sensitivity, and specificity for identifying individuals With parkinson disease who fall. Phys Ther. 2011;91(1):102–113. doi: 10.2522/ptj.20100113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) Scale. J Gerontol A Biol Sci Med Sci. 1995;50A(1):M28–34. doi: 10.1093/gerona/50a.1.m28 [DOI] [PubMed] [Google Scholar]
- 35.Fall Efficacy Scale - International (FES-I) - ScienceDirect. Accessed April 3, 2021. https://www.sciencedirect.com/science/article/pii/S1836955314000265?via%3Dihub
- 36.Fugl-Meyer AR, Jääskö L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med. 1975;7(1):13–31. [PubMed] [Google Scholar]
- 37.Sullivan KJ, Tilson JK, Cen SY, et al. Fugl-Meyer assessment of sensorimotor function after stroke. Stroke. 2011;42(2):427–432. doi: 10.1161/STROKEAHA.110.592766 [DOI] [PubMed] [Google Scholar]
- 38.Liu C, Macedo LD, Finley JM. Conservation of reactive stabilization strategies in the presence of step length asymmetries during walking. Front Hum Neurosci. 2018;12. doi: 10.3389/fnhum.2018.00251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Havens KL, Mukherjee T, Finley JM. Analysis of biases in dynamic margins of stability introduced by the use of simplified center of mass estimates during walking and turning. Gait Posture. 2018;59:162–167. doi: 10.1016/j.gaitpost.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song J, Sigward S, Fisher B, Salem GJ. Altered dynamic postural control during step turning in persons with early-stage parkinson’s disease. Park Dis. Published online 2012. doi: 10.1155/2012/386962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allen JL, Kautz SA, Neptune RR. Step length asymmetry is representative of compensatory mechanisms used in post-stroke hemiparetic walking. Gait Posture. 2011;33(4):538–543. doi: 10.1016/j.gaitpost.2011.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mulroy S, Gronley J, Weiss W, Newsam C, Perry J. Use of cluster analysis for gait pattern classification of patients in the early and late recovery phases following stroke. Gait Posture. 2003;18(1):114–125. doi: 10.1016/s0966-6362(02)00165-0 [DOI] [PubMed] [Google Scholar]
- 43.Akaike H. Likelihood of a model and information criteria. J Econom. 1981;16(1):3–14. doi: 10.1016/0304-4076(81)90071-3 [DOI] [Google Scholar]
- 44.Hocking RR, Leslie RN. Selection of the best subset in regression analysis. Technometrics. 1967;9(4):531–540. doi: 10.2307/1266192 [DOI] [Google Scholar]
- 45.James G, Witten D, Hastie T, Tibshirani R. An Introduction to Statistical Learning: With Applications in R. Springer-Verlag; 2013. Accessed September 9, 2019. https://www.springer.com/gp/book/9781461471370 [Google Scholar]
- 46.Liphart J, Gallichio J, Tilson JK, Pei Q, Wu SS, Duncan PW. Concordance and discordance between measured and perceived balance and the effect on gait speed and falls following stroke. Clin Rehabil. 2016;30(3):294–302. doi: 10.1177/0269215515578294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martelli D, Monaco V, Luciani LB, Micera S. Angular momentum during unexpected multidirectional perturbations delivered while walking. IEEE Trans Biomed Eng. 2013;60(7):1785–1795. doi: 10.1109/TBME.2013.2241434 [DOI] [PubMed] [Google Scholar]
- 48.Krishnan V, Khoo I, Marayong P, DeMars K, Cormack J. Gait training in chronic stroke using walk-even feedback device: A pilot study. Neuroscience Journal. doi: 10.1155/2016/6808319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Awad LN, Palmer JA, Pohlig RT, Binder-Macleod SA, Reisman DS. Walking speed and step length asymmetry modify the energy cost of walking after stroke. Neurorehabil Neural Repair. 2015;29(5):416–423. doi: 10.1177/1545968314552528 [DOI] [PMC free article] [PubMed] [Google Scholar]


