Abstract
Background:
Alcohol use disorder (AUD) is highly comorbid with depression and posttraumatic stress disorder (PTSD) and can complicate their treatment. Transcranial magnetic stimulation is a promising treatment for these disorders, yet prior research often excluded AUD patients out of concern for safety or poorer outcomes. To this end, we revisited a prior study of intermittent theta burst stimulation (iTBS) for PTSD, to evaluate whether mild AUD impacted safety and clinical outcomes.
Methods:
Fifty veterans with PTSD (n=17, with comorbid AUD) received 10 days of sham-controlled iTBS, followed by 10 unblinded sessions. Stimulation was delivered at 80% of the motor threshold for 1800 pulses to the right dorsolateral prefrontal cortex. Safety, PTSD and depressive outcomes were evaluated with repeated measures analysis of variance, to examine the effects of time, treatment group and comorbid AUD.
Results:
iTBS was safe, although AUD patients reported more adverse events, regardless of whether they received active or sham stimulation. Regarding clinical outcomes, patients with AUD who received active stimulation demonstrated a greater rate of improvement in depression symptoms than those without comorbid AUD. The presence of AUD did not impact PTSD symptom change.
Limitations:
Limitations include a modest sample size and use of a categorical, rather than continuous, index of AUD diagnosis.
Conclusion:
While these results require replication, they indicate that iTBS is likely safe in patients with mild comorbid AUD. We propose that comorbid AUD should not preclude clinical use of iTBS, and that iTBS should be further investigated as a novel treatment option for AUD.
Keywords: PTSD, depression, alcohol use, neurostimulation
INTRODUCTION
Alcohol use disorders (AUD) is highly prevalent, and remains one of the leading causes of death in the USA and worldwide1. Furthermore, AUD is often comorbid with other psychiatric disorders, particularly major depressive disorder (MDD) and posttraumatic stress disorder (PTSD), among others1, 2. Furthermore, the relationship between AUD and other psychiatric disorders is often bidirectional. AUD complicates clinical treatment, as AUD can precipitate episodes and prolong the course of depression, while MDD (and other disorders) puts patients at risk for developing AUD3. Due to this complicated relationship, patients with comorbid AUD are often excluded from clinical trials due to concerns related to safety and potential negative impact on outcomes. This leaves our field with important gaps in our knowledge about how to treat these commonly comorbid conditions.
This issue is particularly important when considering the development of new, non-pharmacological options, such as transcranial magnetic stimulation (TMS). TMS is an FDA-cleared treatment for MDD and obsessive-compulsive disorder4–6, with promising data for PTSD and other psychiatric conditions7. A small but growing body of literature indicates TMS may have a therapeutic role in AUD treatment; a number of experiments and randomized controlled trials indicated TMS can be used to reduce craving and improve clinical outcomes8.
However, in daily clinical practice, there remains significant hesitation about the use of brain stimulation for AUD. One major factor leading to this hesitation is the known effect of alcohol on cortical excitability; TMS-evoked measurement of cortical excitability is used to determine TMS treatment intensity (also called the motor threshold). Acute alcohol use can reduce cortical excitability9, whereas alcohol withdrawal can significantly increase excitability (e.g.,10) and lead to seizures. Thus, in the context of alcohol use, motor threshold determination can be incorrect, leading to the risk of under-dosing TMS (i.e., related to artificially low motor threshold) or over-dosing TMS (related to artificially high motor threshold). In both these cases, TMS efficacy and safety can theoretically be impacted. Because of these potential issues, patients with AUD have been systematically excluded from the major randomized controlled trials informing clinical practice with TMS (i.e.,4–6, 11, 12.
To this end, we revisited our prior study of a new kind of TMS, called intermittent theta burst stimulation, where we demonstrated positive outcomes early13 and over the following year after stimulation14. This study, performed in a sample of US veterans utilizing broad inclusion/exclusion criteria, permitted entry of participants as long as they did not have a severe alcohol use disorder, and thus permits a secondary evaluation to evaluate whether iTBS can be used safely and effectively in patients with comorbid PTSD, depression and AUD.
METHODS
Participants
Participants were veterans who participated in a randomized controlled clinical trial examining the effectiveness of iTBS as a treatment for PTSD at the Providence VA Medical Center. Of the 56 veterans who provided informed consent, 50 were randomized to treatment condition between May 2016 to December 2017 (see [16] for a detailed description of these procedures). Participating veterans had a current DSM-5 PTSD diagnosis (assessed via the Structured Clinical Interview for DSM-5,15), a history of trauma exposure (assessed using the Life Events Checklist16), and were between the ages of 18 and 70. Veterans who exhibited PTSD symptoms for at least 6 weeks prior to undergoing study procedures despite psychiatric or psychotherapeutic treatment were also eligible to participate. Participants were permitted to continue regular treatment over the course of the study.
Veterans were excluded if they had implanted medical devices or metal implants above the upper thoracic spine, or medical conditions that could impact safety of iTBS administration (i.e., lifetime history of moderate or severe traumatic brain injury, per VA/DoD Clinical Practice Guidelines; at risk of pregnancy; history of seizure or other significant neurological disorder; history of stroke, CNS tumor, or cerebral aneurysm). Veterans with a primary psychotic disorder, bipolar I disorder, current moderate or severe alcohol and/or substance use disorder, or active suicidality were also excluded from participation. All study procedures were approved by the Institutional Review Board at the Providence VA Medical Center.
Study Design
This study used a modified parallel-group double-blind sham-controlled design. Following informed consent, eligible veterans were randomized to the active (n=25) or sham-controlled (n=25) iTBS condition by a study member uninvolved with TBS delivery. Randomization was performed using a 1:1 design stratified by sex and PTSD symptom severity. Motor threshold was determined by visual inspection of movement in the contralateral hand, and defined as the intensity at which movement was detected in 50% of single pulse TMS trials. Consistent with clinical practice, motor threshold determination was only reassessed when clinical factors occurred that, in the judgment of the treating physician, motor threshold determination needed to be measured. Stimulation intensity was delivered at 80% of the active motor threshold.
During the intervention phase, a staff member uninvolved in iTBS delivery and administration of self-reports set up the appropriate coil (i.e., either active or sham coil) before each session to maintain study blinding. Participants received 10 sessions of active or sham-controlled iTBS. Following the 10 days of sham-controlled iTBS, participants could elect to receive another 10 sessions of unblinded iTBS (n=45). Outcome measures (i.e., PTSD and MDD symptoms) were collected using self-reports at baseline, after the double-blind phase, after the unblinded phase, and at a 1-month follow-up visit. The 1-month follow-up visit was intended to evaluate treatment outcomes during the follow-up period as well as to determine if cumulative dose of iTBS affected clinical outcomes.
Clinical Measures
MDD symptoms.
The Inventory of Depressive Symptomatology- Self-Report (IDS-SR;17) was used to assess depression symptom severity over the past week. Responses range from 0, (i.e., “I do not feel sad”) to 3, (i.e. “I feel sad nearly all of the time”), with higher scores indicating greater symptom severity. Total scores were used to analyze the change in depressive symptoms following each time point.
PTSD symptoms.
The PTSD Checklist (PCL-5;18) was used to assess PTSD symptom severity over the past month. The PCL-5 is a 20-item self-report that corresponds to the DSM-5 symptom criteria for PTSD. Participants were asked to rate how bothered they have been by PTSD symptoms in the past month on a 4-point severity scale (0 = “not at all bothered by” to 4 = “extremely bothered”) for each item (i.e., “Repeated, disturbing, and unwanted memories of the stressful experience”). The total symptom severity scores from each time point were used in analyses.
Alcohol use disorder.
Alcohol use disorder was assessed via the Structured Clinical Interview for DSM-5 [18], in combination with chart review to verify verbal reports.
Safety.
Safety was measured through spontaneously reported events and through systematic query at the end of participation; chi square (and Fischer’s exact) tests were used to compare rates of any adverse event reporting across groups. Breathalyzer and urine toxicology testing was available in the event that a participant reported alcohol (or substance) use at a study visit. Participants were not intoxicated during brain stimulation. Serious adverse events were recorded through participant report and through regular query of the medical record. Motor threshold was recorded in percent of stimulator output, and rates of motor threshold redetermination were counted as simple frequencies.
Intervention
Study design.
This study used a modified parallel-group double-blind sham-controlled design. Following provision of informed consent, eligible veterans were randomized to the active (n=25) or sham-controlled (n=25) iTBS condition by a study member uninvolved with TBS delivery. Randomization was performed using a 1:1 design stratified by sex and PTSD symptom severity. During the intervention phase, a staff member uninvolved in iTBS delivery and administration of self-reports set up the appropriate coil (i.e., either active or sham coil) before each session to maintain study blinding. This study utilized a Magstim sham coil. This system delivers the sensation of TMS (so-called “active” sham stimulation), without sufficient magnetic energy reaching the cortex. This system has been used in numerous TMS trials, and includes specific coil windings to optimize the blinding experience. It is also visually indistinguishable from the active coil to minimize accidental unblinding. Staff who delivered iTBS treatments did not become unblinded during the course of the study.. Participants received 10 sessions of active or sham-controlled iTBS. Following the 10 days of sham-controlled iTBS, participants could elect to receive another 10 sessions of unblinded iTBS (n=45).Participants were asked to guess their group assignment after the double-blind phase to assess effectiveness of the blinding.
Stimulation.
Following randomization, and prior to treatment, each participants’ active motor threshold was determined (i.e., the minimum stimulator output necessary to induce movement in the contralateral hand >50% of the time). Meta-analytic research indicates that right-sided and higher-frequency stimulation yield larger reductions in PTSD symptoms19, 20, and high-frequency TMS administered to the right dorsolateral prefrontal cortex (DLPFC) can reduce amygdala activation to threatening stimuli21. Based on this evidence, TBS was delivered to the right DLPFC using parameters based on previous TBS studies22 (80% active motor threshold, 1,800 pulses, 9.5 minutes)) via a Magstim Rapid 2+1 system (Magstim, Whitland, United Kingdom). At each TBS session, the coil was placed over the right DLPFC using scalp measurements that were checked and monitored at every session to maintain accurate coil placement.
Statistical Analysis
Data in the full sample and within AUD groups were screened for violations of normality. We conducted mixed-model repeated measures ANOVAs (RMANOVAs) to examine the separate and interactive effects of timepoint (double blind, open label, 1-month follow-up), iTBS stimulation group (10 versus 20 active stimulation sessions), and comorbid AUD on PTSD or depressive symptom severity.
RESULTS
Sample Characteristics
Descriptive information for the full sample, and as a function of AUD group (n=25 per group), is displayed in Table 1. Results of t-tests and chi-squared tests indicated that veterans with (n=17) versus without (n=33) a comorbid AUD did not differ on demographic variables, baseline PTSD or depressive symptom severity, or iTBS stimulation group (all ps>.10; sham comorbid AUD in sham group: n=8; comorbid AUD in active group: n=8). Paired t-tests indicated that the severity of both PTSD (t(49)=11.13, p=.000) and depressive (t(49)=7.63, p=.000) symptoms improved from baseline to 1-month follow-up in the full sample.
Table 1.
Demographics and clinical characteristics of participants with and without a comorbid alcohol use disorder.
| Measure | Full Sample (n=50) | Comorbid AUD (n=17) | No Comorbid AUD (n=33) |
|---|---|---|---|
| Age M(SD) | 50.1(12.3) | 54.5(11.4) | 49.0(12.5) |
| Female n(%) | 8(16%) | 4(24%) | 4(12%) |
| Marital status n(%) | |||
| Cohabitating/married | 18(36%) | 5(29%) | 13(39%) |
| Single/never married | 11(22%) | 2(12%) | 9(27%) |
| Divorced/separated | 20(40%) | 9(53%) | 11(33%) |
| Race n %) | |||
| White | 42(84%) | 14(82%) | 28(85%) |
| Black/African American 0 or 2 | 2(4%) | 0(0%) | 2(6%) |
| American Indian/Alaskan Native 4 | 1(2%) | 1(6%) | 0(0%) |
| Multiracial 5 | 3(6%) | 0(0%) | 3(9%) |
| Hispanic n(%) | 2(4%) | 1(6%) | 1(3%) |
| Full active stimulation group n(%) | 25(50%) | 9(53%) | 16(49%) |
| Baseline PCL-5 Score M(SD) | 49.7(10.3) | 50.1(9.0) | 49.5(11.1) |
| Baseline IDS-SR Score M(SD) | 41.0(11.7) | 43.2(13.6) | 39.9(10.7) |
| Clinical relapse at 1 year (%) | 22(44%) | 7(41%) | 15(46%) |
Note. There were no significant differences in demographics between the two groups (all p>.1). AUD = Alcohol Use Disorder; PCL-5 = PTSD Checklist -5; IDS-SR = Inventory of Depressive Symptoms – Self-Report.
Safety
Three serious adverse events (SAE) occurred in the trial; one occurred in a veteran with AUD when he was receiving sham stimulation that developed homicidal ideation, and another SAE in the active group that was associated with alcohol intoxication and suicidal ideation in a veteran with comorbid AUD. The third occurred in a veteran without AUD who required hospitalization for anxiety after he signed informed consent but before he received any stimulation; because of the low rates of SAEs these are provided descriptively. Veterans with AUD reported adverse events more frequently than veterans without AUD (35% versus 11%, respectively; Fischer’s exact test p = .047), although there were no differences in adverse events associated with active or sham stimulation (all p>.1). Consistent with the broader TMS literature, the most commonly reported adverse event was treatment site discomfort (i.e., which was reported more often in the AUD group). There were no seizures, and no participants reported or showed physical signs of intoxication during treatment sessions.
Pretreatment motor thresholds did not differ between groups. The mean motor threshold (SD) in the AUD group was 44.9 (6.3) and non-AUD group was 41.2 (6.7) (all p>.1). Motor threshold redetermination was performed in n=4 participants in the AUD group and n=9 participants in the non-AUD group; upon recheck the motor thresholds were generally stable (i.e., change less than 2% of stimulator output over the entirety of participation).
Severity of PTSD symptoms (PCL-5)
Results of a mixed-model RMANOVA indicated that, as expected, PTSD symptoms improved significantly from baseline to 1-month follow-up, (linear effect; F(1, 46)=143.77, p=.000, ηp2=.76; large effect), with improvement plateauing at the treatment endpoint (quadratic effect; F(1, 46)=30.11, p=.000, ηp2=.40; large effect). Improvement was greatest among Veterans who received the full iTBS dose (Timepoint × iTBS Group; F(1,46)=4.84, p=.03, ηp2=.10; moderate effect). However, there were no significant effects of AUD group on PTSD symptoms over time (AUD Group × Timepoint; F(2.06)=0.94, p=.40, ηp2=.02; small effect), and the AUD Group × Timepoint × iTBS group interaction was also not significant (F(2.06)=0.93, p=.09, ηp2=.05; small effect). Participants in the AUD group also did not differ on PCL-5 scores on average (F(1, 46)=0.20, p=.66, ηp2=.004, very small effect). Taken together, AUD did not impact PTSD symptom trajectories in response to active or sham treatment (see Figures 1A & 1B).
Figure 1.
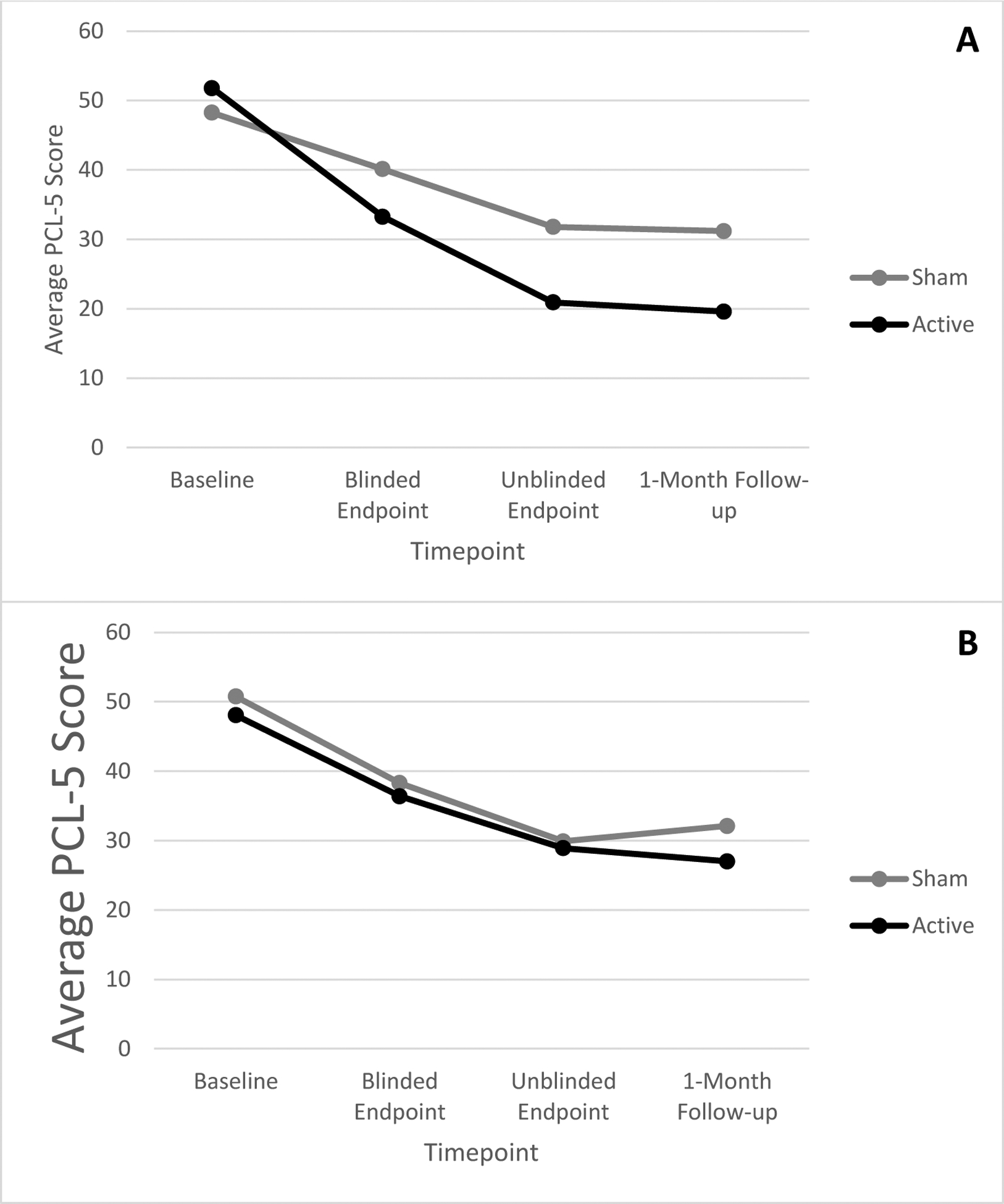
1A. Changes in severity of PTSD symptoms as a function of time and iTBS group among veterans with an Alcohol Use Disorder. 1B. Changes in severity of PTSD symptoms as a function of time and iTBS group among veterans without an Alcohol Use Disorder.
Note. PCL-5 = PTSD Checklist-5.
Severity of depressive symptoms (IDS-SR)
Veterans with an AUD diagnosis did show different patterns of treatment response with regards to depressive symptoms. Overall, as observed in PTSD, depressive symptoms improved from baseline to 1-month follow-up (linear effect; F(1, 46)=67.46, p=.000, ηp2=.60; large effect), and gains levelled off at the treatment endpoint (quadratic effect; F(1, 46)=18.41, p=.000, ηp2=.29; large effect), with improvement greatest among veterans who received the full iTBS dose (Timepoint × iTBS Group; F(1,46)=6.84, p=.01, ηp2=.13; moderate effect). However, unlike the PTSD analysis described above, AUD group moderated the effects of iTBS treatment group on depressive symptoms over time (linear Timepoint × iTBS Group × AUD Group; F(1,46)=7.58, p=.008, ηp2=.14; moderate effect). Patients with an AUD who received the full iTBS dose (versus those who received half the dose) demonstrated a sharper rate of improvement in depression symptoms from baseline to 1-month follow-up (Timepoint × iTBS Group; F(1,15)=8.91, p=.009, ηp2=.37; large effect; see Figure 2A). Patients who did not receive the full dose did not significantly improve in depressive symptoms over time. In contrast, patients without AUD showed similar degrees of improvement in depressive symptoms over time, regardless of iTBS dose (Timepoint × iTBS Group; F(1,31)=0.02, p=.90, ηp2=.00; see Figure 2B).
Figure 2.
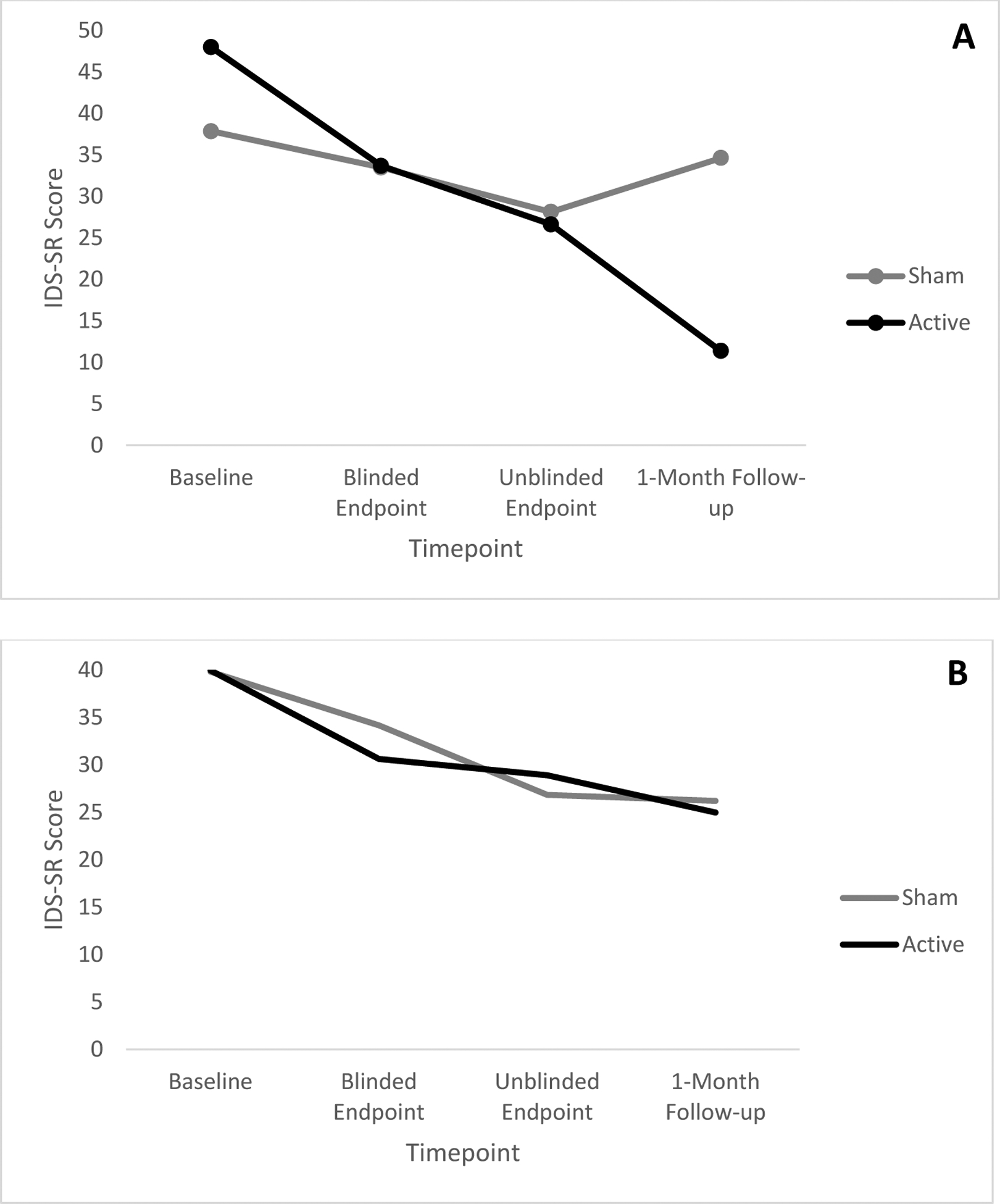
2A. Changes in severity of depressive symptoms as a function of time and iTBS group among veterans with an Alcohol Use Disorder. 1B. Changes in severity of depressive symptoms as a function of time and iTBS group among veterans without Alcohol Use Disorder.
Note. IDS-SR = Inventory of Depressive Symptoms – Self-report.
DISCUSSION
Although neurostimulation is a promising treatment for both depression and posttraumatic stress disorder13, 23, clinical trials often exclude patients with an AUD due to potential concerns about the negative impact of AUD on safety and clinical outcomes. To our knowledge, this study was the first to examine effects of a comorbid AUD diagnosis on PTSD and depressive symptom improvement, drawing on a secondary data analysis of a clinical trial of iTBS for PTSD. Overall, iTBS was safe as delivered, and PTSD symptom trajectories appeared to be orthogonal to AUD diagnosis, in that both groups showed similar trajectories of PTSD improvement across treatment conditions. However, improvement in depressive symptoms varied as a function of treatment dose among patients with an AUD. Taken together, these findings provide initial support for the use of iTBS to treat depression and PTSD among patients with an AUD, and have important implications for clinical practice and future research.
These results suggest that a comorbid AUD is not contraindicated for iTBS treatment for PTSD and depressive symptoms. Indeed, that a comorbid AUD diagnosis was not associated with PTSD symptom trajectories, or symptom improvement as a function of treatment dose, suggests that iTBS can be effectively employed for PTSD treatment among patients regardless of an AUD diagnosis. However, results were more nuanced for treatment of depressive symptoms among patients with an AUD. Specifically, patients with an AUD showed significant reductions in depressive symptom severity over time – but only when given the full iTBS dose. Moreover, patients with an AUD who did not receive the full iTBS dose showed similar levels of depressive symptoms at baseline and 1-month follow-up. This indicates that treatment parameters may be an important consideration when designing future studies of iTBS for depression in AUD patients.
Interestingly, patients with AUD were more likely to report treatment-emergent side effects during their participation, but this was not associated with receiving active or sham stimulation. While our interpretation of this finding is speculative, it indicates that patients with AUD might have a different sensitivity to clinical trial participation more broadly, and as such these findings can help inform the design and conduct of subsequent brain stimulation studies with this patient population.
Furthermore, while the impact of AUD on motor threshold is often a clinical concern, we did not find any concerning patterns. Baseline motor thresholds were nearly identical when comparing AUD and non-AUD patients. Motor threshold redetermination, when clinically indicated, found generally stable thresholds. In fact, participants in the non-AUD group qualitatively required more checking than our AUD participants. Even though motor thresholds were not rechecked regularly as part of the trial, when they were rechecked cortical excitability was largely stable, underscoring the safe nature of the stimulation provided in this patient population.
Limitations
Results of this study should be interpreted in the context of several limitations. Despite leveraging a rigorous, double-blind, sham-controlled study design, the sample size, especially for AUD, used in this study was modest. As such, these results require replication with prospective study. Moreover, as AUD comorbidity was not the primary focus of treatment, we were only able to examine effects of a comorbid AUD diagnosis on study outcomes, and not AUD severity or drinking patterns. We were also unable to examine changes in AUD symptoms over the course of treatment, and alcohol use during stimulation was clinically monitored but not formally quantified. We did not find meaningful differences in motor thresholds or safety reporting between groups over time, but we did not systematically recheck motor thresholds according to a regular schedule and also relied upon spontaneous self-reported side effects. Furthermore, while the parent study included neuroimaging, the sample size of AUD patients was sufficiently small to preclude any rigorous analysis. Since there is a significant overlap in the neurocircuitry of AUD and depression (e.g., reward processing as well as executive control circuits24, 25, future studies should include mechanistic inquiry to identify neurobiological targets to engage. It is important to be mindful that the intensity used here (80% of active MT) represented conservative first use in this patient population. Whether and how the safety observed here corresponds to higher intensity stimulation (e.g., 120% of resting MT) is an important question for further inquiry.
Conclusions
In closing, this analysis indicates that iTBS can be safely used in patients with comorbid AUD, and there is preliminary evidence that stimulation to the right DLPFC may improve clinical outcomes for depression and PTSD. This work provides important data for the design of future randomized controlled studies to prospectively test the efficacy of iTBS in this common patient population.
Highlights.
Comorbid Alcohol Use Disorder (AUD) can complicate treatment of depression and PTSD
We examined whether AUD impacts transcranial magnetic stimulation (TMS) treatment.
AUD was linked with greater depressive symptom improvement from TMS.
A mild comorbid AUD did not impact PTSD symptom change.
These results support the safety and utility of TMS in patients with a comorbid AUD.
ACKNOWLEDGEMENTS
We thank all our participants.
FUNDING AND DISCLOSURE
Effort on this paper was supported in part by the VA RR&D Center for Neurorestoration and Neurotechnology, Department of Veterans Affairs grants I01 RX002450 (NSP, MvWF), I01 HX002572 (MB, NSP), and NIH grant P20 GM130452 (NSP, MvWF). The funders had no role in the conduct of the study, paper preparation, or the decision to submit for publication. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the funders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATIONS OF INTEREST
The authors report no biomedical conflicts of interest related to this work.
References
- 1.Centers for Disease Control and Prevention. Alcoho l& public health: alcohol-related disease impact (ARDI)
- 2.Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, Anthony JCJAogp. Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. 1997;54(4):313–21. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan LE, Fiellin DA, O’Connor PGJTAjom. The prevalence and impact of alcohol problems in major depression: a systematic review. 2005;118(4):330–41. [DOI] [PubMed] [Google Scholar]
- 4.O’Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. 2007;62(11):1208–16. [DOI] [PubMed] [Google Scholar]
- 5.George MS, Lisanby SH, Avery D, McDonald WM, Durkalski V, Pavlicova M, et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. 2010;67(5):507–16. [DOI] [PubMed] [Google Scholar]
- 6.Carmi L, Tendler A, Bystritsky A, Hollander E, Blumberger DM, Daskalakis J, et al. Efficacy and Safety of Deep Transcranial Magnetic Stimulation for Obsessive-Compulsive Disorder: A Prospective Multicenter Randomized Double-Blind Placebo-Controlled Trial. Am J Psychiatry. 2019;176(11):931–8. Epub 2019/05/22. doi: 10.1176/appi.ajp.2019.18101180. [DOI] [PubMed] [Google Scholar]
- 7.Kan RL, Zhang BB, Zhang JJ, Kranz GSJTp. Non-invasive brain stimulation for posttraumatic stress disorder: a systematic review and meta-analysis. 2020;10(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Philip NS, Sorensen DO, McCalley DM, Hanlon CAJN. Non-invasive Brain Stimulation for Alcohol Use Disorders: State of the Art and Future Directions. 2019:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ziemann U, Lönnecker S, Paulus WJB. Inhibition of human motor cortex by ethanol A transcranial magnetic stimulation study. 1995;118(6):1437–46. [DOI] [PubMed] [Google Scholar]
- 10.Nardone R, Bergmann J, Kronbichler M, Caleri F, Lochner P, Tezzon F, et al. Altered motor cortex excitability to magnetic stimulation in alcohol withdrawal syndrome. 2010;34(4):628–32. [DOI] [PubMed] [Google Scholar]
- 11.Blumberger DM, Vila-Rodriguez F, Thorpe KE, Feffer K, Noda Y, Giacobbe P, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. 2018;391(10131):1683–92. [DOI] [PubMed] [Google Scholar]
- 12.Levkovitz Y, Isserles M, Padberg F, Lisanby SH, Bystritsky A, Xia G, et al. Efficacy and safety of deep transcranial magnetic stimulation for major depression: a prospective multicenter randomized controlled trial. 2015;14(1):64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Philip NS, Barredo J, Aiken E, Larson V, Jones RN, Shea MT, et al. Theta-Burst Transcranial Magnetic Stimulation for Posttraumatic Stress Disorder. Am J Psychiatry. 2019;176(11):939–48. Epub 2019/06/25. doi: 10.1176/appi.ajp.2019.18101160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petrosino NJ, Wout-Frank MV, Aiken E, Swearingen HR, Barredo J, Zandvakili A, et al. One-year clinical outcomes following theta burst stimulation for post-traumatic stress disorder. Neuropsychopharmacology. 2020;45(6):940–6. Epub 2019/12/04. doi: 10.1038/s41386-019-0584-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.First M, Williams J, Karg R, Spitzer R. Structured clinical interview for DSM-5—Research version (SCID-5 for DSM-5, research version; SCID-5-RV). Arlington, VA: American Psychiatric Association. 2015:1–94. [Google Scholar]
- 16.Gray MJ, Litz BT, Hsu JL, Lombardo TWJA. Psychometric properties of the life events checklist. 2004;11(4):330–41. [DOI] [PubMed] [Google Scholar]
- 17.Rush AJ, Giles DE, Schlesser MA, Fulton CL, Weissenburger J, Burns C. The inventory for depressive symptomatology (IDS): preliminary findings. Psychiatry research. 1986;18(1):65–87. [DOI] [PubMed] [Google Scholar]
- 18.Weathers FW, Litz BT, Keane TM, Palmieri PA, Marx BP, Schnurr PP. The ptsd checklist for dsm-5 (pcl-5). Scale available from the National Center for PTSD at www ptsd va gov. 2013;10. [Google Scholar]
- 19.Karsen EF, Watts BV, Holtzheimer PEJBS. Review of the effectiveness of transcranial magnetic stimulation for post-traumatic stress disorder. 2014;7(2):151–7. [DOI] [PubMed] [Google Scholar]
- 20.Berlim MT, Van den Eynde FJTCJoP. Repetitive transcranial magnetic stimulation over the dorsolateral prefrontal cortex for treating posttraumatic stress disorder: an exploratory meta-analysis of randomized, double-blind and sham-controlled trials. 2014;59(9):487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baeken C, De Raedt R, Van Schuerbeek P, Vanderhasselt M-A, De Mey J, Bossuyt A, et al. Right prefrontal HF-rTMS attenuates right amygdala processing of negatively valenced emotional stimuli in healthy females. 2010;214(2):450–5. [DOI] [PubMed] [Google Scholar]
- 22.Li C-T, Chen M-H, Juan C-H, Huang H-H, Chen L-F, Hsieh J-C, et al. Efficacy of prefrontal theta-burst stimulation in refractory depression: a randomized sham-controlled study. 2014;137(7):2088–98. [DOI] [PubMed] [Google Scholar]
- 23.Philip NS, Leuchter AF, Cook IA, Massaro J, Goethe JW, Carpenter LLJD, et al. Predictors of response to synchronized transcranial magnetic stimulation for major depressive disorder. 2019;36(3):278–85. [DOI] [PubMed] [Google Scholar]
- 24.Koob GF, Volkow NDJN. Neurocircuitry of addiction. 2010;35(1):217–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koob GF, Volkow NDJTLP. Neurobiology of addiction: a neurocircuitry analysis 2016;3(8):760–73 [DOI] [PMC free article] [PubMed] [Google Scholar]


