Abstract
Inflammasomes play a pivotal role in gastrointestinal homeostasis and inflammation. However, it remains elusive whether the nucleotide-binding oligomerization domain-like receptor (NLR) family inflammasomes, such as NLR family pyrin domain-containing (NLRP) 3, NLRP6, and NLRP12, are involved in the pathogenesis of canine chronic enteropathy (CE), which includes antibiotic-responsive enteropathy (ARE), food-responsive enteropathy (FRE), immunosuppressant-responsive enteropathy (IRE), and non-responsive enteropathy (NRE). Thus, we measured mRNA expression of NLRP3, NLRP6, and NLRP12 in the intestinal mucosa of 35 dogs with CE (ARE, four dogs; FRE, 11 dogs; IRE and NRE, 20 dogs) and seven healthy dogs. As per real-time PCR analysis, significant increases in mRNA expression of NLRP3 and NLRP12 were noted in the colonic but not in the duodenal mucosa of dogs with FRE compared to healthy dogs. These findings suggested that the NLRP3 and NLRP12 inflammasomes might contribute to the development of colitis in dogs with FRE.
Keywords: chronic enteropathy, dog, inflammasome, intestine
Chronic enteropathy (CE) in dogs has been defined as a group of disorders characterized by persistent or recurrent gastrointestinal (GI) signs and mucosal inflammation in the GI tract [2, 5, 26]. CE is comprised of antibiotic-responsive enteropathy (ARE), food-responsive enteropathy (FRE), immunosuppressant-responsive enteropathy (IRE), and non-responsive enteropathy (NRE), depending on the treatment responses [5]. Inflammatory bowel disease (IBD) in dogs includes IRE and NRE [5, 19]. In dogs with CE, intestinal barrier dysfunction, dysbiosis, and inappropriate reactions to dietary components are implicated in the chronic intestinal inflammation [7, 10, 26]. Previous studies have also revealed increased expression of interleukin (IL)-1β protein, a proinflammatory cytokine, in the duodenal and colonic mucosae of dogs with CE [11, 15, 17, 22]. In addition, IL-1β has been identified to have a suppressive effect on mRNA expression of occludin, a tight junction component of intestinal epithelial cells, in the colonic mucosa of healthy dogs [17]. These findings suggest that IL-1β is associated with the development of chronic intestinal inflammation in dogs with CE.
Inflammasomes are identified as intracellular multiprotein complexes composed of a sensor protein, an adaptor protein, and pro-caspase-1 [8, 24]. Inflammasomes play a pivotal role in innate immunity by regulating the secretion of mature IL-1β and IL-18 and the induction of pyroptosis, a form of inflammatory cell death [8, 24]. Among various sensor proteins of inflammasomes, the nucleotide-binding oligomerization domain-like receptor (NLR) family, such as NLR family pyrin domain-containing (NLRP) 1, NLRP3, NLRP6, NLRP12, and NLR family CARD domain containing (NLRC) 4, has been established to regulate intestinal epithelial cell regeneration, microbiota, and mucosal inflammation, thereby maintaining GI homeostasis [1, 3, 16, 24]. In humans with IBD, such as Crohn’s disease (CD) and ulcerative colitis (UC), increases in IL-1β and IL-18 expression are considered key features of the intestinal inflammation [1, 9], pointing towards the potential role of dysregulated activation of these inflammasomes in human IBD pathogenesis. Indeed, increased or decreased mRNA expression of NLRs, as indices of aberrant activation of inflammasomes, was observed in the intestinal mucosa of humans with IBD [4, 20, 21, 25].
Because the pathological and immunological features of CE in dogs are partly similar to those of IBD in humans, dysregulated activation of inflammasomes may contribute to the development of canine CE. However, in a previous study assessing gene expression of inflammasome components in canine intestines, no mRNA transcripts for NLRP1 and NLRC4 were detected in the duodenal and colonic mucosae of healthy dogs and those with CE [22]. In addition, mRNA expression of NLRP3 was observed to decrease in the duodenal mucosa of dogs with CE compared to healthy control dogs [22]. Thus, the significance of inflammasomes in the pathogenesis of canine CE remains elusive.
NLRs of inflammasomes respond to pathogen-associated molecular patterns (PAMPs) and have an important role in the interaction between host and gut microbiota [1, 3, 8, 16, 24]. Given the fact that microbiota are present more abundantly in the large intestine than in the small intestine [6], NLRs of inflammasomes may be activated and expressed aberrantly in the colitis compared to duodenitis in dogs with CE. However, to the best of our knowledge, gene expression of NLRP3 in the colonic mucosa has never been compared between healthy dogs and those with CE. Furthermore, no reports have examined gene expression of other NLRs of inflammasomes in the duodenal and colonic mucosae of dogs with CE. Among the GI tract-related inflammasomes, such as NLRP1, NLRP3, NLRP6, NLRP12, and NLRC4, mRNA transcripts for NLRP1 and NLRC4 could not be identified in the intestinal mucosae of dogs [22]. Therefore, in the present study, we measured mRNA expression of NLRP3, NLRP6, and NLRP12 in the duodenal and colonic mucosae of healthy dogs and those with CE in order to determine the importance of inflammasomes in canine CE.
For this study, we have examined 35 dogs diagnosed with CE at Tokyo University of Agriculture and Technology Animal Medical Center. CE was diagnosed following the methods used by previous studies [2, 17, 18]. Inclusion criteria of CE were chronic GI signs, such as vomiting, small bowel diarrhea (melena, normal fecal frequency, and increased fecal volume), and/or large bowel diarrhea (mucus, hematochezia, tenesmus, increased frequency of defecation, and decreased fecal volume), over a duration of >three weeks, and histopathological evidence of inflammation, such as lymphoplasmacytic enteritis, in the duodenal and/or colonic mucosae obtained by endoscopic biopsy. Other possible causes of chronic GI signs, such as metabolic diseases, infectious diseases including bacterial, viral, and parasitic diseases, exocrine pancreatic insufficiency, hepatic diseases, renal diseases, and neoplasms including alimentary lymphoma were ruled out based on vaccination history and physical and clinical examinations, including blood tests, thoracic and abdominal radiography and ultrasounds, and fecal analysis. Pancreatitis was excluded as a primary cause of vomiting by blood tests and abdominal ultrasound. However, because CE is often accompanied by pancreatitis, it was not completely ruled out in dogs that developed diarrhea without vomiting. After confirmation of CE, FRE and ARE were diagnosed differentially by dietary and antibiotic trails in each dog according to previous reports [17, 18, 23]. In brief, FRE was diagnosed by complete resolution of GI signs with the dietary trial using a hydrolyzed protein diet or a novel protein diet. ARE was diagnosed by complete resolution of GI signs in response to the antibiotic treatment using metronidazole (Flagyl, Shionogi & Co., Ltd., Osaka, Japan; 10–15 mg/kg, per os [PO], q12 hr) or tylosin (Tylan, Eli Lilly Japan K.K., Kobe, Japan; 20 mg/kg, PO, q12 hr). After exclusion of FRE and ARE, IRE was diagnosed by partial or complete resolution of GI signs with prednisolone (Pfizer, Tokyo, Japan; 0.5–2.0 mg/kg, PO, q24 hr). NRE was finally diagnosed by no responses to the immunosuppressants, including prednisolone, budesonide (Zentacoart, Zeria Pharmaceutical Co., Ltd., Tokyo, Japan; 0.2 mg/kg, PO, q24 hr), and chlorambucil (Leukeran, Aspen Pharma, Baar, Switzerland; 3.7–4.7 mg/m2, PO, q24 hr). The clinical severity of 35 dogs with CE was scored according to the canine chronic enteropathy clinical activity index (CCECAI) [2].
Seven healthy intact male beagles (median age, 4.8 years; range, 1.2–6.4 years; median body weight, 11.3 kg; range, 10.1–11.6 kg) were used as a control group (Supplementary Table 1). Dogs were housed in individual cages and fed a commercial diet (Science Diet Adult; Hill’s-Colgate Ltd, Tokyo, Japan). Water was provided ad libitum. All procedures were approved by the institutional animal care and use committee of Tokyo University of Agriculture and Technology (approval numbers: 28-34 and 30-132).
Endoscopic examination and sample collection were performed according to the procedures in previous reports [17, 18]. Dogs were prepared for endoscopy by fasting them for at least 24 hr. Dogs with CE and healthy dogs did not receive any drugs including antibiotics, antidiarrheals, and immunosuppressive agents for at least one week before sample collection. A colon cleansing using saline was conducted under anesthesia. Mucosal biopsy specimens were obtained from all dogs using videoscopes for animals (VQ TYPE 8143B and VQ TYPE 5112B; Olympus Medical Systems, Tokyo, Japan) and endoscopic biopsy forcepses (FB-54Q-1 and VH-142-B52; Olympus Medical Systems) under general anesthesia. Duodenal samples were collected from all dogs. Colonic samples were collected from all healthy control dogs and 33 of 35 dogs with CE. More than six specimens were obtained from each region. The duodenal and colonic biopsy specimens were subjected to histopathological analysis and graded according to the guideline of the World Small Animal Veterinary Association (WSAVA) international gastrointestinal standardization group [26] by a board-certified veterinary anatomic pathologist (HK). A portion of the biopsy sample was stored immediately in RNAlater® Solution (Thermo Fisher Scientific, Waltham, MA, USA), according to the manufacturer’s instruction for preservation until RNA extraction.
After total RNA extraction and cDNA synthesis, real-time PCR analysis for NLRP3, NLRP6, and NLRP12 was performed with a thermal cycling program of 95°C for 30 sec, 40 cycles of 95°C for 5 sec and 60°C for 31 sec, then 95°C for 15 sec, 60°C for 1 min, and 95°C for 15 sec using appropriate reference genes for the duodenum and colon (Supplementary Table 2) as has been described previously [18]. Sequences of primers for real-time PCR (Supplementary Table 2) were designed by a perfect real time support system (Takara Bio, Kusatsu, Japan) and according to the previous report [18]. The fluorescence intensity of PCR products was measured in real-time using the ABI Prism 7000 Sequence Detection System (Thermo Fisher Scientific, Waltham, MA, USA). Relative mRNA expression of target genes was determined by the 2-ΔCt method, wherein each value was presented as an n-fold difference relative to the geometric mean of the three reference genes.
The normality of all data was analyzed by the Shapiro–Wilk test. When the Shapiro–Wilk test showed P>0.05, the distribution was assumed to be normal, and data were analyzed by the parametric tests; when P<0.05, data were analyzed by the nonparametric tests. Data between two groups were compared by the unpaired t-test or the Mann–Whitney U test. Data among more than two groups were compared by the one-way analysis of variance (ANOVA) or the Kruskal–Wallis test. When significant difference was detected among the groups, data between each pair were analyzed by the Tukey–Kramer or the Steel–Dwass test as a post hoc analysis. Correlations between two parameters were evaluated by the Pearson product-moment correlation coefficient or the Spearman’s rank correlation coefficient. The Fisher’s exact test was used to determine an association between two categorical variables. Statistical analyses except for the Fisher’s exact test were performed using BellCurve for Excel software version 3.20 (Social Survey Research Information Co., Ltd., Tokyo, Japan). The Fisher’s exact test was performed using EZR on R commander version 1.42 [13]. P<0.05 was considered to be statistically significant.
The clinical and histopathological characteristics and blood test results of 35 dogs with CE are summarized in Supplementary Tables 3 and 4. Median age of dogs with CE was 8.0 years (range, 1.8–14.1 years), and median body weight was 6.2 kg (range, 1.8–33.6 kg). Median CCECAI at the first visit was 7 (range, 1–16). Median WSAVA scores in the duodenum and colon were 9 (range, 3–15) and 5 (range, 1–9), respectively. Among the 35 dogs with CE, four, 11, 18, and two dogs were diagnosed with ARE, FRE, IRE, and NRE, respectively. Because NRE was diagnosed in only two dogs, IRE and NRE were combined and analyzed as the same group, as reported previously [19].
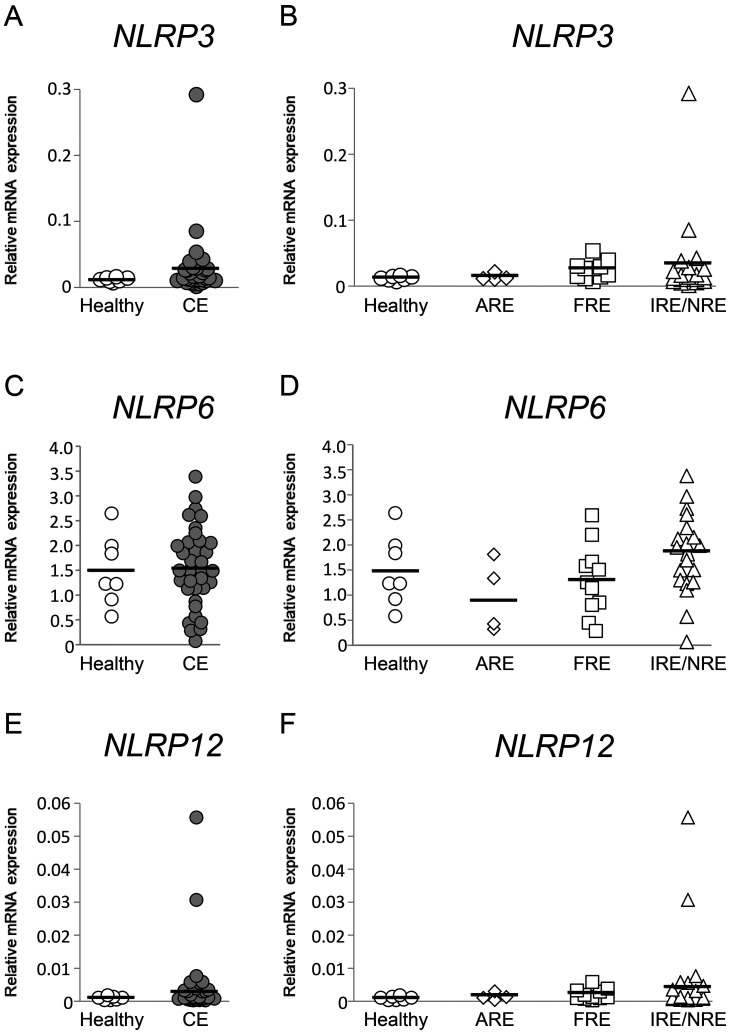
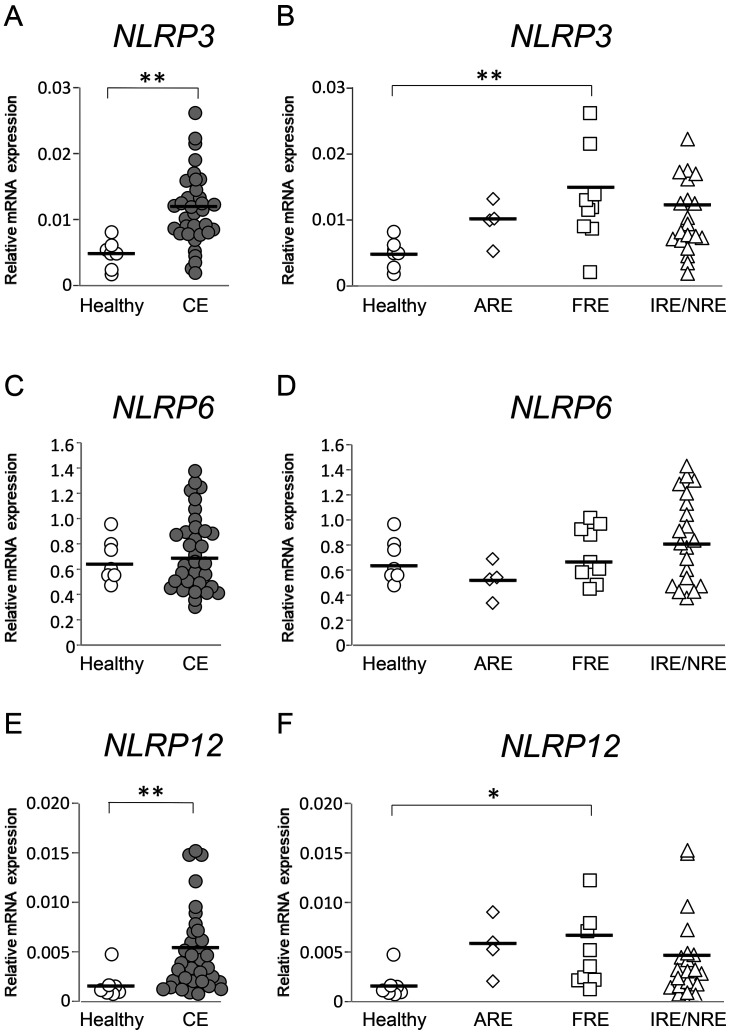
NLRP3, NLRP6, and NLRP12 mRNA expression levels in the duodenal and colonic mucosae were compared between healthy dogs and those with CE. Those results were then compared among healthy dogs and those with ARE, FRE, or IRE/NRE. In the duodenal mucosa, NLRP3, NLRP6, and NLRP12 mRNA expression levels were not significantly different between healthy dogs and those with CE (Fig. 1A, 1C, and 1E; P>0.05, respectively) and among healthy dogs and those with ARE, FRE, or IRE/NRE (Fig. 1B, 1D, and 1F; P>0.05, respectively). In the colonic mucosa, NLRP3 and NLRP12 mRNA expression levels were significantly higher in dogs with CE than in healthy dogs (Fig. 2A and 2E; P<0.01, respectively). Among healthy dogs and those with ARE, FRE, or IRE/NRE, these expression levels were significantly higher in dogs with FRE than in healthy dogs (Fig. 2B and 2F; P<0.05, respectively). A significant correlation between NLRP3 and NLRP12 mRNA expression levels was detected in the colonic mucosa of dogs with CE (Supplementary Table 5; rs=0.3636, P<0.05), whereas it was not found in the colonic mucosa of dogs with FRE (Supplementary Table 5; r=0.3272, P>0.05). In contrast to NLRP3 and NLRP12, NLRP6 mRNA expression levels in the colonic mucosa were not significantly different between healthy dogs and those with CE (Fig. 2C; P>0.05) and among healthy dogs and those with ARE, FRE, or IRE/NRE (Fig. 2D; P>0.05, respectively).
Fig. 1.
Relative mRNA expression of inflammasome sensor subunits in the duodenal mucosa of dogs with chronic enteropathy (CE). Expression levels of nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing (NLRP)3 (A and B), NLRP6 (C and D), and NLRP12 (E and F) mRNA were analyzed by real-time PCR in healthy dogs (n=7) and those with CE (n=35), which included dogs with antibiotic-responsive enteropathy (ARE) (n=4), food-responsive enteropathy (FRE) (n=11), and immunosuppressant-responsive enteropathy (IRE)/non-responsive enteropathy (NRE) (n=20). The horizontal lines in each group represent the mean value. Relative mRNA expression was compared between healthy dogs and those with CE by the Mann–Whitney U test (A and E) or the unpaired t-test (C). Relative mRNA expression was compared among healthy dogs and those with ARE, FRE, or IRE/NRE by the Kruskal–Wallis test (B and F) or the one-way ANOVA (D).
Fig. 2.
Relative mRNA expression of inflammasome sensor subunits in the colonic mucosa of dogs with chronic enteropathy (CE). Expression levels of nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing (NLRP)3 (A and B), NLRP6 (C and D), and NLRP12 (E and F) mRNA were analyzed by real-time PCR in healthy dogs (n=7) and those with CE (n=33), which included dogs with antibiotic-responsive enteropathy (ARE) (n=4), food-responsive enteropathy (FRE) (n=9), and immunosuppressant-responsive enteropathy (IRE)/non-responsive enteropathy (NRE) (n=20). The horizontal lines in each group represent the mean value. Relative mRNA expression was compared between healthy dogs and those with CE by the Mann–Whitney U test (A, C, and E). Relative mRNA expression was compared among healthy dogs and those with ARE, FRE, or IRE/NRE by the one-way ANOVA, followed the Tukey–Kramer test (B), or by the Kruskal-Wallis test, followed the Steel-Dwass test (D and F). *P<0.05, **P<0.01.
Since NLRP3 and NLRP12 mRNA expression levels in the colonic mucosa were significantly higher in dogs with FRE than in healthy dogs, we investigated whether large bowel diarrhea was more frequently observed in dogs with FRE than those with ARE or IRE/NRE. However, there was no significant association between the number of dogs that developed large bowel diarrhea and ARE, FRE, or IRE/NRE (Supplementary Table 6; P>0.05).
To further evaluate whether increased NLRP3 and NLRP12 mRNA expression levels in the colonic mucosa were associated with clinical and histopathological severity, the correlations between gene expression levels and CCECAI or WSAVA score in the colon were examined in dogs with CE and FRE. However, no significant correlations were detected in any combination (Supplementary Table 7; P>0.05, respectively).
Real-time PCR analysis revealed variations of NLRP3, NLRP6, and NLRP12 mRNA expression levels in the duodenal and colonic mucosae of healthy dogs and dogs with CE. Thus, possible influence of sex and age on these expression levels was analyzed in 42 dogs that included seven healthy dogs and 35 dogs with CE. NLRP3 mRNA expression levels were significantly higher in females than males in the duodenal and colonic mucosae (Table 1; P<0.05, respectively). In addition, a significant correlation was detected between age and NLRP12 mRNA expression levels in the duodenal mucosa (Table 2; rs=0.4140, P<0.05).
Table 1. The differences in mRNA expression levels of inflammasome sensor subunits between males and females in 42 dogs that included seven healthy dogs and 35 dogs with chronic enteropathy.
| Intestinal site | Gene | Male | Female | P value |
|---|---|---|---|---|
| Duodenum | NLRP3a) | 0.0122 (0.0010–0.0847) | 0.0259 (0.0071–0.2920) | 0.0134 |
| NLRP6b) | 1.5862 (0.5708–3.3909) | 1.4210 (0.0765–2.9794) | 0.4456 | |
| NLRP12c) | 0.0010 (0.0003–0.0307) | 0.0012 (0.0003–0.0557) | 0.2710 | |
| Colon | NLRP3 | 0.0078 (0.0020–0.0223) | 0.0125 (0.0046–0.0262) | 0.0096 |
| NLRP6 | 0.7128 (0.3728–1.3771) | 0.6537 (0.3197–1.0755) | 0.5956 | |
| NLRP12 | 0.0023 (0.0008–0.0154) | 0.0036 (0.0012–0.0151) | 0.2407 | |
Expression levels of mRNA are shown as median (range). Data were compared between males and females by the Mann-Whitney U test (NLRP3 and NLRP12 in the duodenal mucosa and NLRP6 and NLRP12 in the colonic mucosa) or the unpaired t-test (NLRP6 in the duodenal mucosa and NLRP3 in the colonic mucosa). In the duodenal mucosa, the numbers of males and females were 26 and 16, respectively; in the colonic mucosa, those of males and females were 25 and 15, respectively. a)Nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 3, b)Nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 6, c)Nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 12.
Table 2. The correlations between age and mRNA expression levels of inflammasome sensor subunits in the duodenal and colonic mucosae of 42 dogs that included seven healthy dogs and 35 dogs with chronic enteropathy.
| Duodenum (n=42) |
Colon (n=40) |
||||||
|---|---|---|---|---|---|---|---|
| NLRP3a) | NLRP6b) | NLRP12c) | NLRP3 | NLRP6 | NLRP12 | ||
| Age | rs, r value | rs=0.2432 | r=0.1067 | rs=0.4140 | r=0.0833 | rs=0.2861 | rs=0.2016 |
| P value | 0.1207 | 0.1207 | 0.0064 | 0.092 | 0.0735 | 0.2122 | |
The correlations between age and mRNA expression levels of NLRP3 or NLRP12 in the duodenal mucosa and between age and those of NLRP6 or NLRP12 in the colonic mucosa were analyzed by the Spearman’s rank correlation coefficient (rs). The correlations between age and those of NLRP6 in the duodenal mucosa or NLRP3 in the colonic mucosa were analyzed by the Pearson product-moment correlation coefficient (r). a)Nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 3, b)Nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 6, c)Nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 12.
Our study has demonstrated significant increases in mRNA expression of NLRP3 and NLRP12 in the colonic mucosa of dogs with CE, especially in those with FRE, compared to that in healthy dogs. Significant differences were not detected between healthy dogs and those with CE in expression levels of NLRP3 and NLRP12 mRNA in the duodenal mucosa and NLRP6 mRNA in the duodenal and colonic mucosae. These findings suggest that the NLRP3 and NLRP12 inflammasomes might contribute to the development of colitis in dogs with FRE among CE. However, we could not find significant increase in the number of dogs that developed large bowel diarrhea in FRE among CE. In addition, we could not analyze possible association between large bowel diarrhea and NLRP3 or NLRP12 mRNA expression levels in dogs with FRE due to the small number of dogs with FRE in this study. To clarify clinical significance of the NLRP3 and NLRP12 inflammasomes in FRE, further studies are required.
The NLRP3 inflammasome is mainly expressed in macrophages, dendritic cells, and intestinal epithelial cells in the GI tract of humans, and promotes GI inflammation [1]. The NLRP3 inflammasome is activated by two signals. The first signal, also known as the priming signal, is initiated by the toll-like receptor (TLR) and nuclear factor-κB (NF-κB) pathway, which in turn leads to the upregulation of NLRP3 and pro-IL-1β and pro-IL-18 expressions. The second signal is induced by various pathogens, pore-forming toxins, adenosine triphosphate, and particulate crystals, and it triggers the assembly of the NLRP3 inflammasome components, resulting in the activation of caspase-1, which then promotes cleavage of pro-IL-1β and pro-IL-18 into mature forms [8, 24]. In dogs with lymphocytic-plasmacytic enteritis, a histopathological feature of CE, intestinal permeability was shown to increase [14]. In the barrier-disrupted intestines of dogs with CE, it is plausible that enteric bacteria-derived PAMPs, such as lipopolysaccharide, present in the gut may penetrate the epithelial barrier, which then activates the NLRP3 inflammasome. A previous study reported that mRNA expression of NLRP3 in the duodenal mucosa was significantly lower in dogs with CE than in healthy control dogs [22]. In contrast to the previous finding, in dogs with CE, we did not detect any significant decrease in mRNA expression of NLRP3 in the duodenal mucosa, but instead found a significant increase in mRNA expression of NLRP3 in the colonic mucosa, particularly in dogs with FRE. Similarly, increased mRNA expression of NLRP3 was reported in the colonic mucosa of humans with UC and CD [20, 25]. Upregulated expression of NLRP3 mRNA indicates activation of the NLRP3 inflammasome through the priming signal by the TLR and NF-kB pathway [12]. Therefore, our results suggest that activation of the NLRP3 inflammasome might contribute to the chronic inflammation in the colon, where abundant microbiota are present, in dogs with FRE. In addition, although a significant difference was not detected except for the colonic mucosa of dogs with FRE, mRNA expression of NLRP3 was found to be elevated in the duodenal and colonic mucosae of some dogs with CE. These findings imply that the pathogenesis of canine CE could be at least in part classified by the NLRP3 inflammasome-dependent and -independent mechanisms.
The NLRP12 inflammasome has been determined to exert anti-inflammatory functions by suppressing NF-κB activation [1, 3]. Cell types expressing the NLRP12 inflammasome in the GI tract has not been fully understood, but myeloid cells in the GI tract were shown to express it [1]. A previous study demonstrated that mRNA expression of NLRP12 was significantly downregulated in humans with active UC compared to healthy controls [4]. In addition, NLRP12 deficiency in mice promoted colitis in an experimental model for UC [4]. These results indicate that reduced expression of NLRP12 may cause colonic inflammation in UC. In contrast, we detected increased expression of NLRP12 mRNA in the colonic mucosa of dogs with CE. Similar to our study, upregulation of NLRP12 mRNA was also reported in the colonic mucosa of humans with CD and UC [21, 25]. The mechanisms underlying enhanced expression of NLRP12 mRNA were not determined in the colonic mucosa of dogs with CE and humans with IBD. NLRP12 functions as a negative regulator of inflammatory responses [1]. In addition, the present study revealed the significant correlation between NLRP3 and NLRP12 mRNA expression levels in the colonic mucosa of dogs with CE. These findings suggest that expression of NLRP12 might be stimulated and upregulated during the intestinal inflammation in order to dampen the spread of colitis that might have been promoted by NLRP3 in dogs with CE. This possibility must be addressed and examined in additional studies.
NLRP6 has a regulatory role in GI homeostasis by maintaining gut microbiota and epithelial repair and proliferation [1, 3]. It is currently unknown whether the expression of NLRP6 is reduced in the intestinal mucosa of humans with IBD. Instead, a previous study reported that mRNA expression of NLRP6 was increased in the ileal and colonic mucosae of humans with CD [21]. However, the biological importance of high levels of NLRP6 transcript remains to be determined in humans with IBD. In this study, no significant difference was detected between healthy dogs and those with CE in mRNA expression of NLRP6 in the duodenal and colonic mucosae, suggesting that a pathological role of NLRP6 may be limited in canine CE.
In addition to NLR family inflammasomes, other family inflammasomes, including absent in melanoma 2 and interferon γ-inducible protein 16, were reported to be upregulated in the colonic mucosa of humans with CD and UC [25]. However, since the sequences of canine genes for these inflammasome sensor subunits were not well characterized in the GenBank database, we did not analyze them in this study.
There are three major limitations in this study. First, we measured only mRNA expression of NLRPs in the intestinal mucosae of a small number of dogs with CE. In particular, the number of dogs with ARE was only four, which might not be enough to bring statistical power to compare with others. Thus, further studies should determine the expression and distribution of NLRPs at the protein level in the duodenal and colonic mucosae of dogs with CE, using a larger sample size. It is also necessary to assess the caspase-1 activity for the functional assay of the NLRP inflammasomes in the intestinal mucosae of dogs with CE. Second, we could not examine protein expression of pro-IL-1β, IL-1β, pro-IL-18, and IL-18, because samples for protein analysis were not available. To further clarify the activation status of the NLRP inflammasomes, it might be beneficial to investigate the correlation between the expression levels of NLRPs and those of pro-IL-1β, IL-1β, pro-IL-18, and IL-18 at the protein level. Third, because we found significant influence of sex and age on NLRP3 and NLRP12 mRNA expression levels in the duodenal and colonic mucosae of dogs, sex-, age-, and breed-matched healthy dogs should be used as a control group for CE to exclude the bias. This must be resolved in future studies.
In conclusion, our study revealed that mRNA expression of NLRP3 and NLRP12 increased in the colonic but not in the duodenal mucosa of dogs with FRE among CE. The findings suggest the possibility that duodenitis and colitis were differently regulated by the NLRP3 and NLRP12 inflammasomes in dogs with CE. The imbalance between proinflammatory functions of the NLRP3 inflammasome and anti-inflammatory functions of the NLRP12 inflammasome might be involved in colitis in dogs with FRE. However, it remains unclear how these and other inflammasomes contribute to the development of duodenitis and colitis in dogs with CE. Further studies are necessary to clarify the roles of inflammasomes in the pathogenesis of canine CE.
CONFLICT OF INTEREST
The authors declare no conflicts of interest associated with this manuscript.
Supplementary
REFERENCES
- 1.Aguilera M., Darby T., Melgar S.2014. The complex role of inflammasomes in the pathogenesis of Inflammatory Bowel Diseases - lessons learned from experimental models. Cytokine Growth Factor Rev. 25: 715–730. doi: 10.1016/j.cytogfr.2014.04.003 [DOI] [PubMed] [Google Scholar]
- 2.Allenspach K., Wieland B., Gröne A., Gaschen F.2007. Chronic enteropathies in dogs: evaluation of risk factors for negative outcome. J. Vet. Intern. Med. 21: 700–708. doi: 10.1111/j.1939-1676.2007.tb03011.x [DOI] [PubMed] [Google Scholar]
- 3.Chen G. Y.2014. Role of Nlrp6 and Nlrp12 in the maintenance of intestinal homeostasis. Eur. J. Immunol. 44: 321–327. doi: 10.1002/eji.201344135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen L., Wilson J. E., Koenigsknecht M. J., Chou W. C., Montgomery S. A., Truax A. D., Brickey W. J., Packey C. D., Maharshak N., Matsushima G. K., Plevy S. E., Young V. B., Sartor R. B., Ting J. P.2017. NLRP12 attenuates colon inflammation by maintaining colonic microbial diversity and promoting protective commensal bacterial growth. Nat. Immunol. 18: 541–551. doi: 10.1038/ni.3690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dandrieux J. R.2016. Inflammatory bowel disease versus chronic enteropathy in dogs: are they one and the same? J. Small Anim. Pract. 57: 589–599. doi: 10.1111/jsap.12588 [DOI] [PubMed] [Google Scholar]
- 6.Donaldson G. P., Lee S. M., Mazmanian S. K.2016. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 14: 20–32. doi: 10.1038/nrmicro3552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eissa N., Kittana H., Gomes-Neto J. C., Hussein H.2019. Mucosal immunity and gut microbiota in dogs with chronic enteropathy. Res. Vet. Sci. 122: 156–164. doi: 10.1016/j.rvsc.2018.11.019 [DOI] [PubMed] [Google Scholar]
- 8.Franchi L., Muñoz-Planillo R., Núñez G.2012. Sensing and reacting to microbes through the inflammasomes. Nat. Immunol. 13: 325–332. doi: 10.1038/ni.2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gagliani N., Palm N. W., de Zoete M. R., Flavell R. A.2014. Inflammasomes and intestinal homeostasis: regulating and connecting infection, inflammation and the microbiota. Int. Immunol. 26: 495–499. doi: 10.1093/intimm/dxu066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.German A. J., Hall E. J., Day M. J.2003. Chronic intestinal inflammation and intestinal disease in dogs. J. Vet. Intern. Med. 17: 8–20. doi: 10.1111/j.1939-1676.2003.tb01318.x [DOI] [PubMed] [Google Scholar]
- 11.Hawes M., Riddle A., Kirk J., Jergens A., Allenspach K.2018. Interleukin-1β expression is increased in the duodenum of dogs with idiopathic inflammatory bowel disease. Vet. Rec. 183: 536. doi: 10.1136/vr.104495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jo E. K., Kim J. K., Shin D. M., Sasakawa C.2016. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell. Mol. Immunol. 13: 148–159. doi: 10.1038/cmi.2015.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanda Y.2013. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 48: 452–458. doi: 10.1038/bmt.2012.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobayashi S., Ohno K., Uetsuka K., Nakashima K., Setoguchi A., Fujino Y., Tsujimoto H.2007. Measurement of intestinal mucosal permeability in dogs with lymphocytic-plasmacytic enteritis. J. Vet. Med. Sci. 69: 745–749. doi: 10.1292/jvms.69.745 [DOI] [PubMed] [Google Scholar]
- 15.Maeda S., Ohno K., Nakamura K., Uchida K., Nakashima K., Fukushima K., Tsukamoto A., Goto-Koshino Y., Fujino Y., Tsujimoto H.2012. Mucosal imbalance of interleukin-1β and interleukin-1 receptor antagonist in canine inflammatory bowel disease. Vet. J. 194: 66–70. doi: 10.1016/j.tvjl.2012.02.026 [DOI] [PubMed] [Google Scholar]
- 16.Man S. M.2018. Inflammasomes in the gastrointestinal tract: infection, cancer and gut microbiota homeostasis. Nat. Rev. Gastroenterol. Hepatol. 15: 721–737. doi: 10.1038/s41575-018-0054-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogawa M., Osada H., Hasegawa A., Ohno H., Yanuma N., Sasaki K., Shimoda M., Shirai J., Kondo H., Ohmori K.2018. Effect of interleukin-1β on occludin mRNA expression in the duodenal and colonic mucosa of dogs with inflammatory bowel disease. J. Vet. Intern. Med. 32: 1019–1025. doi: 10.1111/jvim.15117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osada H., Ogawa M., Hasegawa A., Nagai M., Shirai J., Sasaki K., Shimoda M., Itoh H., Kondo H., Ohmori K.2017. Expression of epithelial cell-derived cytokine genes in the duodenal and colonic mucosae of dogs with chronic enteropathy. J. Vet. Med. Sci. 79: 393–397. doi: 10.1292/jvms.16-0451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pierini A., Esposito G., Gori E., Benvenuti E., Ruggiero P., Lubas G., Marchetti V.2021. Platelet abnormalities and platelet-to-lymphocyte ratios in canine immunosuppressant-responsive and non-responsive enteropathy: A retrospective study in 41 dogs. J. Vet. Med. Sci. 83: 248–253. doi: 10.1292/jvms.20-0291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ranson N., Veldhuis M., Mitchell B., Fanning S., Cook A. L., Kunde D., Eri R.2018. NLRP3-Dependent and -Independent Processing of Interleukin (IL)-1β in Active Ulcerative Colitis. Int. J. Mol. Sci. 20: 57. doi: 10.3390/ijms20010057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ranson N., Veldhuis M., Mitchell B., Fanning S., Cook A. L., Kunde D., Eri R.2018. Nod-like receptor pyrin-containing protein 6 (NLRP6) is up-regulated in Ileal Crohn’s Disease and differentially expressed in goblet cells. Cell. Mol. Gastroenterol. Hepatol. 6: 110–112.e8. doi: 10.1016/j.jcmgh.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmitz S., Werling D., Allenspach K.2015. Effects of ex-vivo and in-vivo treatment with probiotics on the inflammasome in dogs with chronic enteropathy. PLoS One 10: e0120779. doi: 10.1371/journal.pone.0120779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simpson K. W., Jergens A. E.2011. Pitfalls and progress in the diagnosis and management of canine inflammatory bowel disease. Vet. Clin. North Am. Small Anim. Pract. 41: 381–398. doi: 10.1016/j.cvsm.2011.02.003 [DOI] [PubMed] [Google Scholar]
- 24.Strowig T., Henao-Mejia J., Elinav E., Flavell R.2012. Inflammasomes in health and disease. Nature 481: 278–286. doi: 10.1038/nature10759 [DOI] [PubMed] [Google Scholar]
- 25.Vanhove W., Peeters P. M., Staelens D., Schraenen A., Van der Goten J., Cleynen I., De Schepper S., Van Lommel L., Reynaert N. L., Schuit F., Van Assche G., Ferrante M., De Hertogh G., Wouters E. F., Rutgeerts P., Vermeire S., Nys K., Arijs I.2015. Strong Upregulation of AIM2 and IFI16 Inflammasomes in the Mucosa of Patients with Active Inflammatory Bowel Disease. Inflamm. Bowel Dis. 21: 2673–2682. doi: 10.1097/MIB.0000000000000535 [DOI] [PubMed] [Google Scholar]
- 26.Washabau R. J., Day M. J., Willard M. D., Hall E. J., Jergens A. E., Mansell J., Minami T., Bilzer T. W., WSAVA International Gastrointestinal Standardization Group.2010. Endoscopic, biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. J. Vet. Intern. Med. 24: 10–26. doi: 10.1111/j.1939-1676.2009.0443.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.