Abstract
A 12-year-old, 3.5-kg, intact female dog was presented with polyuria, polydipsia, and a pendulous abdomen. Laboratory examinations showed elevated hepatobiliary enzyme levels and neutrophilic leukocytosis. The adrenocorticotropic hormone stimulation test confirmed hyperadrenocorticism (HAC). Trilostane therapy managed the clinical condition and cortisol concentration. However, lymphocytosis and nonregenerative anemia developed after HAC remission. Bone marrow aspiration analysis revealed a lymphoproliferative disorder with a clonal T-cell population. Accordingly, the patient was diagnosed with T-cell chronic lymphocytic leukemia (CLL) and concurrent HAC. Thereafter, chemotherapy was initiated, which improved the lymphocytosis. However, euthanasia was performed because of worsening quality of life at 45 weeks after the first presentation. These results suggested that CLL could be masked by excessive endogenous cortisol and discovered after HAC remission.
Keywords: canine, chronic lymphocytic leukemia, hyperadrenocorticism, lymphocytosis
Hyperadrenocorticism (HAC), also known as Cushing’s disease, is a relatively common endocrine disease in middle-aged to older dogs [15]. HAC is caused by a signaling dysfunction along the hypopituitary-adrenal axis that induces hypercortisolemia [7]. Canine patients with HAC commonly show a stress leukogram, which indicates neutrophilia, monocytosis, eosinopenia, and lymphopenia [16, 21]. In particular, up to 80% of dogs with excessive cortisol secretion have lymphopenia due to steroid-induced lympholysis [15]. Another hematological feature of HAC is mild erythrocytosis [16].
Chronic lymphocytic leukemia (CLL) is a type of leukemia characterized by the excessive production of clonal lymphoid cells in the bone marrow, and it generally occurs in middle-aged to older animals [5, 25]. Leukemic cells in patients with CLL are morphologically similar to normal lymphocytes, but they function abnormally [14]. Since mature cells also proliferate under nonneoplastic conditions, clinicians must distinguish chronic leukemias from reactive proliferations [4]. Chronic leukemia is often diagnosed by ruling out other conditions [4]. However, the clinical signs of CLL are nonspecific, including lethargy, inappetence, and gradual weight loss, and the patients are generally asymptomatic [24, 25]. Several reports documented that more than 50% of canine patients with CLL have anemia [25]. Although patients with T-cell CLL have long survival times, those with anemia have a poor prognosis [6].
In human medicine, several studies reported that HAC masked concurrent steroid-responsive disease such as non-Hodgkin’s lymphoma and sarcoidosis [9, 23]. However, no case of CLL masked by co-existing HAC has been reported to date in veterinary or human medicine. To our knowledge, this is the first report in veterinary medicine describing the presentation, diagnosis, clinical course, treatment, and outcome of CLL masked by endogenous cortisol resulting from concurrent HAC.
A 12-year-old, 3.5-kg, intact female Maltese dog was referred to the Veterinary Medical Teaching Hospital of Konkuk University for evaluating suspected HAC. The patient had a history of symmetrical alopecia, polyuria, polydipsia, and panting. On physical examination, thin and erythematous skin, especially at the distended abdomen was noted. A complete blood count (CBC) revealed normal packed cell volume (PCV) (44.9%; reference interval (RI): 37.3–61.7%), thrombocytosis (802 × 103/µl; RI: 143.3–400 × 103/µl), and neutrophilic leukocytosis (30.97 × 109/l; RI: 5.2–13.9 × 109/l). Serum chemical analysis revealed increased levels of aspartate aminotransferase (71 U/l; RI: 15–43 U/l), alanine transaminase (258 U/l; RI: 19–70 U/l), alkaline phosphatase (485 U/l; RI: 15–127 U/l), gamma glutamyltransferase (73 mg/dl; RI: 0–12 mg/dl), lactate (4.5 mg/dl; RI: 0.5–2.5 mg/dl) and symmetrical dimethylarginine (SDMA) (19 µg/dl; RI: 0–14 µg/dl).
Ultrasonography revealed enlarged adrenal glands, and the adrenocorticotropic hormone stimulation test showed cortisol levels of 9 µg/dl (RI: 0.5–10 µg/dl) and 29.8 µg/dl (RI: 6–18 µg/dl) pre and post the test, respectively. Ultrasonography also incidentally revealed hyperechoic renal cortical parenchyma with diffusely hyperechoic foci and an anechoic cystic structure, as well as heterogenous pancreatic parenchyma. Also, SNAP canine pancreas lipase kit (Canine SNAP® cPLTM; IDEXX Laboratories Inc., Westbrook, ME, USA) test showed a positive result. Based on these results, the patient was diagnosed with HAC, chronic pancreatitis, and chronic kidney disease at the first hospital visit. The patient was treated with trilostane (Vetoryl; Dechra Veterinary Products Ltd., Sansaw, UK) 2 mg/kg twice daily per os (PO). Also, considering that neutrophilic leukocytosis is commonly seen in the patient with infection [13], amoxicillin-clavulanate (Amocla; Kuhnil Pharm., Chungnam, Korea) 12.5 mg/kg twice daily PO was prescribed as a prophylactic antibiotic for 4 weeks. A low-fat diet (Royal Canin Gastrointestinal Low-fat; Royal Canin Korea, Gymje, Korea) was recommended as a dietary management of chronic pancreatitis.
After 5 weeks of oral trilostane treatment, the patient’s clinical signs, including polyuria, polydipsia, and panting, disappeared. Moreover, the serum cortisol concentration was well managed (post-test cortisol concentration: 8.7 µg/dl; RI: 1.5–9.0 µg/dl). Trilostane of initial dosage was continuously administered (Fig. 1). Although leukocyte count decreased (white blood cells: 18.6 × 109/l; RI: 5.2–13.9 × 109/l), lymphocytosis (5.9 × 109/l; RI: 1.05–5.1 × 109/l) was newly found on a CBC (Fig. 1). Also, mild nonregenerative anemia (PCV 36.2%, RI: 37.3–61.7%) was coincidentally shown. Therefore, the previously prescribed antibiotic was continued for another 4 weeks in the light of possible chronic infection, but the lymphocytic leukocytosis gradually worsened. At week 12, the patient showed acute anorexia and lethargy, and was diagnosed with acute pancreatitis on the basis of an abnormal SNAP cPL result (Canine SNAP® cPLTM; IDEXX Laboratories Inc.) and enlarged hypoechoic pancreatic parenchyma on abdominal ultrasonography. One week after treating the patient with supportive care, the SNAP cPL result and pancreatic ultrasonography findings normalized. However, the patient’s clinical signs showed no significant improvement, with persistent lethargy and inappetence. Thereafter, serial CBCs performed over the following months showed persistent lymphocytic leukocytosis. At week 34, a CBC showed leukocytosis (33 × 109/l; RI: 5.2–13.9 × 109/l) and lymphocytosis (18.4 × 109/l; RI: 1.05–5.1 × 109/l). Also, persistent anemia (PCV: 35.7%; RI: 37.3–61.7%) was found at the same time. In the next couple of weeks, anemia deteriorated gradually, and PCV dropped to 26% (RI: 37.3–61.7%) at week 37.
Fig. 1.
Sequential total leukocyte and lymphocyte counts according to the medication administered in a dog with hyperadrenocorticism and chronic lymphocytic leukemia. The solid and dotted lines indicate white blood cells and lymphocytes, respectively. Leukocyte count decreased just after initial administration of trilostane (2 mg/kg, orally twice daily). However, after week 5, leukocyte and lymphocyte had gradually increased until initiation of chemotherapy on week 41. WBCs, white blood cells.
To determine the etiology of the lymphocytic leukocytosis, we performed radiography and ultrasonography, but both examinations yielded unremarkable findings. The test for Ehrlichia infection (SNAP® 4Dx; IDEXX Laboratories Inc.) also yielded a negative result. Blood cytological analysis showed well-differentiated, medium-sized lymphocytes (Fig. 2). Since no evidence of infection or other lymphoproliferative disorders was obtained, a tentative diagnosis of CLL was suggested.
Fig. 2.
Peripheral blood smear from a dog with hyperadrenocorticism and chronic lymphocytic leukemia. Medium-sized cells with mature clumped chromatin, nuclear indentation, and rare nucleoli are observed (arrows) (Wright-Giemsa stain; original magnification ×1,000).
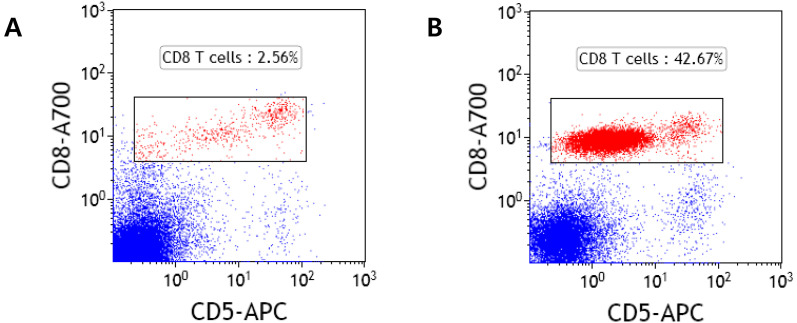
To determine the cell lineage and clonality, bone marrow was aspirated and subjected to the polymerase chain reaction for antigen receptor rearrangement (PARR) assay, immunocytochemical (ICC) staining, and flow cytometry (Clinical Pathology Laboratory of Colorado State University). A high proportion of small to intermediate sized lymphocytes were found on the bone marrow smear, showing more than 40% of all cells (Fig. 3). The PARR assay revealed a clonal T-cell population (Fig. 4). ICC staining revealed that most lymphocytes in the bone marrow were positive for CD3 and only a few were positive for Pax-5 (Fig. 5). Furthermore, flow cytometric immunophenotyping of the bone marrow revealed an expansion of CD8-positive T cells that expressed CD3 and low levels of CD5 (Fig. 6). These findings were compatible with those of T-cell expansion, and taken together with the PARR results, the patient was diagnosed with a T-cell lymphoproliferative disorder. Assuming these were the same cells found in the peripheral blood, this finding would be most consistent with CD8 leukemia. Based on these results, the patient was also diagnosed with T-cell CLL.
Fig. 3.
Bone marrow smear from a dog with hyperadrenocorticism and chronic lymphocytic leukemia. The proportion of lymphocytes appears high, and they appear small to intermediate in size with relatively mature chromatin and scant basophilic cytoplasm (Wright-Giemsa stain, original magnification ×500).
Fig. 4.
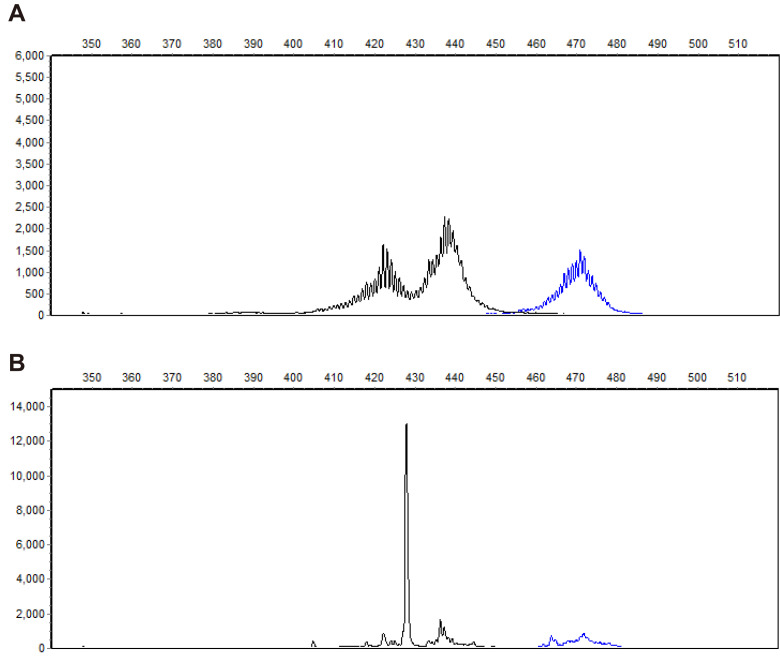
The polymerase chain reaction (PCR) for antigen receptor rearrangement assay findings of a normal canine lymph node (A) and those of a canine patient with hyperadrenocorticism and chronic lymphocytic leukemia (B). A. T-cell clonality shows amplification of T-cell receptor gamma (TRG) genes from the V2 group (blue) and the V3 group (black). B. T-cell clonality reveals amplification of TRG genes showing a single-sized PCR product at 428 base pairs.
Fig. 5.
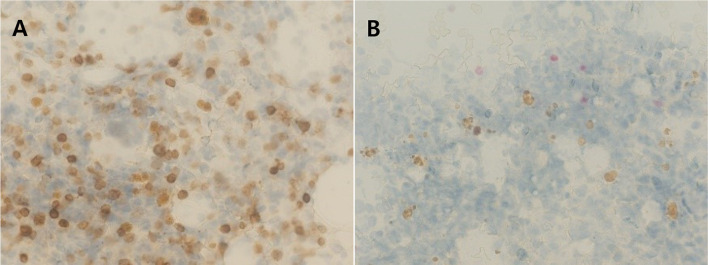
Immunocytochemical staining of the bone marrow cells aspirated from a dog with hyperadrenocorticism and chronic lymphocytic leukemia. A. The cells show strong membranous reactivity for CD3, indicating T-cell leukemia. B. The cells show weak reactivity for PAX-5 (Original magnification ×40; courtesy of Dr. Adam Harris, Colorado State University).
Fig. 6.
Flow cytometry of the bone marrow cells from a normal dog (A) and a dog with hyperadrenocorticism and chronic lymphocytic leukemia (B). A. Flow cytometry shows low percentages of CD8-positive T cells. B. Flow cytometry reveals an expanded population of CD8-positive T cells that express low levels of CD5. Normal ranges are unavailable.
At week 41, the patient was started on metronomic chemotherapy for CLL, after receiving the owner’s consent. The medication included chlorambucil (Lukeran; Samil Pharm., Seoul, Korea) 0.2 mg/kg once daily PO and prednisolone (Solondo; Yuhan Co., Seoul, Korea) 2 mg/kg once daily PO (Fig. 1). After chemotherapy, a gradual decline in the lymphocyte count was observed for 4 weeks. However, the nonregenerative anemia (PCV: 17.8%; RI: 37.3–61.7%) exacerbated simultaneously. Therefore, we subcutaneously injected darbepoetin (NESP Prefilled syringe 60; Kyowa Hakko Kirin Co., Ltd., Tokyo, Japan) at 1 µg/kg twice daily as an erythropoietic agent, but the patient showed no improvement. Owing to the poor prognosis and clinical deterioration because of concurrent underlying diseases, including acute renal failure and acute pancreatitis, the patient was euthanized at the owner’s request at 45 weeks after the first presentation. Unfortunately, the owner did not provide consent for a necropsy.
HAC is a common endocrine disease in dogs [3]. It results from persistent exposure to excessive cortisol, which produces a constellation of biochemical abnormalities and clinical conditions in the patient [16]. The clinical signs include polyuria, polydipsia, pendulous abdomen, and skin lesions, which were present in the current case [10, 12]. Laboratory findings such as eosinopenia and lymphopenia are also common and found in approximately 80% of dogs with HAC [20]. According to the literature, corticosteroids have a lympholytic effect and cause cell apoptosis [25]. Several reports have documented that HAC results in an immunosuppressed condition, making patients susceptible to infection [15, 17]. Considering that dogs with HAC usually have lymphopenia, researchers have speculated that an immunosuppressed condition due to hypercortisolism is associated with the common findings of lymphopenia [15]. Therefore, in the present case, a state of persistent lymphocytosis, which is not usually noted in patients with HAC, strongly suggested that the dog had an infection or another concurrent lymphocytic proliferative disorder.
The differential diagnosis for prolonged lymphocytosis usually includes infectious diseases, autoimmune diseases, hypoadrenocorticism, and neoplastic lymphoproliferative disorders [1]. In particular, chronic Ehrlichia canis infection is a common cause of lymphocytosis in dogs that could elevate the lymphocyte count to 17,000/µl [1]. In the present case, although we continuously performed blood tests, radiography, ultrasonography, and E. canis tests, no probable underlying causes were found. Therefore, tests for immunophenotyping and determining the clonality of the bone marrow cells were needed to distinguish neoplastic from nonneoplastic lymphocytosis [1]. On the basis of the results of flow cytometry, ICC staining, and PARR assay of the bone marrow, the current patient was eventually diagnosed with T-cell CLL. These findings suggest that CLL should be considered a differential diagnosis in patients with HAC and lymphocytosis.
Steroids have been administered to patients with CLL since the 1940s, and the use of high-dose steroids has been proven effective in the treatment of human CLL [22]. However, cases of CLL masked by HAC have not yet been reported in human or veterinary medicine. In human medicine, several studies have demonstrated that endogenous cortisol can suppress coincidental steroid-responsive diseases, including nephrotic syndrome and sarcoidosis [19, 23]. A previous case report also surmised that the progression of non-Hodgkin’s lymphoma in a human was slowed by a concurrent adrenal tumor producing steroids [9]. Kheirandish et al. [11] also reported a clinical case of multicentric lymphoma and concurrent HAC in a dog, in which hepatic tumor-induced hypoglycemia could have been counterbalanced by hyperglycemia secondary to hypercortisolism. However, the findings of that report could not sufficiently prove the correlation between the two disorders because follow-up tests were not conducted during and after HAC remission. In contrast, the present case describes how the clinical course and outcome of CLL varies according to the treatment of concurrent HAC on the basis of biochemical data. Therefore, the present report provides useful evidence to support the associations between the endogenous cortisol produced in HAC and concurrent steroid-responsive disease.
Mild erythrocytosis is a common hematological feature in dogs with HAC [2]. In contrast, mild anemia is found in more than 50% of dogs with CLL [18, 25]. In the present case, after administration of trilostane, anemia has gradually deteriorated as lymphocytosis. Therefore, we speculated that anemia caused by CLL was masked by endogenous glucocorticoids at an early stage and manifested as cortisol suppression. From this perspective, the clinical signs of lethargy and anorexia that the patient showed after week 12 could also have been manifested after HAC remission. However, unexpectedly, anemia deteriorated even after applying chemotherapy to treat CLL. We concluded that the myelosuppression, the main side effect of chlorambucil, could be stronger than the therapeutic effect of CLL in this dog because of her chronic wasting condition.
When compared to acute lymphocytic leukemia, CLL is a relatively indolent form of leukemia [18]. Since almost half of the patients with CLL are asymptomatic, they are usually incidentally diagnosed on routine check-up [18, 24, 25]. Therefore, chemotherapy is not always recommended unless indications such as anemia or other forms of cytopenia are present [25]. Anemia should be considered a poor prognostic factor even in T-cell lymphocytic leukemia, in which the mean survival time is longer than that in other types of lymphocytic leukemia [6]. The development of clinical signs like anorexia and lethargy can also be indications for chemotherapy [25]. Since the patient in this case had both hematological and clinical indications, treatment was conducted. The most commonly recommended therapy for patients with CLL is a combination of prednisone and chlorambucil administered via a continuous or pulse therapy protocol [18, 25]. According to the previous literature, glucocorticoids can be used if dogs with HAC have concurrent steroid-responsive disorders [8]. Therefore, steroid therapy was included in the treatment of CLL in the present case.
To conclude, the present case report provides evidence suggesting that veterinary clinicians should add CLL to the list of differential diagnoses in patients with HAC showing persistent lymphocytosis without any underlying causes. Additionally, we suggest that veterinarians should note the masking effects of corticosteroids in similar steroid-responsive diseases.
CONFLICT OF INTEREST
The authors have nothing to disclose.
REFERENCES
- 1.Avery A. C., Avery P. R.2007. Determining the significance of persistent lymphocytosis. Vet. Clin. North Am. Small Anim. Pract. 37: 267–282, vi. doi: 10.1016/j.cvsm.2006.11.001 [DOI] [PubMed] [Google Scholar]
- 2.Behrend E. N., Kooistra H. S., Nelson R., Reusch C. E., Scott-Moncrieff J. C.2013. Diagnosis of spontaneous canine hyperadrenocorticism: 2012 ACVIM consensus statement (small animal). J. Vet. Intern. Med. 27: 1292–1304. doi: 10.1111/jvim.12192 [DOI] [PubMed] [Google Scholar]
- 3.Bennaim M., Shiel R. E., Mooney C. T.2019. Diagnosis of spontaneous hyperadrenocorticism in dogs. Part 1: Pathophysiology, aetiology, clinical and clinicopathological features. Vet. J. 252: 105342. doi: 10.1016/j.tvjl.2019.105342 [DOI] [PubMed] [Google Scholar]
- 4.Boes K. M., Durham A. C.2017. Bone marrow, blood cells, and the lymphoid/lymphatic system. p.754. In: Pathologic Basis of Veterinary Disease, 6th ed. (Zachary, J. F. ed.), Elsevier, St. Louis. [Google Scholar]
- 5.Comazzi S., Martini V., Riondato F., Poggi A., Stefanello D., Marconato L., Albonico F., Gelain M. E.2017. Chronic lymphocytic leukemia transformation into high-grade lymphoma: a description of Richter’s syndrome in eight dogs. Vet. Comp. Oncol. 15: 366–373. doi: 10.1111/vco.12172 [DOI] [PubMed] [Google Scholar]
- 6.Comazzi S., Gelain M. E., Martini V., Riondato F., Miniscalco B., Marconato L., Stefanello D., Mortarino M.2011. Immunophenotype predicts survival time in dogs with chronic lymphocytic leukemia. J. Vet. Intern. Med. 25: 100–106. doi: 10.1111/j.1939-1676.2010.0640.x [DOI] [PubMed] [Google Scholar]
- 7.Elliott M.2001. Cushing’s disease: a new approach to therapy in equine and canine patients. Br. Homeopath. J. 90: 33–36. doi: 10.1054/homp.1999.0450 [DOI] [PubMed] [Google Scholar]
- 8.Feldman E. C., Nelson R. W.2003. Canine and Feline Endocrinology and Reproduction, 3rd ed., Saunders, Philadelphia. [Google Scholar]
- 9.Greenfield J. R., Moore J., Hill D., Brenner P., Delprado W., Turner J., Taylor J., Campbell L., Wong L. Y.2008. Cushing’s syndrome can precipitate diabetes but mask non-Hodgkin’s lymphoma. Med. J. Aust. 188: 262. doi: 10.5694/j.1326-5377.2008.tb01608.x [DOI] [PubMed] [Google Scholar]
- 10.Hoffman J. M., Lourenço B. N., Promislow D. E. L., Creevy K. E.2018. Canine hyperadrenocorticism associations with signalment, selected comorbidities and mortality within North American veterinary teaching hospitals. J. Small Anim. Pract. 59: 681–690. doi: 10.1111/jsap.12904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kheirandish R., Akhtardanesh B., Askari N., Ghasemi N.2014. Concurrent adrenocortical adenoma and multicentric lymphoma in a German shepherd dog. Online J. Vet. Res. 18: 398–404. [Google Scholar]
- 12.Kooistra H. S., Galac S.2012. Recent advances in the diagnosis of Cushing’s syndrome in dogs. Top. Companion Anim. Med. 27: 21–24. doi: 10.1053/j.tcam.2012.06.001 [DOI] [PubMed] [Google Scholar]
- 13.Kritsepi-Konstantinou M., Oikonomidis I. L.2016. The interpretation of leukogram in dog and cat. Hellenic J. Companion Anim. Med. 5: 54–68. [Google Scholar]
- 14.Leifer C. E., Matus R. E.1985. Lymphoid leukemia in the dog. Acute lymphoblastic leukemia and chronic lymphocytic leukemia. Vet. Clin. North Am. Small Anim. Pract. 15: 723–739. doi: 10.1016/S0195-5616(85)50032-7 [DOI] [PubMed] [Google Scholar]
- 15.Mori A., Lee P., Izawa T., Oda H., Mizutani H., Koyama H., Arai T., Sako T.2009. Assessing the immune state of dogs suffering from pituitary gland dependent hyperadrenocorticism by determining changes in peripheral lymphocyte subsets. Vet. Res. Commun. 33: 757–769. doi: 10.1007/s11259-009-9224-5 [DOI] [PubMed] [Google Scholar]
- 16.Peterson M. E.2007. Diagnosis of hyperadrenocorticism in dogs. Clin. Tech. Small Anim. Pract. 22: 2–11. doi: 10.1053/j.ctsap.2007.02.007 [DOI] [PubMed] [Google Scholar]
- 17.Pivonello R., Isidori A. M., De Martino M. C., Newell-Price J., Biller B. M., Colao A.2016. Complications of Cushing’s syndrome: state of the art. Lancet Diabetes Endocrinol. 4: 611–629. doi: 10.1016/S2213-8587(16)00086-3 [DOI] [PubMed] [Google Scholar]
- 18.Presley R. H., Mackin A., Vernau W.2006. Lymphoid leukemia in dogs. Compendium 28: 831–849. [Google Scholar]
- 19.Paoletta A., Billeci D., Fallo F.2015. A case of nephrotic syndrome hidden by Cushing’s disease. Endocrine 48: 722–724. doi: 10.1007/s12020-014-0455-z [DOI] [PubMed] [Google Scholar]
- 20.Ramsey I., Ristic J.2007. Diagnosis of canine hyperadrenocorticism. In Pract. 29: 446–454. doi: 10.1136/inpract.29.8.446 [DOI] [Google Scholar]
- 21.Sanders K., Kooistra H. S., Galac S.2018. Treating canine Cushing’s syndrome: current options and future prospects. Vet. J. 241: 42–51. doi: 10.1016/j.tvjl.2018.09.014 [DOI] [PubMed] [Google Scholar]
- 22.Smolej L.2012. The role of high-dose corticosteroids in the treatment of chronic lymphocytic leukemia. Expert Opin. Investig. Drugs 21: 1009–1017. doi: 10.1517/13543784.2012.690393 [DOI] [PubMed] [Google Scholar]
- 23.Selek A., Barış S., Çetinaslan B., Cantürk Z., Tarkun İ., Akyay Z.2016. New-onset sarcoidosis after remission of Cushing’s syndrome. Turk. Thorac. J. 17: 35–37. doi: 10.5578/ttj.17.1.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stilwell C. A., Florey J.2020. Peripheral eosinophilia and eosinophilic bronchopneumopathy in a dog with chronic lymphocytic leukemia. Vet. Rec. Case Rep. 8: e000961. doi: 10.1136/vetreccr-2019-000961 [DOI] [Google Scholar]
- 25.Workman H. C., Vernau W.2003. Chronic lymphocytic leukemia in dogs and cats: the veterinary perspective. Vet. Clin. North Am. Small Anim. Pract. 33: 1379–1399, viii. doi: 10.1016/S0195-5616(03)00120-7 [DOI] [PubMed] [Google Scholar]