Abstract
One calf died (No. 1) and another was euthanized following astasia (No. 2). Histopathological examination revealed suppurative meningoencephalitis in these calves. Klebsiella pneumoniae antigens were detected in lesions. Thymocytes were decreased in the thymus cortex in both cases. 16S rRNA gene sequencing of the No. 1 isolate and bacterial extracts from formalin fixed paraffin embedded sections of No. 2 revealed that both samples were K. pneumoniae. The No. 1 isolate showed multidrug resistance against penicillin antibiotics, fosfomycin, streptomycin, macrolide antibiotics, tetracycline antibiotics, and clindamycin. Immunosuppression is a significant septicemic K. pneumoniae infection risk factor. Our study provides new aspects regarding K. pneumoniae infections in cattle, bacterial meningoencephalitis differentiation, and K. pneumoniae and bacterial meningoencephalitis treatments.
Keywords: cattle, Klebsiella pneumoniae, meningoencephalitis, multidrug resistance
Klebsiella pneumoniae is a gram-negative bacterium recognized as a common causative agent of bovine mastitis [6, 24]. It is usually present in the upper respiratory tract of cattle and is isolated from the nasal cavity [5] or tracheobronchial lavage samples [11]. Moreover, K. pneumoniae may cause systemic infections, such as septicemia in humans [18, 23] and pigs [3] and meningoencephalitis in humans [29, 30] and cynomolgus monkey [14]. Although Histophilus somni [21] and Listeria monocytogenes [22] conventionally cause infectious meningoencephalitis in cattle, neonatal bacterial suppurative meningitis (NBSM) is sporadically caused by Streptococcus bovis [28], Mannheimia spp. [2, 4], and Escherichia coli [13, 27]. Generally, NBSM is caused by septicemia from the dissemination of invasive bacteria from the gastrointestinal and respiratory tracts or umbilical cord [19].
Bacterial drug resistance is an emerging problem in humans and livestock, and multidrug resistance (MDR) of K. pneumoniae is commonly reported [18, 20]. Studies on drug resistance, such as colistin resistance [15], β-lactamase production [24], and MDR [1] in bovines have been conducted, however, information on drug resistance of K. pneumoniae isolates, except for those causing mastitis, is limited.
This study reports two fatal cases of meningoencephalitis caused by a K. pneumoniae infection at two epidemiologically unrelated farms in Japan and describes the histopathological and bacterial characteristics of K. pneumoniae in calves.
Clinical symptoms and farm information regarding each case are shown in Supplementary Table 1. Briefly, two calves, the first was a three-day-old Japanese Black beef (No. 1), and the second a 21-day-old crossbred beef (No. 2), had difficulties standing for three days and one week, respectively. Despite artificial milk feeding for treatment (No. 1, two days after birth) or intravenous drip and ursodeoxycholic acid to improve liver function (No. 2, one day), No. 1 calf died, and No. 2 calf was euthanized when moribund. No antimicrobial drugs were administered. Although No. 1 did not want to take colostrum, No. 2 was well fed. Each calf was from farms located in the Miyazaki Prefecture on Kyushu Island and the Aichi Prefecture on the Pacific coast of central Honshu Island, which is the main Island, respectively. The carcass of No. 1 calf (12 hr after death) and No. 2 dying calf were moved to the Miyazaki or Aichi Chuo Prefectural Livestock Hygiene Service Center for necropsy, respectively. No. 2 was euthanized by using xylazine (Bayer Yakuhin Ltd, Osaka, Japan), pentobarbital sodium (Kyoritsu Seiyaku Corp., Tokyo, Japan) and relaxin (Kyorin Pharmaceutical Co., Ltd., Tokyo, Japan) according to euthanasia regulations in the facility of Aichi Prefecture. No clinical symptoms, including astasia, were detected in any of the other cattle in the corresponding farms.
Necropsy revealed congestion in the meningeal vessels in No. 1 (Fig. 1a). Congestion was also detected in the umbilical cord, and petechial hemorrhages were present in the mucous membrane of the urinary bladder with red urine in No. 1. In No. 2 calf, the purulent exudate was filled in the cerebral sulcus, and the meninges were slightly thick (Fig. 1b). Cerebrospinal fluid was increased, and hemorrhages were present on the cut surface of the corpus striatum, diencephalon, and spinal cord. A part of the anterior lung lobe had turned dark red, and petechial hemorrhages were present in the jejunal mucosa. The thymus of both calves was slightly atrophied (Supplementary Fig. 1). No visible lesions were found in any other organ in either calf.
Fig. 1.
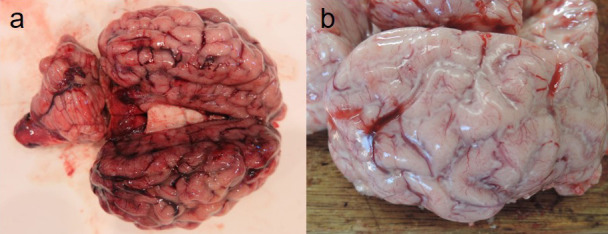
a. At necropsy, congestion was found in the meningeal vessels of No. 1 calf. b. Suppurative exudate was seen in the brain sulcus of No. 2 calf. The meninges were slightly thick.
During necropsy, tissue samples of the liver, spleen, kidney, heart, lung, rumen, reticulum, omasum, abomasum, intestines, cerebrum, cerebellum, spinal cord, hypophysis, urinary bladder, and umbilical cord (only in No. 1) were fixed in 10% neutral-buffered formalin. Fixed tissues were embedded in paraffin wax, sectioned at a thickness of approximately 3-µm, and stained with hematoxylin and eosin (H&E) and gram staining for histological examination. To label the K. pneumoniae antigen, immunohistochemical examination was performed on all sections of both calves according to our previous study [16], with rabbit anti-K. pneumoniae serums (6891, ViroStat, Portland, ME, USA) as primary antibodies.
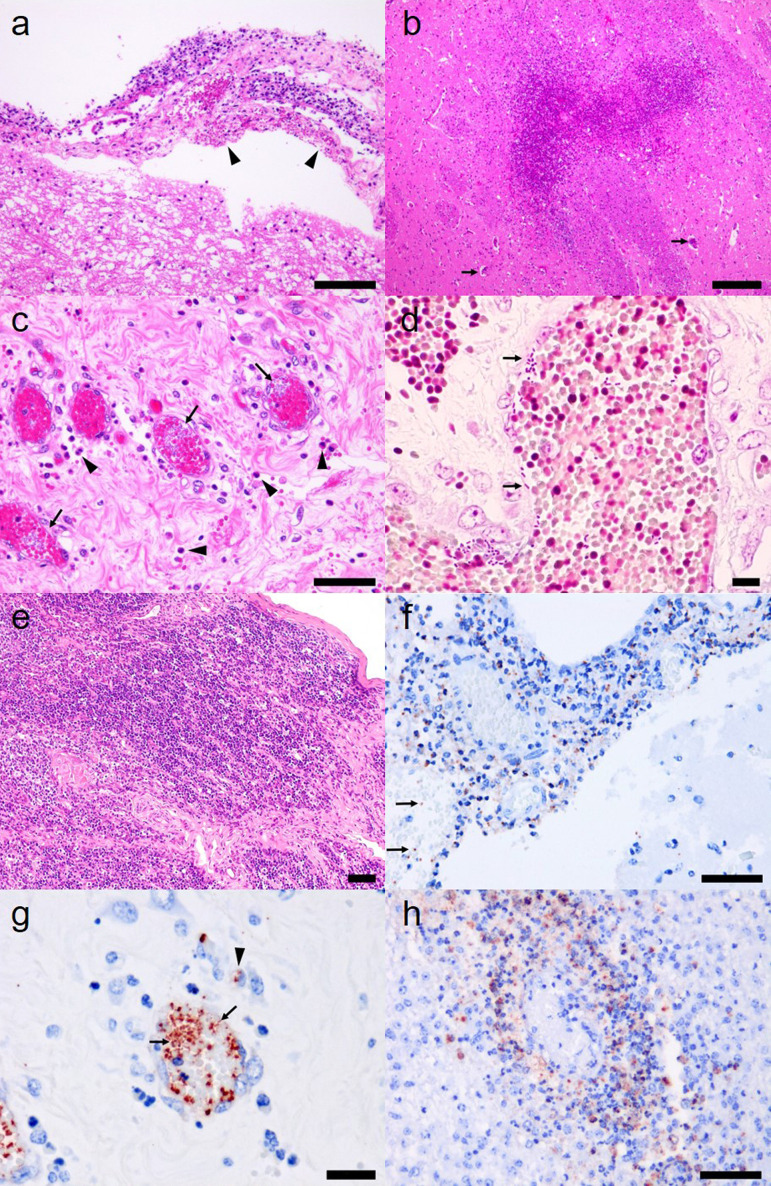
Histological observations are shown in Table 1. Mild to moderate (No. 1) and severe (No. 2) neutrophilic infiltrations were widely detected, and macrophages were scattered in the meninges of the brain and spinal cord in both calves (Fig. 2a). Congestion was detected in lesion vessels. Small areas of necrosis were detected in the cerebral cortex of the frontal lobe of No. 1. Widespread necrosis was detected in the corpus striatum of the cerebrum, brainstem, and dorsal funiculus of the cortex and dorsal horn of the medulla in the spinal cord of No. 2 (Fig. 2b). Gram-negative bacteria, thrombi, perivascular neutrophil infiltrations, and necrosis were detected in the corresponding lesions. Few perivascular neutrophil infiltrations were detected in the midbrain and medulla oblongata, and no inflammation was detected in ependymal cells, brain ventricles, central canal of the spinal cord, or hypophysis. In the other organs of the No .1, mild edema and neutrophilic infiltration was present in the tunica adventitia of the umbilical artery (Fig. 2c), and necrosis was present in the surrounding connective tissue of the umbilical artery. Gram-negative bacteria were detected in the blood vessels of the liver, spleen, kidney, heart, lung, urinary bladder, and umbilical cord (Table 1, Fig. 2d). Many neutrophils infiltrated the lamina propria and tunica muscularis of the urinary bladder, and hemorrhages and gram-negative bacteria were detected in the corresponding lesions. Focal macrophage infiltration and a few neutrophils were observed in the liver parenchyma of No. 2. Many neutrophils were present in the sinusoids of the liver and around the white pulp of the spleen. Suppurative bronchopneumonia was present in the anterior lobe, with a few plant fragments. Thymocytes were decreased in the thymus cortex of both calves (Fig. 2e).
Table 1. Histological observation and specific PCR test results for Klebsiella pneumoniae using FFPE* sections of two calves.
| Suppurative lesionsa) |
Gram-negative bacillib) |
K. pneumoniae antigenc) |
Specific PCRd) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No.1 | No.2 | No.1 | No.2 | No.1 | No.2 | No.1 | No.2 | ||
| CNS** | Cerebrum | +++ | +++ | +++ | +++ | +++ | +++ | + | + |
| Cerebellum | +++ | +++ | +++ | +++ | +++ | +++ | N/A | N/A | |
| Spinal cord | +++ | +++ | +++ | +++ | +++ | +++ | + | + | |
| Liver | - | - | + | - | + | - | + | - | |
| Spleen | - | - | + | - | + | - | + | - | |
| Kidney | - | - | + | - | + | - | + | - | |
| Heart | - | - | + | - | + | - | + | - | |
| Lung | - | + | + | - | + | - | + | - | |
| Urinary Bladder | ++ | - | ++ | - | ++ | - | + | N/A | |
| Umbilical cord | ++ | N/A | ++ | N/A | ++ | N/A | + | N/A | |
a) +, ++, and +++ indicated slight, moderate, and severe infiltration of neutrophils in hematoxylin and eosin staining sections. b) +, ++, and +++ indicated slight, moderate, and severe infiltration of gram-negative bacillus in gram staining sections. c) +, ++, and +++ indicated slight, moderate, and severe reaction of K. pneumoniae antigens in immunohistochemical staining sections. d) The PCR test was conducted according to a past study [9]. *Formalin fixed paraffin embedded. **Central nervous system. N/A, not applicable.
Fig. 2.
a. Mild to moderate neutrophilic infiltrations in the meninges of No. 1. Hemorrhages (arrowheads) were present in brain meninges. Hematoxylin and eosin (H&E) staining. Bar=100 µm. b. Widespread necrosis in the corpus striatum of No. 2. Perivascular infiltrations are scattered in the lesion (arrows). H&E staining. Bar=200 µm. c. Numerous bacteria freely existed in the blood vessels (arrows) of the umbilical cord in No. 1. Neutrophils (arrowheads) and macrophages were infiltrated around vessels. H&E staining. Bar=50 µm. d. Numerous gram-negative bacteria existed freely in the blood vessels (arrows) of the umbilical cord in No. 1. Gram staining. Bar=10 µm. e. Thymocytes were decreased and collagen fibers were increased in thymus cortex in No. 2. H&E staining. Bar=50 µm. f. K. pneumoniae antigen (red) was detected in meninges and blood vessels (arrows) in No. 1. Immunohistochemical staining. Bar=50 µm. g. Many K. pneumoniae antigens were detected in the blood vessels of the umbilical cord (arrows) and cytoplasm of infiltrated macrophages (arrowhead) in No. 1. Immunohistochemical staining. Bar=20 µm. h. K. pneumoniae antigens were detected in the cytoplasm of infiltrated macrophages and infiltrating neutrophils in the perivascular space of the corpus striatum of No. 2. Immunohistochemical staining. Bar=50 µm.
Using immunohistochemical staining, we detected many positive reactions against K. pneumoniae in the cytoplasm of macrophages and neutrophils that infiltrated suppurative lesions in the brain and spinal cord (Fig. 2f). Positive reactions were also detected in the blood vessels of multiple organs, including the umbilical cord of No. 1 (Fig. 2g, Table 1). In contrast with No. 1 calf, the positive reaction was limited to the brain and spinal cord in No. 2 calf (Fig. 2h, Table 1).
For bacterial isolation, tissue blocks of the liver, spleen, kidney, heart, lung, cerebrum, and urine (only No. 1) were stamped and spread onto normal blood, chocolate, and deoxycholate-hydrogen sulfide-lactose agar plates. Inoculated plates were incubated at 37°C in an atmosphere of 5% CO2 under aerobic and anaerobic conditions. Bacterial isolation results are shown in Supplementary Table 2. Gram-negative bacilli with the same colony properties and morphology were isolated from all samples in No. 1 and only from the cerebrum in No. 2. No other bacteria were isolated from the tested samples. The isolate from the cerebrum of No. 1 was subjected to biochemical testing using the API 20 E system (BioMérieux, Inc., Marcy l’Etoile, France) according to the manufacturer’s instructions. The isolate was identified as K. pneumoniae with a profile identical to K. pneumoniae (5215777). To confirm the identification of the isolate of No. 1, PCR assay and a sequence analysis of the 16S rRNA gene using universal primers (16S-F1/16S-R2, 16S-F2/16S-R4, and 16S-F3/16S-R5: Supplementary Table 3) were performed.
As the isolate derived from No. 2 was accidently discarded, to detect gram-negative bacteria, the formalin fixed paraffin embedded (FFPE) section of the brain No. 2 was used for molecular analysis. Genomic DNA was extracted from FFPE tissue sections using the QIAamp DNA FFPE Tissue kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer’s instructions. A PCR assay for the 16S rRNA gene using universal primers (Pag313/Pag1128: Supplementary Table 3) were performed and sequenced.
A basic local alignment search tool (BLAST) analysis revealed that the partial sequences of this region of No. 1 (1,370 bp) and No. 2 (749 bp) calves were identical to K. pneumoniae strains and showed high similarities of 99.85% (Accession Number: CP039524) and 99.87% (Accession Number: MF429166), respectively. Both 16S rRNA gene sequences were deposited in the DNA Data Bank of Japan (Accession Number; No. 1: LC557135, No. 2: LC512010).
A phylogenetic tree analysis based on 16s rRNA gene sequences was performed according to a previous study [17]. Briefly, the sequences in our study and references of K. pneumoniae, K. pneumoniae subsp. ozaenae, and K. pneumoniae subsp. rhinoscleromatis retrieved from the National Center for Biotechnology Information nucleotide database were identical, and a neighbor-joining phylogenetic tree was created using MEGA 7.0 software. Both sequences in this study were classified as K. pneumoniae subsp. pneumoniae (Supplementary Fig. 2).
To confirm the existence of K. pneumoniae and its association with suppurative lesions, a PCR assay specific for K. pneumoniae was also performed according to a previous study [9] using genomic DNA extracted from the abovementioned FFPE sections. The target gene was detected in all tested tissues in No. 1 and only in the cerebrum and spinal cord in No. 2 (Table 1).
To determine the antibiotic susceptibility of K. pneumoniae isolate, disk diffusion method was performed according to the Clinical and Laboratory Standard Institution (CLSI) Guidelines [7]. Antibiotic disks included the BD BBL Sensi-Discs (Becton, Dickinson and Co., Franklin Lakes, NJ, USA) for penicillin (PCG), ampicillin (ABPC), amoxicillin (AMPC), cefazolin (CEZ), cefuroxime, cefotaxime (CTX), cefoxitin, fosfomycin (FOM), streptomycin (SM), gentamicin (GM), kanamycin (KM), azithromycin (AZM), erythromycin (EM), tetracycline (TC), oxytetracycline (OTC), clindamycin (CM), sulfamethoxazole trimethoprim, nalidixic acid, ciprofloxacin (CPFX), norfloxacin, and KB disks (Eiken Chemical Co., Ltd., Tokyo, Japan) for florfenicol. Antibiotic susceptibility test results are presented in Table 2. The isolates of No. 1 were resistant to PCG, ABPC, AMPC, FOM, SM, AZM, EM, TC, OTC, and CM.
Table 2. Results of antimicrobial susceptibility testing.
| Antibiotic disk | µg/disc | Zone diameter (mm) |
|||
|---|---|---|---|---|---|
| Inhibition circle | Resistance | Intermedium | Susceptive | ||
| Penicillin (PCG) | 10 | 6 | 14 | 15 | |
| Ampicillin (ABPC) | 10 | 6 | 13 | 14–16 | 17 |
| Amoxicillin (AMPC) | 25 | 6 | 14 | 15–20 | 21 |
| Cefazolin (CEZ) | 30 | 23 | 19 | 20–22 | 23 |
| Cefuroxime (CXM) | 30 | 22 | 14 | 15–17 | 18 |
| Cefotaxime (CTX) | 30 | 32 | 22 | 23–25 | 26 |
| Cefoxitin (CFX) | 30 | 23 | 14 | 15–17 | 18 |
| Fosfomycin (FOM) | 50 | 11 | 12 | 13–15 | 16 |
| Streptomycin (SM) | 300 | 6 | 11 | 12–14 | 15 |
| Gentamicin (GM) | 120 | 21 | 12 | 13–14 | 15 |
| Kanamycin (KM) | 30 | 22 | 13 | 14–17 | 18 |
| Azithromycin (AZM) | 15 | 13 | 13 | 14–22 | 23 |
| Erythromycin (EM) | 15 | 11 | 13 | 14–22 | 23 |
| Tetracycline (TC) | 30 | 7 | 11 | 12–14 | 15 |
| Oxytetracycline (OTC) | 30 | 6 | 14 | 15–18 | 19 |
| Florfenicol (FF) | 30 | 24 | 14 | 15–18 | 19 |
| Clindamycin (CM) | 2 | 7 | 14 | 15–20 | 21 |
| Sulfamethoxazole/Trimethoprim (ST) | 23.75/1.25 | 23 | 10 | 11–15 | 16 |
| Nalidixic acid (NA) | 30 | 24 | 13 | 14–18 | 19 |
| Ciprofloxacin (CPFX) | 5 | 28 | 15 | 16–20 | 21 |
| Norfloxacin (NRFX) | 10 | 27 | 12 | 13–16 | 17 |
As previously noted, a remarkable aspect of the current study is the evidence for two cases of meningoencephalitis caused by K. pneumoniae in calves. Regarding plural suppurative lesions, No. 1 calf suffered from sepsis. K. pneumoniae conventionally causes mastitis in cattle [6, 24], however, it sometimes causes septic infection in neonatal and elderly humans [23, 29, 30], pigs [3], and cynomolgus monkeys [14]. Although it has been reported that K. oxytoca causes meningitis in a calf [26], to the best of our knowledge, septic infections of K. pneumoniae, including meningoencephalitis, have not been reported in cattle, meaning that our study is the first to report an association between K. pneumoniae and meningoencephalitis.
In this study, meningoencephalitis was a characteristic finding, however, purulent lesions were also detected in the other tissues of both calves. Necrosis was present in the central nervous system (CNS) of both calves and the surrounding connective tissue of the umbilical artery in No. 1. K. pneumoniae antigen and gene fragments were detected in all tested tissues, even in tissues without suppurative lesions, by immunohistochemical staining and PCR using FFPE sections from No. 1. This indicated that the K. pneumoniae infections were initiated from the umbilical cord, spread to multiple organs via the bloodstream, and finally caused meningoencephalitis. In contrast, although neutrophil infiltration in the liver and spleen and suppurative bronchopneumonia was detected in No. 2, specific antigens and genes were negative. This suggested that No. 2 calf had sepsis followed by meningoencephalitis, and the bacteria in the vessels and organs other than those in the CNS could be eliminated by immune cells. The difference between the conditions of the calves might depend on whether they had been inoculated with colostrum or not. Bacterial proliferation in the organs of No. 1 calf during the period between its death and autopsy could also contribute to pathogen detection by PCR and immunohistochemical staining. It was unknown from where K. pneumoniae invaded No. 2, however, based on the neutrophilic infiltration in the liver and spleen, K. pneumoniae would be transferred into the CNS hematogenously [19]. The results of immunohistochemical staining and PCR suggested that K. pneumoniae invaded the CNS of No. 2 calf via the upper respiratory tract [8], umbilical cord and gastrointestinal tracts [12].
Moreover, the thymus was slightly atrophied in both calves. Histology, thymocyte counts in the cortex were decreased, indicative of immunodeficiency. Immunodeficiency was suggested to play an important role in the septicemic infection of K. pneumoniae in this study.
Generally, NBSM can be triggered by septicemic infection caused by passive transfers and insufficient colostrum [10], and these factors might be the cause of the septicemic infection in the first calf.
In this study, two cases of septicemic K. pneumoniae infection occurred independently in distinct farms. No epidemiological similarities were detected in either farm, and the farms had similar management, scale, and area. This suggests that these cases occurred accidentally, however, they are not rare.
The K. pneumoniae isolate from No. 1 showed severe MDR and was resistant to 10 tested drugs. In a previous study, most K. pneumoniae isolates from bovine animals with mastitis in Japan were resistant to AMPC, SM and OTC. However, such isolates were susceptible to CEZ and KM [24]. K. pneumoniae isolates from sick cattle with respiratory manifestations expressed MDR and resistance to ABPC, AMPC, CPFX, CTX, ceftriaxone, GM, ceftazidime, and amikacin in 93.9%, 81.8%, 60.6%, 57.6%, 33.3%, 27.3%, 18.2%, and 9.1% of cases, respectively [5]. It is necessary to monitor the antimicrobial resistance of K. pneumoniae in cattle to control the worldwide spread of MDR K. pneumoniae [20].
Bovine meningoencephalitis associated with bacterial sepsis is common and is mainly caused by H. somni and L. monocytogenes [21, 22]. H. somni most commonly infects calves aged 6–12 months, and thrombotic meningoencephalitis-myelitis occurring in animals aged <4 months is rare [21]. L. monocytogenes generally causes suppurative lesions in the brainstem because of its unique mechanism of infection via axonal migration of the cranial nerve [22]. Moreover, a few reports of NBSM in calves showed that lesions in CNS are limited to the meninges, choroid plexuses, and ventricular walls [4, 26, 28]. Although multifocal perivascular neutrophilic inflammation caused by M. haemolytica has been reported, neutrophil infiltration is also limited in the choroid plexuses [2]. In this study, suppurative meningitis was a characteristic presentation in both calves, which did not have ventriculitis, and particularly widespread necrosis was present in the corpus striatum of the cerebrum, brainstem, and medulla in the spinal cord of calf No. 2. Multiple perivascular neutrophilic infiltrations were scattered in lesions. These facts revealed that necrosis in the brain parenchyma was caused by invasive K. pneumoniae via the blood stream in No. 2. Parenchymal necrosis occurred in three of five calves infected with Escherichia coli in one study [27]. An experimental study on meningitis via hematogenous infection of E. coli demonstrated that E. coli crosses the blood-brain barrier but not the choroid plexus [25]. There are no studies on K. pneumoniae invasion into CNS, and more research is expected to take into account differences in lesion distribution patterns caused by K. pneumoniae. For appropriate treatment, sole K. pneumoniae infections need to be differentiated from K. pneumoniae infections with other infectious diseases.
In conclusion, we reported two cases of bovine meningoencephalitis caused by a K. pneumoniae infection. The MDR bacteria were thought to have invaded the CNS via the blood stream. Thymus hypoplasia and insufficient colostrum were critical factors for septicemic infection. Our study will benefit the diagnosis and differentiation of NBSM in calves, and the establishment of effective treatment.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest associated with this manuscript.
Supplementary
Acknowledgments
The authors thank Mr. M. Kobayashi and Ms. M. Shimada for histopathological assistance and Dr. H. Kobayashi for advice on bacterial identification.
REFERENCES
- 1.Ahmed A. M., Shimamoto T.2011. Molecular characterization of antimicrobial resistance in Gram-negative bacteria isolated from bovine mastitis in Egypt. Microbiol. Immunol. 55: 318–327. doi: 10.1111/j.1348-0421.2011.00323.x [DOI] [PubMed] [Google Scholar]
- 2.Aschenbroich S., Nemeth N., Rech R., Briggs R., Sanchez S., Brown C.2013. Mannheimia haemolytica A1-induced fibrinosuppurative meningoencephalitis in a naturally-infected Holstein-Friesian calf. J. Comp. Pathol. 149: 167–171. doi: 10.1016/j.jcpa.2013.02.001 [DOI] [PubMed] [Google Scholar]
- 3.Bowring B. G., Fahy V. A., Morris A., Collins A. M.2017. An unusual culprit: Klebsiella pneumoniae causing septicaemia outbreaks in neonatal pigs? Vet. Microbiol. 203: 267–270. doi: 10.1016/j.vetmic.2017.03.018 [DOI] [PubMed] [Google Scholar]
- 4.Catry B., Opsomer G., Decostere A., Feyen B., de Kruif A., Haesebrouck F.2004. Fatal meningitis in a calf caused by Mannheimia varigena. Res. Vet. Sci. 77: 187–188. doi: 10.1016/j.rvsc.2004.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng F., Li Z., Lan S., Liu W., Li X., Zhou Z., Song Z., Wu J., Zhang M., Shan W.2018. Characterization of Klebsiella pneumoniae associated with cattle infections in southwest China using multi-locus sequence typing (MLST), antibiotic resistance and virulence-associated gene profile analysis. Braz. J. Microbiol. 49 Suppl 1: 93–100. doi: 10.1016/j.bjm.2018.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng J., Zhang J., Han B., Barkema H. W., Cobo E. R., Kastelic J. P., Zhou M., Shi Y., Wang J., Yang R., Gao J.2020. Klebsiella pneumoniae isolated from bovine mastitis is cytopathogenic for bovine mammary epithelial cells. J. Dairy Sci. 103: 3493–3504. doi: 10.3168/jds.2019-17458 [DOI] [PubMed] [Google Scholar]
- 7.Clinical and Laboratory Standard Institution Guidelines. 2015. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 3rd ed. In: CLSI document VET01S, Clinical and Laboratory Standard Institution, Wayne. [Google Scholar]
- 8.Dao T. T., Liebenthal D., Tran T. K.,, Ngoc Thi Vu, Ngoc Thi Nguyen, Thi Tran H. K., Thi Nguyen C. K., Thi Vu H. L., Fox A., Horby P., Van Nguyen K., Wertheim H. F.2014. Klebsiella pneumoniae oropharyngeal carriage in rural and urban Vietnam and the effect of alcohol consumption. PLoS One 9: e91999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong D., Liu W., Li H., Wang Y., Li X., Zou D., Yang Z., Huang S., Zhou D., Huang L., Yuan J.2015. Survey and rapid detection of Klebsiella pneumoniae in clinical samples targeting the rcsA gene in Beijing, China. Front. Microbiol. 6: 519. doi: 10.3389/fmicb.2015.00519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fecteau G., Smith B. P., George L. W.2009. Septicemia and meningitis in the newborn calf. Vet. Clin. North Am. Food Anim. Pract. 25: 195–208, vii–viii. doi: 10.1016/j.cvfa.2008.10.004 [DOI] [PubMed] [Google Scholar]
- 11.França Dias de Oliveira B. A., Carrillo Gaeta N., Mendonça Ribeiro B. L., Reyes Alemán M. A., Miranda Marques L., Timenetsky J., Melville P. A., Avansi Marques J., Marvulle V., Gregory L.2016. Determination of bacterial aetiologic factor on tracheobronchial lavage in relation to clinical signs of bovine respiratory disease. J. Med. Microbiol. 65: 1137–1142. doi: 10.1099/jmm.0.000345 [DOI] [PubMed] [Google Scholar]
- 12.Fung C. P., Lin Y. T., Lin J. C., Chen T. L., Yeh K. M., Chang F. Y., Chuang H. C., Wu H. S., Tseng C. P., Siu L. K.2012. Klebsiella pneumoniae in gastrointestinal tract and pyogenic liver abscess. Emerg. Infect. Dis. 18: 1322–1325. doi: 10.3201/eid1808.111053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green S. L., Smith L. L.1992. Meningitis in neonatal calves: 32 cases (1983–1990). J. Am. Vet. Med. Assoc. 201: 125–128. [PubMed] [Google Scholar]
- 14.Kasuya K., Takayama K., Bito M., Shimokubo N., Kawashima R., Shibahara T.2017. Septicemic invasive Klebsiella pneumoniae infection in a cynomolgus monkey (Macaca fascicularis) with severe diffused suppurative meningoencephalitis. J. Vet. Med. Sci. 79: 1167–1171. doi: 10.1292/jvms.17-0126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kieffer N., Poirel L., Nordmann P., Madec J. Y., Haenni M.2015. Emergence of colistin resistance in Klebsiella pneumoniae from veterinary medicine. J. Antimicrob. Chemother. 70: 1265–1267. [DOI] [PubMed] [Google Scholar]
- 16.Komatsu T., Inaba N., Watando E., Sugie K., Kimura K., Katsuda K., Shibahara T.2019. Pyelonephritis caused by Mannheimia varigena in a Holstein calf. J. Vet. Med. Sci. 81: 1113–1116. doi: 10.1292/jvms.19-0211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Komatsu T., Kubo T., Kitou R., Kawamoto N., Mase M., Yamamoto Y., Shibahara T.2020. Inclusion body hepatitis caused by Aviadenovirus in a tropical screech owl (Megascops choliba). J. Vet. Med. Sci. 82: 1341–1345. doi: 10.1292/jvms.20-0316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leal H. F., Azevedo J., Silva G. E. O., Amorim A. M. L., de Roma L. R. C., Arraes A. C. P., Gouveia E. L., Reis M. G., Mendes A. V., de Oliveira Silva M., Barberino M. G., Martins I. S., Reis J. N.2019. Bloodstream infections caused by multidrug-resistant gram-negative bacteria: epidemiological, clinical and microbiological features. BMC Infect. Dis. 19: 609. doi: 10.1186/s12879-019-4265-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacKay R. J., Middleton J. R.2019. Disease of the Nervous System. pp. 1042–1045. In: Large Animal Internal Medicine, 6th ed. (Smith, B. P., Van Metre, D. C. and Pusterla, N. eds.), Elsevier, Amsterdam. [Google Scholar]
- 20.Moradigaravand D., Martin V., Peacock S. J., Parkhill J.2017. Evolution and epidemiology of multidrug-resistant Klebsiella pneumoniae in the United Kingdom and Ireland. MBio 8: e01976–e16. doi: 10.1128/mBio.01976-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Toole D., Sondgeroth K. S.2016. Histophilosis as a Natural Disease. Curr. Top. Microbiol. Immunol. 396: 15–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oevermann A., Di Palma S., Doherr M. G., Abril C., Zurbriggen A., Vandevelde M.2010. Neuropathogenesis of naturally occurring encephalitis caused by Listeria monocytogenes in ruminants. Brain Pathol. 20: 378–390. doi: 10.1111/j.1750-3639.2009.00292.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russo T. A., Olson R., Fang C. T., Stoesser N., Miller M., MacDonald U., Hutson A., Barker J. H., La Hoz R. M., Johnson J. R.2018. Identification of biomarkers for differentiation of hypervirulent Klebsiella pneumoniae from classical K. pneumoniae. J. Clin. Microbiol. 56: e00776–e18. doi: 10.1128/JCM.00776-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saishu N., Ozaki H., Murase T.2014. CTX-M-type extended-spectrum β-lactamase-producing Klebsiella pneumoniae isolated from cases of bovine mastitis in Japan. J. Vet. Med. Sci. 76: 1153–1156. doi: 10.1292/jvms.13-0120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwerk C., Tenenbaum T., Kim K. S., Schroten H.2015. The choroid plexus-a multi-role player during infectious diseases of the CNS. Front. Cell. Neurosci. 9: 80. doi: 10.3389/fncel.2015.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seimiya Y., Ohshima K., Itoh H., Murakami R., Haritani M.1993. A case of neonatal calf with meningitis associated with Klebsiella oxytoca infection. J. Vet. Med. Sci. 55: 141–143. doi: 10.1292/jvms.55.141 [DOI] [PubMed] [Google Scholar]
- 27.Seimiya Y., Ohshima K., Itoh H., Ogasawara N., Okutomo M., Tanaka S.1992. Central nervous system lesions due to Escherichia coli infection in neonatal calves. J. Vet. Med. Sci. 54: 767–768. doi: 10.1292/jvms.54.767 [DOI] [PubMed] [Google Scholar]
- 28.Seimiya Y., Ohshima K., Itoh H., Ogasawara N., Okutomo M., Tanaka S.1992. Clinicopathology of meningoventriculitis due to Streptococcus bovis infection in neonatal calves. J. Vet. Med. Sci. 54: 871–874. doi: 10.1292/jvms.54.871 [DOI] [PubMed] [Google Scholar]
- 29.Sundaram V., Agrawal S., Chacham S., Mukhopadhyay K., Dutta S., Kumar P.2010. Klebsiella pneumoniae brain abscess in neonates: a report of 2 cases. J. Child Neurol. 25: 379–382. doi: 10.1177/0883073809338326 [DOI] [PubMed] [Google Scholar]
- 30.Tada M., Toyoshima Y., Honda H., Kojima N., Yamamoto T., Nishikura K., Takahashi H.2006. Multiple gas-forming brain microabscesses due to Klebsiella pneumoniae. Arch. Neurol. 63: 608–609. doi: 10.1001/archneur.63.4.608 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.