Abstract
Background:
Age- and sex-related differences in asthma may be due to changes in sex hormone levels.
Objective:
To examine whether change in free testosterone or free testosterone to estradiol ratio is associated with changes in lung function and eosinophils in Puerto Rican youth.
Methods:
We tested for association between change in sex hormone levels and change in lung function or change in eosinophils in a prospective study of 317 children (with and without asthma) followed from ages 6–14 years to ages 10–20 years (146 females, 171 males) in San Juan (PR). Serum levels of testosterone, estradiol, sex hormone binding globulin (SHBG), and progesterone were measured at two study visits, ~4.9 years apart. Using testosterone and SHBG levels, we derived free testosterone and the free testosterone to estradiol ratio. Multivariable linear regression was used for the analysis of change in lung function and eosinophils, conducted separately by sex.
Results:
In females, each quartile increment in free testosterone to estradiol ratio was associated with a 2.67% increment in FEV1/FVC %predicted between study visits. In males, each quartile increment in free testosterone to estradiol ratio was associated with a 3.13% increment in FEV1 %predicted and a 1.65% increment in FEV1/FVC %predicted between study visits. In females with asthma, an increased free testosterone to estradiol ratio was significantly associated with decreased eosinophils between visits.
Conclusions:
In Puerto Rican youth, an increased free testosterone to estradiol ratio over time was associated with an increased FEV1/FVC in both sexes, and with an increased FEV1 in males.
Keywords: testosterone, estradiol, lung function, eosinophils, asthma
INTRODUCTION
In the United States (U.S.), Puerto Ricans share a disproportionate burden of asthma, whether they live in the continental U.S. or the island of Puerto Rico1, 2.
The prevalence of asthma varies by age groups and sex throughout the lifespan. In the U.S., the prevalence of asthma is higher in males (8.3%) than in females (6.7%) among individuals younger than 18 years, while such prevalence is higher in females (9.8%) than in males (5.5%) among individuals 18 years and older1, 3. Similar variations by age group and sex are observed for asthma mortality1, 4.
Puberty influences asthma. Compared with pre-pubertal females, pre-pubertal males are more likely to be sensitized to allergens, to have asthma symptoms, and to use medications for asthma. After puberty, asthma becomes more severe or difficult to treat in females than in males5–7. In Mendelian randomization studies, early age at menarche or puberty was associated with increased risk of asthma8 and decreased forced vital capacity (FVC) in women9.
Before puberty, sex steroid hormone concentrations are low in both sexes. Two processes occur during the 3–4 years of pubertal maturation. Gonadarche refers to increased hypothalamic-pituitary-gonadal function, leading to increased testicular testosterone secretion in boys and increased ovarian estradiol secretion in girls. Concomitantly, adrenal pubertal maturation characterized by increased adrenal dehydroepiandrosterone sulfate (DHEAS) secretion occurs10. Androgens such as testosterone may protect against asthma by reducing systemic and airway inflammation, while estrogen and progesterone may enhance T-helper cell type 2 (Th2) allergic inflammation11, 12. In mouse models, estrogen has been shown to act through estrogen receptor-alpha upregulated interleukin (IL)-23R expression and increased IL-17 production13.
A cross-sectional study of 68 children with asthma (aged 6–18 years) showed that DHEAS was significantly and positively associated with percent predicted forced expiratory volume in one second (FEV1 %pred) and percent predicted FVC (FVC %pred), and that both DHEAS and testosterone were associated with better symptom control in 45 males. In that study, estradiol was significantly and negatively associated with FEV1 %pred and FVC %pred in 23 females, in whom DHEAS was associated with better symptom control. However, that study was limited by small sample size14.
The testosterone to estradiol ratio reflects the balance between these two key hormones and could thus be a better predictor of health outcomes15, 16. On the basis of prior findings, we hypothesized that increased levels of testosterone and the testosterone to estradiol ratio would lead to better lung function and decreased eosinophils in peripheral blood. To test this hypothesis, we examined whether free testosterone, estradiol, or the ratio of free testosterone to estradiol are associated with changes in lung function and peripheral blood eosinophil count (henceforth called eosinophil count) in a cohort of Puerto Rican youth followed from ages 6 to 14 years to ages 10 to 20 years.
METHODS
Study population
PR-GOAL:
Study design and subject recruitment for the Puerto Rico Genetics of Asthma and Lifestyle Study (PR-GOAL) have been previously described17–19. In brief, participants were recruited from March 2009 through June 2010 from randomly selected households in San Juan and Caguas (Puerto Rico) using a multistage probabilistic sampling design. Based on this design, 7,073 households were selected and 6,401 (91%) were contacted. Of these 6,401 households, 1,111 had ≥1 child who met inclusion criteria (age 6–14 years, four Puerto Rican grandparents, and residence in the same household for ≥ 1 year). Of these 1,111 households, 438 (39.4%) had ≥1 child with asthma (a case, defined as having physician-diagnosed asthma and ≥1 episode of wheeze in the previous year). From these 438 households, one child with asthma was selected (at random if there was more than one such child). Similarly, only one child without asthma (a control subject, having neither physician-diagnosed asthma nor wheeze in the previous year) was randomly selected from the remaining 673 households. To reach a target sample size of 700 children, we attempted to enroll 783 of the 1,111 eligible children. Parents of 105 (13.4%) of these 783 households refused to participate or could not be reached, leaving 678 study participants (351 cases and 327 controls). There were no significant differences in age, sex, or area of residence between eligible children who did (n=678) and did not (n=105) participate in PR-GOAL.
EVA-PR:
Study design and subject recruitment for the Epigenetic Variation and Childhood Asthma in Puerto Ricans study (EVA-PR) were similar to those for PR-GOAL and have been previously described20. In brief, subjects were recruited from San Juan and Caguas (Puerto Rico) from February 2014 through May 2017. Of the 1,111 households that were randomly selected for PR-GOAL (see above), 1,045 still had current and correct contact information and were thus screened for EVA-PR. Of these 1,045 households, 180 were unreachable despite several efforts. Of the 865 households that were contacted, 720 had at least one child who met the following inclusion criteria: age 9 to 20 years, four Puerto Rican grandparents, and living in the same residence for at least one year. To reach a sample size of ~550 subjects, we attempted to enroll 638 of the 720 eligible children. Of the 638 youth whom we attempted to enroll, 543 (85.1%) agreed to participate. There were no significant differences in age, sex, or area of residence between subjects who did (n=543) and did not (n=95) agree to participate in EVA-PR.
Participants included in the current prospective analysis:
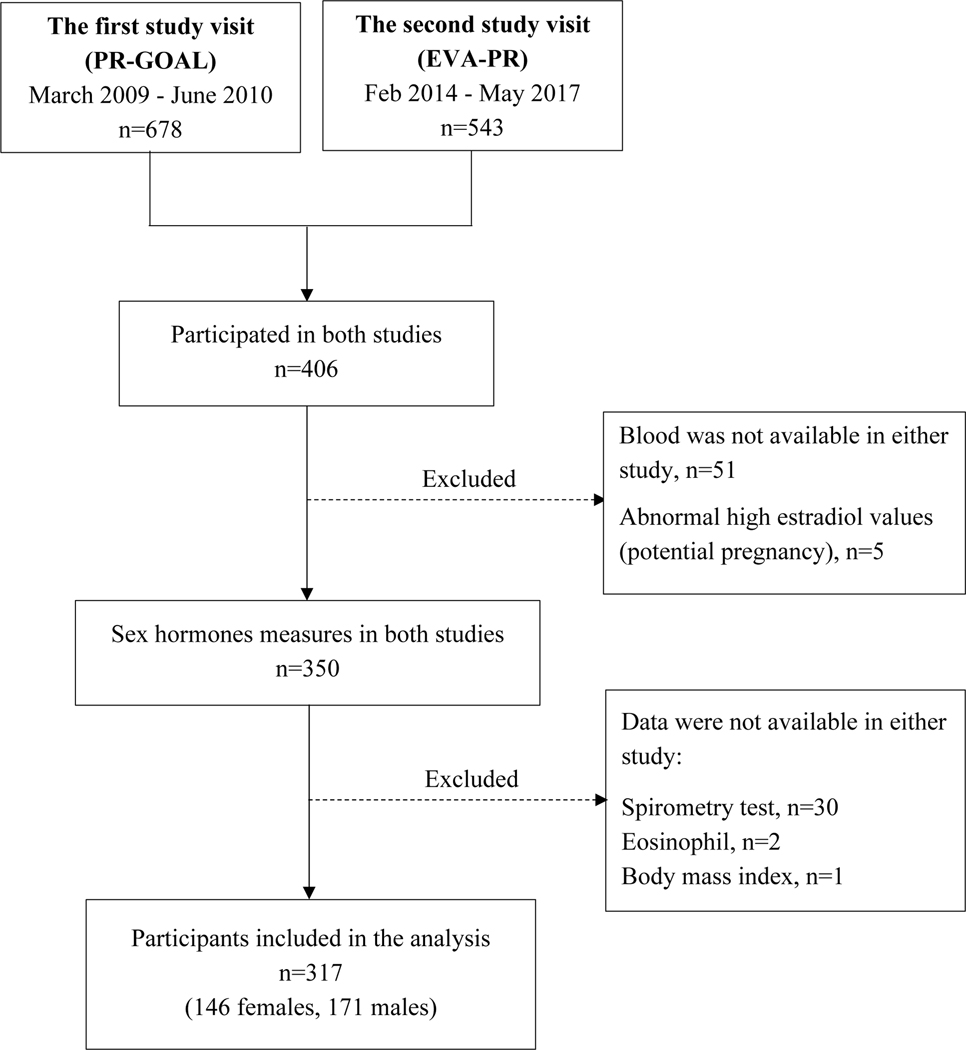
Of the 543 participants in EVA-PR, 406 had previously participated in PR-GOAL. Of these 406 subjects, 317 (146 females and 171 males) had sex hormone measurements and complete data on the covariates and outcomes of interest in both PR-GOAL and EVA-PR, and were thus included in the current analysis (Figure 1). The average time interval between the first (in PR-GOAL) and second (in EVA-PR) study visits was approximately 5.3 years. eTable 1 (see eSupplement) shows a comparison of the main baseline characteristics of the 317 subjects who participated in PR-GOAL (the first study visit) and who were included in the current analysis against the 363 subjects who participated in PR-GOAL but were not included in this analysis because they did not participate in EVA-PR (n=272) or had missing data (n=89), separately in females and males. Both female and male subjects excluded from this analysis were slightly older than those included. In males, subjects excluded from this analysis were more likely to be post-pubertal and had a lower eosinophil count than those included.
Figure 1 -.
Flowchart for selection of the participants included in the current analysis.
Study procedures
All 317 participants completed a protocol including questionnaires and spirometry. At both study visits, a questionnaire was administered to one of the child’s caretakers (usually [>93%] the mother) to obtain information about demographics, socioeconomic status, family history, and the child’s respiratory health21. At the second study visit, all participants completed an adapted version of the validated Pubertal Development Scale (PDS)22, 23. This scale contains questions about body hair growth (armpit and pubic) for both sexes, facial hair growth for males, and breast growth for females. Each question in the PDS has five answers (scored from 0 to 4): “not yet started” (1), “barely started” (2)”, “definitely started” (3)”, “seems complete” (4), and “I don’t know” or “missing” (0). Females were also asked whether they had begun to menstruate (scored as 1 for “no” and as 4 for “yes”)22, 23. For both males and females, the scores for all questions were first added and then divided by the number of questions, so that the summary PDS score ranged from 0 to 4 points. Among female participants with data on menarche, the median age of onset was 12 years, and thus puberty was defined as age ≥12 years for all female participants. Since a PDS of 2 corresponded to an age of 12.2 in girls, we used that PDS score as cut-off for puberty in males. In males, a PDS score of 2 corresponded to an age of 13 years, which was used as the proxy for pubertal onset for all male participants. For each sex, puberty onset was defined as having reached the age proxy for puberty between study visits.
Spirometry was conducted at both study visits using an EasyOne spirometer (NDD Medical Technologies, Andover, MA), following American Thoracic Society/European Respiratory Society recommendations for children24. The best FEV1 and FVC were selected for data analysis. Percent-of-predicted (%pred) FEV1, FVC, and FEV1/FVC were calculated using Global Lung Initiative (GLI) equations that account for age, sex, race/ethnicity, and height25. Body mass index (BMI) z-scores were calculated based on the 2000 Centers for Disease Control (CDC) growth charts26.
Blood samples were collected at both study visits. In those samples, eosinophils were counted using Counter/Coulter techniques. Serum estradiol, progesterone, and total testosterone (TT) were measured using the UniCel DxI 800 Access Immunoassay System (Beckman Coulter, Inc. Brea, CA), and sex hormone binding globulin (SHBG) was measured using UniCel DxI 600 Access Immunoassay System (Beckman Coulter, Inc. Brea, CA), following the manufacturers’ recommendations. Because testosterone circulates highly bound to SHBG, free testosterone was estimated using the empirical free testosterone (EFT) formula, as follows27:
EFT-low (TT < 5 nM) = −6.593 + 19.304 × TT + 0.056 × SHBG - 0.0959 × TT × SHBG
EFT-high (TT ≥ 5 nM) = −52.65 + 24.4 × TT - 0.704 × SHBG - 0.0782×T×SHBG - 0.0584×TT2
Our exposure of interest was change in the levels of each sex hormone (free testosterone, estradiol, and progesterone) and the ratio of free testosterone to estradiol between the first and second study visits. Our outcomes of interest were: 1) change in percent predicted lung function measures (FEV1, FVC, and FEV1/FVC), and 2) eosinophil count (log-transformed for data analysis) between the first and second study visits.
Written parental consent was obtained for participating children, from whom written assent was also obtained. PR-GOAL was approved by the Institutional Review Boards of the University of Puerto Rico (San Juan, PR), Brigham and Women’s Hospital (Boston, MA) and the University of Pittsburgh (Pittsburgh, PA). EVA-PR was approved by the Institutional Review Boards of the University of Puerto Rico and the University of Pittsburgh.
Statistical analysis
All analyses were conducted separately in females and in males. Bivariate analyses were conducted using chi-squared tests for binary variables and two-tailed t tests for pairs of categorical and continuous variables. For ease of interpretation, quartiles of free testosterone to estradiol ratio were used in the multivariable linear regression analysis. All models were adjusted for potential confounders including age, type of health insurance (private vs. others) at the baseline visit; asthma status and puberty onset; and change in BMI z-score, change in progesterone level, and time interval between study visits. All analyses were conducted using SAS 9.4 software (SAS Institute, Inc., Cary, NC).
RESULTS
Table 1 shows a comparison of the main characteristics of subjects with (cases) and without (controls) asthma at the first or baseline study visit, separately in males and females. Compared with female controls, female cases had a higher eosinophil count. There were no significant differences in sex hormone levels or any other characteristic between females with and without asthma. Compared with male control subjects, male cases had higher eosinophil count but lower FEV1 %pred and FVC %pred. There were no significant differences in sex hormone levels or any other characteristic between male cases and male controls.
Table 1-.
Characteristics of study participants at the baseline study visit
| Females (n=146) | Males (n=171) | |||
|---|---|---|---|---|
| Controls (n=75) | Cases (n=71) | Controls (n=74) | Cases (n=97) | |
| Age | 10.3 ± 2.8 | 9.8 ± 2.5 | 9.9 ± 2.7 | 9.7 ± 2.4 |
| Pre-pubertal status | 46 (61.3) | 50 (70.4) | 56 (75.7) | 81 (83.5) |
| Private health insurance | 30 (40.0) | 23 (32.4) | 22 (29.7) | 34 (35.1) |
| Annual household income > $15,000 | 29 (39.7) | 22 (31.0) | 20 (29.0) | 38 (41.3) |
| Body mass index, z-score | 0.7 ± 1.1 | 1.0 ± 1.0 | 0.4 ± 1.2 | 0.7 ± 1.2 |
| Log10 eosinophil count (cell/μL) | 2.2 ± 0.5 | 2.5 ± 0.3* | 2.4 ± 0.4 | 2.6 ± 0.4* |
| % predicted FEV1 | 96.0 ± 14.8 | 92.8 ± 15.1 | 98.9 ± 16.9 | 91.3 ± 14.5* |
| % predicted FVC | 103.7 ± 17.5 | 102.7 ± 16.3 | 106.2 ± 16.0 | 100.0 ± 16.0* |
| % predicted FEV1/FVC | 92.6 ± 8.8 | 90.4 ± 10.0 | 93.0 ± 11.6 | 91.4 ± 9.5 |
| Inhaled steroids in the 6 months | - | 27 (38.6) | - | 29 (29.9) |
| prior to the second study visit | ||||
| Total testosterone (nmol/L) | 0.8 ± 0.6 | 1.0 ± 0.9 | 2.8 ± 4.7 | 2.6 ± 4.3 |
| Free testosterone (pmol/L) | 8.8 ± 9.3 | 10.6 ± 12.9 | 38.9 ± 74.0 | 32.1 ± 62.4 |
| Estradiol (pmol/L) | 117.4 ± 127.8 | 122.6 ± 172.3 | 40.7 ± 6.2 | 41.5 ± 8.9 |
| Free testosterone to estradiol ratio | 0.08 ± 0.08 | 0.12 ± 0.15 | 0.89 ± 1.58 | 0.66 ± 1.19 |
| Progesterone (nmol/L) | 2.9 ± 6.5 | 3.4 ± 8.3 | 1.3 ± 1.7 | 1.3 ± 1.7 |
| Sex hormone binding globulin (nmol/L) | 61.5 ± 39.4 | 51.9 ± 28.6 | 68.6 ± 38.3 | 71.4 ± 42.1 |
| Time interval between visits (years) | 5.5 ± 0.8 | 5.5 ± 1.1 | 5.2 ± 0.8 | 5.1 ± 0.8 |
Data are presented as number (percentage) for categorical variables or mean ± standard deviation for continuous variables.
P<0.05 for comparison between controls and case within each sex (conducted using 2-sample t-tests for continuous variables, and chi-squared tests for binary variables).
eTable 2 shows the results of the multivariable analysis of changes in both the level of each sex hormone (free testosterone, estradiol, and progesterone) and lung function measures between the two study visits. In males, each quartile increment in free testosterone was significantly associated with a 3.35% increment in FEV1 %pred, but not with FVC %pred or FEV1/FVC %pred. In females, there was no significant association between changes in any sex hormone level and lung function.
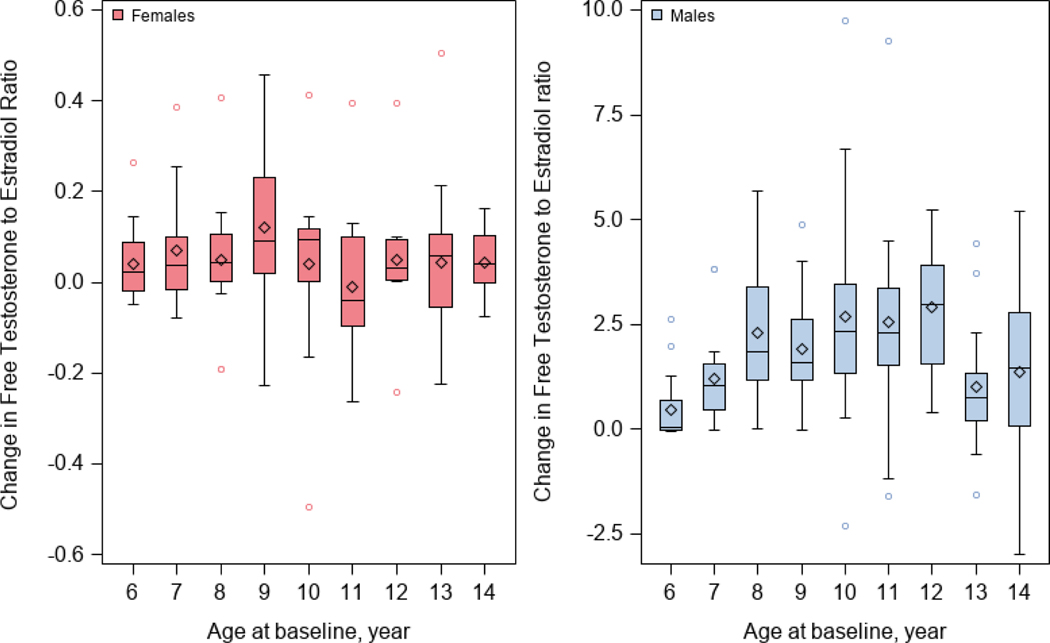
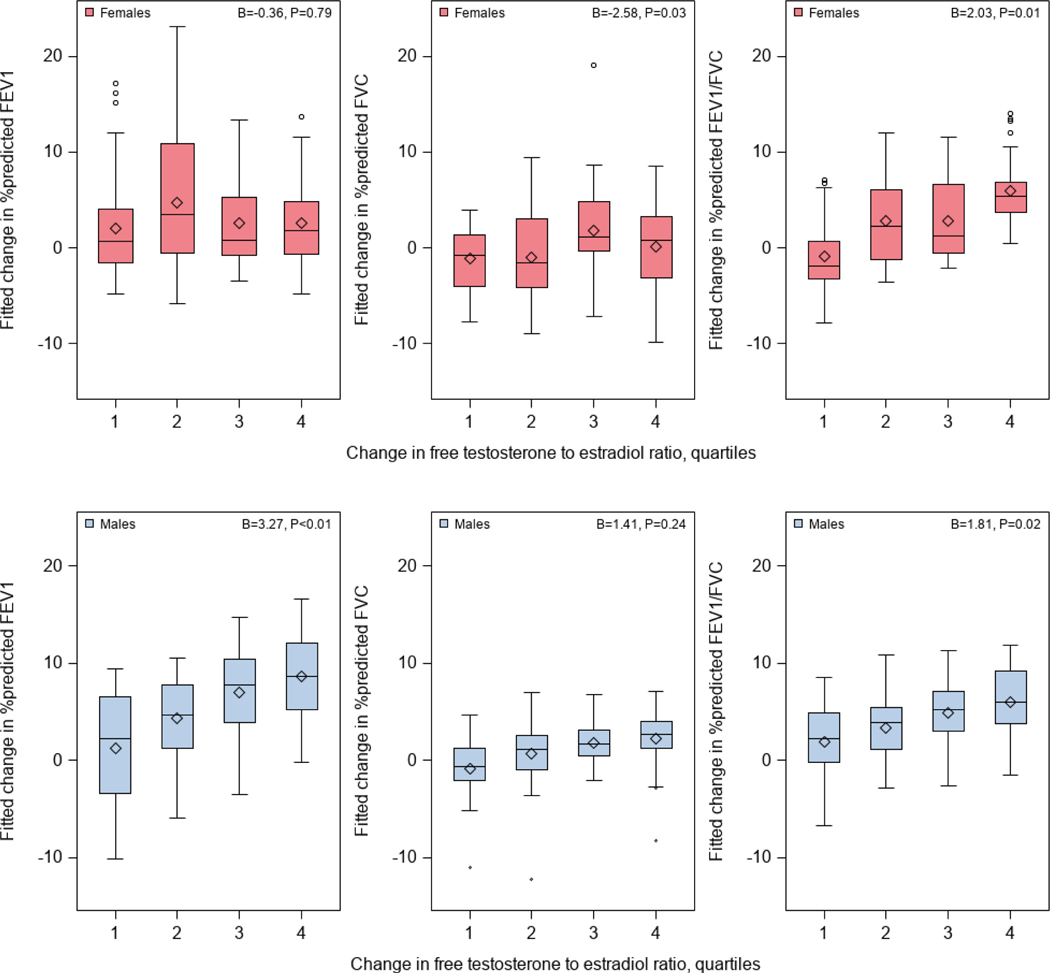
As expected, changes in free testosterone to estradiol ratio between two study visits were much greater in males than in females (Figure 2). Table 2 shows the results of the analysis of change in free testosterone to estradiol ratio and change in lung function measures between the two study visits. Among females, an analysis adjusted for age, asthma (case-control) status, type of health insurance, BMI z-score, and the time interval between visits (Model 1) showed that each quartile increment in free testosterone to estradiol ratio was significantly associated with a decrement of 2.40% in FVC %pred but a 1.80% increment in FEV1/FVC %pred ). After additional adjustment for puberty onset and change in progesterone level between study visits (Model 2), each quartile increment in free testosterone to estradiol ratio was significantly associated with a decrement of 2.58% in FVC %pred but a 2.03% increment in FEV1/FVC %pred ( see Figure 3). Among males, an analysis adjusted for age, asthma status, type of health insurance, BMI z-score, and the time interval between visits (Model 1) showed that each quartile increment in the free testosterone to estradiol ratio was significantly associated with a 3.25% increment in FEV1 %pred (95% CI= 1.14% to 5.36%) and a 1.90% increment in FEV1/FVC %pred (95%=0.43% to 3.38%). After additional adjustment for puberty onset and changes in progesterone levels between visits (Model 2), each quartile increment in free testosterone to estradiol ratio was significantly associated with an increment of 3.27% in FEV1 %pred and an increment of 1.81% in FEV1/FVC %pred (Figure 3). We found no significant modification of the estimated effect of change in the free testosterone to estradiol ratio on change in lung function measures by asthma status in males or females (P for interaction >0.10 in all instances).
Figure 2 -.
Changes in free testosterone to estradiol ratio between the two study visits, by age and sex.
Table 2-.
Multivariable analysis of change in free testosterone to estradiol ratio and change in lung function measures between the two study visits
| Females (n=146) | Males (n=171) | |
|---|---|---|
| Change (Δ) in free testosterone to estradiol ratio, per quartile increment | β (95% confidence interval) | |
| Model 1 | ||
| Δ % predicted FEV1 | −0.36 (−2.04, 1.32) | 3.25 (1.14, 5.36)† |
| Δ % predicted FVC | −2.40 (−4.61, −0.19)* | 1.26 (−0.94, 3.46) |
| Δ % predicted FEV1/FVC | 1.80 (0.29, 3.31)* | 1.90 (0.43, 3.38)* |
| Model 2 | ||
| Δ % predicted FEV1 | −0.23 (−1. 95, 1.50) | 3.27 (1.01, 5.52)† |
| Δ % predicted FVC | −2.58 (−4.88, −0.29)* | 1.40 (−0.93, 3.74) |
| Δ % predicted FEV1/FVC | 2.03 (0.47, 3.59)* | 1.81 (0.24, 3.38)* |
Model 1 was adjusted for age and type of health insurance at baseline, asthma status and puberty onset, and change in BMI z-score and time interval between study visits. Model 2 was adjusted for all covariates in Model 1 plus puberty onset and change in progesterone level between the two study visits.
P <0.05
P<0.01
Figure 3 -.
Fitted changes in lung function measures and free testosterone to estradiol ratio in quartile between the two study visits (females in upper panels, males in lower panels).
All models adjusted for age and type of health insurance at baseline; asthma status and puberty onset; and change in BMI z-score, change in progesterone, and time interval between study visits.
We ran a sensitivity analysis for lung function measures after additional adjustment for use of inhaled steroids, obtaining similar results (Model 1 in eTable 3). We conducted another sensitivity analysis after excluding children who had reached puberty at the first visit, obtaining similar results despite smaller sample size (Model 2 in eTable 3).
Next, we analyzed the relationship between change in free testosterone to estradiol ratio and change in eosinophil count. In a multivariable analysis adjusting for asthma status, age, type of health insurance, BMI z-score, puberty onset, change in progesterone level, and the time interval between visits, there was no significant association between change in free testosterone to estradiol ratio and eosinophils in males or females. However, there was a significant interaction between asthma status and change in free testosterone to estradiol ratio on changes in eosinophil count in females (P = 0.02). We thus conducted a multivariable analyses stratified by asthma status (Table 3). Among females with asthma, each quartile increment in free testosterone to estradiol ratio was significantly associated with decrements in eosinophil count (P=0.03). Among males, free testosterone to estradiol ratio was not significantly associated with eosinophil count, regardless of asthma status.
Table 3-.
Multivariable analysis of change in free testosterone to estradiol ratio and change in eosinophil count between the two study visits
| Model 1 | Model 2 | |
|---|---|---|
| Change (Δ) in free testosterone to estradiol ratio, per quartile increment | β (95% confidence interval) | |
| Δ Log10 eosinophils | ||
| Females | ||
| With asthma (n=71) | −0.09 (−0.17, −0.02)* | −0.08 (−0.15, −0.01)* |
| Without asthma (n=75) | 0.01 (−0.07, 0.10) | 0.02 (−0.07, 0.11) |
| Males | ||
| With asthma (n=97) | −0.01 (−0.09, 0.06) | −0.04 (−0.11, 0.04) |
| Without asthma (n=74) | 0.003 (−0.06, 0.07) | 0.01 (−0.06, 0.08) |
Model 1 adjusted for age and type of health insurance at baseline, and change in BMI z-score and time interval between study visits.
Model 2 adjusted for all covariates in Model 1 plus puberty onset and change in progesterone level between the two study visits
P <0.05
P<0.01
DISCUSSION
In this study of Puerto Rican youth, an increased free testosterone to estradiol ratio over ~5 years was associated with positive changes in FEV1 and FEV1/FVC in males and a positive change in FEV1/FVC in females. Among females with asthma, an increased free testosterone to estradiol ratio was associated with decreased eosinophils. To our knowledge, this is the first longitudinal study of sex hormones and lung function or eosinophil count in children and adolescents.
A few cross-sectional studies have examined testosterone and lung function14,28,29, 30. In a U.S. study, total testosterone was positively correlated with FEV1 in subjects with asthma older than 11 years (regardless of sex), but not in younger subjects28. In two studies of adult males, free or total testosterone and dihydrotestosterone (DHT) have been positively associated with FEV1 and FVC29, 30. In one of those studies, men with severe airflow obstruction (FEV1 <50% of predicted ) had lower free testosterone levels30. Our longitudinal findings in youth are thus consistent with and expand those of prior cross-sectional studies, as we show a positive association between change in free testosterone to estradiol and FEV1/FVC (a marker of airflow obstruction) in males and females, and FEV1 in males. The observed negative effect of change in free testosterone to estradiol ratio on FVC (but not on FEV1 or FEV1/FVC) in females is intriguing and should be further examined in future studies.
Atopic asthma is more common in males than in females before puberty, but this ratio reverses after puberty31–33. Consistent with a role of sex hormones in sex-specific differences in atopic asthma, a higher free testosterone to estradiol ratio was associated with lower eosinophils in females with asthma. The negative findings for free testosterone to estradiol ratio and eosinophils in males may be partly explained by unmeasured androgens (e.g., DHT or DHNE-S) or androgen receptors34. Alternatively, this may be explained by selection bias, as males with a lower eosinophil count were more likely to be excluded from the current analysis (eTable 1).
Findings from previous cross-sectional studies suggest protective effects of an increased testosterone level on asthma or its intermediate phenotypes in adults (e.g., lung function and eosinophils)35, 36. In British adults, an elevated free testosterone was associated not only with lower odds of asthma in females and males, but also with higher FEV1 and FVC in males36. Moreover, a human phase II clinical trial with small sample size showed that nebulized DHEA-S improved asthma control and symptoms in adults with poorly controlled moderate to severe asthma on inhaled corticosteroids and long-acting ß2-agonists37. In support of findings from those human studies, testosterone upregulated expression of the gene for the β2-adrenergic receptor (ADRB2) in airway smooth muscle (ASM) and increased salbutamol-induced ASM relaxation in healthy male guinea pigs38 and reduced reactivity of ASM in male guinea pigs39. Because female guinea pigs were not included, further study is needed to determine whether testosterone affects ASM in females.
A higher testosterone to estradiol ratio may reduce systemic and airway inflammation40–43. In two studies of adult males, total or free testosterone and total testosterone to estradiol molar ratio were inversely associated with C-reactive protein (CRP) level, while estradiol was positively associated with CRP level40, 41. In a murine model, testosterone decreased dust mite-induced eosinophilic and neutrophilic inflammation in the lungs, as well as Th2 and Th17 cytokines, partially through androgen receptor (AR) signaling42. In another mouse model, 5α-DHT decreased Alternaria-induced IL-5, IL-13, and lung eosinophils by attenuating group 2 innate lymphoid cells (ILC2)43. In contrast to those findings for testosterone, estradiol has been shown to increase eosinophils in the lung and upregulate Th2 genes in male mice44, as well as to increase IL-33 release and ILC2-mediated airway inflammation via the estrogen receptor-alpha in mice45. Consistent with findings in rodents, an in vitro study in human subjects with asthma showed that estradiol upregulated IL-17, IL-23 and TGF-β expression in peripheral blood mononuclear cells46.
We acknowledge additional study limitations. First, we had limited statistical power to detect modest associations. Second, recall bias and misclassification of puberty onset are possible because we lack data on Tanner stage in study participants. However, prior studies have shown that puberty onset, rather than puberty stage, is associated with asthma occurrence or symptoms47, 48. Third, data on the phase of menstrual cycle (luteal or follicular) was not available in girls. Nonetheless, change in progesterone was not associated with any outcome, and our results were unchanged in analyses adjusted for such change. Fourth, though with much lower binding affinities, SHBG can also bind to estradiol. However, we obtained similar results in analyses adjusted for change in SHBG between the study visits. Fifth, we lack data on potential confounders such as use of oral contraceptives or sex hormones, growth hormone level, and environmental exposure to endocrine disruptors or air pollution49. Finally, the generalizability of our findings to other racial or ethnic groups requires further investigation.
In summary, our findings suggest that an increased testosterone to estradiol ratio may contribute to changes in lung function after puberty in males and females. Larger longitudinal studies are needed to validate our results.
Supplementary Material
Acknowledgments
Funding Sources: This work was supported by grants HL079966, HL117191, and MD011764 from the U.S. National Institutes of Health (NIH) to J.C.C. Dr. Forno’s contribution was supported by grant HL149693 from the U.S. NIH. The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Abbreviations:
- %pred
percent of predicted
- BMI
body mass index
- CI
confidence interval
- CRP
C-reactive protein
- DHEAS
dehydroepiandrosterone sulfate
- DHT
dihydrotestosterone
- EFT
empirical free testosterone
- EVA-PR
Epigenetic Variation and Childhood Asthma in Puerto Ricans study
- FEV1
forced expiratory volume in one second
- FVC
forced vital capacity
- GLI
Global Lung Initiative
- IL
interleukin
- PDS
pubertal development scale
- PR-GOAL
Puerto Rico Genetics of Asthma and Lifestyle Study
- SHBG
sex hormone binding globulin
- Th2
T-helper cell type 2
- TT
total testosterone
Footnotes
Conflicts of interest: Dr. Celedón has received research materials from GSK and Merck (inhaled steroids) and Pharmavite (vitamin D and placebo capsules), to provide medications free of cost to participants in NIH-funded studies, unrelated to this work. The other authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2016–2018 National Health Interview Survey (NHIS): Most Recent National Asthma Data. Atlanta, Georgia: 2018. [Google Scholar]
- 2.Rosser FJ, Forno E, Cooper PJ, Celedon JC. Asthma in Hispanics. An 8-year update. Am J Respir Crit Care Med. 2014;189:1316–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Postma DS. Gender differences in asthma development and progression. Gend Med. 2007;4 Suppl B:S133–146. [DOI] [PubMed] [Google Scholar]
- 4.Pennington E, Yaqoob ZJ, Al-Kindi SG, Zein J. Trends in Asthma Mortality in the United States: 1999 to 2015. Am J Respir Crit Care Med. 2019;199:1575–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yung JA, Fuseini H, Newcomb DC. Hormones, sex, and asthma. Ann Allergy Asthma Immunol. 2018;120:488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hohmann C, Keller T, Gehring U, et al. Sex-specific incidence of asthma, rhinitis and respiratory multimorbidity before and after puberty onset: individual participant meta-analysis of five birth cohorts collaborating in MeDALL. BMJ Open Respir Res. 2019;6:e000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Almqvist C, Worm M, Leynaert B, working group of GALENWPG. Impact of gender on asthma in childhood and adolescence: a GA2LEN review. Allergy. 2008;63:47–57. [DOI] [PubMed] [Google Scholar]
- 8.Minelli C, van der Plaat DA, Leynaert B, et al. Age at puberty and risk of asthma: A Mendelian randomisation study. PLoS Med. 2018;15:e1002634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gill D, Sheehan NA, Wielscher M, et al. Age at menarche and lung function: a Mendelian randomization study. Eur J Epidemiol. 2017;32:701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plant TM, Terasawa E, Witchel SF. Puberty in Non-human Primates and Man. In: Plant TM, Zeleznik AJ, eds. Knobil and Neill’s Physiology of Reproduction: Academic Press Books - Elsevier; 2015. [Google Scholar]
- 11.Fuseini H, Newcomb DC. Mechanisms Driving Gender Differences in Asthma. Curr Allergy Asthma Rep. 2017;17:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuentes N, Silveyra P. Endocrine regulation of lung disease and inflammation. Exp Biol Med (Maywood). 2018;243:1313–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuseini H, Cephus JY, Wu P, et al. ERalpha Signaling Increased IL-17A Production in Th17 Cells by Upregulating IL-23R Expression, Mitochondrial Respiration, and Proliferation. Front Immunol. 2019;10:2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeBoer MD, Phillips BR, Mauger DT, et al. Effects of endogenous sex hormones on lung function and symptom control in adolescents with asthma. BMC Pulm Med. 2018;18:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao D, Guallar E, Ouyang P, et al. Endogenous Sex Hormones and Incident Cardiovascular Disease in Post-Menopausal Women. J Am Coll Cardiol. 2018;71:2555–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim C, Halter JB. Endogenous sex hormones, metabolic syndrome, and diabetes in men and women. Curr Cardiol Rep. 2014;16:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brehm JM, Acosta-Perez E, Klei L, et al. Vitamin D insufficiency and severe asthma exacerbations in Puerto Rican children. Am J Respir Crit Care Med. 2012;186:140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forno E, Acosta-Perez E, Brehm JM, et al. Obesity and adiposity indicators, asthma, and atopy in Puerto Rican children. J Allergy Clin Immunol. 2014;133:1308–1314, 1314 e1301–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han YY, Forno E, Brehm JM, et al. Diet, interleukin-17, and childhood asthma in Puerto Ricans. Ann Allergy Asthma Immunol. 2015;115:288–293 e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forno E, Wang T, Qi C, et al. DNA methylation in nasal epithelium, atopy, and atopic asthma in children: a genome-wide study. Lancet Respir Med. 2019;7:336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blumenthal MN, Banks-Schlegel S, Bleecker ER, Marsh DG, Ober C. Collaborative studies on the genetics of asthma--National Heart, Lung and Blood Institute. Clin Exp Allergy. 1995;25 Suppl 2:29–32. [DOI] [PubMed] [Google Scholar]
- 22.Pompeia S, Zanini GAV, Freitas RS, et al. Adapted version of the Pubertal Development Scale for use in Brazil. Rev Saude Publica. 2019;53:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carskadon MA, Acebo C. A self-administered rating scale for pubertal development. J Adolesc Health. 1993;14:190–195. [DOI] [PubMed] [Google Scholar]
- 24.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. [DOI] [PubMed] [Google Scholar]
- 25.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention National Center for Health Statistics. 2000 CDC Growth Charts for the United States: Methods and Development. In: Department of Health and Human Services, ed. Vol 11. Hyattsville, Maryland: 2002. [Google Scholar]
- 27.Ly LP, Handelsman DJ. Empirical estimation of free testosterone from testosterone and sex hormone-binding globulin immunoassays. Eur J Endocrinol. 2005;152:471–478. [DOI] [PubMed] [Google Scholar]
- 28.Bulkhi AB, Shepard KV 2nd, Casale TB, Cardet JC. Elevated testosterone is associated with decreased likelihood of current asthma regardless of sex. J Allergy Clin Immunol Pract. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohan SS, Knuiman MW, Divitini ML, et al. Higher serum testosterone and dihydrotestosterone, but not oestradiol, are independently associated with favourable indices of lung function in community-dwelling men. Clin Endocrinol (Oxf). 2015;83:268–276. [DOI] [PubMed] [Google Scholar]
- 30.Svartberg J, Schirmer H, Medbo A, Melbye H, Aasebo U. Reduced pulmonary function is associated with lower levels of endogenous total and free testosterone. The Tromso study. Eur J Epidemiol. 2007;22:107–112. [DOI] [PubMed] [Google Scholar]
- 31.Bonds RS, Midoro-Horiuti T. Estrogen effects in allergy and asthma. Curr Opin Allergy Clin Immunol. 2013;13:92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leynaert B, Sunyer J, Garcia-Esteban R, et al. Gender differences in prevalence, diagnosis and incidence of allergic and non-allergic asthma: a population-based cohort. Thorax. 2012;67:625–631. [DOI] [PubMed] [Google Scholar]
- 33.Hansen S, Probst-Hensch N, Keidel D, et al. Gender differences in adult-onset asthma: results from the Swiss SAPALDIA cohort study. Eur Respir J. 2015;46:1011–1020. [DOI] [PubMed] [Google Scholar]
- 34.Becerra-Diaz M, Strickland AB, Keselman A, Heller NM. Androgen and Androgen Receptor as Enhancers of M2 Macrophage Polarization in Allergic Lung Inflammation. J Immunol. 2018;201:2923–2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han YY, Forno E, Celedon JC. Sex Steroid Hormones and Asthma in a Nationwide Study of U.S. Adults. Am J Respir Crit Care Med. 2020;201:158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han YY, Yan Q, Yang G, Chen W, Forno E, Celedon JC. Serum free testosterone and asthma, asthma hospitalisations and lung function in British adults. Thorax. 2020;75:849–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wenzel SE, Robinson CB, Leonard JM, Panettieri RA Jr. Nebulized dehydroepiandrosterone-3-sulfate improves asthma control in the moderate-to-severe asthma results of a 6-week, randomized, double-blind, placebo-controlled study. Allergy Asthma Proc. 2010;31:461–471. [DOI] [PubMed] [Google Scholar]
- 38.Carbajal-Garcia A, Reyes-Garcia J, Casas-Hernandez MF, et al. Testosterone augments beta2 adrenergic receptor genomic transcription increasing salbutamol relaxation in airway smooth muscle. Mol Cell Endocrinol. 2020;510:110801. [DOI] [PubMed] [Google Scholar]
- 39.Montano LM, Flores-Soto E, Reyes-Garcia J, et al. Testosterone induces hyporesponsiveness by interfering with IP3 receptors in guinea pig airway smooth muscle. Mol Cell Endocrinol. 2018;473:17–30. [DOI] [PubMed] [Google Scholar]
- 40.Tsilidis KK, Rohrmann S, McGlynn KA, et al. Association between endogenous sex steroid hormones and inflammatory biomarkers in US men. Andrology. 2013;1:919–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kupelian V, Chiu GR, Araujo AB, Williams RE, Clark RV, McKinlay JB. Association of sex hormones and C-reactive protein levels in men. Clin Endocrinol (Oxf). 2010;72:527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fuseini H, Yung JA, Cephus JY, et al. Testosterone Decreases House Dust Mite-Induced Type 2 and IL-17A-Mediated Airway Inflammation. J Immunol. 2018;201:1843–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cephus JY, Stier MT, Fuseini H, et al. Testosterone Attenuates Group 2 Innate Lymphoid Cell-Mediated Airway Inflammation. Cell Rep. 2017;21:2487–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lauzon-Joset JF, Mincham KT, Abad AP, et al. Oestrogen amplifies pre-existing atopy-associated Th2 bias in an experimental asthma model. Clin Exp Allergy. 2020;50:391–400. [DOI] [PubMed] [Google Scholar]
- 45.Cephus JY, Gandhi VD, Shah R, et al. Estrogen receptor-alpha signaling increases allergen-induced IL-33 release and airway inflammation. Allergy. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahmadi-Vasmehjani A, Baharlou R, Atashzar MR, Raofi R, Jafari M, Razavi FS. Regulatory Effects of Estradiol on Peripheral Blood Mononuclear Cells Activation in Patients with Asthma. Iran J Allergy Asthma Immunol. 2018;17:9–17. [PubMed] [Google Scholar]
- 47.Fu L, Freishtat RJ, Gordish-Dressman H, et al. Natural progression of childhood asthma symptoms and strong influence of sex and puberty. Ann Am Thorac Soc. 2014;11:939–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vink NM, Postma DS, Schouten JP, Rosmalen JG, Boezen HM. Gender differences in asthma development and remission during transition through puberty: the TRacking Adolescents’ Individual Lives Survey (TRAILS) study. J Allergy Clin Immunol. 2010;126:498–504 e491–496. [DOI] [PubMed] [Google Scholar]
- 49.Fuentes N, Nicoleau M, Cabello N, et al. 17beta-Estradiol affects lung function and inflammation following ozone exposure in a sex-specific manner. Am J Physiol Lung Cell Mol Physiol. 2019;317:L702–L716. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.