Summary
A multicomponent approach for the treatment of pediatric overweight/obesity, which includes behavioral strategies to alter diet and physical activity/sedentary behavior, has graded recommendations for its use. Dietary interventions to be used within this approach do not. In adults, research indicates that strongly graded dietary interventions providing greater structure (or more control over the types/amount of food consumed) produce better weight outcomes. For this critical review, dietary interventions recommended by the Expert Committee for the treatment of pediatric overweight/obesity were categorized according to their potential degree of dietary structure, and their impact on weight outcomes was described. Four levels of dietary structure were reviewed, operationalized as alterations to the following: food groups, such as fruits and vegetables (low structure); daily eating occasions, such as meals (moderate structure); large nutrients, such as energy (high structure); and energy plus additional dietary alterations (very high structure). In total, 24 interventions (four low, three moderate, five high, and 12 very high structure structure) were identified and reviewed. Reductions in standardized body mass index increased with increasing structure, and interventions ≥6 months had better outcomes than interventions <6 months. Future research should empirically test dietary intervention structure to determine its impact on weight status during pediatric overweight/obesity treatment.
Keywords: childhood, diet, treatment, weight loss
1 |. INTRODUCTION
The high prevalence of overweight and obesity in children and adolescents remains a public health concern in the United States.1 Pediatric overweight and obesity increases the likelihood of children and adolescents experiencing overweight and obesity as adults.2,3 Children and adolescents with overweight or obesity are also at greater risk for developing associated comorbidities, such as prediabetes and hypercholesterolemia, during childhood, as well as adulthood.2,4 Finally, children and adolescents with overweight and obesity also experience more teasing and bullying than their healthy weight counterparts, which puts them at greater risk for experiencing psychosocial distress.5
As a consequence of the deleterious effects of pediatric overweight and obesity, professional organizations have developed recommendations for treatment, such as the recommendations from the Expert Committee6 (endorsed by 15 national health care organizations) and the clinical practice guidelines from the American Psychological Association.7 A recent scoping review of guidelines for the dietary management of childhood obesity found that, in general, recommendations encourage a multicomponent approach, in which diet and physical activity (PA) and/or sedentary behaviors are targeted to alter energy balance using family-based behavioral strategies.8 The US Preventive Services Task Force also provides similar recommendations for pediatric overweight and obesity.9 Additionally, current recommendations support treatment to begin as early as 2 years of age and to provide at least 26 h of contact time, as consistent, longer term support leads to greater improvements weight outcomes compared with low-intensity (or low-contact) interventions.7,9
Although there are graded recommendations regarding the multicomponent approach (grading: strong),7 and there is some grading of the quality of the evidence for dietary interventions (grading: consistent evidence to suggest),6 there are no graded recommendations for dietary interventions to be used within this multicomponent approach. In adults, graded dietary recommendations for the treatment of overweight and obesity generally focus on changing the overall diet (i.e., reducing overall energy intake, altering macronutrient intake, and/or changing intake from all food groups in the diet).10 Moreover, dietary interventions in adult obesity treatment that provide greater structure, or more control over the types and amount of foods and beverages consumed, result in greater weight loss (e.g., a low-kilocalorie [kcal] diet using meal replacements versus a low-kcal diet using conventional foods).11 In contrast, the nongraded dietary interventions recommended for the treatment of overweight and obesity in children and adolescents6 are more broad, ranging from altering only specific parts of the diet (e.g., sugar-sweetened beverages [SSBs] and breakfast) to the overall diet (e.g., the Traffic Light Diet,12 which includes daily energy goals and daily servings from food groups that correspond to the colors of the traffic light). Thus, due to the lack of grading and the broadness of the recommendations, the purpose of this critical review was to describe the relative effectiveness of the nongraded dietary interventions recommended for the treatment of overweight and obesity in children and adolescents on improving weight status relative to the degree of structure imposed on the diet. A critical review, which is a narrative review that reinterprets the reviewed literature to identify a new concept, theory, or framework, was conducted to develop an innovative conceptual framework, based upon dietary structure, by which existing pediatric dietary interventions could be interpreted.13 This dietary structure framework has not been directly examined within the field of overweight or obesity treatment in children and adolescents.
2 |. METHODS
2.1 |. Search strategy and study selection
To increase the rigor of this critical review, a methodical search was conducted in PubMed and SCOPUS to identify dietary interventions for the treatment of overweight and obesity in children and adolescents published between January 1, 1980 (the approximate period when pediatric obesity rates began to rise in the United States14) and July 7, 2020. Dietary interventions recommended by the Expert Committee6 were used to develop broad search terms intended to capture all relevant studies. Key search terms (Table S1) included fruit, vegetable*, sugar-sweetened beverages, breakfast, meal timing, energy density, calorie restrict*, meal plan, structured meals, energy restrict*, low energy, low calorie, very low calorie, DASH, Mediterranean, stoplight diet, traffic light diet, very low energy diet, eating pattern, and meal replacement. Two authors (LG and SD) completed a title/abstract review of all unduplicated search results. For references not excluded during the title/abstract screening, a full text of the article was obtained and reviewed by the same authors. For both stages of review, the third author (HR) was consulted when consensus could not be reached.
2.2 |. Inclusion criteria
Inclusion criteria were finalized through an iterative review of articles identified in the database searches until the final sample included only studies that contained at least one intervention that could be classified according to its dietary structure and could be compared by child weight outcomes. All articles included in this review had at least one intervention that met the following criteria: tested an intervention conducted in any setting (clinic, research facility, etc.) for children and adolescents (aged 2 to 19 years) with overweight or obesity, as defined by study authors (e.g., Centers for Disease Control and Prevention body mass index [BMI]-for-age cutoffs,6 International Obesity Task Force BMI cutoffs,15 percent overweight); used an experimental design in which a dietary intervention was implemented and there was a pre-post assessment; included at least one dietary intervention that directly targeted food, nutrient, and/or eating occasion intake recommended by the Expert Committee6 for the treatment of overweight or obesity in children and adolescents; was at least 8 weeks (2 months) in length; contained at least one behavioral strategy designed to assist with changing energy balance behaviors (goals and planning, comparison of outcomes, self-monitoring of diet, self-monitoring of outcome, reward and threat, stimulus control, modeling of healthy lifestyle behaviors by parents, problem solving, motivational interviewing, general parenting skills [e.g., positive parenting] or family conflict management); did not specifically recruit children and adolescents with underlying disorders or issues related to metabolism, growth, appetite, or feeding or health conditions that required specific dietary restrictions; assessed standardized body mass index (ZBMI) as an outcome of the intervention (to allow for comparisons between interventions); was conducted within a developed country16; and was published in English. Articles were excluded if the intervention(s) included medications or surgery as the primary intervention or if at least one of the recommended dietary interventions did not report specific dietary goals (e.g., reduce energy intake vs. 1200 to 1500 kcal/day), as the ability to determine how much structure was imposed on the diet became less accurate.
3 |. CONCEPTUALIZING DIETARY STRUCTURE
The Expert Committee recommends nine dietary interventions for the treatment of pediatric overweight and obesity that directly target food, nutrient, and/or eating occasion intake.6 These include decreasing SSBs, increasing fruits and vegetables (FVs), eating breakfast, eating structured daily meals and snacks, limiting energy-dense foods and/or reducing energy density, consuming a macronutrient-balanced diet, reducing energy intake, very-low-kcal diets, and meal replacements.6 For this review, an additional dietary intervention, energy restriction plus additional dietary interventions, was included to allow for the combination of a commonly prescribed recommendation (reducing energy intake) with other recommendations.
These recommended dietary interventions were organized according to their potential degree of imposing dietary structure, or control over the types and amount of foods and beverages consumed (Figure 1). This method of dietary organization proposes that as more or larger parts of the diet are targeted, greater control over the types and amounts of foods and beverages consumed are expected. Structure was operationalized as having four levels: alterations to food and beverage groups, such as FVs (low structure); alterations to the structure or size of daily eating occasions, such as breakfast or structured daily meals (moderate structure); alterations in nutrients, such as energy or macronutrients (high structure); and alterations to energy intake combined with any additional dietary alterations, such as macronutrient goals (very high structure). For example, targeting FVs alone (low structure) is focused on changing the type and amount of one food group. In comparison, targeting energy intake (high structure) can change the type and amount of most foods and beverages consumed, influencing more areas of the diet (e.g., servings in multiple food groups could be reduced, snacking occasions could diminish, and total energy could decrease).
FIGURE 1.
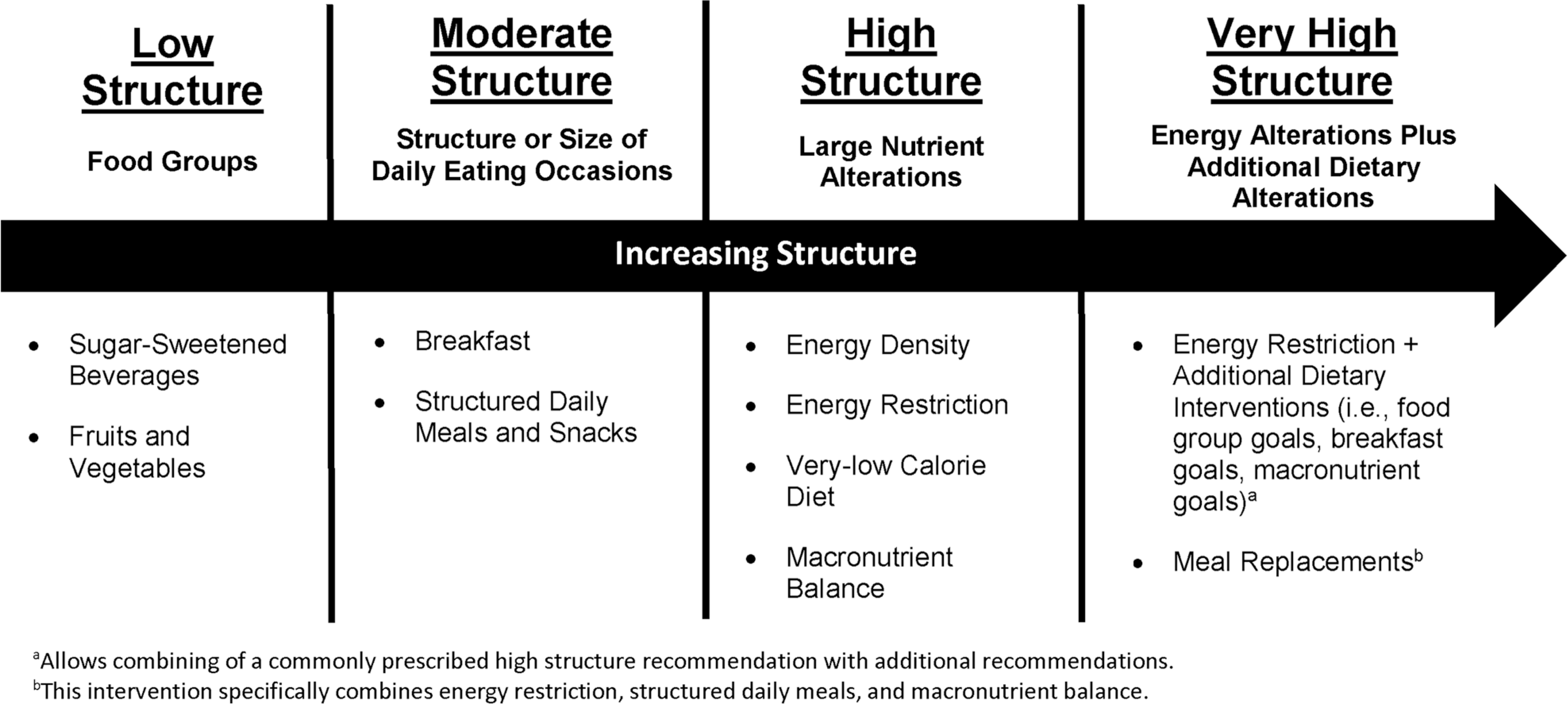
Dietary interventions recommended by the Expert Committee6 for the treatment of overweight and obesity for children and adolescents organized by degree of dietary structure
4 |. RESULTS
A total of 8196 unduplicated citations were identified, of which 1517–31 included at least one intervention meeting eligibility criteria. These 15 articles contained a total of 38 interventions, of which 13 did not meet eligibility criteria17,18,20,23,24,27–29,31 (nine were control conditions or standard care interventions17,18,20,23,24,29,31 and four were dietary interventions not meeting inclusion criteria21,27,28) and one did not implement a consistent dietary intervention across time (meal replacements followed by a conventional diet at 4 months).21 Thus, this study reviews a total of 24 interventions. Each included intervention was assigned to a structure category based on the component of the dietary intervention with the highest structure. For example, if a dietary intervention had goals to increase FVs (low structure recommendation) and eat structured daily meals (moderate structure recommendation), the intervention was assigned to the moderate structure category. The 24 included interventions were classified by structure as follows: four low structure,17,18 three moderate structure,19,20 five high structure,21–24 and 12 very high structure dietary interventions.21,25–31 Interventions were primarily conducted in the United States,17,18,20–30 with three interventions conducted in Australia,23,31 and two interventions conducted in Norway.19 Interventions were conducted in a variety of settings, with 11 in unreported settings,17,21–23,25–27 five in outpatient settings,28,30,31 three in research settings,18 two in a school setting,19,20 one in a camp setting,19 one in a clinic and home setting,24 and one in a primary care setting.29 See Table 1 for a description of reviewed dietary interventions.
TABLE 1.
Multicomponent interventions for the treatment of overweight or obesity in children and adolescents using a dietary intervention recommended by the Expert Committee6 categorized by the degree of structure implemented in the diet
| Author/year (country/setting) | Study design: interventions | Sample descriptiona (n, age range, demographics) | Intervention characteristics (length, frequency of contactb, contact hours, length of follow-upc) | Dietary interventions | Leisure-time activity interventions | Behavioral strategies | Dietary intake assessment | ΔZBMId |
|---|---|---|---|---|---|---|---|---|
| Low structure | ||||||||
| Looney 201417 (USA/NR) | RCT: N + GM + BC, N + GM,e Ne |
N ± GM ± BC: n = 7 4–10 years 86% female 71% White 14% Hispanic |
N ± GM ± BC: 6-month intervention Monthly contact (3 in-person mtgs, 3 phone calls) 2.5 contact hours Follow up at 6 months |
N ± GM ± BC: <3 svgs/week of SSBs ≥1½ cups/day of whole vegetables ≥1 cup/day of whole fruit |
N ± GM ± BC: ≥60 min/day of MVPA <2 h/day of television |
N ± GM ± BC: Self-monitoring, modeling, stimulus control, positive reinforcement |
3-day food records (all dietary goals assessed) |
N ± GM ± BC: −0.16 (p value NR) |
| Raynor 201218 Trial 1 (USA/research) | RCT: Decrease, Increase, Growth Monitoringe |
Decrease and Increase: n = 68 4–9 years 62% female 84% White 18% Hispanic |
Decrease and Increase: 6-month intervention Two mtgs/month for 2 months, monthly mtgs for months 3–6 6 contact hours Follow-up at 6 and 12 months |
Decrease: ≤3 svgs/week of sweet and salty snack foods ≤3 svgs/week of SSBs Increase: 2 svgs/day of whole fruit 3 svgs/day of vegetables 2 svgs/day of LF dairy |
Decrease and Increase: None |
Decrease and Increase: Self-monitoring, pre-planning, problem-solving, shaping, goal setting, positive reinforcement, stimulus control, parental modeling |
3-day food records (all dietary goals assessed) |
Decreased:f 6 months: −0.08 (p value NR) 12 months: −0.10 (p value NR) Increased:f 6 months: −0.10 (p value NR) 12 months: −0.13 (p value NR) |
| Raynor 201218 Trial 2 (USA/research) | RCT: Traditional, Substitutes,e Growth Monitoringe |
Traditional: n = 26 4–9 years 65% female 85% White 12% Hispanic |
Traditional: 6-month intervention Two mtgs/month for 2 months, then monthly mtgs for months 3–6 6 contact hours Follow-up at 6 and 12 months |
Traditional: ≤3 svgs/week of SSBs |
Traditional: 60 min/day of MVPA on most day/week |
Traditional: Self-monitoring, pre-planning, problem-solving, shaping, goal setting, positive reinforcement, stimulus control, parental modeling |
3-day food records (all dietary goals assessed) |
Traditional:f 6 months: −0.11 (p value NR) 12 months: −0.17 (p value NR) |
| Moderate structure | ||||||||
| Benestad 201719 (Norway/camp, school) | RCT: Summer Camp, Lifestyle School |
Summer Camp and Lifestyle School: n = 90 7–12 years 50% female 86% White Ethnicity NR |
Summer Camp: 24-month intervention Monthly contact with local coordinator; group mtgs at months 0, 12, 24; 2-week inpatient Camp at months 2; 4, 2-day inpatient weekends at months 6, 12, 18, 24 Contact hours could not be calculated Follow-up at 24 months Lifestyle School: 24-month intervention Monthly contact with local coordinator; group mtgs at months 0, 12, 24; 4 days of outpatient school delivered in 2, 2-day mtgs at month 5 Contact hours could not be calculated Follow-up at 24 months |
Summer Camp and Lifestyle School: ≥5 portions/day of FV Reduce/avoid sugary drinks (goal not specified) Eat breakfast (goal not specified) Eat whole grain products, fish, lean meats (goal not specified) |
Summer Camp and Lifestyle School: ≤2 h/day of screen time ≥60 min/day of PA Reduce sedentary time (goal not specified) |
Summer Camp and Lifestyle School: Motivational interviewing, parenting skills, goal setting |
Not assessed |
Summer Camp: −0.44 (95% Cl = −0.85 to 0.04) (p value NR) Lifestyle School: −0.33 (95% Cl = −0.64 to 0.01) (p value NR) |
| Pbert 201320 (USA/school) | Cluster RCT: Intervention, Controle |
Intervention: n =42 Grades 9–11 64% female 74% White 14% Hispanic |
Intervention: 2-month intervention 6 sessions over 2 months ~2.5 contact hours Follow-up at 2 and 6 months |
Intervention: ≥5 svgs/day of FV 3 structured meals/day, including breakfast 0 svgs/day SSBs Limit portions (goal not specified) |
Intervention: ≥60 min of PA on ≥5 days/week ≤2 h/day of screen time |
Intervention: Self-monitoring, goal setting, problem solving, pre-planning |
24-h dietary recall 8-item instrument to assess healthful/unhealthful dietary behaviors targeted by Intervention (all dietary components with goals assessed) |
Intervention: 2 months: 0.00 (95% Cl: −0.05 to 0.05) (p value NR) 6 months: 0.00 (95% Cl: −0.07 to 0.07) (p value NR) |
| High structure | ||||||||
| Berkowitz 201121 (USA/NR) |
RCT: CDg MR/MRh MR/CDi |
CD: n =42 13–17 years 79% female 26% White Ethnicity NR |
CD:12-month intervention Weekly mtgs for months 1–4, 2 mtgs/months for months 5–7, then monthly mtgs for months 8–12 Contact hours could not be calculated Follow-up at 12 months |
CD: 1300–1500 kcal/day |
CD: ≥30 min/day of PA Reduce sedentary behavior (goal not specified) |
CD: Self-monitoring, stress management, stimulus control, problem solving, contingency management, cognitive restructuring, social support |
Not assessed |
CD: −0.09 (p value NR) |
| Jelalian 201022 (USA/NR) | RCT: CBT + EXER, CBT+ PEAT |
All Groups: n = 118 13–16 years 68% female 76% White 9% Latino |
All Groups: 16-week intervention Weekly mtgs 16 contact hours Follow-up at 4 and 12 months |
All Groups: 1400–1600 kcal/day |
All Groups: 60 min/day of PA on most day/week CBT ± EXER: An additional 35 min/week supervised PA |
All Groups: Cognitive behavioral therapy, self-monitoring, goal setting, stimulus control, relapse prevention |
Not assessed |
CBT ± EXER: 4 months: −0.16 (p < 0.01) 12 months: −0.11 (p < 0.01) CBT ± PEAT: 4 months: −0.21 (p < 0.01) 12 months: −0.17 (p < 0.01) |
| Jensen 201323 (Australia/NR) | RCT: Intervention, Wait-list Controle |
Intervention: n=13j 8–17 years 23% female Race/ethnicity NR |
Intervention: 10-week intervention Weekly contact (in-person weeks 0, 1, 2, 4, 6, 8, 10 and phone calls weeks 3, 5, 7, 9) Contact hours could not be calculated Follow-up at 10 weeks |
Intervention: Energy deficit of 500 kcal/day |
Intervention: None |
Intervention: Goal setting, self-monitoring |
Not assessed |
Intervention: −0.20 (p < 0.05) |
| Stark 201124 (USA/clinic and home) | RCT: LAUNCH, Standard Caree |
LAUNCH: n = 8 2–5 years 25% female 75% White 25% Hispanic |
LAUNCH: 6-month intervention Weekly mtgs for 12 weeks, then every other week for 12 weeks ~22 contact hours Follow-up at 6 and 12 months |
LAUNCH: 1000–1200 kcal/day |
LAUNCH: ≤2 h/day of screen time 60 min/day of active play 5000 steps/day |
LAUNCH: Self-monitoring, child behavior management skills (praise and attention; ignoring and time-out to manage tantrums; contingency management; modeling) |
24-h dietary recall (all dietary goals assessed) |
LAUNCH: 6 months: −0.49 (p value NR) 12 months: −0.37 (p value NR) |
| Very high structure | ||||||||
| Berkowitz 201121(USA/NR) | RCT: CD,g MR/MR,h MR/CDi |
MR/MRand MR/CDk: n = 71 13–17 years 82% female 26% White Ethnicity NR |
MR/MR: 12-month intervention Weekly mtgs for months 1–4, 2 mtgs/month for months 5–7, then monthly mtgs for months 8–12 Contact hours could not be calculated Follow-up at 12 months |
MR/MR: For months 1–4: 1300–1500 kcal/day 3 MR/day 1 prepackaged meal/day 2 svgs/day of fruit 3 svgs/day of vegetables For months 5–12: 1300–1500 kcal/day 2 MR/day 1 prepackaged meal/day breakfast of conventional foods 5 svgs/day of FV |
MR/MR: ≥30 min/day of PA Reduce sedentary behavior (goal not specified) |
MR/MR: Self-monitoring, stress management, stimulus control, problem solving, contingency management, cognitive restructuring, and social support |
Questionnaire on MR and prepackaged meal use |
MR/MR: −0.10 (p value NR) |
| Epstein 200525 (USA/NR) | RCT: Behavioral Economic Treatment, Standard Treatment |
All Groups: n =41 8–12 years 56% female Race/ethnicity NR |
All Groups: 12-month intervention Eight mtgs in first 7 weeks, followed by 4 biweekly mtgs and 2 bimonthly mtgs in first 6 months; monthly mtgs in months 6–12 Contact hours could not be calculated Follow-up at 12 and 24 months |
All Groups: 1000–1500 kcal/day ≤ 14 svgs/week of RED foods |
All Groups: ≥30 min of MVPA accumulated in increments of ≥10 min bouts ≥6 days/week Decrease sedentary behavior (goal not specified) |
All Groups: Praise and reinforcement, self-monitoring, pre-planning |
4-day food record (dietary goals not assessed) |
Behavioral Economic Treatment:l 12 months: −0.63 (p value NR) 24 months: −0.84 (p value NR) Standard Treatment:l 12 months: −0.85 (p value NR) 24 months: −0.86 (p value NR) |
| Epstein 200826 (USA/NR) |
RCT: Increase Healthy Food, Reduce High-energy-dense Food |
All Groups: n =41 8–12 years 44% female Race/ethnicity NR |
All Groups: 6-month intervention Weekly mtgs for 2 months, biweekly mtgs for 2 months, and 1 monthly mtg 31.5 contact hours Follow-up at 6 and 24 months |
Increase Healthy Food: 1000–1500 kcal/day ≥5 svgs/day of FV ≥2 svgs/day of LF dairy Reduce High-energy-dense Food: 1000–1500 kcal/day ≤2 RED foods/day |
All Groups: ≥60 min of MVPA accumulated in increments of ≥15 min bouts ≥6 days/week |
All Groups: Self-monitoring, stimulus control, parental role modeling, positive reinforcement, preplanning, problem-solving |
Food intake questionnaire (FV, LF dairy, and RED food intake assessed) |
Increase Healthy Food: 6 months: −0.25 (p value NR) 24 months: −0.27 (p value NR) Reduce High-energy-dense Food: 6 months: −0.31 (p value NR) 24 months: −0.11 (p value NR) |
| Kirk 201227 (USA/NR) | RCT: Portion-controlled, Low-CHO,e Reduced Glycemic Loade |
Portion-controlled: n = 31 7–12 years 74% female 71% White Ethnicity NR |
Portion-controlled: 3-month intervention Weekly mtgs 12 contact hours Follow-up at 3 and 12 months |
Portion-controlled: 55–60% kcal CHO, 10–15% kcal protein, and 30% kcal fat with 500-kcal deficit |
Portion-controlled: ≥30 min/day of PA on most day/week |
Portion-controlled: Contracted goals, self-monitoring, positive reinforcement |
3-day food records (all dietary goals assessed) |
Portion-controlled:l 3 months: −0.21 (p ≤ 0.0001) 12 months: −0.32 (p ≤ 0.0001) |
| Krebs 201028 (USA/outpatient clinic) | RCT: LF, HPLCe |
LF: n = 22 12–18 years 55% female Race and ethnicity NR |
LF: 12-week intervention Followed at 2-week intervals Contact hours could not be calculated Follow-up at weeks 12 and 36 |
LF: Energy goal of 70% of resting EEE and ≤30% kcal fat |
LF: ≥30 min/day MVPA |
LF: Self-monitoring |
3-day food records (all dietary goals assessed) |
LF: 12 weeks: −0.14 (p ≤ 0.05)l 36 weeks: −0.15 (p = 0.002) |
| Saelens 200229 (USA/ primary care) |
RCT: HH, TCe |
HH: n = 23 12–16 years Sex, race, and ethnicity NR |
HH: 4-month intervention Weekly phone calls for 8 weeks, 3 biweekly calls Contact hours could not be calculated Follow-up at 4 and ~11 months |
HH: 1200 to 1500 kcal/day 40 svgs/week of GREEN foods ≤15 svgs/week of RED foods |
HH: ≥60 min/day of MVPA on 5 days/week |
HH: Self-monitoring, goal setting, problem solving, stimulus control, self-reward, preplanning |
2-day dietary recall (kcal assessed) |
HH: 4 months: −0.05 (p value NR) ~11 months: −0.06 (p value NR)l |
| Saelens 201130 (USA/ outpatient clinic) |
RCT: Added, Standard |
All Groups: n = 29 7–11 years 55% female 66% White 14% Hispanic |
All Groups: 14-week intervention Weekly mtgs ~16 contact hours Follow-up at 14 weeks |
All Groups: 1000–1200 kcal/day ≥ 5 days/week ≤15 svgs/week of RED foods |
Added: ≥90 min of MVPA on at ≥6 days/week Standard: ≥60 min of PA on most day/week |
All Groups: Monitoring, goal setting, contingency management, stimulus control |
3-day food records (kcal assessed) |
Added: −0.21 (p value NR) Standard: −0.32 (p value NR) |
| Truby 201631 (Australia/outpatient clinic) | RCT: Structured Modified CHO, Structured Modified LF, Controle |
Structured Modified CHO and Structured Modified LF: n = 73 10–17 years 73% female 92% White Ethnicity NR |
Structured Modified CHO and Structured Modified LF: 12-week intervention In-person mtgs for weeks 0, 2, 4, 8, 12 and phone calls for weeks 6 and 10 Contact hours could not be calculated Follow-up at 12 weeks |
Structured Modified CHO: 20% reduction in energy from EEE, 35% kcal CHO, 30% kcal protein, and 35% kcal fat Structured Modified LF: 20% reduction in energy from EEE, 55% kcal CHO, 20% kcal protein, and 25% kcal fat |
Structured Modified CHO and Structured Modified LF: Decrease sedentary behavior (goal not specified) |
Structured Modified CHO and Structured Modified LF: Goal setting, problem solving, self-monitoring |
Percent kcal from macronutrients reported but dietary assessment method not described |
Structured Modified CHO: −0.13 (p ≤ 0.001) Structured Modified LF: −0.12 (p ≤ 0.001) |
Abbreviations: CBT + EXER, cognitive-behavioral treatment with exercise; CBT + PEAT, cognitive-behavioral treatment with peer-enhanced adventure therapy; CD, conventional diet; CHO, carbohydrate; CI, confidence interval; EEE, estimated energy expenditure; FC, Family Connections; FC-IVR, Family Connections and interactive voice response; FV, fruits and vegetables; HH, Healthy Habits; HPLC, high-protein, low-carbohydrate; kcal, kilocalories; LAUNCH, Learning about Activity and Understanding Nutrition for Child Health; LF, low fat; MR, meal replacement; mtg(s), meeting(s); MVPA, moderate-to vigorous-intensity physical activity; n, number; N, newsletter; N + GM, newsletter and growth monitoring; N + GM + BC, newsletter and growth monitoring plus family-based behavioral counseling condition; NR, not reported; PA, physical activity; RCT, randomized controlled trial; SSBs, sugar sweetened beverages; svgs, servings; TC, typical care; USA, United States of America; ZBMI, standardized body mass index.
Sample description is for participants receiving an intervention that met inclusion/exclusion criteria.
Meetings occurred in-person unless otherwise specified.
Follow-up at end of intervention. If additional follow-up is reported, it is the longest reported follow-up assessment.
Δ denotes within-group change from baseline to follow-up.
Condition that did not meet inclusion/exclusion criteria.
Data obtained from author, as it was not available in the publication.
Conventional Diet: This group received the conventional diet throughout the 12 months of intervention.
Meal Replacement/Meal Replacement: This group received meal replacement throughout the 12 months of the intervention.
Meal Replacement/Conventional Diet: This group received meal replacements for the first 4 months of the intervention, and then the conventional diet for months 5 to 12 of the intervention. No results are presented as the dietary intervention was not consistent across the intervention.
Sixteen participants were randomized to this condition, but demographics were provided on 13 participants.
Demographics for the sample are reported on all participants randomized to a meal replacement condition, rather than reporting demographics for the two meal replacement conditions separately.
ZBMI change estimated from Figures through consensus of two authors.
4.1 |. Low structure interventions
Three investigations (published in two articles), representing four interventions, were identified that fit the criteria for the low structure category.17,18 All of the investigations were randomized controlled trials (RCTs) that predominantly enrolled non-Hispanic, White children in early and middle childhood (age range 4–10 years). Two of the four interventions included goals to increase FV intake,17,18 and three of the four interventions had goals to decrease SSB consumption.17,18 Other dietary goals that were targeted included low-fat dairy18 and sweet and salty snack foods.18 Specific goals were reported for all dietary interventions. All interventions, except for the intervention in Trial 2 by Raynor et al.,18 targeted at least two broadly defined foods groups. Two of the interventions17,18 also included goals to engage in the recommended32 60 min/day of moderate- to vigorous-intensity PA for children, and one intervention17 additionally included a goal to reduce television watching. All interventions included the behavioral strategies of self-monitoring, modeling, stimulus control, and positive reinforcement, with the total number of implemented behavioral strategies ranging from four17 to eight.18 Initial frequency of contact ranged from twice per month18 to once a month.17 None of the interventions began with weekly contact. Contact time ranged from 2.517 to 6 h.18
Changes in ZBMI from preintervention to postintervention were relatively small (Table 2), ranging from −0.0818 to −0.16.18 As none of the low structure interventions met contact time recommendations, all are considered low-intensity contact interventions (2.517 to 6 h18). All four interventions were 6 months in length.17,18
TABLE 2.
Minimum and maximum change in ZBMI from preintervention to postintervention by structure category and length of intervention
| ZBMI change at end of treatment |
||
|---|---|---|
| Intervention structure category | Interventions <6 months in length | Interventions ≥6 months in length |
| Lowa (n = 4) | N/A | −0.0818 to −0.1617 |
| Moderateb (n = 3) | 0.020 | −0.3319 to −0.4419 |
| High (n = 5) | −0.1622 to −0.2122 | −0.0921 to −0.4924 |
| Very high (n = 12) | −0.0529 to −0.3230 | −0.1021 to −0.8525 |
No low structure interventions were <6 months in length.
Only one moderate structure intervention was <6 months in length.
4.2 |. Moderate structure interventions
Two investigations, representing three interventions, were identified that fit the criteria for the moderate structure category.19,20 Study designs included one RCT19 and one cluster RCT20 that predominantly enrolled White (ethnicity unclear) children in middle childhood through adolescence (age range 7–17 years). The moderate structure dietary interventions included breakfast consumption19,20 and structured daily meals.20 Other dietary goals that were targeted included increasing FV intake19,20; decreasing SSB consumption19,20; eating whole grain products, fish, and lean meats19; and limiting portions.20 One intervention20 reported specific dietary goals for all dietary targets except limiting portions. The remaining two interventions19 reported specific goals for FV intake only (goals not specified for reducing SSBs; eating breakfast; or eating whole grain products, fish, and lean meats). All interventions also included goals to engage in at least 60 min/day of PA. Other leisure-time activity interventions included limiting screen time19,20 and/or reducing sedentary time.19 All interventions included the behavioral strategy of goal setting, with total the total number of implemented behavioral strategies ranging from three19 to four.20 Initial frequency of contact ranged from just less than weekly20 (six sessions over 2 months) to monthly.19 Contact hours could not be determined for two intensive interventions,19 whereas the third intervention provided approximately 2.5 contact hours.20 Two interventions were at least 6 months in length.19
Changes in ZBMI from preintervention to postintervention ranged from 0.0020 to −0.4419 (Table 2). The study reporting no change in ZBMI provided a short length of treatment (2 months) and only 2.5 contact hours.20 The intervention with the greatest ZBMI change (−0.44) provided 24 months of treatment (exact contact hours could not be calculated), which included time spent in an in-patient camp.19
4.3 |. High structure interventions
Four investigations, representing five interventions, were identified that fit the criteria for the high structure category.21–24 All high structure dietary interventions were RCTs that predominantly enrolled non-Hispanic, White children (only two investigations22,24 reported ethnicity) in early childhood through adolescence (age range 2–17 years). All interventions targeted energy restriction and reported specific dietary goals (daily intake goals ranging from 1000–1600 kcal/day21,22,24 or a 500-kcal reduction/day23), with no other dietary interventions prescribed. Three interventions also included goals to engage in 3021 to 60 min/day of PA.22,24 Other leisure-time activity interventions included weekly supervised PA,22 step goals,24 and limiting screen time.24 All interventions included the behavioral strategy of self-monitoring, with the total number of implemented behavioral strategies ranging from two23,24 to seven.21 Frequency of initial contact for all high structure interventions was weekly. Contact time could only be determined for three interventions,22,24 with none providing at least 26 h of contact time. Two of the interventions were at least 6 months in length.21,24
Changes in ZBMI from preintervention to postintervention ranged from −0.0921 to −0.4924 (Table 2). The intervention21 reporting the smallest decrease in ZBMI was the longest in length, but exact contact hours could not be calculated. The intervention24 reporting the greatest change in ZBMI reported the most contact time (approximately 22 h) and was 6 months in length.
4.4 |. Very high structure interventions
Eight investigations, representing 12 interventions, were identified that fit the criteria for the very high structure category.21,25–31 All very high structure dietary interventions were RCTs that predominantly enrolled White (ethnicity unclear) children in middle childhood through adolescence (age range 7–18 years). All interventions included goals for energy restriction, including calorie goals of 1000 to 1500 kcal/day,21,25,26,29,30 500 kcal/day reduction,27 or 20%–30% reduction in energy intake from estimated energy expenditure.28,31 One intervention21 provided meal replacements, along with goals for FVs, and also breakfast consumption as meal replacements was decreased over time. Six interventions used the Traffic Light Diet,12 which included goals for decreasing non-nutrient-dense, high-energy-dense foods (RED foods)25,26,30 or goals for decreasing RED foods and increasing nutrient-dense, low-energy-dense foods (GREEN foods).29 Four interventions included macronutrient distribution goals,27,28,31 and one intervention had goals for FVs and low-fat dairy.26 Specific goals were reported for all dietary interventions. Ten interventions21,25–30 also included goals to engage in 3021,25,27,28 to 9030 min/day of PA, whereas five interventions21,25,31 included unspecified goals to decrease sedentary behavior. All interventions included the behavioral strategy of self-monitoring, with the total number of implemented behavioral strategies ranging from one28 to seven.21 Nine21,25–27,29,30 of the 12 interventions had an initial frequency of contact of at least once per week. Most of the interventions did not report the number of contact hours for the intervention,21,25,28,29,31 but two interventions had ≥26 h of contact time.26 Five of the interventions21,25,26 were at least 6 months in length.
Changes in ZBMI from preintervention to postintervention ranged from −0.0529 to −0.8525 (Table 2). The intervention29 with the smallest change in ZBMI (−0.05) was less than 4 months in length (exact contact time not reported). The greatest decrease in ZBMI (−0.85) was observed after the completion of a 12-month intervention (exact contact time not reported).25
5 |. DISCUSSION
A multicomponent intervention, which includes behavioral strategies to alter dietary intake and time in PA and/or sedentary behavior, is recommended for the treatment of overweight and obesity in children and adolescents.7,9 Although there is some grading of the evidence for pediatric dietary interventions,6 there are no graded recommendations for the diet to be used within this multicomponent approach. Suggested dietary interventions for pediatric overweight and obesity treatment are broad,6 and it is not clear what impact these dietary interventions have on weight status in children and adolescents. As dietary interventions that provide greater structure have produced better weight loss outcomes in adults,11 the purpose of this critical review was to classify dietary interventions recommended for the treatment of overweight and obesity in children and adolescents by the degree of structure that the intervention imposes on the overall diet and examine the impact on changes in ZBMI. In general, results suggest that dietary interventions that impose more structure demonstrate the ability to produce larger improvements in weight status. Similar to findings in adults,11 the greatest reduction in ZBMI at the end of treatment for each structure category was as follows: low structure, −0.1617; moderate structure, −0.4419; high structure, −0.4924; and very high structure, −0.85.25
While the purpose of this critical review was to assist with the development of an innovative conceptual framework by which existing pediatric dietary interventions could be interpreted, because the proposed framework is novel and, to date, has not been tested in an intentional or systematic way, it is important to note that the ability to draw strong conclusions from this critical review is limited. In particular, the reviewed interventions have substantial heterogeneity in participant age, intervention contact time, and length of treatment among the included interventions. The low structure interventions targeted younger children (4–10 years),17,18 whereas the moderate to very high structure interventions targeted older children and adolescents (7–18 years),19–23,25–31 with the exception of one high structure intervention in preschoolers.24 Intervention contact time ranged from 2.517,20 to 31.5 h,26 with contact time not reported in 1119,21,23,25,28,29,31 of the 24 interventions. For interventions in which contact time was provided, only low17,18 and moderate20 structure interventions were <12 h, usually with more time between contacts (e.g., monthly versus weekly contact). Additionally, there were substantial differences in intervention length, which ranged from 220 to 2419 months (all interventions reported length of intervention). While all low structure interventions17,18 were at least 6 months in length, more than half of the high22,23 and very high27–31 structure interventions were less than 6 months in length. In addition to the potential influence of dietary structure on weight outcomes, the heterogeneity in treatment length suggests that, in general, outcomes were improved with increased intervention length. Interventions of at least 6 months17–19,21,24–26 reported larger reductions in ZBMI compared with interventions of less than 6 months20,22,23,27–31 in each structure category (except low, for which there were no interventions less than 6 months). Although contact time could not be calculated for all interventions, longer intervention length may generally reflect increased contact hours and allow greater length of time for ZBMI change. Thus, the improved outcomes seen with increased intervention length align with the findings that have been used to support recommendations to provide at least 26 h of contact time in pediatric overweight and obesity interventions.7,9
Although very high structure interventions (i.e., energy alterations plus additional dietary alterations) appear to have the potential to achieve the greatest reductions in ZBMI, a very high structure dietary intervention may not be ideal for all situations in which a multicomponent intervention is implemented. Dietary interventions with higher structure are considered more complex and thus may be most appropriately delivered by dietitians or other nutrition specialists. Higher structure dietary interventions may also require greater initial contact time to assist children and families with understanding the more complex intervention (the high21–24 and very high21,25–27,29,30 structure dietary interventions were commonly delivered with weekly contact, whereas low17,18 and moderate19,20 structure dietary interventions were not). These two factors, specialized staff and greater initial frequency of contact, may limit the settings in which this type of dietary intervention can be implemented, as only specialty clinics and research settings may support these factors. These types of settings limit accessibility of the intervention to all families. Finally, higher structure dietary interventions may also be better suited to older children and adolescents who can understand more sophisticated dietary concepts. For example, one of the most highly studied pediatric dietary interventions, the Traffic Light Diet,12 requires participants to understand caloric intake, as well as energy density and the classification of foods into traffic light colors. Being able to self-monitor dietary intake for this type of dietary intervention and identify whether intervention goals are met requires a fair degree of health and math literacy that younger children do not possess.
While dietary interventions of lower structure may not produce as significant reductions in ZBMI as those of higher structure, these interventions may be more suited for younger children and may be delivered by nonspecialist providers. Dietary interventions of lower structure include less complex dietary goals. This may allow more flexibility in who can provide the intervention (e.g., community workers or nurses versus dietitians) and may not require as much initial contact time for children and families to understand. Thus, this type of intervention could be provided in community or primary care settings, which may increase the accessibility of the intervention. The dietary intervention messaging may also be simpler due to reduced complexity of dietary goals, which may make the intervention more suited to younger children. Still, the low and moderate structure interventions reported nonexistent to modest ZBMI reductions (−0.020 to −0.1617), with the exception of two interventions19 that appeared to provide high intensity of contact time (exact hours could not be calculated) and long intervention length (24 months) (ZBMI reductions of −0.33 and −0.44). As it is currently hypothesized that greater than −0.16 reduction in ZBMI is needed to improve cardiometabolic outcomes in children,33 additional investigation into the efficacy of low structure interventions, with consideration for the roles of frequency of contact, total contact hours, and intervention length, is warranted.
As previously mentioned, there are graded recommendations for a multicomponent approach to pediatric weight loss,7 but there are no graded recommendations for dietary interventions to be used within this multicomponent approach.9 Research that specifically examines the degree of structure implemented by pediatric dietary interventions could help to address this gap. Trials that directly compare different degrees of dietary intervention structure (while controlling participant age, intervention length, contact hours, PA goals, and behavioral strategies) are needed to confirm whether higher structure dietary interventions really do produce greater improvements in weight-related outcomes compared with lower structure dietary interventions. Additionally, future research should examine the use of a stepped-care approach, similar to the recommendations of the Prevention Plus model,6 that increases dietary intervention structure if lower degrees of structure do not produce desired improvements in weight status. The use of contemporary study designs that consider not only weight-related outcomes but also optimization of treatment delivery, such as sequential, multiple assignment, randomized trials (also known as SMARTs),34 may be advantageous for testing such a stepped-structure approach. Finally, pragmatic trials that test the alignment or misalignment of different degrees of dietary intervention structure with differences in participant characteristics (i.e., age) intervention length, contact hours, settings, and providers are needed to determine which dietary interventions will be most successful in which circumstances.
Strengths of this review include its novel focus on dietary structure in the treatment of overweight and obesity treatment in children and adolescents, the methodical approach to reviewing the literature, and the examination of dietary interventions specifically recommended by the Expert Committee.6 Additionally, the authors self-assessed the quality of this review using the Scale for the Assessment of Narrative Review Articles, or SANRA, and determined it to be high quality (12 out of 12 possible points).35 Limitations include study variability in participant ages, intervention length, contact hours, intensity of the PA goals or sedentary behavior component, and the robustness of the included behavioral component. Also, outcomes were only examined at the end of the intervention. Long-term follow-up was not examined (the ability to do this was limited in the interventions that met inclusion criteria); thus, conclusions cannot be drawn about which interventions are better long term (i.e., do the high/very high structure interventions have greater weight regain over time). Additionally, examining only interventions implementing Expert Committee dietary recommendations may have excluded other potentially effective pediatric dietary interventions (e.g., intermittent energy restriction36). Further, to allow for comparison between interventions, only those investigations reporting ZBMI were included. Although this allowed for comparisons between the interventions as they all reported the same commonly utilized outcome, it is important to note that extrapolation of ZBMI beyond the 97th percentile can lead to compressed z scores,37 which may have occurred within the reviewed studies. Furthermore, several investigations did not report any dietary adherence,19,21–23,25 whereas others reported adherence to only some of prescribed dietary interventions.21,26,29–31 Only eight17,18,20,24,27,28 of the 24 investigations reviewed reported on dietary adherence on all aspects of the dietary interventions. More consistent reporting of adherence to the prescribed interventions is needed to improve the body of evidence published on pediatric dietary interventions to be able to draw strong conclusions regarding the influence of any diet intervention on weight outcomes.
Finally, it is important to note that all studies in which race and ethnicity were reported were conducted in predominantly non-Hispanic, White populations, despite children from racial and ethnic minorities experiencing disproportionately high rates of overweight and obesity.38 As a greater percentage of children from racial and ethnic minorities are of lower socioeconomic status and are underserved, they may experience a greater number of barriers, such as competing demands, lack of health care access, and food insecurity, which may hinder ability to engage in child or adolescent interventions for overweight or obesity intervention.39,40 This is particularly true for interventions that may be designed to provide a large amount of contact, which may compete with parental work responsibilities and present a need for reliable transportation, require specialty settings that can only be covered by health insurance or be paid out-of-pocket, and/or require large changes in the diet, which may increase the cost of the diet. Due to the lack of diversity within the samples of the investigations reviewed and these issues, more research is needed to determine if factors such as race, ethnicity, and socioeconomic status may be moderators in the relationship between dietary structure and improved weight status in children during overweight or obesity treatment.
6 |. CONCLUSION
This critical review methodically appraised the pediatric overweight and obesity intervention literature to examine the relationship between the amount of structure imposed by dietary interventions and subsequent weight outcomes to develop a new conceptual framework. Findings indicate that as dietary interventions for the treatment of overweight and obesity during childhood and adolescence increased in structure, larger improvements in weight status may be achievable. To better establish the relationship between dietary intervention structure and weight outcomes in overweight and obesity interventions in children and adolescence, trials that empirically test different degrees of dietary intervention structure, while maintaining consistency in other aspects of the intervention, are needed.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number R01DK121360. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- FVs
fruits and vegetables
- kcal
kilocalories
- PA
physical activity
- RCTs
randomized controlled trials
- SSBs
sugar-sweetened beverages
- ZBMI
standardized body mass index
Footnotes
CONFLICT OF INTEREST
Hollie A. Raynor has been a consultant for Dietitians of Canada. Lauren Griffiths and Steve M. Douglas have no conflicts of interest to disclose.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity Among Adults and Youth: United States, 2015–2016. NCHS data brief, no. 288. Hyattsville, MD: National Center for Health Statistics; 2017. https://stacks.cdc.gov/view/cdc/49223 [Google Scholar]
- 2.Goldhaber-Fiebert JD, Rubinfeld RE, Bhattacharya J, Robinson TN, Wise PH. The utility of childhood and adolescent obesity assessment in relation to adult health. Med Decis Making. 2013;33(2):163–175. 10.1177/0272989X12447240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh AS, Mulder C, Twisk JW, van Mechelen W, Chinapaw MJ. Tracking of childhood overweight into adulthood: a systematic review of the literature. Obes Rev 2008;9(5):474–488. 10.1111/j.1467-789X.2008.00475.x [DOI] [PubMed] [Google Scholar]
- 4.Imperatore G, Boyle JP, Thompson TJ, et al. Projections of type 1 and type 2 diabetes burden in the U.S. population aged <20 years through 2050: dynamic modeling of incidence, mortality, and population growth. Diabetes Care. 2012;35(12):2515–2520. 10.2337/dc12-0669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Geel M, Vedder P, Tanilon J. Are overweight and obese youths more often bullied by their peers? A meta-analysis on the correlation between weight status and bullying. Int J Obes (Lond). 2014;38(10): 1263–1267. 10.1038/ijo.2014.117 [DOI] [PubMed] [Google Scholar]
- 6.Barlow SE, The Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(Supplement 4):S164–S192. [DOI] [PubMed] [Google Scholar]
- 7.American Psychological Association, Clinical Practice Guideline Panel. Clinical practice guideline for multicomponent behavioral treatment of obesity and overweight in children and adolescents: current state of the evidence and research needs. 2018. http://www.apa.org/obesity-guideline/obesity.pdf
- 8.Alman KL, Lister NB, Garnett SP, Gow ML, Aldwell K, Jebeile H. Dietetic management of obesity and severe obesity in children and adolescents: a scoping review of guidelines. Obes Rev 2021;22(1): e13132. 10.1111/obr.13132 [DOI] [PubMed] [Google Scholar]
- 9.Grossman DC, Bibbins-Domingo K, Curry SJ, et al. Screening for obesity in children and adolescents: US Preventive Services Task Force recommendation statement. JAMA. 2017;317(23): 2417–2426. [DOI] [PubMed] [Google Scholar]
- 10.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol 2014;63(25 Pt B):2985–3023. 10.1016/j.jacc.2013.11.004 [DOI] [PubMed] [Google Scholar]
- 11.Raynor HA, Champagne CM. Position of the Academy of Nutrition and Dietetics: interventions for the treatment of overweight and obesity in adults. J Acad Nutr Diet 2016;116(1):129–147. 10.1016/j.jand.2015.10.031 [DOI] [PubMed] [Google Scholar]
- 12.Epstein LH, Squires S. The Stoplight Diet for Children. Little, Brown and Co; 1988. [Google Scholar]
- 13.Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Info Libr J 2009;26(2):91–108. 10.1111/j.1471-1842.2009.00848.x [DOI] [PubMed] [Google Scholar]
- 14.Ogden CL, Carroll MD, Division of Health and Nutrition Surveys. Prevalence of obesity among children and adolescents: United States, trends 1963–1965 through 2007–2008. National Center for Health Statistics. June 2010. https://www.cdc.gov/nchs/data/hestat/obesity_child_07_08/obesity_child_07_08.htm#:~:text=Table%201%20shows%20the%20increase,18.1%25%20during%20the%20same%20period [Google Scholar]
- 15.Cole TJ, Lobstein T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr Obes 2012;7 (4):284–294. 10.1111/j.2047-6310.2012.00064.x [DOI] [PubMed] [Google Scholar]
- 16.United Nations. World Economic Situation and Prospects. 2020:165. https://www.un.org/development/desa/dpad/wp-content/uploads/sites/45/WESP2020_FullReport.pdf
- 17.Looney SM, Raynor HA. Examining the effect of three low-intensity pediatric obesity interventions: a pilot randomized controlled trial. Clin Pediatr (Phila). 2014;53(14):1367–1374. 10.1177/0009922814541803 [DOI] [PubMed] [Google Scholar]
- 18.Raynor HA, Osterholt KM, Hart CN, Jelalian E, Vivier P, Wing RR. Efficacy of US paediatric obesity primary care guidelines: two randomized trials. Pediatr Obes 2012;7(1):28–38. 10.1111/j.2047-6310.2011.00005.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benestad B, Lekhal S, Smastuen MC, et al. Camp-based family treatment of childhood obesity: randomised controlled trial. Arch Dis Child. 2017;102(4):303–310. 10.1136/archdischild-2015-309813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pbert L, Druker S, Gapinski MA, et al. A school nurse-delivered intervention for overweight and obese adolescents. J Sch Health. 2013; 83(3):182–193. 10.1111/josh.12014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berkowitz RI, Wadden TA, Gehrman CA, et al. Meal replacements in the treatment of adolescent obesity: a randomized controlled trial. Obesity (Silver Spring). 2011;19(6):1193–1199. 10.1038/oby.2010.288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jelalian E, Lloyd-Richardson EE, Mehlenbeck RS, et al. Behavioral weight control treatment with supervised exercise or peer-enhanced adventure for overweight adolescents. J Pediatr 2010;157(6): 923–928.e1. 10.1016/j.jpeds.2010.05.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen ME, Gibson PG, Collins CE, Hilton JM, Wood LG. Diet-induced weight loss in obese children with asthma: a randomized controlled trial. Clin Exp Allergy. 2013;43(7):775–784. 10.1111/cea.12115 [DOI] [PubMed] [Google Scholar]
- 24.Stark LJ, Spear S, Boles R, et al. A pilot randomized controlled trial of a clinic and home-based behavioral intervention to decrease obesity in preschoolers. Obesity (Silver Spring). 2011;19(1):134–141. 10.1038/oby.2010.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Epstein LH, Roemmich JN, Stein RI, Paluch RA, Kilanowski CK. The challenge of identifying behavioral alternatives to food: clinic and field studies. Ann Behav Med 2005;30(3):201–209. 10.1207/s15324796abm3003_4 [DOI] [PubMed] [Google Scholar]
- 26.Epstein LH, Paluch RA, Beecher MD, Roemmich JN. Increasing healthy eating vs. reducing high energy-dense foods to treat pediatric obesity. Obesity (Silver Spring). 2008;16(2):318–326. 10.1038/oby.2007.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirk S, Brehm B, Saelens BE, et al. Role of carbohydrate modification in weight management among obese children: a randomized clinical trial. J Pediatr 2012;161(2):320–327e1. 10.1016/j.jpeds.2012.01.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krebs NF, Gao D, Gralla J, Collins JS, Johnson SL. Efficacy and safety of a high protein, low carbohydrate diet for weight loss in severely obese adolescents. J Pediatr 2010;157(2):252–258. 10.1016/j.jpeds.2010.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saelens BE, Sallis JF, Wilfley DE, Patrick K, Cella JA, Buchta R. Behavioral weight control for overweight adolescents initiated in primary care. Obes Res 2002;10(1):22–32. 10.1038/oby.2002.4 [DOI] [PubMed] [Google Scholar]
- 30.Saelens BE, Grow HM, Stark LJ, Seeley RJ, Roehrig H. Efficacy of increasing physical activity to reduce children’s visceral fat: a pilot randomized controlled trial. Int J Pediatr Obes 2011;6(2):102–112. 10.3109/17477166.2010.482157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Truby H, Baxter K, Ware RS, et al. A randomized controlled trial of two different macronutrient profiles on weight, body composition and metabolic parameters in obese adolescents seeking weight loss. PLoS ONE. 2016;11(3):e0151787. 10.1371/journal.pone.0151787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. JAMA. 2018;320(19):2020–2028. 10.1001/jama.2018.14854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kolsgaard ML, Joner G, Brunborg C, Anderssen SA, Tonstad S, Andersen LF. Reduction in BMI z-score and improvement in cardiometabolic risk factors in obese children and adolescents. The Oslo Adiposity Intervention Study—a hospital/public health nurse combined treatment. BMC Pediatr 2011;11:47. 10.1186/1471-2431-11-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Almirall D, Nahum-Shani I, Sherwood NE, Murphy SA. Introduction to SMART designs for the development of adaptive interventions: with application to weight loss research. Transl Behav Med 2014;4(3): 260–274. 10.1007/s13142-014-0265-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baethge C, Goldbeck-Wood S, Mertens S. SANRA—a scale for the quality assessment of narrative review articles. Res Integr Peer Rev 2019;4:5. 10.1186/s41073-019-0064-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jebeile H, Gow ML, Lister NB, et al. Intermittent energy restriction is a feasible, effective, and acceptable intervention to treat adolescents with obesity. J Nutr 2019;149(7):1189–1197. 10.1093/jn/nxz049 [DOI] [PubMed] [Google Scholar]
- 37.Wei R, Ogden CL, Parsons VL, Freedman DS, Hales CM. A method for calculating BMI z-scores and percentiles above the 95th percentile of the CDC growth charts. Ann Hum Biol 2020;47(6):514–521. 10.1080/03014460.2020.1808065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS Data Brief, no 219. Hyattsville, MD: National Center for Health Statistics; 2015. https://www.ncbi.nlm.nih.gov/pubmed/26633046 [Google Scholar]
- 39.Byrd AS, Toth AT, Stanford FC. Racial disparities in obesity treatment. Curr Obes Rep 2018;7(2):130–138. 10.1007/s13679-018-0301-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.George S, Duran N, Norris K. A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. Am J Public Health. 2014;104(2):e16–e31. 10.2105/ajph.2013.301706 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


