Abstract
Objective:
This study evaluates the 24 month follow up for the NICHD Neonatal Research Network (NRN) Inositol for Retinopathy Trial.
Study Design:
Bayley Scales of Infants Development-III and a standardized neurosensory examination were performed in infants enrolled in the main trial. Moderate/severe NDI was defined as BSID-III Cognitive or Motor composite score <85, moderate or severe cerebral palsy, blindness, or hearing loss that prevents communication despite amplification were assessed.
Results:
Primary outcome was determined for 605/638 (95%). The mean gestational age was 25.8 ±1.3 weeks and mean birthweight was 805 ± 192 grams. Treatment group did not affect the risk for the composite outcome of death or survival with moderate/severe NDI (60% vs 56%, p=0.40).
Conclusions:
Treatment group did not affect the risk of death or survival with moderate/severe NDI. Despite early termination, this study represents the largest RCT of extremely preterm infants treated with myo-inositol with neurodevelopmental outcome data.
ClinicalTrials.gov ID
INS-3: NCT01954082
Introduction
Randomized clinical trials (RCTs) are crucial to determine the effect of promising neonatal interventions on the improving but still high rate of death or impairment among extremely preterm infants. One such intervention is administration of myo-inositol, an important component of membrane phospholipids with an important role in signal transduction and surfactant synthesis.1 Myo-inositol was reported in small clinical trials during the 1990s to decrease the risk for death, respiratory distress syndrome (RDS) and bronchopulmonary dysplasia (BPD).2,3 In a Cochrane meta-analysis of the 4 previously published RCTs of myo-inositol treatment in preterm infants, inositol supplementation was associated with a decreased risk of death, retinopathy of prematurity (ROP), and intraventricular hemorrhage (IVH) Grade 3 and 4, with no difference in BPD.4 Only one of those studies evaluated neurodevelopmental outcome, and the assessment was limited in scope and not standardized.2 In that study, Hallman and colleagues clinically evaluated outcomes at one year and found no statistically significant difference in major neurologic findings between the groups (9% in inositol-treated children compared to 18% in the placebo group), though the study was underpowered for that outcome. Given that previous studies were small and included limited or no long-term follow-up data, a larger RCT was indicated to examine these findings in a trial adequately powered to evaluate long term neurologic outcome.
To address this gap in knowledge, the Neonatal Research Network (NRN) of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) performed an RCT to evaluate the safety and efficacy of myo-inositol to reduce the risk of type I ROP in infants born < 28 weeks gestation. Study enrollment was terminated early due to increased mortality risk in the myo-inositol exposed infants. 5 The succeeding Cochrane review that included these findings identified no overall benefit of inositol supplementation at hospital discharge but emphasized the importance of follow-up assessments to identify any effects on long‐term outcomes in early childhood.6 This analysis evaluates the risk of death or survival with moderate/severe NDI at 24 months corrected age in enrolled infants.
Methods
Study Design:
This study was a double-blind placebo controlled RCT conducted under an Investigational New Drug application (IND) from the Food and Drug Administration and registered on clinicaltrials.gov (#NCT01954082). The study protocol was approved by the NRN Data Safety Monitoring Committee (DSMB) and the Institutional Review Board for each clinical site. Informed consent was obtained from the parent/legal guardian at enrollment which included consent to participate in the pre-specified 24-month corrected age follow-up visit. Detailed study methods were previously published5. There were no changes in the protocol except for the early termination of enrollment in the trial and no further treatment of enrolled patients based on the recommendation of the DSMB. The analyses completed for the manuscript also are consistent with the approaches detailed in the protocol and statistical analysis plan with the exception of the added ad-hoc analysis of outcomes by study drug lot given the manufacturing issues identified. Standardized protocols for the neurodevelopmental follow-up assessment and annual certification of examiners were utilized throughout the study period.7 Sample size estimates were outlined in the primary outcome publication.5
Patient population:
Infants born between April 17, 2014 and September 4, 2015 who were less than 28 and 0/7 weeks gestation and survived for at least 12 hours at one of the participating NRN centers were eligible for inclusion in the primary study. Infants with major congenital anomalies, any eye anomaly or those with a moribund condition were excluded.
Study Participants:
Participants were stratified by center and gestational age (GA) (<26 0/7 vs 26 0/7 to 27 6/7 weeks) and randomized in a 1:1 ratio to receive either placebo or 40 mg/kg/dose every 12 hours of myo-inositol parenterally or enterally for up to 10 weeks. Enrollment was discontinued due to an unexpected higher mortality rate in the myo-inositol treated group.
Outcomes:
Mortality or moderate/severe NDI at 24 months corrected age was a pre-specified primary outcome for this study. Secondary outcomes at 24 months included death, any ROP, the composite of any ROP or death, ophthalmologic outcomes (including nystagmus, strabismus and need for surgery); as well as the individual components of NDI.
Study definitions:
Neonatal morbidities were classified based on definitions outlined in the NRN Generic Database Protocol.8 At the 24 months follow-up visit a standardized neurosensory examination was performed and the Bayley Scales of Infant and Toddler Development, 3rd Edition (BSID III) was administered by certified examiners who completed annual training to ensure inter-rater reliability.7,9 Predefined criteria were specified for each of the following neurosensory examination categories: normal, suspect, neurologically abnormal/non-Cerebral Palsy (CP), and CP. According to the Pearson Corporation that developed the Bayley Scales, the mean value of the scaled scores is 100 and the standard deviation is 15.9 Children who were untestable were assigned a cognitive score of 54, a composite language score of 46, and a composite motor score of 46. The Gross Motor Function Classification System (GMFCS) was used to distinguish severity of CP such that mild CP was defined as GMFCS Level 1, moderate CP as GMFCS Level 2–3, and severe CP as GMFCS Level 4–5.10 Neurodevelopmental impairment was defined as a BSID III Cognitive or Motor composite <85, or moderate or severe CP, or blindness (unilateral or bilateral), or hearing loss that does not permit the child to communicate despite amplification.
ROP status was diagnosed by a primary ophthalmologist at each center trained and certified on the 2006 International Classification of ROP.11 Eye exams were performed up to 55 weeks postmenstrual age (PMA) until either eye met criteria for type 1 ROP, both eyes were fully vascularized, or two consecutive exams showed vessels to be in zone III.12 ROP, for this neurodevelopmental follow-up study, was defined as having any ROP diagnosis in either eye by an ophthalmologist during the initial study, or having any ROP diagnosis or surgical/treatment intervention in either eye during the 2-year evaluation.
Statistical Analysis
Baseline characteristics and early neonatal morbidities were analyzed controlling for center where possible using the appropriate Cochran-Mantel-Haenszel tests for categorical and ordinal data and multivariable linear regression for continuous data. Comparisons were made between the inositol vs. placebo groups completing study follow-up, as well as between the total follow-up completers vs. those that died prior to follow-up vs. those that were lost-to-follow-up.
Analyses of the outcomes were performed on the intention-to-treat population with missing data treated as missing completely at random and excluded from the analysis. Robust Poisson regression estimated adjusted relative risks (RRs). A generalized linear model with a binomial distribution and identity link estimated the adjusted absolute risk difference (and associated 95% CIs) for myo-inositol vs. placebo for the main outcome and for the binary secondary outcomes. Growth at follow-up variables (height, weight, and head circumference) were analyzed with an analysis of covariance model. The analyses were controlled for the randomization factors of center and gestational age strata as fixed effects for the main outcome of moderate/severe NDI or death and its components as well as for all secondary outcomes where possible. A Kaplan-Meier curve and log-rank test were used to compare difference in survival probability between the inositol and placebo group. Individuals surviving through their last follow-up assessment were right censored. Because of manufacturing issues reported for the parent trial, a supplemental analysis of the outcomes was also performed by comparing the inositol lot 1 vs. inositol lot 2 groups.
All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc.). As this follow-up analysis was a secondary objective of the clinical trial, all analyses are exploratory and not intended to be formal test of hypotheses; therefore, no adjustments were made for multiplicity.
Results
Population characteristics
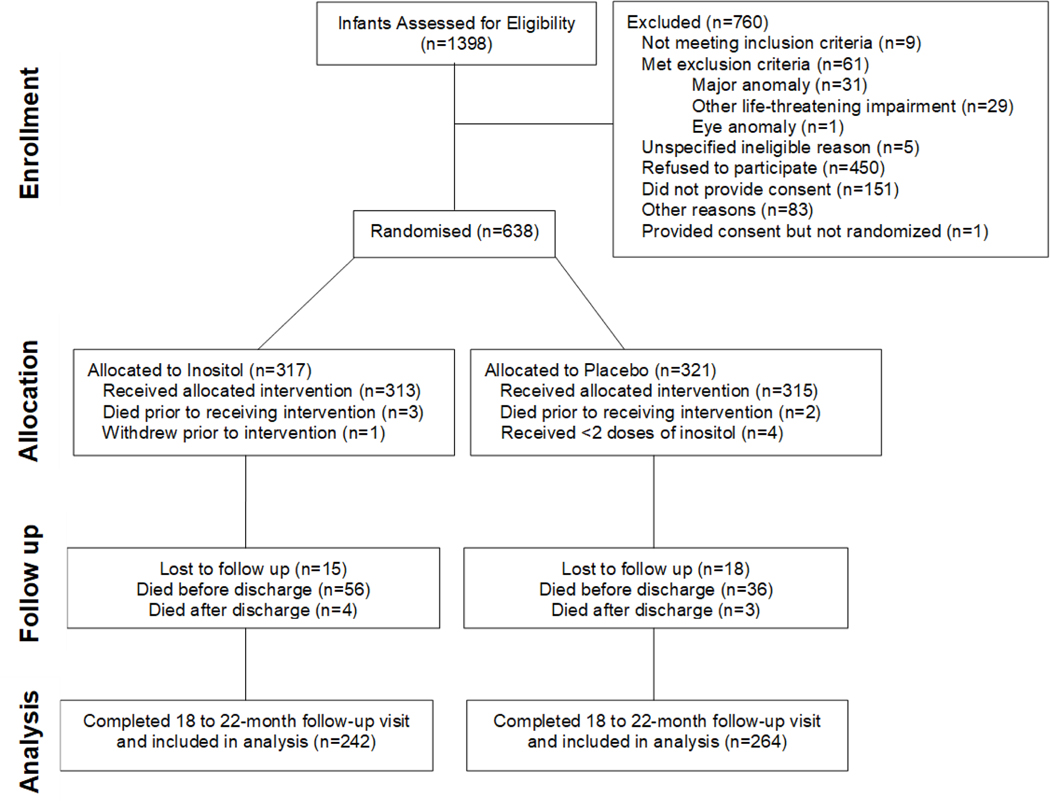
Analyses of the primary outcome (death or type I ROP) and of mortality have been previously published.5,13 These data represent a pre-planned analysis of mortality and NDI outcome at 24 months corrected age for children enrolled in this RCT prior to termination of the study. As previously reported,5 treatment groups were similar at randomization. Of the 638 infants randomized in the phase III RCT, 539 survived to follow-up, of whom 506 (94%) were evaluated. (Figure 1) Demographic characteristics of the treatment and placebo groups of randomized infants subsequently completing 24 month follow--up were similar (Table 1). The mean GA±SD was 25.8 ±1.3 weeks in both treatment groups (p=0.50) with a similar distribution above or below 26 weeks. The 33 children lost to follow-up had a similar demographic and morbidity profile as those evaluated. Those who died prior to follow-up were of lower birthweight and gestational age and were more likely to have neonatal morbidities, including late onset sepsis, BPD, necrotizing enterocolitis, severe IVH, and periventricular leukomalacia (PVL) (Table 1 and Table 2). Early onset sepsis occurred infrequently and rates were similar in all groups.
Figure 1:
Consort Diagram
Table 1:
Baseline Characteristics and Demographics
| Study Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Inositol Follow-up Completers (N=242) | Placebo Follow-up Completers (N=264) | P1 | Total Follow-up Completers (N=506) | Death Prior to Follow-up (N=99) | LTF Prior to Follow-up (N=33) | P: Total vs. Death1 | P: Total vs. LTF1 | P: Death vs. LTF1 |
| Gestational Age (wks) | |||||||||
| Mean (SD) | 25.8 (1.3) | 25.8 (1.3) | 0.50 | 25.8 (1.3) | 24.8 (1.5) | 26.1 (1.5) | <.001 | 0.18 | <.001 |
| <26 0/7 wk | |||||||||
| <26 | 119 (49.2%) | 131 (49.6%) | 0.88 | 250 (49.4%) | 74 (74.7%) | 15 (45.5%) | <.001 | 0.48 | 0.002 |
| >= 26 | 123 (50.8%) | 133 (50.4%) | 256 (50.6%) | 25 (25.3%) | 18 (54.5%) | ||||
| Birthweight (gms) | |||||||||
| Mean (SD) | 801.2 (193.9) | 808.0 (190.9) | 0.67 | 804.8 (192.1) | 639.9 (155.9) | 777.8 (196.3) | <.001 | 0.64 | <.001 |
| Sex | |||||||||
| Male | 119 (49.2%) | 127 (48.1%) | 0.72 | 246 (48.6%) | 59 (59.6%) | 16 (48.5%) | 0.10 | 0.79 | 0.26 |
| Female | 123 (50.8%) | 137 (51.9%) | 260 (51.4%) | 40 (40.4%) | 17 (51.5%) | ||||
| Race | |||||||||
| Non-Hispanic White | 100 (43.7%) | 107 (43.9%) | 0.58 | 207 (43.8%) | 41 (43.6%) | 17 (56.7%) | 0.52 | 0.79 | 0.36 |
| Non-Hispanic Black | 91 (39.7%) | 91 (37.3%) | 182 (38.5%) | 40 (42.6%) | 10 (33.3%) | ||||
| Asian | 8 (3.5%) | 6 (2.5%) | 14 (3.0%) | 3 (3.2%) | |||||
| Hispanic or Latino | 30 (13.1%) | 40 (16.4%) | 70 (14.8%) | 10 (10.6%) | 3 (10.0%) | ||||
| Antenatal Steroids | |||||||||
| Yes | 218 (90.1%) | 238 (90.2%) | 0.98 | 456 (90.1%) | 81 (81.8%) | 27 (84.4%) | 0.02 | 0.30 | 0.74 |
| No | 24 (9.9%) | 26 (9.8%) | 50 (9.9%) | 18 (18.2%) | 5 (15.6%) | ||||
| Cesarean Delivery | |||||||||
| Yes | 165 (68.5%) | 186 (70.7%) | 0.53 | 351 (69.6%) | 48 (48.5%) | 24 (72.7%) | <0.001 | 0.17 | 0.02 |
| No | 76 (31.5%) | 77 (29.3%) | 153 (30.4%) | 51 (51.5%) | 9 (27.3%) | ||||
| Apgar - 1 minute | |||||||||
| Median (IQR) | 4.0 (2.0, 6.0) | 3.0 (1.0, 5.0) | 0.02 | 4.0 (2.0, 6.0) | 2.0 (1.0, 5.0) | 4.0 (2.0, 5.0) | 0.02 | 0.89 | 0.02 |
| Apgar - 5 minute | |||||||||
| Median (IQR) | 7.0 (5.0, 8.0) | 6.0 (5.0, 8.0) | 0.06 | 7.0 (5.0, 8.0) | 6.0 (3.0, 7.0) | 6.0 (5.0, 8.0) | 0.02 | 0.65 | 0.04 |
| Chorioamnionitis | |||||||||
| Yes | 37 (15.4%) | 37 (14.1%) | 0.60 | 74 (14.7%) | 13 (13.3%) | 2 (6.1%) | 0.75 | 0.21 | 0.35 |
| No | 204 (84.6%) | 226 (85.9%) | 430 (85.3%) | 85 (86.7%) | 31 (93.9%) | ||||
| Early Onset Sepsis | |||||||||
| Yes | 6 (2.5%) | 8 (3.0%) | 0.71 | 14 (2.8%) | 2 (2.0%) | 0 (0.0%) | >.99 | >.99 | >.99 |
| No | 236 (97.5%) | 256 (97.0%) | 492 (97.2%) | 97 (98.0%) | 33 (100.0%) | ||||
LTF, Lost to Follow-up; P, P-value
Analyses adjusted for center were possible.
Table 2:
Early Neonatal Morbidities
| Study Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Inositol Follow-up Completers (N=242) | Placebo Follow-up Completers (N=264) | P1 | Total Follow-up Completers (N=506) | Death Prior to Follow-up (N=99) | LTF Prior to Follow-up (N=33) | P: Total vs. Death1 | P: Total vs. LTF1 | P: Death vs. LTF1 |
| Bronchopulmonary Dysplasia (BPD)2 | 140/242 (58%) | 149/263 (57%) | 0.90 | 289/505 (57%) | 19/ 22 (86%) | 16/ 33 (48%) | 0.008 | 0.61 | 0.004 |
| Postnatal Steroid for BPD/CLD2 (oxygen at 36 weeks)3 | 53/225 (24%) | 56/241 (23%) | 0.91 | 109/466 (23%) | 20/ 96 (21%) | 6/ 30 (20%) | 0.52 | 0.80 | 0.92 |
| Patent Ductus Arteriosus, Receiving Surgery | 37/242 (15%) | 39/264 (15%) | 0.63 | 76/506 (15%) | 5/ 98 (5%) | 2/ 33 (6%) | 0.004 | 0.25 | 0.66 |
| Late Onset Sepsis | 53/242 (22%) | 47/264 (18%) | 0.16 | 100/506 (20%) | 39/ 92 (42%) | 8/ 33 (24%) | <.001 | 0.31 | 0.06 |
| NEC - Suspected or Proven | 18/242 (7%) | 21/264 (8%) | 0.83 | 39/506 (8%) | 16/ 99 (16%) | 4/ 33 (12%) | 0.008 | 0.32 | 0.78 |
| NEC, Receiving Surgery | 7/242 (3%) | 4/264 (2%) | 0.17 | 11/506 (2%) | 11/ 99 (11%) | 1/ 33 (3%) | 0.005 | >.99 | 0.26 |
| Spontaneous Intestinal Perforation without Necrotizing Enterocolitis | 13/242 (5%) | 13/264 (5%) | 0.82 | 26/506 (5%) | 13/ 99 (13%) | 2/ 33 (6%) | 0.02 | 0.69 | 0.35 |
| Any Intraventricular Hemorrhage | 73/241 (30%) | 67/263 (25%) | 0.23 | 140/504 (28%) | 45/ 91 (49%) | 7/ 33 (21%) | <.001 | 0.31 | <.001 |
| Intraventricular Hemorrhage, Grade 3 or 4 | 35/241 (15%) | 32/263 (12%) | 0.38 | 67/504 (13%) | 32/ 91 (35%) | 2/ 33 (6%) | <.001 | 0.22 | 0.001 |
| Periventricular Leukomalacia | 14/242 (6%) | 12/263 (5%) | 0.53 | 26/505 (5%) | 5/ 91 (5%) | 1/ 33 (3%) | 0.80 | >.99 | >.99 |
| Type 1 ROP w/adjunction | 35/238 (15%) | 28/264 (11%) | 0.16 | 63/502 (13%) | 5/ 16 (31%) | 6/ 32 (19%) | 0.04 | 0.26 | 0.33 |
ROP, retinopathy of prematurity; BPD, Bronchopulmonary dysplasia; VPS, VP Shunt; VAD, Ventricular access device; NEC, Necrotizing enterocolitis; CLD, Chronic lung disease; P, P-value
Analyses adjusted for center were possible.
Bronchopulmonary dysplasia (BPD) is a form of chronic lung disease (CLD) most commonly seen in premature infants who require mechanical ventilation and oxygen therapy for acute respiratory distress. For the study, it is defined as supplemental oxygen required to maintain an oxygenation saturation of >90% at 36 weeks postmenstrual age (PMA) (NICHD physiologic definition)
46 participants were removed from analysis as they received a trial treatment that could have either been an active treatment or placebo.
Neonatal morbidities
Early neonatal morbidities were similar among those seen and those lost to follow-up. Neonatal morbidities were similar between the myo-inositol and placebo groups (Table 2). Any ROP was common and was diagnosed in 70% of enrolled patients by the 24 month follow-up visit. However, a much smaller percentage received either ophthalmologic treatment (Inositol 22% vs 17% placebo, p=0.20) or surgery (Inositol 8% v 6%, p=0.45). (Tables 3 and 4). Type 1 ROP through early follow-up was present in 13% of patients and risk did not differ by treatment group5 (Table 2). BPD was common in both groups and present in 57% of all those who completed follow-up. Growth parameters were similar between the two groups at 24 month follow-up (Table 4).
Table 3:
Composite Outcome Of Death Or Survival With Moderate/Severe Neurodevelopmental Impairment Among Randomized Infants1
| Study Group | |||||
|---|---|---|---|---|---|
| Outcome | Inositol (N=302) | Placebo (N=303) | Adjusted Risk Diff (95% CI) | Adjusted RR (95% CI) | P-value |
| Corrected age at follow-up; Median (IQR) | 24 (23,26) | 24 (22,26) | N/A | N/A | |
| NDI or Death Prior to Follow-up | 172/287 (60%) | 161/289 (56%) | 2 (−5 to 10) | 1.06 (0.93 to 1.21) | 0.40 |
| Any ROP or Death Prior to Follow-up | 231/302 (76%) | 224/303 (74%) | 1 (−6 to 7) | 1.03 (0.94 to 1.12) | 0.53 |
| Death Prior to Follow-up | 60/302 (20%) | 39/303 (13%) | 4 (−2 to 11) | 1.53 (1.08 to 2.18) | 0.02 |
| Moderate or Severe NDI | 112/227 (49%) | 122/250 (49%) | −1 (−9 to 8) | 1.01 (0.85 to 1.21) | 0.88 |
| Moderate NDI2 | 66/227 (29%) | 79/250 (32%) | |||
| Severe NDI3 | 46/224 (21%) | 43/246 (17%) | |||
| Any ROP4 | 181/260 (70%) | 191/271 (70%) | −2 (−10 to 5) | 0.99 (0.90 to 1.10) | 0.92 |
NDI, neurodevelopmental impairment; ROP, retinopathy of prematurity
All analyses adjusted for center and gestational age strata
Excludes lost to follow-up participants
Occurrence of any of the following: GMFCS level II or III, Bayley III cognitive composite score between and including 70 and 84, Bayley III motor composite score between and including 70 and 84, unilateral blind or bilateral blind, permanent hearing loss that does not permit child to understand directions of the examiner and communicate despite amplification with cochlear implant or hearing aid
Occurrence of any of the following: GMFCS level IV or V, Bayley III cognitive composite score < 70, Bayley III motor composite score < 70, unilateral blind or bilateral blind, permanent hearing loss that does not permit child to understand directions of the examiner and communicate despite amplification with cochlear implant or hearing aid
Includes any report of ROP in the GDB NG-03 form, Follow-up NF-04 form, INS 3–14 Follow-up form (ophthalmologist diagnosed or treated ROP), or a documented finding of ROP on any ophthalmologic exam during the initial INS follow-up
Table 4:
Neurodevelopment Outcomes Among Follow-up Completers
| Study Group | |||||
|---|---|---|---|---|---|
| Outcome | Inositol Follow-up Completers (N=242) | Placebo Follow-up Completers (N=264) | Adjusted Risk Diff (95% CI) | Adjusted RR (95% CI) | P-value |
| Growth at Follow-up | |||||
| Weight - kg, mean (std)1 | 11.8 (2.2) | 11.7 (1.7) | 0.01 (−.32 to 0.34) | 0.94 | |
| Head Circumference - cm, mean (std)1 | 47.5 (2.3) | 47.6 (2.4) | −.12 (−.49 to 0.24) | 0.50 | |
| Height - cm, mean (std)1 | 85.3 (4.1) | 85.6 (4.3) | −.41 (−1.1 to 0.31) | 0.26 | |
| Components of NDI | |||||
| Cerebral Palsy, Any2 | 39/240 (16%) | 39/259 (15%) | 1 (−5 to 7) | 1.08 (0.72 to 1.62) | 0.71 |
| Cerebral Palsy, moderate or severe2 | 22/239 (9%) | 16/258 (6%) | 2 (−2 to 7) | 1.49 (0.80 to 2.76) | 0.21 |
| GMFCS >=22 | 25/238 (11%) | 24/258 (9%) | 1 (−4 to 6) | 1.12 (0.66 to 1.90) | 0.67 |
| BSID III Cognitive <702 | 31/231 (13%) | 38/258 (15%) | −1 (−7 to 5) | 0.91 (0.59 to 1.41) | 0.66 |
| BSID III Cognitive <851 | 78/231 (34%) | 83/258 (32%) | 2 (−6 to 10) | 1.05 (0.82 to 1.34) | 0.72 |
| BSID III Motor <702 | 37/227 (16%) | 34/253 (13%) | 3 (−3 to 9) | 1.20 (0.78 to 1.85) | 0.40 |
| BSID III Motor <851 | 94/227 (41%) | 107/253 (42%) | −1 (−10 to 7) | 0.99 (0.81 to 1.22) | 0.94 |
| Bilateral Blindness3 | 3/240 (1%) | 4/259 (2%) | 0 (−2 to 2) | 0.81 (0.18 to 3.58) | 0.78 |
| Unilateral Blindness3,4 | 4/240 (2%) | 5/259 (2%) | 0 (−3 to 2) | 0.86 (0.23 to 3.18) | 0.83 |
| Bilateral Hearing Loss2 | 11/238 (5%) | 6/252 (2%) | 3 (−3 to 9) | 1.93 (0.73 to 5.10) | 0.19 |
| Ophthalmologic Outcomes | |||||
| Any ROP through follow-up2,5 | 171/242 (71%) | 185/264 (70%) | 0 (−7 to 8) | 1.02 (0.92 to 1.13) | 0.75 |
| Any Ophthalmologic treatment1,6 | 42/195 (22%) | 37/221 (17%) | 4 (−3 to 12) | 1.30 (0.87 to 1.92) | 0.20 |
| Any Ophthalmologic surgery1,7 | 15/195 (8%) | 13/221 (6%) | 0 (−5 to 5) | 1.32 (0.64 to 2.70) | 0.45 |
| Strabismus1,8 | 35/239 (15%) | 39/263 (15%) | −1 (−7 to 5) | 0.98 (0.66 to 1.47) | 0.93 |
| Nystagmus2,9 | 11/239 (5%) | 8/263 (3%) | 1 (−2 to 5) | 1.51 (0.62 to 3.70) | 0.36 |
NDI, neurodevelopmental impairment; GMFCS, gross motor function classification system; BSID III, Bayley Scales of Infant Development-III; ROP, retinopathy of prematurity
Analyses adjusted for center and gestational age strata
Analyses adjusted for gestational age strata
Analyses not adjusted due to low cell count
Includes bilateral blindness
Includes any report of ROP in the GDB NG-03 form, Follow-up NF-04 form, INS 3–14 Follow-up form (ophthalmologist diagnosed or treated ROP), or a documented finding of ROP on any ophthalmologic exam during the initial INS follow-up
Includes any reported eye surgery or medical treatment (in either eye) on the INS 3–14 Follow-up form
Includes any reported eye surgery (in either eye) on the INS 3–14 Follow-up form
Includes any report of Strabismus in the Follow-up NF-04 or NF-05 forms
Includes any report of Nystagmus in the Follow-up NF-04 or NF-05 forms
24 Month Outcome of Death or Neurodevelopmental Impairment:
There was no difference in the risk of the composite outcome of death or survival with moderate/severe NDI for infants in this clinical trial [60% in myo-inositol treated vs 56% in placebo; ARR 1.06 (95% CI=0.93–1.21; p=0.40)]. Neurodevelopmental outcomes were similar among survivors in the two treatment groups. The increased mortality rate in the myo-inositol treated infants noted prior to hospital discharge remained stable through 24 month follow-up (Figure 2). Two different lots of study drug were used during the study period. No differences were seen for the composite neurodevelopmental outcome between those receiving myo-inositol or placebo by lot number (Table 5).
Figure 2:
Kaplan Meier Curve through 24 month follow-up
Table 5:
Composite Outcome Of Death Or Survival With Major Morbidity For LOT 1 Only Vs LOT 2 Only1
| Lot 1 Use Only | Lot 2 Use Only | Inositol Lot | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | Inositol (N=124) | Placebo (N=119) | RR (95% CI) | P | Inositol (N=137) | Placebo (N=148) | RR (95% CI) | P | RR (95% CI) | P |
| NDI or Death Prior to Follow-up | 75/116 (65%) | 59/115 (51%) | 1.29 (1.05 to 1.60) | 0.02 | 72/132 (55%) | 80/140 (57%) | 0.93 (0.76 to 1.14) | 0.49 | 0.85 (0.69 to 1.04) | 0.11 |
| Any ROP or Death Prior to Follow-up | 99/124 (80%) | 84/119 (71%) | 1.14 (1.00 to 1.29) | 0.05 | 103/137 (75%) | 110/148 (74%) | 1.01 (0.88 to 1.15) | 0.91 | 0.88 (0.76 to 1.02) | 0.09 |
| Death Prior to Follow-up | 31/124 (25%) | 19/119 (16%) | 1.50 (0.91 to 2.46) | 0.11 | 27/137 (20%) | 20/148 (14%) | 1.45 (0.86 to 2.45) | 0.17 | 0.80 (0.52 to 1.25) | 0.34 |
| Moderate or Severe NDI2 | 44/ 85 (52%) | 40/ 96 (42%) | 1.23 (0.90 to 1.69) | 0.19 | 45/105 (43%) | 60/120 (50%) | 0.86 (0.65 to 1.14) | 0.29 | 0.83 (0.61 to 1.11) | 0.21 |
| Any ROP3 | 74/101 (73%) | 67/102 (66%) | 1.10 (0.92 to 1.31) | 0.31 | 79/119 (66%) | 94/133 (71%) | 0.95 (0.80 to 1.12) | 0.52 | 0.91 (0.77 to 1.08) | 0.27 |
NDI, neurodevelopmental impairment; ROP, retinopathy of prematurity; P, P-value
Robust Poisson regression adjusting for center where possible and GA strata were used to obtain p-values and estimated adjusted relative risks and 95% confidence intervals. Lot 1 was used as the reference group for the Inositol lot comparison.
Excludes lost to follow-up participants
Occurrence of any of the following: GMFCS level II or higher, Bayley III cognitive composite score < 85, Bayley III motor composite score < 85, unilateral blind or bilateral blind, permanent hearing loss that does not permit child to understand directions of the examiner and communicate despite amplification with cochlear implant or hearing aid
Includes any report of ROP in the GDB NG-03 form, Follow-up NF-04 form, INS 3–14 Follow-up form (ophthalmologist diagnosed or treated ROP), or a documented finding of ROP on any ophthalmologic exam during the initial INS follow-up
At a median age of 24 months corrected age, 49% of children in both groups met criteria for moderate or severe NDI at follow-up (Table 4). Among those with NDI, moderate impairment was more common than severe impairment in both treatment groups. BSID III Motor score < 85 (myo-inositol treated 41% v placebo 42%) and BSID III Cognitive score < 85 (myo-inositol treated 34% v placebo 32%) were the most frequent abnormal findings on psychometric testing but scores did not differ between the treatment groups (Table 4). Rates of severe motor and cognitive impairment, defined as having BSID III Cognitive and Motor scores <70 respectively, were also similar between the treatment groups (Table 4). The rate of any CP or moderate/severe CP was similar between groups. At follow-up, 95.2% were sitting without support, 89.6% were walking independently and 2.6% were walking but required a device or hands held.
Secondary outcomes:
Although ROP was present in 70% of patients in both groups, the majority did not receive ophthalmologic or surgical treatment. Treatment with myo-inositol did not change the risk of developing type 1 ROP. Among those seen for follow-up, children in the treatment and placebo group had similar rates of developing ROP or receiving medical or surgical treatment for ROP prior to the follow-up visit. Strabismus was noted at follow-up in 15% of children in both groups. Nystagmus was present in a smaller percentage (myo-inositol 5% v placebo 3%, p=0.36). Ocular outcomes were similar within and between the two lots of study drug (Table 6).
Table 6:
Neurodevelopment Outcome For LOT 1 Only Vs LOT 2 Only Among Follow-up Completers1
| Lot 1 Use Only | Lot 2 Use Only | Inositol Lot Comparison | |||||
|---|---|---|---|---|---|---|---|
| Outcome | Inositol Follow-up Completers (N=93) | Placebo Follow-up Completers (N=100) | P | Inositol Follow-up Completers (N=110) | Placebo Follow-up Completers (N=128) | P | P |
| Growth at Follow-up | |||||||
| Weight - kg, mean (std) | 11.9 (2.1) | 11.9 (1.7) | 0.83 | 11.8 (2.4) | 11.5 (1.6) | 0.22 | 0.62 |
| Head Circumference - cm, mean (std) | 47.5 (2.2) | 47.7 (2.1) | 0.33 | 47.7 (2.3) | 47.5 (2.5) | 0.69 | 0.45 |
| Height - cm, mean (std) | 85.2 (4.0) | 85.7 (4.1) | 0.35 | 85.3 (4.2) | 85.7 (4.5) | 0.43 | 0.48 |
| Components of NDI | |||||||
| Cerebral Palsy, Any | 12/ 92 (13%) | 12/ 99 (12%) | 0.88 | 17/109 (16%) | 24/124 (19%) | 0.46 | 0.61 |
| Cerebral Palsy, moderate or severe | 9/ 92 (10%) | 4/ 99 (4%) | 0.14 | 9/109 (8%) | 10/124 (8%) | 0.94 | 0.69 |
| GMFCS >=2 | 9/ 92 (10%) | 7/ 99 (7%) | 0.54 | 11/107 (10%) | 13/123 (11%) | 0.94 | 0.93 |
| BSID III Cognitive <70 | 12/ 87 (14%) | 9/ 99 (9%) | 0.33 | 13/106 (12%) | 22/123 (18%) | 0.24 | 0.74 |
| BSID III Cognitive <85 | 28/ 87 (32%) | 23/ 99 (23%) | 0.18 | 35/106 (33%) | 44/123 (36%) | 0.67 | 0.92 |
| BSID III Motor <70 | 14/ 85 (16%) | 7/ 96 (7%) | 0.07 | 14/105 (13%) | 20/121 (17%) | 0.50 | 0.54 |
| BSID III Motor <85 | 39/ 85 (46%) | 36/ 96 (38%) | 0.26 | 33/105 (31%) | 52/121 (43%) | 0.08 | 0.04 |
| Bilateral Blindness | 0/ 92 (0%) | 1/ 99 (1%) | 1/109 (1%) | 3/124 (2%) | 0.40 | ||
| Unilateral Blindness1 | 0/ 92 (0%) | 2/ 99 (2%) | 2/109 (2%) | 3/124 (2%) | 0.76 | ||
| Bilateral Hearing Loss | 4/ 92 (4%) | 1/ 98 (1%) | 0.20 | 5/108 (5%) | 5/119 (4%) | 0.88 | 0.93 |
| Ophthalmologic Outcomes | |||||||
| Any ROP through follow-up2 | 68/ 93 (73%) | 65/100 (65%) | 0.26 | 76/110 (69%) | 90/128 (70%) | 0.91 | 0.52 |
| Any Ophthalmologic treatment3 | 15/ 72 (21%) | 14/ 82 (17%) | 0.54 | 19/ 91 (21%) | 16/108 (15%) | 0.24 | 0.90 |
| Any Ophthalmologic surgery4 | 5/ 72 (7%) | 4/ 82 (5%) | 0.58 | 9/ 91 (10%) | 6/108 (6%) | 0.24 | 0.60 |
| Strabismus5 | 12/ 92 (13%) | 12/ 99 (12%) | 0.87 | 17/108 (16%) | 22/128 (17%) | 0.80 | 0.61 |
| Nystagmus6 | 3/ 92 (3%) | 4/ 99 (4%) | 0.76 | 6/108 (6%) | 3/128 (2%) | 0.21 | 0.45 |
NDI, neurodevelopmental impairment; GMFCS, gross motor function classification system; BSID III, Bayley Scales of Infant Development-III; ROP, retinopathy of prematurity; P, P-value
Robust Poisson regression adjusting for center where possible and GA strata were used to obtain p-values and estimated adjusted relative risks and 95% confidence intervals.
Includes bilateral blindness
Includes any report of ROP in the GDB NG-03 form, Follow-up NF-04 form, INS 3–14 Follow-up form (ophthalmologist diagnosed or treated ROP), or a documented finding of ROP on any ophthalmologic exam during the initial INS follow-up
Includes any reported eye surgery or medical treatment (in either eye) on the INS 3–14 Follow-up form
Includes any reported eye surgery (in either eye) on the INS 3–14 Follow-up form
Includes any report of Strabismus in the Follow-up NF-04 or NF-05 forms
Includes any report of Nystagmus in the Follow-up NF-04 or NF-05 forms
Discussion
In this study, myo-inositol treatment in the neonatal period in infants born < 28 weeks gestation was not associated with a statistically significant difference in the combined risk of death or survival with moderate/severe neurodevelopmental impairment at 24 months corrected age. The increased rate of death seen in the original trial persisted until 2 year follow up. Although small clinical trials performed during the pre-surfactant era reported a decreased risk of early adverse morbidities including BPD, sepsis, and severe IVH, it was necessary to determine if treatment with myo-inositol demonstrated the same benefits in a contemporary group of preterm neonates cared for during the surfactant era. Furthermore, previous clinical trials reported very limited or no neurodevelopmental outcome data for study subjects. This study represents the largest clinical trial evaluating the neurodevelopmental outcome of children born extremely preterm treated with myo-inositol, including its impact on survival, neonatal morbidities, and neurologic sequelae.
Differences in the patient populations and the risk for associated morbidities may explain the differences in our results compared to previous studies published in the 1990s. Over 50% of patients in both treatment groups were <26 weeks GA. The mean GA for infants enrolled in the previous studies by Hallman was 29 weeks GA.2,3 Shifts in neonatal practice patterns, including increased antenatal steroid use, routine administration of surfactant, and lung protective strategies, may also explain some of the differences in our outcomes.
The findings of this trial were sufficiently compelling to prompt the recent Cochrane meta-analysis to conclude that inositol supplementation should not be a part of the routine nutritional management of preterm children and that further inositol trials are unwarranted based on the lack of reduction in mortality or early neonatal morbidities, including ROP, sepsis, BPD or severe Grade 3 or 4 IVH.6 Furthermore, they stressed the importance of completing longer term follow-up on the children enrolled in previous clinical trials.
Neurodevelopmental outcome is strongly correlated with the presence of various neonatal morbidities, including IVH, PVL, late onset sepsis, and BPD.14–17 The morbidity profile was similar for children in both treatment groups in our study. The rate of moderate/severe NDI was high (49%) but similar between the two treatment groups. (Tables 3 and 4) Based on concern that the BSID III may underestimate NDI, we analyzed our data using a cutoff of <85 to define moderate NDI and <70 to define severe NDI for the cognitive and motor scales of this instrument.18 Treatment group did not affect the risk for moderate or severe NDI.
Hallman reported the neurodevelopmental outcome of 169 preterm infants at one year of age (73 in placebo group and 96 inositol treated),2 and found no statistically significant difference in the rate of major handicap (18% vs 9%; p=ns) or CP ( 10% v 7%; p=ns) in the placebo compared to inositol treated infants. Information regarding severity of CP was not included in the analysis. In our RCT, rates of all severity levels of CP were similar between the two treatment groups. The slightly higher rate of any CP in our study is likely related to the lower GA of our patient population. Previous studies reported that inositol treated patients were less likely to have Grade 3 or 4 IVH. In our overall study population, there was no difference between groups, with 16% of patients in each group developing Grade 3 or 4 IVH. 5 Similarly, among those who completed follow-up, 13% of infants had a Grade 3 or 4 IVH and rates were similar between treatment groups.
The need to halt enrollment early reduced the sample size and thereby increased the possibility of either false positive or false negative findings. It was also not possible to adjust for center or gestational age stratum in all analyses. However, this study is the largest randomized trial of myo-inositol for premature infants and has multiple strengths to enhance its validity, including pharmacokinetic studies to determine the appropriate dose,13 a high follow-up rate, and standardized neurodevelopmental assessments by certified masked examiners.
Conclusion
We found no difference between groups in the risk for death or survival with moderate or severe neurodevelopmental impairment at 24 months corrected age. Further studies of myo-inositol in this population are not warranted.
Article Summary
There was no difference in the risk for moderate/severe neurodevelopmental impairment in preterm infants enrolled in this RCT of treatment with myo-inositol.
What is known on this subject
There are limited data regarding the neurodevelopmental outcome of preterm infants treated with myo-inositol
What this study adds
In the largest randomized clinical trial of treatment with myo-inositol in preterm infants, treatment did not affect the risk for death or survival with moderate/severe neurodevelopmental impairment in early childhood
Acknowledgements
Ira-Adams Chapman died following writing the manuscript. The study team appreciates her efforts in leading the follow up study, data analysis and completion of the manuscript. She is a very special person and will be missed by all of the team. Mina Chung, MD, was the research ophthalmologist at the University of Rochester and conducted primary outcome examinations for study participants recruited at this center. Dr. Chung provided important contributions to the study concept and design and she performed ophthalmologic evaluations on the 63 infants enrolled at this site. Dr. Chung died prior to preparation of the final manuscript. The study team which like to extend a special acknowledgement of her efforts on this project.
The National Institutes of Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) through the Neonatal Research Network, and the National Eye Institute (NEI) provided grant support for the Inositol Trial. While NICHD staff had input into the study design, conduct, analysis, and manuscript drafting, the comments and views of the authors do not necessarily represent the views of NICHD, NEI, the National Institutes of Health, the Department of Health and Human Services, or the U.S. Government.
Participating NRN sites collected data and transmitted it to RTI International, the data coordinating center (DCC) for the network, which stored, managed and analyzed the data for this study. On behalf of the NRN, RTI International had full access to all of the data in the study, and with the NRN Center Principal Investigators, takes responsibility for the integrity of the data and accuracy of the data analysis.
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study. The following investigators, in addition to those listed as authors, participated in this study:
NRN Steering Committee Chair: Richard A. Polin, MD, Division of Neonatology, College of Physicians and Surgeons, Columbia University, (2011-present).
Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island (UG1 HD27904) – Abbot R. Laptook, MD; Martin Keszler, MD; Elisabeth C. McGowan, MD; Angelita M. Hensman, PhD RNC-NIC; Barbara Alksninis, PNP; Mary Lenore Keszler, MD; Andrea M. Knoll; Theresa M. Leach, MEd Emilee Little, RN BSN; Elisabeth C. McGowan, MD; Michael R. Muller, PharmD; Elisa Vieira, RN BSN; Victoria E. Watson, MS CAS.
Case Western Reserve University, Rainbow Babies & Children’s Hospital (UG1 HD21364) – Michele C. Walsh, MD MS; Anna Maria Hibbs, MD MSCE; Nancy S. Newman, BA RN; Michael Banchy, RPH; Monika Bhola, MD; Jeffrey L. Blumer, MD; Allison H. Payne, MD MS; Bonnie S. Siner, RN; Elizabeth Ross, MS; Eileen K. Stork, MD; H. Gerry Taylor, PhD; Gulgun Yalcinkaya, MD; Arlene Zadell, RN.
Children’s Mercy Hospital, University of Missouri Kansas City School of Medicine (UG1 HD68284) – William E. Truog, MD; Eugenia K. Pallotto, MD MSCE; Prabhu S. Parimi, MD; Cheri Gauldin, RN BSN CCRC; Lisa Gaetano, MSN RN; Anne M. Holmes, RN MSN MBA-HCM CCRC; Kathy Johnson RN, CCRC; Allison Knutson, BSN RNC-NIC.
Cincinnati Children’s Hospital Medical Center, University of Cincinnati Medical Center, and Good Samaritan Hospital (UG1 HD27853, UL1 TR77) – Kurt Schibler, MD; Cathy Grisby, BSN CCRC; Patricia Cobb, MS; Teresa L. Gratton, PA; Kristin Kirker, CRC; Stacey Tepe, BS; Sandra Wuertz, RN-BSN CCRP CLC; Kimberly Yolton, PhD.
Duke University School of Medicine, University Hospital, University of North Carolina, Duke Regional Hospital, and WakeMed Health and Hospitals (UG1 HD40492, UL1 TR1117) – Ronald N. Goldberg, MD; Joanne Finkle, RN JD; Kimberley A. Fisher, PhD FNP-BC IBCLC; William F. Malcolm, MD; Patricia L. Ashley, MD PHD; Deesha Mago-Shah, MD; Chi Dang-Hornik, PharmD BCPS; Sharon F. Freedman, MD; Kathryn E. Gustafson, PhD; Mary Miller-Bell, PharmD RPh; Sasapin Grace Prakalapakorn, MD MPH; Matthew M. Laughon, MD MPH; Carl L. Bose, MD; Janice Bernhardt, MS RN; Cindy Clark, RN; Diane D. Warner, MD MPH; Michael T. O’Shea, MD MPH; Janice Wereszczak CPNP-AC/PC; Jennifer Talbert, MS RN; Stephen D. Kicklighter, MD; Sofia Aliaga, MD, MPH; Jeffery Board, MD; Kevin Gertsch, MD; Jerry Magolan, MD; Linda Manor, RPh; Jan Niklas Ulrich, MD; Ginger Rhodes-Ryan, ARNP MSN NNP-BC; Donna White, BSN, RN-BC BSN; Alexandra Bentley, MD; Laura Edwards, MD.
Emory University, Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory University Hospital Midtown (UG1 HD27851, UL1 TR454) – David P. Carlton, MD; Barbara J. Stoll, MD; Ellen C. Hale, RN BS CCRC; Yvonne Loggins, RN; Diane I. Bottcher, RN MSN; Sheena L. Carter, PhD; Colleen Mackie, BS RT; Maureen Mulligan LaRossa, RN; Lynn C. Comerford, NNP; Gloria Smike, PNP MSN; Salathiel Kendrick-Allwood, MD; Angela Leon-Hernandez, MD.
Eunice Kennedy Shriver National Institute of Child Health and Human Development – Stephanie Wilson Archer, MA.
Indiana University, Riley Hospital for Children and Methodist Hospital at Indiana University Health (UG1 HD27856) – Gregory M. Sokol, MD; Susan Gunn, NNP CCRC; Dianne E. Herron, RN CCRC; Abbey C. Hines, PsyD; Elizabeth Hynes, RNC-NIC; Lu-Ann Papile, MD; Lucy Smiley CCRC.
McGovern Medical School at The University of Texas Health Science Center at Houston and Children’s Memorial Hermann Hospital (UG1 HD87229) – Jon E. Tyson, MD MPH; Kathleen A. Kennedy, MD MPH; Amir M. Khan, MD; Andi Duncan, MD, Ricardo Mosquera, MD, MS, Elizabeth Allain, MS; Julie Arldt-McAlister, MSN APRN; Shanti Brown, RCPhT; Allison G. Dempsey, PhD; Elizabeth Eason, MD; Farida El-Ali, RPH; Carmen Garcia, RN BSN; Kartik Kumar, MD; Janice John, CPNP; Patrick M. Jones, MD MA; M. Layne Lillie, RN BSN; Karen Martin, RN; Sara C. Martin, RN; Georgia E. McDavid, RN; Shannon McKee EdS; Hatice Ozsoy, PhD RPh; Shawna Rodgers, RN; Daniel Sperry, RN; Emily K. Stephens, RN BSN; Vu Ta, PharmD; Christine Wong, PharmD; Sharon L. Wright, MT (ASCP).
Nationwide Children’s Hospital and The Ohio State University Wexner Medical Center (UG1 HD68278) – Pablo J. Sánchez, MD; Leif D. Nelin, MD; Sudarshan R. Jadcherla, MD; Amanda E. Graf, MD; Patricia Luzader, RN; Christine A. Fortney, PhD RN; Gail E. Besner; Nehal A. Parikh, MD; David L. Rogers, MD; Richard P. Golden, MD; Catherine Olson Jordan, MD.
RTI International (U10 HD36790) – Dennis Wallace, PhD; Marie G. Gantz, PhD; Carla M. Bann, PhD; Jeanette O’Donnell Auman, BS; Margaret M. Crawford, BS CCRP; Jenna Gabrio, MPH CCRP; Carolyn M. Petrie Huitema, MS CCRP; James W. Pickett II, BS; Annie M. VonLehmden, BS.
Stanford University and Lucile Packard Children’s Hospital (UG1 HD27880, UL1 TR93) – Krisa P. Van Meurs, MD; David K. Stevenson, MD; M. Bethany Ball, BS CCRC; Steven Chinn, PharmD; Melinda S. Proud, RCP; Barbara Bentley, PsychD MSEd; Maria Elena DeAnda, PhD; Anne M. DeBattista, RN PNP PhD; Beth Earhart, PhD; Lynne C. Huffman, MD; Casey E. Krueger, PhD; Ryan Lucash, PhD; Hali E. Weiss, MD.
University of Alabama at Birmingham Health System and Children’s Hospital of Alabama (UG1 HD34216) – Waldemar A. Carlo, MD; Namasivayam Ambalavanan, MD; Monica V. Collins, RN BSN MaEd; Shirley S. Cosby, RN BSN; Rebecca J. Quinn, PharmD; Brenda Reed Denson, PharmD; Ann Marie Arciniegas-Bernal, MD; Fred J. Biasini, PhD; Kristen C. Johnston, MSN CRNP; Cryshelle S. Patterson, PhD; Vivien A. Phillips, RN BSN; Sally Whitley, MA OTR-L FAOTA.
University of California - Los Angeles, Mattel Children’s Hospital, Santa Monica Hospital, Los Robles Hospital and Medical Center, and Olive View Medical Center (UG1 HD68270) – Uday Devaskar, MD; Meena Garg, MD; Teresa Chanlaw, MPH; Rachel Geller, RN BSN.
University of Iowa (UG1 HD53109, UL1 TR442) – Edward F. Bell, MD; Jane E. Brumbaugh, MD; Karen J. Johnson, RN BSN; Jacky R. Walker, RN; Claire A. Goeke, RN; Kristine M. Johnson, BSPharm RPh; Angela Merriss, BA CPhT; Joanna L. Nohr, PharmD BCPS; Susannah Q. Longmuir, MD; Arlene V. Drack, MD; Diane L. Eastman, RN CPNP MA; Scott A. Larson, MD; Kevin R. Gertsch, MD; Vikki P. Bell.
University of New Mexico Health Sciences Center (UG1 HD53089, UL1TR41) – Robin K. Ohls, MD; Sandra Sundquist Beauman, MSN RNC; Tara Dupont, MD; Mary Ruffaner Hanson, RN BSN; Carol H, Hartenberger, MPH RN; Elizabeth Kuan, RN BSN; Susan J. Kunkel, PharmD; Jean Lowe, PhD; Nancy A. Morgan, RPh MBA.
University of Oulu, and Oulu University Hospital, Oulu, Finland – Mikko K. Hallman, MD.
University of Pennsylvania, Hospital of the University of Pennsylvania, Pennsylvania Hospital, and Children’s Hospital of Philadelphia (UG1 HD68244) – Barbara Schmidt, MD MSc; Haresh Kirpalani, MB MSc; Soraya Abbasi, MD; Aasma S. Chaudhary, BS RRT; Toni Mancini, RN BSN CCRC; William V. Anninger, MD; Judy C. Bernbaum, MD; Gil Binenbaum, MD MSCE; Noah Cook, MD; Stefanie L. Davidson, MD; Marsha Gerdes, PhD; Hallam Hurt, MD; Monte D. Mills, MD; Mina Ricciardelli, PharmD; Kenneth Rockwell, Jr., PharmD MS; Jonathan Snyder, RN BSN; Sze Man Yau, RPh.
University of Rochester Medical Center, Golisano Children’s Hospital, and the University of Buffalo Women’s and Children’s Hospital of Buffalo (UG1 HD68263, UL1 TR42) – Carl D’Angio, MD; Satyan Lakshminrusimha, MD; Anne Marie Reynolds, MD MPH; Stephen A. Bean, PharmD; Melissa F. Carmen, MD; Patricia R. Chess, MD; Rosemary Jensen; Rajeev S. Ramchandran, MD; Ann Marie Turner, PharmD; Ashley Williams, MS Ed; Michael G. Sacilowski, MAT; Holly Wadkins, MA; Julianne Hunn; Aimee Horan, LPN; Melissa Bowman, RN NP; Michele Hartley-McAndrew, MD; William Zorn, PhD; Osman Farooq, MD; Kelley Yost, PhD; Joan Merzbach, LMSW; Cait Fallone, MA; Kyle Binion, BS; Constance Orme; Premini Sabaratnam, MPH.
University of Texas Southwestern Medical Center, Parkland Health & Hospital System, and Children’s Medical Center Dallas (UG1 HD40689) – Myra H. Wyckoff, MD; Luc P. Brion, MD; Diana M. Vasil, RNC-NIC; Sally S. Adams, MS RN CPNP; Christine Cha, PharmD; Juana Cisneros, RN; Maria M. De Leon, BSN RN; Frances Eubanks, BSN RN; Lynda Godowic, PharmD RPh; Laura Grau, RN; Alicia Guzman; Elizabeth Heyne, PsyD PA-C; Lizette E. Lee, RN; Helen C. Lira, PharmD; Azadeh Mozaffari, PharmD RPh; Lara Pavageau, MD; Catherine Twell Boatman, MS CIMI; Reshma Wright, RPh.
Wayne State University, Hutzel Women’s Hospital and Children’s Hospital of Michigan (UG1 HD21385) – Seetha Shankaran, MD; Beena G. Sood, MD MS; Rebecca Bara, RN BSN; Prashant Agarwal, MD; Monika Bajaj, MD; Sanjay Chawla, MD; Kirsten Childs, RN BSN; Melissa February, MD; Laura A. Goldston, MA; Mary E. Johnson, RN BSN; Mirjana Lulic-Botica, RPh; Bogdan Panaitescu, MD; Eunice Woldt, RN MSN.
Data and Safety Monitoring Committee – Christine A. Gleason, MD, chair, University of Washington; Marilee C. Allen, MD, Johns Hopkins University School of Medicine; Robert J. Boyle, MD, University of Virginia Health System; Traci Clemons, PhD, The EMMES Corporation; Mary E. D’Alton, MD, Columbia Ob/Gyn Midtown; Abhik Das (ex officio), PhD, RTI International; Donald Everett, MA (non-voting member), National Eye Institute; Ralph E. Kauffman, MD, University of Missouri-Kansas City, Medical Research Department at Children’s Mercy Hospital; Menachem Miodovnik, MD, Washington Hospital Center; T. Michael O’Shea, MD MPH, Wake Forest University School of Medicine; Lois Smith, MD, Harvard University Children’s Hospital; Steven J. Weiner, MS, The George Washington University; Marian Willinger (ex officio), PhD, Eunice Kennedy Shriver National Institute of Child Health and Human Development.
The BOOST Study ROP Credentialing site, www.boostnz.info/ROP/[boostnz.info, allowed NRN ophthalmologists to use their online system to certify their ROP training.
Funding
Funded by the National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)( U10 HD36790, UG1 HD27904, UG1 HD21364, UG1 HD68284, UG1 HD27853, UG1 HD40492, UG1 HD27851, UG1 HD27856, UG1 HD87229, UG1 HD68278, UG1 HD27880, UG1 HD34216, UG1 HD68270, UG1 HD53109, UG1 HD53089, UG1 HD68244, UG1 HD68263, UG1 HD40689, UG1 HD21385), the National Eye Institute (via co-funding to NICHD), and the National Center for Advancing Translational Sciences (UL1 TR41, UL1 TR42, UL1 TR77, UL1 TR93, UL1 TR442, UL1 TR454, UL1 TR1117).
Abbreviations
- BSID-III
Bayley Scales of Infants Development-III
- BPD
Bronchopulmonary Dysplasia
- CP
Cerebral Palsy
- DSMB
Data Safety Monitoring Board
- FU PI
Follow-up Principal Investigator
- GA
Gestational Age
- GMFCS
Gross Motor Functional Classification System
- IVH
Intraventricular Hemorrhage
- IND
Investigational New Drug
- NDI
Neurodevelopmental Impairment
- NICHD
National Institute of Child Health and Human Development
- NRN
Neonatal Research Network
- PVL
Periventricular Leukomalacia
- RCT
Randomized Controlled Trial
- RDS
Respiratory Distress Syndrome
- ROP
Retinopathy of Prematurity
Footnotes
Data Sharing
Data reported in this paper may be requested through a data use agreement. Further details are available at https://neonatal.rti.org/index.cfm?fuseaction=DataRequest.Home
Conflicts of Interest and Disclosures
None of the authors report any commercial, proprietary, or financial interest in any of the products described in this article. NICHD is the sponsor of the study and holds the investigational new drug (IND) application.
Abbott Nutrition Division, Abbott Laboratories, Columbus, OH, provided the inositol product. They had no role in the: design of the trial; the analyses, interpretation, or writing of the manuscript; or the decision to submit the manuscript for publication. They provided on-site monitoring to assist in quality assurance of the data collection.
REFERENCES
- 1.Hasan SH, Nishigaki I, Tsutsui Y, Yagi K. Studies on myoinositol. IX. Morphological examinaiton of the effect of massive doses of myoinositol on the liver and kidney of rat. Journal of Nutritional Science and Vitaminology. 1974;20(1):55–58. [PubMed] [Google Scholar]
- 2.Hallman M, Bry K, Hoppu K, Lappi M, Pohjavuori M. Inositol Supplementation in Premature Infants with Respiratory Distress Syndrome. New England Journal of Medicine. 1992;326(19):1233–1239. [DOI] [PubMed] [Google Scholar]
- 3.Hallman M, Järvenpää AL, Pohjavuori M. Respiratory distress syndrome and inositol supplementation in preterm infants. Archives of Disease in Childhood. 1986;61(11):1076–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howlett A, Ohlsson A, Plakkal N. Inositol in preterm infants at risk for or having respiratory distress syndrome. Cochrane Database Syst Rev. 2015(2):CD000366. [DOI] [PubMed] [Google Scholar]
- 5.Phelps DL, Watterberg KL, Nolen TL, Cole CA, Cotten CM, Oh W, et al. Effects of Myo-inositol on Type 1 Retinopathy of Prematurity Among Preterm Infants <28 Weeks’ Gestational Age: A Randomized Clinical Trial. JAMA. 2018;320(16):1649–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howlett A, Ohlsson A, Plakkal N. Inositol in preterm infants at risk for or having respiratory distress syndrome. Cochrane Database Syst Rev. 2019;7:CD000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newman JE, Bann CM, Vohr BR, Dusick AM, Higgins RD. Improving the Neonatal Research Network annual certification for neurologic examination of the 18–22 month child. J Pediatr. 2012;161(6):1041–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal Outcomes of Extremely Preterm Infants From the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bayley N. Manual for the Bayley Scales of Infant and Toddler Development. 3 San Antonio, Texas: Harcourt Assessment; 2006. [Google Scholar]
- 10.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Developmental Medicine and Child Neurology. 1997;39(4):214–223. [DOI] [PubMed] [Google Scholar]
- 11.Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2006;117(2):572–576. [DOI] [PubMed] [Google Scholar]
- 12.Early Treatment For Retinopathy Of Prematurity Cooperative G. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121(12):1684–1694. [DOI] [PubMed] [Google Scholar]
- 13.Phelps DL, Ward RM, Williams RL, Nolen TL, Watterberg KL, Oh W, et al. Safety and pharmacokinetics of multiple dose myo-inositol in preterm infants. Pediatr Res. 2016. August;80(2):209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, Higgins RD. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292(19):2357–2365. [DOI] [PubMed] [Google Scholar]
- 15.Serenius F, Ewald U, Farooqi A, Fellman V, Hafström M, Hellgren K, et al. Neurodevelopmental outcomes among extremely preterm infants 6.5 years after active perinatal care in sweden. JAMA Pediatrics. 2016;170(10):954–963. [DOI] [PubMed] [Google Scholar]
- 16.Adams-Chapman I, DeMauro SB. Neurodevelopmental Outcomes of the Preterm Infant. Clin Perinatol. 2018;45(3):xvii–xviii. [DOI] [PubMed] [Google Scholar]
- 17.Ancel P, Goffinet F, and the E-WG. Survival and morbidity of preterm children born at 22 through 34 weeks’ gestation in france in 2011: Results of the epipage-2 cohort study. JAMA Pediatrics. 2015;169(3):230–238. [DOI] [PubMed] [Google Scholar]
- 18.Anderson PJ, De Luca CR, Hutchinson E, Roberts G, Doyle LW. Underestimation of developmental delay by the new Bayley-III Scale. Arch Pediatr Adolesc Med.164(4):352–356. [DOI] [PubMed] [Google Scholar]