Abstract
Context:
Sodium azide is a highly toxic chemical. Its production has increased dramatically over the last 30 years due to its widespread use in vehicular airbags, and it is available for purchase online. Thus, accidental exposure to azide or use as a homicidal or suicidal agent could be on the rise, and secondary exposure to medical personnel can occur. No antidote exists for azide poisoning. We conducted a systematic review of azide poisoning to assess recent poisoning reports, exposure scenarios, clinical presentations, and treatment strategies.
Methods:
We searched both medical and newspaper databases to review the literature between 01/01/2000 and 12/31/2020, pairing the controlled vocabulary and keyword terms “sodium azide” or “hydrazoic acid” with terms relating to exposures and outcomes, such as “ingestion,” “inhalation,” “exposure,” “poisoning,” and “death.” We included all peer-reviewed papers and news articles describing human azide poisoning cases from English and non-English publications that could be identified using English keywords. Data abstracted included the number, age, and gender of cases, mode of exposure, exposure setting, azide dose and route of exposure, symptoms, outcome, and treatment modalities.
Results:
We identified 663 peer-reviewed papers and 303 newspaper articles. After removing duplicated and non-qualifying sources, 54 publications were reviewed describing 156 cases, yielding an average of 7.8 reported azide poisoning cases per year. This rate is three times higher than in a previous review covering the period of 1927 to 1999. Poisoning occurred most commonly in laboratory workers, during secondary exposure of medical personnel, or from a ripped airbag. Hypotension occurred commonly, in some cases requiring vasopressors and one patient received an intra-aortic ballon pump. Gastric lavage and/or activated charcoal were used for oral azide ingestion, and sodium nitrite, sodium thiosulfate, and/or hydroxocobalamin were used in severely poisoned patients.
Conclusions:
Recent increases in azide poisoning reports may stem from greater commercial use and availability. Treatment of systemic poisoning may require aggressive hemodynamic support due to profound hypotension. Based on mechanistic considerations, hydroxocobalamin is a rational choice for treating azide poisoning.
Keywords: Sodium azide (NaN3), hydrazoic acid (HN3), poisoning, treatment, review
Introduction
Sodium azide (NaN3) and its conjugate acid, hydrazoic acid (HN3), are toxic compounds. We refer to both agents generically as “azide.” Fortunately, azide poisoning is relatively rare, but this means most medical personnel may have not encountered a case and possess limited knowledge of azide poisoning. This may prove detrimental in a mass casualty event, for example, an industrial accident or terrorist attack. The latter is possible, because sodium azide is easily available through online retailers and NaN3 is listed as a potential weapon of mass destruction [1,2]. Indeed, azide has been used in several planned and/or executed terrorist attacks [3–7].
Azide has been most notably used as a propellant in vehicular airbags and airplane safety chutes. Following an automobile crash, an igniter generates high temperatures that rapidly decompose NaN3 into sodium metal and nitrogen gas. Due mainly to its use in airbags, NaN3 production surged beginning in 1990, and at least 1,000 tons are produced annually [8,9]. The fate of azide pellets in old airbags is generally unknown, posing a potential environmental problem [10–12]. Azide is also used in chemical laboratories to facilitate synthetic reactions, and in biomedical laboratories to inhibit microbial growth.
Within a year of its discovery, azide was shown to be toxic to plants and animals [13]. The mouse LD50 is 19 mg/kg by intravenous injection, and the human lethal dose is estimated to be ≥700 mg total or ~10 mg/kg [14,15]. Humans can be exposed to azide through three major routes: ingestion, transdermal or transmucosal absorption, or inhalation of hydrazoic acid vapors or sodium azide dust particles. At low doses, azide causes dizziness, nausea, vomiting, and restlessness. At high doses, it causes seizures, hypotension, metabolic acidosis, coma, and respiratory failure. Symptoms occur within minutes of exposure.
Azide has several mechanisms of toxicity. At the cellular level, it inhibits mitochondrial cytochrome C oxidase and catalase [16,17]. The former enzyme is part of complex IV in the mitochondrial electron transport chain and the latter enzyme detoxifies hydrogen peroxide to water and oxygen. Thus, azide can reduce ATP synthesis and cause oxidative stress, the latter due both to mitochondrial electron leakage and reduced catabolism of reactive oxygen species. Cyanide also inhibits cytochrome C oxidase, and azide and cyanide are especially toxic to cells with high respiratory rates, such as neurons and cardiomyocytes. At the organismal level, azide is a potent vasodilator and inhibits platelet aggregation, likely via conversion to nitric oxide. Azide generates nitric oxide in vitro in erythrocytes, platelets, and isolated blood vessels, and recently, nitrosyl-hemoglobin was found in the blood of mice that had received azide [18]. Cytochrome C oxidase inhibition and nitric oxide generation likely underlie the hypotension, myocardial and respiratory failure, and metabolic acidosis that occur in azide poisoning.
A previous systematic review examined human azide poisonings between 1927 and 1999 [14]. Due to the marked increase in azide production over the last 30 years and the potential of new treatments, we thought it timely to perform an updated review of human azide poisoning, concentrating on contemporary exposure settings, clinical presentations, and treatment.
Methods
Search strategy
We conducted the study in accordance with the Preferred Reporting Item for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. We searched all publications from January 1, 2000 to December 31, 2020 that described human cases of azide exposure and toxicity. We conducted separate searches of peer-reviewed papers and newspaper articles.
KH led the literature searches. For peer-reviewed papers, we searched PubMed (pubmed.gov), Embase (embase.com), and Academic Search Complete (ProQuest) using “sodium azide” and “hydrazoic acid’ as controlled vocabulary and keyword terms, pairing them with terms relating to exposure, settings, or outcomes, such as “ingestion,” “inhalation,” “exposure,” “poisoning,” and “death.” For news articles, we searched Access World News and U.S. Major Dailies using a similar search strategy and keywords, without a controlled vocabulary. Full search strategies for all databases are in Appendix 1. Supplemental Google searches were conducted for both peer-reviewed papers and newspaper articles using the same search terms. All records were imported into EndNote and de-duplication followed the Wichor Bramer process [19].
Inclusion and exclusion criteria
All articles describing human azide exposure were included. Reports of physical trauma due to airbag deployment alone were excluded as were articles on azides other than sodium, since NaN3 is the principal azide species produced and it is available for purchase online [2,20,21]. Finally, we excluded articles that were duplicated cases, had insufficient data, or irrelevant account, such as no discussion of human azide poisoning.
JT and SS evaluated the titles and abstracts, and full-text of the articles, with GRB arbitrating conflicts. To increase the international scope and relevance, non-English publications were included, when English language search terms led to their identification. If an English translation was unavailable, native language speakers translated appropriate sections of the papers.
Data collection
JT and SS abstracted the following data, when available: the number, age, and gender of cases, mode of exposure (accident, suicide, or homicide), exposure setting (automobile accident, or industrial, laboratory, or secondary exposure of medical personnel), azide dose received and route of exposure (ingestion, inhalation, or dermal contact), clinical symptoms, outcome including hospitalization and survival of the victim(s), and treatment modalities. The level of evidence for each article was determined. If a case was reported in both a peer-reviewed paper and a news article, only the peer-reviewed paper is included.
Results
For peer-reviewed papers, the initial search identified 663 publications. Based on the Wichor Bramer process, 175 duplications were removed. Of the remaining 488 papers, we excluded 432 after screening the titles and abstracts and finding the same case published in multiple journals, or absence of reference to human exposure. We performed full-text review of the remaining 56 eligible papers, excluding an additional 19 papers based on grounds of duplication, irrelevant account, and/or insufficient data. Thus, 37 papers met our inclusion criteria and were reviewed (Figure 1). Among the 37 papers, a native Chinese speaker reviewed one Chinese paper, and a native Japanese speaker (SS) reviewed two Japanese papers.
Figure 1.
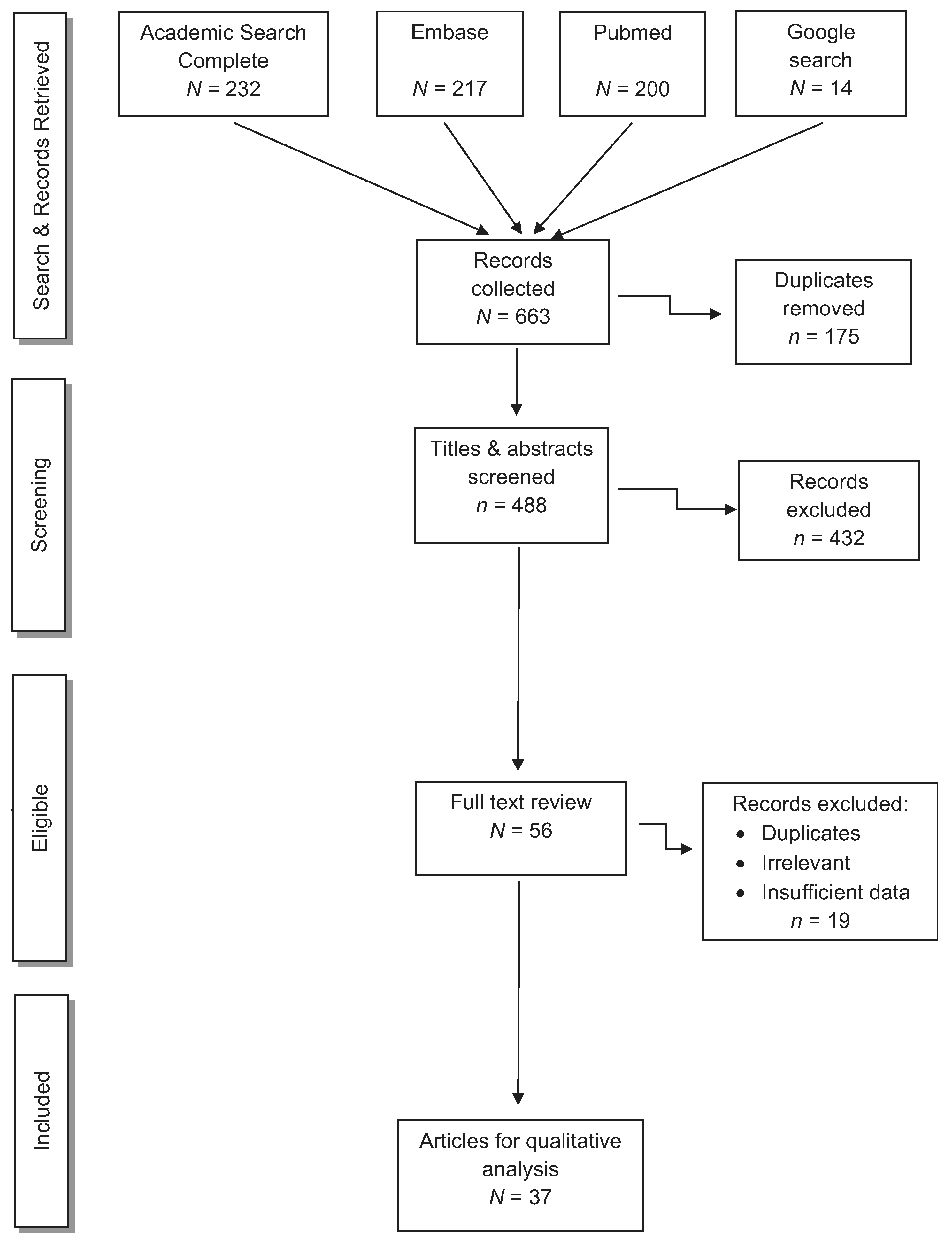
Peer-reviewed articles concerning human cases of NaN3 or HN3 exposure.
For news articles, the initial search identified 303 articles. Applying the same screening methods as for the peer-reviewed papers, we removed 133 duplications, and then excluded 107 articles after screening the titles and abstracts. Of the remaining 63 articles eligible for full-text review, 46 articles were excluded, producing 17 news publications that met our inclusion criteria (Figure 2).
Figure 2.
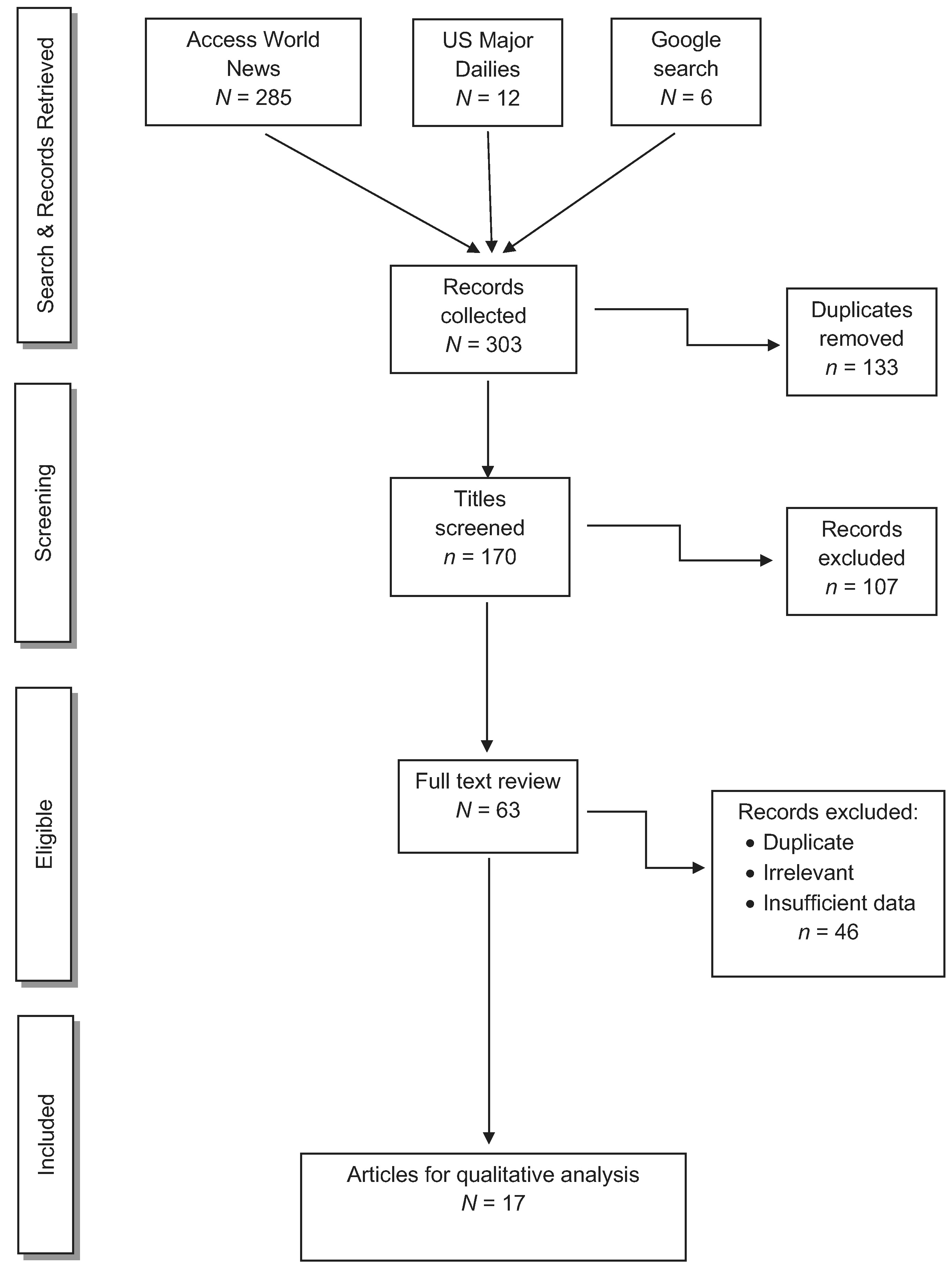
News articles concerning human cases of NaN3 or HN3 exposure.
Cases are grouped based on the mode of azide exposure, accidental, intentional (suicidal or homicidal), secondary, or unknown, and sub-grouped based on exposure setting. We found a total of 156 unique cases: 106 cases in the 37 peer-reviewed papers (Table 1), and 50 cases in the 17 news articles (Table 2). Of the 37 peer-reviewed papers, 36 were case reports that met level VII evidence: opinions of authorities and/or reports of expert committees, and, one was an observational study that met level IV evidence [22,23]. We did not perform quantitative bias analysis on these publications, because no quantitative data were available in either the peer-reviewed papers or the news articles that would allow us to correlate outcome to exposure location, route, or mode or treatment modalities. The median age of the patients was 32 with a range of 18 to 73 years old. The gender distribution was about 17% females and 31% males, with 52% unknown.
Table 1.
Review of 37 peer-reviewed papers yielded 106 cases.
| 1A-1. Accidental exposure due to airbag deployment | |||||||||
| Publication | Level of evidence | Number of cases | Gender | Age | Exposure route | Clinical features | Hospitalization | Treatment | Survival |
| Corazza et al. [24] | VII | 1 | Male | 35 | Dermal contact | First-degree burns Irritant contact dermatitis | Yes | Topical steroids | Yes |
| Foley and Helm [25] | VII | 1 | Female | 32 | Dermal contact | First-degree burns | Yes | Topical antibiotics | Yes |
| Corazza et al. [33] | VII | 1 | Male | 36 | Dermal contact | First and second-degree burns | Yes | Topical antibiotics | Yes |
| Wu et al. [26] | VII | 1 | Female | 29 | Dermal contact | Irritant contact dermatitis | Yes | Topical and oral steroids | Yes |
| Suhr and Kreusch [27] | VII | 1 | Female | 73 | Dermal contact | Second-degree burns | Yes | Topical antibiotics | Yes |
| Hambrook and Fink [28] | VII | 1 | Male | 47 | Inhalation | Asthma Chest tightness Cough | Yes | p-agonists Inhaled steroids | Yes |
| Caudle et al. [29] | VII | 1 | Male | 18 | Inhalation | Chemical pneumonitis Dyspnea Hemoptysis | Yes | Oral steroids Oxygen | Yes |
| Belhadj-Tahar et al. [32] | VII | 1 | Male | 37 | Inhalation and dermal contact | Burning eyes Dyspnea | Yes | Ocular irrigation Supplemental oxygen | Yes |
| Francis et al. [9] | VII | 1 | Male | 22 | Inhalation and dermal contact | Alkaline ocular injury Erythematous supraglottis Stridor Tachypnea | Yes | Mechanical ventilation Ocular irrigation Steroids | Yes |
| Govindarajan et al. [30] | VII | 1 | Male | 56 | Inhalation | Chemical pneumonitis | Yes | N/A | Yes |
| Sever et al. [31] | VII | 3 | Female Male |
21 39 33 |
Dermal contact | All three had second-degree burns One patient had alkaline ocular injury | Yes | Standard burn care Victim with ocular burn received additional ophthalmic care | Yes |
| 1A-2. Accidental exposure due to industrial work | |||||||||
| Publication | Level of evidence | Number of cases | Gender | Age | Exposure route | Clinical features | Hospitalization | Treatment | Survival |
| Pham et al. [36] | VII | 1 | Male | 29 | Inhalation and dermal contact | Bradycardia Hypotension Metabolic acidosis Third-degree burns | Yes | Intravenous fluids Mechanical ventilation Sodium bicarbonate Vasopressors | No |
| Miljours and Braun [34] | IV | 41 | Unknown | Median age not available | Inhalation | Burning eyes Dizziness Headache Palpitations | No | Unknown | Yes |
| Fang et al. [35] | VII | 1 | Male | 32 | Inhalation and dermal contact | Diplopia Dizziness Paresthesias Reduced muscle strength |
Yes | Hyperbaric oxygen
Steroids Traditional Chinese medicines Vitamins B1, B12, and C |
Yes |
| 1A-3. Accidental exposure in a laboratory | |||||||||
| Publication | Level of evidence | Number of cases | Gender | Age | Exposure route | Clinical features | Hospitalization | Treatment | Survival |
| Angelotti et al. [39] | VII | 1 | Male | 33 | Dermal contact (13 g) | Burns Hypotension Lacerations |
Yes | Amputation Mechanical ventilation Vasopressors Wound care | Yes |
| 1A-4. Accidental exposure in a medical facility | |||||||||
| Publication | Level of evidence | Number of cases | Gender | Age | Exposure route | Clinical features | Hospitalization | Treatment | Survival |
| Watanabe et al. [47] | VII | 1 | Female | 53 | Ingestion (1 g) | Generalized seizures Hypotension Metabolic acidosis | Yes | Hemodialysis Gastric lavage with activated charcoal Intra-aortic balloon pump Intravenous steroids Mechanical ventilation Vasopressors | Yes |
| Dermican et al. [46] | VII | 1 | Female | 25 | Ingestion (0.1 g) | Generalized seizures Headache Vomiting |
Yes | Gastric lavage | Yes |
| 1B-1. Suicide in a laboratory setting | |||||||||
| Publication | Level of evidence | Number of cases | Gender | Age | Exposure route | Clinical features | Hospitalization | Treatment | Survival |
| Spadafora et al. [40] | VII | 1 | Female | Unknown | Ingestion (>10 g) | Bradycardia Coma Hypoxia Metabolic acidosis |
Yes | Unknown | No |
| Senda et al. [50] | VII | 1 | Female | 25 | Ingestion | Acute respiratory distress syndrome Arrythmia Cardiac arrest Coma Metabolic acidosis |
Yes | Gastric lavage Intravenous steroids Mechanical ventilation Sodium bicarbonate | No |
| Fuyuno and Cyranoski [51] | VII | 1 | Male | 42 | Ingestion | N/A | No (found dead) | N/A | No |
| Łopaciński et al. [52] | VII | 2 | Male | 30 | Ingestion (>0.18g) | Dizziness Metabolic acidosis Tachycardia |
Yes | Inhaled amyl nitrite Intravenous sodium nitrite Intravenous sodium | Yes |
| 23 | Ingestion (10 g) | Arrythmia Cardiac arrest Coma Metabolic acidosis |
Yes | thiosulfate | No | ||||
| Meatherall and Palatnick [53] | VII | 1 | Male | 59 | Ingestion | Arrythmia Cardiogenic shock Coma Hypotension Metabolic acidosis |
Yes | Exchange transfusion Inhaled amyl nitrite Intravenous sodium nitrite Intravenous sodium thiosulfate Mechanical ventilation Sodium bicarbonate Vasopressors | No |
| French et al. [54] | VII | 1 | Male | 28 | Ingestion (0.1 g) | Bradycardia Hypotension Metabolic acidosis | Yes | Intravenous fluids Gastric lavage Sodium bicarbonate | Yes |
| Kostek et al. [55] | VII | 1 | Female | 55 | Ingestion (0.6 g) | Metabolic acidosis | Yes | Gastric lavage | Yes |
| Le Blanc-Louvry et al. [56] | VII | 1 | Male | 35 | Ingestion (6g) | N/A | No (found dead) | N/A | No |
| Bartecka-Mino et al. [57] | VII | 1 | Female | 25 | Ingestion | Coma Hypotension Metabolic acidosis |
Yes | Hydroxocobalamin Vasopressors |
Yes |
| Downes et al. [58]a | VII | 1 | Male | 32 | Ingestion | Hypotension Metabolic acidosis | Yes | Intravenous fluids Vasopressors | No |
| Gao et al. [60]b | VII | 1 | Male | 23 | Ingestion (1.38 g) | Chest pain Hypotension Metabolic acidosis Nausea and vomiting | Yes | Hemodialysis Intravenous crystalloid Norepinephrine | Yes |
| Muvalia et al. [59] | VII | 1 | Female | 19 | Ingestion (50 g) | Coma Hypotension Metabolic acidosis Nausea and vomiting |
Yes | Mechanical ventilation Vasopressors | No |
| 1B-2. Suicide in a non-laboratory setting | |||||||||
| Publication | Level of evidence | Number of cases | Gender | Age | Exposure route | Clinical features | Hospitalization | Treatment | Survival |
| Wiergowski et al. [61] | VII | 1 | Male | 19 | Ingestion (~20 g) | N/A | No (found dead) | N/A | No |
| Meatherall and Oleschuk [53] | VII | 1 | Male | 35 | Ingestion | N/A | No (found dead) | N/A | No |
| Overtchouk et al. [63] | VII | 1 | Female | 69 | Ingestion (15g) | Myocardial dysfunction Metabolic acidosis | Yes | Gastric lavage Intravenous fluids | Yes |
| Rojek et al. [64] | VII | 1 | Female | 50 | Ingestion | Bradycardia Coma Hypotension |
Yes | Mechanical ventilation Vasopressors | No |
| Ciesla et al. [65] | VII | 1 | Male | 24 | Ingestion | Coma | Yes | Unknown | No |
| Leonard et al. [2] | VII | 1 | Male | 22 | Ingestion (40 g) | Arrhythmia Cardiac arrest Hypotension Metabolic acidosis | Yes | Mechanical ventilation | No |
| 1C. Secondary exposure of emergency medical personnel | |||||||||
| Publication | Level of evidence | Number of cases | Gender | Age | Exposure route | Clinical features | Hospitalization | Treatment | Survival |
| Hirose et al. [73]c | VII | 6 | Unknown | Unknown | Inhalation | Burning eyes Dizziness Dyspnea Headache | No | Unknown | Yes |
| Downes et al. [58]a | VII | 10 | 5 Males 5 Females |
39 (median) | Inhalation and dermal contact | 1 case reported fatigue 1 case reported stress | No | Time off from work for the two cases | Yes |
| 1D. Unknown exposure | |||||||||
| Publication | Level of evidence | Number of cases | Gender | Age | Exposure route | Clinical features | Hospitalization | Treatment | Survival |
| Hirose et al. [73]c | VII | 7 | 1 Female 6 Males |
22 20 24 24 43 55 |
Ingestion | Dizziness Hypotension Palpitations Paresthesias Syncope |
Yes | Gastric lavage Inhaled amyl nitrite Intravenous sodium nitrite Intravenous sodium thiosulfate Vasopressors | Yes |
| Schwarz et al. [74] | VII | 5 | 3 Females 2 Males |
Median age not available | Ingestion | Arrhythmia Headache Hypotension |
Yes | Intravenous fluids Oral antiemetics | Yes |
They are grouped based on mode of azide exposure (i.e., accidental, suicidal, secondary, and unknown, letters A through D, respectively) and sub-grouped based on exposure setting (numbers 1 through 4). Unless indicated, an entry is a primary patient.
Downes et al. [58] reported a total of 11 patients. The primary victim committed suicide by azide ingestion and is described in Table 1(B-1). Ten medical personnel who treated the patient are described in Table 1(C).
Although not in the abstract, we contacted the corresponding author who confirmed the patient ingested azide to commit suicide.
Ten patients were exposed to azide and hospitalized. Hirose worked at the hospital where seven of the patients were treated. Hence, the paper provided data on only those seven victims; they are summarized in Table 1(D) (information was not provided on whether poisoning was intentional or accidental). Six medical personnel who treated the seven azide-poisoned patients presented symptoms consistent with low-dose azide exposure, likely via inhaling HN3 gas released when performing gastric lavage on the patients. They are described in Table 1(C).
Table 2.
Review of 17 news articles yielded 50 cases.
| 2A-1. Accidental exposure due to industrial work | |||||||
| Publication | Number of cases | Gender | Age | Exposure route | Clinical features | Hospitalization | Survival |
| Perkins [37] | 1 | Male | 45 | Dermal contact | Burned >15% of body | Yes | No |
| 2A-2. Accidental exposure in a laboratory | |||||||
| Publication | Number of cases | Gender | Age | Exposure route | Clinical features | Hospitalization | Survival |
| Green [41] | 1 | Male | Unknown | Dermal Contact (65 g) | Burns | Yes | Yes |
| Author unknown [44] | 1 | Male | Unknown | Ingestion | Unknown | Yes | Yes |
| Lillington [40] | 11 | Unknown | Unknown | Inhalation | Unknown | Yes | Yes |
| Crabbe [42]a | 1 | Male | 27 | Dermal contact | Facial burns Glass embedded in chest and abdomen Minor lacerations | Yes | Yes |
| Kemsley [43] | 1 | Male | Unknown | Dermal contact (200 g) | Second-degree burns Minor lacerations | Yes | Yes |
| Author unknown [45] | 1 | Male | Unknown | Dermal contact | Minor lacerations | Yes | Yes |
| 2A-3. Accidental exposure in a medical facility | |||||||
| Publication | Number of cases | Gender | Age | Exposure route | Clinical features | Hospitalization | Survival |
| Author unknown [48] | 1 | Male | 66 | Ingestion (1 –5 g) | Rapid deterioration Vomiting | Yes | No |
| 2B. Suicide in a non-laboratory setting | |||||||
| Publication | Number of cases | Gender | Age | Exposure route | Clinical features | Hospitalization | Survival |
| DeMare [67]b | 1 | Female | 32 | Ingestion | Unknown | No (found dead) | No |
| Stout [68]c | 1 | Female | 25 | Ingestion | Unknown | Yes | No |
| Bender [66] | 1 | Female | 71 | Ingestion | N/A | No (found dead) | No |
| Singh [69] | 1 | Male | 21 | Ingestion | Unknown | Yes | No |
| Hicks [70]d | 1 | Male | 27 | Unknown | N/A | No (found dead) | No |
| 2C. Homicide | |||||||
| Publication | Number of cases | Gender | Age | Exposure route | Clinical features | Hospitalization | Survival |
| Author unknown [48] | 3 | Unknown | Unknown | Ingestion (20 g) |
Unknown | 1 Yes 2 No |
Yes |
| State of Arizona, Appellee, v. Wendi Elizabeth Adriano, Appellant [72] | 1 | Male | 33 | Ingestion (possibly 21 g) | N/A | No (found dead) | No |
| 2D. Secondary occupational exposure of medical personnel | |||||||
| Publication | Number of cases | Gender | Age | Exposure route | Clinical features | Hospitalization | Survival |
| DeMare [67]b | 5 | Unknown | Unknown | Unknown | Minor respiratory symptoms | Yes | Yes |
| Crabbe [42]a | 1 | Male | 25 | Dermal contact | Facial and corneal burns | Yes | Yes |
| Stout [68]c | 6 | Unknown | Unknown | Unknown | Unknown | Yes | Yes |
| Hicks [70]d | 1 | Unknown | Unknown | Unknown | Unknown | Yes | Yes |
| 2E. Unknown exposure | |||||||
| Publication | Number of cases | Gender | Age | Exposure route | Clinical features | Hospitalization | Survival |
| Author unknown [75] | 6 | 1 Male 1 Female 4 Unknown |
Unknown | Ingestion | Dizziness Syncope Tinnitus |
Yes | Yes |
| Author unknown [76] | 4 | Unknown | Unknown | Ingestion | Dizziness Lightheadedness |
Yes | Yes |
They are grouped based on mode of azide exposure (i.e., accidental, suicidal, homicidal, secondary, and unknown, letters A through E, respectively) and sub-grouped based on exposure setting (numbers 1 through 3). Unless indicated, an entry is a primary patient.
Crabbe [42] reported two patients. The primary victim was exposed to azide in a laboratory explosion and is described Table 2(A-2). A firefighter was exposed to azide while responding to the primary victim and is described in Table 2(D).
DeMare [67] reported six patients. The primary victim committed suicide by ingesting azide and is described Table 2(B). Five emergency response personnel who came into contact with the primary victim are described in Table 2(D).
Stout [68] reported seven patients. The primary victim committed suicide by ingesting azide and is described Table 2(B). Six emergency response personnel who came into contact with the primary victim are described in Table 2(D).
Hicks [70] reported two patients. The primary victim committed suicide with azide via an unknown route and is described Table 2(B). Among personnel who responded to the victim, one officer was hospitalized for observation and is described in Table 2(D).
Accidental exposure to azide
In 77 cases, victims were accidentally exposed to NaN3, either from airbag deployment after a vehicular accident, or in an industrial, laboratory, or medical setting. Three victims died.
Airbag deployment: peer-reviewed papers
In 13 patients, azide exposure occurred from airbag deployment (Table 1(A-1) [9,24–33]). The patients developed symptoms after either inhaling or having dermal or ocular contact with undecomposed NaN3 released when an airbag ripped during a vehicular accident. Seven people had chemical burns and one person was diagnosed with contact dermatitis [24–27,31,33]. Five patients without prior history of respiratory disease developed pulmonary symptoms such as dyspnea or stridor, and two were diagnosed with chemical pneumonitis [9,28–30,32]. Three patients sustained ocular injury [9,31,32].
Industrial exposure: peer-reviewed papers
Three papers described 43 patients who were accidentally exposed to azide at work, presenting symptoms ranging from mild to severe, including one death (Table 1(A-2) [34,35,36]). One paper was a case-control survey of 41 workers who consistently inhaled more than the legal limit of 0.3mg/m3 of NaN3 over a five-year period. The case subjects reported more events of burning eyes, dizziness, headache, and palpitations than control subjects [34]. In the second paper, a patient working in a poorly ventilated NaN3 packing factory was hospitalized for diplopia, dizziness, paresthesias, and severely reduced muscle strength that immobilized him for three months [35]. He partially recovered muscle strength, but was left with moderate neurological dysfunction. The last paper described a patient who sustained third-degree burns when his forklift accidently struck a 50-gallon barrel containing NaN3 waste, resulting in an explosion [36]. He subsequently developed hypotension and metabolic acidosis, and died, likely from both the azide exposure and the severe burns.
Industrial exposure: news articles
A worker died in an industrial explosion. He was cleaning out a filter drum used to produce NaN3 when the drum exploded, causing burns to >15% of his body (Table 2(A-1) [37]). Less than five years earlier, another azide-induced explosion at the same plant injured four workers, one of whom died [38].
Laboratory exposure: peer-reviewed paper
A chemist was exposed to the equivalent of 13 g (185 mg/kg) of azide while performing an experiment using NaN3 that led to an explosion (Table 1(A-3) [39]). He suffered thermal and chemical burns and lacerations. On hospitalization, he rapidly deteriorated with profound hypotension. He survived, but his left hand required amputation. Again, his clinical presentation was likely secondary to the combination of azide poisoning and severe burns.
Laboratory exposure: news articles
Six articles described 16 cases of accidental exposure among laboratory workers (Table 2(A-2) [40–45]). A clinical immunology technician dropped a 200-mL bottle containing a weak solution of NaN3 that generated volatile HN3. Out of concern, 11 workers were hospitalized but subsequently returned to work without complications [40]. The other five cases involved chemists and were less benign. A graduate student did not follow proper safety procedures, and ingested an azide solution; he became ill, but survived [44]. In four cases, chemical reactions with NaN3 resulted in explosions, leaving the operators with superficial burns and minor lacerations. Three explosions were caused by negligence using azide amounts that exceeded safety limits [41,43,45].
Exposure in a medical facility: peer-reviewed papers
Two papers described accidental azide ingestion in a medical facility (Table 1(A-4)). In one case, a dentist ingested ~10 mg NaN3. At this low dose, she experienced generalized seizures, headache, and vomiting within minutes [46]. In the second case, a hospitalized patient drank a solution containing 1 g NaN3 that was meant as a urine preservative [47]. She quickly developed generalized seizures, hypotension, and metabolic acidosis. Both patients survived, likely due to a relatively low azide dose in the first case and a rapid and aggressive medical response in the second case.
Exposure in a medical facility: news article
A patient died after swallowing an azide tablet thought to be a painkiller (Table 2(A-3) [48]). He started vomiting and rapidly deteriorated 30 min after ingesting the tablet, and died the next day. The source of the azide tablet was unknown.
Intentional exposure to azide
We found 24 cases of suicidal ingestion of NaN3 (Tables 1(B-1), 1(B-2), and 2(B)). Thirteen cases occurred inside a laboratory (Table 1(B-1)). We also found four cases of homicidal exposure to NaN3 (Table 2(C)). Of these 28 cases, 19 of the patients died.
Suicide in a laboratory setting: peer-reviewed papers
Of 13 people who intentionally ingested NaN3, two were found dead, and the remaining 11 were hospitalized, of whom six later died, leaving five survivors (Table 1(B-1) [49–60]). Many of the patients were hypotensive with metabolic acidosis, consistent with high-dose azide exposure. When the amount of ingested azide could be identified, the highest survivable dose was 1.38 g (patient’s weight was unknown) [60].
Suicide in a non-laboratory setting: peer-reviewed papers
Of six cases of suicidal ingestion of NaN3, two of the patients were found dead (Table 1(B-2) [2,61–65]). The remaining four patients were hospitalized, and three died, of whom two were hypotensive and two had metabolic acidosis. The one surviving patient was particularly notable: a 69 year-old female who ingested ~5 g of NaN3, about 20-fold more than the estimated lethal human dose, was not hypotensive but had reduced myocardial contraction with a left ventricular ejection fraction of 30% that returned to normal three weeks later [63].
Suicide in a non-laboratory setting: news articles
Five people who ingested NaN3 in a non-laboratory setting all died, three were found dead, while the other two died after hospitalization (Table 2(B) [66–70]). In one case, NaN3 was found in the victim’s car, but it was unclear if he died due to ingesting azide or inhaling hydrazoic acid, which could have been created by mixing NaN3 with water [70].
Homicides: news articles
Two articles described four homicidal cases (Table 2(C)). A chemist hoping to cause workplace havoc poisoned three of his colleagues’ coffee with ~20 g of NaN3 [71]. Due to an unusual smell, the victims immediately spat out the coffee, but two of them became mildly ill and the third fell unconscious. In the fourth case, a woman bought NaN3 to poison her husband to claim his life insurance policy [72]. She also stabbed and bludgeoned him. It was unclear how much azide contributed to his death, but it was found in his blood.
Secondary occupational exposure of medical personnel
We found 29 cases of medical personnel becoming secondarily poisoned while treating azide-poisoned victims. All of the personnel lived.
Secondary exposure of medical personnel: peer-reviewed papers
Ten medical workers were likely exposed to azide from a suicidal patient who was not decontaminated prior to hospitalization (Table 1(C) [58]). Three non-hospital staff who either provided prehospital care or transported the patient to the hospital were admitted to the emergency room for evaluation and were subsequently discharged, presumably without symptoms. But, of seven hospital personnel who were in direct care of the patient, two required time off. One person took a day off on supervisory advice to rest due to fatigue. The second person required several weeks off due to psychological stress from exposure to a toxic chemical. In a separate incident, six hospital personnel likely inhaled HN3 gas liberated while performing gastric lavage on patients who had drank an azide-poisoned beverage at work (Table 1(C) [73]). They developed ocular irritation, dizziness, dyspnea, and headache, symptoms consistent with low-dose azide exposure, but promptly recovered.
Secondary exposure of medical personnel: news articles
Thirteen medical workers were secondarily exposed (Table 2(D) [42,67,68,70]). One case was particularly notable: a firefighter had to be treated for facial and eye burns after responding to an azide-related university laboratory explosion [42]. Although he was wearing a protective face mask, it was postulated that azide powder contacted his face above the mask, where it reacted with sweat that then dripped down onto his eye.
Unknown exposure
Peer-reviewed papers
Seven workers had to be hospitalized after becoming ill from drinking tea or coffee made from azide-contaminated water, and five people at a restaurant drank azide-tainted iced-tea (Table 1(D) [73,74]). Symptoms occurred within minutes, which included arrhythmias, headache, and hypotension. There were no fatalities.
News articles
A total of 10 people at two different medical schools drank from an azide-tainted coffee pot or coffee machine (Table 2(E) [75,76]). All of the victims survived, although they reported symptoms consistent with low-dose azide exposure.
Treatment
No specific azide antidote currently exists. Therefore, treatments were largely supportive and in response to symptoms. Gastric lavage, with or without activated charcoal, was used in 13 patients, one of whom died [46,47,50,54,55,63,73]. The NaN3 dose in these cases ranged from 0.1 g to 1.38 g, with the outlier being the one patient who survived 15 g [63].
Twenty-three patients presented with hypotension [2,36,39,47,53,54,57–60,64,73,74]. They received intravenous fluids and/or vasopressors, with some patients requiring large amounts of fluids, e.g., up to 32 L over 12 h [36]. The hypotension was presumably secondary to azide conversion to nitric oxide. Fifteen patients presented with metabolic acidosis, likely due to profound hypotension and anaerobic metabolism to compensate for mitochondrial inhibition [2,36,47,49,50,52–55,57–60,63]. In some cases, acidosis was severe enough that sodium bicarbonate was administered [36,50,53,54]. Two patients underwent hemodialysis, one of whom required an intra-aortic ballon pump to maintain blood pressure; both patients survived [47,60]. One patient who underwent exchange transfusion died [53].
Due to mechanistic similarities between azide and cyanide, many patients were given cyanide antidote(s). Ten patients were treated with sodium thiosulfate, amyl nitrite, and/or sodium nitrite, of whom two died [52,53,73]. One patient treated with hydroxocobalamin survived [57].
Persons who had respiratory distress due to inhaling hydrazoic acid or sodium azide dust were treated with corticosteroids and β-agonists [28,29]. People who had azide contact to skin or eyes received general treatment for a chemical exposure, which included steroids and antibiotics [9,24–27,31–33].
Discussion
We found 156 cases in 37 peer-reviewed papers and 17 news articles over the 20-year period between 2000 and 2020, providing a mean of 7.8 cases per year. For comparison, Chang and Lamm found 185 cases over a 72-year period, or an average of 2.6 cases per year. Our rate could be higher due to better reporting and/or more cases, the latter presumably because of greater azide production in the last 30 years and/or the ease of purchasing sodium azide online.
In the Chang and Lamm review, three people were exposed to sodium azide in vehicular accidents. We found 13 people exposed to sodium azide during airbag deployment. The increase in reported cases is likely because all automobiles sold in the U.S. after September 1, 1998 had to have airbags around the front seat. Five persons developed respiratory symptoms after airbag deployment. These five cases are consistent with reports that both sodium azide and hydrazoic acid can cause respiratory distress in animals [15]. Although azide was the likely cause of the respiratory problems, airbags contain other substances such as talcum powder, sodium carbonate, and sodium hydroxide that could cause pulmonary injury. Seven patients exposed to azide during airbag deployment had evidence of dermal burns and three of the patients had evidence of ocular injury. Even though many airbag manufacturers have stopped using azide, it still persists in airbags in many cars; thus, physicians need to be aware of potential respiratory, cutaneous, and ocular injuries due to azide exposure from airbag deployment.
Accidental industrial exposure to azide is almost inevitable, since numerous factories either produce sodium azide, or use it in manufacturing processes. Two of the 44 cases of industrial exposure died from azide-related explosions. Azide is shock-sensitive, and vibrations occurring when azide-containing drums were moved may have led to the explosions. Several patients developed neurological symptoms, and neurotoxicity secondary to azide exposure has occurred in other human cases and in animal models [77,78]. Care should be taken to minimize azide inhalation through use of face coverings and working in well-ventilated spaces, as well as minimizing mechanical friction that could trigger azide explosions.
Azide-related laboratory accidents occurred in 17 cases. Several cases seem to have been the result of safety negligence. While no death occurred, several victims sustained serious injury, highlighting the need to be vigilant when working with azide.
Three cases of accidental azide ingestion occurred in a medical facility; one of whom died. Sodium azide is an ordinary white powder that when dissolved in water, becomes a clear, odorless solution. It is thus possible that sodium azide could be confused as medicine and that azide solutions could be mistaken for drinking water. While accidental ingestion of azide in a medical facility is rare, azide is commonly used as a preservative, and its use must be monitored closely.
Chang and Lamm reported azide was used as a suicidal agent by 13 people, all of whom died. We found 24 suicidal cases, of whom 18 died. Death due to azide is dependent on dose and the time between exposure and treatment onset. The latter parameter was impossible to ascertain in many cases. Thirteen of the 24 cases were scientific workers who ingested azide in their laboratories and likely had direct access to the chemical. This, in conjunction with 17 cases of accidental laboratory exposure to azide, indicates laboratory workers constitute a high-risk group with regards to azide poisoning.
We found four attempted homicides using azide. Three cases were laboratory workers who did not sustain serious injury. In another incident, the victim was poisoned by his wife who purchased azide online. These cases highlight the danger of azide in the hands of those with malicious intentions.
Our findings reveal medical personnel are at high risk for azide exposure, 29 people became secondarily poisoned while caring for azide-poisoned patients. Although the risk of serious injury is low, several providers reported dizziness, dyspnea, headache, ocular irritation, and fatigue, symptoms compatible with low dose azide exposure. Medical response personnel need to be aware of the consequences of azide poisoning, both in caring for azide-poisoned victims and to protect themselves from secondary exposure.
Twenty-two people were poisoned after drinking from communal beverage sources. While no long-term sequalae occurred due to low exposure dose and rapid care, it was unclear if the poisoning events were accidental or intentional. The latter highlights the potential threat of large-scale azide exposure due to a terrorist attack, especially considering azide’s online availability and ease of synthesis.
Treatment varied, depending on the scenario. Gastric lavage with or without activated charcoal may not be effective, because it is not known how long sodium azide, which is water soluble and generally ingested as a solution, would remain in the stomach, and it is unclear if azide actually binds to charcoal [79,80].
Since azide can cause profound hypotension, supportive care with vasopressors and large amounts of intravenous fluids is frequently needed. Two patients underwent hemodialysis, one of whom also received an intra-aortic balloon pump to maintain blood pressure [47,60]. The former treatment may be useful, because NaN3 is a small molecule (65 kDa).
Although both azide and cyanide inhibit cytochrome C oxidase, it seems unlikely that sodium thiosulfate, amyl nitrite, or sodium nitrite would be useful against azide poisoning. Sodium thiosulfate detoxifies cyanide by converting it to thiocyanate; it seems unlikely that sodium thiosulfate would react with azide. Amyl nitrite and sodium nitrite generate methemoglobin, which scavenges cyanide, but methemoglobin binds azide only weakly (log Kobserved = 5.427 at pH 7.02) [81]. Moreover, nitrites could be detrimental, because they generate nitric oxide, which could exacerbate azide-induced hypotension [82,83].
Hydroxocobalamin appears to be a reasonable treatment for azide poisoning, because cobalamin binds nitric oxide, and high doses of cobalamin raise blood pressure in control subjects, likely via reducing plasma nitric oxide concentrations [84,85]. Thus, cobalamin could potentially raise the blood pressure in azide-poisoned hypotensive patients.
Limitations
While two-thirds of the sources we included were peer-reviewed, all but one paper were case reports, which are prone to publication bias and therefore limited generalizability. The single case-control study relied on a work-medical history questionnaire, which is susceptible to recall bias.
As in other reviews of clinical cases, we were limited by whether someone chose to publish a case and whether a diagnosis was made appropriately. The former is exemplified in Hirose et al. where data on only seven of 10 azide-poisoned victims were provided [73]. The latter may be particularly problematic for azide poisoning, since it can be mistaken for cyanide poisoning due to mechanistic and symptomatic similarities. Taken together, the actual incidence of azide poisoning is likely to be greater than that reported. The cases reported in newspaper articles generally did not describe treatment strategies, limiting our ability to tie treatment to outcome.
Conclusions
Multiple routes of azide exposure exist, resulting in a variety of symptoms and range of disease severity. Treatment depends largely on the type, site, and degree of injury, and success can be ambiguous. Preventing or minimizing exposure, especially among high-risk persons, is the best strategy.
Laboratory workers should perform risk assessments to identify potential areas of concern prior to working with azide, and consideration should be given to limiting access to azide to prevent accidental or intentional ingestion. Concentrated azide solutions should not be exposed to shock or friction, or come into contact with heavy metals due to the possibility of explosion.
For medical personnel, the most likely exposure routes are inhalation of hydrazoic acid expired by (or lavaged from) a patient, dermal contact from sodium azide dust left on patients or their clothing, or eye exposure to hydrazoic acid or sodium azide dust. First responders should therefore wear safety goggles and gloves, and use CPR barrier devices where azide poisoning is possible. Moreover, any person suspected of azide poisoning should be decontaminated prior to hospitalization according to standard Hazmat protocols.
For severe systemic exposures to azide, hemodynamic support is clearly important, with some patients requiring aggressive therapy. Quantitative information on appropriate drugs is lacking, but, hydroxocobalamin is a rational choice based on its mechanism of action and relatively good safety profile.
Funding
This work was supported in part by the NIH CounterACT Program, Office of the Director, NIH, and NINDS grants U01NS058030 and U01NS087964 to GRB, and partial support from NIH/NIGMS IRACDA K12 grant GM068524 to JT. The authors thank Dr. S. Wang for Chinese translation.
Footnotes
Disclosure statement
The authors report no conflict of interest.
References
- [1].Holstege CP, Bechtel LK, Reilly TH, et al. Unusual but potential agents of terrorists. Emerg Med Clin North Am. 2007;25(2): 549–566. [DOI] [PubMed] [Google Scholar]
- [2].Leonard J, Ripple M, Hines EQ. Prime eligible and only $17.00 on Amazon (R): fatal sodium azide poisoning. Clin Toxicol. 2018;56: 991–992. [Google Scholar]
- [3].The Japan Times. Poisonings uncover lax sodium azide controls. The Japan Times. 1998. Oct 30. Available from: https://www.japan-times.co.jp/news/1998/10/30/national/poisonings-uncover-lax-sodium-azide-controls/#.W1YN3NJKjIU.
- [4].Zambito T. Man admits making deadly toxins Jersey City expharmacist also stockpiled cache of weapons. The Star-Ledger. 2014. Available from: https://infoweb.newsbank.com/apps/news/document-view?p=AWNB&docref=news/14E2312D8C23ECE8
- [5].Agence France-Presse. Czech embassy in Slovakia gets poisoned mail. Agence France-Presse; 2014. [cited 2019 Nov 12]. Available from: https://infoweb.newsbank.com/apps/news/document-view?p=AWNB&docref=news/1522EAF0A6ED0AC8. [Google Scholar]
- [6].Pankratz H. Raided building evacuated again Chemist may have poured compounds in sink. The Denver Post, (Denver, CO); 2002. [cited 2019 Nov 13]; B-02. Available from: https://infoweb.newsbank.com/apps/news/document-view?p=AWNB&docref=news/0F575FDC8AC5FBC4.
- [7].US Fed News (USA). Pipe bomb crafted from vehicle airbag gets birmingham man 10 years in federal prison. US Fed News (USA); 2014. [cited 2019 Nov 13]. Available from: https://infoweb.newsbank.com/apps/news/document-view?p=AWNB&docref=news/14FFB2D572480648. [Google Scholar]
- [8].McKeen S. High Production Volume (HPV) chemicals. Paris (France): The Organization for Economic Co-Operation and Development; Environment Directorate; 2010. [Google Scholar]
- [9].Francis D, Warren SA, Warner KJ, et al. Sodium azide-associated laryngospasm after air bag deployment. J Emerg Med. 2010;39(3): e113–e115. [DOI] [PubMed] [Google Scholar]
- [10].Breslin M. Recylers face challenges with non-deployed OEM airbags; 2014. Available from: https://americanrecycler.com/8568759/index.php/news/category-news-2/543-recyclers-face-challenges-with-non-deployed-oem-airbags.
- [11].Hazardous Waste Experts. Airbag hazardous waste: unintended consequence to a decades-old safety regulation; 2017. [updated 2017 Mar 01]. Available from: https://www.hazardouswasteexperts.com/airbag-hazardous-waste/.
- [12].Betterton EA. Environmental fate of sodium azide derived from automobile airbags. Crit Rev Environ Sci Technol. 2003;33(4): 423–458. [Google Scholar]
- [13].Loew O. Ueber das Verhalten des Azoimids zu lebenden Organismen. Ber Deut Chem Ges. 1891;24:2947–2953. [Google Scholar]
- [14].Chang S, Lamm SH. Human health effects of sodium azide exposure: a literature review and analysis. Int J Toxicol. 2003;22(3): 175–186. [DOI] [PubMed] [Google Scholar]
- [15].Graham JDP, Li DMF. Actions of sodium azide. Br J Pharmacol. 1973;49(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Keilin D, Hartree E. Inhibitors of catalase reaction. Nature. 1934; 134(3398):933–933. [Google Scholar]
- [17].Stannard J, Horecker B. The in vitro inhibition of cytochrome oxidase by azide and cyanide. J Biol Chem. 1948;172(2):599–608. [PubMed] [Google Scholar]
- [18].Frawley KL, Totoni SC, Bae Y. A comparison of potential azide antidotes in a mouse model. Chem Res Toxicol. 2020;33(2): 594–603. [DOI] [PubMed] [Google Scholar]
- [19].Bramer WM, Giustini D, de Jonge GB, et al. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc. 2016;104(3):240–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jobelius HH, Scharff HD. Hydrazoic acid and azides. Ullmann’s encyclopedia of industrial chemistry. New York: Wiley; 2000. [Google Scholar]
- [21].Bräse S, Mende M, Jobelius HH, et al. Hydrazoic acid and azides. Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim, Germany: Wiley-VCH; 2015. p. 1–11. [Google Scholar]
- [22].Ackley BJ. Evidence-based nursing care guidelines: medical-surgical interventions. St. Louis (MO): Elsevier Health Sciences; 2008. [Google Scholar]
- [23].Mazurek Melnyk B. A focus on adult acute and critical care. Worldviews Evid Based Nurs. 2004;1(3):194–197. [Google Scholar]
- [24].Corazza M, Bacilieri S, Morandi P. Airbag dermatitis. Contact Derm. 2000;42(6):367–368. [PubMed] [Google Scholar]
- [25].Foley E, Helm TN. Air bag injury and the dermatologist. Cutis. 2000;66(4):251–252. [PubMed] [Google Scholar]
- [26].Wu JJ, Sanchez-Palacios C, Brieva J, et al. A case of air bag dermatitis. Arch Dermatol. 2002;138(10):1383–1384. [DOI] [PubMed] [Google Scholar]
- [27].Suhr M, Kreusch T. Burn injuries resulting from (accidental) airbag inflation. J Craniomaxillofac Surg. 2004;32(1):35–37. [DOI] [PubMed] [Google Scholar]
- [28].Hambrook DW, Fink JN. Airbag asthma: a case report and review of the literature. Ann Allergy Asthma Immunol. 2006;96(2): 369–372. [DOI] [PubMed] [Google Scholar]
- [29].Caudle JM, Hawkes R, Howes DW, et al. Airbag pneumonitis: a report and discussion of a new clinical entity. CJEM. 2007;9(6): 470–473. [DOI] [PubMed] [Google Scholar]
- [30].Govindarajan R, Ferrer G, Smolley LA, et al. Airbag pneumonitis. Case Rep Med. 2010;2010:498569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sever C, Külahçi Y, Öksüz S, et al. Airbag-related burns. Akademik Acil Tip Olgu Sunumlari Dergisi. 2013;12(4):225–228. [Google Scholar]
- [32].BelhadJ-Tahar H, Coulais Y, Sadeg N. Sodium azide-related chemical burn after air-bag deployment. Proceedings of the IVth Mediterranean Academy of Forensic Sciences Meeting; 2009; Antalya, Turkey. [Google Scholar]
- [33].Corazza M, Trincone S, Virgili A. Cutaneous lesions induced by air bags. Annali Italiani di Dermatologia Allergologica. Clinica e Sperimentale. 2002;56(1):27–29. [Google Scholar]
- [34].Miljours S, Braun CM. A neuropsychotoxicological assessment of workers in a sodium azide production plant. Int Arch Occup Environ Health. 2003;76(3):225–232. [DOI] [PubMed] [Google Scholar]
- [35].Fang L, Shao Z-H, Yu Q-Q. One case of occupational sodium azide poisoning. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2011;29(5):397–398. [PubMed] [Google Scholar]
- [36].Pham T, Palmieri TL, Greenhalgh DG. Sodium azide burn: a case report. J Burn Care Rehabil. 2001;22(3):246–248. [DOI] [PubMed] [Google Scholar]
- [37].Perkins N. Iron County chemical plant safety under investigation. The Deseret News. 2002. Apr 16 [cited 2019 Nov 13]; B07. Available from: https://infoweb.newsbank.com/apps/news/document-view?p=AWNB&docref=news/0F37F22E5F8E9583.
- [38].Reece ML. Victim of Cedar explosion knew risks. Deseret News; 1997. [1997 Sep 02]. Available from: https://www.deseret.com/1997/8/2/19326965/victim-of-cedar-explosion-knew-risks.
- [39].Angelotti T, Mireles S, McMahon D. Anesthetic implications of a near-lethal sodium azide exposure. Anesth Analg. 2007;104(1): 229–230. [DOI] [PubMed] [Google Scholar]
- [40].Lillington C. 11 Screened for chemical spill at uni. Birmingham Mail (England). 2010. Sep 05 [cited 2019 Nov 13]; 11. Available from: https://infoweb.newsbank.com/apps/news/document-view?p=AWNB&docref=news/1316AD4092CA3610.
- [41].Green P. Manchester evening news: blast probe uncovers danger chemical store. Manchester Evening News (England). 2008. Sep 10 [cited 2019 Nov 13]. Available from: https://infoweb.newsbank.com/apps/news/document-view?p=AWNB&docref=news/12322D22B6D98CB8.
- [42].Crabbe N. Glass embedded in student’s chest, abdomen in lab explosion. The Gainsville Sun (FL) 2012. Jan 18. [Google Scholar]
- [43].Kemsley J. C&EN talks safety with Minnesota Chemistry Chair William B. Tolmane. Chem Eng News. 2014. 34. [Google Scholar]
- [44].Author unknown. KU grad student critically injured after ingesting toxic chemical. The Kansas City Star (MO). 2009. Nov 05 [cited 2019 Nov 13]. Available from: https://infoweb.newsbank.com/apps/news/document-view?p=AWNB&docref=news/12C856B2E0399E58.
- [45].Author unknown. Lessons learned: azide synthesis explosion: The University of Akron; 2017. Available from: https://www.uakron.edu/cpspe/documents/LessonsLearned-1117.pdf. [Google Scholar]
- [46].Demircan A, Özsarac M, Karamercan MA, et al. Following accidental low dose sodium azide ingestion-case report. Akademik Acil Tip Olgu Sunumlari Dergisi. 2011;10(1):41–42. [Google Scholar]
- [47].Watanabe K, Hirasawa H, Oda S, et al. A case of survival following high-dose sodium azide poisoning. Clin Toxicol (Phila) 2007; 45(7):810–811. [DOI] [PubMed] [Google Scholar]
- [48].Author unknown. Drug mix-up kills heart patient. The Yomiuri Shimbun/Daily Yomiuri (Tokyo, Japan). 2002. Mar 3 [cited 2019 Nov 13]. Available from: https://infoweb.newsbank.com/apps/news/document-view?p=AWNB&docref=news/0F26E6A3837546AC.
- [49].Spadafora M, Gummin D, Cichon M, et al. Case report: case discussion. Internet J Med Tox. 2000;3(3):19. [Google Scholar]
- [50].Senda T, Nishio K, Hori Y, et al. A case of fatal acute sodium azide poisoning. Chudoku Kenkyu: Chudoku Kenkyukai Jun Kikanshi. 2001;14(4):339–342. [PubMed] [Google Scholar]
- [51].Fuyuno I, Cyranoski D. Mystery surrounds lab death. Nature. 2006;443(7109):253. [DOI] [PubMed] [Google Scholar]
- [52].Łopaciński B, Kołacinski Z, Winnicka R. Sodium azide–clinical course of the poisoning and treatment. Przegl Lek. 2007;64(4–5): 326–330. [PubMed] [Google Scholar]
- [53].Meatherall R, Palatnick W. Convenient headspace gas chromatographic determination of azide in blood and plasma. J Anal Toxicol. 2009;33(8):525–531. [DOI] [PubMed] [Google Scholar]
- [54].French L, Hendrickson R, Horowitz B. Sodium azide ingestion associated with qrs prolongation. Clin Toxicol; 2012;50:273–366. [Google Scholar]
- [55].Kostek H, Sawiniec J, Lewandowska-Stanek H, et al. [Sodium azide poisoning–a rare reason of hospitalization in toxicological units-case report]. Przeglad Lekarski. 2012;69(8):565–567. [PubMed] [Google Scholar]
- [56].Le Blanc-Louvry I, Laburthe-Tolra P, Massol V, et al. Suicidal sodium azide intoxication: an analytical challenge based on a rare case. Forensic Sci Int. 2012;221(1–3):e17–e20. [DOI] [PubMed] [Google Scholar]
- [57].Bartecka-Mino K, Schiel H, Holzer A, et al. Hydroxocobalamin: an antidote for sodium azide poisoning? Clin Toxicol. 2014;52: 295–443. [Google Scholar]
- [58].Downes MA, Taliana KE, Muscat TM, et al. Sodium azide ingestion and secondary contamination risk in healthcare workers. Eur J Emerg Med. 2016;23(1):68–70. [DOI] [PubMed] [Google Scholar]
- [59].Muvalia G, Ekka M, Jamshed N, et al. Fatal suicidal toxicity by sodium azide: a case report. Clin Toxicol. 2020;58(4):304–354. [Google Scholar]
- [60].Gao HT, McGlone SEM, Ly BT. Refractory vasoplegic shock after sodium azide ingestion treated with hemodialysis. J Med Toxicol. 2020;16(2):116–168. [Google Scholar]
- [61].Wiergowski M, Galer-Tatarowicz K, Krzyzanowski M, et al. Suicidal intoxication with sodium azide–a case report. Przegl Lek. 2012; 69(8):568–571. [PubMed] [Google Scholar]
- [62].Meatherall R, Oleschuk C. Suicidal fatality from azide ingestion. J Forensic Sci. 2015;60(6):1666–1667. [DOI] [PubMed] [Google Scholar]
- [63].Overtchouk P, Poissy J, Thieffry C, et al. Surviving a massive sodium azide poisoning with toxic cardiomyopathy. Int Cardiovascular Forum J. 2015;4:90–91. [Google Scholar]
- [64].Rojek S, Hydzik P, Gomółka E, et al. Clinical and analytical problems of sodium azide poisonings as exemplified by a case of fatal suicidal poisoning. Arch Med Sadowej Kryminol. 2015;65(3): 145–157. [DOI] [PubMed] [Google Scholar]
- [65].Ciesla MM, Calello DP, Nelson LS. When the poisoned risk poisoning others: fatal sodium azide overdose. Emerg Med. 2018;50(6): 132–134 [Google Scholar]
- [66].Bender KJ. Police evacuate Berkeley City Club after hazardous materials call. Alameda Times-Star (CA) 2014. Mar 19 [cited 2019 Nov 13]. Available from: https://infoweb.newsbank.com/apps/news/document-view?p=AWNB&docref=news/14CAADC3345BC730. [Google Scholar]
- [67].DeMare C. 5 treated for chemical exposure. The Times Union (Albany, NY) 2005. Sep 30 [cited 2019 Nov 13]; B9. Available from: https://infoweb.newsbank.com/apps/news/document-view?p=AWNB&docref=news/10CF6B9DE5594780.
- [68].Stout M. Deadly mix easy to find online - suspected in student suicide. Boston Herald (MA) 2012. [cited 2019 Nov 13]; 25. Available from: https://infoweb.newsbank.com/apps/news/document-view?p=AWNB&docref=news/13E17C5AF3B0BE08.
- [69].Singh D. 21-year-old IIT-Bombay student found dead in suspected suicide case. Indian Express (India) 2015. [cited 2019 Nov 12]. Available from: https://infoweb.newsbank.com/apps/news/document-view?p=AWNB&docref=news/1551ADB8430A8C98.
- [70].Hick JP. Body, toxic chemicals found at beach. Ann Arbor News (MI). 2018. Nov 05 [cited 2019 Nov 12]; 003. Available from: https://infoweb.newsbank.com/apps/news/document-view?p=AWNB&docref=news/16F84ADAD2A963B8.
- [71].Author unknown. Chemist held after colleagues poisoned. The Yomiuri Shimbun/Daily Yomiuri (Tokyo, Japan) 2002. Aug 30 [cited 2019 Nov 13]. Available from: https://infoweb.newsbank.com/apps/news/document-view?p=AWNB&docref=news/0F5D3741D01CCE30.
- [72].State Arizona, Appellee, v. Wendi Elizabeth Andriano, Appellant, 2007. https://caselaw.findlaw.com/az-supreme-court/1015530.html
- [73].Hirose Y, Hata K, Honda H, et al. Clinical study of a sodium azide poisoning cluster. Nihon Kyukyu Igakukai Zasshi. 2001;12(3): 125–129. [Google Scholar]
- [74].Schwarz ES, Wax PM, Kleinschmidt KC, et al. Multiple poisonings with sodium azide at a local restaurant. J Emerg Med. 2014;46(4): 491–494. [DOI] [PubMed] [Google Scholar]
- [75].Author unknown. Harvard: Lab workers poisoned by tainted coffee. Times Argus, The (Montpelier-Barre, VT) 2009. Oct 26 [cited 2019 Nov 13]. Available from: https://infoweb.newsbank.com/apps/news/document-view?p=AWNB&docref=news/12B9718F2062C968.
- [76].Author unknown. Sodium azide may have caused illness at Yale School of Medicine in New Haven, officials say. New Haven Register (CT) 2017. Mar 08 [cited 2019 Nov 12]. Available from: https://infoweb.newsbank.com/apps/news/document-view?p=AWNB&docref=news/16305CE60C373280.
- [77].Graham J, Rogan J, Robertson D. Observations on hydrazoic acid. J Ind Hyg Toxicol. 1948;30(2):98–102. [PubMed] [Google Scholar]
- [78].Smith RP, Louis CA, Kruszyna R, et al. Acute neurotoxicity of sodium azide and nitric oxide. Toxicol Sci. 1991;17(1):120–127. [DOI] [PubMed] [Google Scholar]
- [79].Dyer J. Sodium Azide. In: Olson KR, Anderson IB, Benowitz NL, et al. , editor. Poisoning & Drug Overdose. New York (NY): McGraw-Hill; 2012. [Google Scholar]
- [80].Albertson T, Reed S, Siefkin A. A case of fatal sodium azide ingestion. J Toxicol Clin Toxicol. 1986;24(4):339–351. [DOI] [PubMed] [Google Scholar]
- [81].Beetlestone J, Irvine D. Reactivity differences between haemoglobins. Part VII. The effect of ionic strength on the affinity of methaemoglobins A and C for azide ion. A test of the dielectric cavity model for methaemoglobins. J Chem Soc, A. 1968:951–959. [Google Scholar]
- [82].Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7(2):156–167. [DOI] [PubMed] [Google Scholar]
- [83].Klein-Schwartz W, Gorman RL, Oderda GM, et al. Three fatal sodium azide poisonings. Med Toxicol Adverse Drug Exp. 1989; 4(3):219–227. [DOI] [PubMed] [Google Scholar]
- [84].Sharma VS, Pilz RB, Boss GR, et al. Reactions of nitric oxide with vitamin B12 and its precursor, cobinamide. Biochemistry. 2003; 42(29):8900–8908. [DOI] [PubMed] [Google Scholar]
- [85].Uhl W, Nolting A, Golor G, et al. Safety of hydroxocobalamin in healthy volunteers in a randomized, placebo-controlled study. Clin Toxicol. 2006;44(sup1):17–28. [DOI] [PubMed] [Google Scholar]


