Abstract
Background:
Estrogen fluctuations throughout the lifespan may contribute to major depressive disorder (MDD) risk in women through effects on brain networks important in stress responding, and mood regulation. Although there is evidence to support ovarian hormone treatment for peri-menopausal depression, postmenopausal use has not been well examined. The objective of this study was to investigate whether estrogen modulation of the neural and emotional cognitive responses to stress differs between postmenopausal women with and without MDD history.
Methods:
60 postmenopausal women completed an fMRI psychosocial stress task, after receiving no drug or 3 months of daily estradiol (E2). fMRI activity and subjective mood response were examined.
Results:
In women without a history of MDD, E2 was associated with a more negative mood response to stress and less activity in emotional regulation regions. In women with a history of MDD, E2 was associated with a less negative mood response to stress and less activity in emotion perception regions.
Limitations:
This study was limited by open-label estradiol administration and inclusion of participants using antidepressants.
Conclusions:
These results support a differential effect of estrogen on emotional and neural responses to psychosocial stress in postmenopausal women with MDD history and may reflect a shift in brain activity patterns related to emotion processing following menopause.
Keywords: stress, depression, menopause, estrogen, fMRI
Introduction
Between puberty and menopause, women have greater rates of major depressive disorder (MDD) than men (Bromet et al., 2011; Kessler et al., 2010), supporting that changes in estrogen may play a role in mood dysregulation in some women. However, the majority of women do not experience MDD. Moreover, most women who experience depression exhibit normal ovarian hormone levels (Schmidt & Rubinow, 2009; Young et al., 2000), indicating that depression is not simply associated with abnormal estrogen changes, but rather that estrogen fluctuations may contribute to mood disorders in women who are at increased risk for MDD from other vulnerability factors. Exposure to psychosocial stressors is one such vulnerability factor, but it is unclear whether estrogen fluctuations alter emotional and neural responses to stress, in turn contributing to depression vulnerability.
In young women, estrogen fluctuations across the menstrual cycle affect activity in brain areas important for emotional processing and responding to stress, including the insula, ventral anterior cingulate, and prefrontal regions (for review see Sundström-Poromaa, 2018; Toffoletto et al., 2014). We previously demonstrated that menstrual phase is associated with differential neural and mood responses to psychosocial stress, with less subjective stress and greater hippocampal activity observed during a stress task administered in a phase with high circulating estrogen (Albert et al., 2015). In young cycling women, estrogen supports emotion regulation and decreases negative mood responses to psychosocial stress.
Estrogen effects may differ after menopause. The menopausal transition and accompanying estrogen withdrawal present a time of increased depression vulnerability (Freeman et al., 2014). Estrogen replacement during the perimenopause has shown some success in reducing depressive symptoms (Rubinow & Schmidt, 2018). The effectiveness of estrogen replacement for depression in post-menopausal women has been less well-studied with few randomized controlled studies in women with current or past depression (Rubinow & Schmidt, 2018). In contrast to our work in young women, we previously found that estrogen administration in post-menopausal women with no history of MDD was associated with a more negative mood response to psychosocial stress (Dumas et al., 2012; Newhouse et al., 2008). It may be that women who are resilient to depression experience some mood lability during the menopause transition but adapt to the low estrogen state following menopause. In healthy women, estrogen administration may disrupt the post-menopause homeostatic state. However, in post-menopausal women with a history of peri-menopausal depression estrogen administration is associated with reduced depressive symptoms (Schmidt et al., 2015). This suggests that women with a vulnerability to depression may remain sensitive to the beneficial mood effects of estrogen following menopause.
Previously, we found that in women with no MDD history estrogen administration was associated with greater negative mood following psychosocial stress. We did not see this effect in women with a history of MDD, and instead estrogen was associated with less negative mood following experimental psychosocial stress (Albert et al., 2020). The current study sought to cross-sectionally examine the effects of MDD history and estrogen administration on brain activity measured with fMRI during experimentally induced stress. We hypothesized that women without a history of MDD would show greater stress-related activity in emotional control brain regions compared to women with a history of MDD and this activity would be reduced in women who received estrogen. Conversely, women with a history of MDD would show greater stress-related activity in emotional processing brain areas that would be reduced in women who received estrogen.
Material and Methods
Participants
Sixty euthymic postmenopausal women, including participants with no psychiatric history and a history of remitted MDD, were enrolled. Enrollment criteria were designed to include currently euthymic women with a past personal history of MDD and women with no history of MDD (see Supporting Information for enrollment criteria and screening procedures).
This study was approved by the Vanderbilt University Institutional Review Board and all participants provided informed consent. Participants were not informed that the study focused on psychosocial stress. Upon study activity completion participants were debriefed and re-consented; no participants withdrew consent following debriefing.
Estradiol Administration
Women were recruited to received open-label oral estradiol (E2) for 3 months or did not receive E2. This resulted in four participant groups: women with a history of depression who did (1: MDD+/E2+, n = 10) or did not (2: MDD+/E2-, n =12 ) receive E2; women without a history of depression who did (3: MDD-/E2+, n = 20 ) or did not (4: MDD-/E2-, n =18 ) receive E2. Participants in the E2 arm of the study received oral 17β-estradiol (Estrace) at a dose of 2.0 mg/day (see Supporting Information for estradiol administration).
Stress Task
For psychosocial stress induction, we employed the Montreal Imaging Stress Task (MIST) (Albert et al., 2015; Dedovic et al., 2005; Pruessner et al., 2008). The MIST produces moderate psychosocial stress through a combination of motivated performance and social-evaluative threat (see Albert et al. 2020 and Supporting Information for MIST procedures). The MIST was presented in a block design during fMRI; each condition was presented twice per 3 runs (“stress”: 100 seconds, “control”: 50 seconds, ‘rest”: 30 seconds). The block lengths were designed to allow time during the “stress” condition for the development of psychosocial stress and during the “control” condition for psychosocial stress to be reduced.
Subjective Measures
Before and after the MIST, participants completed self-rated measures of stress-related distress and arousal. This included the Stress Arousal Checklist (SACL), a self-rated measure that provides separate scores for current subjective stress (SACL-Stress) and current arousal (SACL-Arousal) (Duckro et al., 1989). Mood changes were assessed using the Profile of Mood States (POMS) (King et al., 1983; McNair et al., 1989). Results for the subjective measures have been reported previously (Albert et al., 2020). As SACL-Stress score showed interaction effects of MDD history and E2 administration, change in SACL-Stress score (post MIST score – pre MIST score) was examined as a covariate of interest for brain activity during the MIST.
fMRI Analysis:
Participants completed the MIST during an fMRI session (see Supporting Information for MRI parameters and preprocessing procedures). In first level analysis, T-maps were created from linear contrasts for task conditions (“stress” – “control”). Analyses compared average activity during the “control” condition in runs 1 and 2 of the MIST (when stress would be relatively low) and average activity during the “stress” condition in runs 2 and 3 of the MIST (when stress would be relatively high). As the experience of stress likely continues into the “control” condition, this approach was used to better separate the contrast between the “control” and “stress” condition by examining the “control” condition during lower stress runs and the “stress” during higher stress runs. T-maps resulting from the first-level analysis were used in the second level analyses.
Analyses were adjusted for multiple comparisons with p < 0.005 with FDR < 0.05 (k= 102) from alpha simulation in the REST (REST_V1.8_130615, Song et al., 2011) toolbox for SPM, using a whole brain gray matter mask. Percent signal change (PSC, condition – “rest”) was extracted to conduct post-hoc tests to compare activity change in significant clusters for the four groups and correlations with SACL-Stress score.
fMRI analyses and results are reviewed in Table 1: A) Brain activity difference between the “stress” and “control” MIST conditions in all participants to assess the task effects regardless of participant group (paired t-test); B) The effect of MDD history and E2 administration (2 X 2 ANOVA) on activity and post-hoc correlation analysis of the relationship between clusters that showed significant MDD history, E2, or interaction effects, and SACL-Stress score; C) The relationship between SACL-Stress score and brain activity in all participants (full factorial regression) D) Group differences in the relationship between SACL-Stress score ( full factorial regression). Models controlled for age, time since last menstrual period (time since menopause), and current antidepressant use. As the sample size was relatively small, we conducted both parametric (Pearson) and non-parametric (Spearman) correlation analyses for post-hoc results.
Table 1.
fMRI Analyses and Results
| fMRI Analysis | Results | Regions |
|---|---|---|
|
A) Effect of MIST Task “Stress” Condition: “Stress” – “Control” All Participants |
Increased Activity During “Stress” | Right Superior / Middle Temporal Right Middle Occipital / Cuneus |
| Decreased Activity During “Stress” | Left Middle Frontal / Anterior Cingulum Left Caudate / Inferior Frontal / Insula Right Caudate Left Angular |
|
|
B) Effects of MDD History and E2: “Stress” – “Control” MDD History and E2 as factors |
MDD History, E2 Interaction | Left Inferior Frontal Left Angular Gyrus |
| Correlations between activity and SACL-Stress Score | Left Angular Gyrus: Negative Correlation in MDD-/E2+ Left Angular Gyrus Positive Correlation in MDD-/E2- |
|
|
C) Correlation between Activity and Subjective Stress: “Stress” – “Control” All Participants SACL-Stress Score as Covariate |
Positive Correlation between Activity and SACL-Stress Score | No Significant Regions |
| Negative Correlation between Activity and SACL-Stress Score | Left Precentral Posterior Cingulum / Precuneus Left Angular / Supramarginal Left Precentral Gyrus Left Inferior Temporal Left Superior Temporal Left Superior Temporal Anterior Cingulum Left Inferior / Middle Temporal Supplementary Motor Area Right Superior / Middle Temporal |
|
|
D) Group Differences in Correlations between Activity and Subjective Stress: “Stress” – “Control” Group as factor SACL-Stress Score as Covariate |
Correlations between Activity and SACL-Stress Score | Right Inferior Frontal: Positive correlation in MDD+/E2+ Right Temporal/ Insula: Positive Correlation in MDD+/E2- |
Results
There were no group differences observed for screening measures, age, or time since menopause (Table S1). One participant in the MDD+/E2+ and 6 participants in the MDD+/E2- group were taking antidepressant medication; no MDD- participants were taking antidepressant medication. As previously reported, MDD- women who received E2 exhibited greater subjective stress (higher SACL-Stress score) in response to the MIST than MDD- women who did not receive E2 (Albert et al., 2020). This effect was reversed in women with a history of MDD such that MDD+ women who received E2 reported lower subjective stress than women who did not receive E2 in response to the MIST procedure. There were no main or interactive effects of MDD history or E2 on the change score for SACL-Arousal or the POMS total mood disturbance (Albert et al., 2020).
Change in brain activity during experimentally induced stress with the MIST
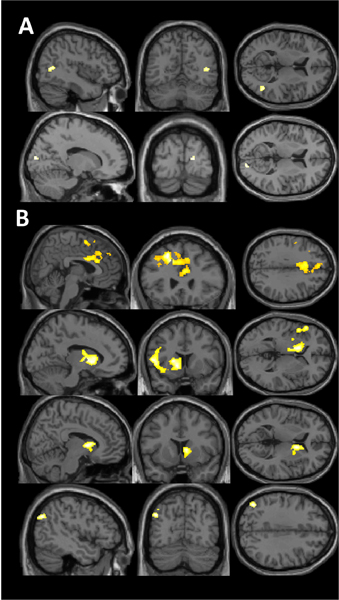
Significant differences in brain activity between the “stress” and “control” conditions of the MIST were observed in the initial analysis including all participants (Figure 1, Table 1A, Table S2). We observed greater activity during the “stress” condition compared to the “control” in the right superior and middle temporal region and the middle occipital/ cuneal regions. We observed reduced activity in the “stress” condition compared to the “control” condition in left middle frontal, bilateral caudate, and left angular gyrus regions
Figure 1. Change in brain activity during experimentally induced stress with the MIST.
A) Positive activity (“Stress” > “Control”) in superior temporal and occipital regions. B) Negative activity (“Stress” < “Control”) in left middle frontal, bilateral caudate, and left angular regions.
Effects of MDD History and E2 on change in brain activity with stress exposure during the MIST
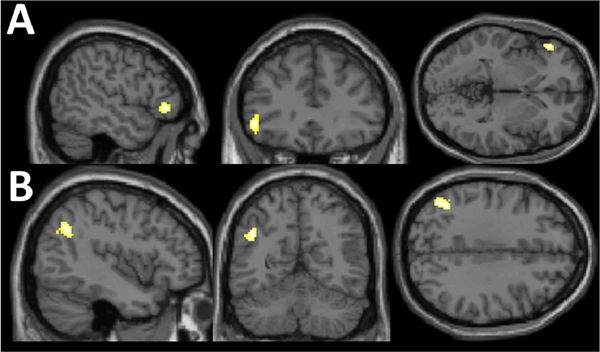
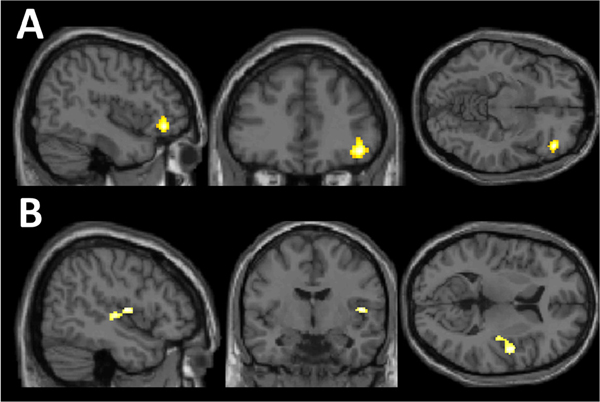
The primary analysis examined the effects of MDD history and E2 administration on brain activity during the MIST. This analysis demonstrated an interactive effect of MDD history and E2 in 2 clusters in the left inferior frontal region and angular gyrus (Figure 2, Table 1B, Table S3).
Figure 2. Effects of MDD History and E2 on change in brain activity with stress exposure during the MIST.
Interactive effect of major depression disorder history and estradiol administration in A) left inferior frontal region and B) angular activity change between the “stress” and “control” conditions of the Montreal Imaging Stress Task.
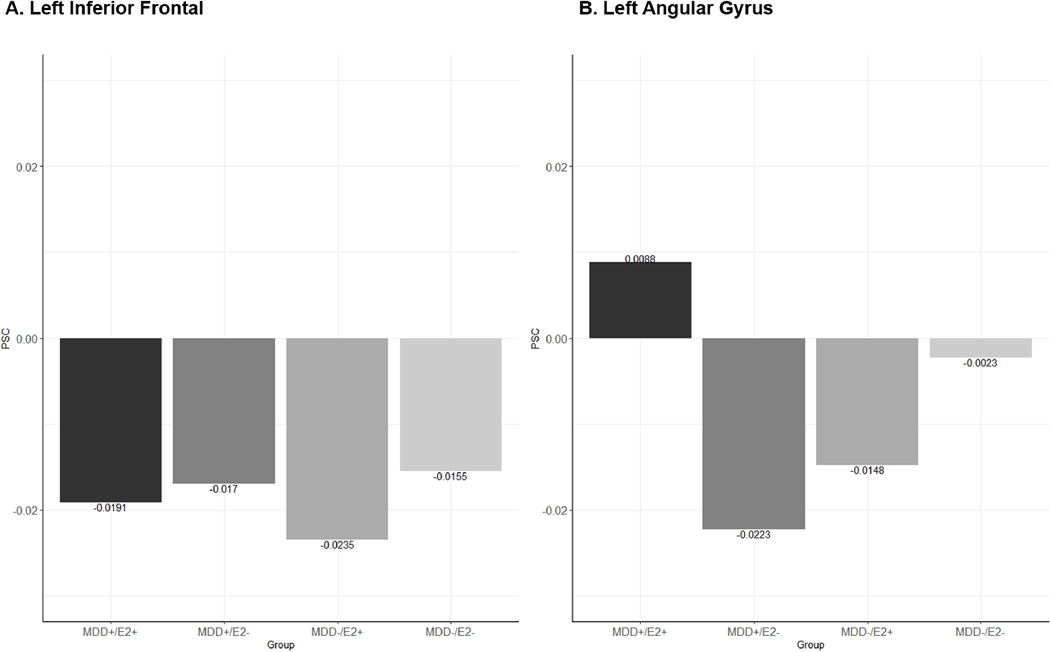
Post-hoc analyses with extracted PSC demonstrated that in all groups left inferior frontal activity decreased during the “stress” condition relative to the “control” condition, however this decrease was largest in the MDD-/E2+ group. Similarly, the MDD+/E2- and MDD-/E2+ groups showed decreased activity in the left angular gyrus during the “stress” condition compared to the “control” condition, while the MDD+/E2+ group showed less deactivation in this region and the MDD-/E2- group showed little change in activity across conditions (Figure 3).
Figure 3. Percent Signal Change “Stress” – “Control”: MDD History – E2 Interaction.
Interactive effect of major depression disorder history and estradiol administration in left inferior frontal region and angular percent signal change (PSC) between the “stress” and “control” conditions of the Montreal Imaging Stress Task.
We conducted exploratory analyses to examine whether activity change in these regions was associated with the SACL-Stress measure. Left angular gyrus activity was significantly associated with SACL-Stress change score in the two groups without MDD history. For the MDD-/E2+ group there was a negative correlation (r = − 0.50, p = 0.03; rs = −0.38, p = 0.09, non-parametric correlation not significant) such that greater activity in the left angular gyrus was associated with lower subjective stress while in the MDD-/E2- group there was a positive correlation (r = 0.53, p = 0.02; rs = 0.51, p = 0.03) with greater activity associated with higher subjective stress. There were no main effects of MDD history or E2.
Correlation between Change in Activity during the MIST and Subjective Stress
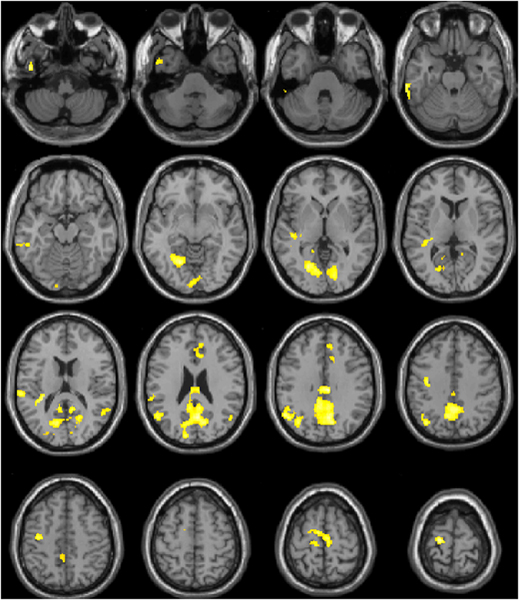
Regression analysis using SACL-Stress change score showed no significant positive associations, and 11 clusters with negative correlations. Greater subjective stress was associated with decreased activity in cingulate, temporal, and motor regions during the “stress” condition of the task (Figure 4, Table 1C, Table S4).
Figure 4. Correlation between Change in Activity during the MIST and Subjective Stress.
Brain areas including cingulate, temporal, and motor regions where activity change between the “stress” and “control” condition of the Montreal Imaging Stress Task (MIST) was negatively correlated with Stress-Arousal Checklist Stress score change across the stress task. Greater Activity in these areas during the “stress” condition of the MIST was associated with less subjective stress.
Effects of MDD History and E2 on the correlation between Change in Activity during the MIST and Subjective Stress
ANOVA analysis showed 2 clusters (right inferior frontal and right temporal/insula) where the correlation between SACL-Stress change score and MIST brain activity differed between the groups (Figure 5, Table 1D. Table S5).
Figure 5. Effects of MDD History and E2 on the correlation between Change in Activity during the MIST and Subjective Stress.
A) Women with a history of MDD who received estradiol showed a negative correlation between activity in the right inferior frontal region during the “stress” condition (compared to “rest”) of the Montreal Imaging Stress Task (MIST)and Stress-Arousal Checklist (SACL) Stress change score with less activity associated with greater subjective stress. B) Women with a history of MDD who did not receive estradiol showed a positive correlation between activity in the right temporal/ insula region during the “stress” condition of the MIST (compared to “rest) and SACL-Stress change score and also between activity change between the “control” and “stress” conditions and SACL-Stress change score with greater activity associated with greater subjective stress.
Post-hoc analyses with extracted PSC demonstrated that for the right inferior frontal region the MDD+/E2+ group showed a significant negative correlation between activity during the “stress” condition of the MIST (compared to “rest”) and SACL-Stress change score (r = −0.67, p = 0.04; rs = −0.69, p = 0.03). Greater deactivation during the “stress” condition in the right inferior frontal region was associated with greater subjective stress. For the right temporal/ insula region the MDD+/E2- group showed significant positive correlation between activity during the “stress” condition of the MIST (compared to “rest) and SACL-Stress change score (r = 0.67, p = 0.02; rs = 0.45, p = 0.14, non-parametric correlation not significant) and also between activity change between the “control” and “stress” conditions and SACL-Stress change score (r = 0.67, p = 0.02; rs = 0.63, p = 0.03). In the MDD+/E2- group greater activity in the right temporal/ insula region during the “stress” condition of the MIST and a greater change from the “control” condition were associated with greater subjective stress. All other correlations for groups and conditions with the SACL-Stress change score did not reach statistical significance.
Discussion
Our study had several key findings related to the neural response to experimental stress and how estradiol exposure and depression history interact to modify that neural response.
Observed Response to Stress
During stress, brain activity increased in temporal and occipital areas but also exhibited widespread decreases in frontal regions, striatum, and the left angular gyrus (Table 1A). The increased activity in superior and middle temporal and occipital regions during stress may reflect emotion regulation (Morawetz et al., 2016), and processing (Kumfor et al., 2014) functions. This is consistent with Kogler and colleagues work demonstrating greater superior temporal lobe activity during the “stress” condition of the MIST, particularly in female participants (Kogler et al., 2015).
The pattern of widespread deactivation is similar to previous results using the MIST, where the “stress” condition is generally associated with decreased activity in limbic and paralimbic regions (Albert et al., 2013; Dedovic et al., 2009; Orem et al., 2019; Pruessner et al., 2010; Wheelock et al., 2016). This deactivation pattern may reflect reduced salience network activity related to emotional control (Ziaei et al., 2014). Salience network activity decreases during demanding cognitive tasks (Luo et al., 2014) and the MIST task presents competing cognitive demands; to engage in the challenging arithmetic task and cognitive emotion regulation. Reduced salience network activity may reflect decreased top-down modulation of attention during cognitive tasks with emotional stimuli as attention becomes primarily driven by bottom-up emotional processing (Luo et al., 2014).
Effects of MDD History and E2 on Inferior Frontal Gyrus Activity
While all groups exhibited decreased activity in the left inferior frontal gyrus during the “stress” condition of the MIST (Figure 3a), the MDD-/E2+ group showed the largest change (Table 1B). The inferior frontal gyrus is involved in top-down emotion regulation (Cromheeke & Mueller, 2014) that may modulate limbic activity and emotional processing of negatively valenced stimuli. The MIST may engage emotion regulation as participants attempt to maintain arithmetic performance while experiencing psychosocial stress and a negative affective state. Hypoactivation of the left inferior frontal gyrus to emotional stimuli has been seen in adults with early childhood trauma (Quidé et al., 2018) and post-traumatic stress disorder (Hayes et al., 2012) and is associated with impaired emotional response inhibition (Kohn et al., 2014). Golde and colleagues recently found that following stress induced by the MIST, women with a history of childhood trauma showed worse emotional inhibition and less inferior frontal activity to negative faces than women without trauma history (Golde et al., 2019). This supports that reduced inferior frontal activity during stress may reflect diminished emotion regulation capacity.
In the current study, the MDD-/E2+ group reported greater subjective stress to the MIST than the MDD-/E2- group, which replicated our previous work showing a negative effect of E2 administration on mood response to stress in healthy postmenopausal women (Dumas et al., 2012; Newhouse et al., 2008). Similarly, the MDD-/E2+ group had the largest decrease in inferior frontal activity during stress while the MDD-/E2- group had the smallest decrease according with the pattern of E2 effects on the subjective measures. This finding is supported by previous work showing estrogen reduces inferior frontal activity for non-verbal tasks (Comasco et al., 2014; Resnick et al., 1998; Stevens et al., 2005; Toffoletto et al., 2014). Reduced inferior frontal activity following estrogen administration in post-menopausal women with no history of MDD may result in decreased emotion regulation function and thus more negative mood response to psychosocial stress.
Effects of MDD History and E2 on Angular Gyrus Activity
The groups showing the greatest increase in subjective stress (MDD+/E2- and MDD-/E2+) also exhibited the greatest deactivation in the left angular gyrus during the “stress” condition of the MIST (Table 1B). Additionally, in women without a history of MDD we observed an effect of E2 administration on the behavioral correlate of angular gyrus activity (Table 1B). During stress the MDD-/E2+ group showed greater left angular deactivation, which was in turn associated with greater subjective stress. By comparison, the MDD-/E2- group showed less change in left angular activity and less subjective stress.
The angular gyrus is part of a default mode network system involved in the regulation of internal state (Picó-Pérez et al., 2017). Reduced angular gyrus activity and functional connectivity has been reported in anxiety and mood disorders (Picó-Pérez et al., 2017) and is associated with negative stress coping styles (Santarnecchi et al., 2018). Estrogen replacement in post-menopausal women reduces left angular activity to visual distractors (Stevens et al., 2005), supporting that estrogen may affect left angular function in allocating attention. Alterations in attentional control may be particularly important in the type of stress modeled by the MIST. During the MIST participants attempt to maintain cognitive function while experiencing psychosocial stress and a negative affective state. Successfully responding to the psychosocial stress such that cognitive performance is maintained may include emotion regulation processes that involve allocating attention to external task-related stimuli rather than allocating attention to internal affective information. In women without a history of depression, E2 administration may reduce the angular gyrus’ function of appropriately allocating attention away from affective information which accords with a greater subjective stress response to the MIST in these women compared to women who did not receive E2.
Integration of Findings in Context of Menopause and Aging
The differential association of left angular activity with subjective stress response may reflect disruptive effects of E2 on emotion regulation networks in healthy post-menopausal women (Rubinow & Schmidt, 2018). This replicates previous work demonstrating that estrogen administration in post-menopausal women without a history of MDD results in a more negative subjective response to psychosocial stress (Newhouse et al., 2008) and contrasts with the protective mood effects seen in pre-menopausal women (Albert et al., 2015). This pattern suggests that healthy women may adapt to the low estrogen state following menopause and shift emotion regulation function to brain areas that are less dependent on estrogen modulation. In young women estrogen reduces activity in limbic and ventral emotional processing areas (Goldstein, 2005) and is associated with greater hippocampal activity and lower subjective stress response (Albert et al., 2015). Following menopause, women with no psychiatric history show shifts in brain activity patterns for emotional processing, including greater prefrontal, posterior cingulate, and temporal-parietal activity in response to negatively valenced images (Berent-Spillson et al., 2017) and reduced amygdala activity during emotion regulation (Frey et al., 2010) compared to pre-menopausal women. Negative mood responses to stress following E2 administration may reflect disruptive effects in brain networks that have adapted to maintain emotion regulation in the absence of estrogen.
A number of shifts in brain activity pattern have been demonstrated in aging and have been posited as compensatory changes to maintain normal function (Cabeza, 2002; Davis et al., 2008). The positivity effect is a well-established change in emotional processing, attention, and memory bias from negative information in youth towards positive information in the elderly (Carstensen & DeLiema, 2018). The positivity effect may be accompanied by shifts in functional patterns such that emotional information more strongly engages cognitive control regions that modulate limbic activity (Nashiro et al., 2012). Similar to other age-related changes in brain activity patterns, menopause may be accompanied by a change in emotion regulation and processing network activity that supports a successful transition by shifting activity patterns to areas that are less affected by ovarian hormones (Frey et al., 2010).
Women vulnerable to mood disorders may not show this adaptive shift in brain activity after menopause, resulting in continued risk for depressive episodes following menopause (Freeman et al., 2014). Our analyses found that greater levels of subjective stress were associated with reduced activity in anterior and posterior cingulate, motor planning and temporal-parietal junction regions (Table 1C), according with the proposal that activity in cognitive control regions may moderate subjective stress response following menopause (Berent-Spillson et al., 2017; Frey et al., 2010). However, in women with a history of MDD, greater subjective stress was associated with activity in inferior frontal, inferior temporal, and insular regions (Table 1D). This supports our hypothesis that individuals with a history of MDD continue to rely on ventral and salience network regions for emotional processing and mood regulation. This model suggests that the effects of ovarian-hormone based anti-depressant interventions may have unique mechanisms of action in women with a history of MDD and future work may benefit from considering this difference.
Strengths and Limitations
The strengths of this study include the use of an established, in-scanner psychosocial stress task and the experimental administration of E2. The production of psychosocial stress is a challenge in the fMRI environment as the experience of stress is likely prolonged and thus brain activity related to stress may appear in the “control” condition. We used relatively long and uneven block lengths to allow time for the production of psychosocial stress during the “stress” condition and the reduction of stress during the “control” condition. We also attempted to reduce the amount of stress-related brain activity during the “control” condition and maximize this activity during the “stress” condition by examining the “control” condition earlier in the task and the “stress” condition later in the task. The goal of this approach was to enhance the contrast between “control” and “stress”. Although we controlled for motion and signal drift in our preprocessing, these approaches may have introduced additional noise.
E2 administration allowed us to examine the effects of increasing estrogen in postmenopausal women and control the dose and duration of E2. Limiting this approach was the use of a cross-sectional design and open label E2 administration. As the stress task used deception to invoke psychosocial stress, the MIST could not be repeated and thus we were not able to compare activity during the MIST following E2 administration to baseline (without E2) activity in the same women. As women were not aware that the stress response was being examined, the use of deception likely limited placebo effects, however there may be a selection bias in the women who chose to enroll in the drug arm of the study. All participants were euthymic with low depression or anxiety symptoms and there were no differences in the groups in these measures at baseline suggesting that the groups were similar and the women who enrolled in the drug arm did not have different depression or anxiety symptoms than the women in the control arm.
This study is further limited in the characterization of menopause and depression history. We controlled analyses for time since final menstrual period and required women to have non-surgical menopause with no ovarian hormone use in the last year. We also verified menopausal status using FSH level but did not include past ovarian hormone replacement use in our statistical models. Likewise, we required women to be euthymic at the time of the study, with low depression and anxiety symptoms. Additionally, we required anti-depressant use to be at a stable regimen and dose for at least 3 months prior to study participation and included antidepressant use in our statistical models. However, the use of retrospective participant reported MDD history limits conclusions regarding the effects of MDD history. Future work should examine the effects of prior ovarian hormone use and the severity and duration of depression history.
Conclusions
The results of this study demonstrated that in women without a history of MDD estrogen administration is associated with decreased activity in brain areas involved in emotion regulation during psychosocial stress. The effect of estradiol administration was reversed in women with a history of MDD; women who did not receive E2 had a more negative mood response to psychosocial stress and decreased activity related to emotion regulation. We propose that in healthy women the menopause transition is accompanied by a shift in neural activity patterns that support emotional regulation despite estrogen withdrawal. Administration of estrogen in these women may result in disrupted emotion regulation-related activity and negative mood responses to psychosocial stress. This shift may not be seen in women with MDD vulnerability. This suggests that women with a history of MDD, even while currently euthymic, have both a different mood and neural responses to psychosocial stress and may show a beneficial mood effect of E2 that is not seen in women without a history of MDD. These findings help develop a model of mood regulation in healthy post-menopausal women and may be useful in future work examining the use of ovarian hormone approaches to maintaining healthy mood in post-menopausal women with a history of MDD.
Supplementary Material
Highlights.
Estradiol effects on subjective psychosocial stress differ by depression history
Estradiol effects on brain activity to stress differ by depression history
In women with no depression history estradiol reduces emotion regulation activity
In women with depression history estradiol reduces emotion perception activity
Acknowledgments
Funding
This work was supported by the National Institutes of Aging R01AG021476, the National Institutes of Mental Health H110598, and Vanderbilt CTSA grant UL1 TR002243 from National Center for Advancing Translational Sciences.
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albert K, Pruessner J, Rabinowitz T, & Newhouse P. (2013). Menstrual phase is associated with differences in brain activity during psychosocial stress. Biological Psychiatry. [Google Scholar]
- Albert K, Ledet T, Taylor W, & Newhouse P. (2020). Estradiol administration differentially affects the response to experimental psychosocial stress in post-menopausal women with or without a history of major depression. Journal of Affective Disorders. 10.1016/j.jad.2019.09.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert K, Pruessner J, & Newhouse P. (2015). Estradiol levels modulate brain activity and negative responses to psychosocial stress across the menstrual cycle. Psychoneuroendocrinology, 59, 14–24. 10.1016/j.psyneuen.2015.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berent-Spillson A, Marsh C, Persad C, Randolph J, Zubieta JK, & Smith Y. (2017). Metabolic and hormone influences on emotion processing during menopause. Psychoneuroendocrinology, 76, 218–225. 10.1016/j.psyneuen.2016.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromet E, Andrade LH, Hwang I, Sampson NA, Alonso J, de Girolamo G, de Graaf R, Demyttenaere K, Hu C, Iwata N, Karam AN, Kaur J, Kostyuchenko S, Lépine JP, Levinson D, Matschinger H, Mora MEM, Browne MO, Posada-Villa J, … Kessler RC (2011). Cross-national epidemiology of DSM-IV major depressive episode. BMC Medicine. 10.1186/1741-7015-9-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R. (2002). Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychology and Aging, 17(1), 85–100. 10.1037//0882-7974.17.1.85 [DOI] [PubMed] [Google Scholar]
- Carstensen LL, & DeLiema M. (2018). The positivity effect: a negativity bias in youth fades with age. In Current Opinion in Behavioral Sciences (Vol. 19, pp. 7–12). Elsevier Ltd. 10.1016/j.cobeha.2017.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comasco E, Frokjaer VG, & Sundström-Poromaa I. (2014). Functional and molecular neuroimaging of menopause and hormone replacement therapy. In Frontiers in Neuroscience (Vol. 8, Issue DEC). Frontiers Media S.A. 10.3389/fnins.2014.00388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromheeke S, & Mueller SC (2014). Probing emotional influences on cognitive control: An ALE meta-analysis of cognition emotion interactions. Brain Structure and Function, 219(3), 995–1008. 10.1007/s00429-013-0549-z [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, & Cabeza R. (2008). Que PASA? The Posterior-Anterior Shift in Aging. Cerebral Cortex, 18(5), 1201–1209. 10.1093/cercor/bhm155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedovic K, D’Aguiar C, & Pruessner JC (2009). What stress does to your brain: A review of neuroimaging studies. In Canadian Journal of Psychiatry. 10.1177/070674370905400104 [DOI] [PubMed] [Google Scholar]
- Dedovic K, Renwick R, Mahani NK, Engert V, Lupien SJ, & Pruessner JC (2005). The Montreal Imaging Stress Task: Using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. Journal of Psychiatry and Neuroscience. 10.1007/s00066-017-1186-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckro PN, Korytnyk NX, & Vandenberg BR (1989). The Stress-Arousal Checklist as a measure of situational stress versus simple arousal. Psychological Reports, 64(1), 239–242. 10.2466/pr0.1989.64.1.239 [DOI] [PubMed] [Google Scholar]
- Dumas JA, Albert KM, Naylor MR, Sites CK, Benkelfat C, & Newhouse PA (2012). The effects of age and estrogen on stress responsivity in older women. American Journal of Geriatric Psychiatry, 20(9), 734–743. 10.1097/JGP.0b013e31825c0a14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman EW, Sammel MD, Boorman DW, & Zhang R. (2014). Longitudinal pattern of depressive symptoms around natural menopause. JAMA Psychiatry, 71(1), 36–43. 10.1001/jamapsychiatry.2013.2819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey BN, Hall GB, Attard S, Yucel K, Skelin I, Steiner M, & Soares CN (2010). Shift in the brain network of emotional regulation in midlife women: Is the menopausal transition the turning point? Menopause. 10.1097/gme.0b013e3181df840f [DOI] [PubMed] [Google Scholar]
- Golde S, Wingenfeld K, Riepenhausen A, Schröter N, Fleischer J, Prüssner J, Grimm S, Fan Y, Hellmann-Regen J, Beck A, Gold SM, & Otte C. (2019). Healthy women with severe early life trauma show altered neural facilitation of emotion inhibition under acute stress. Psychological Medicine, 1–10. 10.1017/S0033291719002198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM (2005). Hormonal Cycle Modulates Arousal Circuitry in Women Using Functional Magnetic Resonance Imaging. Journal of Neuroscience, 25(40), 9309–9316. 10.1523/JNEUROSCI.2239-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JP, Hayes SM, & Mikedis AM (2012). Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biology of Mood & Anxiety Disorders, 2(1). 10.1186/2045-5380-2-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Birnbaum H, Bromet E, Hwang I, Sampson N, & Shahly V. (2010). Age differences in major depression: Results from the national comorbidity survey replication (NCS-R). Psychological Medicine. 10.1017/S0033291709990213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MG, Burrows GD, & Stanley GV (1983). Measurement of stress and arousal: validation of the stress/arousal adjective checklist. British Journal of Psychology (London, England : 1953), 74 (Pt 4), 473–479. 10.1111/j.2044-8295.1983.tb01880.x [DOI] [PubMed] [Google Scholar]
- Kogler L, Gur RC, & Derntl B. (2015). Sex differences in cognitive regulation of psychosocial achievement stress: Brain and behavior. Human Brain Mapping, 36(3), 1028–1042. 10.1002/hbm.22683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn N, Eickhoff SB, Scheller M, Laird AR, Fox PT, & Habel U. (2014). Neural network of cognitive emotion regulation - An ALE meta-analysis and MACM analysis. NeuroImage, 87, 345–355. 10.1016/j.neuroimage.2013.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumfor F, Irish M, Hodges JR, & Piguet O. (2014). Frontal and temporal lobe contributions to emotional enhancement of memory in behavioral-variant frontotemporal dementia and Alzheimer’s disease. Frontiers in Behavioral Neuroscience, 8(JUNE), 225. 10.3389/fnbeh.2014.00225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Qin S, Fernández G, Zhang Y, Klumpers F, & Li H. (2014). Emotion perception and executive control interact in the salience network during emotionally charged working memory processing. Human Brain Mapping, 35(11), 5606–5616. 10.1002/hbm.22573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair D, Lorr M, & Droppleman LF (1989). Profile of mood states (POMS). In Douglas M. McNair, Maurice Lorr, and Leo F. Droppleman (p. Douglas M. McNair, Maurice Lorr, and Leo F. Droppl). 10.1007/978-1-4419-9893-4_68 [DOI] [Google Scholar]
- Morawetz C, Bode S, Baudewig J, Jacobs AM, & Heekeren HR (2016). Neural representation of emotion regulation goals. Human Brain Mapping, 37(2), 600–620. 10.1002/hbm.23053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashiro K, Sakaki M, & Mather M. (2012). Age differences in brain activity during emotion processing: Reflections of age-related decline or increased emotion regulation? In Gerontology. 10.1159/000328465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhouse PA, Dumas J, Hancur-Bucci C, Naylor M, Sites CK, Benkelfat C, & Young SN (2008). Estrogen administration negatively alters mood following monoaminergic depletion and psychosocial stress in postmenopausal women. Neuropsychopharmacology. 10.1038/sj.npp.1301530 [DOI] [PubMed] [Google Scholar]
- Orem TR, Wheelock MD, Goodman AM, Harnett NG, Wood KH, Gossett EW, Mrug S, Knight DC, & Granger DA (2019). Amygdala and Prefrontal Cortex Activity Varies with Individual Differences in the Emotional Response to Psychosocial Stress. Behavioral Neuroscience, 133(2), 203–211. 10.1037/bne0000305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picó-Pérez M, Radua J, Steward T, Menchón JM, & Soriano-Mas C. (2017). Emotion regulation in mood and anxiety disorders: A meta-analysis of fMRI cognitive reappraisal studies. In Progress in Neuro-Psychopharmacology and Biological Psychiatry (Vol. 79, pp. 96–104). Elsevier Inc. 10.1016/j.pnpbp.2017.06.001 [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Khalili-Mahani N, Engert V, Pruessner M, Buss C, Renwick R, Dagher A, Meaney MJ, & Lupien S. (2008). Deactivation of the Limbic System During Acute Psychosocial Stress: Evidence from Positron Emission Tomography and Functional Magnetic Resonance Imaging Studies. Biological Psychiatry, 63(2), 234–240. 10.1016/j.biopsych.2007.04.041 [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Pruessner M, Lord C, Buss C, Collins L, Dagher A, & Lupien SJ (2010). Stress regulation in the central nervous system: evidence from structural and functional neuroimaging studies in human populations - 2008 Curt Richter Award Winner. In Psychoneuroendocrinology. 10.1016/j.psyneuen.2009.02.016 [DOI] [PubMed] [Google Scholar]
- Quidé Y, O’Reilly N, Watkeys OJ, Carr VJ, & Green MJ (2018). Effects of childhood trauma on left inferior frontal gyrus function during response inhibition across psychotic disorders. Psychological Medicine, 48(9), 1454–1463. 10.1017/S0033291717002884 [DOI] [PubMed] [Google Scholar]
- Resnick SM, Maki PM, Golski S, Kraut MA, & Zonderman AB (1998). Effects of estrogen replacement therapy on PET cerebral blood flow and neuropsychological performance. Hormones and Behavior, 34(2), 171–182. 10.1006/hbeh.1998.1476 [DOI] [PubMed] [Google Scholar]
- Rubinow DR, & Schmidt PJ (2018). Is there a role for reproductive steroids in the etiology and treatment of affective disorders? In Dialogues Clin Neurosci (Vol. 20). www.dialoguescns.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarnecchi E, Sprugnoli G, Tatti E, Mencarelli L, Neri F, Momi D, Di Lorenzo G, Pascual-Leone A, Rossi S, & Rossi A. (2018). Brain functional connectivity correlates of coping styles. Cognitive, Affective and Behavioral Neuroscience, 18(3), 495–508. 10.3758/s13415-018-0583-7 [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Ben Dor R, Martinez PE, Guerrieri GM, Harsh VL, Thompson K, Koziol DE, Nieman LK, & Rubinow DR (2015). Effects of estradiol withdrawal on mood in women with past perimenopausal depression: A randomized clinical trial. JAMA Psychiatry. 10.1001/jamapsychiatry.2015.0111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PJ, & Rubinow DR (2009). Sex hormones and mood in the perimenopause. Annals of the New York Academy of Sciences, 1179, 70–85. 10.1111/j.17496632.2009.04982.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X-W, Dong Z-Y, Long X-Y, Li S-F, Zuo X-N, Zhu C-Z, He Y, Yan C-G, & Zang Y-F (2011). REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PloS One, 6(9), e25031. 10.1371/journal.pone.0025031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC, Clark VP, & Prestwood KM (2005). Low-dose estradiol alters brain activity. Psychiatry Research - Neuroimaging. 10.1016/j.pscychresns.2005.04.004 [DOI] [PubMed] [Google Scholar]
- Sundström-Poromaa I. (2018). The Menstrual Cycle Influences Emotion but Has Limited Effect on Cognitive Function. In Vitamins and Hormones. 10.1016/bs.vh.2018.01.016 [DOI] [PubMed] [Google Scholar]
- Toffoletto S, Lanzenberger R, Gingnell M, Sundström-Poromaa I, & Comasco E. (2014). Emotional and cognitive functional imaging of estrogen and progesterone effects in the female human brain: A systematic review. In Psychoneuroendocrinology (Vol. 50, pp. 28–52). Elsevier Ltd. 10.1016/j.psyneuen.2014.07.025 [DOI] [PubMed] [Google Scholar]
- Wheelock MD, Harnett NG, Wood KH, Orem TR, Granger DA, Mrug S, & Knight DC (2016). Prefrontal cortex activity is associated with biobehavioral components of the stress response. Frontiers in Human Neuroscience, 10(NOV2016). 10.3389/fnhum.2016.00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EA, Midgley AR, Carlson NE, & Brown MB (2000). Alteration in the Hypothalamic-Pituitary-Ovarian Axis in Depressed Women. Archives of General Psychiatry, 57(12), 1157. 10.1001/archpsyc.57.12.1157 [DOI] [PubMed] [Google Scholar]
- Ziaei M, Peira N, & Persson J. (2014). Brain systems underlying attentional control and emotional distraction during working memory encoding. NeuroImage, 87, 276–286. 10.1016/j.neuroimage.2013.10.048 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.