Abstract
Infants born very preterm (PT), prior to 32 weeks gestation, are at increased risk of developing cerebral palsy. Children with spastic cerebral palsy have impaired selective leg joint movement which contributes to lifelong walking limitations. We investigated whether infants born PT generated more selective hip-knee joint movement (e.g., hip flexes as knee extends) while participating in a scaffolded mobile task. Infants born PT and infants born full-term (FT) at 4 months corrected age participated in a scaffolded mobile task for 2–3 consecutive days. The scaffolded mobile task required infants to raise their legs vertically over a virtual threshold. Three threshold heights (low, middle, high) were used to test if the middle and high heights encourage infants to move their legs more selectively. Fifteen infants born FT learned the task and showed more selective hip-knee movement at each of the three threshold heights on the day that they learned, compared to their baseline spontaneous kicking. Thirteen infants born PT learned the task and showed more selective hip-knee movement on their learning day, but only when the middle and high thresholds were used. The results show that the scaffolded mobile task effectively encouraged infants to generate more selective hip-knee joint movement.
Keywords: Infant, Mobile task, Prematurity, Scaffolding, Selective leg movement
Introduction
Cerebral palsy is a disorder of posture and movement caused by damage to the developing brain (Rosenbaum et al., 2007). Children with spastic cerebral palsy have impaired selective leg joint movement which contributes to lifelong walking limitations (Farmer, 2003; Farmer, Pearce, & Stewart, 2008; Fowler, Staudt, & Greenberg, 2010). Selective joint movement is defined as the ability to isolate the movement of one joint from the movement of the other joints within the limb (e.g., extending the knee while flexing the hip) (Chen, Fetters, Holt, & Saltzman, 2002; Fetters, Chen, Jonsdottir, & Tronick, 2004; Fetters, Sapir, Chen, Kubo, & Tronick, 2010; Fowler & Goldberg, 2009; Sargent, Kubo, & Fetters, 2018; Sargent, Schweighofer, Kubo, & Fetters, 2014). Impaired selective leg movement results in excessively coupled hip and knee flexion and extension during the gait of children with spastic cerebral palsy, contributing to short stride length, slow walking speed, and excessive energy consumption (Farmer, 2003; Fowler & Goldberg, 2009).
Infants born very preterm (PT), prior to 32 weeks gestation, are at increased risk of developing cerebral palsy, with estimates indicating that 6–15% will develop cerebral palsy (Himpens, Van den Broeck, Oostra, Calders, & Vanhaesebrouck, 2008; Pascal et al., 2018). Infants born PT with brain lesions are at highest risk of developing cerebral palsy (Gopagondanahalli et al., 2016; Hagberg, Hagberg, Beckung, & Uvebrant, 2001), and show impaired selective leg movement during spontaneous kicking as early as one month of age, corrected for prematurity (Fetters et al., 2004). In typical development, newborns spontaneously kick with coupled leg movements (Heriza, 1988), but over the first 10 months of life they demonstrate more selective leg movements (Sargent, Scholz, Reimann, Kubo, & Fetters, 2015; Thelen, 1985); this is the same time frame that infants are learning to move their legs to participate in tasks. It is not known if infants born PT with brain lesions have the capacity to produce more selective leg movement when a task reinforces them to move more selectively. As a first step, an infant kick-activated mobile task was developed to encourage selective leg movement of infants born PT without brain lesions (Sargent et al., 2018; Sargent et al., 2014).
In the infant kick-activated mobile task, which occurs on two consecutive days, 3- to 4-month-old infants are supine under an infant mobile that rotates and plays music when the infants lift their legs vertically above a specific threshold height. Three-month-old infants born full-term (FT) who learned the association between their leg movements and mobile activation demonstrated coupled hip and knee movements when interacting with the mobile on the first day, but demonstrated more selective hip-knee movements on the second day, compared to their baseline spontaneous kicking (Sargent et al., 2014). The authors speculated that the infants born FT demonstrated more selective hip-knee movements by learning to activate the mobile more efficiently by maintaining their hip flexed while bending and straightening their knee joints to keep their feet right around the threshold. Three-month-old infants born PT did not learn the association, but when they participated in the task again at 4 months of age, the infants born PT who learned the association continued to demonstrate coupled hip and knee movements on both days (Sargent et al., 2018).
Infants born PT may need further support during the task, or scaffolding, to produce more selective hip-knee movement. Here, we define “scaffolding” as modifying a task so that the infant is first reinforced for an easily attainable goal, then reinforced for progressively more challenging goals, until the infant is finally reinforced for the most challenging, but still achievable task goal; this has also been referred to as task structuring (Kim, Fetters, Kubo, Eckel, & Sargent, 2021; Reiser, 2004).
In the present study, we scaffolded the mobile task to motivate selective hip-knee movement for infants born PT (Kim et al., 2021). Unlike the previous studies that only used a single, low threshold (Sargent et al., 2018; Sargent et al., 2014), we designed the task to encourage infants to kick high by using three heights of threshold (low, middle, high; dotted lines in Figure 1). The infant mobile played music and rotated when the threshold was crossed by a specific foot marker on each of the infant’s feet (the solid circle in Figure 1). On the first day, only the low threshold was used to support learning of the association between their leg movements and mobile activation. On the second and third days, the threshold was systematically increased from low to middle to high to encourage infants to kick higher. Four-month-old infants born FT and infants born PT both demonstrated learning of the scaffolded mobile task and increased kick heights when the high threshold was used. We hypothesize that this task using high thresholds may require infants born PT to extend the knee while flexing the hip to move their foot above the high threshold, thereby generating more selective hip-knee movement.
Figure 1.
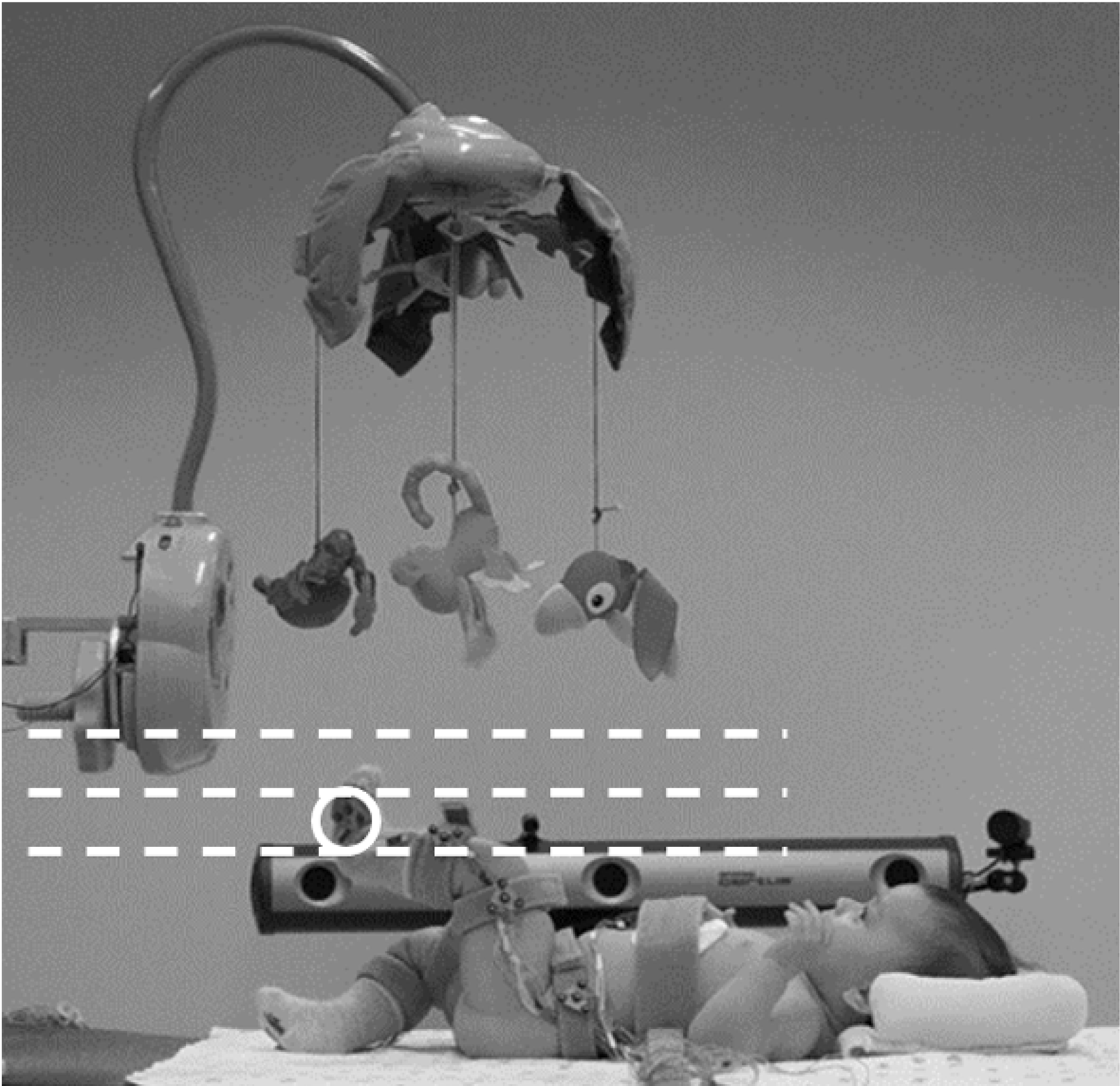
Scaffolded mobile task. One infrared light-emitting diode marker (circled) on each foot activated the mobile once it crossed a virtual threshold. The dotted lines depict the three heights of the virtual threshold (low, middle, high) that systematically increased during the task.
Therefore, the objective of this study was to assess if infants born FT and infants born PT who learned the scaffolded mobile task demonstrated more selective hip-knee joint movement when interacting with the mobile, compared to baseline spontaneous kicking. We tested three hypotheses: 1) On the first day, infants born FT and infants born PT would not demonstrate more selective hip-knee movement when interacting with the mobile compared to baseline kicking, based on previous research (Sargent et al., 2018; Sargent et al., 2014). 2) On the day that infants born FT learned the task, they would exhibit more selective hip-knee movement while interacting with the mobile during the conditions using the low, middle, and high threshold heights, compared to baseline kicking, based on previous research (Sargent et al., 2014). 3) On the day that infants born PT learned the task, they would not exhibit more selective hip-knee movement during the low threshold height as shown in previous research (Sargent et al., 2018), but would exhibit more selective hip-knee movement during the middle and high threshold heights, compared to baseline kicking. It is important to note that our overall goal is to develop a task to promote selective hip-knee movement, therefore, our primary objective is to determine if selective hip-knee control emerges as infants learn the scaffolded mobile task. Our objective is not to determine the efficacy of scaffolding; this is an area for future research.
Methods
Participants
The present study was conducted according to guidelines laid down in the Declaration of Helsinki, with written informed consent obtained from a parent for each child before any assessment or data collection. All procedures involving human subjects in this study were approved by the University of Southern California (USC) Health Sciences Institutional Review Board (#HS-17–00119). Infants born PT (≤32 weeks gestation) and infants born FT (>37 weeks gestation) between the ages of 4 months 0 day to 4 months 15 days corrected age, corrected for prematurity, were recruited from USC and the Los Angeles County + USC Medical Center from July 2016 to August 2019. Infants were excluded based on parent report if they were ill or had congenital malformations, chromosomal abnormalities, orthopedic impairments, or vision or hearing impairments that would hinder their ability to see or hear the mobile. Refer to Kim et al. (2021) for details of protocol.
Experimental Protocol
Data were collected in the Development of Infant Motor Performance Laboratory at USC. Throughout data collection, the parent sat next to the infant, out of the infant’s sight, and was instructed to not touch the infant and stay quiet, but use soothing words (e.g., “you are okay”) if the infant fussed or cried.
Scaffolded Mobile Task.
All infants participated in the 10-min scaffolded mobile task for 2 to 3 consecutive days. Each day, infants were supine on a testing table with their feet under an infant mobile. An Optotrak Certus Motion Capture System sensor (Northern Digital Inc., Waterloo, ON, Canada) was on each side of the testing table. Custom, rigid marker arrays with 4-embedded infrared light-emitting diodes (IREDs) were attached to the infants’ pelvis, thigh, shank, and foot bilaterally using Velcro straps. A custom marker array with two embedded IREDs was attached to the infants’ sternum using collar tape.
On Day 1, during the 2-min baseline condition, the mobile did not move but each infant kicked spontaneously. The computer used position data from the center IRED on each foot to compute an individualized threshold for mobile activation at a height that was one standard deviation (sd) above the average height of both feet during the 2-min baseline; refer to equation in Supporting Information. During the following 8-min low threshold condition, the musical mobile rotated only if the infant lifted either foot IRED vertically higher than the low threshold (mean plus 1sd baseline foot height). Once either foot crossed the threshold, the musical mobile rotated for the duration the IRED stayed above the threshold to a maximum of 3 sec. To reactivate the mobile after 3 sec, infants were required to lower the foot below the threshold and re-cross the threshold. This 3-sec setting was used to reinforce infants to move their legs rather than hold their feet above the threshold.
On Day 2, the 10-min mobile task consisted of 2-min low, 4-min middle, and 4-min high threshold conditions in which the threshold height was systematically increased. For the first 2-min low threshold condition, the threshold was consistent with the low threshold on Day 1 (mean plus 1sd baseline foot height). For the following 4-min middle threshold condition, the threshold was increased to the mean baseline foot height plus 1.5sd. For the last 4-min high threshold condition, the threshold was increased to the mean baseline foot height plus 2sd. Individual infants were identified as learners if the percentage of time that the infant activated the mobile during the entire Day 2 task was ≥1.5 times the percentage of time that the infant would have activated the mobile during the Day 1 baseline condition, assuming the mobile could be activated (Sargent et al., 2018; Sargent et al., 2014). Since learning is regarded as a relatively permanent change in behavior that is typically assessed the next day (Schmidt & Lee, 2011), the percentages of time that activated the mobile on Days 2 and 3, but not Day 1, were used to define learning. This 1.5 individual learning criteria has been used in mobile paradigms for over 50 years to indicate infant learning (Chen et al., 2002; Rovee-Collier, 1997; Sargent et al., 2018; Sargent et al., 2014). The parents of infants that did not meet this individual learning criterion were asked to participate in the identical task on the next day (Day 3). Refer to Kim et al. (2021) for details of the learning measure.
Please note, statistically, at the 1sd threshold height the infant’s spontaneous kicks would activate the mobile approximately 16% of the time, which is a reasonable reinforcement schedule to learn a task. The 1.5sd and 2sd threshold heights were chosen as a systematic way to increase the height of the threshold while assuring that the threshold remained within each infant’s capability. Statistically at the 1.5sd threshold height, the infant’s spontaneous kicks would activate the mobile approximately 10% of the time, and at the 2sd threshold height the infant’s spontaneous kicks would activate the mobile approximately 3% of the time. We expected the 1.5sd and 2sd threshold heights would encourage more selective hip-knee coordination as infants flexed their hips and extended their knees to move their feet over the higher threshold heights, but felt that systematically increasing the threshold heights was necessary to promote learning of the mobile task since activating the mobile 3 or 10% of the time does not appear to be a reasonable reinforcement schedule for an infant to learn a task.
Video data.
During the mobile task, a video camera (Canon Inc., VIXIA-R700 Camcorder) was positioned overhead to record infants’ facial expressions and eye gaze.
Kinematic data.
The mobile task was followed by a 5-sec static calibration trial for each leg, which defined the hip, knee and ankle angles at 0° (Sargent et al., 2014). During this trial, an additional 5 individual IREDs were attached to the infants’ skin bilaterally at the lateral midline of the trunk below the 10th rib, greater trochanter of the hip, lateral knee joint line, ankle lateral malleolus, and distal end of the 5th metatarsal using double-sided collar tape. During the static trial, the infants’ straight leg positions were held by an experimenter for 5 seconds.
Anthropometric and developmental measures.
On Day 2, after the mobile task, an experimenter measured infants’ weights, heights, lengths of the lower extremity, and widths of knee and ankle. Infants were also evaluated using the motor subtest of the Bayley Scales of Infant and Toddler Development, 3rd ed. (BSITD-III) (Bayley, 2006).
Data Reduction
A custom Matlab R2019b program (The Mathworks, Inc., Natick, MA) was used to process the position data of the IREDs as follows: (a) interpolate missing position data up to 20 consecutive frames using a cubic spline, (b) extract segments >25 frames without missing position data of all IREDs, (c) filter position data using a fourth-order Butterworth with a cut-off frequency of 5 Hz, (d) compute hip and knee joint angles of flexion and extension, and (e) extract kicks (Sargent et al., 2018; Sargent et al., 2014). A kick onset was defined as a change into hip or knee flexion/extension angle of >11.5° and the termination of a kick was defined as the peak flexion or extension amplitude following a movement in the opposite direction (Fetters et al., 2004; Fetters et al., 2010; Jensen, Schneider, Ulrich, Zernicke, & Thelen, 1994; Sargent et al., 2018; Sargent et al., 2014).
Selective movement was quantified for each kick using Pearson correlation coefficients (CC) between hip and knee joint angles throughout the kick. The CC values were converted to Fisher Z scores for statistical comparisons among infants (Hip-Knee ZCC), but then converted back to Hip-Knee CC when results were graphed. A more negative Hip-Knee CC was interpreted as more selective movement, and a more positive Hip-Knee CC was interpreted as less selective movement (Chen et al., 2002; Fetters et al., 2010; Sargent et al., 2018; Sargent et al., 2014). Differences in hip-knee selective movement between the FT group and PT group and across conditions were assessed statistically (Sargent et al., 2018; Sargent et al., 2014). Individually, infants were categorized as demonstrating considerably more selective hip–knee movement during each threshold condition if there was at least a −0.25 difference in the Hip–Knee CC during that threshold condition compared to the Day 1 baseline condition. The −0.25 value was chosen as it was the minimal mean within-group difference in Hip–Knee CC that resulted in a statistically significant difference between a threshold condition and the Day 1 baseline condition; it occurred in the PT group during the learning day high threshold condition.
To further investigate selective movement, relative phase was measured between hip and knee angles (Hip-Knee RP) at joint reversal for each kick. The absolute value of RP was computed to analyze the magnitude of selective movement. The mean Hip-Knee RP of all kicks was computed for each infant. A Hip-Knee RP approaching 0° was interpreted as less selective movement, and a Hip-Knee RP approaching 180° was interpreted as more selective movement.
Statistical Analysis
Demographic data.
Independent t-tests and Chi-square tests were used to test group differences using SPSS v.25.0 (IBM Corp., Armonk, NY).
Kinematic data.
For each Hip-Knee ZCC and RP, mixed regression models using repeated measures were performed to test between- and within-group differences using SAS v.9.0 (SAS Institute Inc., Cary, NC). An autoregressive covariance structure was selected because it was consistent with the study design and was the best fit based on Akaike Information Criteria and Bayesian Information Criteria. The fixed effects of Group (FT, PT) and Condition (Day 1 baseline, Day 1 low threshold, learning day low threshold, learning day middle threshold, learning day high threshold) and its interaction (Group*Condition) were tested for Hip-Knee ZCC and RP, respectively. The Hip-Knee ZCC and RP, respectively, were compared 1) between the baseline and low threshold conditions on Day 1 and 2) between the baseline condition on Day 1 and each of the threshold conditions (low, middle, high) on the learning day. Also, the Hip-Knee ZCC and RP during each threshold condition were compared between groups. Post-hoc comparisons were performed using Bonferroni correction to adjust for multiple comparisons with an adjusted alpha of .0011 (.05/45 comparisons).
Results
Participants
Thirty-six infants (18 FT, 18 PT) completed the study. Of those, fifteen infants born FT and 13 infants born PT learned the scaffolded mobile task on either Day 2 (13 FT, 8 PT) or Day 3 (2 FT, 5 PT), defined as the learning day. Demographics of infants who learned the task are in Table 1 and Table S1 in Supporting Information. There were significant differences between the two infant groups for gestational age and birth weight, but no significant group differences for sex, age, ponderal index, and scores on the motor subset of the BSITD-III. Refer to Kim et al. (2021) for details on the learning results of the task including: learning, kick height, arousal, and visual attention.
Table 1.
Demographics of Participants
| Characteristics, Mean (SD) | Full-term learner (n=15) | Preterm learner (n=13) | p |
|---|---|---|---|
| Sex, N (%) | .06 | ||
| Female | 5 (33%) | 9 (69%) | |
| Male | 10 (67%) | 4 (31%) | |
| Gestational age, week | 39.0 (0.9) | 27.9 (2.1) | < .001 |
| Birth weight, kg | 3.5 (0.3) | 1.2 (0.3) | < .001 |
| Testing age, days corrected age | 123.7 (4.8) | 126.4 (4.7) | .15 |
| Ponderal index, g/cm3 | 2.45 (0.28) | 2.54 (0.29) | .42 |
| BSITD-III motor, percentile | 77.9 (17.3) | 69.5 (20.3) | .25 |
Note. BSITD-III motor, Bayley Scale of Infant/Toddler Development 3rd edition motor subtest. A Chi-square (χ2) test was used for sex. Independent t-tests were used for other variables.
Selective Hip-Knee Movement
There were significant main effects of Group (FT, PT) [F(1,26)=39.72, p<.0001] and Condition (Day 1 baseline, Day 1 low threshold, learning day low threshold, learning day middle threshold, learning day high threshold) [F(4,104)=20.41, p<.0001], and a significant interaction effect of Group*Condition [F(4,104)=8.09, p<.0001] on the Hip-Knee ZCC. Means and standard errors of the Hip-Knee CC are in Figure 2. Means and standard errors of the Hip-Knee CC and the number of kicks analyzed are in Table 2. Refer to Table S2 in Supporting Information for details on the Hip-Knee RP results. Figure 3 (colored version in Figure S1) shows the change in the Hip-Knee CC of each infant between the Day 1 baseline condition and each threshold condition.
Figure 2.
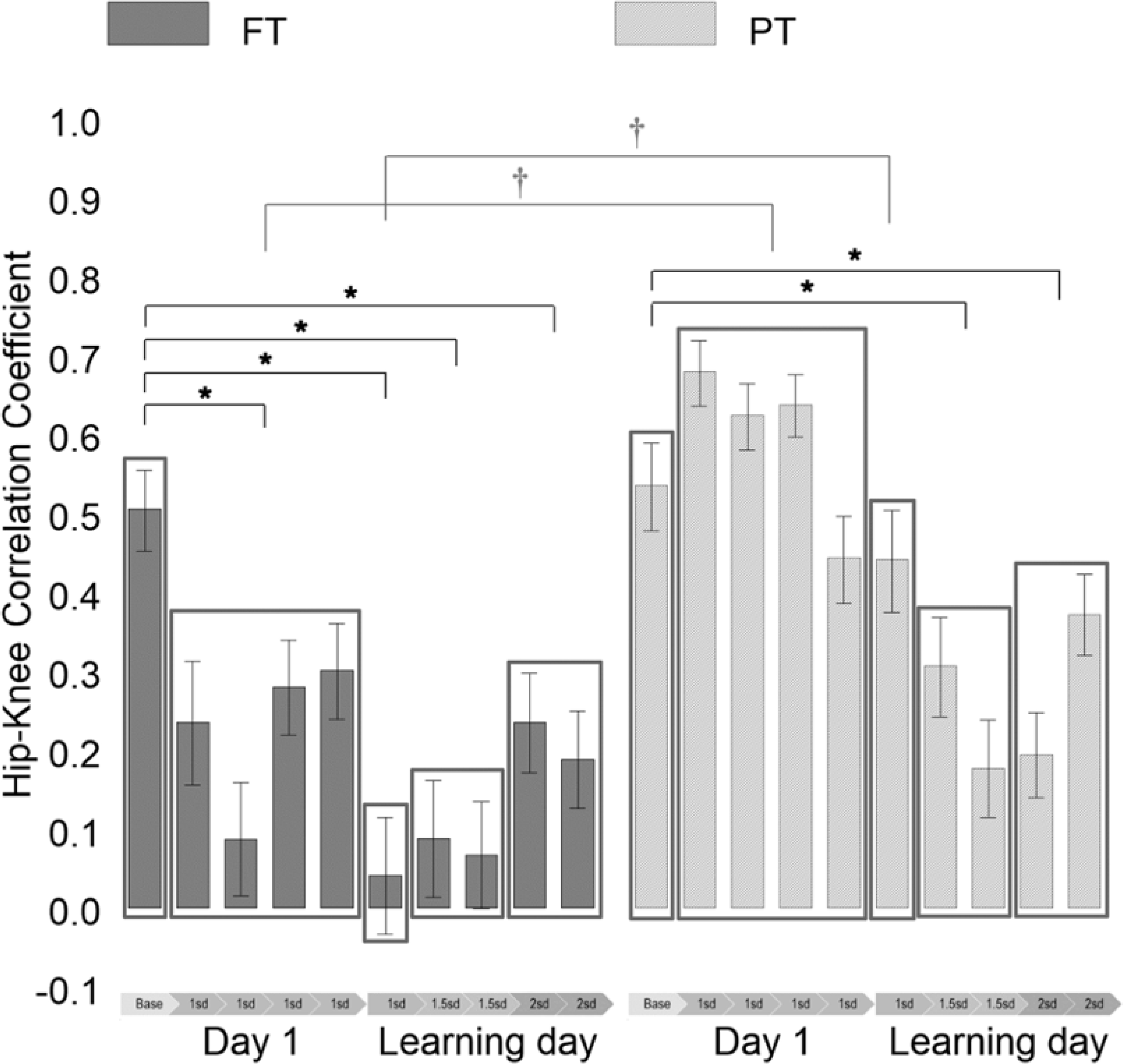
Hip-Knee correlation coefficient (CC) of the kicks of the full-term (FT) and preterm (PT) groups in 2-min intervals on Day 1 and the learning day. Base = baseline condition; 1sd = mean+1sd threshold condition; 1.5sd = mean+1.5sd threshold condition; 2sd = mean+2sd threshold condition. *Significant within-group differences compared to the Hip-Knee CC of the Day 1 baseline condition (adjusted p < 0.05). †Significant between-group differences (adjusted p < .05). Error bars denote standard errors. The Hip-Knee CC are plotted for each 2-min interval to show the change in the time series, but for statistical analysis, the mean values of each threshold condition were compared to the Day 1 baseline condition.
Table 2.
Hip-Knee Correlation Coefficient (CC) by Condition
| Condition | CC of full-term learners, means (SE) | Number of kicks | CC of preterm learners, means (SE) | Number of kicks | |
|---|---|---|---|---|---|
| Day 1 | Baseline | 0.51 (0.05) | 673 | 0.53 (0.06) | 528 |
| Low (+1sd) | 0.23 (0.03)*† | 2,230 | 0.60 (0.02) | 2,548 | |
| Learning Day | Low (+1sd) | 0.04 (0.07)*† | 526 | 0.44 (0.06) | 483 |
| Middle (+1.5sd) | 0.08 (0.05)* | 1,232 | 0.24 (0.04)* | 1,431 | |
| High (+2sd) | 0.21 (0.04)* | 1,472 | 0.28 (0.04)* | 1,890 |
Note. sd, standard deviation; SE, standard error.
Significant within-group differences compared to the Hip-Knee CC of the Day 1 baseline condition (adjusted p <. 05).
Significant between-group differences (adjusted p < .05).
Figure 3.
Change in Hip-Knee correlation coefficient (CC) between the baseline condition and each threshold condition on Day 1 and the learning day (L Day) of the infants born full-term and preterm. Circles denote the mean change in Hip-Knee CC of each infant. Circles located below dotted horizontal lines at −0.25 denote considerable changes into more selective hip-knee movement and the numbers of infants were indicated.
Day 1.
On Day 1, there was no group difference during the baseline condition (t=−0.39, adjusted p>.05), but there was a significant group difference during the low threshold condition (t=−8.95, adjusted p<.05), with a lower Hip-Knee ZCC in the FT group, compared to the PT group. In the FT group, the Hip-Knee ZCC was decreased during the low threshold condition, compared to the baseline condition (t=4.12, adjusted p<.05), but was not significantly different in the PT group (t=−1.11, adjusted p>.05). Based on the individual −0.25 criteria to identify a considerable change in Hip-Knee CC, 5 infants born FT and 2 infants born PT demonstrated a considerable decrease in Hip-Knee CC during the low threshold condition compared to the baseline spontaneous kicking condition.
These results suggest that both infant groups demonstrated similar hip-knee movement during baseline spontaneous kicking. However, the infants born FT generated more selective hip-knee movement when interacting with the mobile on Day 1, compared to baseline spontaneous kicking, but the infants born PT did not. Hip-Knee RP results show a similar trend, more selective movement during the low threshold condition in the FT group, but not PT, although the results were not statistically significant.
Learning day.
On the learning day, there was a significant group difference only during the low threshold condition (t=−3.97, adjusted p<.05), with a lower Hip-Knee ZCC in the FT group, compared to the PT group. There was no group difference during the middle threshold condition (t=−2.38, adjusted p>.05) or high threshold condition (t=−1.20, adjusted p>.05). In the FT group, the Hip-Knee ZCC was decreased during all three threshold conditions, compared to the Day 1 baseline; low (t=5.09, adjusted p<.05), middle (t=5.61, adjusted p<.05), and high (t=4.12, adjusted p<.05). In the PT group, the Hip-Knee ZCC was also decreased during the middle (t=3.91, adjusted p<.05) and high (t=3.50, adjusted p<.05) threshold conditions, but not during the low threshold condition (t=1.07, adjusted p>.05). Based on the individual −0.25 criteria, 8 infants born FT and 6 infants born PT demonstrated a considerable decrease in Hip-Knee CC during the learning day low threshold condition, 9 infants born FT and 6 infants born PT during the middle threshold condition, and 7 infants born FT and 7 infants born PT during the high threshold condition.
These results suggest that the FT group generated more selective hip-knee movement on the learning day at each of the three threshold conditions, and the PT group generated more selective movement only when the middle and high thresholds were used, but not the low threshold. In the FT group, Hip-Knee RP results show a similar trend, more selective movement during all three threshold conditions, but the significant difference was found only during the middle threshold condition. In the PT group, Hip-Knee RP results were consistent with ZCC results.
Discussion
The current study investigated whether 4-month-old infants who learned the scaffolded mobile task demonstrated more selective hip-knee joint movement. Infants born FT, contrary to our hypothesis, demonstrated more selective hip-knee movement on the first day during the low threshold condition, compared to their baseline spontaneous kicking. This finding is different from a previous finding from a similar mobile task of 3-month-old infants born FT in which the infants did not demonstrate more selective hip-knee movement on the first day (Sargent et al., 2014). We speculate that the infants born FT at 4 months in the present study were able to better uncouple their joint movement than at a younger age (Thelen, 1985). Infants born PT at 4 months corrected age, as hypothesized, did not exhibit more selective hip-knee movement on the first day during the low threshold condition, compared to their baseline spontaneous kicking. This is consistent with a previous finding from a similar mobile task of infants born PT at 4 months corrected age (Sargent et al., 2018).
On the learning day, as hypothesized, the infants born FT demonstrated more selective hip-knee movement at each of the three threshold heights, compared to baseline spontaneous kicking. This finding is similar to that of Sargent et al. (2014) in that 3-month-old infants born FT who learned the mobile task demonstrated more selective hip-knee movement on the second day, compared to their spontaneous kicking on the first day. The authors speculated that the infants born FT changed their coordination to activate the mobile more efficiently by holding their hips flexed while bending and straightening their knee joints to keep their feet right around the threshold. This previous study, however, only used the low threshold throughout the task which could still be crossed without moving selectively by lifting the feet against gravity using coupled hip and knee flexion (Sargent et al., 2014). The present study added the middle and high thresholds on the second and third days. We expected that the middle and high threshold heights would more directly motivate infants to move selectively as they needed to flex their hips and extend their knees to move their feet across the higher threshold heights. Our results show that the infants born FT exhibited more selective joint movement at each of the three threshold heights. This finding may imply that infants born FT can generate more selective hip-knee movement without task scaffolding.
The infants born PT, as hypothesized, exhibited more selective hip-knee movement on the learning day when the middle and high threshold heights were used, compared to baseline spontaneous kicking. This result highlights the positive effect of task scaffolding because the previous study using only the low threshold found that 4-month-old infants born PT who learned the task did not change their hip-knee coordination (Sargent et al., 2018). In the current study, the infants born PT also did not change their coordination using the low threshold as expected, but they generated more selective hip-knee movement when the middle and high thresholds were used. This may indicate that the scaffolded mobile task effectively reinforced more selective hip-knee movement of infants born PT.
Scaffolding may provide a means to support the early learning and motor control of infants born PT, at increased risk of cerebral palsy. Due to their early differences in motor control (Dionisio, Santos, & Tudella, 2017; Sargent, Reimann, Kubo, & Fetters, 2017) and increased risk for learning disabilities (Haley, Grunau, Oberlander, & Weinberg, 2008; Lobo & Galloway, 2013), infants born PT may require systematic scaffolding of tasks to generate age-appropriate movement. This study provides an example of how to systematically scaffold a task for infants born PT to generate more selective hip-knee movement. Specifically, we scaffolded the task to reinforce progressively higher kicks to encourage infants born PT to extend the knee while flexing the hip, thereby generating more selective movement.
This study also provides a scientific foundation to develop a task to support more selective movement of infants with brain lesions, at highest risk of cerebral palsy. Scaffolding for infants with brain lesions may include extra days of practice to learn the task (Sargent et al., 2020) and customized threshold heights and timing based on the infants’ real-time performance of the task (Pulido et al., 2017). For example, if the infant demonstrated more selective movement at one height but then demonstrated less selective movement at a higher height, the threshold could be slightly lowered to support the infant’s ability to generate more selective movement. Further study is required to develop this task for infants with brain lesions.
The main limitation of this study is that we do not differentiate the effect of using a high threshold without a gradual increase in height from using a high threshold with a gradual increase (i.e., scaffolding). In the present study, we refer to the term ‘scaffolding’, but note that we cannot ensure that the progressive aspect of the threshold increase is a key factor in explaining our findings. Our second limitation is the lack of a ‘non-scaffolded’ control group. Since this study did not have a control group, conclusions cannot be drawn about coordination change without scaffolding. However, based on the previous research, which found that 4-month-old infants born PT did not change their movement when they participated in a similar mobile task using only the low threshold (Sargent et al., 2018), it seems that the middle and high thresholds used in the present study reinforced the infants born PT to generate more selective hip-knee movement. Further study including a control group is required to confirm the effect of task scaffolding. Specifically, an additional group with a randomized order of threshold conditions could be used to separate the effect of a gradual increase in threshold height from the effect of the high threshold height itself and from the effect of time.
Conclusions
The infants born FT and the infants born PT who learned the scaffolded mobile task demonstrated more selective hip-knee movement at the middle and high threshold heights. This finding provides insight into the effect of task scaffolding on encouraging selective leg movement of infants born PT and may have implications for clinical practice for infants born PT, at increased risk of cerebral palsy.
Supplementary Material
Acknowledgements
We are grateful to Hyeshin Park, Jeremy Welch, Yukikazu Hidaka, Younggeun Choi, and Nicolas Schweighofer who provided technical resources for the development of the infant kick-activated mobile system, and to Jessica Nguyen, Maggie Ridenhour, Manjima Sarkar, Sophia Zhou, Nicole Crisan, Alisa Kokanoutranon, Aubrey Baker, Joshua Limlingan, Emily Puzo, Jamie Proffitt, Vivian Pae, Stephanie Horwitz, Carlos Marroquin, Brandon Mamou, Andres Caballero, and Christine Lee who assisted with data collection and analysis. Special thanks to the parents and infants who participated in the study.
Declarations of Interest
The authors declare no conflicts of interest with regard to the funding source for this study. This research was supported by the USC Division of Biokinesiology and Physical Therapy, the American Physical Therapy Association (APTA) Academy of Pediatric Physical Therapy Mentored Grant to Jeong Ah Kim, a Southern California Clinical and Translational Science Institute (SC CTSI) grant under award number UL1TR001855 and UL1TR000130 from the National Center for Advancing Translational Science (NCATS) of the U.S. National Institutes of Health (NIH), and by the NIH Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) under award number K12-HD055929 (PI: Ottenbacher) to Barbara Sargent. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- Bayley N (2006). Bayley scales of infant and toddler development–third edition: Technical manual. San Antonio, TX: Harcourt Assessment. [Google Scholar]
- Chen YP, Fetters L, Holt KG, & Saltzman E (2002). Making the mobile move: Constraining task and environment. Infant Behavior & Development, 25(2), 195–220. doi: 10.1016/S0163-6383(02)00121-2 [DOI] [Google Scholar]
- Dionisio J, Santos GLD, & Tudella E (2017). Influence of additional ankle weights on kinematic variables of late preterm infants aged 3–4 months. Journal of Motor Behavior, 49(3), 306–311. doi: 10.1080/00222895.2016.1204264 [DOI] [PubMed] [Google Scholar]
- Farmer SE (2003). Key factors in the development of lower limb co-ordination: Implications for the acquisition of walking in children with cerebral palsy. Disability and Rehabilitation, 25(14), 807–816. doi: 10.1080/0963828031000106148 [DOI] [PubMed] [Google Scholar]
- Farmer SE, Pearce G, & Stewart C (2008). Developing a technique to measure intra-limb coordination in gait: Applicable to children with cerebral palsy. Gait and Posture, 28(2), 217–221. doi: 10.1016/j.gaitpost.2007.12.005 [DOI] [PubMed] [Google Scholar]
- Fetters L, Chen YP, Jonsdottir J, & Tronick EZ (2004). Kicking coordination captures differences between full-term and premature infants with white matter disorder. Human Movement Science, 22(6), 729–748. doi: 10.1016/j.humov.2004.02.001 [DOI] [PubMed] [Google Scholar]
- Fetters L, Sapir I, Chen YP, Kubo M, & Tronick E (2010). Spontaneous kicking in full-term and preterm infants with and without white matter disorder. Developmental Psychobiology, 52(6), 524–536. doi: 10.1002/dev.20455 [DOI] [PubMed] [Google Scholar]
- Fowler EG, & Goldberg EJ (2009). The effect of lower extremity selective voluntary motor control on interjoint coordination during gait in children with spastic diplegic cerebral palsy. Gait and Posture, 29(1), 102–107. doi: 10.1016/j.gaitpost.2008.07.007 [DOI] [PubMed] [Google Scholar]
- Fowler EG, Staudt LA, & Greenberg MB (2010). Lower-extremity selective voluntary motor control in patients with spastic cerebral palsy: Increased distal motor impairment. Developmental Medicine & Child Neurology, 52(3), 264–269. doi: 10.1111/j.1469-8749.2009.03586.x [DOI] [PubMed] [Google Scholar]
- Gopagondanahalli KR, Li J, Fahey MC, Hunt RW, Jenkin G, Miller SL, & Malhotra A (2016). Preterm hypoxic–ischemic encephalopathy. Frontiers in Pediatrics, 4(114). doi: 10.3389/fped.2016.00114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg B, Hagberg G, Beckung E, & Uvebrant P (2001). Changing panorama of cerebral palsy in Sweden. VIII. Prevalence and origin in the birth year period 1991–94. Acta Paediatrica, 90(3), 271–277. doi: 10.1111/j.1651-2227.2001.tb00303.x [DOI] [PubMed] [Google Scholar]
- Haley DW, Grunau RE, Oberlander TF, & Weinberg J (2008). Contingency learning and reactivity in preterm and full-term infants at 3 months. Infancy, 13(6), 570–595. doi: 10.1080/15250000802458682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heriza CB (1988). Comparison of leg movements in preterm infants at term with healthy full-term infants. Physical Therapy, 68(11), 1687–1693. doi: 10.1093/ptj/68.11.1687 [DOI] [PubMed] [Google Scholar]
- Himpens E, Van den Broeck C, Oostra A, Calders P, & Vanhaesebrouck P (2008). Prevalence, type, distribution, and severity of cerebral palsy in relation to gestational age: A meta-analytic review. Developmental Medicine & Child Neurology, 50(5), 334–340. doi: 10.1111/j.1469-8749.2008.02047.x [DOI] [PubMed] [Google Scholar]
- Jensen JL, Schneider K, Ulrich BD, Zernicke RF, & Thelen E (1994). Adaptive dynamics of the leg movement patterns of human infants: I. The effects of posture on spontaneous kicking. Journal of Motor Behavior, 26(4), 303–312. doi: 10.1080/00222895.1994.9941686 [DOI] [PubMed] [Google Scholar]
- Kim JA, Fetters L, Kubo M, Eckel SP, & Sargent B (2021). Infants born full term and preterm increase the height of anti-gravity leg movements during a kick-activated mobile task using a scaffolded task environment. Infancy, 26(1), 168–183. doi: 10.1111/infa.12379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MA, & Galloway JC (2013). Assessment and stability of early learning abilities in preterm and full-term infants across the first two years of life. Research in Developmental Disabilities, 34(5), 1721–1730. doi: 10.1016/j.ridd.2013.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascal A, Govaert P, Oostra A, Naulaers G, Ortibus E, & Van den Broeck C (2018). Neurodevelopmental outcome in very preterm and very-low-birthweight infants born over the past decade: A meta-analytic review. Developmental Medicine & Child Neurology, 60(4), 342–355. doi: 10.1111/dmcn.13675 [DOI] [PubMed] [Google Scholar]
- Pulido JC, González JC, Suárez-Mejías C, Bandera A, Bustos P, & Fernández F (2017). Evaluating the child–robot interaction of the NAOTherapist platform in pediatric rehabilitation. International Journal of Social Robotics, 9(3), 343–358. doi: 10.1007/s12369-017-0402-2 [DOI] [Google Scholar]
- Reiser BJ (2004). Scaffolding complex learning: The mechanisms of structuring and problematizing student work. Journal of the Learning Sciences, 13(3), 273–304. doi: 10.1207/s15327809jls1303_2 [DOI] [Google Scholar]
- Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, Damiano D, . . . Jacobsson B. (2007). A report: The definition and classification of cerebral palsy April 2006. Developmental Medicine & Child Neurology Supplement, 109, 8–14. [PubMed] [Google Scholar]
- Rovee-Collier C (1997). Dissociations in infant memory: Rethinking the development of implicit and explicit memory. Psychological Review, 104(3), 467–498. doi: 10.1037/0033-295X.104.3.467 [DOI] [PubMed] [Google Scholar]
- Sargent B, Havens KL, Wisnowski JL, Wu TW, Kubo M, & Fetters L (2020). In-home kicking-activated mobile task to motivate selective motor control of infants at high risk of cerebral palsy: A feasibility study. Physical Therapy, 100(12), 2217–2226. doi: 10.1093/ptj/pzaa174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent B, Kubo M, & Fetters L (2018). Infant discovery learning and lower extremity coordination: Influence of prematurity. Physical & Occupational Therapy in Pediatrics, 38(2), 210–225. doi: 10.1080/01942638.2017.1357065 [DOI] [PubMed] [Google Scholar]
- Sargent B, Reimann H, Kubo M, & Fetters L (2017). Infant intralimb coordination and torque production: Influence of prematurity. Infant Behavior & Development, 49, 129–140. doi: 10.1016/j.infbeh.2017.08.009 [DOI] [PubMed] [Google Scholar]
- Sargent B, Scholz J, Reimann H, Kubo M, & Fetters L (2015). Development of infant leg coordination: Exploiting passive torques. Infant Behavior & Development, 40, 108–121. doi: 10.1016/j.infbeh.2015.03.002 [DOI] [PubMed] [Google Scholar]
- Sargent B, Schweighofer N, Kubo M, & Fetters L (2014). Infant exploratory learning: Influence on leg joint coordination. PLoS One, 9(3), e91500. doi: 10.1371/journal.pone.0091500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RA, & Lee TD (2011). Chapter 10: Motor learning concepts and research methods, In Motor control and learning: A behavioral emphasis (5th ed., pp. 327–346). Champaign, IL: Human Kinetics. [Google Scholar]
- Thelen E (1985). Developmental origins of motor coordination: Leg movements in human infants. Developmental Psychobiology, 18(1), 1–22. doi: 10.1002/dev.420180102 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



