Abstract
Objective:
The goal of the present study was to determine if spike and wave discharges (SWDs) and spike and wave discharges with superimposed fast ripples (SWDFRs) could be biomarkers of post-traumatic epileptogenesis.
Methods:
Fluid percussion injury was conducted on 13–14 week old male Sprague-Dawley rats. Immediately after traumatic brain injury (TBI), they were implanted with microelectrodes in the neocortex, hippocampus and striatum bilaterally. Age matched sham rats with the same electrode implantation montage acted as controls. Wide band brain electrical activity was recorded intermittently from day 1 of TBI, and continued from 2 to 21 weeks after TBI. SWD and SWDFR analysis was performed during the first two weeks to investigate if the occurrence of this pattern predicted development of epilepsy. The remaining 3 to 21 weeks were used for identifying which rats became epileptic (E+ group) and which did not (E− group).
Results:
The E+ group (n= 9) showed a significant increase in SWD rate in prefrontal cortex during weeks 1 and 2 after TBI. E− group showed a significant increase of SWD rate only in the 2nd week. 100% of rats in the E+ group displayed SWDFRs beginning from the first week after TBI. The SWDFR pattern was observed in all recorded brain areas: prefrontal and perilesional cortices, hippocampus and striatum. None of rats in the E− group showed co-existence of FR with SWD.
Significance:
Occurrence of SWDFR after TBI, but not an increase in the rate of SWD, could be a noninvasive EEG biomarker of post-traumatic epileptogenesis.
Keywords: post-traumatic epilepsy, traumatic brain injury, epilepsy, biomarker, spike and wave discharges, fast ripples
1. INTRODUCTION
Acquired epilepsies have a potential for preventive treatment, if reliable biomarkers can indicate which subjects have a high probability to develop epilepsy after an initial insult.1–3 Currently there are numerous attempts to identify molecular, imaging, EEG and behavioral biomarkers, however, we are only at the beginning of the process of finding them. Most likely several biomarkers of different modalities will provide high sensitivity and specificity for determination of subjects who require emergent preventive antiepileptogenic treatment.1,3–7 One of the potential electrographic biomarkers of ongoing epileptogenesis is pathological high frequency oscillations (pHFOs),5,8,9 however this pattern of electrical activity requires invasive implantation of recording electrodes. If pHFOs are consistently associated with other patterns of brain electrical activity that can be recorded noninvasively, it may be possible to distinguish EEG patterns containing pHFOS from those which do not. This will provide an additional tool for potential clinical application. Earlier we have described that pHFOs are associated with spindle like oscillations (SLO),10 which could be recorded by routine clinical EEG and could be analyzed during the post insult period in subjects that later develop and do not develop epilepsy.
During the last several years there has been debate about the role of spike and wave discharges (SWDs), an intrinsic electrophysiological pattern, in post-traumatic epileptogenesis. Earlier studies indicated that the spiky activity in the range of 7–12Hz should be considered epileptiform discharges,11,12 while others found this pattern to be present in normal laboratory rats, and even in wild rats,13–16 and considered it a reflection of normal animal brain activity.14,15 The single SWD pattern can, therefore, reflect both physiological and pathological conditions, while combined patterns, such as pHFOs-SLO (Bragin et al., 201610) are more likely to predict epileptic pathology. In this study, we further extend our focus to investigate the SWDs superimpose on fast ripples (SWDFRs) during posttraumatic epileptogenesis. We hypothesized that the co-occurrences of SWDs and FRs can be observed in animals that later develop epilepsy but not in the lesioned-control animals. By exploring the associations of FRs with this intrinsic, yet easily-detected, electrophysiological pattern, we hope to study the role of SWDFRs and ultimately improve the translational value of non-invasive EEG in epilepsy prevention and cure.
2. METHODS
2.1. Animals
Data were collected from male Sprague-Dawley rats (13–14 weeks old, 350–400g in weight). The animals were purchased from Charles River Laboratories, MA, USA and were maintained on 12–12 hour light-dark cycle with food and water ad libitum. All procedures were approved by the University of California, Los Angeles Animal Research Committee and complied with NIH guideline.
2.2. TBI and electrode Implantation
The TBI model was adopted from the method developed at the UCLA Brain Injury Research Center.17,18 The animals were allowed to acclimate to the laboratory environment for at least 7 days prior to the surgery. They were randomly divided into two groups: a sham group (n= 8) and a TBI group (n= 44), but the final TBI group includes 21 rats by excluding 23 rats (52% mortality rate) which died after lateral fluid percussion injury (FPI). The surgeries were performed on 13–14 weeks old rat. The animals were anesthetized with isoflurane (4–5% for induction, 1.5–2% for maintenance) after which flunixin meglumine (2.5 mg/kg) were given systemically and lidocaine (2%) was applied subcutaneously to the skull. After, the skull was exposed and cleaned with sterile saline, 5.0 mm diameter craniotomy was performed over the left sensorimotor cortex, centered 5.0 mm posterior to the bregma, 3.0 mm lateral to the midline. An injury cap (5.0 mm inner diameter, made from a 1ml syringe barrel) was glued outside the craniotomy edge above the skull and fixed with dental cement. The percussion intensity from the custom-made pneumatic device, 3.2 to 3.5 atm percussion force, considered to cause severe TBI, was adopted from FPI protocol described in our previous publications.10,19 Specifically, the isoflurane anesthesia was discontinued and FPI was induced after the animal was responsive to toe pinch. Following TBI or sham injury, the animals were kept on the heating pad which was maintained at 37°C until first response to toe pinch. The animals were then implanted with 50um thick tungsten electrodes in bilateral sites of (1) prefrontal cortex; AP = 3.7, ML = ±0.5, DV = 3.0 (2) striatum; AP = 1.6, ML = ±1.8, DV = 6.0 (3) anterior and posterior perilesional area; AP = −2.5 and −7.5, ML = 3.0, DV = 1.0 and (4) hippocampus AP = −5.2, ML = ± 5.2, DV = 7.4. In addition, two micro-screws were fixed onto the skull above the cerebellum for reference and ground electrodes. All the electrodes were later secured to the skull with dental acrylic cement and the animals were allowed to recover in individual cages. Sham‐injured rats received identical anesthesia and craniotomy but were not exposed to brain injury. After implantation surgery, the animals were monitored every day for signs of distress, including; stress, loss of appetite and immobility. The animals which did not show voluntary locomotor behavior and which showed signs of disparity were excluded from the study.
2.3. Electrophysiological recordings and data analysis
Local field potentials (LFP) with a wide bandwidth ranging from 0.1 to 3.0 kHz (16 channels) were recorded using the Intan RHD 2000 digital system (Intan Technologies, Los Angeles, CA, USA). One hour after surgery, the rats were plugged into the data acquisition system and wide band electrical activity was recorded continuously for the first three days. On 4th day the rats were unplugged, and re-plugged on 7th day after TBI for 24 hours. From week 2, the EEG was recorded two days a week (48 hours), till week 21 of TBI. For analyzing SWDs and SWDFRs, EEG data from week 1 (days; 1, 2, 3 and 7) and week 2 was used. The remaining data of weeks, 3 to 21 were used for identification of epileptic and non-epileptic rats. Initially, all recorded data were reviewed by two reviewers of the student research program students, who were trained to recognize seizures on the raw data based on the criteria described in our laboratory earlier (Bragin et al. 201628). These data were verified by the one of the co-authors (UK). Based on the existence of seizures, the animals were divided into two groups; those which became epileptic (E+ group) and those which did not (E− group). Though manual reviewing is a time-consuming process, it has advantages over automated methods of checking and asserting true positive events.
Later analysis was performed blindly for the animals in the E+ and E− groups. Three-hour duration raw signal during immobility, free of artifacts, was selected for experimental day (12h) for SWD analyses for each animal. The period of immobility in EEG was characterized by the occurrence of higher amplitude slow waves in prefrontal cortex, striatum, perilesional area and hippocampus and absence of theta rhythm activity. During the awake state or rapid eye movement sleep the theta waves were more prominent in the above recording areas. The accuracy of selected files was verified by reviewing their time frequency plots and by calculating the ratio between delta (1–3Hz) and theta (4–10Hz) frequency bands on power spectrograms. With EDF (European data format) browser software, the SWD episodes were manually detected in 30 second windows from the LFP signals recorded from the prefrontal cortex, striatum, perilesional area (sensorimotor cortex) and hippocampus. The SWD and the SLOs were distinguished in raw wide-band signal based on the following criteria;
In a 30 second epoch window, the amplitude threshold of SWD was three times higher than the background amplitude. The amplitude threshold for SLOs was one to less than three times higher.
The duration of SWDs should be two seconds or greater. The SLOs were 0.5 second to 1.5 seconds in duration.
The peak amplitude in the power spectrogram was 7–12Hz for SWD and 9–14Hz for SLO (Figure 1).
Existence of a spike component duration of 50–100ms in the SWD event.
After calculation of the SWD rate and the duration at each recording site, the data were analyzed for statistical significance between E+, E− and sham groups.
FIGURE 1.
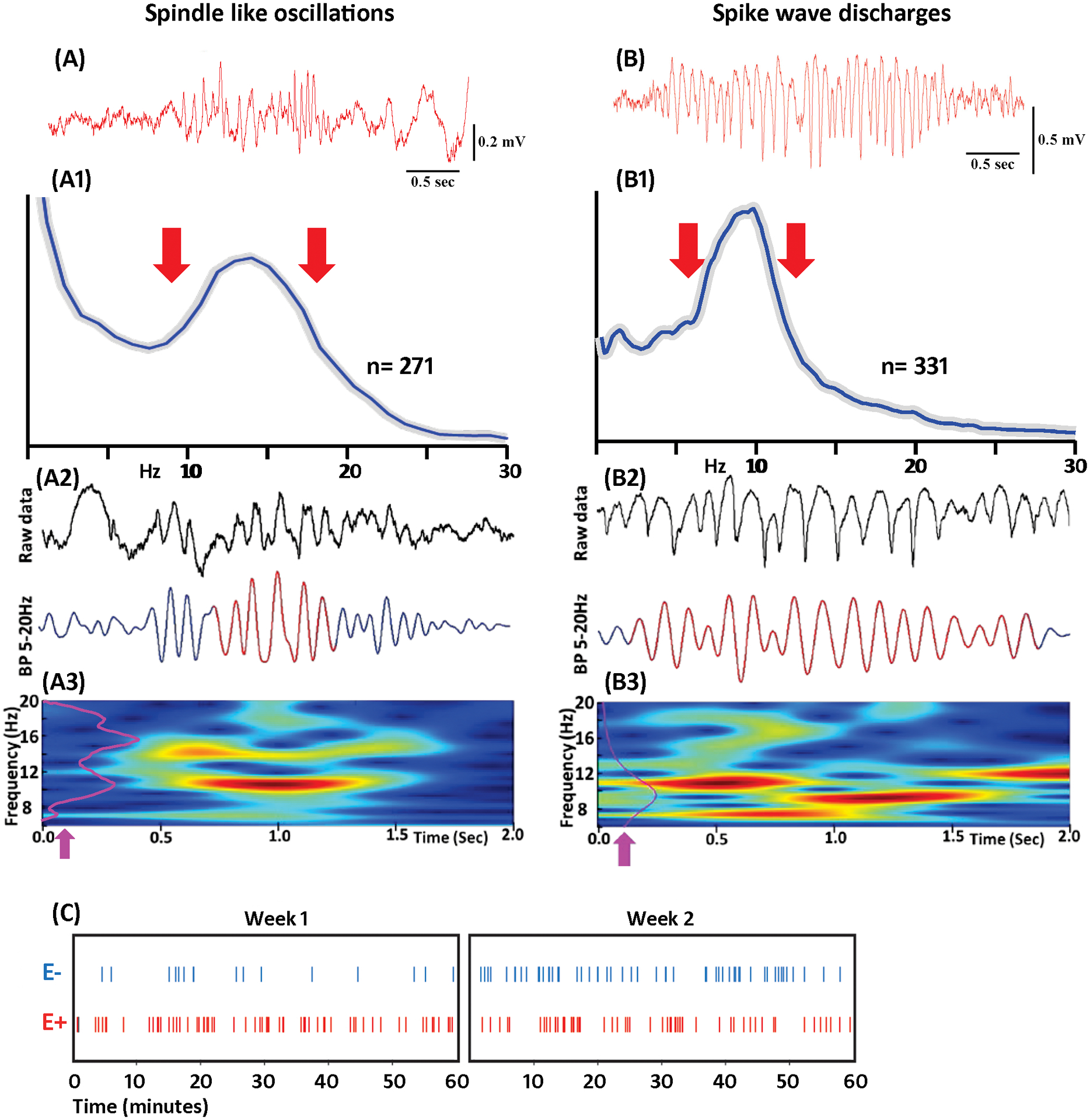
Comparison of spindle like oscillations (SLOs) and spike and wave discharges (SWDs) during slow wave EEG period from TBI group. A, Example of SLO. A1, Average power spectrogram of 271 SLOs. Gray line indicates SD. A2, Example of SLO detected by Ripple lab software; top – raw data and bottom band pass (5–20Hz) filtered data. A3, Time frequency plot, where the purple line (arrow) is the normalized power histogram of the event. B, Example of SWD. B1, Average power histogram of 331 SWDs. B2, Example of SWD detected by the Ripple lab software with raw data on the top and band pass filtered data at the bottom. B3, Time frequency plot. C, Demonstration of SWDs rate in one-hour duration EEG of E+ and E− groups. SWDs were combined for all subjects at each week period. Results indicate that animals from E+ group displayed more SWDs than the animals in E− group. The increase in SWDs in E+ group was seen in week 1 and week 2 period of TBI.
For detecting fast ripple oscillations (FRs), the same three hour duration wideband EDF files from each animal per experimental day were imported to MATLAB (MathWorks, Natick, MA, USA) RIPPLELAB toolbox. The fast ripples (250Hz to 500Hz) were identified based on the criteria described by Staba et al.20 Specifically, the EEG data were bandpass filtered (250Hz to 500Hz), the root mean square of the band pass signal (3 millisecond window) was calculated, and events containing minimum 4 oscillations and the values of root mean square value greater than 3 standard deviation with longer than 6 millisecond duration were selected. Finally, these RIPPLELAB detected fast ripple events were visually cross-examined with raw wideband EEG data to ascertain their true positivity.
2.4. Magnetic resonance Imaging
After completion of EEG recording, the animals were perfused, and its brain were fixed for an ex-vivo MRI scan to validate the electrode tracks. The MRI was performed on a 7T horizontal bore magnet (Bruker, Billerica, MA, USA) with 14mm diameter H radiofrequency coils. One structure scan was performed using T2-weighted Rapid-Relaxation-with-Enhancement (RARE). The data was acquired with 50×0.5mm slices, 128×128 in a 35×35mm field-of-view; TR/TE=5000, 60, RARE factor 8, 2 averages (Supplementary Figure 1).
2.5. Statistical analysis
Statistical analysis was performed using the Prism 7.0 software (GraphPad Software, San Diego, CA). After passing D’Agostino-Pearson normality test, statistical analysis was performed using one-way ANOVA for the comparison of total SWD rate and duration among E+, E− and sham groups. This was followed by Sidak test for the comparison between E+ and the other two groups in each week period. For SWD occurrence side in E+ and E− group, after Shapiro-Wilk normality test, unpaired T-test was performed for comparison of SWD numbers between ipsilateral and contralateral sides in each recording period. For FR associated with SWD, Shapiro-Wilk normality and T-tests were performed for comparison between the recording weeks in E+ group. For all the tests a confidence level of 95% was accepted as significant.
3. RESULTS
3.1. General description
Electrographic recordings were conducted on all the 21 TBI rats, in addition to 8 sham rats. Out of 21 rats which survived TBI, 9 became epileptic displaying recurrent spontaneous seizures between weeks 2 and 21, and 12 rats did not develop epilepsy. In total, 435 hours of recordings from week 1 to week 2 were analyzed for detecting SWDs and SWDFRs, in epileptic, non-epileptic and sham rats. On average 15 hours of recordings were reviewed per animal.
SWDs were observed in prefrontal cortex, striatum, perilesional area (sensorimotor cortex) and hippocampus. The analysis was performed on a total 1316 SWD events from E+ (895), E− (399) and sham (22) rats. There was no significant difference in the rate and duration of SWD between brain areas located ipsi- and contralateral to the TBI side. The highest amplitude of SWD was in the prefrontal cortex and we focused our analysis on SWD recorded in this area.
3.2. Rate and duration of SWD after TBI
The mean duration of SWDs in E+, E− and sham group was correspondingly 2.5 ± 0.3, 2.3 ± 0.1 and 2.1 ± 0.1 seconds. There were no significant differences between the groups by this parameter (P> 0.05). We did not observe SWD durations longer than 3 seconds in these experiments, although SWD durations of 5 seconds and longer were noticed in rats 6 month of age and older (data not shown). In 40% of the sham group (n= 3) SWD were not observed at all. In 60% (n= 5) of the sham group SWDs were observed at a rate of one SWD per 1–2 hours.
In all animals after TBI, SWD appeared during the first week and remained during 2 weeks period. The total SWD rate in the E+ group varied between 3 to 10 per hour, and in the E− group between 2 to 7 per hour (Figure 1C). The SWD rate was 81% and 28% higher in the E+ group during weeks 1 and 2 respectively, in comparison with the E− group (Figure 2A). Statistical analysis shows a significant increase in SWD rate in both the E+ and E− groups compared to the sham group during the two weeks period after TBI (Table 1). Specifically, the Sidak test revealed that in the E+ group, the total SWD rate and the total duration per hour (Figure 2B) was significantly higher during the week 1 and 2 than in the E− and sham group [(SWD rate:- week 1: P< 0.001; week 2: P< 0.001). (SWD duration: - week 1: P< 0.001; week 2: P< 0.001)].
FIGURE 2.
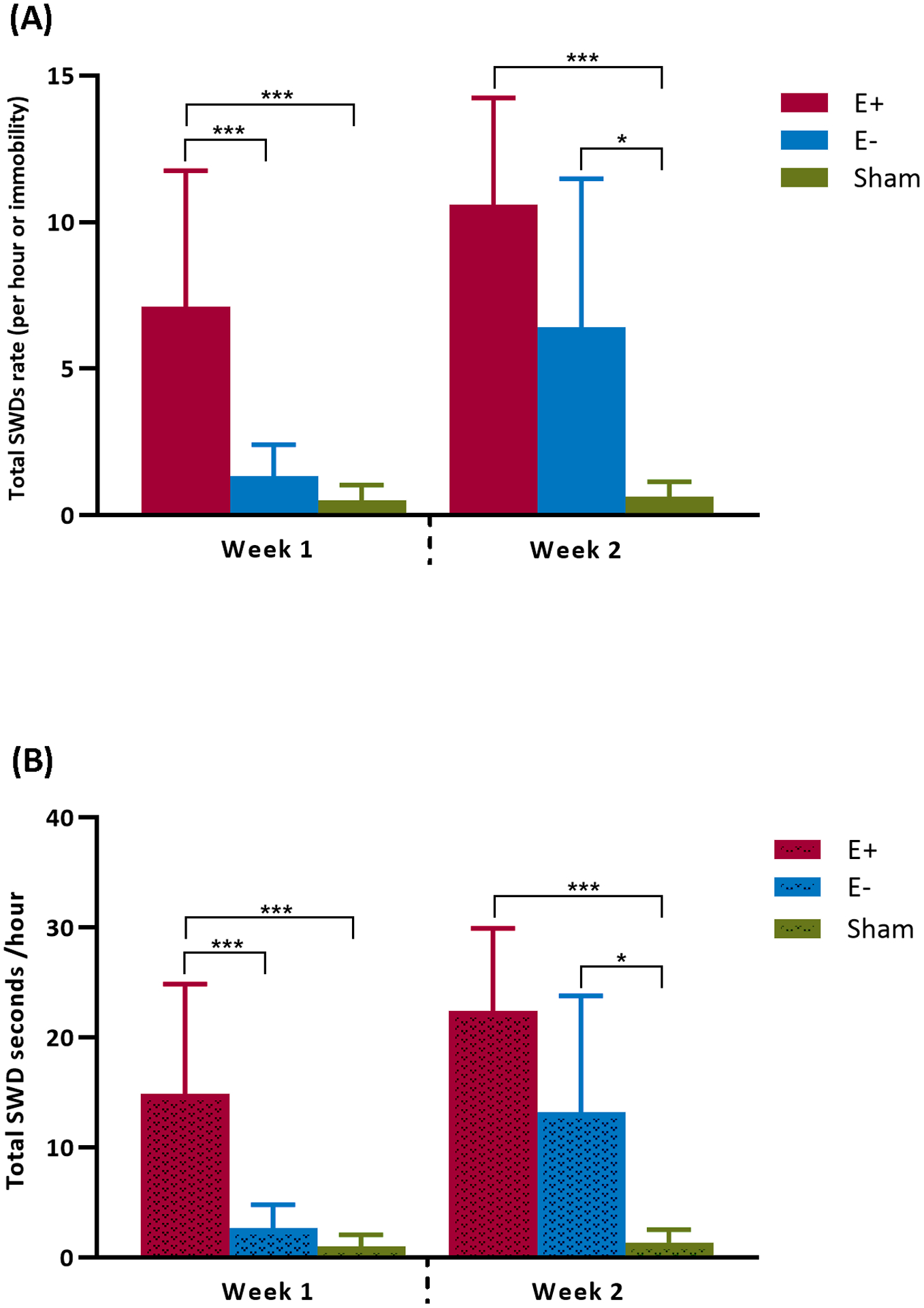
Comparison of total SWDs rate (A) and total duration (B) per hour in E+, E− and sham groups. Data are presented as mean and standard deviation. ***Significant difference (P < 0.001) and *Significant difference (P < 0.05).
Table 1.
Total SWD rate per hour (mean ± SD)
| Week 1 | Week 2 | |
|---|---|---|
| E+ (n= 9) | 7.1 ± 4.6*** | 10.6 ± 3.6*** |
| E− (n= 12) | 1.3 ± 1.0 | 6.4 ± 5.0* |
| Sham (n= 8) | 0.5 ± 0.5 | 0.6 ± 0.5 |
Sidak test revealed, in E+ group total SWD rate was significantly higher on week 1 and week 2 when compared to E− and sham groups. On week 2, a significant difference in total SWD rate was noticed in E− group in comparison to sham group. Asterisks represent statistical significance;
Significant difference (P < 0.001)
Significant difference (P < 0.05).
3.3. Co-occurrence of Fast Ripples with SWD after TBI
In epileptic brain fast ripples (250–500Hz) are considered as a pathologic high frequency oscillations.21,22 Similar to our previous publications (Bragin et al., 201610; Reid et al., 201619) in the current study fast ripples were initially found in the perilesional area. They occurred by itself, or were associated with EEG spikes or SLOs. Fast Ripples were also associated with SWDs throughout the two-week recording period in the prefrontal cortex, striatum, perilesional neocortical area and hippocampus, in the animals that later developed epilepsy (Figure 3). We have termed this pattern Spike Wave Discharges with superimposed Fast Ripples (SWDFR). 100% (n= 9) of rats in the E+ group displayed SWDFRs from week 1 to week 2. FR oscillations were associated with almost all spike components of the SWD (Figures 3 and 4A). No fast ripples were seen on SWDs ≥ 2 second long in E− or sham groups. Figure 4A, B, C shows an example of SWDFR event in the prefrontal cortex, of the E+ group. Spike triggered average (Figure 4D) and perievent histogram (Figure 4E) confirm that the majority of fast ripples occurred on the spike component of the SWD event. 90% of SWD recorded in the prefrontal cortex and striatum were associated with the FR oscillations, while only 10% of SWDs in the perilesional area and hippocampus were associated with the FR component. SWDFRs were found both in ipsilateral and contralateral sides of TBI. Detailed analysis of spatial distribution of SWD and FR is a subject of other study.
FIGURE 3.
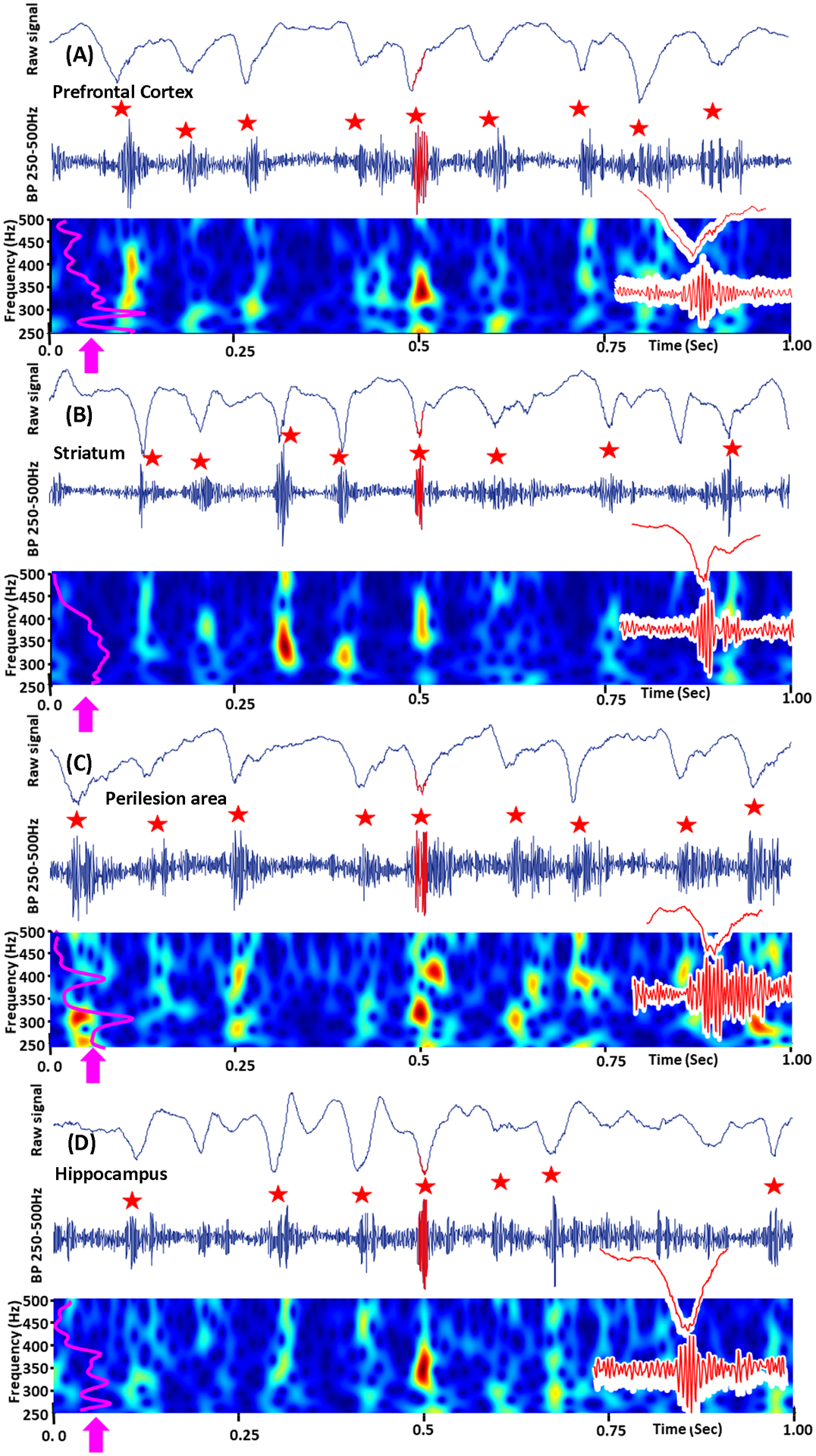
Examples of SWDFR from E+ group. A, EEG from prefrontal cortex, recorded on week 1 of TBI show the presence of FRs (red star) in ≥ 2 second SWD event. B, C and D are examples of SWDFRs in striatum, perilesion area (somatosensory cortex) and hippocampus. The top image in A, B, C and D is 1 second epoch window with raw SWD event. Middle is the 250–500Hz band pass filtered data. Bottom is the time-frequency plots, were purple line (arrow) is normalized power histogram of HFO events. The insets on the right are extended examples of HFOs with raw frequency band recorded LFPs. Fast ripples associated with SWD spikes are indicated by red stars.
FIGURE 4.
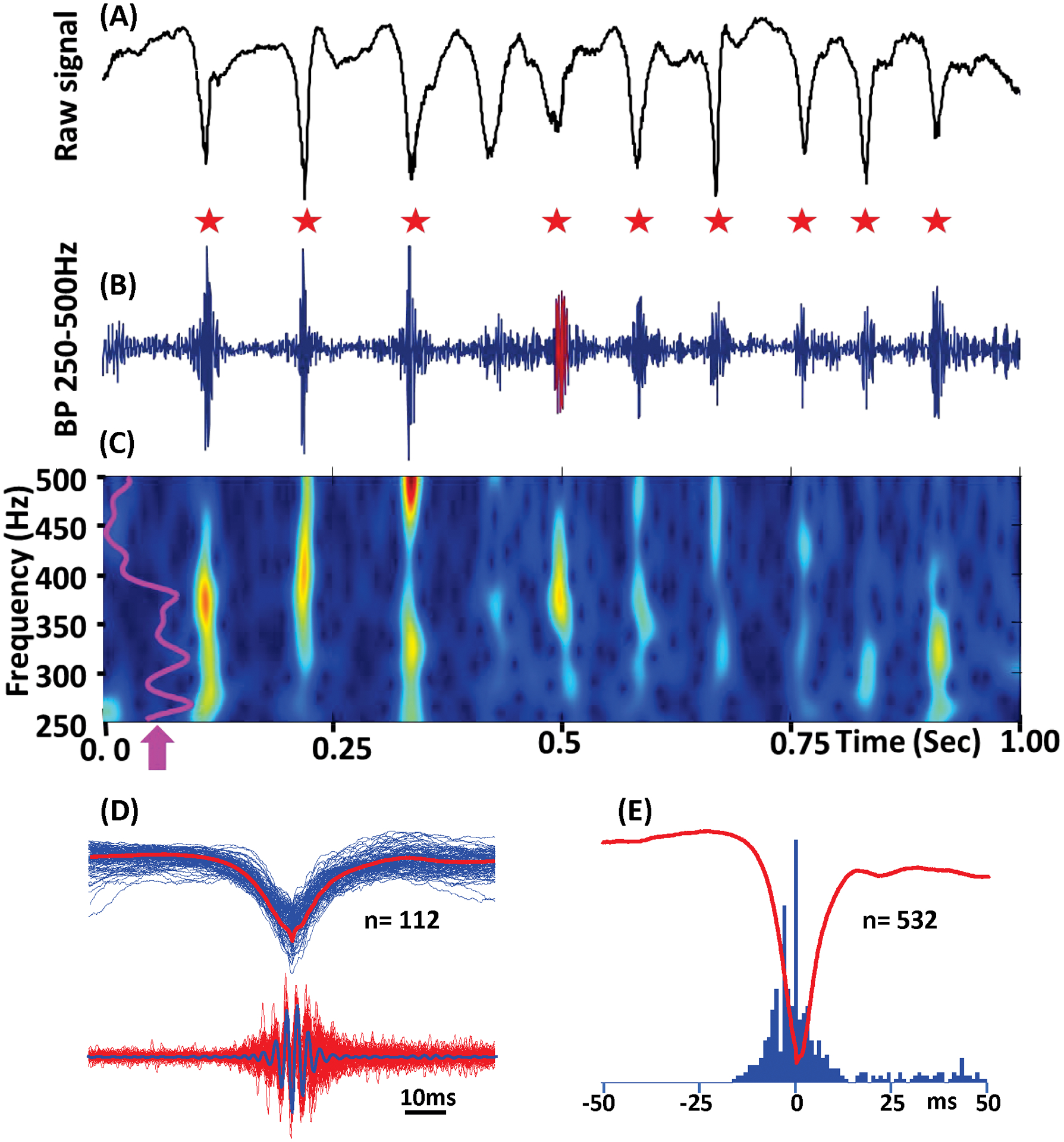
Example of SWDFR from E+ group. A, Raw SWD event. B, Band pass (250–500Hz) signal. Fast ripples associated with the SWD spikes are indicated by red stars. C, Time frequency plot of the event presented above. Purple line (arrow) represents normalized power histogram. D, Superposition of 112 SWD spikes (top) and FR oscillations (bottom). The red line on the top and the blue line at the bottom are average of SWD spikes and FR oscillations. E, Perievent histogram of FR oscillations triggered by SWD local field potential spikes.
3.4. MRI analysis of brain images
An example of MRI images with electrode localizations is presented in the Supplement Figure 1. MRI inspection of brains after perfusion did not reveal any sign of abscess or other infection. In the coronal sections of the brain, at a plane including the injury and the posterior hippocampus, showed that in both E+ and E− groups there was greater tissue loss and cortical thinning, ventricular enlargement, and deformation of the hippocampus ipsilateral to the side of injury. No lesions were noted in any sham-injured animals.
4. DISCUSSION
Our data are close to the results described by Pearce et al. 201416 that SWD are essentially absent in the Sprague-Dawley males within a period 2–6 months. Although we did observe SWD in the sham group, the rate was low 0.5–0.6/ hour and observed in 60% of animals. Our results contradict publications of Rodgers et al. 201513 and Taylor et al. 201715 which found SWD in normal conditions in the same strain of rats during periods of 2–6 months. The reason for these differences is unclear and we think that one of the explanations could be that rats from different vendors were used in these experiments. Rodgers et al. 2015 and Taylor et al. 2017 used rats from Harlan and Pearce et al. 2014 and our data were obtained from rats purchased from Charles River. The percentage of rats that developed epilepsy after TBI varied in different publications from zero13,15 to 25%.11,23 In our study, 42% of animals developed epilepsy, consistent with our earlier publications10,19 and publications from other laboratories.24,25 This difference may be explained by the specifics of the TBI procedure in our experiments. The diameter of the TBI area in our experiments was 5mm compared to 3–4mm in other publications. We have used our homemade device, which was based on the schematics described by Frey et al. 200926, and TBI was performed at the time when animals just recovered after isoflurane anesthesia. The mortality rate in our experiments was very high 52% compare to 25–30% in other studies.11,27,28
The first finding of this study is that TBI significantly increases the rate of occurrence of SWD in all brain areas both in the E+ and E− groups. An increase in the rate of SWD during the 1st week after TBI is significantly higher in the group that later developed epilepsy. We speculate that the process of occurrence of SWD is similar to what is observed during the aging process when spindle oscillations are transformed into high voltage spindles.29–31 This process is believed to be related to the death of interneurons and an increase in synchronization of neuronal discharges in the thalamo-cortical circuitry and specifically may depend on pathological changes in the cholinergic nucleus basalis.
The second finding of this study is that in the E+ group SWD discharges were associated with high frequency oscillations in the fast ripple frequency range (250–500Hz), which are consider as pathological high frequency oscillations (pHFOs). The association of HFOs with SWDs was described in older rats by Kandel and Buzsaki 1997.31 Based on similarities between Kandel and Buzaski’s data, we can suggest that TBI might not only trigger epileptogenesis but also accelerate a processes similar to aging. The association of SWD with pHFO indicates higher synchrony of neuronal discharges compared to those SWD where pHFOs are not visible. The degree of neuronal discharge synchrony may be critical for later development of the epileptogenic neuronal network. TBI related occurrence of SWD during later periods is difficult to investigate because of the age-related occurrence of normal SWD. Furthermore, we cannot say with confidence that our E− group will not later develop epilepsy.
As it was shown in the KA chronic model of epilepsy32,33 these pHFOs occurred in multiple brain areas. In our previous publication10 we showed that FR are associated with SLO and we termed this pattern repetitive HFOs and spikes (rHFOSs). pHFOs can occur independently or be associated with other EEG pattern such as interictal spikes or slow waves34–36,21 or, as shown in our earlier and current experiments with SLO and SWD. Association of FR with other EEG patterns should change their shape and more careful investigation of this parameter in clinical recordings may provide indirect information about the existence of pHFOs in the recorded area.
The third finding of the current study is that SWDFRs are found in multiple brain areas: perilesional cortex, prefrontal cortex, striatum and hippocampus bilaterally. Considering that SWDFRs are generated by clusters of pathologically interconnected neurons (PIN-clusters),22,37 the occurrence of SWDFR could indicate the formation of an epileptogenic PIN cluster network during TBI related epileptogenesis. Detailed investigation of the properties of PIN cluster networks is a subject of future studies.
Supplementary Material
SUPPLEMENTRY FIGURE 1 Ex-vivo MRI scan indicate the tracks of implanted depth electrodes in, prefrontal cortex (A), striatum (B), and CA1 of hippocampus (C). Dots represent the tip of electrode in each brain area. Green dots represent the electrode tip in prefrontal cortex, orange dots represents striatum and blue dots represent the electrode tip in hippocampus.
MRI scan protocol: At the termination of EEG recording, ex-vivo MRI was performed on rat brain to validate the electrode tracks. MRI was performed on 7T horizontal bore magnet (Bruker, Billerica, MA) with 14mm diameter H radiofrequency coils. Structure scan was performed using T2-weighted Rapid-Relaxation-with-Enhancement (RARE). The data was acquired with 50×0.5mm slices, 128×128 in a 35×35mm field-of-view; TR/TE=5000, 60, RARE factor 8, 2 averages.
SUPPLEMENTRY FIGURE 2 Examples of electrographic seizures captured on continuous EEG recording from epileptic rats after traumatic brain injury. A, Example of a generalized seizure observed on the week 3 after lateral fluid percussion injury. B, Example of a focal seizure observed on the week 5 after lateral fluid percussion injury. LPC/RPC= left/right prefrontal cortex, LSTR/RSTR= left/right striatum, APLC/PPLC= anterior/posterior perilesional cortex, LHP/RHP= left/right hippocampus.
SUPPLEMENTRY FIGURE 3 Total SWDFRs rate per hour in E+ group. After TBI, E+ group demonstrated continuous fast ripples (250–500 Hz) superimposed in ≥ 2 second SWD event during week 1 and week 2 period. Data are presented as mean and standard deviation. SWDFRs= spike and wave discharge with superimposed fast ripples; SWD= spike and wave discharge.
Key points.
Post-traumatic epilepsy is a serious consequence of traumatic brain injury, highlighting a dire need for novel biomarkers.
An increased rate of spike and wave discharges after TBI does not necessarily reflect the process of epileptogenesis.
Increased rate of spike and wave discharges with superimposed fast ripples could be a noninvasive biomarker of post-traumatic epileptogenesis.
ACKNOWLEDGMENTS
This study was supported by research grants R01NS033310, U54NS100064, and R01NS065877 from the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
REFERENCES
- 1.Pitkanen A, Engel J Jr. Past and present definitions of epileptogenesis and its biomarkers Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2014. April;11:231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pitkanen A, Halonen T. Prevention of epilepsy Trends Pharmacol Sci. 1998. July;19:253–255. [DOI] [PubMed] [Google Scholar]
- 3.Pitkanen A, Lukasiuk K. Mechanisms of epileptogenesis and potential treatment targets Lancet neurology. 2011. February;10:173–186. [DOI] [PubMed] [Google Scholar]
- 4.Simonato M, Agoston DV, Brooks-Kayal A, Dulla C, Fureman B, Henshall DC, et al. Identification of clinically relevant biomarkers of epileptogenesis - a strategic roadmap Nat Rev Neurol. 2021. February 16. [DOI] [PubMed] [Google Scholar]
- 5.Engel J. Epileptogenesis, traumatic brain injury, and biomarkers Neurobiology of Disease. 2019;123:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engel J Jr, Bragin A, Staba R. Nonictal EEG biomarkers for diagnosis and treatment Epilepsia Open. 2018;3:120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pitkänen A Therapeutic approaches to epileptogenesis—Hope on the horizon Epilepsia. 2010;51 (suppl. 3):2–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bragin A, Engel JJ, Staba RJ. High-frequency oscillations in epileptic brain Current Opinion in Neurology. 2010;23:151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engel J Jr., Pitkanen A, Loeb JA, Dudek FE, Bertram EH 3rd, Cole AJ, et al. Epilepsy biomarkers Epilepsia. 2013. August;54 Suppl 4:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bragin A, Li L, Almajano J, Alvarado-Rojas C, Reid AY, Staba RJ, et al. Pathologic electrographic changes after experimental traumatic brain injury Epilepsia. 2016;57:735–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Ambrosio R, Fairbanks JP, Fender JS, Born DE, Doyle DL, Miller JW. Post-traumatic epilepsy following fluid percussion injury in the rat Brain. 2004. February 1, 2004;127:304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Ambrosio R, Fender JS, Fairbanks JP, Simon EA, Born DE, Doyle DL, et al. Progression from frontal-parietal to mesial-temporal epilepsy after fluid percussion injury in the rat Brain. 2005. January 1, 2005;128:174–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodgers KM, Dudek FE, Barth DS. Progressive, Seizure-Like, Spike-Wave Discharges Are Common in Both Injured and Uninjured Sprague-Dawley Rats: Implications for the Fluid Percussion Injury Model of Post-Traumatic Epilepsy The Journal of Neuroscience. 2015. June 17, 2015;35:9194–9204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor JA, Reuter JD, Kubiak RA, Mufford TT, Booth CJ, Dudek FE, et al. Spontaneous Recurrent Absence Seizure-like Events in Wild-Caught Rats The Journal of Neuroscience. 2019;39:4829–4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor JA, Rodgers KM, Bercum FM, Booth CJ, Dudek FE, Barth DS. Voluntary Control of Epileptiform Spike-Wave Discharges in Awake Rats J Neurosci. 2017. June 14;37:5861–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pearce PS, Friedman D, LaFrancois JJ, Iyengar SS, Fenton AA, MacLusky NJ, et al. Spike-wave discharges in adult Sprague-Dawley rats and their implications for animal models of temporal lobe epilepsy Epilepsy and Behavior. 2014;32:121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giza CC, Prins ML, Hovda DA, Herschman HR, Feldman JD. Genes preferentially induced by depolarization after concussive brain injury: effects of age and injury severity J Neurotrauma. 2002. April;19:387–402. [DOI] [PubMed] [Google Scholar]
- 18.Prins ML, Lee SM, Cheng CL, Becker DP, Hovda DA. Fluid percussion brain injury in the developing and adult rat: a comparative study of mortality, morphology, intracranial pressure and mean arterial blood pressure Brain Res Dev Brain Res. 1996. September 2;95:272–282. [DOI] [PubMed] [Google Scholar]
- 19.Reid AY, Bragin A, Giza CC, Staba RJ, Engel J. The progression of electrophysiologic abnormalities during epileptogenesis after experimental traumatic brain injury Epilepsia. 2016;57:1558–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Staba RJ, Wilson CL, Bragin A, Fried I, Engel J Jr. Quantitative analysis of high-frequency oscillations (80–500 Hz) recorded in human epileptic hippocampus and entorhinal cortex J Neurophysiol. 2002. October;88:1743–1752. [DOI] [PubMed] [Google Scholar]
- 21.Bragin A, Engel J Jr., Wilson CL, Fried I, Mathern GW. Hippocampal and entorhinal cortex high-frequency oscillations (100--500 Hz) in human epileptic brain and in kainic acid-treated rats with chronic seizures Epilepsia. 1999;40:127–137. [DOI] [PubMed] [Google Scholar]
- 22.Bragin A, Mody I, Wilson CL, Engel J Jr. Local Generation of Fast Ripples in Epileptic Brain J Neuroscience. 2002;22:2012–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andrade P, Ciszek R, Pitkänen A, Ndode-Ekane XE. A web-based application for generating 2D-unfolded cortical maps to analyze the location and extent of cortical lesions following traumatic brain injury in adult rats Journal of Neuroscience Methods. 2018;308:330–336. [DOI] [PubMed] [Google Scholar]
- 24.Kharatishvili I, Immonen R, Grohn O, Pitkanen A. Quantitative diffusion MRI of hippocampus as a surrogate marker for post-traumatic epileptogenesis Brain. 2007. December;130:3155–3168. [DOI] [PubMed] [Google Scholar]
- 25.Kharatishvili I, Nissinen JP, McIntosh TK, Pitkanen A. A model of posttraumatic epilepsy induced by lateral fluid-percussion brain injury in rats Neuroscience. 2006;140:685–697. [DOI] [PubMed] [Google Scholar]
- 26.Frey LC, Hellier J, Unkart C, Lepkin A, Howard A, Hasebroock K, et al. A novel apparatus for lateral fluid percussion injury in the rat Journal of Neuroscience Methods. 2009;177:267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andrade P, Nissinen J, Pitkanen A. Generalized Seizures after Experimental Traumatic Brain Injury Occur at the Transition from Slow-Wave to Rapid Eye Movement Sleep J Neurotrauma. 2017. April 1;34:1482–1487. [DOI] [PubMed] [Google Scholar]
- 28.Mishra AM, Bai X, Sanganahalli BG, Waxman SG, Shatillo O, Grohn O, et al. Decreased resting functional connectivity after traumatic brain injury in the rat PloS one. 2014;9:e95280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buzsaki G, Bickford RG, Armstrong DM, Ponomareff G, Chen KS, Ruiz R, et al. Electric activity in the neocortex of freely moving young and aged rats Neuroscience. 1988;26:735–744. [DOI] [PubMed] [Google Scholar]
- 30.Buzsaki G, Laszlovszky I, Lajtha A, Vadasz C. Spike-and-wave neocortical patterns in rats: genetic and aminergic control Neuroscience. 1990;38:323–333. [DOI] [PubMed] [Google Scholar]
- 31.Kandel A, Buzsaki G. Cellular-synaptic generation of sleep spindles, spike-and-wave discharges, and evoked thalamocortical responses in the neocortex of the rat. J of Neurosci. 1997; 17:6783–6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li L, Patel M, Almajano J, Engel J, Bragin A. Extrahippocampal high-frequency oscillations during epileptogenesis Epilepsia. 2018;59:epi.14041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheybani L, Birot G, Contestabile A, Seeck M, Kiss JZ, Schaller K, et al. Electrophysiological Evidence for the Development of a Self-Sustained Large-Scale Epileptic Network in the Kainate Mouse Model of Temporal Lobe Epilepsy J Neurosci. 2018. April 11;38:3776–3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ewell LA, Fischer KB, Leibold C, Leutgeb S, Leutgeb JK. The impact of pathological high-frequency oscillations on hippocampal network activity in rats with chronic epilepsy Elife. 2019. February 22;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kobayashi K, Yoshinaga H, Toda Y, Inoue T, Oka M, Ohtsuka Y. High-frequency oscillations in idiopathic partial epilepsy of childhood Epilepsia. 2011;52:1812–1819. [DOI] [PubMed] [Google Scholar]
- 36.Levesque M, Shiri Z, Chen LY, Avoli M. High-frequency oscillations and mesial temporal lobe epilepsy Neurosci Lett. 2018. February 22;667:66–74. [DOI] [PubMed] [Google Scholar]
- 37.Bragin A, Wilson CL, Engel J Jr. Chronic epileptogenesis requires development of a network of pathologically interconnected neuron clusters: a hypothesis Epilepsia. 2000;41:S144–152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTRY FIGURE 1 Ex-vivo MRI scan indicate the tracks of implanted depth electrodes in, prefrontal cortex (A), striatum (B), and CA1 of hippocampus (C). Dots represent the tip of electrode in each brain area. Green dots represent the electrode tip in prefrontal cortex, orange dots represents striatum and blue dots represent the electrode tip in hippocampus.
MRI scan protocol: At the termination of EEG recording, ex-vivo MRI was performed on rat brain to validate the electrode tracks. MRI was performed on 7T horizontal bore magnet (Bruker, Billerica, MA) with 14mm diameter H radiofrequency coils. Structure scan was performed using T2-weighted Rapid-Relaxation-with-Enhancement (RARE). The data was acquired with 50×0.5mm slices, 128×128 in a 35×35mm field-of-view; TR/TE=5000, 60, RARE factor 8, 2 averages.
SUPPLEMENTRY FIGURE 2 Examples of electrographic seizures captured on continuous EEG recording from epileptic rats after traumatic brain injury. A, Example of a generalized seizure observed on the week 3 after lateral fluid percussion injury. B, Example of a focal seizure observed on the week 5 after lateral fluid percussion injury. LPC/RPC= left/right prefrontal cortex, LSTR/RSTR= left/right striatum, APLC/PPLC= anterior/posterior perilesional cortex, LHP/RHP= left/right hippocampus.
SUPPLEMENTRY FIGURE 3 Total SWDFRs rate per hour in E+ group. After TBI, E+ group demonstrated continuous fast ripples (250–500 Hz) superimposed in ≥ 2 second SWD event during week 1 and week 2 period. Data are presented as mean and standard deviation. SWDFRs= spike and wave discharge with superimposed fast ripples; SWD= spike and wave discharge.


