Abstract
This Letter describes the synthesis and optimization of a series of heteroaryl-pyrrolidinone positive allosteric modulators (PAMs) of the muscarinic acetylcholine receptor M1 (mAChR M1). Through the continued optimization of M1 PAM tool compound VU0453595, with a focus on replacement of the 6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one with a wide variety of alternative 4,5-dihydropyrrolo-fused heteroaromatics, the generation of M1 PAMs with structurally novel chemotypes is disclosed. Two compounds from these subseries, 8b (VU6005610) and 20a (VU6005852), show robust selectivity for the M1 mAChR, and no M1 agonism. Both compounds have favorable preliminary PK profiles in vitro; 8b additionally demonstrates high brain exposure in a rodent IV PK cassette model.
Keywords: muscarinic acetylcholine receptor, positive allosteric modulator, structure-activity relationship
Graphical Abstract
The muscarinic acetylcholine receptors (mAChRs, subtypes M1-M5) are Class A G protein-coupled receptors (GPCRs) with important roles in a variety of biological processes. The mAChRs are expressed in both peripheral and central nervous system (CNS) tissues, and all 5 subtypes have emerged as attractive targets for drug discovery.1,2 In particular, the M1 mAChR has seen a steady increase in interest from the scientific community. A growing body of evidence suggests that M1 plays a crucial role in the modulation of biological pathways relating to cognition, learning and memory.3-5 Specifically, reduction in M1 expression in the prefrontal cortex has been noted in populations of schizophrenic patients4, and allosteric M1 modulators such as benzylquinolone carboxylic acid (BQCA) have been shown to restore discrimination reversal learning in rodent models of Alzheimer’s disease.5 As such, the need for novel (and selective) tool compounds to further probe M1 biology has never been more important.
Xanomeline is an extensively characterized muscarinic agonist that showed promise in treating the cognitive deficits related to Alzheimer’s disease (AD) and schizophrenia.6 The compound is often referred to in the literature as “M1/M4 preferring”, a fitting description given the reports highlighting the roles of these two subtypes in xanomeline’s pro-cognitive efficacy.6-8 Although xanomeline ultimately proved intolerable to patients due to severe gastrointestinal side effects, the drug’s promise as a CNS therapeutic continues to this day. Recently, promising phase 2 clinical data with a combination of xanomeline and trospium (a peripheral muscarinic antagonist) have been disclosed.9
Inspired by the efficacy of xanomeline and other early muscarinic agonists, both industrial10-13 and academic14-20 groups have disclosed a variety of novel and selective M1 positive allosteric modulator (PAM) chemotypes. Although the structure activity relationships (SAR) necessary for receptor activity are relatively well understood for M1 PAMs, novel chemotypes continue to emerge. In general, M1 PAM compounds are comprised of a flexible tail pendant and a substituted amide or lactam (often substituted with a trans-1,2-aminohydroxycyclohexane or tetrahydropyran), linked by a heteroaryl core (Figure 1).
Figure 1.
A common chemotype for M1 PAMs, and structures of selected compounds.
In SAR campaigns around such compounds, it has proven crucial to monitor the agonist and/or “ago-PAM” activity of the given chemotypes; high M1 agonist activity has been linked to severe side effects including seizures.21 Indeed, compounds that were initially reported to have negligible M1 agonist activity have been shown to act as robust allosteric agonists in native systems,22 highlighting the necessity of detailed characterization of these ligands.
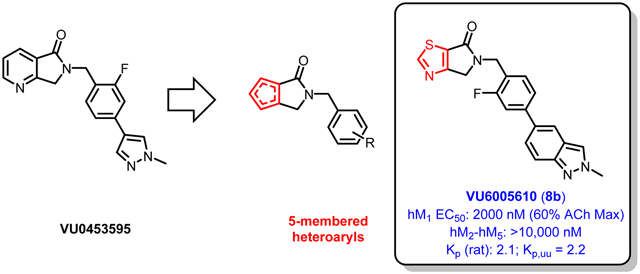
As part of our ongoing medicinal chemistry efforts toward selective M1 PAMs devoid of agonist activity, we have previously disclosed VU0453595 (3, Figure 1), a highly subtype-selective M1 PAM with favorable properties both in vitro and in vivo, and further showed that the compound reverses both the negative and cognitive symptoms in a rodent model of schizophrenia.23 Importantly, the pro-cognitive effects of 3 are not mediated through direct agonism at M1; the compound functions as a “pure PAM”.24 VU0453595 features a chemically distinct aza-isoindolinone scaffold, a template that has proven fruitful for our group and others in follow up medicinal chemistry efforts on M1 PAMs (Figure 1).10,12,25,26 This “reversed lactam” chemotype was originally derived from a class of M1,3,5-preferring isatin compounds,28 and an exploration of positional lactam isomers has allowed for further elaboration (Figure 2).25,26 Molecules derived from the VU0453595 chemotype are reported to have minimal ago-PAM activity, a profile that has crippled the development of compounds in the alternate series.12,21,26
Figure 2.
The VU0453595 aza-isoindolinone chemotype allows for extensive SAR exploration.
The present work describes a novel class of 5-substituted, fused heteroaryl-pyrrolidinones based on the VU0453595 aza-isoindolinone structure (Figure 2). In holding the γ-lactam moiety constant, along with historically M1-preferring pendant tail pieces, we have developed a series of novel 5,5-bicyclic lactams, featuring a wide variety of 5-membered heteroaryls. Importantly, compounds in this series are devoid of agonist activity and display robust selectivity for the M1 mAChR.
Compounds were synthesized as shown in Schemes 1-5.29 For bicyclic imidazoles, thiazoles and thiophenes 8, commercially available aldehydes 6 were condensed with substituted benzylamines29 to give intermediates 7, which were then hydrolyzed and cyclized under standard amide forming conditions to give final analogs 8 (Scheme 1). The synthesis of substituted oxazoles and furans is shown in Scheme 2. Commercially available esters of type 9 were brominated under radical conditions to give intermediates 10 (oxazoles; methyl 2-(bromomethyl)furan-3-carboxylate is available commercially). Bromides 10 were then subjected to reductive amination, hydrolysis, and lactam-formation conditions analogously to Scheme 1 to give final analogs 12. Substituted 4,5-dihydropyrrolo[3,4-b]pyrrol-6(1H)-ones and substituted 4,5-dihydropyrrolo[3,4-c]pyrazol-6(1H)-ones were synthesized in a similar fashion (Schemes 3 and 4), with N-alkylation giving final products 16 and 20, respectively. The pyrazolo analogs (20a-j) were generally alkylated at the beginning of the sequence, as the resulting alkyl isomers were readily separable and identifiable by NOESY at this stage (Scheme 4) (alkylations can be also conducted and separated at the end of the sequence after deprotection of a para-methoxybenzyl intermediate, as in the case of analog 20a (see the experimental section for further synthetic details)). Lastly, methyl pyrazole 25a (Scheme 5) was synthesized by route of a silyl protection-Vilsmeier-Haack reaction sequence,30 and carried through to give final analog 25a in a similar fashion. This chemistry generally proved quite facile, although certain ester hydrolyses required elevated temperatures and/or microwave irradiation to proceed. These routes were also found to be amenable to re-ordering – substituted tail pieces could be generated in a late-stage library format through substitution of fluorinated (4-bromophenyl)methanamines under standard Suzuki-Miyaura conditions.26
Scheme 1. Reagents and conditions:
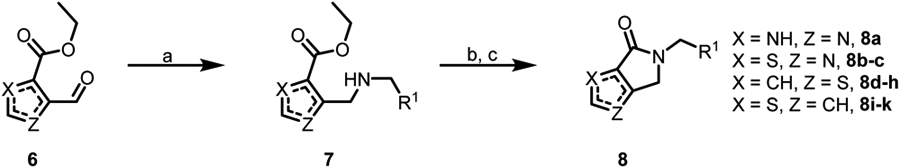
(a) R1 benzylamine, NaBH(OAc)3, AcOH, r.t. or 80 °C; (b) NaOH, MeOH, THF, H2O, r.t. or 80 °C; (c) HATU, DIPEA, DMF, r.t.
Scheme 5. Reagents and conditions:

(a) TIPS-Cl, NaH, THF, 0 °C to r.t.; (b) DMF, oxalyl chloride, DMF, 0 °C to r.t.; (c) R1 benzylamine, NaBH(OAc)3, DCE, r.t.; (d) NaOH, THF, H2O, 70 °C; (e) HATU, DIPEA, DMF, r.t.
Scheme 2. Reagents and conditions:

(a) NBS, benzoyl peroxide, DCE, 80 °C (oxazoles); (b) R1 benzylamine, DIPEA, MeCN, r.t.; (c) LiOH or NaOH, MeOH, THF, H2O, r.t. or 80 °C; (d) HATU, DIPEA, DMF, r.t.
Scheme 3. Reagents and conditions:

(a) R1 benzylamine, NaBH(OAc)3, DCE, r.t.; (b) LiOH, THF, H2O, 120 °C, microwave; (c) HATU, DIPEA, DMF, r.t.; (d) R2 alkyl chloride or bromide, Cs2CO3, DMF, 70 °C.
Scheme 4. Reagents and conditions:

(a) R2 alkyl chloride or bromide, K2CO3, DMF, 50 °C, chromatographic separation of isomers; (b) R1 benzylamine, NaBH(OAc)3, DCE, r.t.; (c) LiOH, THF, H2O, r.t.; (d) HATU, DIPEA, DMF, r.t.
hM1 potency data for all analogs is listed in Table 1. Imidazole 8a was found to be inactive at hM1 (Table 1), while thiazoles 8b and 8c displayed activities in the micromolar range. N-methylindazole compound 8b was found to have superior potency within the thiazole series, a trend that has been previously observed for similar indazole tail pieces in related M1 reports.26 Both thiophene regioisomers (8d-k) demonstrated similar micromolar hM1 potency to 8b, and N-methylimidazole compound 8j displayed remarkably high potency (EC50 246 nM, although ACh Max was relatively low (40%)).
Table 1.
Structures and Activities of Cyclic Pyrrolidinone M1 PAMsa
| Compound | Structure | hM1 EC50 (nM) | hM1 ACh Max |
|---|---|---|---|
| 8a | 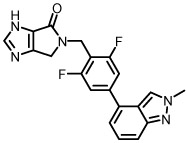 |
>10,000 | - |
| 8b | 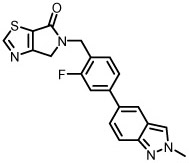 |
2000b | 60 |
| 8c |  |
8000 | 30 |
| 8d |  |
1920b | 75 |
| 8e |  |
1020 | 59 |
| 8f |  |
2870 | 60 |
| 8g | 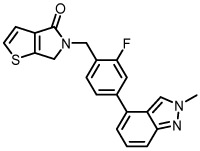 |
3970 | 67 |
| 8h |  |
2780 | 51 |
| 8i |  |
2164b | 66 |
| 8j |  |
246 | 40 |
| 8k |  |
4900 | 67 |
| 12a |  |
>10,000 | - |
| 12b |  |
>10,000 | - |
| 12c |  |
992 | 45 |
| 16a |  |
6450 | 58 |
| 16b |  |
5580 | 67 |
| 16c |  |
2920 | 56 |
| 20a | 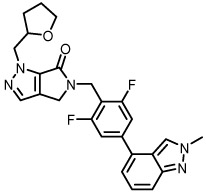 |
1120c | 85 |
| 20b |  |
3540b | 61 |
| 20c |  |
3930 | 84 |
| 20d |  |
739b | 87 |
| 20e |  |
5180 | 57 |
| 20f |  |
1510c | 84 |
| 20g |  |
1660b | 79 |
| 20h | 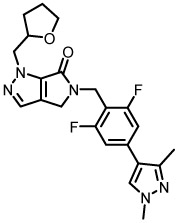 |
3220 | 77 |
| 20i |  |
6890 | 66 |
| 20j |  |
3580 | 65 |
| 25a |  |
>10,000 | - |
Calcium mobilization assays with hM1-CHO cells performed in the presence of an EC20 fixed concentration of acetylcholine for PAM, no exogenous acetylcholine (ACh) for the agonist EC50; values represent means from one (n = 1)
two (n = 2)
three (n = 3) independent experiments performed in triplicate.
Interestingly, both oxazole regioisomers (12a and 12b) were found to be inactive, while furan (12c) was approximately equipotent to its thiophene congener 8e. Substituted pyrroles (16a-c) and pyrazoles (20a-j) proved even more fruitful, and compounds in these series consistently displayed activities in the low micromolar range for hM1. Within the pyrazole series, 1-substituted pyrazoles (i.e. 20a) were generally more potent than the 2-substituted analogs (20b). Methyl pyrrolo compound 25a was found to be inactive, further demonstrating that subtle changes in ring substituents and/or electronics can have notable effects on potency for M1 PAMs; this kind of SAR has been previously established in the field.26
Because of their acceptable potencies, distinct chemotypes, and relatively high ACh max values, thiazole 8b (VU6005610) and pyrazole 20a (VU6005852) were selected for further profiling. Both compounds displayed robust selectivity for hM1 over the other 4 mAChR subtypes (>10,000 nM hM2-hM5) at the concentrations tested (Table 2). Both compounds were also devoid of any hM1 agonist activity, an encouraging profile for the further development of this series. Additionally, 8b displayed no species disconnect between human and rat M1 (rM1 EC50 = 2080 nM). Both compounds also displayed good free fraction and low to moderate predicted hepatic clearance across species (human and rat, see Table 3).20 After IV administration in a rat cassette model (0.20 mg/kg, brain sampling 0.25 h post dose), 8b (VU6005610) was also found to be highly brain penetrant (Kp = 2.1, Kp,uu = 2.2 (average of 3 experiments)). In contrast, 20a was found to have much lower brain exposure in this model (Kp = 0.12).
Table 2.
Muscarinic Selectivity Data for Compounds 8b and 20a.a
| Compound | hM1 EC50 (nM) |
hM2 EC50 (nM) |
hM3 EC50 (nM) |
hM4 EC50 (nM) |
hM5 EC50 (nM) |
|---|---|---|---|---|---|
| 8b | 2000 | >10,000 | >10,000 | >10,000 | >10,000 |
| 20a | 1120 | >10,000 | >10,000 | >10,000 | >10,000 |
Calcium mobilization assays with hM1- or hM5-CHO cells performed in the presence of an EC20 fixed concentration of acetylcholine for PAM, no exogenous ACh for the agonist EC50; values represent means from one (n = 1) independent experiment performed in triplicate
Table 3.
Physicochemical Properties; Human and Rat Hepatic Clearance and Free Fraction Data for Compounds 8b and 20a.a
| Compound | MW | tSPA | hClhep(mL/min/kg) | rClhep(mL/min/kg) |
fu (plasma, rat) |
fu (brain, rat) |
fu (plasma, human) |
|---|---|---|---|---|---|---|---|
| 8b | 378.4 | 48.3 | 10.4 | 43 | 0.040 | 0.042 | 0.050 |
| 20a | 463.5 | 60.7 | 18.6 | 58.7 | 0.027 | 0.019 | 0.025 |
See ref. 20 for experimental details.
In summary, we have developed a series of structurally novel and highly mAChR subtype-selective M1 PAMs devoid of agonist activity. Preliminary PK data for VU6005610 (8b) was particularly encouraging. The favorable muscarinic selectivity, lack of species potency disconnect, and high brain exposure should facilitate the use of this compound as a tool to further probe M1 biology in rodent models. Additional synthetic efforts within these series are underway in our laboratory and will be reported in due course.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank the NIMH (U19MH106839, RO1MH082867) for funding this work, as well as the William K. Warren Foundation for Endowing the Warren Center for Neuroscience Drug Discovery.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES AND NOTES
- 1.Langmead CJ; Watson J; Reavill C Pharm. Ther 2008, 117, 232–243. [DOI] [PubMed] [Google Scholar]
- 2.Kruse AC; Kobilka BK; Gautam D; Sexton PM; Christopoulos A; Wess J Nat. Rev. Drug Dis 2014, 13, 549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melancon BJ; Tarr JC; Panarese JD; Wood MR; Lindsley CW Drug Disc. Today 2013, 18, 1185–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erskine D; Taylor J-P; Bakker G; Brown AJH; Tasker T; Nathan PJ Drug Discov. Today. 2019, 24, 2307–2314. [DOI] [PubMed] [Google Scholar]
- 5.Shirey JK; Brady AE; Jones PJ; Davis AA; Bridges TM; Jadhav SB;> Menon U; Christain EP; Doherty JJ; Quirk M;C; Snyder DH; Leavy AI; Watson ML; Nicolle MM; Lindsley CW; Conn PJ J. Neurosci 2009, 29, 14271–14286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bender AM; Jones CK; Lindsley CW ACS Chem. Neurosci 2017, 8, 435–443. [DOI] [PubMed] [Google Scholar]
- 7.Heinrich JN; Butera JA; Carrick T; Kramer A; Kowal D; Lock T; Marquis KL; Pausch MH; Popiolek M; Sun S-C; Tseng E; Uveges AJ; Mayer SC Eur. J. Pharmacol 2009, 605, 53–56. [DOI] [PubMed] [Google Scholar]
- 8.Woolley ML; Carter HJ; Gartlon JE; Watson JM; Dawson LA Eur. J. Pharmacol 2009, 603, 147–149. [DOI] [PubMed] [Google Scholar]
- 9.Brannan S; Sawchak S; Miller A; Paul SM; Breier A Biol. Psych 2020, 87, S169. [Google Scholar]
- 10.Davoren JE; Lee C-W; Garnsey M; Brodney MA; Cordes J; Dlugolenski K; Edgerton JR; Harris AR; Helal CJ; Jenkinson S; Kauffman GW; Kenakin TP; Lazzaro JT; Lotarski SM; Mao Y; Nason DM; Northcott C; Nottebaum L; O’Neil SV; Pettersen B; Popiolek M; Reinhart V; Salomon-Ferrer R; Steyn SJ; Webb D; Zhang L; Grimwood S J. Med. Chem 2016, 59, 6313–6328. [DOI] [PubMed] [Google Scholar]
- 11.Davoren JE; Garnsey M; Pettersen B; Brodney MA; Edgerton JR; Fortin J-P; Grimwood S; Harris AR; Jenkinson S; Kenakin T; Lazzaro JT; Lee C-W; Lotarski SM; Nottebaum L; O’Neil SV; Popiolek M; Ramsey S; Steyn SJ; Thorn CA; Zhang L; Webb D J. Med. Chem 2017, 60, 6649–6663. [DOI] [PubMed] [Google Scholar]
- 12.Yang Z-Q; Shu Y; Ma L; Wittmann M; Ray WJ; Seager MA; Koeplinger KA; Thompson CD; Hartman GD; Bilodeau MT; Kuduk SD ACS Med. Chem. Lett 2014, 5, 604–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beshore DC; Di Marco CN; Chang RK; Greshock TJ; Ma L; Wittman M; Seager MA; Koeplinger KA; Thompson CD; Fuerst J; Hartman GD; Bilodeau MT; Ray WJ; Kuduk SD ACS Med. Chem. Lett 2018, 9, 652–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mistry SN; Jörg M; Lim H; Vinh NB; Sexton PM; Capuano B; Christopoulos A; Lane JR; Scammells PJ J. Med. Chem 2016, 59, 388–409. [DOI] [PubMed] [Google Scholar]
- 15.Mistry SN; Lim H; Jörg M; Capuano B; Christopoulos A; Lane JR; Scammells PJ ACS Chem. Neurosci 2016, 7, 647–661. [DOI] [PubMed] [Google Scholar]
- 16.Dallagnol JCC; Khajehali E; van der Westhuizen ET; Jörg M; Valant C; Gonçalves AG; Capuano B; Christopoulos A; Scammells PJ J. Med. Chem 2018, 61, 2875–2849. [DOI] [PubMed] [Google Scholar]
- 17.Jorg M; van der Westhuizen ET; Khajehali E; Burger WAC; White JM; Choy KHC; Tobin AB; Sexton PM; Valant C; Capuano B; Christopoulos A; Scammells PJ ACS Chem. Neurosci 2019, 10, 1099–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tarr JC; Turlington ML; Reid PR; Utley TJ; Sheffler DJ; Cho HP; Klar R; Pancani T; Klein MT; Bridges TM; Morrison RD; Blobaum AL; Xiang Z; Daniels JS; Niswender CM; Conn PJ; Wood MR; Lindsley CW ACS Chem. Neurosci 2012, 3, 884–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rook JM; Bertron JL; Cho HP; Garcia-Barrantes PM; Moran SP; Maksymetz JT; Nance KD; Dickerson JW; Remke DH; Chang S; Harp JM; Blobaum AL; Niswender CM; Jones CK; Stauffer SR; Conn PJ; Lindsley CW ACS Chem. Neurosci 2018, 9, 2274–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engers JL; Childress ES; Long MF; Capstick RA; Luscombe VB; Cho HP; Dickerson JW; Rook JM; Blobaum AL; Niswender CM; Engers DW; Conn PJ; Lindsley CW ACS Med. Chem. Lett 2018, 9, 917–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rook JM; Abe M; Cho HP; Nance KD; Luscombe VB; Adams JJ; Dickerson JW; Remke DH; Garcia-Barrantes PM; Engers DW; Engers JL; Chang S; Foster JJ; Blobaum AL; Niswender CM; Jones CK; Conn PJ; Lindsley CW ACS Chem. Neurosci 2017, 8, 866–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moran SP; Cho HP; Maksymetz J; Remke DH; Hanson RM; Niswender CM; Lindsley CW; Rook JM; Conn PJ ACS Chem. Neurosci 2018, 9, 2218–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghoshal A; Rook JM; Dickerson JW; Roop GN; Morrison RD; Jalan-Sakrikar N; Lamsal A; Noetzel MJ; Poslusney MS; Wood MR; Melancon BJ; Stauffer SR; Xiang Z; Daniels JS; Niswender CM; Jones CK; Lindsley CW; Conn PJ Neuropsychopharmacology 2016, 41, 598–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moran SP; Dickerson JW; Cho HP; Xiang Z; Maksymetz J; Remke DH; Lv X; Doyle CA; Rajan DH; Niswender CM; Engers DW; Lindsley CW; Rook JM; Conn JP Neuropsychopharmacology 2018, 43, 1763–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panarese JD; Cho HP; Adams JJ; Nance KD; Garcia-Barrantes PM; Chang S; Morrison RD; Blobaum AL; Niswender CM; Stauffer SR; Conn PJ; Lindsley CW Bioorg. Med. Chem. Lett 2016, 26, 3822–3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engers JL; Bender AM; Kalbfleisch JJ; Cho HP; Lingenfelter KS; Luscombe VB; Han C; Melancon BJ; Blobaum AL; Dickerson JW; Rook JM; Niswender CM; Emmitte KA; Conn PJ; Lindsley CW ACS Chem. Neurosci 2019, 10, 1035–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bridges TM; Kennedy JP; Cho HP; Breininger ML; Gentry PR; Hopkins CR; Conn PJ; Lindsley CW Bioorg. Med. Chem. Lett 2010, 20, 558–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bridges TM; Kennedy JP; Noetzel MJ; Breininger ML; Gentry PR; Conn PJ; Lindsley CW Bioorg. Med. Chem. Lett 2010, 20, 1972–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.For further synthetic description, including the synthesis of the substituted benzylamines, see: Lindsley CW; Conn PJ; Engers DW; Spearing PK; Bender AM 2019, US Patent Application 20190330226.
- 30.Hasvold LA; Sheppard GS; Wang L; Fidanze SD; Liu D; Pratt JK; Mantei RA; Wada CK; Hubbard R; Shen Y; Lin X; Huang X; Warder SE; Wilcox D; Li L; Buchanan FG; Smithee L; Albert DH; Magoc TJ; Park CH; Petros AM; Panchal SC; Sun C; Kovar P; Soni NB; Elmore SW; Kati WM; McDaniel KF Bioorg. Med Chem. Lett 2017, 27, 2225–2233. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




