Graphical abstract

Keywords: Heavy metals, Fish, Health risk, Bioaccumulation, Monte carlo simulation
Highlights
-
•
Concentration of Arsenic and Cadmium in the fish tissues exceed the limits set by the European Union.
-
•
THQ values indicated likely adverse effects during a person’s lifetime with continuous exposure to Arsenic and Cadmium.
-
•
As, Cd, and Nickel may pose cancer risk to consumer of fish from the two rivers over longtime exposure.
-
•
Cancer risk due to long time consumption of fish from the rivers can be a major concern.
Abstract
Ogun and Eleyele Rivers are in the Western part of Nigeria with a potential risk of heavy metal pollution because of many industrial wastes channeling through their courses. Therefore, in this study, the concentration of heavy metals and the possible human health risk of consuming Clarias gariepinus and Sarotherodon melanotheron collected from industrially polluted Ogun and Eleyele Rivers in Nigeria were evaluated.
The concentration of arsenic (As), cadmium (Cd), cobalt (Co), chromium (Cr), manganese (Mn), nickel (Ni), and lead (Pb) in tissues (gill, muscle, and liver) of fish was measured using Atomic Absorption Spectroscopy (AAS) and compared with the maximum permissible. The Estimated Daily Intake (EDI), Targeted Hazard Quotient (THQ), and Carcinogenic Risk (CR) of the metals were estimated for the determination of human health risk. Probabilistic predictions of the health risk were performed with Oracle Crystal Ball software.
Results of this study showed that the dry weight concentrations of the metals in the gills, liver, and muscle of the two fish species from the two sites were well below the permissible limits set by the joint FAO/WHO Expert Committee. Only the EDI for arsenic in gills of C. gariepinus obtained from the Ogun River exceeded the set limit. The THQ was >1 for As in the gills and liver of C. gariepinus and S. melanotheron obtained from the Ogun river suggesting non-carcinogenic risk to the consumers. The carcinogenic risk above 10−6 obtained for As, Cd, and Ni in the tissues of the two fish species suggested cancer risk to the consumers of fish from the two rivers. Consequent to our observation, consumption of fish from the study site presents some public health concerns. Therefore, this study advises routine heavy metal monitoring of fish along these rivers to implement regulatory standards by the government environmental health management agencies.
1. Introduction
The global increase in fish consumption is attributable to the nutritional benefits of fish consumption [[1], [2], [3]]. They serve as a good source of proteins, vitamins, omega-3-fatty acids, and essential minerals [4]. Owing to the advancing industrialization and increasing population, the turnover of industrial waste and anthropogenic activities have heightened the contamination rate of the aquatic environment [5]. Globally, aquatic environments are being polluted with toxicants and heavy metals. Studies have shown accumulation of heavy metals in different aquatic biodiversities such as prawn, crayfish, crabs, bivalve, and fish [[6], [7], [8], [9], [10]]. Consequently, there is a concomitant increase in the human health risk through consumption of fish contaminated with heavy metals [[11], [12], [13], [14], [15]].
Heavy metals are important aquatic pollutants with non-biodegradable and bioaccumulation properties, high toxicity, and long-time persistence [3,16]. The major source of heavy metal pollution into the river is the earth's crust. Additionally, heavy metals are known to be transmitted into the river through industrial effluents directed to the rivers. Sea organisms accumulate these contaminants primarily by uptake through the skin and gills via surface contacts with sediments, industrial effluent, and wastewater, as well as via the food they consume [17]. Thus serving as a secondary source of exposure to humans [18].
These heavy metals upon entry through the fish’s gills and other organs are accumulated in different parts of the fish body up to a toxic level [19]. Previous studies have confirmed that heavy metals may enter into the food chain through the natural and anthropogenic processes and cause toxicity in the aquatic organisms even at low concentrations [19,20]. The long period of intake of heavy metals through foodstuffs such as fish may lead to chronic accumulation, which consequently can cause damages to organs and tissues [21]. Consequential effects of metal accumulation are mutagenesis, carcinogenesis, teratogenesis, deformation, and breakdowns of organs [22].
Thus, this study selected the Ogun and Eleyele Rivers located in the Western part of Nigeria because they are key commercial rivers for fishing and are prone to industrial wastes pollution. For instance, the Ogun River is a recipient of a huge amount of waste including industrial and domestic effluents as well as a recipient of urban and agricultural sewage wastewaters. Ogun state is highly populated and industrialized, making it rivers pollution prone. About six different industries discharge their wastes into the river. The industries include Vitabiotics, Nestle, Glaxos, Smith kline, Sona Breweries, and Nigerian German chemicals. [23]. The Lafenwa Agoka fishing unit is a commercial fishing hub of the Ogun River, surrounded at the bank by lafenwa cow butcher, lafenwa market, and sawmill. Eleyele River on the other hand is located northeast of Ibadan, Oyo state. It extends northward to Awotan, Westward to Ologun eru, and Eastward to Ijokodo. The Apete River links the Eleyele River and Oba-dam that is located within the University of Ibadan. Agricultural farm runoff, human wastes, waste from automobile workshops, and other anthropogenic activities are constantly polluting the water. The fish hunted in the Ogun and Eleyele Rivers are consumed as the main protein source by the residents of these cities as well as the other cities in the western parts of Nigeria. Therefore, the assessment of heavy metals in the tissue of fish is considered highly important as they are highly accumulated in aquatic organisms and pose significant health risks over prolonged exposure. We determined the concentration of heavy metals and the health risk associated with two highly consumed fish species collected from the Ogun and Eleyele rivers in Nigeria (Fig. 1).
Fig. 1.
Map of the sampling Sites (A: Eleyele river in Ibadan (Site 2), B: Agoka fishing units of Ogun river (Site 1)).
2. Materials and methods
2.1. Study area
Collection of fish was done in May 2020 with Ogun River, Abeokuta, and Eleyele River, Ibadan was chosen as sites 1 and 2 respectively. Ogun River originates in Sepeteri, Oyo state near Shaki, it flows through Ogun state via Ogun-oyan dam and discharges into the Lagos lagoon. In addition, Lafenwa Agoka fishing unit (Latitude 7.16 °N, Longitude 3.33 °E) (Fig. 1B) is a commercial fishing hub of the Ogun River, surrounded at the bank by Lafenwa cow butcher, Lafenwa market, and sawmill. The fishing unit serves as a source of livelihood for many people in the area.
Eleyele River is located northeast of Ibadan, Oyo state (Latitude 7.42 °N, Longitude 3.86 °E) (Fig. 1A). It extends northward to Awotan, Westward to Ologun eru, and Eastward to Ijokodo.
2.2. Collection of fish samples
Five replicates totalling 20 each of two fish species; Clarias gariepinus, and Sarotherodon melanotheron (Fig. 2) were collected from sites 1 and 2 in May and November 2020. Upon collection, the fish were immediately transferred into the icebox and conveyed into the laboratory. Before dissection, they were allowed to thaw and anthropometric measurements were taken. The gills, liver, and muscle were harvested and prepared for metal analysis. Each of the tissue was oven-dried at 80 °C and was monitored until a constant weight was reached.
Fig. 2.
Samples of Fish Species collected in this study (a: Clarias gariepinus, b: Sarotherodon melanotheron).
2.3. Sample digestion
Dry gills, muscle, and the liver (0.1 g) were ground to powder. The samples were then transferred into a digestion flask containing a mixture of Conc. Sulphuric acid (H2SO4) and Conc. Nitric acid prepared in a 3:1 (v/v) ratio and heated in a water bath. The heating was followed by repeated addition of ¾ drops of hydrogen peroxide (H2O2) until the solution became clear. H2O2 was added to reduce nitrous vapours and accelerates digestion by raising the temperature [24,25]. After 20 min of additional heating at 150 °C, the samples were allowed to cool to room temperature. The gills and muscle samples were diluted with deionized water up to 50 mL and 25 mL for liver samples. The diluted samples were filtered through No 1 Whatman filter paper.
2.4. Atomic absorption spectroscopic analysis
The resultant filtrates obtained from the digestion process were subjected to Atomic Absorption Spectroscopic Analysis (AAS) of heavy metals. The concentration of Arsenic, Cobalt, Chromium, Cadmium, Lead, Manganese, and Nickel was measured in all the samples on PG instrument (AA990) AAS that uses air-acetylene flame with UV-detector and automatic zero to compensate the blank. A hydride generator was used along with the flame AAS for Arsenic detection. The working standard solutions of lead (Pb), Arsenic (As), cobalt (Co), chromium (Cr), and cadmium (Cd), Nickel (Ni), and Manganese (Mn) were the product of Loba Chemie PVT. Ltd. Mumbai, India. The calibration curves for each standard were prepared individually by applying linear correlation using the least square method.
2.5. Human health risk assessment
2.5.1. Estimated daily intake (EDI)
The daily intake by consuming the fish sample was estimated using equation 1 below:
([26],Matouke and Abdullahi, [5].
| (i) |
The daily intake of heavy metals was estimated based on the concentration in the muscle samples of the fish species.
C is the average concentration of heavy metals in fish (mg/kg dry weight); IR (Fish consumption ratio: 24.7 g/person/day described by [27] and reported by Taiwo et al. [26]); ED is the exposure duration (70.65 years as average lifetime) [28]; EFr is the exposure frequency (365 days/year). BWa is the average adult body weight (60 kg; [29]); and ATn is the average exposure time for non-carcinogens (assuming 70 years)
2.5.2. Non-Carcinogenic Risk (THQ)
The non-carcinogenic risk of consuming the fish species was determined using the Targeted Hazard Quotient (THO). The THO gives indications of the human health risk level due to exposure to pollutants. Higher THQ values indicate there is a higher probability of experiencing long-term non-carcinogenic effects. If THQs <1, toxic effects are not expected to occur [71]. If the THQ =/>1, there is a potential health hazard [30].
| (ii) |
Where; RFD is the oral reference dose. The RFD for metals obtained from Integrated Risk [[31], [32], [33]].
2.5.3. Hazard Index (total THQ)
The combined non-carcinogenic effect of the metals was assessed to determine the risk of multiple metal contaminations.
| (iii) |
2.5.4. Carcinogenic risk (CR)
Carcinogenic risk is the incremental probability to develop cancer over a lifetime of an individual when exposed to a potential carcinogen [11,34]. The acceptable limit set for lifetime exposure to carcinogens ranged from 10–4 (risk of developing cancer over a lifetime is 1 in 10,000) to 10–6 (risk of developing cancer over a lifetime is 1 in 1,000,000) [35,36]. When CR values are above 10−5, the probability that an individual will develop cancer would be > 1 over 100,000 [37,38]. The oral slope factor of carcinogens (mg/kg/day) derived from the Integrated Risk Information System provided [39,40]) is accessible for the carcinogenic metals (As, Pb, Ni, Cr, and Cd).
| (IV) |
2.5.5. Probabilistic risk modeling using monte carlo simulation (MCS)
The use of single-point values for the estimation of risk exposure to a pollutant is usually accompanied by high uncertainties. To accommodate these uncertainties, a probabilistic method involving the Monte Carlo simulation technique was used. The Oracle Crystal Ball Software (version 11.1.2.4, Oracle, Inc., USA) considering 10,000 iterations was used for the simulation, and percentile 95 % of THQ and CR was benchmarked as a value that could endanger the health of consumers [[41], [42], [43], [44]].
3. Results
3.1. Concentration of Metals in the tissues of two different fish species obtained from Eleyele and Ogun River
Concentration of heavy metals in the tissues of the fish collected from the two selected rivers was reported in Table 1. The metals concentration was estimated in mg/kg/ dry weight. In all the tissues and fish species, the concentration of As was highest for both the fish collected from the Eleyele and Ogun rivers. Clarias gariepinus collected from the Ogun river had the highest concentration of As in the gills followed by the liver, whereas, the Pb (7.93 ± 1.43 mg/kg) content was highest in the muscle. Ni was next after As with a concentration range of 5.9 ± 4.2–12.0 ± 2.1 mg/kg. Similarly, the As concentration in Sarotherodon melanotheron supersedes every other metal analysed. The concentration in the tissues; gills (190.0 ± 62.0 mg/kg), liver (230.0 ± 128.0 mg/kg) and muscle (130.0 ± 28.0 mg/kg) indicated the highest content was in the liver. As clearly indicated in Table 2, the concentration of As, Cd, Co, and Ni were highest in the liver whereas the Pb and Cr concentration were highest in the gills. Only Mn concentration was found to be higher in the muscle of Sarotherodon melanotheron.
Table 1.
Metal concentration in three different tissues of fish species obtained from Ogun and Eleyele Rivers.
| Site | Specie | Organ | Heavy Metals (10−5 mg/kg dw) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| As | Cd | Co | Cr | Mn | Ni | Pb | |||
| Ogun River | Clarias gariepinus | Liver | 46.0 ± 33.0 | 3.05 ± 1.60 | 1.58 ± 1.5 | BDL | BDL | 5.9 ± 4.2 | 3.47 ± 2.15 |
| Muscle | 35.0 ± 30.0 | BDL | 1.55 ± 0.01 | 0.29 ± 0.10 | BDL | 7.8 ± 0.2 | 7.93 ± 1.43 | ||
| Gills | 580 ± 25.0 | 0.81 ± 0.42 | 2.58 ± 0.76 | 0.39 ± 0.00 | BDL | 12.0 ± 2.1 | 6.87 ± 4.99 | ||
| Sarotherodon melanotheron | Gills | 190.0 ± 62.0 | 3.2 ± 0.44 | 3.23 ± 2.45 | 1.17 ± 0.78 | 0.64 ± 0.59 | 10.0 ± 1.2 | 9.36 ± 0.00 | |
| Liver | 230..0 ± 128.0 | 4.2 ± 1.01 | 3.48 ± 0.13 | 0.39 ± 0.00 | 1.25 ± 0.93 | 17.0 ± 2.7 | 8.76 ± 1.87 | ||
| Muscle | 130.0 ± 28.0 | 3.2 ± 0.13 | BDL | 0.78 ± 0.39 | 1.48 ± 0.70 | 9.0 ± 0.8 | 5.01 ± 0.61 | ||
| Eleyele River | Clarias gariepinus | Gills | 69.0 ± 3.0 | 3.3 ± 0.11 | 2.05 ± 0.00 | 1.17 ± 0.00 | 1.82 ± 0.94 | 8.7 ± 0.7 | 8.13 ± 1.24 |
| Liver | BDL | 2.6 ± 0.70 | 1.82 ± 0.00 | 0.39 ± 0.00 | 1.93 ± 1.28 | 8.7 ± 0.1 | 6.87 ± 1.25 | ||
| Muscle | 130.0 ± 13.0 | 2.1 ± 0.63 | 3.48 ± 0.65 | 1.17 ± 0.78 | BDL | 4.3 ± 3.4 | 4.41 ± 3.72 | ||
| Sarotherodon melanotheron | Gills | 21.0 ± 17.0 | 2.8 ± 0.46 | 2.83 ± 0.26 | 0.12 ± 0.08 | BDL | 1.0 ± 0.1 | 1.12 ± 0.19 | |
| Liver | BDL | BDL | 0.08 ± 0.03 | BDL | BDL | 8.8 ± 7.6 | 2.53 ± 0.59 | ||
| Muscle | BDL | 0.65 ± 0.22 | 0.27 ± 0.17 | 0.16 ± 0.04 | BDL | 4.1 ± 2.3 | 7.85 ± 7.78 | ||
NB: BDL means Below Detection Limit.
Table 2.
Estimated daily intake of the metals identified in various tissues of two fish species obtained from Eleyele and Ogun Rivers.
| Site | Specie | Organ | Expected Daily Intake (10−5 mg/kg/dw) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| As | Cd | Co | Cr | Mn | Ni | Pb | |||
| Ogun River | Clarias gariepinus | Liver | 19.0 | 1.25 | 0.65 | – | – | 2.42 | 1.43 |
| Muscle | 14.0 | – | 0.64 | 0.04 | – | 3.21 | 3.26 | ||
| Gills | 24.0 | 0.33 | 1.06 | 0.16 | – | 4.9 | 2.83 | ||
| Sarotherodon melanotheron | Gills | 78.0 | 1.32 | 1.33 | 0.48 | 0.27 | 4.15 | 3.85 | |
| Liver | 94.0 | 1.71 | 1.43 | 0.16 | 0.52 | 4.78 | 3.61 | ||
| Muscle | 52.0 | 1.30 | – | 0.32 | 0.61 | 3.72 | 2.06 | ||
| Eleyele River |
Clarias gariepinus | Gills | 28.0 | 1.34 | 0.84 | 0.48 | 0.75 | 3.6 | 3.34 |
| Liver | – | 1.06 | 0.75 | 0.16 | 0.80 | 3.59 | 2.83 | ||
| Muscle | 56.0 | 0.88 | 1.43 | 0.48 | – | 1.77 | 1.81 | ||
| Sarotherodon melanotheron | Gills | 8.3 | 1.16 | 1.16 | 0.05 | – | 0.41 | 4.63 | |
| Liver | – | – | 0.03 | – | – | 3.63 | 1.04 | ||
| Muscle | – | 0.27 | 0.11 | 0.06 | – | 1.7 | 3.23 | ||
For the fish obtained from Eleyele River, the arsenic concentration was 69.0 ± 3.0 mg/kg and 130.0 ± 13.0 mg/kg in the gills and muscle respectively while it was below the detectable limit (BDL) in the liver. The concentration of Pb in the muscle was 4.41 ± 3.72 mg/kg, 6.87 ± 1.25 mg/kg in the liver, and 8.13 ± 1.24 mg/kg in the gills. The same concentration of Ni was found in the gills (8.7 ± 0.7 mg/kg) and liver (8.7 ± 0.1 mg/kg) of Clarias gariepinus while the concentration in the muscle was 4.3 ± 3.4. Cd in the gills, liver, and muscle was 3.3 ± 0.11 mg/kg, 2.6 ± 0.70 mg/kg, and 2.1 ± 0.63 mg/kg respectively while the same amount of Cr was found in the gills (1.17 ± 0.00 mg/kg) and muscle (1.17 ± 0.78 mg/kg). Mn was below the detectable limit in the muscle but the concentration was 1.82 ± 0.94 mg/kg and 1.93 ± 1.28 mg/kg in the gills and liver respectively. The muscle of Clarias gariepinus contains the highest amount of Co followed by the gills and the liver. The trend of metal concentration in Sarotherodon melanotheron obtained from the Eleyele River varies with the tissues. Arsenic in the liver and muscle was below the detectable limit while the concentration in the gills was 21.0.0 ± 17.0 mg/kg. The distribution pattern of Cd and Co, in Sarotherodon melanotheron followed the pattern gills > muscle > liver. Cd, and Cr, were below detectable limits in the liver of Sarotherodon melanotheron. In all the tissues, the concentration of Mn was below the detectable limit. Lead concentration in the muscle was the highest having the value of 7.85 ± 7.78 mg/kg and 2.53 ± 0.59 mg/kg in the liver while the concentration in the gills was 1.12 ± 0.19 mg/kg. Nickel on the other hand was highest in the liver with the concentration being 8.8 ± 7.6, followed by muscle (4.1 ± 2.3 mg/kg) and gills (1.0 ± 0.1 mg/kg).
3.2. Human Health Risk Assessment of heavy metals detected in two different fish species collected from Eleyele and Ogun River
3.2.1. Estimated Daily Intake (EDI) of heavy metals detected in two different fish species collected from Eleyele and Ogun River
The estimated daily ingestion values of the identified metals through consumption of two species of fish from two locations are shown in Table 2. The calculation was based on the United states-Environment and Protection Agency risk analysis. The values obtained are dependent on both the concentration of the metal in fish tissue and reported protein ingestion rate when the average adult body weight was considered at 60 kg. The trend of the EDI for the heavy metals in the fish species is tissue-dependent. The EDI obtained for Cd, Pb and As were lower than the provisional tolerable weekly intake (PTWI), only the EDI for As in gills of Clarias gariepinus obtained from the Ogun river exceeds the set limit. The reported PTWI for As, Pb, and Cd is 0.015, 0.025, and 0.007 mg/kg body weight respectively
3.2.2. Non-carcinogenic and carcinogenic risks of consuming fish obtained from Eleyele and Ogun River
3.2.2.1. Non-carcinogenic risks of consuming fish obtained from Eleyele and Ogun River
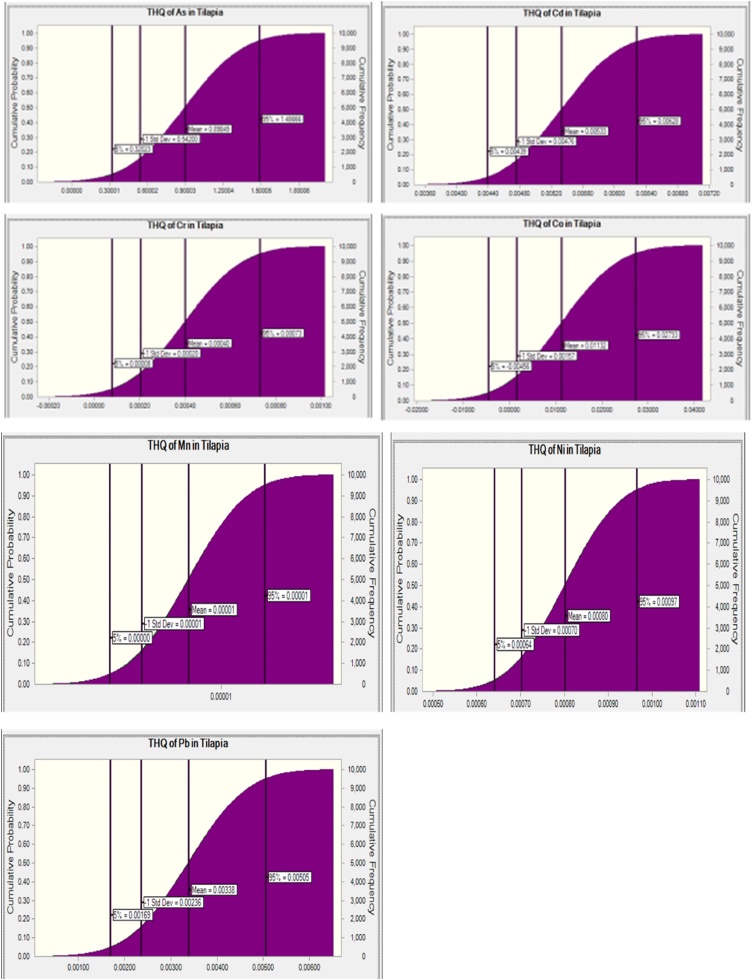
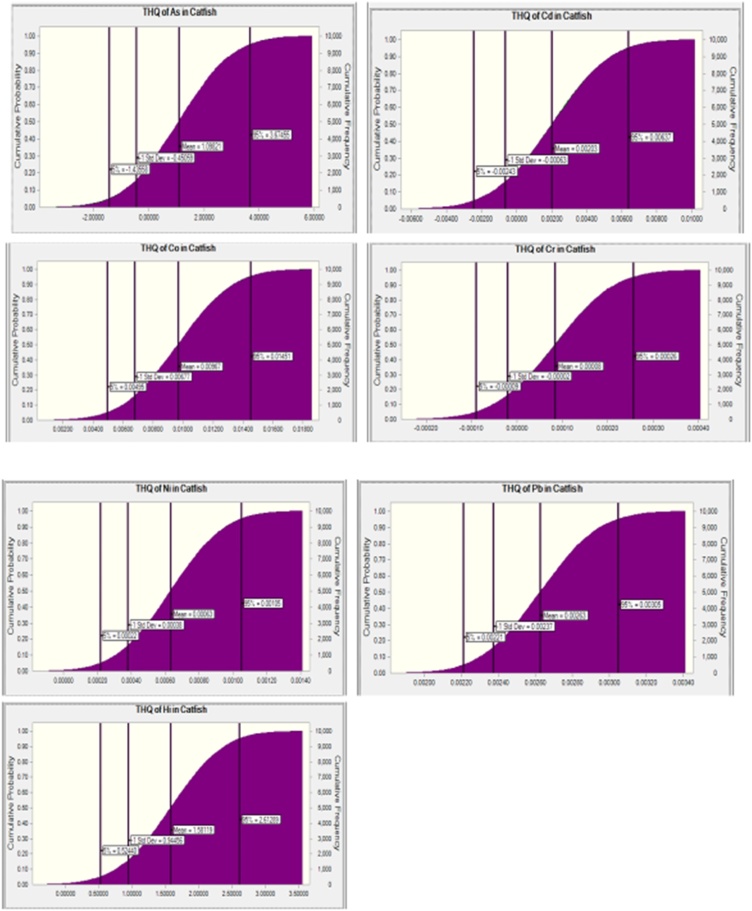
Target Hazard Quotient (THQ) and Hazard Index (HI) > 1, is an indication for probable adverse health effects whereas when the THQ is ≤ 1, adverse health effects are not probable. The THQ and HI due to ingestion of As, Cd, Cr, Pb, Ni, and Co in different species as well as when consumed in the gills, liver, and muscle of fish obtained from the Eleyele and Ogun rivers are depicted in Table 3 and Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8 respectively. The THQ values of As through consumption of gills and liver of Clarias gariepinus and Sarotherodon melanotheron obtained from the Ogun river exceed the non-carcinogenic threshold value of 1 indicating non-carcinogenic risk to the consumers. The THQ values for the other heavy metals (Cd, Co, Cr, Ni, and Pb) show an absence of non-carcinogenic risk with THQ < 1. TTHQ (Hazard index) of two fish species obtained from the Eleyele River suggest the absence of non-carcinogenic combined effects, however, HI through consumption of Sarotherodon melanotheron obtained from the Eleyele River is above 0.6, suggesting caution. The HI through consumption of the two fish species obtained from the Ogun River may pose some non-carcinogenic risk. Overall, Arsenic was the highest non-cancer risk contributor of all the fish species (Table 3).
Table 3.
THQ and Hazard Index for different heavy metals from consumption of two fish species obtained from Ogun and Eleyele River.
| Site | Specie | Organ | Target Hazard Quotient (THQ) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| As | Cd | Co | Cr | Mn | Ni | Pb | HI | |||
| Ogun River | Clarias gariepinus | Liver | 0.232 | 0.246 | 0.008 | – | – | 0.0004 | 0.0015 | 0.246 |
| Muscle | 0.177 | 0.188 | 0.008 | 0.00005 | – | 0.0006 | 0.0034 | 0.188 | ||
| Gills | 2.907 | 2.925 | 0.013 | 0.0002 | – | 0.0009 | 0.003 | 2.925 | ||
| Sarotherodon melanotheron | Liver | 1.15 | 0.006 | 0.018 | 0.0002 | 0.00001 | 0.0009 | 0.0038 | 1.179 | |
| Gills | 0.958 | 0.005 | 0.016 | 0.0006 | 0.000007 | 0.0008 | 0.0041 | 0.984 | ||
| Muscle | 0.643 | 0.005 | – | 0.0004 | 0.00002 | 0.0007 | 0.0022 | 0.651 | ||
| Eleyele River | Clarias gariepinus | Gills | 0.346 | 0.005 | 0.01 | 0.0006 | 0.00002 | 0.0007 | 0.0035 | 0.366 |
| Liver | – | 0.004 | 0.009 | 0.0002 | 0.00002 | 0.0007 | 0.003 | 0.017 | ||
| Muscle | 0.682 | 0.003 | 0.018 | 0.0006 | – | 0.0003 | 0.0019 | 0.705 | ||
| Sarotherodon melanotheron | Gills | 0.101 | 0.004 | 0.014 | 0.00006 | – | 0.00008 | 0.0005 | 0.121 | |
| Liver | – | – | 0.0004 | – | – | 0.0007 | 0.0011 | 0.003 | ||
| Muscle | – | 0.001 | 0.001 | 0.00008 | – | 0.0003 | 0.0034 | 0.006 | ||
Fig. 3.
THQ for the consumer of Sarotherodon melanotheron obtained from Ogun River due to its heavy metal content.
Fig. 4.
THQ for the consumer of Clarias gariepinus obtained from Ogun River due to its heavy metal content.
Fig. 5.
THQ for the consumer of Sarotherodon melanotheron obtained from Eleyele River, Ibadan due to its heavy metal content.
Fig. 6.
THQ for the consumer of Clarias gariepinus obtained from Eleyele River, due to its heavy metal content.
Fig. 7.
THQ in consumers due to heavy metals content in Muscle of Clarias gariepinus and Sarotherodon melanotheron obtained from Eleyele River, Ibadan.
Fig. 8.
THQ in consumers due to heavy metals content in Muscles of Clarias gariepinus and Sarotherodon melanotheron obtained from Ogun River.
3.2.2.2. Carcinogenic risk of consuming fish obtained from Eleyele and Ogun River
The carcinogenic risk values of different metals identified in different tissues of fish obtained from the Eleyele and Ogun River are shown in Table 4. The accepted range (10−4-10-6) was exceeded by As, Cd, and Ni from all the fish species. When CR value is less than 10-6 it is insignificant and the cancer risk can be neglected while values above 10-4 is considered harmful and pose a cancer risk. Among all the studied heavy metals, As has the highest chance of cancer risk while Cd and Ni have the lowest chance of cancer risk. Cr and Pb had no chance of cancer risk when consumed in the fish obtained from the Eleyele and Ogun rivers.
Table 4.
The cancer risk for heavy metals from consumption of two fish species obtained from Ogun and Eleyele River.
| Site | Specie | Organ | Cancer Risk (10−5) |
||||
|---|---|---|---|---|---|---|---|
| As | Cd | Cr | Ni | Pb | |||
| Ogun River | Clarias gariepinus | Liver | 10.0 | 2.91 | – | 1.52 | 0.0045 |
| Muscle | 7.94 | – | 0.074 | 2.01 | 0.010 | ||
| Gills | 130.0 | 0.78 | 0.030 | 3.07 | 0.0089 | ||
| Sarotherodon melanotheron | Liver | 43.0 | 3.06 | 0.089 | 2.6 | 0.012 | |
| Gills | 52.0 | 3.97 | 0.030 | 2.99 | 0.011 | ||
| Muscle | 29.0 | 3.01 | 0.059 | 2.33 | 0.0065 | ||
| Eleyele River | Clarias gariepinus | Gills | 16.0 | 3.12 | 0.089 | 2.25 | 0.011 |
| Liver | – | 2.47 | 0.030 | 2.25 | 0.0089 | ||
| Muscle | 31.0 | 2.03 | 0.089 | 1.11 | 0.0057 | ||
| Sarotherodon melanotheron | Gills | 4.56 | 2.70 | 0.0089 | 2.56 | 0.0015 | |
| Liver | – | – | – | 2.27 | 0.0033 | ||
| Muscle | – | 0.62 | 0.012 | 1.07 | 0.011 | ||
3.2.3. Non-cancer risk characterization based on Monte Carlo simulation
Monte Carlo simulation was employed to handle the uncertainties in heavy metal concentration and the variability in toxic response among individuals, body weight, and ingestion rate of fish.
The THQ due to ingestion of As, Cd, Cr, Pb, Ni, and Co in different species as well as when consumed in the muscle of fish obtained from the Eleyele and Ogun rivers based upon Monte Carlo simulation are depicted in Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8. The rank order of non-cancer risk for each metal across species, organs, and Collection site was based on the 95th percentile of the THQ and HI.
The order was As (1.49)>Co(0.02)>Ni (0.00097)>Cd (0.0064)>Pb (0.005)>Cr (0.00026)>Mn (0.00001) in Sarotherodon melanotheron, and As(3.67)>Co(0.015))>Cd (0.006)>Pb (0.0031)>Ni(0.0011) and HI (2.61) in Clarias gariepinus obtained from the Ogun River (Fig. 3, Fig. 4). For the fish obtained from the Eleyele, Ibadan, the THQ in adult consumer of Sarotherodon melanotheron was As (0.13)>Co (0.018)>Cd (0.007)>Pb (0.0042)>Ni (0.0009)>Cr (0.00009) and HI (0.047). Whereas in Clarias gariepinus, it was As (0.90)>Co (0.021)>Cd(0.0056)>Pb(0.0042)>Ni(0.00095)>Cr(0.00085)>Mn(0.00003) and HI (0.85) (Fig. 5, Fig. 6). Overall, consumption of fish from the Eleyele River does not pose non-cancer risk to consumers as the THQ is less than 1 for all metals whereas greater than 1 for As in the two fish species obtained from the Ogun River.
The simulation prediction of the non-cancer risk of consuming heavy metals from the muscle of fish obtained from the Ogun River and Eleyele River, Ibadan are depicted in (Fig. 7, Fig. 8). The THQ rank order for the heavy metals in the muscle of the fish obtained from the Eleyele River, Ibadan was, As (1.13)>Co(0.029)>Pb(0.00438)>Cd(0.0044)>Cr(0.00094)>Ni(0.00031) (Fig. 7). While the order was As (0.82)>Cd(0.011)>Co(0.01)>Ni(0.008)>Cr(0.005)>Pb(0.004) (Fig. 8) for the fish obtained from Ogun river.
3.2.4. Carcinogenic risk characterization based on Monte Carlo simulation
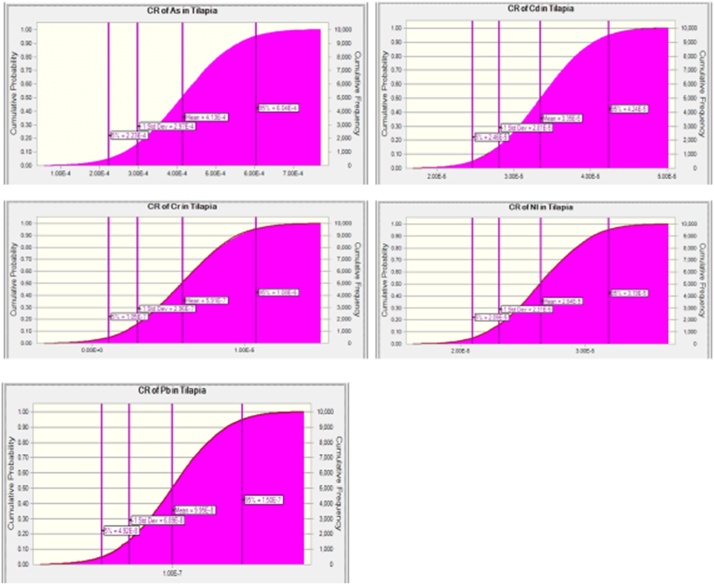
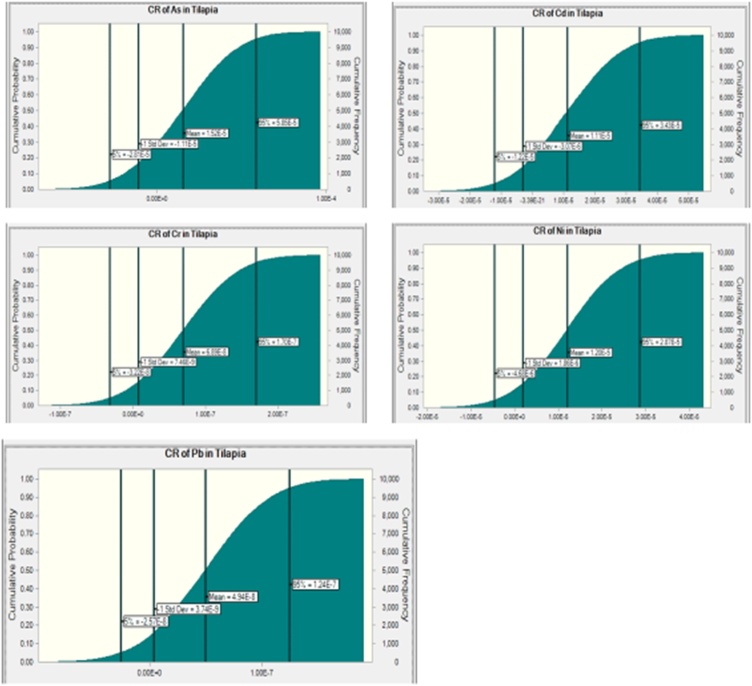
The percentile 95th of the Cancer Risk in consumers of fish obtained from the Eleyele and Ogun Rivers are shown in Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8, Fig. 9, Fig. 10, Fig. 11, Fig. 12, Fig. 13, Fig. 14. The acceptable limit set for lifetime exposure to carcinogens ranged from 10–4 (risk of developing cancer over a lifetime is 1 in 10,000) to 10–6 (risk of developing cancer over a lifetime is 1 in 1,000,000). Using the acceptable limit of 10–4, As pose cancer risk to consumers of Clarias gariepinus and Sarotherodon melanotheron obtained from the Ogun River and Clarias gariepinus obtained from the Eleyele River, as the CR values were higher than the set limit (Table 3). Also, the probabilistic prediction of the cancer risk of consuming As in the muscle of fish obtained from the Eleyele River (Fig. 13) and muscle of fish obtained from the Ogun River (Fig. 14) showed value greater than the set limit.
Fig. 9.
CR of As, Cd, Cr, Ni and Pb In Adult Consumer of Clarias gariepinus obtained from Ogun River.
Fig. 10.
CR of As, Cd, Cr, Ni and Pb In Adult Consumer of Sarotherodon melanotheron obtained from Ogun River.
Fig. 11.
Cancer Risk of As, Cd, Cr, Ni and Pb In Adult Consumer of Clarias gariepinus obtained from Eleyele River, Ibadan.
Fig. 12.
Cancer Risk of As, Cd, Cr, Ni and Pb In Adult Consumer of Clarias gariepinus obtained from Eleyele River, Ibadan.
Fig. 13.
Cancer risk of As, Cd, Cr, Ni and Pb In Adult Consumer of the edible portion of Clarias gariepinus and Sarotherodon melanotheron obtained from Eleyele River, Ibadan.
Fig. 14.
CR of consuming As, Cd, Cr, Ni, and Pb in the Muscles of Clarias gariepinus and Sarotherodon melanotheron obtained from Ogun River.
4. Discussion
The concentration of As, Cd, Co, Pb, Ni, Mn, and Cr analysed in this study suggested that the metals are from similar sources of pollution related to the study site viz., agricultural waste, human waste and anthropogenic activities, biometric characteristics, and as well, the behavioural pattern of the fish [45]. Similar to the observation of Arulkumar et al. [9], our result also suggested variation in the tissue accumulation pattern of the heavy metals.
Metal accumulation in fish is a complex process contributed mainly by endogenous and exogenous factors [46,47]. The exogenous factors could include parameters such as bioavailability of a particular metal, alkalinity, and temperature of the environment [47], whereas, the endogenous factor could include the feeding habit of the fish species. Surface contact with water, food chain, and respiratory activity are the three major means through which metals enter into the fish (Malik et al., 2010). For metal accumulation studies in fish, the gills which are in direct contact with the contaminated medium (water) and have the thinnest epithelium of all the organs making it penetrable for metals [48], and the liver being the organ of metabolism are best target organs [24]. The level in gills will reflect the concentration of elements in the water where the fish lives while the level in the liver will correspond to storage in the fish body [49]. According to Kalay et al. [50], a contaminant will accumulate in the muscle of fish only after exceeding the body defence barriers. In other words, the muscle may serve as a good indicator for chronic exposure [51]. In this study, more accumulation was observed in the gills for some of the metals. This is consistent with previous observations where a higher accumulation of some of the metals was observed in the gills than the other tissues analysed [52]. The variation in metal accumulation suggested that both exogenous and endogenous factors contribute to metal accumulation in the fish. From the observation of Jovanović et al. [53], direct transfer of heavy metals from sediments is the major route of passage in many aquatic species i.e. metals accumulate in bottom feeders and transfer up the food chain through biomagnification. Arsenic for instance is perpetual in the sediment thus continually accumulates in benthic fish [54]. This could therefore explain why As accumulate more in the fish species studied.
The concentration of all the identified metals in the tissues (gills, liver, and muscle) of the fish species is below the suggested consumption limit ([55,56], USFDA, 1993 [57];). Overall, the fish species collected from the two sites contained detectable levels of the metals analysed and the accumulation of these heavy metals may present health risk to the population of consumers based on calculation and simulation obtained in this study.
The estimated daily intake or tolerable intake was used to describe the safe levels of the heavy metals [58]. The values described in this study were dependent on the dry weight concentration of metals in the fish muscle and the fish ingestion rate considering the average adult weight at 60 kg [5]. Only Arsenic in the gills of Clarias gariepinus harvested from the Ogun River exceeded the provisional intake level sets by WHO, [59], therefore, consumption of these fish species harvested from the two sites might not pose any health risk since the provisional limits were not exceeded in the muscle of fish.
The THQ as a measure of health risk may signify some levels of concern [60]. When THQ > 1, it flags a concern alert whereas a THQ < 1 indicate that the level of exposure is lower than the reference dose in which daily exposure at this level is unlikely to cause any adverse health effect during an individual’s life time [45]. In this study, long time consumption of fish from the Ogun River might pose some health concern suggesting that consumers of the fish from the sites might experience significant non-carcinogenic health risk and therefore must exercise some caution.
Risk associated with carcinogenic effects of a metal is expressed as excess probability of cancer incidence over a 70 years lifetime [61]. Consumption of the two fish species from the two sites over a long period may have carcinogenic effects as the cancer Risk (CR) values obtained for Arsenic, Cadmium, and Nickel were greater than the acceptable guideline value of 10−6 [58]. This observation is in agreement with Nduka et al. [62] who reported similar health risks for As, Cd, Cr, Ni, and Pb in different painkiller drugs locally manufactured in Nigeria. The aggressive accumulation through consumption of these metals from various sources, therefore, raises concern and requires a prompt response from the necessary regulatory agencies in Nigeria.
The toxic nature of arsenic has been said to be dependent on its chemical form [63]. Arsenic is a metalloid widely reported with adverse health effects in humans. Chronic exposure has been shown with a variety of health concerns including many types of cancer, neurological, dermatitis, and cardiovascular diseases [[64], [65], [66], [67]]. Cadmium is another heavy metal reported with a high risk of cancer, nephrotoxicity, hepatotoxicity, reproductive and skeletal damages [68], while nickel is a natural constituent of earth crust assumed to be useful as a cofactor during iron absorption [69]. Chronic exposure may however be involved in circulatory system disorders in humans. Chromium is a metal irritant of the respiratory tract with the potential to cause pulmonary sensitization. Chronic exposure can also lead to bronchial carcinoma, auric deficiency (renal diseases), lung cancer, and the hepatocellular deficiency [70]. Based on the observation of this study, the toxic potentials of As, Cd, Ni, and Cr are likely to be manifested in the consumers of fish harvested from the two sites especially the Ogun River if continually consumed over a long period.
4.1. Conclusion and recommendation
This study analysed the concentration of As, Cd, Co, Cr, Ni, Mn, and Pb in the tissues of two fish species collected from the Ogun and Eleyele Rivers. We observed that arsenic was most prominent among the metals in the tissue of all the fish species. Although the concentration of metals in the fish was below the permissible limit, consumption of the fish from the Ogun River over a long period might present some non-carcinogenic risk while consumption of As, Cd, Ni, and Cr might predispose consumer of fish from the two sites to cancer risk. Therefore, proper awareness must be created and necessary government environmental health management agencies must put in place necessary regulations.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor: Aristidis Tsatsakis
References
- 1.Adel M., Dadar M., Fakhri Y., Conti G.O., Ferrante M. Heavy metal concentration in muscle of pike (Esox Lucius Linnaeus, 1758) from Anzali international wetland, southwest of the Caspian Sea and their consumption risk assessment. Toxin Rev. 2016;35:217–223. [Google Scholar]
- 2.FAO . Contribuer à la sécurité alimentaire et à la nutrition de tous; Rome: 2016. La Situation Mondiale Des Pêches Et De L′aquaculture 2016. [Google Scholar]
- 3.Varol M., Kaya G.K., Alp A. Heavy metal and arsenic concentrations in rainbow trout (Oncorhynchus mykiss) farmed in a dam reservoir on the Firat (Euphrates) River: risk-based consumption advisories. Sci. Total Environ. 2017;599–600:1288–1296. doi: 10.1016/j.scitotenv.2017.05.052. [DOI] [PubMed] [Google Scholar]
- 4.Varol M., Sünbül M.R. Macroelements and toxic trace elements in muscle and liver of fish species from the largest three reservoirs in Turkey and human risk assessment based on the worst-case scenarios. Environ. Res. 2020;184(2020):1–8. doi: 10.1016/j.envres.2020.109298. [DOI] [PubMed] [Google Scholar]
- 5.Matouke M.M., Abdullahi K.L. 2020. Assessment of Heavy Metals Contamination and Human Health Risk in Clarias gariepinus [Burchell, 1822] Collected From Jabi Lake, Abuja, Nigeria. Scientific Afican. [DOI] [Google Scholar]
- 6.Ali H., Khan E., Nasir M.J. Bioaccumulation of Some Potentially Toxic Heavy Metals in Freshwater Fish of River Shah Alam, Khyber Pakhtunkhwa. Pakistan. Pakistan Journal Zool. 2020;52(2):603–608. [Google Scholar]
- 7.Anandkumar A., Nagarajan R., Prabakaran K., Bing C.H., Rajaram R., Li J., Du D. Bioaccumulation of trace metals in the coastal Borneo (Malaysia) and health risk assessment. Mar. Pollut. Bull. 2019;145:56–66. doi: 10.1016/j.marpolbul.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Anandkumar A., Li J., Prabakaran K., Jia Z.I., Leng Z., Nagarajan R., Du D. Accumulation of toxic elements in an invasive crayfish species (Procambarus clarkii) and its health risk assessment to human. J. Food Compos. Anal. 2020;88(103449) [Google Scholar]
- 9.Arulkumar A., Paramasivam S., Rajaram R. Toxic heavy metals in commercially important food fishes collected from Palk Bay, Southeastern India. Mar. Pollut. Bull. 2017;2017 doi: 10.1016/j.marpolbul.2017.03.045. [DOI] [PubMed] [Google Scholar]
- 10.Rawtani D., Parmar T.K., Agrawal Y.K. Bioindicators: the natural indicator of environmental pollution. Front. Life Sci. 2016;9:110–118. doi: 10.1080/21553769.2016.1162753. [DOI] [Google Scholar]
- 11.Ahmed M.K., Baki M.A., Kundu G.K., Islam M.S., Islam M.M., Hossain M.M. Human health risks from heavy metals infish of Buriganga River. Bangladesh. Springer Plus. 2016;5:1697. doi: 10.1186/s40064-016-3357-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ali M.M., Ali M.L., Islam M.S., Rahman M.Z. Preliminary assessment of heavy metals in water and sediment of Karnaphuli River, Bangladesh. Environ. Nanotechnol. Monit. Manag. 2016;5:27–35. [Google Scholar]
- 13.Kibria G., Hossain M.M., Mallick D., Lau T.C., Wu R. Trace/heavy metal pollution monitoring in estuary and coastal area of Bay of Bengal, Bangladesh and implicated impacts. Mar. Pollut. Bull. 2016;105:393–402. doi: 10.1016/j.marpolbul.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 14.Kibria G., Hossain M.M., Mallick D., Lau T.C., Wu R. Monitoring of metal pollution in waterways across Bangladesh and ecological and public health implications of pollution. Chemosphere. 2016;165:1–9. doi: 10.1016/j.chemosphere.2016.08.121. [DOI] [PubMed] [Google Scholar]
- 15.Liang H., Wu W.L., Zhang Y.H., Zhou S.J., Long C.Y., Wen J., Liu N. Levels, temporal trend and health risk assessment offive heavy metals in fresh vegetables marketed in Guangdong Province of China during 2014–2017. Food Control. 2018;92:107–120. [Google Scholar]
- 16.Uysal K., Köse E., Bülbül M., Dönmez M., Erdoğan Y., Koyun M., Ömeroğlu Ç., Özmal F. The comparison of heavy metal accumulation ratios of some fish species in Enne Dame Lake (Kütahya/Turkey) Environ. Monit. Assess. 2009;157:355–362. doi: 10.1007/s10661-008-0540-y. [DOI] [PubMed] [Google Scholar]
- 17.Soltani N., Moore F., Keshavarzi B., Sorooshian A., Javid R. Potentially toxic elements (PTEs) and polycyclic aromatic hydrocarbons (PAHs) in fish and prawn in the Persian Gulf, Iran Ecotoxicology. and Environmental Safety. 2019;173(2019):251–265. doi: 10.1016/j.ecoenv.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Dang V.D., Kroll K.J., Supowit S.D., Halden R.U., Denslow N.D. Tissue distribution of organochlorine pesticides in largemouth bass (Micropterus salmoides) from laboratory exposure and a contaminated lake. Environ. Pollut. 2016;216:877–883. doi: 10.1016/j.envpol.2016.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar K.A., Achyuthan H. Heavy metal accumulation in certain marine animals along the East Coast of Chennai Tamil Nadu. India J. Env. Biol. 2005;28:637–643. [PubMed] [Google Scholar]
- 20.Harte J., Holdren C., Schneider R., Shirley C. University of California Press; Oxford England: 1991. Toxics A to Z A Guide to Everyday Pollution Hazards. 1991. [Google Scholar]
- 21.Gwimbi P., Kotelo T., Selimo M.J. Heavy metal concentrations in sediments and Cyprinus carpio from Maqalika Reservoir–Maseru, Lesotho: An analysis of potential health risks to Fish consumers. Toxicol. Program Tech. Rep. Ser. 2020;7:475–479. doi: 10.1016/j.toxrep.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghasemidehkordi B., Malekirad A.A., Nazem H., Fazilati M., Salavati H., Shariatifar N., Rezaei M., Khaneghah A.M., Fakhri Y. Concentration of lead and mercury in collected vegetables and herbs from Markazi province, Iran: Non-carcinogenic risk assessment. Food 447 and Chemical Toxicology. 2018;2018 doi: 10.1016/j.fct.2018.01.048. [DOI] [PubMed] [Google Scholar]
- 23.Farombi E.O., Adelowo O.A., Ajimoko Y.R. Biomarkers of Oxidative Stress and Heavy Metal Levels as Indicators of Environmental Pollution in African Cat Fish (Clarias gariepinus) from Nigeria Ogun River. Int. J. Environ. Res. Public Health. 2007;4(2):158–165. doi: 10.3390/ijerph2007040011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taweel A., Shuhaimi-Othman M., Ahmad A.K. Heavy metals concentration in different organs of tilapia fish (Oreochromis niloticus) from selected areas of Bangi, Selangor, Malaysia. African J. Biotechnol. 2011;10(55):1 1562–1 1566. [Google Scholar]
- 25.Saha N., Mollah M.Z.I., Alam M.F., Safiur M.Rahman. Seasonal investigation of heavy metals in marine fishes captured from the Bay of Bengal and the implications for human health risk assessment. Food Control. 2016;70(2016):110–118. [Google Scholar]
- 26.Taiwo A.M., Oyebode A.O., Salami F.O., Okewole I., Gbogboade A.S., Agim C., Oladele T.O., Kamoru T.A., Abdullahi K.L., Davidson N. Carcinogenic and non-carcinogenic evaluations of heavy metals in protein foods from southwestern Nigeria. J. Food Compos. Anal. Xxx. 2018;(2018) xxx. [Google Scholar]
- 27.FAO . FAO Fisheries and Aquaculture Department. Food and Agriculture Organization of the UN; Rome: 2008. The Sate of World Fisheries and Aquaculture 2008. [Google Scholar]
- 28.The World Fact Book . 2014. CIA (Central Intelligence Agency). July 2014. Retrieved 10 August 2014. [Google Scholar]
- 29.FAO (Food and Agriculture Organization) FAO Regional Office for the Asia and Pacific; Bangkok, Thailand: 2006. Arsenic Contamination of Irrigation Water, Soil and Crops in Bangladesh: Risk Implication for Sustainable Agriculture and Food Safety in Asia. [Google Scholar]
- 30.Wang X., Sato T., Xing B. Health risks of heavy metals to the general public in Tianjin, China via consumption of vegetables and fish. Sci. Total Environ. 2005;350:28–37. doi: 10.1016/j.scitotenv.2004.09.044. [DOI] [PubMed] [Google Scholar]
- 31.Nadal M., Ferre-Huget N., Martí-Cid R. Exposure to metals through the consumption of fish and seafood by population living near the Ebro River in Catalonia, Spain: health risks. Hum. Ecol. Risk Assess. 2008;14:780–795. [Google Scholar]
- 32.USEPA . 2010. Risk-Based Concentration Table. Available at http://www.epa.gov/reg3hwmd/risk/human/index.htm(Accessed April 15, 2014. [Google Scholar]
- 33.Islam M.S., Ahmed M.K., Mamun M.H.A. Determination of heavy metals in fish and vegetables in Bangladesh and health implications. Hum. Ecol. Risk Assess. 2015;21(4):986–1006. [Google Scholar]
- 34.Zhong W., Zhang Y., Wu Z., Yang R., Chen X., Yang J., Zhu L. Health risk assessment of heavy metals in freshwaterfish in the central and eastern North China. Ecotoxicol. Environ. Saf. 2018;157:343–349. doi: 10.1016/j.ecoenv.2018.03.048. [DOI] [PubMed] [Google Scholar]
- 35.FAO . FAO Fisheries and Aquaculture Dept; 2014. The State of the World Fisheries and Aquaculture. [Google Scholar]
- 36.Yin S., Feng C., Li Y., Yin L., Shen Z. Heavy metal pollution in the surface water of the Yangtze Estuary: a 5-year follow-up study. Chemosphere. 2015;138:718–725. doi: 10.1016/j.chemosphere.2015.07.060. [DOI] [PubMed] [Google Scholar]
- 37.Traina A., Bono G., Bonsignore M., Falco F., Giuga M., Quinci E.M., Sprovieri M. Heavy metals concentrations in some commercially key species from Sicilian coasts (Mediterranean Sea): potential human health risk estimation. Ecotoxicol. Environ. Saf. 2019;168:466–478. doi: 10.1016/j.ecoenv.2018.10.056. [DOI] [PubMed] [Google Scholar]
- 38.Vieira C., Morais S., Ramos S., Delerue-Matos C., Oliveira M.B.P.P. Mercury, cadmium, lead, and arsenic levels in three pelagicfish species from the Atlantic Ocean: intra-and inter-specific variability and human health risks for consumption. Food Chem. Toxicol. 2011;49:923–932. doi: 10.1016/j.fct.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 39.USEPA . United States Environmental Protection Agency; Washington, DC, USA: 2010. Integrated Risk Information System (IRIS) Available online.www.epa.gov/ncea/iris/index.html, Accessed date: 15 September 2018. [Google Scholar]
- 40.USEPA . 2010. Risk-based Concentration Table.http://www.epa.gov/reg3hwmd/risk/human/index.htm Available from. [Google Scholar]
- 41.Chen M.-J., Hsu H.-T., Lin C.-L., Ju W.-Y. A statistical regression model for the estimation of acrylamide concentrations in French fries for excess lifetime cancer risk assessment. Food Chem. Toxicol. 2012;50:3862–3876. doi: 10.1016/j.fct.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 42.Park J.D., Zheng W. Human exposure and health effects of inorganic and elemental mercury. J. Prev. Med. Public Health. 2012;45:344. doi: 10.3961/jpmph.2012.45.6.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qu C., Ma z., Yang j., Liu Y., Bi J., Huang L. Human exposure pathways of heavy metals in a lead-zinc mining area, Jiangsu Province. China. PLoS ONE. 2012;7(11):46793. doi: 10.1371/journal.pone.0046793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu B., Jia X., Hu J., Xu D., Xia F., Li Y. Assessment of heavy metal pollution and health risks in the soil-plant-human system in the Yangtze River delta. China. Int J Environ Res Public Health. 2017;14:1042. doi: 10.3390/ijerph14091042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yi Y., Yang Z., Zhang S. Ecological risk assessment of heavy metals in sediment and human health risk assessment of heavy metals in fishes in the middle and lower reaches of the Yangtze River basin. Nitrogen Depos. Crit. Loads Biodivers. 2011;159:2575–2585. doi: 10.1016/j.envpol.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 46.Griboff J., Wunderlin Daniel A., Monferran Magdalena V. Metals, As and Se determination by inductively coupled plasma-mass spectrometry (ICP-MS) in edible fish collected from three eutrophic reservoirs. Their consumption represents a risk for human health? Microchem. J. 2016 doi: 10.1016/j.microc.2016.09.013. [DOI] [Google Scholar]
- 47.Jabeen F., Chaudhry A.S. Environmental impacts of anthropogenic activities on the mineral uptake in Oreochromis mossambicus from Indus River in Pakista. Environ. Monit. Assess. 2010;166:641–651. doi: 10.1007/s10661-009-1029-z. [DOI] [PubMed] [Google Scholar]
- 48.Bebianno M.J., Geret F., Hoarau P., Serafim M.A., Coelho M.R., Gnassia-Barelli M., Romeo M. Biomarkers in Ruditapes decussatus: a potential bioindicator species. Biomarkers. 2004;9:305–330. doi: 10.1080/13547500400017820. [DOI] [PubMed] [Google Scholar]
- 49.Roméo M., Siau Y., Sidoumou Z., Gnassia-Barelli M. Heavy metal distribution in different fish species from the Mauritania Coast. Sci. Total Environ. 1999;232(3):169–175. doi: 10.1016/s0048-9697(99)00099-6. [DOI] [PubMed] [Google Scholar]
- 50.Kalay M., Ay Ö., Canli M. Heavy metal concentrations in fish tissue from the northeast Mediterranean Sea. Bull. Environ. Contam. Toxicol. 2000;63:673–681. doi: 10.1007/s001289901033. [DOI] [PubMed] [Google Scholar]
- 51.Ballesteros M.L., Wunderlin D.A., Bistoni M.A. Oxidative stress responses in different organs of Jenynsia multidentata exposed to Endosulfan. Ecotoxicol. Environ. Saf. 2009;72:199–205. doi: 10.1016/j.ecoenv.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 52.Anandkumar A., Nagarajan R., Prabakaran K., Bing C.H., Rajaram R. Human health risk assessment and bioaccumulation of trace metals infish species collected from the Miri coast, Sarawak, Borneo. Marine Pollut. Bull. 2018;133:655–663. doi: 10.1016/j.marpolbul.2018.06.033. [DOI] [PubMed] [Google Scholar]
- 53.Jovanović D.A., Marković R.V., Teodorović V.B., Šefer D.S., Krstić M.P., Radulović S.B., Ćirić J.S.I., Janjić J.M., Baltić M.Ž. Determination of heavy metals in muscle tissue of sixfish species with different feeding habits from the Danube River, Belgrade—public health and environmental risk assessment. Environ. Sci. Pollut. Res. 2017;24(12):11383–11391. doi: 10.1007/s11356-017-8783-1. [DOI] [PubMed] [Google Scholar]
- 54.Noël L., Chekri R., Millour S., Merlo M., Leblanc J.C., Guérin T. Distribution and relationships of As, Cd, Pb and Hg in fresh water fish from five French fishing areas. Chemosphere. 2013;90:1900–1910. doi: 10.1016/j.chemosphere.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 55.European Union (EU) 2001. Commission Regulation As Regards Heavy Metals. Directive 2001/22/EC, No: 466/2001. [Google Scholar]
- 56.European Union (EU) 2008. Commission Regulation (EC) No. 629/2008. Setting Maximum Levels for Certain Contaminants in Foodstuffs. Official Journal of the European Union L 173. [Google Scholar]
- 57.FAO/WHO . 1987. Principles of the Safety Assessment of Food Additives and Contaminants in Food Environmental Health Criteria, Geneva. No: 70. Geneva. [Google Scholar]
- 58.Keshavarzi B., Hassanaghaei M., Moore F., Mehr M.R., Soltanian S., Lahijanzadeh A.Z., Sorooshian A. Heavy metal contamination and health risk assessment in three commercial fish species in the Persian Gulf. Mar. Pollut. Bull. 2018;129:245–252. doi: 10.1016/j.marpolbul.2018.02.032. [DOI] [PubMed] [Google Scholar]
- 59.WHO . 2007. Exposure of Children to Chemical Hazards in Food FACT SHEET NO. 4.4. May 2007 · CODE: RPG4_Food_Ex1. [Google Scholar]
- 60.Bassey F.I., Oguntunde F.C., Iwegbue C.M.A., Osabor V.N., Edem C.A. Effects of processing on the proximate and metal contents in three fish species from Nigerian coastal waters. Food Sci. Nutr. 2014;2(3):272–281. doi: 10.1002/fsn3.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bhupander K., Mukherjee D.P. Assessment of human health risk for arsenic, copper, nickel, mercury, and zinc in fish collected from tropical wetlands in India. Adv. Life. Sci. Technol. 2012;2:13–25. [Google Scholar]
- 62.Nduka J.K., Kelle H.I., Ogoko E.C. Hazards and risk assessment of heavy metals from consumption of locally manufactured painkiller drugs in Nigeria. Toxicol. Program Tech. Rep. Ser. 2020;7:1066–1074. doi: 10.1016/j.toxrep.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Medeiros R.J., Dos Santos L.M.G., Freire A.S., Santelli R.E., Braga A.M.C., Krauss T.M., Jacob S.D.C. Determination of inorganic trace elements in edible marinefish from Rio de Janeiro State. Brazil. Food Control. 2012;23:535–541. [Google Scholar]
- 64.Hopenhayn-Rich C., Browning S.R., Hertz-Picciotto I., Ferreccio C., Peralta C., Gibb H. Chronic arsenic exposure and risk of infant mortality in two areas of Chile. Environ. Health Perspect. 2000;108(7):667–673. doi: 10.1289/ehp.00108667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ikem A., Egiebor N.O. Assessment of trace elements in canned fishes (mackerel, tuna, salmon, sardines and herrings) marketed in Georgia and Alabama (united States of America) J. Food Anal. 2005;18(8):771–787. [Google Scholar]
- 66.Ashraf M., Maah M., Yusoff I. Bioaccumulation of heavy metals in fish species collected from former tin mining catchment. Int. J. Environ. Res. 2011;6:209–218. [Google Scholar]
- 67.Bardach A.E., Ciapponi A., Soto N., Chaparro M.R., Calderon M., Briatore A., Cadoppi N., Tassara R., Litter M.L. Epidemiology of chronic disease related to arsenic in Argentina: a systematic review. Sci. Total Environ. 2015;538:802–816. doi: 10.1016/j.scitotenv.2015.08.070. [DOI] [PubMed] [Google Scholar]
- 68.Behbahani M., Bagheri A., Amini M.M., Sadeghi O., Salarian M., Najafi F., Taghizadeh M. Application of multiwalled carbon nanotubes modified by diphenylcarbazide for selective solid phase extraction of ultra-traces Cd (II) in water samples and food products. Food Chem. 2013;141(1):48–53. doi: 10.1016/j.foodchem.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 69.Das K., Das S., Dhundasi S. Nickel, its adverse health effects, & oxidative stress. Indian J. Med. Res. 2008;128(4):412. [PubMed] [Google Scholar]
- 70.Baruthio F. Toxic effects of chromium and its compounds. Biol. Trace Elem. Res. 1992;32(1992):145–153. doi: 10.1007/BF02784599. [DOI] [PubMed] [Google Scholar]
- 71.Chien L.C., Hung T.C., Choang K.Y. Daily intake of TBT, Cu, Zn, Cd and As for fishermen in Taiwan. Sci. Total Environ. 2002;285:177–185. doi: 10.1016/s0048-9697(01)00916-0. [DOI] [PubMed] [Google Scholar]