Abstract
Pleuropulmonary blastoma is a rare and highly aggressive pulmonary malignancy in children. Clinically, the malignancy is often mistaken for symptoms of respiratory tract infection or pneumothorax. The neoplasm is histologically characterized by primitive blastema and a malignant mesenchymal stroma that demonstrates multidirectional differentiation. The patients with PPB are managed by multimodal therapy. We present a report of 3 cases of histopathologically diagnosed pleuropulmonary blastoma. The patients presented with chief complaints of difficulty in breathing, cough, fever and chest pain. Radiographs of the patients showed partial to complete opacification of hemithorax. Contrast enhanced computed tomography scans revealed large well defined heterogenously enhancing solid mass lesions in the hemithorax. Knowledge of types, imaging findings, staging and association with other tumors is crucial for correct diagnosis of pleuropulmonary blastoma and subsequent adequate management.
Keywords: Pleuropulmonary Blastoma (PPB), Contrast Enhanced Computed Tomography (CECT)
Introduction
Pleuropulmonary blastoma (PPB) is a rare and highly aggressive neoplasm. It was earlier classified with pulmonary blastoma, which constitutes 0.25%-0.50% of primary lung malignancies in all age groups [1]. PPB is the most common primary lung malignancy in children. It occurs primarily in children younger than 6 years. PPB is of 3 types – purely cystic (type I), mixed cystic and solid (type II) and purely solid (type III) [2]. Another variant (type I-regressed) was added in 2006 [3]. Type I tumors can progress with time to more aggressive type II and type III tumors. Moreover, pleuropulmonary blastoma is associated with mutation in DICER 1 gene and recognized as part of DICER syndrome comprising multiple other neoplasms [4]. Thus, the correct diagnosis of pleuropulmonary blastoma and imaging surveillance to detect other commonly associated neoplasms is necessary. Being a rare neoplasm, only a few articles and case reports have detailed the imaging appearance of pleuropulmonary blastoma [2,5]. Our report describe 3 cases of pleuropulmonary blastoma with review of current literature on pathogenesis, clinical features and imaging findings of the malignancy.
Case presentations
Case 1
A 5-year-old female, presented to the out-patient department with chief complaints of difficulty in breathing and cough for past 3 months. On examination, tachypnea was present and breath sounds were absent on the right side. The child had normal term vaginal delivery with birth weight of 2.7 kgs. There were no reported anomalies in the antenatal ultrasound. She had no neonatal complication. No past history of any chronic illness or developmental delay was present.
Chest X ray was done which showed complete homogenous opacification of right hemithorax. There was marked tracheal deviation and mediastinal shift to the left side (Fig. 1). Focal ill defined lytic sclerotic area was noted in the anterior 4th rib. Contrast enhanced CT revealed a large well defined heterogeneously enhancing solid mass lesion in the right hemithorax. Few ill-defined non enhancing hypodense areas likely necrosis were noted within the lesion. The mass was seen to compress and displace the mediastinal structures to the left. It was seen to cause erosions with reactive sclerosis in anterolateral part of right 4th rib and extend into soft tissues of right posterolateral chest wall (Figs. 2A and B). Right lobe of liver was compressed and displaced inferiorly by the lesion. Mild pleural effusion was seen (Fig 2C). No calcification was seen within the lesion. Right lung is not aerated and appears collapsed (likely due to compression by mass) and displaced medially by the mass lesion (Fig 2D). There was no evidence of vertebral destruction, neural foramen widening or extension into spinal canal.
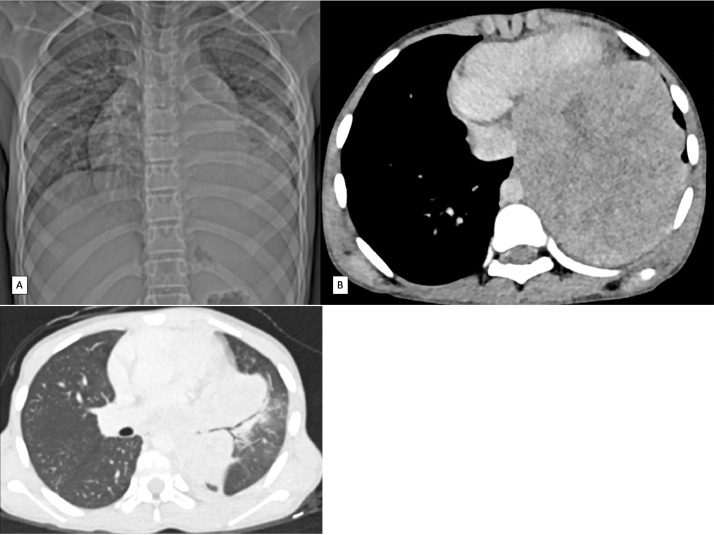
Fig. 1.
Case 1- Chest radiograph of 5-year-old female. Complete homogenous opacification of right hemithorax with contralateral mediastinal and tracheal deviation. Focal irregularity in outline of 4th rib (black arrow).
Fig. 2.
Case 1- Axial CECT chest images of 5-year-old female. (A) a large well defined solid mass with heterogenous post contrast enhancement in right hemithorax with invasion of soft tissues of overlying right chest wall. (B) Bone reconstruction image showed cortical irregularity and erosions with reactive sclerosis of right 4th rib (black arrow). (C) Sagittal reformatted image showed extension of the mass and mild basal pleural effusion (black arrow). (D) Axial lung window image shows complete opacification of hemithorax by mass lesion and non-visualized lung parenchyma. Right lobar bronchi are seen medially.
Based on the above features of large solid mass lesion completely occupying the right hemithorax causing right lung collapse and medial displacement, with no calcification within and no intraspinal extension, possibility of Chest wall based mass was considered and provisional diagnosis of pleuropulmonary blastoma was made. Other Differential diagnosis were Ewing's sarcoma, rhabdomyosarcoma and inflammatory myofibroblastic tumor, because the mass showed features of rib erosion and chest wall invasion. CT guided biopsy was done which confirmed the diagnosis of pleuropulmonary blastoma.
The patient underwent successful surgical excision of the mass and adjuvant chemoradiotherapy. Post-operative course was uneventful.
Case 2
A 4 year old male presented with chief complaints of difficulty in breathing and on and off fever for past 1 month. On examination, there was significant reduced air entry on left side with respiratory distress. No abnormality was reported in antenatal ultrasonogram. No past history of any chronic illness or developmental delay was present.
Chest X ray showed radio-opaque mass lesion in the left lower lung zone with broad base towards mediastinum. The lateral border of the mass and its interface with lung was ill defined. There was loss of silhouette of left hemidiaphragm (Fig. 3A). Contrast enhanced CT scan showed a large sharply marginated heterogenously enhancing solid mass in left lower hemithorax. The mass was compressing the left chambers of the heart anteromedially and partially encasing descending aorta medially (Fig.3B). There was invasion of adjacent lung parenchyma laterally (Fig 3C). No evidence of rib erosion, chest wall invasion or intraspinal extension was noted.
Fig. 3.
Case 2- (A) Chest radiograph of 4-year-old male showed a radio-opaque mass lesion in left lower lung zone with broad base towards mediastinum and ill-defined lateral margin. (B) Axial CECT chest image showed well defined heterogenously enhancing solid mass in left lower lobe compressing the heart anteromedially. (C) axial lung window image shows irregular interface with lung parenchyma suggestive of parenchymal involvement by the mass.
The lesion was located medially, involving the posterior mediastinum, causing displacement of mediastinal structures and showing invasion of adjacent lung parenchyma laterally. Based on the features of medial pleuropulmonary location of the mass lesion, no evidence of rib erosion, chest wall invasion or intraspinal extension, the provisional diagnosis of pleuropulmonary blastoma was made. Differential diagnosis of neuroblastoma was kept because of the posterior mediastinal location of the mass lesion. Patient underwent CT guided biopsy and histopathological analysis confirmed the diagnosis. The patient was lost to follow up.
Case 3
A 2-year-old female presented to the emergency department with complaints of chest pain, difficulty in breathing and low grade fever for past 2 months. Clinical examination revealed respiratory distress and absent breath sounds in right hemithorax. The child had normal term vaginal delivery with birth weight of 2.6 kgs. Antenatal ultrasonogram was normal. No past history of any chronic illness or developmental delay was present.
Chest X ray revealed a similar picture to case 1 showing complete homogenous opacification of right hemithorax (Fig. 4). Marked tracheal deviation and mediastinal shift was noted to the left side. No obvious rib erosion, vertebral destruction or chest wall involvement was noted. Findings in the chest X ray were mistaken for massive right pleural effusion. Intercostal drainage tube was inserted, however there was no symptomatic relief. On contrast enhanced CT scan, a massive heterogenous mass lesion with peripherally enhancing solid component and large central non enhancing necrotic area was noted in right hemithorax. The lesion was noted extending across the midline to the left side (Fig 5 A and B). It was seen to cause marked contralateral displacement of mediastinal structures. Mild pleural effusion was present. No calcification was seen within. Right lung is not aerated and appears collapsed (likely due to compression by mass) and displaced medially by the mass lesion (Fig 5C). No rib erosion, chest wall invasion, vertebral destruction or intraspinal extension was noted.
Fig. 4.
Case 3- Chest radiograph of 2-year-old female. Complete homogenous opacification of right hemithorax with contralateral mediastinal and tracheal deviation. ICD tube seen in situ.
Fig. 5.
Case 3- CECT chest of 2-year-old female. (A) Axial image showed a large heterogenous mass lesion with peripherally enhancing solid component and central necrosis in right hemithorax and extending across the midline with pleural effusion medially (yellow arrow). (B) Coronal reformatted image showed extension and mass effect of the mass on mediastinal structures and liver (black arrow). (C) axial lung window image shows medially displaced right lobar bronchi with non-visualized lung parenchyma, likely collapsed due to compression.
Based on the features of heterogenous mass lesion occupying entire right hemithorax causing collapse and displacement of lung medially, chest wall based mass lesion was considered. Since the mass lesion showed no rib erosion, chest wall invasion, intraspinal extension or intratumoral calcification, the provisional diagnosis of pleuropulmonary blastoma was made.
CT guided Biopsy was done from the solid part. Microscopically, there were variable blastematous foci in which the constituent cells had scanty cytoplasm, round to ovoid nuclei with granular chromatin, inconspicuous nucleoli and frequent mitoses. Their stroma was less dense and fibroblastic that blended with sarcomatous component (Fig. 6A). The sarcomatous component was composed of spindle cell proliferation arranged in fascicular pattern; their nuclei showed anisonucleosis and hyperchromasia with scattered pleomorphic bizarre giant cells. There were scattered polygonal rhabdomyoblasts occurring singly (Fig. 6B). Small proportion of immature to mature hyaline cartilage was noted. Areas of infarction and necrosis in the cartilaginous, blastematous and sarcomatous component producing pseudocysts were noted. Thus, histopathology confirmed the diagnosis of type III pleuropulmonary blastoma. The patient underwent complete surgical excision and post-operative adjuvant chemoradiotherapy. Post-operative course was uneventful.
Fig. 6.
Histopathology images of Case 3 (A) Nodules of blastema like cells. H&E x100. (B) Pleomorphic bizarre giant cells. H&E x 100.
No evidence of distant metastasis was seen in all the cases. Bone scan was normal in all 3 cases. Ultrasonography abdomen was done in all 3 cases and commonly co-occurring cystic nephroma and other tumors associated with PPB were ruled out.
Discussion
Pleuropulmonary blastoma is a rare intrathoracic tumor seen in children. Spencer first coined the term in 1961 and suggested that the tumor arose from mesodermal blastema [6]. In 1988, Manivel et al. described pleuropulmonary blastoma in children as distinct tumor from the biphasic epithelial-stromal morphology of the classic adult type [7]. In 2015, World Health Organization classified pleuropulmonary blastoma as distinct from adult pulmonary blastoma and now falls under the category of mesenchymal neoplasms [8]. It is primarily seen in children less than 6 years age [1]. According to International Pleuropulmonary Blastoma Registry, it is mainly of 3 types – type I, II, and III (Table 1). These types form a continuum and there is progression from type I to type III over time [2,5].
Type I PPB has a median age of 8 months and the best prognosis among all types - 91% 5-year overall survival rate. On histopathology multiloculated cystic thin walled structure with fibrous septa containing immature cells is seen. Type Ir is a subtype added in 2006, with a median age of 48 months and histopathological features of cystic areas with few spindle cells and foci of dystrophic calcification.
Type II PPB has a median age of 35 months and 71% 5-year overall survival rate. On histopathology solid nodules and polypoid tissue extending into cysts are seen. Type III PPB has a median age of 44 months and worst prognosis among all types – 53% 5 year overall survival rate. On histopathology it appears completely solid. Solid tissue in type II and III PPB contains mixed blastematous and sarcomatous elements which is different from adult type pulmonary blastoma (contains malignant epithelial and mesenchymal tissue)
In recent studies, the malignancy has been recognized as part of DICER1 syndrome or PPB familial tumor and dysplasia syndrome [1,4]. Around 66 % patients with the malignancy have heterozygous mutation in DICER1 gene located on chromosome 14q13.2 [5]. A total of 25% of cases have association with other neoplasms in themselves or their families. Neoplasms associated with DICER1 syndrome include cystic nephroma, pineoblastoma, pituitary blastoma, ovarian sex cord stromal tumors, embryonal rhabdomyosarcomas and other tumors [4]. Thus, it is essential to screen a patient diagnosed with pleuropulmonary blastoma for DICER1 mutation and associated neoplasms.
Clinical presentation is usually with nonspecific respiratory complaints such as difficulty in breathing, dyspnea, chest pain, hemoptysis. Fever, malaise and anorexia are associated with type II and type III PPB. On imaging, chest radiography is the first modality used for evaluation. Pleuropulmonary blastoma is most commonly seen on right side. It appears as hemi-opaque thorax with contralateral tracheal and mediastinal deviation [1]. Other patterns are partial opacification of lung, benign appearing cystic lung disease, pleural effusion, tension pneumothorax and mediastinal shift [3]. Contrast enhanced computed tomography of chest shows the morphology, enhancement pattern, complete tumor extension and invasion of adjacent structures. These features help in differentiating pleuropulmonary blastoma from more commonly occurring lesions such as neuroblastoma, congenital pulmonary airway malformation (CPAM). Type I lesions appear as single cyst or multicystic lesions with fluid or air within. Type II PPB shows cystic lesion with air or fluid within and solid component which enhances on contrast administration. Type III lesions appear as heterogenously enhancing solid lesions with areas of hemorrhage or necrosis within [1,3]. Pleural effusion and pneumothorax can be commonly seen. Calcification is not a common feature. The lesion usually presents late because of the nonspecific complaints of respiratory distress which are often ignored in early disease. Due to this reason, the mass at presentation causes complete opacification of hemithorax (most commonly right side). Such a presentation was seen in 2 of our patients. This can lead to a misdiagnosis of massive pleural effusion based on chest radiograph appearance. This happened with patient 3 in our series, where Intercostal drainage tube was inserted.
All the patients in our report showed imaging features of type III pleuropulmonary blastoma with the presence of rib erosion and chest wall invasion in case 1. Invasion of adjacent structures has also been reported in previous case series [2,5]. Metastasis is seen mostly in type II and type III PPB with predominant involvement of bones and central nervous system [1]. In our cases, no distant metastasis was detected.
The differential diagnosis of type I pleuropulmonary blastoma is congenital pulmonary airway malformation (CPAM) which can't be distinguished by imaging. However, Type I PPB is multifocal and occurs postnatally compared to congenital cystic lesions which are unifocal and usually seen in second trimester antenatal ultrasonography [9]. The differential diagnosis for type II and type III PPB include commonly occurring pediatric solid tumors such as neuroblastoma, Ewing's sarcoma, rhabdomyosarcoma and inflammatory myofibroblastic tumor. The absence of calcifications, vertebral destruction and intraspinal extension can differentiate pleuropulmonary blastoma from these tumors [1]. Infantine Fibrosarcoma is another probable differential diagnosis, which occurs exclusively in neonates and infants.
The imaging recommendations for staging in patients with suspicion of pleuropulmonary blastoma are contrast enhanced CT of chest and abdomen (to detect commonly associated cystic nephroma) for type I; contrast enhanced CT chest and abdomen, brain MRI, Bone scan for type II and type III pleuropulmonary blastomas [3].
The recommended treatment for type I tumors consists of surgical excision and adjuvant chemotherapy. For type Ir tumors, only follow-up and no chemotherapy are recommended. For type II and type III tumors, the treatment consists of aggressive surgery and chemotherapy. For large tumors, neoadjuvant chemotherapy is instituted to reduce the tumor size followed by surgical resection. Radiation therapy is reserved for patients with known but nonresectable tumors or for residual tumors after chemotherapy. For brain metastasis, all 3 treatment modalities - surgery, chemotherapy and radiation therapy are recommended. For recurrent tumors, high dose consolidation therapy (HDCT) with autologous stem cell rescue (ASCR) is recommended [3].
Conclusion
Pleuropulmonary blastoma is a highly aggressive malignancy of early childhood seen primarily in children younger than 6 years. The supporting features for a diagnosis of pleuropulmonary blastoma are right sided mass lesion, complete hemithorax opacification, absence of intralesional calcification, no evidence of chest wall invasion/rib erosion/intraspinal extension. Type I PPB should be differentiated from CPAM; type II and type III PPB need to be differentiated from common solid pediatric tumors such as neuroblastoma, Ewing's sarcoma, infantile myofibroblastic tumor etc. PPB can be associated with DICER1 syndrome, thus a diagnosis of PPB should lead to screening and surveillance for commonly associated neoplasms such as cystic nephroma in the patient, their siblings and their first degree relatives. The patients diagnosed with the malignancy should undergo radical surgery, even in cases with microscopic residual disease.
Footnotes
Name of the department to which the work is attributed: Department of Radiodiagnosis, ABVIMS and Dr RML Hospital, New Delhi.
"Written informed consent was obtained from the patients’ guardian for publication of this case review, including accompanying images".
Acknowledgments: Self finance.
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Lichtenberger JP, Biko DM, Carter BW, Pavio MA, Huppmann AR, Chung EM. Primary lung tumors in children: radiologic-pathologic correlation from the radiologic pathology archives. RadioGraphics. 2018;38(7):2151–2172. doi: 10.1148/rg.2018180192. [DOI] [PubMed] [Google Scholar]
- 2.Priest JR, McDermott MB, Bhatia S, Watterson J, Manivel JC, Dehner LP. Pleuropulmonary blastoma: a clinicopathologic study of 50 cases. Cancer. 1997;80(1):147–161. [PubMed] [Google Scholar]
- 3.The International Pleuropulmonary Blastoma (PPB) /DICER1 Registry [Internet]. [cited 2020 Nov 24 ]. Available from: https://www.ppbregistry.org/.
- 4.Priest JR, Watterson J, Strong L, Huff V, Woods WG, Byrd RL. Pleuropulmonary blastoma: a marker for familial disease. J Pediatr. 1996;128(2):220–224. doi: 10.1016/s0022-3476(96)70393-1. [DOI] [PubMed] [Google Scholar]
- 5.Messinger YH, Stewart DR, Priest JR, Williams GM, Harris AK, Schultz KAP. Pleuropulmonary blastoma: a report on 350 central pathology-confirmed pleuropulmonary blastoma cases by the International Pleuropulmonary Blastoma Registry. Cancer. 2015;121(2):276–285. doi: 10.1002/cncr.29032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spencer H. Pulmonary blastoma. J Pathol Bacteriol. 1961;82:161–165. [Google Scholar]
- 7.Manivel JC, Priest JR, Watterson J, Steiner M, Woods WG, Wick MR. Pleuropulmonary blastoma. The so-called pulmonary blastoma of childhood. Cancer. 1988;62(8):1516–1526. doi: 10.1002/1097-0142(19881015)62:8<1516::aid-cncr2820620812>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 8.Mlika M, Anjum F, El Mezni F., In . StatPearls [Internet] StatPearls Publishing; Treasure IslandFL: 2020. Pleuropulmonary Blastoma.http://www.ncbi.nlm.nih.gov/books/NBK534211/ [cited 2020 Nov 24]. Available from: [Google Scholar]
- 9.Orazi C, Inserra A, Schingo PMS, De Sio L, Cutrera R, Boldrini R. Pleuropulmonary blastoma, a distinctive neoplasm of childhood: report of three cases. Pediatr Radiol. 2007;37(4):337–344. doi: 10.1007/s00247-006-0402-0. [DOI] [PubMed] [Google Scholar]