Highlight
We report that gastric cancer patients exhibiting high levels of heparanase 2 (Hpa2) survive longer. Similarly, mice administrated with gastric carcinoma cells engineered to overexpress Hpa2 produced smaller tumors and survived longer than mice administrated with control cells. These beneficial effects were found to associate with increased phosphorylation of AMP-activated protein kinase (AMPK) that play an instrumental role in cell metabolism and is situated at the center of a tumor suppressor network. We also found that MG132, an inhibitor of the proteasome that results in proteotoxic stress, prominently enhances Hpa2 expression. Notably, Hpa2 induction by MG132 appeared to be mediated by AMPK, thus establishing a loop that feeds itself where Hpa2 enhances AMPK phosphorylation that, in turn, induces Hpa2 expression, possibly leading to attenuation of gastric tumorigenesis.
Keywords: Heparanase 2, Gastric cancer, Stress, AMPK, Gene expression
Abstract
Heparanase is highly implicated in tumor metastasis due to its capacity to cleave heparan sulfate and, consequently, remodel the extracellular matrix underlying epithelial and endothelial cells. In striking contrast, only little attention was given to its close homolog, heparanase 2 (Hpa2), possibly because it lacks heparan sulfate-degrading activity typical of heparanase. We subjected sections of gastric carcinoma to immunostaining and correlated Hpa2 immunoreactivity with clinical records, including tumor grade, stage and patients' status. We over-expressed Hpa2 in gastric carcinoma cell lines and examined their tumorigenic properties in vitro and in vivo. We also evaluated the expression of Hpa2 by gastric carcinoma cells following inhibition of the proteasome, leading to proteotoxic stress, and the resulting signaling responsible for Hpa2 gene regulation. Here, we report that gastric cancer patients exhibiting high levels of Hpa2 survive longer. Similarly, mice administrated with gastric carcinoma cells engineered to over-express Hpa2 produced smaller tumors and survived longer than mice administrated with control cells. This was associated with increased phosphorylation of AMP-activated protein kinase (AMPK), a kinase that is situated at the center of a tumor suppressor network. We also found that MG132, an inhibitor of the proteasome that results in proteotoxic stress, prominently enhances Hpa2 expression. Notably, Hpa2 induction by MG132 appeared to be mediated by AMPK, and AMPK was found to induce the expression of Hpa2, thus establishing a loop that feeds itself where Hpa2 enhances AMPK phosphorylation that, in turn, induces Hpa2 expression, leading to attenuation of gastric tumorigenesis. These results indicate that high levels of Hpa2 in some tumors are due to stress conditions that tumors often experience due to their high rates of cell proliferation and high metabolic demands. This increase in Hpa2 levels by the stressed tumors appears critically important for patient outcomes.
Introduction
Gastric adenocarcinoma is the fifth most commonly diagnosed cancer and the fourth leading cause of cancer-related death worldwide; over a million new cases of gastric cancer are diagnosed each year [1]. Despite the use of multiple treatment modalities, including surgery, combined with radiation therapy, chemotherapy, or targeted chemo-immune therapy, the disease often progresses, relapses, or metastasizes and has a 5-year survival rate of less than 35% overall, and only 2% for cases of peritoneal metastases [2]. Thus, better understanding of the disease and the development of new treatment modalities are required to offer patients more effective treatment options.
Heparanase is an endo-β-D-glucuronidase proficient in cleaving heparan sulfate (HS) side chains of heparan sulfate proteoglycans (HSPG). These macromolecules are highly abundant in the extracellular matrix (ECM) underlying epithelial and endothelial cells, where they bind and assemble the major protein constituents of the ECM (i.e., laminin, fibronectin, collagen-IV, etc.). This HS bridge between ECM components assists in establishing a 3-dimensional, non-soluble, thick matrix that provides structural support and biochemical cues to many types of cells [[3], [4], [5]]. Cleavage of HS by heparanase thus results in remodeling of the ECM, most notably associating with sprouting of new blood vessels (angiogenesis) and cell dissemination associated with tumor metastasis and transmigration of immune cells [[6], [7], [8]]. Also, heparanase can release a wide variety of enzymes, growth factors, cytokines and chemokines that are bound to HS as a storage depot [9], converting them into active biological mediators. Heparanase is, therefore, considered pro-tumorigenic in many types of cancers, and patients that exhibit high levels of heparanase survive less time than patients with low levels of heparanase [6,10,11], thus encouraging the development of heparanase inhibitors. Few such inhibitors are currently under clinical evaluation [12,13]. Similar considerations are also noted in gastric cancer [14]. Meta-analyses revealed that high expression of heparanase mRNA and protein are correlated with increased depth of invasion, lymph node metastasis, tumor size and TNM stage [15], associating with poor prognosis [16].
Unlike the intense research effort devoted to exploring the significance of heparanase in human diseases, very little attention was given to heparanase 2 (Hpa2), a close homolog of heparanase [17]. Alignment of the coding region of heparanase and Hpa2 reveals an overall identity of 40% and sequence resemblance of 59% [17]. Importantly, Hpa2 lacks intrinsic HS-degrading activity, the hallmark of heparanase [18]. Hpa2 retains, nonetheless, the capacity to bind heparin/HS [18] and exhibits even higher affinity towards HS than heparanase, thus competing for HS binding and inhibiting heparanase enzymatic activity [18]. Moreover, co-immunoprecipitation studies revealed physical association between Hpa2 and heparanase [18], providing additional explanation for the inhibition of heparanase enzymatic activity by Hpa2. Both heparanase and Hpa2 are glycoproteins that are targeted to the ER by their signal peptide, run through the Golgi apparatus, and secreted. Subsequently, heparanase rapidly and efficiently interacts with cell membrane HS (i.e., syndecans), followed by internalization of the heparanase-syndecan complex. The heparanase containing endosomes are then converted to lysosomes, where heparanase is subjected to proteolytic processing and activation by cathepsin L [19,20]. Hpa2 similarly interacts with cell membrane HSPG, but unlike heparanase, Hpa2 is retained for a relatively long period on the cell membrane and fails to get internalized [18]. The consequences of this high-affinity, prolonged, interaction of Hpa2 with plasma membrane HSPG are not entirely clear but seem to exert a prominent effect on cell adhesion and migration [21]. The role of Hpa2 in cancer has not been sufficiently explored. Clinical and experimental results suggest, nevertheless, that Hpa2 functions to attenuate tumor growth. Hpa2 is readily detected in normal epithelium of the bladder, breast, and ovarian tissues whereas its expression is markedly decreased in the resulting carcinomas [20,22,23], an expression pattern typical of a tumor suppressor. Moreover, head and neck and pancreatic cancer patients expressing high levels of Hpa2 survived longer than patients endowed with low levels of Hpa2 [18,24]. https://kmplot.com/analysis Experimentally, overexpression of Hpa2 in head and neck and pancreatic carcinoma cells resulted in a prominent decrease in tumor growth, mediated by lower vascular density (blood and lymph vessels) [25], likely due to reduced Id1 expression, a transcription factor highly implicated in VEGF-A and VEGF-C gene regulation [26]. Tumors produced by cells overexpressing Hpa2 were not only smaller but also exhibited a higher degree of cell differentiation (i.e., cytokeratin expression) [25,27], thus strengthening the significance of Hpa2 as a tumor suppressor [20]. Likewise, high levels of Hpa2 were associated with lower tumor grade [28,29], further associating Hpa2 with cell differentiation. More recently, we have reported that Hpa2 enhances the expression of Sox2 which, in head and neck cancer, functions to suppress tumor growth [27], and increase ER stress response that, once present constitutively, leads to enhanced rate of apoptotic cell death [24]. The role of Hpa2 in gastric cancer has not been elucidated yet. Here, we report that gastric cancer patients exhibiting high levels of Hpa2 survive longer. Similarly, mice administrated with gastric carcinoma cells engineered to overexpress Hpa2 produced smaller tumors and survived longer than mice administrated with control cells. These beneficial effects were found to associate with increased phosphorylation of AMP-activated protein kinase (AMPK) that plays an instrumental role in cell metabolism. Also, AMPK is situated at the center of a tumor suppressor network known to attenuate the growth of many types of cancer including gastric cancer [30]. Interestingly, AMPK was found to induce the expression of Hpa2, thus establishing a loop that feeds itself where Hpa2 enhances AMPK phosphorylation that, in turn, induces Hpa2 expression, leading to attenuation of gastric tumorigenesis.
Materials and methods
Gastric tissue arrays
Two gastric tissue arrays were purchased from Outdo Biotech Co., Ltd. (http://www.superchip.com.cn/) including 90 (HStma180Su09) and 98 (HStmA180Su15) gastric carcinoma patients and adjacent normal gastric tissue. The array provided clinicopathological information in accordance with the American Joint Committee on Cancer (AJCC) 2010, including TNM classification and overall survival data. After immunostaining, 135 tissue samples were available for clinical analyses. Gastric carcinoma tissue array (ST2084b; 96 patients), was also purchased from US Biomax (Rockville, MD).
Cells and cell culture
Human AGS, BGC-832, MKN-45, SGC-7901, and MGC-803 gastric carcinoma and mouse Lewis lung carcinoma (LLC) and mouse embryonic fibroblasts (MEF) cells have been described previously [[31], [32], [33]] and were grown in Dulbecco's modified Eagle's medium (Biological Industries, Beit Haemek, Israel) supplemented with 10% FCS and antibiotics.
Antibodies and reagents
Anti-Hpa2 antibody (#58) has been described previously [18]. Anti-phospho-acetyl CoA carboxylase (ACC; Ser79), anti-phospho-AMPK (Thr172), anti-phospho-JNK (T183/Y185), anti-JNK, anti-phospho-p70S6K (Thr389), anti-cleaved caspase3, and anti-cleaved PARP antibodies were purchased from Cell Signaling (Danvers, MA). Anti-AMPK, anti-p70S6K, and anti-heat shock factor (HSF) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-phospho-HSF1 (Ser326), and anti-tubulin antibodies were from Abcam (Cambridge, UK). MG132, KRIBB 11, KNK-437, rapamycin, dorsomorphin, 17-AAG, bortezomib, metformin, and phenformin were purchased from Calbiochem (Sigma-Aldrich).
Real time-PCR
Real-time-PCR (qPCR) analyses were performed using ABI PRISM 7000 Sequence Detection System employing SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA), essentially as described [25,31]. The primers sets utilized in this study are summarized in Suppl. Table 1. Data are expressed as the mean level of expression normalized to actin, and represent the mean±SEM of triplicate samples; results are representative of 3 independent experiments.
Cell proliferation
Relative cell viability upon Hpa2 over-expression was assessed using MTT proliferation assay, essentially as described [34]. Briefly, 2 × 103 cells/well were seeded in 96 wells plate in medium supplemented with 2.5% FCS. At the indicated time points, 20 µl of MTT were added for 3 hours and absorbance at 570 nm was measured by a plate reader. Reduction of the MTT was calculated and presented as relative cell viability according to the manufacturer's (Bio-Rad; Hercules, CA) instructions.
Cell migration and invasion
Migration and invasion assays were performed using modified CEBoyden chambers with polycarbonate Nucleopore membrane (6.5 mm in diameter, 8 µm pore-size; Corning, Corning, NY), coated with fibronectin (30 µl; 10 µg/ml; cell migration) or Matrigel (30 µl; cell invasion) essentially as described [35,36].
Colony formation
Colony formation in soft agar was performed as described [36]. Briefly, Dulbecco's modified Eagle's medium (DMEM) (3 ml) containing 0.5% low-melt agarose (Bio-Rad) and 10% FCS was poured into 60-mm Petri dishes. The layer was covered with cell suspension (2 × 103 cells) in 1.5 ml DMEM containing 0.3% low-melt agarose and 10% FCS, followed by the addition of 2 ml DMEM containing 10% FCS. Medium was exchanged once a week. Colonies were visualized and counted under a microscope 2 to 5 weeks after seeding, as described [34,37].
Cell lysates and protein blotting
Preparation of cell lysates and protein blotting were carried out essentially as described [25,38].
Immunohistochemistry and immunofluorescent staining
Staining of formalin-fixed, paraffin-embedded 5-micron sections and immunofluorescent staining were performed essentially as described [39,40]. Specimens that were similarly stained with normal rabbit serum or by applying the above procedure but lacking the primary antibody yielded no detectable staining.
Tumorigenicity
Control (Vo) and Hpa2 overexpressing MGC-803 cells were detached with trypsin/EDTA, washed with PBS, and brought to a concentration of 5 × 106 cells/ml. Cell suspension (5 × 105/0.1 ml) was inoculated intra-peritoneal (i.p) to 6 to 10 week-old male NOD/SCID mice and the survival of mice was followed over time. All experiments were performed in accordance with the Technion's Institutional Animal Care and Use Committee (IL-080-08-2018; OPRR-A5026-01).
Statistics
Data are presented as means ± SE. Statistical significance was analyzed by a 2-tailed t test. Values of P< 0.05 were considered significant. Data sets passed D'Agostino-Pearson normality (GraphPad Prism 5 utility software). All experiments were repeated at least 3 times with similar results.
Results
Clinical significance and pre-clinical studies
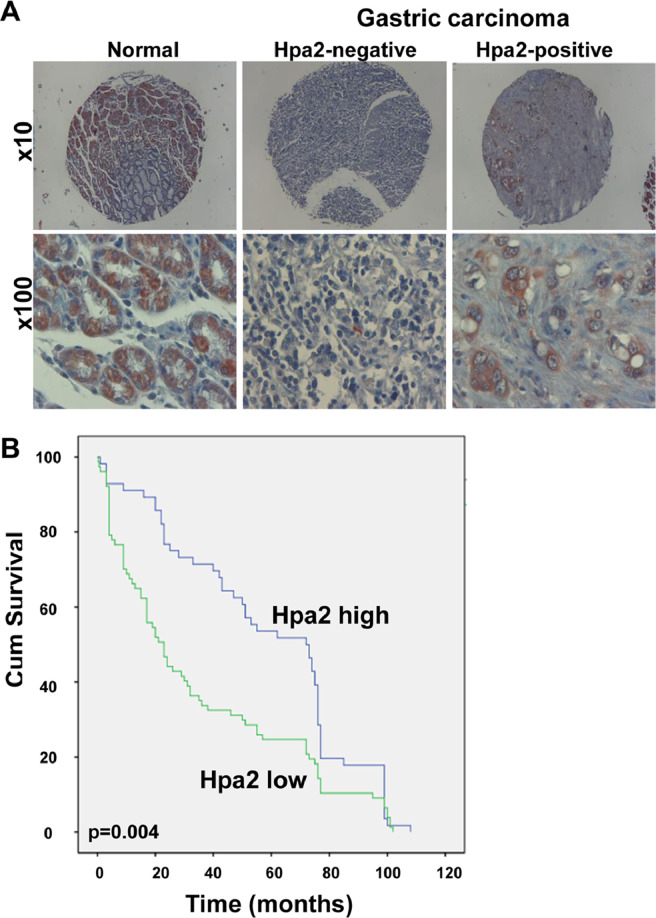
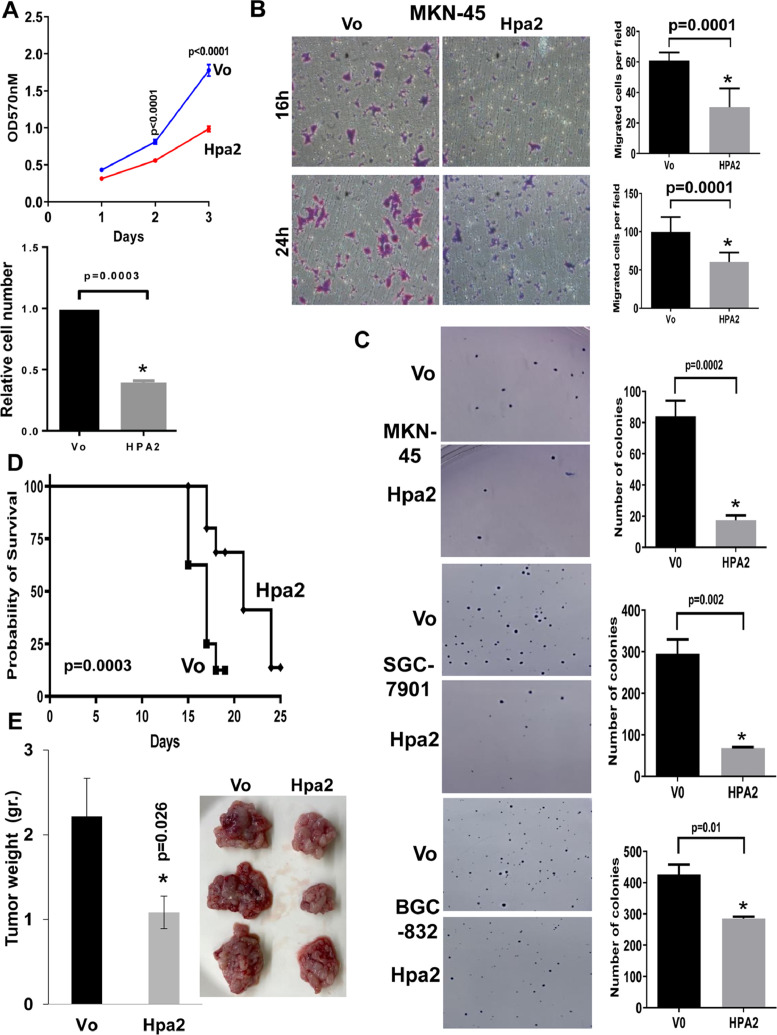
To reveal the clinical significance of Hpa2 in gastric cancer, we subjected tissue array of gastric carcinoma biopsies and adjacent normal gastric tissue to immunostaining applying anti-Hpa2 antibody. We found that 94% (87/93) of the normal gastric tissues were stained positive for Hpa2 (Fig. 1A, left panels) compared with 57% (50/87) of the gastric carcinomas (Table 1), differences that are statistically highly significant (P= 0.001). This implies that about half of the gastric carcinoma cases lose, or exhibit reduced Hpa2 expression (Fig. 1A, middle and right panels), an expression pattern typical of a tumor suppressor. Notably, gastric tumors that were stained strongly for Hpa2 were diagnosed as low grade whereas Hpa2-negative tumors were of high grade (Fig. 1A, lower panels; P= 0.02). Moreover, Hpa2 staining intensity correlated inversely with tumor stage (P= 0.04; Table 2) and tumor metastasis to lymph nodes (N). Thus, most (54%) of the tumors that were stained strongly for Hpa2 had only a few infected lymph nodes (N1-N2), whereas patients with low levels of Hpa2 were mostly (67%) diagnosed with multiple infected lymph nodes (N3-N4), differences that are statistically highly significant (P= 0.01; Table 2). Such a correlation between Hpa2 and tumor metastasis to lymph nodes was noted earlier in head and neck carcinoma [25]. Importantly, patients that exhibited high levels of Hpa2 (n = 56, median survival time of 72 months) survived longer than patients with low Hpa2 levels (n = 79, median survival time of 23 months) (Fig. 1B; P= 0.004). This suggests that in gastric cancer, Hpa2 functions to restrain tumor growth. To further explore the role of Hpa2 in gastric cancer we next transfected gastric carcinoma cell lines with Hpa2 gene construct (Suppl. Fig. 1A) and examined their tumorigenic capacities in vitro. We found that proliferation of MKN-45 cells was attenuated markedly following Hpa2 overexpression (Fig. 2A; P< 0.0001). Likewise, migration of MKN-45-Hpa2 cells was prominently reduced compared with control (Vo) cells (Fig. 2B; P< 0.0001). Furthermore, the capacity of MKN-45 (Fig. 2C, upper panel), SGC-7901 (Fig. 2C, middle panels), and BGC-823 (Fig. 2C, lower panels) to form colonies in soft agar was attenuated following Hpa2 overexpression compared with control (Vo) cells. We next implanted control (Vo) and Hpa2 overexpressing MGC-803 cells intra-peritoneal (i.p) and the survival time of the mice was followed. This model is most relevant because peritoneal metastases occur in 55% to 60% of patients with gastric cancer, associating with a low (2%) 5-year overall survival rate [2]. Notably, mice implanted with Hpa2 cells survived significantly longer than mice inoculated with control (Vo) cells (Fig. 2D; P= 0.0003). In a subsequent experiment, mice were similarly implanted with control (Vo) and Hpa2 cells, sacrificed 14 days later, and tumor lesions were collected. Remarkably, mice implanted with Hpa2 cells exhibited reduced tumor mass collected from the peritoneum, mostly associating with the colon and related organs (Fig. 2E), also evident by reduced amounts of ascites fluids collected (Suppl. Fig. 1B). While the morphology of tumors produced by control (Vo) and Hpa2 cells appeared similar (Suppl. Fig. 1C, lower panels), Hpa2 tumors were decorated with far more fat cells (Suppl. Fig. 1C, upper panels). Together, these results suggest that Hpa2 attenuates the tumorigenic properties of gastric carcinoma cells.
Fig. 1.
High levels of Hpa2 are associated with longer survival of gastric cancer patients. (A) Immunostaining. Tumor biopsies and adjacent normal gastric tissues were subjected to immunostaining applying anti-Hpa2 antibody. Shown are representative images of Hpa2 staining in normal gastric tissue (left) and gastric carcinoma stained negative (middle) and positive (right) for Hpa2. Original magnifications: x10 (upper panels), x100 (lower panels). (B) Kaplan-Meier survival plot. Kaplan-Meier survival analysis of gastric cancer patients according to their Hpa2 staining intensities (high vs low). Gastric carcinoma patients exhibiting strong staining of Hpa2 (n = 56) survive significantly longer than patients that show low levels of Hpa2 (n = 79; P= 0.004). Hpa2, heparanase 2.
Table 1.
Hpa2 levels are decreased in gastric carcinoma vs normal gastric tissue.
| Hpa2 |
Total | ||
|---|---|---|---|
| Negative (%) | Positive (%) | ||
| Normal | 6 (6) | 87 (94) | 93a |
| Malignant | 37 (43) | 50 (57) | 87b |
| P < 0.001 | 43 | 137 | 180 |
Hpa2 = heparanase 2.
Data on 3 cases was missing.
Data on 9 cases was missing.
Table 2.
Pathological characterization of gastric carcinoma patients in correlation with Hpa2 staining intensity.
| HPA2 |
|||||
|---|---|---|---|---|---|
| Total | Low (%) | High (%) | χ2 value | P value | |
| Gendera | |||||
| Male | 101 | 57 (56) | 44 (44) | ||
| Female | 33 | 21 (63) | 12 (37) | 0.53 | 0.4 |
| Age | |||||
| <65 | 62 | 27 (43) | 35 (57) | ||
| ≥65 | 73 | 52 (71) | 21 (29) | 10.585 | 0.001 |
| Volumeb | |||||
| <5cm | 54 | 24 (44) | 30 (56) | ||
| ≥5cm | 77 | 52 (67) | 25 (33) | 6.946 | 0.008 |
| Tc | |||||
| T1-T2 | 26 | 12 (46) | 14 (54) | ||
| T3-T4 | 95 | 57 (60) | 38 (40) | 1.597 | 0.2 |
| Na | |||||
| N1-N2 | 58 | 27 (46) | 31 (54) | ||
| N3-N4 | 76 | 51 (67) | 25 (33) | 5.713 | 0.01 |
| M | |||||
| M0 | 128 | 74 (57) | 54 (43) | ||
| M1 | 7 | 5 (71) | 2 (29) | 0.507 | 0.4 |
| Staged | |||||
| I-II | 54 | 26 (48) | 28 (52) | ||
| III-IV | 73 | 48 (65) | 25 (35) | 3.956 | 0.04 |
Hpa2 = heparanase 2.
Information on 1 patient was missing.
Information on 4 patients was missing.
Information on 14 patients was missing.
Information on 8 patients was missing.
Fig. 2.
Overexpression of Hpa2 attenuates the pro-tumorigenic characteristics of gastric carcinoma cells. (A) Cell proliferation. Control (Vo) and Hpa2 overexpressing MKN-45 cells (2 × 103/well) were seeded in a 96-well plate and relative cell numbers were examined over time as described under “Materials and Methods” (upper panel). The relative number of Hpa2 cells at day 3 is shown graphically vs control (Vo) cells set arbitrarily to a value of 1 (lower panel). (B) Cell migration. Control (Vo) and Hpa2 overexpressing MKN-45 cells were seeded on fibronectin-coated inserts and cell migration was examined 16 hours (upper panels) and 24 hours (lower panels) afterward. Shown are representative images taken at x20 magnification. The number of migrating cells is shown graphically in the right panels. (C) Colony formation. Control (Vo) and Hpa2 overexpressing MKN-45 (upper panels), SGC-7901 (middle panels), and BGC-832 (lower panels) cells were grown in soft agar as described under “Materials and Methods” After 3 to 4 weeks, dishes were fixed with formalin and cell colonies were stained with Crystal violet. Representative photomicrographs are shown in the left panels (original magnifications x10). Quantification of the number of colonies per dish is shown graphically in the right panels. (D, E) Survival times and tumor growth. Control (Vo) and Hpa2 overexpressing MGC-803 cells (0.5 × 106) were injected intraperitoneal (i.p) into NOD/SCID mice (n = 7) and survival times recorded (D). Control and Hpa2 MGC-803 cells were similarly inoculated ip into NOD/SCID mice (n = 7). After 14 days mice were sacrificed and all the tumor lesions from each mouse were collected, weighed (E, left) and photographed. Shown are representative images of the tumor lesions collected from mice implanted with control (Vo) and Hpa2 cells (E, right). Hpa2, heparanase 2.
Hpa2 expression is induced by stress, involving HSF1 and AMPK
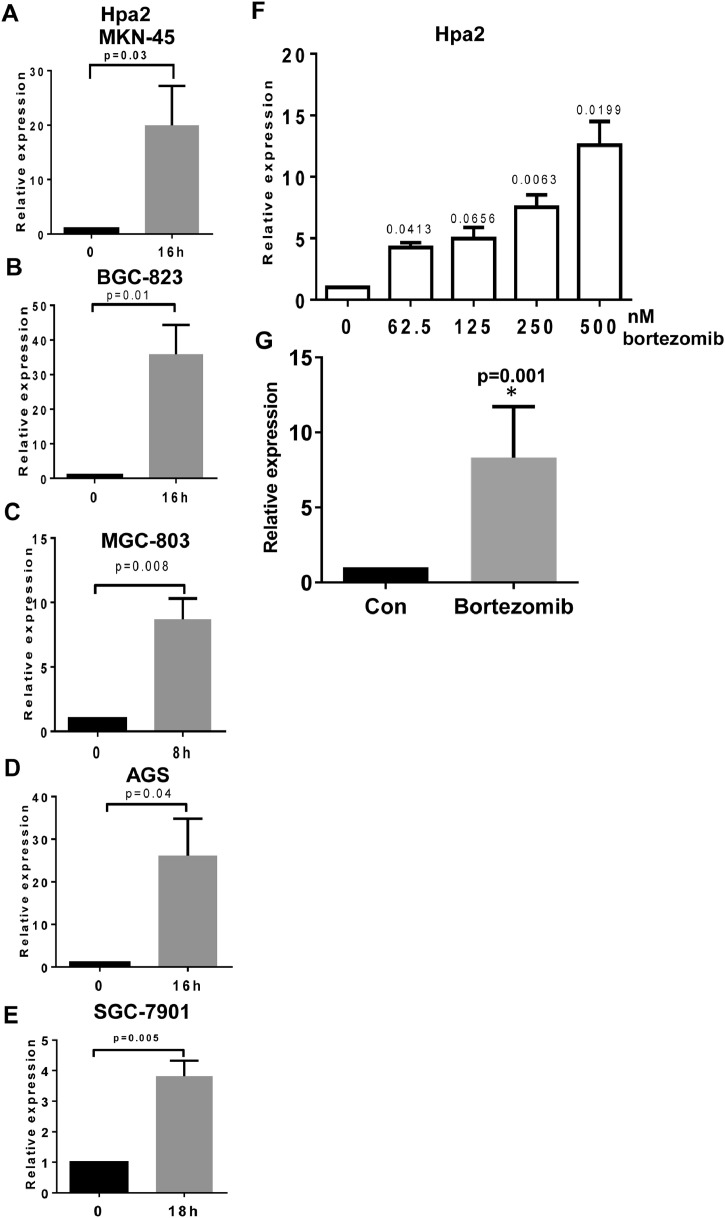
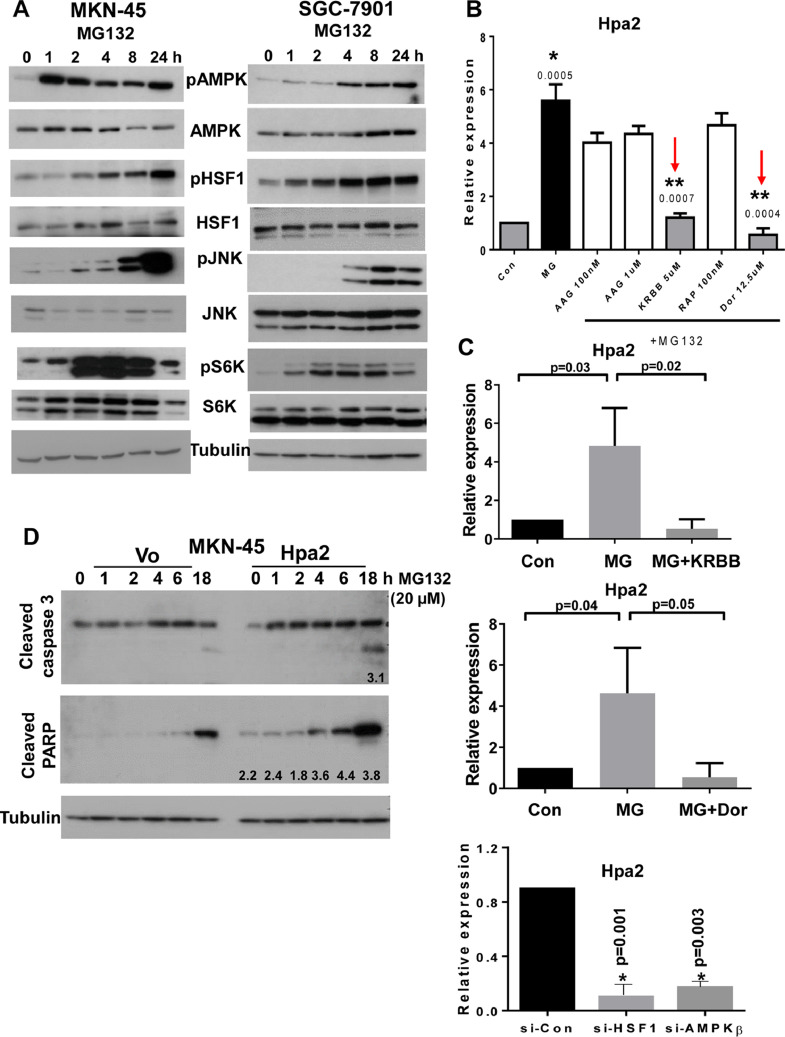
Reduced Hpa2 expression in some of the gastric carcinomas and its high expression in others (Fig. 1A) suggests that Hpa2 expression is tightly regulated. However, mechanisms that regulate Hpa2 gene expression are largely unknown. We hypothesized that conditions of stress, which are often associated with the fast-growing tumor are involved in Hpa2 gene regulation. To examine this possibility, we focused on the proteasome that is often dysregulated in human malignancies [41]. We exposed gastric carcinoma cell lines to MG132, an inhibitor of the proteasome, resulting in massive accumulation of proteins tagged for degradation, leading to severe proteotoxic stress [42]. Consistently, we found that Hpa2 expression was induced 10-30 folds by MG132 in MKN-45, BGC-823, MGC-803, AGS, and SGC-7901 cells (Fig. 3A−E). Moreover, Hpa2 expression was induced to a comparable extent by bortezomib (Velcade) (Fig. 3E, F), a proteasome inhibitor that is most effective in the treatment of multiple myeloma patients and is also effective in gastric cancer [43]. Hpa2 gene induction was similarly observed in non-transformed cells such as MEF (Suppl. Fig. 2A, upper panel), and in mouse Lewis lung carcinoma cells (Suppl. Fig. 2B, upper panel). In these cells, as well as in gastric carcinoma cells, the stress conditions also induced the expression of cytokines such as MIP2 (Suppl. Fig. 2A, B middle panels) and TNF-α (Suppl. Fig. 2A, B, lower panels) that have a profound impact on the immune system and the tumor microenvironment. To examine the molecular mechanism underlying Hpa2 induction, we subjected gastric carcinoma cells to MG132 for increasing periods of time, and protein extracts were subjected to immunoblotting. Consistently, we found that MG132 treatment resulted in a profound increase in the phosphorylation levels of AMPK (Fig. 4A, upper panels), JNK (Fig. 4A, fifth panels), and p70S6K (pS6K; Fig. 4A, seventh panels), the latter is indicative of mTOR activation. We also found that MG132 enhances the phosphorylation of heat shock factor-1 (HSF1; Fig. 4A, third panels), while HSF1 expression was not affected (Fig. 4A, fourth panels). Notably, the increased phosphorylation of HSF1 was associated with a profound increase in the expression levels of heat shock protein (HSP) 40, 105, 27, and 70 (Suppl. Fig. 3A), a consequence of HSF1 activation because this increase in HSP expression was abrogated by HSF1 inhibitors KRIBB 11 and KNK-437 (Suppl. Fig. 3B). To tie between Hpa2 induction and the signaling pathways elicited by the stress, we treated MKN-45 cells with MG132 in the absence or presence of inhibitors specific for each signaling pathway. We found that Hpa2 induction by MG132 was attenuated markedly by KRIBB 11, an inhibitor of HSF1, and by dorsomorphin (Dor), an inhibitor of AMPK (Fig. 4B, red arrows; Fig. 4C, upper and middle panels). Hpa2 gene induction by MG132 was also modestly affected by sp600125 (a JNK inhibitor; Suppl. Fig. 4A), but not by rapamycin (inhibitor of mTOR) (Fig. 4B), thus pointing to AMPK, HSF1 and JNK as modulators of Hpa2 expression. Moreover, silencing of HSF and AMPK-beta resulted in reduced Hpa2 expression (Fig. 4C, lower panel), further supporting the significance of these pathways in Hpa2 gene regulation. Notably, subjecting control (Vo) and Hpa2 overexpressing MKN-45 cells to MG132 revealed increased levels of cleaved caspase 3 and cleaved PARP in Hpa2 cells vs control (Vo) cells (Fig. 4D), suggesting that Hpa2 cells are more sensitive to proteotoxic stress conditions, resulting in increased apoptosis.
Fig. 3.
Hpa2 expression is induced by MG132. (A-E) The indicated gastric carcinoma cell lines were treated with MG132 (20 µM) for the indicated time. Control cells (0) were treated with vehicle (DMSO). Total RNA was then extracted and subjected to qPCR applying primers specific for Hpa2. Hpa2 expression (fold increase) in response to MG132 treatment is shown graphically vs control untreated cells (0), set arbitrarily to a value of 1, and after normalization to actin. (F-G) Bortezomib treatment. MKN-45 cells were treated with the indicated concentration of bortezomib for 16 hours and Hpa2 expression was quantified by qPCR (F). Average Hpa2 expression (fold-increase) by bortezomib treated cells vs control (0) at a concentration of 500 nM is shown graphically (G). Hpa2, heparanase 2.
Fig. 4.
Hpa2 induction by MG132 involves AMPK and HSF1. (A) Immunoblotting. MKN-45 (left) and SFC-7901 (right) gastric carcinoma cells were left untreated (0) or were treated with MG132 (20 µM) for the time indicated. Cell extracts were then prepared and subjected to immunoblotting applying antibodies directed to phospho-AMPK (pAMPK; upper panels), AMPK (second panels), phospho-HSF1 (pHSF1; third panels), HSF1 (fourth panels), phospho-JNK (fifth panels), JNK (sixth panels), phospho-p70S6K (pS6K; seventh panels), S6K (8 panels), and tubulin (lower panels). (B) Inhibitors. MKN-45 cells were similarly treated with vehicle (DMSO; Con) or MG132 without (MG) or with the indicated concentrations of 17-AAG (AAG) (HSP90 inhibitor), KRIBB 11 (KRBB; HSF1 inhibitor), rapamycin (Rap; mTOR inhibitor), and dorsomorphin (Dor; AMPK inhibitor). Total RNA was extracted after 24 hours and subjected to qPCR applying primers specific for Hpa2. Note that Hpa2 induction by MG132 is attenuated by inhibitors of AMPK and HSF1 (red arrows).* P= 0.0005 for MG vs Con; ** P= 0.0007 and P= 0.0004 for MG+KRBB vs MG and MG+Dor vs MG, respectively. (C) Quantitation of Hpa2 induction by MG132 without or with KRIBB 11 (upper panel) and dorsomorphin (middle panel). Hpa2 expression is similarly decreased following silencing of HSF and AMPK-beta (C, lower panel). (D) Hpa2 cells are more sensitive to stress conditions. Control (Vo) and Hpa2 overexpressing MKN-45 cells were treated with MG132 (20 µM) for the time indicated. Cell extracts were then prepared and subjected to immunoblotting applying antibodies directed against cleaved caspase 3 (upper panel), cleaved PARP (second panel) and tubulin (lower panel). Note increased levels of the apoptotic markers in Hpa2 cells. Numbers underneath each blot specify band intensity in Hpa2 cells in relation to the same time point in control (Vo) cells. (color version of figure is available online). AMPK, activated protein kinase; Hpa2, heparanase 2; HSF1, heat shock factor-1.
Hpa2 enhances AMPK phosphorylation
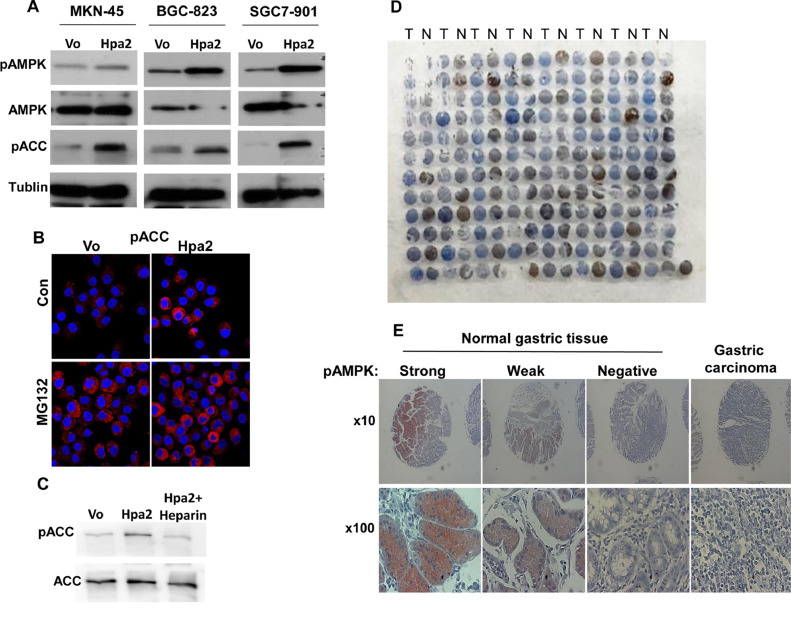
We next attempted to reveal signaling pathways that are modulated by Hpa2 and may be responsible for the observed reduced tumorigenic properties (Figs. 1, 2) and higher sensitivity to conditions of stress (Fig. 4D). Immunoblot analyses of cell extracts derived from control and Hpa2 overexpressing MKN-45 (Fig. 5A, left panels), BGC-823 (Fig. 5A, middle panels) and SGC-7901 (Fig. 5A, right panels) cells revealed a consistent increase in the phosphorylation of AMPK (Fig. 5A, upper panels) and its substrate, acetyl CoA carboxylase (pACC; Fig. 5A, third panels). Increased ACC phosphorylation by Hpa2 was also evident by immunofluorescent staining (Fig. 5B). Moreover, we found that the increase in ACC phosphorylation in Hpa2 cells was reversed by adding heparin to the cell culture medium (Fig. 5C), suggesting that this effect involves the interaction of Hpa2 with cell membrane or secreted HS [18]. Enhanced phosphorylation of AMPK is relevant to the anti-tumorigenic properties of Hpa2 because AMPK activation is found in correlation with good prognosis of cancer patients, including gastric cancer patients [30,44].
Fig. 5.
Increased AMPK phosphorylation in cells overexpressing Hpa2. (A) Lysate samples of control (Vo) and Hpa2 overexpressing MKN-45 (left panels), BGC-823 (middle panels), and SGC-7901 (right panels) cells were subjected to immunoblotting applying the indicated antibody. (B) Immunofluorescent staining. Control (Vo) and Hpa2 overexpressing SGC-7901 cells were treated with vehicle (Con) or MG132 (20 µM) for 16 hours. Cells were then fixed with cold methanol for 10 minutes and subjected to immunofluorescent staining with anti-phospho-ACC antibody (red). Shown are representative images together with nuclear counter-staining (blue). Note stronger staining in Hpa2 cells before (upper right) and after MG132 treatment (lower right) vs control (Vo) cells. (C) Increased ACC phosphorylation by Hpa2 cells is HS-dependent. Hpa2 overexpressing SGC-7901 cells were left untreated or were treated with heparin (20 µM) added to the culture medium. Cell extracts were then prepared and subjected to immunoblotting applying anti-phospho-ACC (upper panel) and anti-ACC (lower panel) antibodies. (D, E) AMPK phosphorylation is decreased in gastric cancer. A tissue array that includes biopsies of gastric carcinoma tumors (T) and adjacent normal gastric tissue (N) was subjected to immunostaining applying anti-phospho-AMPK antibody (D). Higher magnification of the staining in normal and tumor biopsies is shown in (E). Note decreased AMPK phosphorylation in tumor samples vs normal gastric tissue (see also Table 3). AMPK, activated protein kinase; Hpa2, heparanase 2.
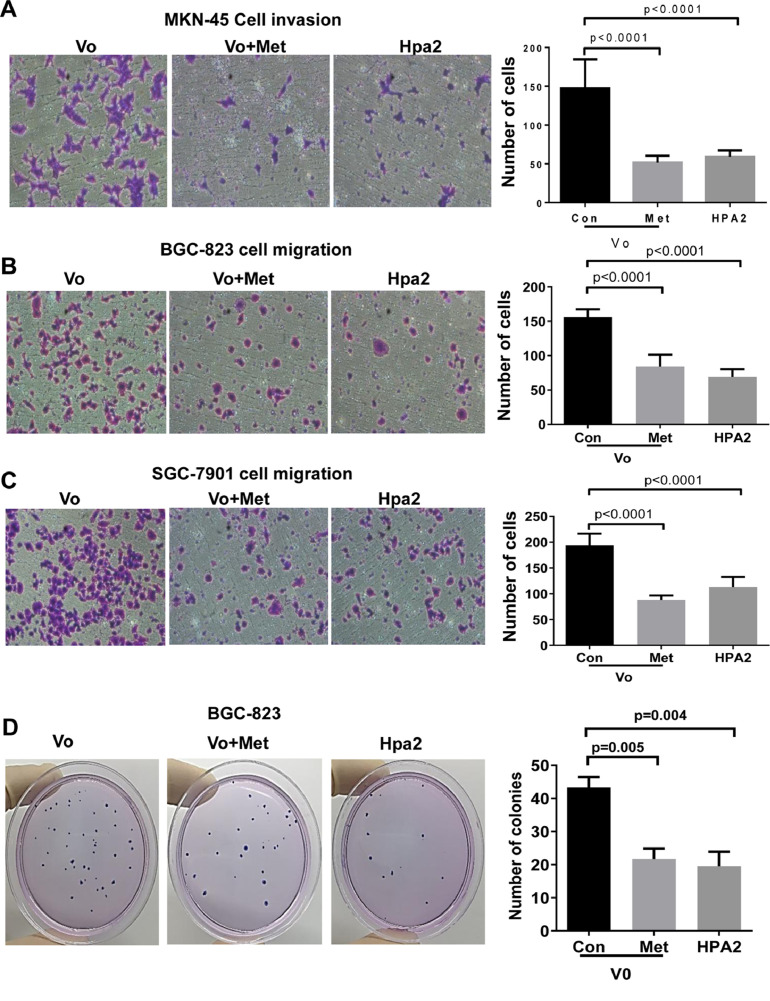
In order to study the significance of AMPK in gastric carcinoma, we applied metformin, a drug that induces the phosphorylation of AMPK (Suppl. Fig. 4B) and is used in the clinic to treat diabetic patients [45,46]. Notably, the proliferation of MKN-45 cells was attenuated markedly by metformin in a dose-dependent manner (Suppl. Fig. 4C). Furthermore, expression of Hpa2 was induced by phenformin (Suppl. Fig. 4D), an analog of metformin that exerts a more potent effect than that of metformin. Furthermore, we found that metformin treatment reduced the invasion (Fig. 6A, Suppl. Fig. 4E), migration (Fig. 6B, C), and colony formation (Fig. 6D) by gastric carcinoma cells to an extent comparable to Hpa2 (Fig. 6A−D, Vo+Met vs Hpa2). These results suggest that the anti-tumorigenic properties of Hpa2 in gastric carcinoma are mediated, at least in part, by enhancing the phosphorylation of AMPK.
Fig. 6.
(A-D) AMPK activation by metformin decreases the motility and colony formation by gastric carcinoma cells to levels comparable with Hpa2 overexpression. Cellular invasion and migration by control (Vo), Vo+metformin (0.5 mM), and Hpa2 overexpressing MKN-45 (A), BGC-823 (B), and SGC-7901 (C) cells were evaluated. Shown are representative images of invading (A) and migrating (B, C) cells taken 24 hours after cell seeding. Quantifications of the number of invading and migrating cells are shown graphically in the right panels. Colony formation by BGC-823 cells untreated or treated with metformin, and overexpressing Hpa2 is shown in (D). Quantification of colony number per dish is shown graphically at the right panel. AMPK, activated protein kinase; Hpa2, heparanase 2.
Given that Hpa2 is highly expressed in normal gastric tissue (Fig. 1A, left), we next examined the correlation between Hpa2 levels and AMPK phosphorylation in this tissue. We found that AMPK is phosphorylated in about half of the gastric tissues examined (43/93; Table 3) and, like Hpa2, is decreased prominently in gastric cancer (Fig. 5D; N = normal, T = Tumor). Importantly, gastric tissues that exhibit high levels of AMPK phosphorylation (Fig. 5D, E) are also stained positive for Hpa2 (Table 3; P= 0.02). These results imply that Hpa2 functions to modulate AMPK phosphorylation and metabolic aspects in normal gastric epithelial cells.
Table 3.
Hpa2 levels correlate with AMPK phosphorylation in normal gastric tissue.
| Hpa2 |
|||
|---|---|---|---|
| AMPK | Negative (%) | Positive (%) | Total |
| Negative | 6 (12) | 44 (88) | 50 |
| Positive | 0 (0) | 43 (100) | 43 |
| P = 0.02 | 6 | 87 | 93 |
AMPK = activated protein kinase; Hpa2 = heparanase 2.
Discussion
Hpa2 was cloned shortly after the cloning of heparanase [17] but acquired very little attention, possibly because it lacks HS-degrading activity typical of heparanase [18]. The emerging role of Hpa2 in a rare autosomal recessive congenital disease called urofacial syndrome (UFS) [47−49] indicated for the first time that Hpa2 plays an important role in human disorders. In UFS, biallelic mutations of Hpa2 mostly result in frameshifts that lead to an early stop codon and a truncated Hpa2 protein, resulting in a loss-of-function phenotype [47,48]. The lack of Hpa2 appears to be responsible for peripheral neuropathy of the bladder, typical of UFS [50,51] and likely affects facial nerves because people with UFS have a characteristic grimace upon smiling, indicating that Hpa2 plays a critical role in neuronal function.
The role of Hpa2 in normal epithelial cells has not been investigated yet. Here, we found that increased AMPK phosphorylation in human gastric epithelial cells correlates with high levels of Hpa2 (Table 3), suggesting that Hpa2 functions to modulate AMPK phosphorylation in normal epithelia. This capacity is of high importance, given the immense role of AMPK in energy sensing and cell metabolism [52,53], thus implying that Hpa2 regulates metabolic processes. Maintaining high levels of AMPK phosphorylation in normal epithelia is also important for tumorigenesis because AMPK was reported to protect normal epithelia from oncogenic transformation [54]. It is yet to be demonstrated whether changes in AMPK activity are also relevant to UFS and the neuropathies associated with Hpa2-deficient neurons [51].
Given the tumor-suppressive properties of AMPK [54−56], increased AMPK phosphorylation by human gastric carcinoma cells overexpressing Hpa2 (Fig. 5) supports the notion that Hpa2 functions to attenuate tumor growth. This notion is reinforced by the following observations. Hpa2 expression was noted to be substantially decreased in breast, ovarian, bladder [20,22,23] and gastric (Fig. 1; Table 1) carcinomas vs adjacent normal tissues, expression pattern typical of tumor suppressors. In bladder [28] and gastric (Fig. 1A) cancers, tumors that retain high levels of Hpa2 are diagnosed as low grade, indicating that Hpa2 likely functions to maintain epithelial cell differentiation. Moreover, in head and neck and pancreatic cancer, patients who scored to express high levels of Hpa2 survived longer than patients exhibiting low levels of Hpa2 [18,57]. Prolonged survival is also shown here for gastric cancer patients exhibiting high levels of Hpa2 (Fig. 1B), associating with reduced lymph node metastasis and lower tumor stage of Hpa2-high tumors (Table 2), together suggesting that Hpa2 functions to suppress tumorigenesis. The cellular and molecular mechanism(s) exerted by Hpa2 to attenuate tumorigenicity are largely obscure. Previously, we have reported that head and neck cancer patients expressing high levels of Hpa2 show reduced lymph node metastasis [18], and overexpression of Hpa2 in FaDu pharyngeal carcinoma cells resulted in smaller tumors that exhibited a marked decrease in tumor vascularity (blood and lymph vessels) [25]. Thus, reduced lymph node metastasis may result from decreased lymphatic vasculature responsible for dissemination of metastatic cells. Anti-metastatic effect of Hpa2 is also found in gastric cancer patients (Table 2), suggesting that this effect is more general among different types of cancer. Our results suggest an additional molecular mechanism exerted by Hpa2 to restrain tumor growth. Namely, we report here for the first time that Hpa2 enhances the phosphorylation of AMPK and its activity, reflected by increased phosphorylation of its substrate, ACC, in gastric carcinoma cells (Fig. 5A, B). Even stronger increase in AMPK phosphorylation by Hpa2 was observed in tumor xenografts produced by pharyngeal carcinoma cells (Suppl. Fig. 4F), suggesting that modulation of AMPK phosphorylation by Hpa2 is not restricted to gastric cancer. This finding is both important and relevant because cancer patients, including gastric cancer patients, with high levels of AMPK phosphorylation, are endowed with good prognosis [30]. The anti-tumorigenic properties of AMPK are best exemplified by an intense effort to develop compounds that activate AMPK as anti-cancer medications [55]. A prototype of such AMPK activators is metformin [45] that is evaluated as anti-cancer drug for many types of cancer [55]. Notably, metformin attenuated cell proliferation, migration, invasion, and colony formation by gastric carcinoma cells to an extent comparable with Hpa2 (Fig. 6, Suppl. Fig. 4), suggesting that these properties of Hpa2 are mediated, at least in part, by AMPK. The role of AMPK in Hpa2-mediated tumor attenuation awaits stringent confirmation in a subsequent study, employing gastric, head and neck, pancreatic [25,27,57], or other types of cancer that are affected by Hpa2 and show increased levels of AMPK phosphorylation.
The mechanism by which Hpa2 promotes AMPK phosphorylation is not clear but seems to involve HS because the increased ACC phosphorylation levels in Hpa2 cells was reversed by the addition of heparin to the cell culture medium (Fig. 5C). Similarly, it was reported that antithrombin protects the heart from ischemia and reperfusion injury, and this cardioprotection effect was mediated by interaction of antithrombin with HS, leading to activation of AMPK [58]. Support for this possibility emerges from the study of decorin, a small leucine-rich proteoglycan. Decorin was noted to down-regulate the expression of VEGF and, consequently, inhibit tumor angiogenesis and tumor growth [59], a functional repertoire that resembles Hpa2 functions [24,25]. Notably, decorin was found to promote AMPK activation, leading to inhibition of mTOR and, consequently, elevation of autophagy in endothelial cells [59]. It is yet to be revealed whether a similar mechanism also occurs in gastric carcinoma cells and whether Hpa2 can interact with decorin and modulate its activity. Interestingly, AMPK was found to regulate the levels of glycosaminoglycans sulfation [60], which likely affect Hpa2-glycosaminoglycan interaction, thus providing another layer of regulation of the Hpa2-AMPK axis. Heparanase and Hpa2 are secreted glycoproteins; both proteins exhibit high affinity to HS (the affinity of Hpa2 to HS is 10-fold higher than that of heparanase) but, unlike heparanase, Hpa2 is not internalized and is not subjected to proteolytic processing. Given that AMPK resides in the cytoplasm of the cell, Hpa2 and AMPK do not seem to physically interact. It appears more likely that a signal initiated at the cell membrane by Hpa2 is translated to enhanced AMPK phosphorylation. The nature of this signaling pathway is yet to be resolved.
Substantial changes in Hpa2 staining intensities between normal and tumor lesions (Fig. 1) [20,22,61,62] strongly suggest that Hpa2 expression is tightly regulated, but mechanisms that regulate Hpa2 expression have not been sufficiently elucidated yet. Zhang et al have reported that the Hpa2 gene is methylated, and gene methylation results in decreased Hpa2 expression [63]. Importantly, hypermethylation of Hpa2 was associated with poor prognosis of colorectal cancer patients [63], thus further supporting the notion that Hpa2 functions to suppress tumorigenesis. Recently, we have reported that Hpa2 expression by pancreatic carcinoma cells is induced markedly by compounds that elicit ER stress [57]. Here, we support and further extend the notion that Hpa2 expression is induced by conditions of stress. Notably, Hpa2 expression was prominently induced by stress elicited by inhibition of the proteasome. Thus, treatment of normal (MEF) and tumor-derived cells with MG132, an inhibitor of the proteasome that results in proteotoxic stress, prominently enhances Hpa2 expression (Fig. 3, Suppl. Fig. 2). Inhibition of the proteasome that leads to massive accumulation of proteins tagged for degradation, is likely translated to several stress pathways. This is evident by a noticeable induction of HSP (Suppl. Fig. 3), along with induction of the stress arm of the MAPK pathway (i.e., JNK phosphorylation; Fig. 4A) in response to treatment with MG132. Induction of ER stress following inhibition of the proteasome has also been reported, but we did not find an increase in ER stress markers (i.e., Bip, phospho eIF2) in the MG132-treated gastric cancer cells (data not shown). Notably, Hpa2 induction by MG132 appeared to be mediated by AMPK and HSF1 because specific inhibitors of AMPK (dorsomorphin) and HSF1 (KRIBB 11) prevented Hpa2 induction by MG132 (Fig. 4). Moreover, Hpa2 expression was induced by metformin analog, phenformin. This drug is more effective than metformin due to the way it enters the cell. Metformin is a very hydrophobic compound and requires organic cation transporters to pass through the cell membrane. In contrast, phenformin does not require transport protein to enter the cell, leading to higher concentrations of the drug inside cells. These results imply the occurrence of a cycle that feeds itself, by which Hpa2 enhances AMPK phosphorylation that, in turn, induces Hpa2 expression. A similar cycle was observed in pancreatic cancer, where Hpa2 elicits ER stress response which, in turn, induces the expression of Hpa2 [57]. The mechanisms by which conditions of stress, and AMPK, induce the expression of Hpa2 are presently unclear and deserve a separate study. Preliminary results suggest the involvement of activating transcription factor 3 (ATF3) in Hpa2 gene regulation, but this awaits in-depth investigation. Importantly, pancreatic and gastric carcinoma cells overexpressing Hpa2 are more sensitive to external stress, resulting in increased features of apoptotic cell death (i.e., cleaved caspase3, cleaved PARP; Fig. 4D) [57]. Given that tumors are frequently experiencing stress conditions due to their high proliferative nature and high metabolic demands [64,65], tumors that exhibit high levels of Hpa2 expression will become more prone to these conditions, and the consistency of the stress will likely result in cell death and reduced tumor growth. This, and the lower rate of lymph node metastasis (Table 2) may explain the prolonged survival of gastric cancer patients that express high levels of Hpa2 (Fig. 1).
Conclusions
Our results indicate that Hpa2 positivity is associated with good prognosis of gastric cancer patients, supporting the notion that Hpa2 functions as a tumor suppressor. Induction of Hpa2 expression by stress suggests that high levels of Hpa2 in some tumors but not in others are due to stress conditions that tumors often experience due to their high rates of cell proliferation and high metabolic demands. This increase in Hpa2 levels converts the cells more sensitive to conditions of stress, likely leading to decreased tumor growth and prolonged survival of gastric cancer patients, possibly involving increased AMPK phosphorylation. Thus, compounds designed to elicit stress may turn beneficial therapeutics in gastric cancer patients exhibiting high levels of Hpa2.
Author contributions
IV, NI, SY concepted and designed the work; JL, IK, MG-C, HJ, WS, TL, NI performed, acquisited, analyzed, and/or interpreted the data; NI, IV, SY drafted the work and/or substantively revised it. All authors read and approved the final manuscript.
Institutional review board statement
Ethics approval and consent to participate
Human data. Approval is waived given that we have used commercial tissue arrays purchased from Outdo Biotech Co., Ltd. (http://www.superchip.com.cn/) and from US Biomax (Rockville, MD) as detailed under “Materials & Methods.” Samples are not collected by interacting or intervening with living people. None of the investigators or collaborators can identify the subjects through coded private information or specimens (NIH exemption #4).
Helsinki approval for the use of human tissues (0667-19-RMC) was issued on 21/11/2019 by Rambam Health Care Campus, Helsinki Committee. Animals. All experiments were performed in accordance with the Technion's Institutional Animal Care and Use Committee (IL-080-08-2018; OPRR-A5026-01). See “Materials & Methods.”
Informed consent statement
Patient consent was waived given that we have used commercial tissue arrays purchased from Outdo Biotech Co., Ltd. (http://www.superchip.com.cn/) and from US Biomax (Rockville, MD) as detailed under “Materials & Methods.” Samples are not collected by interacting or intervening with living people. None of the investigators or collaborators can identify the subjects through coded private information or specimens (NIH exemption #4).
Data availability
The datasets obtained and/or analyzed during the current study are available from the corresponding author on reasonable request. All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Acknowledgments
Not applicable.
Footnotes
Funding: These studies were generously supported by research grants awarded by the Israel Science Foundation (ISF grant 1021/19), the NSFC-ISF cooperation program (grant 2572/16) between the National Natural Science Foundation of China (NSFC) and the Israel Science Foundation (ISF), NFSC grant # 81661148050, and the Israel Cancer Research Fund (ICRF). I. Vlodavsky is a Research Professor of the ICRF.
Conflicts of interest: The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neo.2021.07.010.
Appendix. Supplementary materials
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Yao X, Ajani JA, Song S. Molecular biology and immunology of gastric cancer peritoneal metastasis. Transl Gastroenterol Hepatol. 2020;5:57. doi: 10.21037/tgh.2020.02.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Ann Rev Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- 4.Iozzo RV, Sanderson RD. Proteoglycans in cancer biology, tumour microenvironment and angiogenesis. J Cell Mol Med. 2011;15:1013–1031. doi: 10.1111/j.1582-4934.2010.01236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karamanos NK, Piperigkou Z, Theocharis AD, Watanabe H, Franchi M, Baud S, Brezillon S, Gotte M, Passi A, Vigetti D. Proteoglycan chemical diversity drives multifunctional cell regulation and therapeutics. Chem Rev. 2018;118:9152–9232. doi: 10.1021/acs.chemrev.8b00354. [DOI] [PubMed] [Google Scholar]
- 6.Coombe DR, Gandhi NS. Heparanase: a challenging cancer drug target. Front Oncol. 2019;9:1316. doi: 10.3389/fonc.2019.01316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khanna M, Parish CR. Heparanase: historical aspects and future perspectives. Adv Exp Med Biol. 2020;1221:71–96. doi: 10.1007/978-3-030-34521-1_3. [DOI] [PubMed] [Google Scholar]
- 8.Vlodavsky I, Ilan N, Sanderson RD. Forty years of basic and translational heparanase research. Adv Exp Med Biol. 2020;1221:3–59. doi: 10.1007/978-3-030-34521-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Billings PC, Pacifici M. Interactions of signaling proteins, growth factors and other proteins with heparan sulfate: mechanisms and mysteries. Connect Tissue Res. 2015;56:272–280. doi: 10.3109/03008207.2015.1045066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rivara S, Milazzo FM, Giannini G. Heparanase: a rainbow pharmacological target associated to multiple pathologies including rare diseases. Future Med Chem. 2016;8:647–680. doi: 10.4155/fmc-2016-0012. [DOI] [PubMed] [Google Scholar]
- 11.Vlodavsky I, Singh P, Boyango I, Gutter-Kapon L, Elkin M, Sanderson RD, Ilan N. Heparanase: from basic research to therapeutic applications in cancer and inflammation. Drug Resist Updat. 2016;29:54–75. doi: 10.1016/j.drup.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dredge K, Brennan TV, Hammond E, Lickliter JD, Lin L, Bampton D, Handley P, Lankesheer F, Morrish G, Yang Y. A Phase I study of the novel immunomodulatory agent PG545 (pixatimod) in subjects with advanced solid tumours. Br J Cancer. 2018;118:1035–1041. doi: 10.1038/s41416-018-0006-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galli M, Chatterjee M, Grasso M, Specchia G, Magen H, Einsele H, Celeghini I, Barbieri P, Paoletti D, Pace S. Phase I study of the heparanase inhibitor roneparstat: an innovative approach for ultiple myeloma therapy. Haematologica. 2018;103:e469–e472. doi: 10.3324/haematol.2017.182865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang B, Yang S. Involvement of heparanase in gastric cancer progression and immunotherapy. Adv Exp Med Biol. 2020;1221:351–363. doi: 10.1007/978-3-030-34521-1_13. [DOI] [PubMed] [Google Scholar]
- 15.Li HL, Gu J, Wu JJ, Ma CL, Yang YL, Wang HP, Wang J, Wang Y, Chen C, Wu HY. Heparanase mRNA and protein expression correlates with clinicopathologic features of gastric cancer patients: a meta- analysis. Asian Pac J Cancer Prev. 2015;16:8653–8658. doi: 10.7314/apjcp.2015.16.18.8653. [DOI] [PubMed] [Google Scholar]
- 16.Takaoka M, Naomoto Y, Ohkawa T, Uetsuka H, Shirakawa Y, Uno F, Fujiwara T, Gunduz M, Nagatsuka H, Nakajima M. Heparanase expression correlates with invasion and poor prognosis in gastric cancers. Lab Invest. 2003;83:613–622. doi: 10.1097/01.lab.0000067482.84946.bd. [DOI] [PubMed] [Google Scholar]
- 17.McKenzie E, Tyson K, Stamps A, Smith P, Turner P, Barry R, Hircock M, Patel S, Barry E, Stubberfield C. Cloning and expression profiling of Hpa2, a novel mammalian heparanase family member. Biochem Biophys Res Commun. 2000;276:1170–1177. doi: 10.1006/bbrc.2000.3586. [DOI] [PubMed] [Google Scholar]
- 18.Levy-Adam F, Feld S, Cohen-Kaplan V, Shteingauz A, Gross M, Arvatz G, Naroditsky I, Ilan N, Doweck I, Vlodavsky I. Heparanase 2 interacts with heparan sulfate with high affinity and inhibits heparanase activity. J Biol Chem. 2010;285:28010–28019. doi: 10.1074/jbc.M110.116384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ilan N, Elkin M, Vlodavsky I. Regulation, function and clinical significance of heparanase in cancer metastasis and angiogenesis. Intl J Biochem Cell Biol. 2006;38:2018–2039. doi: 10.1016/j.biocel.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Vlodavsky I, Gross-Cohen M, Weissmann M, Ilan N, Sanderson RD. Opposing functions of heparanase-1 and heparanase-2 in cancer progression. Trends Biochem Sci. 2018;43:18–31. doi: 10.1016/j.tibs.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gross-Cohen M, Feld S, Arvatz G, Ilan N, Vlodavsky I. Elucidating the consequences of heparan sulfate binding by heparanase 2. Front Oncol. 2020;10 doi: 10.3389/fonc.2020.627463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu J, Khaybullin R, Zhang Y, Xia A, Qi X. Gene expression profiling leads to discovery of correlation of matrix metalloproteinase 11 and heparanase 2 in breast cancer progression. BMC Cancer. 2015;15:473. doi: 10.1186/s12885-015-1410-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ilan N, Bhattacharya U, Barash U, Boyango I, Yanku Y, Gross-Cohen M, Vlodavsky I. Heparanase-The message comes in different flavors. Adv Exp Med Biol. 2020;1221:253–283. doi: 10.1007/978-3-030-34521-1_9. [DOI] [PubMed] [Google Scholar]
- 24.Kayal Y, Singh P, Naroditsky I, Ilan N, Vlodavsky I. Heparanase 2 (Hpa2) attenuates the growth of pancreatic carcinoma. Matrix Biol. 2021;98:21–31. doi: 10.1016/j.matbio.2021.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Gross-Cohen M, Feld S, Doweck I, Neufeld G, Hasson P, Arvatz G, Barash U, Naroditsky I, Ilan N, Vlodavsky I. Heparanase 2 attenuates head and neck tumor vascularity and growth. Cancer Res. 2016;76:2791–2801. doi: 10.1158/0008-5472.CAN-15-1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fong S, Debs RJ, Desprez PY. Id genes and proteins as promising targets in cancer therapy. Trends Mol Med. 2004;10:387–392. doi: 10.1016/j.molmed.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 27.Gross-Cohen M, Yanku Y, Kessler O, Barash U, Boyango I, Cid-Arregui A, Neufeld G, Ilan N, Vlodavsky I. Heparanase 2 (Hpa2) attenuates tumor growth by inducing Sox2 expression. Matrix Biol. 2021;99:58–71. doi: 10.1016/j.matbio.2021.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Gross-Cohen M, Feld S, Naroditsky I, Nativ O, Ilan N, Vlodavsky I. Heparanase 2 expression inversely correlates with bladder carcinoma grade and stage. Oncotarget. 2016;7:22556–22565. doi: 10.18632/oncotarget.8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia B, Garcia-Suarez O, Fernandez-Vega I, Vallina A, Astudillo A, Quiros LM. Heparanase and heparanase 2 display differently deregulation in neuroendocrine tumors, depending on their differentiation grade. Histol Histopathol. 2016;31:73–81. doi: 10.14670/HH-11-650. [DOI] [PubMed] [Google Scholar]
- 30.Kim JG, Lee SJ, Chae YS, Kang BW, Lee YJ, Oh SY, Kim MC, Kim KH, Kim SJ. Association between phosphorylated AMP-activated protein kinase and MAPK3/1 expression and prognosis for patients with gastric cancer. Oncology. 2013;85:78–85. doi: 10.1159/000351234. [DOI] [PubMed] [Google Scholar]
- 31.Gutter-Kapon L, Alishekevitz D, Shaked Y, Li JP, Aronheim A, Ilan N, Vlodavsky I. Heparanase is required for activation and function of macrophages. Proc Natl Acad Sci USA. 2016;113:E7808–E7817. doi: 10.1073/pnas.1611380113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hao NB, Tang B, Wang GZ, Xie R, Hu CJ, Wang SM, Wu YY, Liu E, Xie X, Yang SM. Hepatocyte growth factor (HGF) upregulates heparanase expression via the PI3K/Akt/NF-kappaB signaling pathway for gastric cancer metastasis. Cancer Lett. 2015;361:57–66. doi: 10.1016/j.canlet.2015.02.043. [DOI] [PubMed] [Google Scholar]
- 33.Tang B, Xie R, Qin Y, Xiao YF, Yong X, Zheng L, Dong H, Yang SM. Human telomerase reverse transcriptase (hTERT) promotes gastric cancer invasion through cooperating with c-Myc to upregulate heparanase expression. Oncotarget. 2015;7:11364–11379. doi: 10.18632/oncotarget.6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barash U, Spyrou A, Liu P, Vlodavsky E, Zhu C, Luo J, Su D, Ilan N, Forsberg-Nilsson K, Vlodavsky I. Heparanase promotes glioma progression via enhancing CD24 expression. Intl J Cancer. 2019;145:1596–1608. doi: 10.1002/ijc.32375. [DOI] [PubMed] [Google Scholar]
- 35.Arvatz G, Barash U, Nativ O, Ilan N, Vlodavsky I. Post-transcriptional regulation of heparanase gene expression by a 3′ AU-rich element. Faseb J. 2011;24:4969–4976. doi: 10.1096/fj.10-156372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barash U, Zohar Y, Wildbaum G, Beider K, Nagler A, Karin N, Ilan N, Vlodavsky I. Heparanase enhances myeloma progression via CXCL10 downregulation. Leukemia. 2014;28:2178–2187. doi: 10.1038/leu.2014.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barash U, Lapidot M, Zohar Y, Loomis C, Moreira A, Feld S, Goparaju C, Yang H, Hammond E, Zhang G. Involvement of heparanase in the pathogenesis of mesothelioma: basic aspects and clinical applications. J Natl Cancer Inst. 2018;110:1102–1114. doi: 10.1093/jnci/djy032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhattacharya U, Gutter-Kapon L, Kan T, Boyango I, Barash U, Yang SM, Liu J, Gross-Cohen M, Sanderson RD, Shaked Y. Heparanase and chemotherapy synergize to drive macrophage activation and enhance tumor growth. Cancer Res. 2020;80:57–68. doi: 10.1158/0008-5472.CAN-19-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shteingauz A, Ilan N, Vlodavsky I. Processing of heparanase is mediated by syndecan-1 cytoplasmic domain and involves syntenin and alpha-actinin. Cell Mol Life Sci. 2014;71:4457–4470. doi: 10.1007/s00018-014-1629-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weissmann M, Arvatz G, Horowitz N, Feld S, Naroditsky I, Zhang Y, Ng M, Hammond E, Nevo E, Vlodavsky I. Heparanase-neutralizing antibodies attenuate lymphoma tumor growth and metastasis. Proc Natl Acad Sci USA. 2016;113:704–709. doi: 10.1073/pnas.1519453113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fhu CW, Ali A. Dysregulation of the ubiquitin proteasome system in human malignancies: a window for therapeutic intervention. Cancers (Basel) 2021;13:1513. doi: 10.3390/cancers13071513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee DH, Goldberg AL. Proteasome inhibitors: valuable new tools for cell biologists. Trends Cell Biol. 1998;8:397–403. doi: 10.1016/s0962-8924(98)01346-4. [DOI] [PubMed] [Google Scholar]
- 43.Nakata W, Hayakawa Y, Nakagawa H, Sakamoto K, Kinoshita H, Takahashi R, Hirata Y, Maeda S, Koike K. Anti-tumor activity of the proteasome inhibitor bortezomib in gastric cancer. Int J Oncol. 2011;39:1529–1536. doi: 10.3892/ijo.2011.1141. [DOI] [PubMed] [Google Scholar]
- 44.Kuhajda FP. AMP-activated protein kinase and human cancer: cancer metabolism revisited. Int J Obes (Lond) 2008;32(Suppl 4):S36–S41. doi: 10.1038/ijo.2008.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piskovatska V, Storey KB, Vaiserman AM, Lushchak O. The use of metformin to increase the human healthspan. Adv Exp Med Biol. 2020;1260:319–332. doi: 10.1007/978-3-030-42667-5_13. [DOI] [PubMed] [Google Scholar]
- 46.Aljofan M, Riethmacher D. Anticancer activity of metformin: a systematic review of the literature. Future Sci OA. 2019;5:FSO410. doi: 10.2144/fsoa-2019-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daly SB, Urquhart JE, Hilton E, McKenzie EA, Kammerer RA, Lewis M, Kerr B, Stuart H, Donnai D, Long DA. Mutations in HPSE2 cause urofacial syndrome. Am J Hum Genet. 2010;86:963–969. doi: 10.1016/j.ajhg.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pang J, Zhang S, Yang P, Hawkins-Lee B, Zhong J, Zhang Y, Ochoa B, Agundez JA, Voelckel MA, Fisher RB. Loss-of-function mutations in HPSE2 cause the autosomal recessive urofacial syndrome. Am J Hum Genet. 2010;86:957–962. doi: 10.1016/j.ajhg.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stuart HM, Roberts NA, Hilton EN, McKenzie EA, Daly SB, Hadfield KD, Rahal JS, Gardiner NJ, Tanley SW, Lewis MA. Urinary tract effects of HPSE2 mutations. J Am Soc Nephrol. 2015;26:797–804. doi: 10.1681/ASN.2013090961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roberts NA, Hilton EN, Lopes FM, Singh S, Randles MJ, Gardiner NJ, Chopra K, Coletta R, Bajwa Z, Hall RJ. Lrig2 and Hpse2, mutated in urofacial syndrome, pattern nerves in the urinary bladder. Kidney Int. 2019;95:1138–1152. doi: 10.1016/j.kint.2018.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roberts NA, Woolf AS. Heparanase 2 and urofacial syndrome, a genetic neuropathy. Adv Exp Med Biol. 2020;1221:807–819. doi: 10.1007/978-3-030-34521-1_35. [DOI] [PubMed] [Google Scholar]
- 52.Hardie DG, Schaffer BE, Brunet A. AMPK: an energy-sensing pathway with multiple inputs and outputs. Tren Cell Biol. 2016;26:190–201. doi: 10.1016/j.tcb.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ross FA, MacKintosh C, Hardie DG. AMP-activated protein kinase: a cellular energy sensor that comes in 12 flavours. FEBS J. 2016;283:2987–3001. doi: 10.1111/febs.13698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vara-Ciruelos D, Russell FM, Hardie DG. The strange case of AMPK and cancer: Dr Jekyll or Mr Hyde? (dagger) Open Biol. 2019;9 doi: 10.1098/rsob.190099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Steinberg GR, Carling D. AMP-activated protein kinase: the current landscape for drug development. Nat Rev Drug Discov. 2019;18:527–551. doi: 10.1038/s41573-019-0019-2. [DOI] [PubMed] [Google Scholar]
- 56.Zhang J, Wen L, Zhou Q, He K, Teng L. Preventative and therapeutic effects of metformin in gastric cancer: a new contribution of an old friend. Cancer Manag Res. 2020;12:8545–8554. doi: 10.2147/CMAR.S264032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kayal Y, Singh P, Naroditsky I, Ilan N, Vlodavsky I. Heparanase 2 (Hpa2) attenuates the growth of pancreatic carcinoma. Matrix Biol. 2021;98:21–31. doi: 10.1016/j.matbio.2021.03.002. [DOI] [PubMed] [Google Scholar]
- 58.Ma Y, Wang J, Gao J, Yang H, Wang Y, Manithody C, Li J, Rezaie AR. Antithrombin up-regulates AMP-activated protein kinase signalling during myocardial ischaemia/reperfusion injury. Thromb Haemost. 2015;113:338–349. doi: 10.1160/TH14-04-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gubbiotti MA, Neill T, Iozzo RV. A current view of perlecan in physiology and pathology: a mosaic of functions. Matrix Biol. 2017;57-58:285–298. doi: 10.1016/j.matbio.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shrikanth CB, Jagannath S, Chilkunda ND. AMPK differentially alters sulphated glycosaminoglycans under normal and high glucose milieu in proximal tubular cells. J Biochem. 2021;169:75–86. doi: 10.1093/jb/mvaa094. [DOI] [PubMed] [Google Scholar]
- 61.Doweck I, Feibish N. Opposing effects of heparanase and heparanase-2 in head & neck cancer. Adv Exp Med Biol. 2020;1221:847–856. doi: 10.1007/978-3-030-34521-1_37. [DOI] [PubMed] [Google Scholar]
- 62.Ilan N, Bhattacharya U, Barash U, Boyango I, Yanku Y, Gross-Cohen M, Vlodavsky I. Heparanase-The message comes in different flavors. Adv Exp Med Biol. 2020;1221:253–283. doi: 10.1007/978-3-030-34521-1_9. [DOI] [PubMed] [Google Scholar]
- 63.Zhang H, Xu C, Shi C, Zhang J, Qian T, Wang Z, Ma R, Wu J, Jiang F, Feng J. Hypermethylation of heparanase 2 promotes colorectal cancer proliferation and is associated with poor prognosis. J Transl Med. 2021;19:98. doi: 10.1186/s12967-021-02770-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta. 2013;1833:3460–3470. doi: 10.1016/j.bbamcr.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen X, Cubillos-Ruiz JR. Endoplasmic reticulum stress signals in the tumour and its microenvironment. Nat Rev Cancer. 2021;21:71–88. doi: 10.1038/s41568-020-00312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets obtained and/or analyzed during the current study are available from the corresponding author on reasonable request. All data generated or analyzed during this study are included in this published article [and its supplementary information files].