Abstract
Early life stress (ELS) is a well-established risk factor for psychopathology across the lifespan. Cognitive vulnerability to stress-induced cortisol may explain risk and resilience. The current study aimed to elucidate a psychobiological pathway linking stress to altered memory for affective words among youth with and without exposure to ELS. One hundred and fifteen youth (ages 9–16, 47% female) were randomized either to a psychosocial stressor or a control condition. Immediately following the stress or control condition, participants completed a memory task for affective words. Change in salivary cortisol from immediately before to 25 min after stress onset were used to predict memory for affective words. Exposure to the acute laboratory stressor led to activation of the HPA axis. Greater cortisol reactivity was associated with less accurate recognition of negative valence words. Among youth exposed to ELS, greater cortisol reactivity to acute stress was associated with poorer recognition of dysphoric and neutral words. Acute increases in cortisol may interfere with negatively-valenced information processing that has implications for memory. Youth exposed to high ELS may be particularly vulnerable to the effects of cortisol, which may explain one pathway through which stress leads to psychopathology among at-risk youth.
Keywords: affective science, cortisol, developmental psychopathology, early life stress, memory, stress
1 |. INTRODUCTION
Stress activates the hypothalamic-pituitary-adrenal axis (HPA axis), which results in the circulation of glucocorticoids, cortisol in humans. Cortisol has profound effects on attention, learning, and memory (Roozendaal, 2002), and has been implicated in the atypical processing of affective information associated with stress-related diseases (Ellenbogen et al., 2002; Erickson et al., 2003). Yet, examinations of the association between cortisol and processing of affectively-valenced information among youth are limited. This is an important gap in our understanding because about 50% of all mental health problems begin by age 14, and 75% by age 18 (Kessler et al., 2005). Characterizing the role of stress in memory for affective information among youth may clarify the pathophysiology of stress-related diseases like depression, particularly among high-risk populations. Individuals exposed to 4 or more adversities during childhood are at four times greater risk for depression (Chapman et al., 2004; Green et al., 2010; Kessler et al., 2010; McLaughlin et al., 2010). A better understanding of psychobiological pathways linking stress to disorders like depression in this group is needed.
Several studies have shown that experimentally manipulating glucocorticoids leads to changes in memory for affective information (Kuhlmann et al., 2005; Quesada et al., 2012). In adults, exogenous administration of cortisol impedes short-term retrieval of negatively-valenced but not neutral words (Kuhlmann et al., 2005). Cortisol may support adaptive navigation of stressful environments at the temporary expense of memory. This is biologically plausible due to the high density of glucocorticoid receptors in neural structures essential to processing affective information, such as the hippocampus and the amygdala (Herman et al., 2005; Kim & Diamond, 2002). These biases in memory may have implications for mood, coping, and risk for psychopathology, particularly during adolescence which is a period of enhanced neural sensitivity to internal (e.g., stress hormones) and external influences (e.g., social evaluation) (Fuhrmann et al., 2015; Sumter et al., 2010). Indeed, individuals who go on to develop depression tend to encode memories with less specificity (Sumner et al., 2010), particularly among youth exposed to chronic interpersonal stress (Sumner et al., 2011).
Some individuals may be more susceptible to the effects of stress than others, such as individuals with a history of early life stress (ELS). ELS may alter the structure and function of the brain (Teicher et al., 2016) and sensitivity of the brain to glucocorticoids (De Kloet et al., 2005; Lupien et al., 2009). Thus, two individuals with the same magnitude of cortisol response to stress may differ in the cognitive and behavioral sequelae. This may explain why individuals exposed to ELS exhibit impairments in valenced-information processing that persist into adulthood (Pechtel & Pizzagalli, 2010).
In this study, we examined how acute changes in cortisol were associated with performance on a memory task for affective words. We hypothesized that (1) acute psychological stress would activate the HPA axis, (2) acute increases in cortisol would be associated with less accurate memory for negatively-valenced words, and (3) that the effect of cortisol on memory would be exaggerated for youth exposed to ELS.
2 |. METHOD
2.1 |. Participants
Participants in the present study were 115 youth (54 females) between 9 and 16 years of age, recruited from the local community. Participants were excluded from the study if they were currently taking medications known to influence the HPA axis, had ever been diagnosed with Autism Spectrum Disorder, had psychotic symptoms, or had a major medical condition. Data for the present analyses were collected as part of a multi-visit study examining the affective and neuroendocrine mechanisms underlying pediatric anxiety and depression (Lopez-Duran et al., 2015). Participants were 75.7% Caucasian, 11.3% Biracial, 4.3% African-American, 2.6% Asian, 2.6% Latino, and the remaining identified as “Other.” Participants in the sample lived in largely well-educated and financially-stable households: only 2.6% of the sample reported welfare or social aid as a major source of household income, and in about half of families (51.3%) both parents had a college degree or higher.
2.2 |. Procedures
All participating youth provided written, informed assent, and their parents provided written, informed consent at the initial study visit. All participants came to the lab between 1:00 and 4:00 p.m. to reduce the impact of circadian rhythms on our results, and were asked to refrain from eating or drinking for 1 h prior to lab arrival. At initial study enrollment, participants were randomized to either a stress or control condition. The socially-evaluative cold pressor task (SE-CPT) (Schwabe et al., 2008) was administered to participants in the stress condition. In the control condition, participants waited in the waiting room for an additional 5 min. All participants then completed the affective memory task. Participants in both conditions then watched a neutral film (i.e., National Geographic) in a private office for an hour. Participants provided six saliva samples throughout these procedures.
2.3 |. Measures
2.3.1 |. Early life stress
Parents completed the 50-item Early Trauma Inventory (ETI) (Bremner et al., 2000). Parents (81% mothers) indicated whether their child (yes or no) had ever been exposed to a series of potentially traumatic events including physical abuse (being hit to the point of bruising), sexual abuse (being forced to engage in sexual acts), emotional abuse (often put down or ridiculed), or non-intentional traumatic events (witnessing an accident, natural disaster). The number of items marked yes for each participant was summed to create an index of ELS. Cumulative exposure to more than three adversities in childhood is associated with significantly greater health disparities relative to individuals with three or fewer reported adversities (Felitti et al., 1998). This occurs in 5%–12% of the population and places them on a lifelong trajectory of health disparity (Anda et al., 2006; Dube et al., 2003; Kessler et al., 2010), including almost a five-fold increase in prevalence of depressive disorders and a 12-fold increase in suicidality (Chapman et al., 2004), as well as accounts for 1/3 of all adolescent-onset mood disorders (Kessler et al., 2010). For this reason, youth with a score ≥4 were considered to have high exposure to ELS (31.6%). See Kuhlman, Geiss, et al. (2015), Kuhlman, Vargas, et al. (2015) for more details on overall ELS exposure in this sample.
2.3.2 |. Acute laboratory stress
The SE-CPT (Schwabe et al., 2008) was selected as the standardized stress task in this study because it involves uncontrollability and social evaluation which reliably activate the human HPA axis (Dickerson & Kemeny, 2004), including in youth (Sumter et al., 2010). The task involves having the participant place their hand in a bucket of ice water (34–36°F) for up to 3 min. A research assistant stood in front of the participant with a stopwatch, positioned a video camera 12-inches from their face, and told the participant to look directly into the camera so they could “monitor your facial expressions.” If the participant removed their hand from the bucket before 3 min had elapsed, the research assistant instructed the participant to remain seated, and continue to look into the camera for the remainder of the time. The experimenter’s presence and use of the video camera added an evaluative component, which leads to greater stress reactivity when compared to the cold pressor alone (Schwabe et al., 2008; Schwabe & Schächinger, 2018). HPA axis activation to this task is similar to that of other common laboratory stressors (e.g., TSST), but takes <5 min to administer making it optimal for studies interested in high resolution timing for stress onset (Buske-Kirschbaum et al., 1997; Schwabe & Schächinger, 2018).
2.3.3 |. Cortisol reactivity
Cortisol reactivity was measured in saliva collected via passive drool directly into sterile salivettes (Sarstedt, Inc.). Saliva samples were collected upon arrival to the laboratory; the participant then waited for 30 min to acclimate to the laboratory setting before taking their baseline sample. Subsequent saliva samples were collected +25, +35, +45, +55, and +65 min after the stress or control procedure began. All cortisol samples were used to determine whether stress-induced HPA-activation. Cortisol is significantly elevated within the first 10 min post-stress relative to baseline (Dickerson & Kemeny, 2004), and peak salivary cortisol concentrations occur 21–30 min post-stress. It was important to our study design that the memory task occurred during the window of time in which glucocorticoids were actively binding to their receptors as opposed to when cortisol was declining (suggesting negative feedback had initiated). Furthermore, sustained elevations in cortisol at +35 through +65 min post-stress could reflect sustained elevations that occurred as a consequence of the memory task. For these reasons, only acute change in cortisol from immediately before stress initiation and immediately after completing the computer tasks (+25 min after stress initiation) was used as the cortisol reactivity predictor in our models. All samples were stored at −20°C on the day of collection and kept frozen until the day of assay. Samples remained frozen during transport to the University of Michigan Core Assay Facility, located in the same building as the laboratory, where salivary cortisol was assayed via enzyme-linked immunosorbent assay (ELISA) using commercially available assay kits (Salimetrics, Inc.). All samples for an individual were assayed together, in duplicate. The inter-assay and intra-assay coefficients of variability were 5% and 9% respectively.
2.3.4 |. Affective memory
The memory task included an encoding and recognition block. During the encoding block, participants viewed 80 words (20 neutral, positive, dysphoric, and anxious). Words for this task were validated for use in participants with a 2nd grade reading level (John, 1988). Positive words included cheer and joke; dysphoric words included sad and funeral; anxious words included afraid and bomb; and neutral words included bread and replace. Each word was displayed for 5000 ms. During this time, the participant was instructed to think about how much the word applied to their life and indicate their response according to a 3-point Likert scale (1 “not at all,” 2 “a little,” 3 “a lot”). Participants who did not respond within the 5000 ms allotted had missing data for these encoding trials. In the recognition block, participants saw 80 words: 40 target words (shown during the encoding phase) and 40 novel words. Participants indicated whether they recognized each word from the encoding phase. This task took 15.64 min (SD = 1.80). Performance on this task was measured by the number of accurate trials the participant completed during the recognition block.
2.4 |. Data analysis
All analyses were conducted using SPSS v25. Continuous variables were assessed for normality. Raw baseline cortisol, MBaseline Cortisol = 0.12, SDBaseline cortisol = 0.07, was highly kurtotic, skewness = 1.98, excess kurtosis = 5.03. This variable was transformed using the natural log transformation, which adjusted the excess kurtosis to within an acceptable range (≤±4), MBaseline Cortisol (log) = −2.28, SDBaseline cortisol = 0.55, skewness = 0.06, excess kurtosis = 0.62. Raw cortisol reactivity, MReactivity = 0.005, SDReactivity = 0.05, was highly kurtotic, skewness = 1.27, excess kurtosis = 4.05. This variable was transformed using a square root transformation, which adjusted the kurtosis to within an acceptable range, MReactivity (sqrt) = 0.37, SDReactivity = 0.07, skewness = −0.04, excess kurtosis = 3.50. Raw ELS, METI Total = 3.11, SDETI Total = 3.36, was both skewed and kurtotic, skewness = 2.10, excess kurtosis = 6.00. There were no transformations that would bring this variable within acceptable ranges to meet assumptions for normality and heteroscedasticity as a continuous variable.
First, we tested whether randomization to the stress condition effectively led to cortisol reactivity in our sample. We then fit multiple linear regression models predicting memory performance from cortisol reactivity (change in cortisol from 0 to 25 min post-stress initiation). All models included baseline cortisol, age, and gender as covariates. In order to minimize the variance in our outcome that could be attributable to inattention or variability in processing speed during encoding trials, we also covaried for the number of encoding trials in which the participant failed to respond. To test whether ELS moderated the association between cortisol reactivity and affective processing, we conducted moderation analyses using the SPSS PROCESS macro (Hayes, 2013).
For all regression models, we computed influence statistics (DFFIT) to determine the potential role of outliers in our results. Based upon our adjusted models and sample size, participants were influential on the results if DFFIT >0.42. There were four individuals who met this criterion when predicting memory for neutral words, 5 for positive words, 5 for dysphoric words, and 2 for anxiety-related words. The pattern of results did not change when these individuals were excluded from the models.
3 |. RESULTS
3.1 |. Effect of acute laboratory stress on HPA-activation
There were no differences between participants randomized to stress (n = 52) or control in age, F(1, 113) = 1.39, p = .24, sex, χ2 = 2.11, p = .15, ELS, F(1,112) =1.86, p = .18, current depression, χ2 = 0.03, p = .87, history of depression, χ2 = 1.55, p = .21, presence of an anxiety disorder, χ2 = 1.10, p = .30, maternal education, χ2 = 7.01, p = .43, the size of family home (number of rooms), F(1, 109) = 0.26, p = .61, or salivary cortisol concentrations at baseline, F(1,113) = 0.22, p = .64.
Youth randomized to the laboratory stressor exhibited, on average, a 23% increase in cortisol from baseline to 25 min post-stress initiation, SE = 0.08, 95% CI [0.06, 0.39], and youth randomized to the control condition exhibited, on average, a 3% increase in cortisol, SE = 0.05, 95% CI [−0.07, 0.14], F(1,113) = 4.27, p = .041. See Figure 1a for average (±SE) cortisol concentrations by condition.
FIGURE 1.
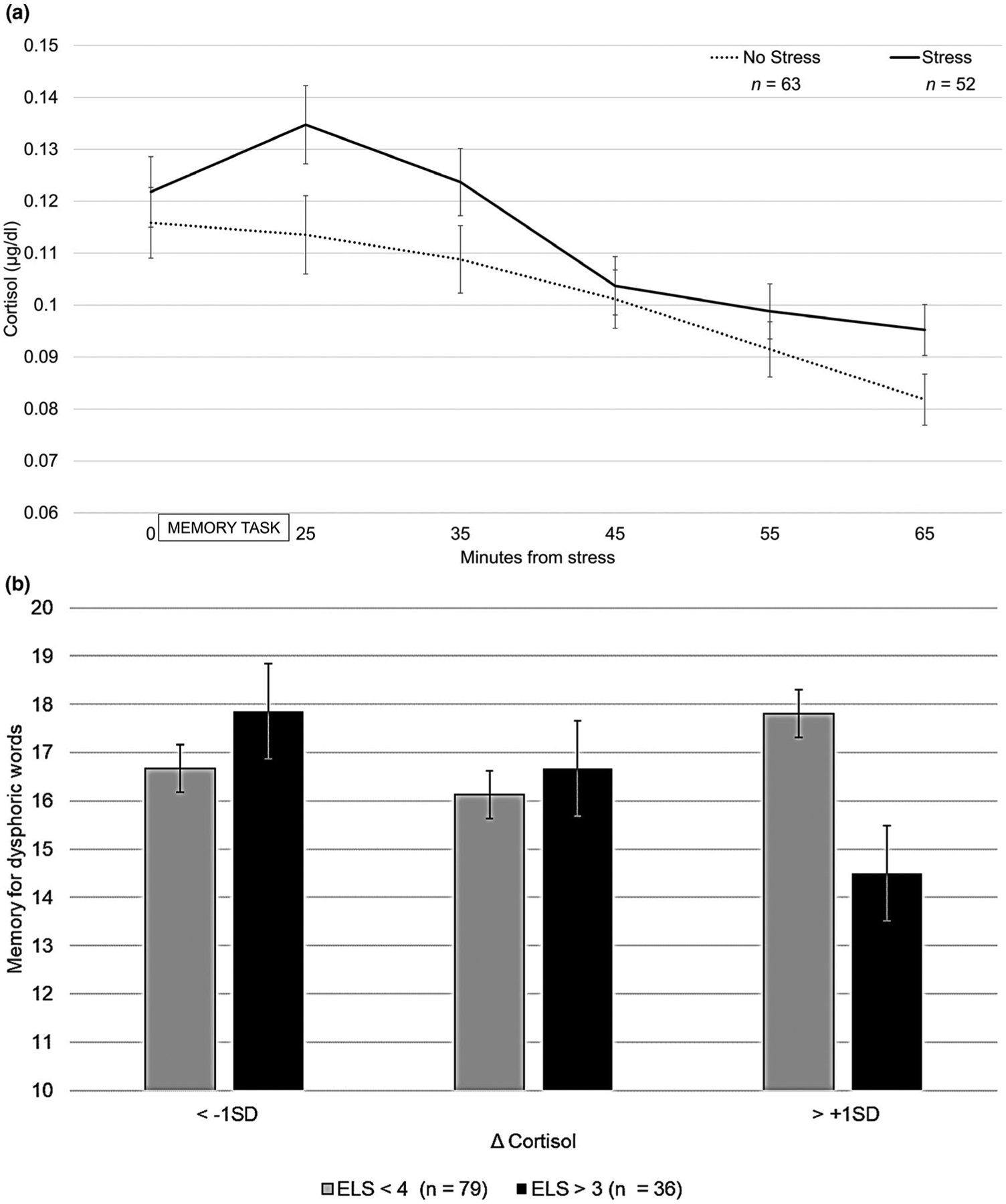
Mean (±SE) salivary cortisol for stress and control groups (a), and mean (±SE) memory accuracy for dysphoric words by exposure to early life stress and Δ cortisol reactivity (b)
3.2 |. HPA axis activation and memory for affective information
Table 1 provides descriptive statistics and bivariate correlations between all key study variables. Greater increases in cortisol were associated with less accurate memory for anxiety-related words, b = −12.16, SE = 4.30, p = .006, and dysphoric words, b = −8.96, SE = 4.02, p = .028, but not neutral words, b = −7.10, SE = 4.88, p = .15, or positive words, b = −4.04, SE = 4.98, p = .42.
TABLE 1.
Means, standard deviations, and bivariate correlations between all continuous study variables (n = 115)
| Correlations | ||||||||
|---|---|---|---|---|---|---|---|---|
| M (SD) | 1. | 2. | 3. | 4. | 5. | 6. | 7. | |
| 1. Age | 12.78 (2.28) | 1.0 | ||||||
| 2. Early life stress | 3.11 (3.36) | .13 | 1.0 | |||||
| 3. Baseline cortisol (μg/dl)a | 0.12 (0.07) | .24** | .20* | 1.0 | ||||
| 4. Δ Cortisolb | 0.005 (0.05) | −.12 | .12 | −.29** | 1.0 | |||
| Memory performance | ||||||||
| 5. Neutral words | 18.52 (3.51) | .16+ | .06 | .15 | −.14 | 1.0 | ||
| 6. Positive words | 16.65 (3.55) | .21* | −.01 | −.06 | −.08 | .17+ | 1.0 | |
| 7. Dysphoric words | 16.34 (2.93) | .10 | .04 | .01 | −.21* | .02 | .24** | 1.0 |
| 8. Anxious words | 15.22 (3.17) | .27** | −.07 | .10 | −.26** | .14 | .30** | .16+ |
Raw mean is reported, log transformed for all non-univariate analyses.
Change in cortisol from baseline to +25 min (μg/dl) in both stress and control groups, raw mean is reported, square root transformed for all non-univariate analyses.
p < .01,
p < .05.
p < .10.
We then tested whether acute change in cortisol following the stress manipulation was associated with variability in memory when accounting for key covariates. See Table 2 for the estimated effects of cortisol change on memory for affective words. Greater increases in cortisol while the participant was completing the affective tasks was associated with less accurate memory for both anxiety-related and dysphoric words.
TABLE 2.
Adjusted estimates predicting memory performance from cortisol reactivity (n = 115)
| Memory for affective words | ||||
|---|---|---|---|---|
| Neutral | Positive | Dysphoric | Anxious | |
| R 2 | R 2 | R 2 | R 2 | |
| .07 | .07 | .06 | .15** | |
| b (SE) | b (SE) | b (SE) | b (SE) | |
| Intercept | 22.68 (2.28)** | 16.56 (2.30)** | 18.77 (1.91)** | 20.31 (1.96)** |
| Baseline cortisol | 0.54 (0.64) | −0.90 (0.64) | −0.42 (0.53) | −0.14 (0.55) |
| Δ Cortisol | −4.74 (5.11) | −4.71 (5.15) | −9.49 (4.27)* | −10.97 (4.39)** |
| Age | 0.20 (0.15) | 0.37 (0.15)* | 0.13 (0.13) | 0.33 (0.13)** |
| Gender (female) | −0.41 (0.66) | −0.44 (0.66) | −0.03 (0.55) | −0.76 (0.57) |
| Number of missing encoding trials | −0.03 (0.03) | 0.001 (0.03) | 0.01 (0.02) | −0.03 (0.03) |
p ≤ .01,
p ≤ .05.
3.3 |. ELS moderates the link between HPA axis activation and memory
ELS moderated the association between cortisol and memory for dysphoric words, b = −20.71, SE = 8.16, p = .01, such that greater cortisol reactivity was associated with less accurate memory for dysphoric words among youth exposed to high ELS, b = −20.16, SE = 5.84, p = .001, but not among youth exposed to low ELS, b = 0.55, SE = 5.93, p = .93. See Figure 1b. Importantly, there was also a significant main effect of ELS on memory for dysphoric words in this model, b = 7.93, SE = 3.14, p = .013, which was not observed for any other word type.
ELS also moderated the association between cortisol and memory for neutral words, b = −19.09, SE = 9.87, p = .05, such that greater cortisol reactivity was associated with less accurate memory for neutral words among youth exposed to high ELS, b = −14.86, SE = 7.06, p = .03, but not among youth exposed to low ELS, b = 4.22, SE = 7.17, p = .56. This pattern of results did not change when accounting for current MDD. ELS did not moderate the association between cortisol reactivity and memory for anxiety-related, p = .26, or positive-valence words, p = .95.
4 |. DISCUSSION
Overall, stress-induced increases in cortisol were associated with less accurate recognition of negatively-valenced words, both dysphoric and anxiety-related. The association between cortisol reactivity and memory was moderated by ELS, such that greater cortisol responses to stress were associated with reduced memory for dysphoric and neutral words in individuals with high ELS. These findings provide evidence that acute changes in cortisol may have an impact on memory for affective information in youth exposed to ELS.
Acute increases in cortisol were associated with less accuracy for dysphoric and anxiety-related words. This observation is consistent with previous studies in both children and adults, which have shown that glucocorticoids impede short-term memory for negatively-valenced words (Kuhlmann et al., 2005; Quesada et al., 2012). The consistency of our findings with that of a previous study using exogenous administration of cortisol (Quesada et al., 2012) may suggest that this physiological phenomenon generalizes to other manipulations that circulate cortisol and activate glucocorticoid receptors. Glucocorticoids enhance encoding of information but tend to impede its retrieval (Wolf, 2009). Affective information appears to be most sensitive to these effects because its encoding and retrieval are mediated by the amygdala (Roozendaal et al., 2009), which is densely populated with glucocorticoid receptors. Glucocorticoid-induced memory impairment may give rise to overgeneralized memory, which prospectively predicts depression-onset (Sumner et al., 2010, 2013). Overgeneralized memory is a phenomenon exhibited among chronically stressed or depressed individuals where autobiographical memories are recalled without specificity. Low accuracy in our experiment may reflect difficulty with memory specificity (i.e., seeing the word “sad”, but remembering the word “miserable”) when the HPA axis is activated, particularly for negatively-valenced information. Indeed, experimental models have shown that the presence of threat increases overgeneralization of episodic memory (Starita et al., 2019). Here, glucocorticoids may have facilitated overgeneralized encoding of negatively-valenced words, and may indicate how autobiographical experiences are encoded in the daily lives of these youth. The lack of specificity of these memories, particularly in the context of continued life stress, may impede the ability to avoid similar stressors in the future or recall effective coping strategies for use in those contexts. This may exacerbate stressors and lead to depression or other stress-related psychopathology over time.
The effects of acute cortisol reactivity on memory for dysphoric and neutral words were specific to youth exposed to ELS. Youth exposed to ELS may also be more sensitive to the link between cortisol and memory, presumably via glucocorticoid receptor sensitivity in neural circuits that govern affective information processing. This is particularly interesting given that ELS has been linked to misattribution of neutral information as negatively-valenced (Pechtel & Pizzagalli, 2010; Pollak et al., 2000). Modest reactivity to acute stress is likely to reflect adaptive cognitive, neurobiological, and behavioral coping with stress (Koolhaas et al., 1999; Lewis et al., 2017; Salovey et al., 2002; Sladek et al., 2016). It is, therefore, plausible that the average association between cortisol and memory is indicative of normative processes of coping with stress that may only contribute to pathology with repeated exposure.
It is important to consider the design of our memory task when interpreting the observations made in this study. Youth were prompted to reflect on the degree to which the presented word was relevant to them during the encoding trials. The 76%–92% accuracy on our recognition trials across all valences suggests that this was an effective way of engaging our participants’ attention during encoding. However, it is plausible that this encoding phase activated self-referential biases in some participants, such as those with a history of ELS or internalizing disorders. Negative self-referential biases are negative representations about the self that influence the way in which individuals attend to, interpret, and recall emotional information (Beck, 1967, 1987). These tendencies prospectively predict depressive episodes during adolescence (LeMoult et al., 2017). If so, the negatively-valenced words may have activated diffuse mental representations for negative words, which interfered with accuracy during recognition. Our study design does not allow us to disentangle whether cortisol reactivity may have influenced memory for negatively-valenced words directly or indirectly through self-referential biases, rumination, or negative affect during the encoding trials. Importantly, glucocorticoids can induce depressed mood and ruminative tendencies observed in depressed individuals (Lupien et al., 2009), which may be one pathway through which stress leads to depression (Hamlat et al., 2015). This warrants further investigation as a behavioral intervention target given that the vast majority of depressive episodes occur in the wake of a major life stressor (Hammen, 2015).
The results of this study should be viewed within the context of its limitations. Only cortisol was assayed from our saliva samples. Stress activates a number of physiological processes (e.g., autonomic nervous system, immune system), which may have played a role in affective attention and memory in this sample. Indeed, ELS has recently been linked to greater behavioral sensitivity to inflammation (Kuhlman et al., 2020). Additionally, HPA axis functioning and reactivity is robustly modulated by gonadal hormones such as estrogens and testosterone (Heck & Handa, 2019). Whether the role of gonadal hormones in memory during acute stress is direct or indirect through effects on the HPA axis is important to determine, given that estradiol has been directly linked to memory and specifically cognitive functioning after chronic stress (e.g., Luine, 2016). The memory task in this study does not inform whether cortisol reactivity interfered with encoding or retrieval of negatively-valenced words. Cortisol can have different effects depending on the timing of cortisol increases in relation to encoding versus retrieval (Het et al., 2005). Separation of the processes is needed given that individuals may benefit more from a memory system that favors encoding over retrieval to facilitate future avoidance and active coping. Finally, ELS in this sample was assessed via retrospective, parent-report of their child’s exposure, and dichotomized based upon exposure to 4 or more stressful events. While the rates of exposure in our sample were comparable to nationally representative samples, it is likely that some forms of ELS such as those perpetrated on children by their parents were under-reported in this sample. Furthermore, this approach gives equal weight to all stressors. Attention to whether it is cumulative life stress or specific types of ELS that foster this sensitization is needed. Furthermore, the distribution of ELS in our sample precluded us from testing ELS as a continuous moderator. Corroboration of this finding in a sample recruited with more even distribution of ELS is needed.
The results of this study offer preliminary insight into the complex interplay of acute stress, a neuroendocrine biomarker, and a primary cognitive process during a vulnerable phase of development. Specifically, acute increases in cortisol following stress alter specific domains of cognition (i.e., memory for affective words) and youth with a history of ELS may be more sensitive to these effects. While individuals exposed to high ELS only represent a small percent of the population (Anda et al., 2006; Dube et al., 2003; Kessler et al., 2010), they are disproportionately represented among individuals with psychopathology (Kessler et al., 2010), and importantly, empirically supported treatments for depression are less effective for individuals with high ELS (Lewis et al., 2010; Nanni et al., 2012; The TADS Team, 2007). Thus, a better understanding of the short- and long-term biobehavioral correlates of ELS may lead to a more nuanced understanding of the pathways of risk through which ELS leads to stress-related disorders.
ACKNOWLEDGEMENTS
This research would not be possible without the team at MichiganPAL (http://www.michiganpal.org/), the families who generously gave their time in order to improve our understanding of the underpinnings of anxiety and depression, and the following organizations for their financial support of this research: Blue Cross Blue Shield of Michigan Foundation, Barbara A. Oleshansky Memorial Award, American Psychological Foundation, and Rackham Graduate School at University of Michigan. The composition of this manuscript was made possible by the National Institute of Mental Health via a Mentored Clinical Scientist Research Career Development Award granted to Dr. Kuhlman (K08MH112773), as well as the National Institute of Aging via a Career Development Award granted to Dr. Mayer (K99AG062778).
Footnotes
CONFLICT OF INTEREST
Drs. Kuhlman, Mayer, Vargas, and Lopez-Duran have no potential conflicts of interest to report.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Anda R, Felitti V, Bremner J, Walker J, Whitfield CH, Perry B, Dube SH, & Giles W (2006). The enduring effects of abuse and related adverse experiences in childhood. European Archives of Psychiatry and Clinical Neuroscience, 256(3), 174–186. 10.1007/s00406-005-0624-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT (1967). Depression: Clinical, experimental, and theoretical aspects. Hoeber Medical Division, Harper & Row. [Google Scholar]
- Beck AT (1987). Cognitive models of depression. Journal of Cognitive Psychotherapy, 1, 5–37. [Google Scholar]
- Bremner JD, Vermetten E, & Mazure CM (2000). Development and preliminary psychometric properties of an instrument for the measurement of childhood trauma: The Early Trauma Inventory. Depression and Anxiety, 12(1), 1–12. 10.1002/1520-6394(2000)12 [DOI] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Jobst S, Wustmans A, Kirschbaum C, Rauh W, & Hellhammer D (1997). Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosomatic Medicine, 59(4), 419–426. 10.1097/00006842-199707000-00012 [DOI] [PubMed] [Google Scholar]
- Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, & Anda RF (2004). Adverse childhood experiences and the risk of depressive disorders in adulthood. Journal of Affective Disorders, 82(2), 217–225. 10.1016/j.jad.2003.12.013 [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Joëls M, & Holsboer F (2005). Stress and the brain: From adaptation to disease. Nature Reviews Neuroscience, 6(6), 463–475. 10.1038/nrn1683 [DOI] [PubMed] [Google Scholar]
- Dickerson SS, & Kemeny ME (2004). Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin, 130(3), 355–391. 10.1037/0033-2909.130.3.355 [DOI] [PubMed] [Google Scholar]
- Dube SR, Felitti VJ, Dong M, Giles WH, & Anda RF (2003). The impact of adverse childhood experiences on health problems: Evidence from four birth cohorts dating back to 1900. Preventive Medicine, 37(3), 268–277. 10.1016/S0091-7435(03)00123-3 [DOI] [PubMed] [Google Scholar]
- Ellenbogen MA, Schwartzman AE, Stewart J, & Walker C-D (2002). Stress and selective attention: The interplay of mood, cortisol levels, and emotional information processing. Psychophysiology, 39(6), 723–732. 10.1111/1469-8986.3960723 [DOI] [PubMed] [Google Scholar]
- Erickson K, Drevets W, & Schulkin J (2003). Glucocorticoid regulation of diverse cognitive functions in normal and pathological emotional states. Neuroscience & Biobehavioral Reviews, 27(3), 233–246. 10.1016/S0149-7634(03)00033-2 [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, & Marks JS (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) Study. American Journal of Preventive Medicine, 14(4), 245–258. 10.1016/S0749-3797(98)00017-8 [DOI] [PubMed] [Google Scholar]
- Fuhrmann D, Knoll LJ, & Blakemore SJ (2015). Adolescence as a sensitive period of brain development. Trends in Cognitive Sciences, 19(10), 558. 10.1016/j.tics.2015.07.008 [DOI] [PubMed] [Google Scholar]
- Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, & Kessler RC (2010). Childhood adversities and adult psychiatric disorders in the National Comorbidity Survey Replication I: Associations with first onset of DSM-IV disorders. Archives of General Psychiatry, 67(2), 113–123. 10.1001/archgenpsychiatry.2009.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlat EJ, Connolly SL, Hamilton JL, Stange JP, Abramson LY, & Alloy LB (2015). Rumination and overgeneral autobiographical memory in adolescents: An integration of cognitive vulnerabilities to depression. Journal of Youth and Adolescence, 44(4), 806–818. 10.1007/s10964-014-0090-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen CL (2015). Stress and depression: Old questions, new approaches. Current Opinion in Psychology, 4, 80–85. 10.1016/j.copsyc.2014.12.024 [DOI] [Google Scholar]
- Hayes AF (2013). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Guilford Press. [Google Scholar]
- Heck AL, & Handa RJ (2019). Sex differences in the hypothalamic–pituitary–adrenal axis’ response to stress: An important role for gonadal hormones. Neuropsychopharmacology, 44(1), 45–58. 10.1038/s41386-018-0167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, & Figueiredo H (2005). Limbic system mechanisms of stress regulation: Hypothalamo-pituitary-adrenocortical axis. Progress in and Biological Psychiatry, 29(8), 1201–1213. 10.1016/j.pnpbp.2005.08.006 [DOI] [PubMed] [Google Scholar]
- Het S, Ramlow G, & Wolf OT (2005). A meta-analytic review of the effects of acute cortisol administration on human memory. Psychoneuroendocrinology, 30(8), 771–784. 10.1016/j.psyneuen.2005.03.005 [DOI] [PubMed] [Google Scholar]
- John CH (1988). Emotionality ratings and free-association norms of 240 emotional and non-emotional words. Cognition & Emotion, 2(1), 49–70. [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, & Walters EE (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62(6), 593. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, Aguilar-Gaxiola S, Alhamzawi AO, Alonso J, Angermeyer M, Benjet C, Bromet E, Chatterji S, de Girolamo G, Demyttenaere K, Fayyad J, Florescu S, Gal G, Gureje O, … Williams DR (2010). Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. The British Journal of Psychiatry, 197(5), 378–385. 10.1192/bjp.bp.110.080499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, & Diamond DM (2002). The stressed hippocampus, synaptic plasticity and lost memories. Nature Reviews Neuroscience, 3(6), 453–462. 10.1038/nrn849 [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, De Jong IC, Ruis MAW, & Blokhuis HJ (1999). Coping styles in animals: Current status in behavior and stress-physiology. Neuroscience & Biobehavioral Reviews, 23(7), 925–935. 10.1016/S0149-7634(99)00026-3 [DOI] [PubMed] [Google Scholar]
- Kuhlman KR, Geiss EG, Vargas I, & Lopez-Duran NL (2015). Differential associations between childhood trauma subtypes and adolescent HPA-axis functioning. Psychoneuroendocrinology, 54, 103–114. 10.1016/j.psyneuen.2015.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman KR, Robles TF, Haydon MD, Dooley LN, Boyle CC, & Bower JE (2020). Early life stress sensitizes individuals to the psychological correlates of mild fluctuations in inflammation. Developmental Psychobiology, 62(3), 400–408. 10.1002/dev.21908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman KR, Vargas I, Geiss EG, & Lopez-Duran NL (2015). Age of trauma onset and HPA Axis dysregulation among trauma-exposed youth. Journal of Traumatic Stress, 28(6). 10.1002/jts.22054/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann S, Kirschbaum C, & Wolf OT (2005). Effects of oral cortisol treatment in healthy young women on memory retrieval of negative and neutral words. Neurobiology of Learning and Memory, 83(2), 158–162. 10.1016/j.nlm.2004.09.001 [DOI] [PubMed] [Google Scholar]
- LeMoult J, Kircanski K, Prasad G, & Gotlib IH (2017). Negative self-referential processing predicts the recurrence of major depressive episodes. Clinical Psychological Science, 5(1), 174–181. 10.1177/2167702616654898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CC, Simons AD, Nguyen LJ, Murakami JL, Reid MW, Silva SG, & March JS (2010). Impact of childhood trauma on treatment outcome in the treatment for adolescents with depression study (TADS). Journal of the American Academy of Child & Adolescent Psychiatry, 49(2), 132–140. 10.1016/j.jaac.2009.10.007 [DOI] [PubMed] [Google Scholar]
- Lewis EJ, Yoon KL, & Joormann J (2017). Emotion regulation and biological stress responding: Associations with worry, rumination, and reappraisal. Cognition & Emotion, 32(7), 1487–1498. 10.1080/02699931.2017.1310088 [DOI] [PubMed] [Google Scholar]
- Lopez-Duran NL, McGinnis EG, Kuhlman KR, Geiss EG, Vargas I, & Mayer SE (2015). HPA-axis stress reactivity in youth depression: Evidence of impaired regulatory processes in depressed boys. Stress, 18(5), 545–553. 10.3109/10253890.2015.1053455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V (2016). Estradiol: Mediator of memories, spine density and cognitive resilience to stress in female rodents. The Journal of Steroid Biochemistry and Molecular Biology, 160, 189–195. 10.1016/j.jsbmb.2015.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, & Heim C (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience, 10(6), 434–445. 10.1038/nrn2639 [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, & Kessler RC (2010). Childhood adversities and adult psychiatric disorders in the National Comorbidity Survey Replication II: Associations with persistence of DSM-IV disorders. Archives of General Psychiatry, 67(2), 124–132. 10.1001/archgenpsychiatry.2009.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanni V, Uher R, & Danese A (2012). Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: A meta-analysis. American Journal of Psychiatry, 169(2), 141–151. 10.1176/appi.ajp.2011.11020335 [DOI] [PubMed] [Google Scholar]
- Pechtel P, & Pizzagalli DA (2010). Effects of early life stress on cognitive and affective function: An integrated review of human literature. Psychopharmacology, 214(1), 55–70. 10.1007/s00213-010-2009-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak SD, Cicchetti D, Hornung K, & Reed A (2000). Recognizing emotion in faces: Developmental effects of child abuse and neglect. Developmental Psychology, 36(5), 679–688. 10.1037/0012-1649.36.5.679 [DOI] [PubMed] [Google Scholar]
- Quesada AA, Wiemers US, Schoofs D, & Wolf OT (2012). Psychosocial stress exposure impairs memory retrieval in children. Psychoneuroendocrinology, 37(1), 125–136. 10.1016/j.psyneuen.2011.05.013 [DOI] [PubMed] [Google Scholar]
- Roozendaal B (2002). Stress and memory: Opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiology of Learning and Memory, 78(3), 578–595. 10.1006/nlme.2002.4080 [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, & Chattarji S (2009). Stress, memory and the amygdala. Nature Reviews Neuroscience, 10(6), 423. [DOI] [PubMed] [Google Scholar]
- Salovey P, Stroud LR, Woolery A, & Epel ES (2002). Perceived emotional intelligence, stress reactivity, and symptom reports: Further explorations using the Trait Meta-Mood Scale. Psychology & Health, 17(5), 611–627. 10.1080/08870440290025812 [DOI] [Google Scholar]
- Schwabe L, Haddad L, & Schachinger H (2008). HPA axis activation by a socially evaluated cold-pressor test. Psychoneuroendocrinology, 33(6), 890–895. 10.1016/j.psyneuen.2008.03.001 [DOI] [PubMed] [Google Scholar]
- Schwabe L, & Schächinger H (2018). Ten years of research with the Socially Evaluated Cold Pressor Test: Data from the past and guidelines for the future. Psychoneuroendocrinology, 92, 155. [DOI] [PubMed] [Google Scholar]
- Sladek MR, Doane LD, Luecken LJ, & Eisenberg N (2016). Perceived stress, coping, and cortisol reactivity in daily life: A study of adolescents during the first year of college. Biological Psychology, 117, 8–15. 10.1016/j.biopsycho.2016.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starita F, Kroes MCW, Davachi L, Phelps EA, & Dunsmoor JE (2019). Threat learning promotes generalization of episodic memory. Journal of Experimental Psychology: General, 148(8), 1426–1434. 10.1037/xge0000551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner JA, Griffith JW, & Mineka S (2010). Overgeneral autobiographical memory as a predictor of the course of depression: A meta-analysis. Behaviour Research and Therapy, 48(7), 614–625. 10.1016/j.brat.2010.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner JA, Griffith JW, Mineka S, Rekart KN, Zinbarg RE, & Craske MG (2011). Overgeneral autobiographical memory and chronic interpersonal stress as predictors of the course of depression in adolescents. Cognition and Emotion, 25(1), 183–192. 10.1080/02699931003741566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner JA, Mineka S, & McAdams DP (2013). Specificity in autobiographical memory narratives correlates with performance on the Autobiographical Memory Test and prospectively predicts depressive symptoms. Memory, 21(6), 646–656. 10.1080/09658211.2012.746372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumter SR, Bokhorst CL, Miers AC, Van Pelt J, & Westenberg PM (2010). Age and puberty differences in stress responses during a public speaking task: Do adolescents grow more sensitive to social evaluation? Psychoneuroendocrinology, 35(10), 1510–1516. 10.1016/j.psyneuen.2010.05.004 [DOI] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, Anderson CM, & Ohashi K (2016). The effects of childhood maltreatment on brain structure, function and connectivity. Nature Reviews Neuroscience, 17(10), 652–666. 10.1038/nrn.2016.111 [DOI] [PubMed] [Google Scholar]
- The TADS Team. (2007). The Treatment for Adolescents with Depression Study (TADS): Long-term effectiveness and safety outcomes. Archives of General Psychiatry, 64(10), 1132–1143. 10.1001/archpsyc.64.10.1132 [DOI] [PubMed] [Google Scholar]
- Wolf OT (2009). Stress and memory in humans: Twelve years of progress? Brain Research, 1293, 142–154. [DOI] [PubMed] [Google Scholar]


