Abstract
BACKGROUND:
Benign paroxysmal torticollis (BPT) is characterized by attacks of head tilt associated with vomiting, irritability, and/or ataxia in early childhood. BPT is associated with migraine but risk factors are unknown. Impact on quality of life is also unknown.
METHODS:
Parents/caregivers of children with ongoing or resolved BPT participated in telephone interviews (n = 73). Those with ongoing BPT completed the Infant Toddler Quality of Life questionnaire (ITQoL).
RESULTS:
Median age of children at the time of interview was 2.9 years (range 0.25–23). BPT was ongoing in 52% (n = 38). Nineteen percent (n = 14) developed migraine (median age 9.25 years, range 2.5–23) and 63% (n = 46) developed another episodic syndrome associated with migraine. Proportion of patients who developed migraine was higher among those with certain migrainous symptoms during BPT attacks vs. those without: phonophobia (58 vs. 21%, p = 0.02), photophobia and phonophobia (55 vs. 23%, p = 0.05), and photophobia, phonophobia, and motion sensitivity (60 vs. 22%, p = 0.02). ITQoL results showed significant impact of BPT on quality of life.
CONCLUSIONS:
Children with BPT may develop migraine or other episodic syndromes associated with migraine. Presence of migrainous features during BPT episodes may increase likelihood of developing migraine. Though characterized as “benign,” BPT can significantly impact children and families.
INTRODUCTION
Benign paroxysmal torticollis (BPT) is a rare and underdiagnosed disorder of early childhood characterized by recurrent episodes of head tilt associated with malaise, pallor, irritability, ataxia, and nausea/vomiting.1–3 BPT is considered one of the “episodic syndromes that may be associated with migraine” (hereafter referred to as “episodic syndromes”) in the International Classification of Headache Disorders, Third Edition (ICHD-3)3 and may represent a form of dystonia.4 Current understanding of the clinical phenotype of BPT is based largely on case reports and case series.5 Treatment is also based on case reports, with one small series suggesting topiramate to be helpful in preventing episodes.6 Children with BPT often have a family history of migraine,5–8 and mutations in genes associated with familial hemiplegic migraine including CACNA1A,7,9–13 PRRT2,14 and ATP1A27 have been identified in families with BPT. Some children with BPT go on to develop migraine and/or other episodic syndromes later in childhood.15 Risk factors for developing these other conditions are unknown. The extent of developmental impact of BPT is also debated.5,7,12
The goals of this study were to characterize the phenotype of BPT in a larger population; identify which treatments are utilized by families with BPT; evaluate the association of BPT with migraine and other episodic syndromes; and understand the impact of BPT on children and families.
METHODS
Study design and participants
Parents/caregivers of children with BPT or history of BPT were recruited to participate in an approximately 30-min semi-structured phone interview with a pediatric headache specialist (K.A.G., W.Q., or A.A.G.) from the University of California, San Francisco (UCSF) Child and Adolescent Headache Program.
Permissions
The UCSF IRB approved this observational cohort study. All subjects provided written informed consent. Children aged 7–17 years provided assent, and those who had reached the age of 18 years provided consent for their parent/caregiver to be interviewed about their history of BPT. Families provided consent for use of photographs/videos for publication.
Recruitment and screening
Recruitment methods included social media advertisements on Twitter and Facebook pages for BPT support groups, UCSF pediatric headache clinic providers recruiting their own patients, word-of-mouth from the UCSF general pediatric neurology clinic, and search of the UCSF electronic medical records for a diagnosis of “benign paroxysmal torticollis.” A recruitment flyer was mailed to patients identified through medical record search.
Potential participants were screened using an online questionnaire based on key features of the ICHD-33 diagnostic criteria for BPT. Subjects met inclusion criteria if their child: (1) experienced episodes of head tilt (i.e., not persistent torticollis) and (2) during attacks experienced at least one of: pallor, irritability, malaise, vomiting, or ataxia. Subjects were considered to have “ongoing BPT” if the child had had an episode of head tilt within the past year; otherwise they were considered to have “resolved BPT.”
Data collection
Subjects meeting inclusion were scheduled for a phone interview. Interviewers utilized a standardized data-collection form in REDCap (Research Electronic Data Capture).16
Interviewers first ascertained whether the child met key criteria for BPT based on the ICHD-3,3 including experiencing recurrent attacks of head tilt in a young child, with or without slight rotation, remitting spontaneously after minutes to days; subjects were excluded if the child did not meet these criteria. The ICHD-3 criteria additionally states that the child should have a normal neurological exam, and other diagnoses have been excluded. Due to the nature of the phone interview, it was not possible to perform a neurological exam.3 Exclusion of other diagnoses as specified in the ICHD-33 was necessarily based on parental/caregiver report of the child’s medical evaluation and test results; if the child had not had a formal medical evaluation, exclusion of other diagnoses was at the interviewer’s discretion based on clinical features and comorbidities.
Interview questions additionally addressed demographics, clinical phenotype of BPT, family history, developmental history, medical history, development of other episodic syndromes that may be associated with migraine and/or migraine, and treatment trials and outcomes. Parents/caregivers were asked about any medical evaluations, diagnostic testing, and results; these data were based on parental report as medical record review was beyond the scope of this study. Frequency of episodes was defined as the interval from the start of one head tilt episode to the start of the next. Duration of episodes was defined as time from the start of head tilt until resolution of head tilt, regardless of duration of associated symptoms.
Parents were asked whether the child had developed migraine and/or episodic syndromes, including infantile colic, benign paroxysmal vertigo (BPV), cyclic vomiting syndrome, and abdominal migraine.3 If the patient did not have a specific ICHD-3 diagnosis from a medical provider and/or episodes were atypical, these were considered “other episodic syndrome not otherwise specified (NOS).” The child was considered to have migraine if they had been diagnosed with migraine by a clinician or if the parent reported the child had migraine or headaches associated with nausea/vomiting or photophobia and phonophobia.
Parents were asked whether they had ever given their child acute or preventive treatments for BPT and whether they perceived each treatment to be effective. Medications were considered “helpful” if families reported definite benefit for BPT episodes, “possibly helpful” if families expressed uncertainty of benefit or were unable to recall benefit, and “unhelpful” if families reported that medication was definitively unhelpful for BPT episodes.
Subjects were queried regarding the presence or absence of the symptoms listed within the diagnostic criteria for BPT, i.e., pallor, fussing/irritability, vomiting, and ataxia; however “malaise” was considered difficult to operationalize. Subjects were also asked about truncal or limb dystonia during attacks. Given the association of BPT with migraine, they were also asked about phonophobia, phonophobia, and movement sensitivity and decreased oral intake as a possible indicator of nausea. They were asked whether there were any other symptoms their child experienced during attacks and which symptom the parent perceived to be the “most bothersome symptom,” an increasingly important treatment outcome in migraine research.17
Parents of children with ongoing BPT were asked to complete the Infant Toddler Quality of Life questionnaire (ITQoL-SF47),18 a validated instrument for measuring the impact of health conditions on quality of life in children ages 2 months–5 years and their families.
Data analysis
Descriptive statistics were used to describe the clinical phenotype of BPT, diagnostic testing, medication use, and concern for developmental delay. In reporting family history among sibling pairs, the older sibling was used as the index case. As a post hoc exploratory analysis, we compared proportion (Chi-square test) who developed migraine if migrainous features were present during BPT attacks; this analysis was restricted to children ≥29 months of age as this was the earliest age when migraine was reported. The ITQoL-SF47 questionnaire was scored according to proprietary scoring rules and results from the BPT cohort were compared to normative data.19,20 Analyses were performed using Excel 2016 and SAS 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Subjects
Seventy-one parents of 74 individuals with BPT were interviewed (Fig. 1). One was excluded prior to analysis as the child had a continuous head tilt in the setting of a congenital fourth cranial nerve palsy. The remaining 73 children, from 70 families, were included in the analysis. There were three sibling pairs, including one set of identical twins. Participants lived in 12 countries on 4 continents. Ninety-two percent (n = 67) were recruited from Facebook and the other 6 from within the UCSF pediatric neurology program. Thirty-eight (52%) had ongoing BPT; of these, 35 (92%) completed the ITQoL questionnaire. Ninety-two percent of subjects (67/73) had received a formal diagnosis of BPT from a medical provider; of these, 64% (43/67) had been diagnosed by a neurologist, 22% (15/37) had been diagnosed by a general pediatrician, and 13% (9/67) had been diagnosed by another type of provider. Parents of 56 subjects (77%) reported that their child had additional medical issues (see Supplemental Table S1 (online)).
Fig. 1. CONSORT diagram.
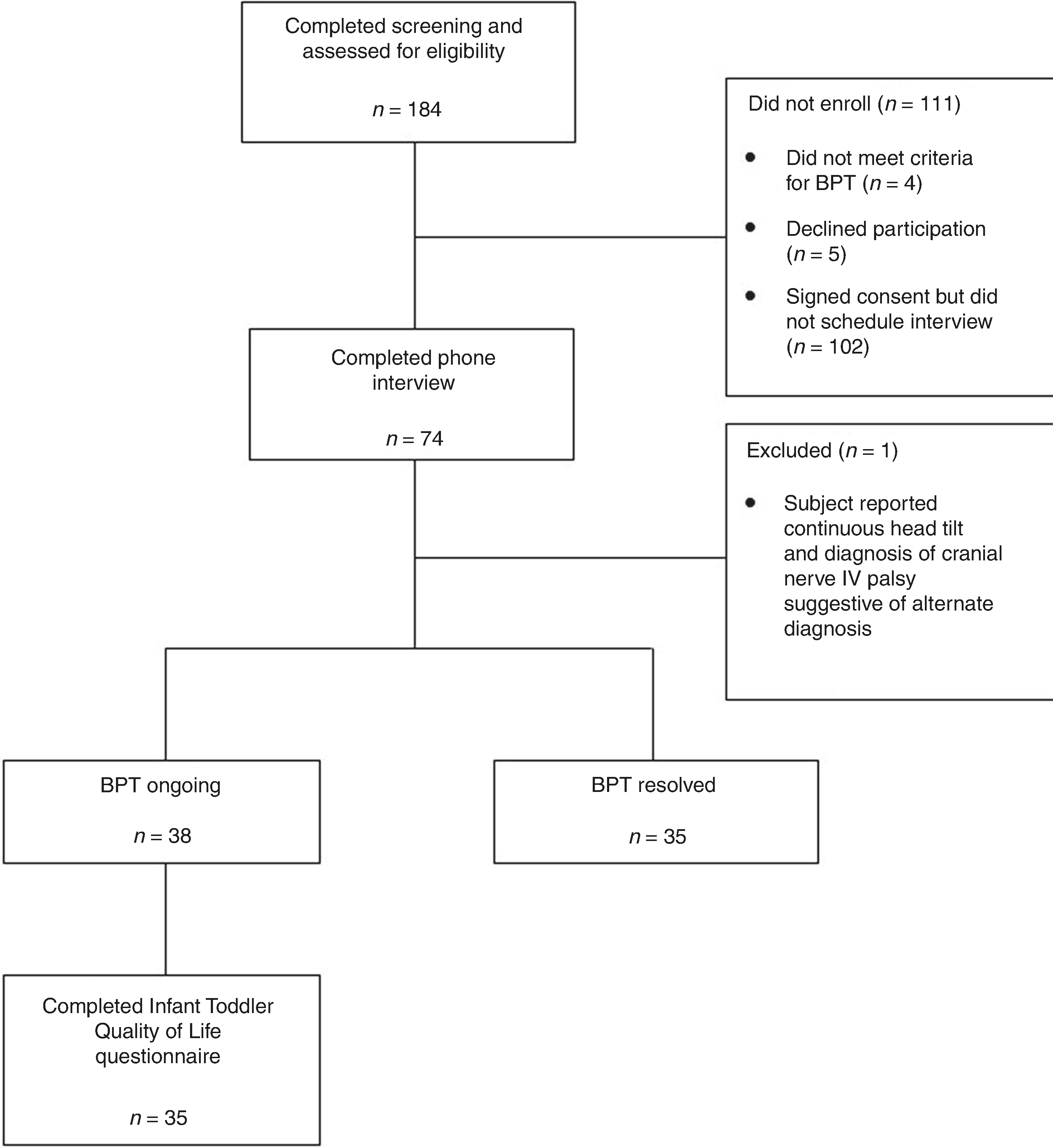
Flow diagram of patients who were screened, enrolled and completed phone interview, and among those with ongoing BPT, who completed the ITQoL survey.
Clinical phenotype
The clinical characteristics of the n = 73 individuals with BPT are summarized in Table 1. Appearance of head tilt during BPT episodes at different ages is shown in Fig. 2. A video of an infant during a BPT episode demonstrating head tilt and associated irritability is provided in the supplemental materials.
Table 1.
Clinical characteristics of n = 73 children with benign paroxysmal torticollis (BPT).
| n = 73 | |
|---|---|
|
| |
| % With ongoing BPT | 52% |
| % Male | 34% |
| Current age [years; median (range)] | 2.9 (0.3–23) |
| BPT ongoing | 2.0 (0.3–4.5) |
| BPT resolved | 5.0 (4.5–23) |
| Age at onset of BPT [months; median (range)] | 3.5 (0.1–20) |
| % Diagnosed by medical provider | 92% (n = 67) |
| Neurologist | 66% |
| Pediatrician | 21% |
| Other | 13% |
| Age at diagnosis of BPT [months; median (range)] | 12 (2–48) |
| Time to diagnosis [months; median (range)] | 7 (1–45) |
| Age at resolution of BPT (n = 35) [years; median (range)] | 2.5 (0.5–13) |
| Duration of episodes for each patient [median (range)] | |
| “Typical” | 6 days (1 min-28 days) |
| Shortest | 2 days (1 min-10 days) |
| Longest | 9 days (2 min-42 days) |
| Frequency of episodes at onset (every “x” weeks; median (range)] | 3 (0.5–12) |
| Frequency of episodes at offset (every “x” weeks; median (range)] | 6.0 (0.5–208) |
Fig. 2. Photo of a study participant at 4 months of age with left head tilt (left) and same participant around 10 months of age with right head tilt (right).

Family provided consent for use of photograph for publication.
The most commonly reported associated symptoms during attacks are shown in Fig. 3. Additional symptoms reported by more than one family included abnormal eye movement (n = 15), sleepiness or sleeping more than usual (n = 8), change in eye appearance (redness, swelling, glazed, or sunken; n = 7), abnormal head or body movements (n = 4), clinginess (n = 5), diaphoresis (n = 3), dizziness (n = 3), altered responsiveness (n = 6), and decreased muscle tone (n = 2).
Fig. 3. Associated symptoms.
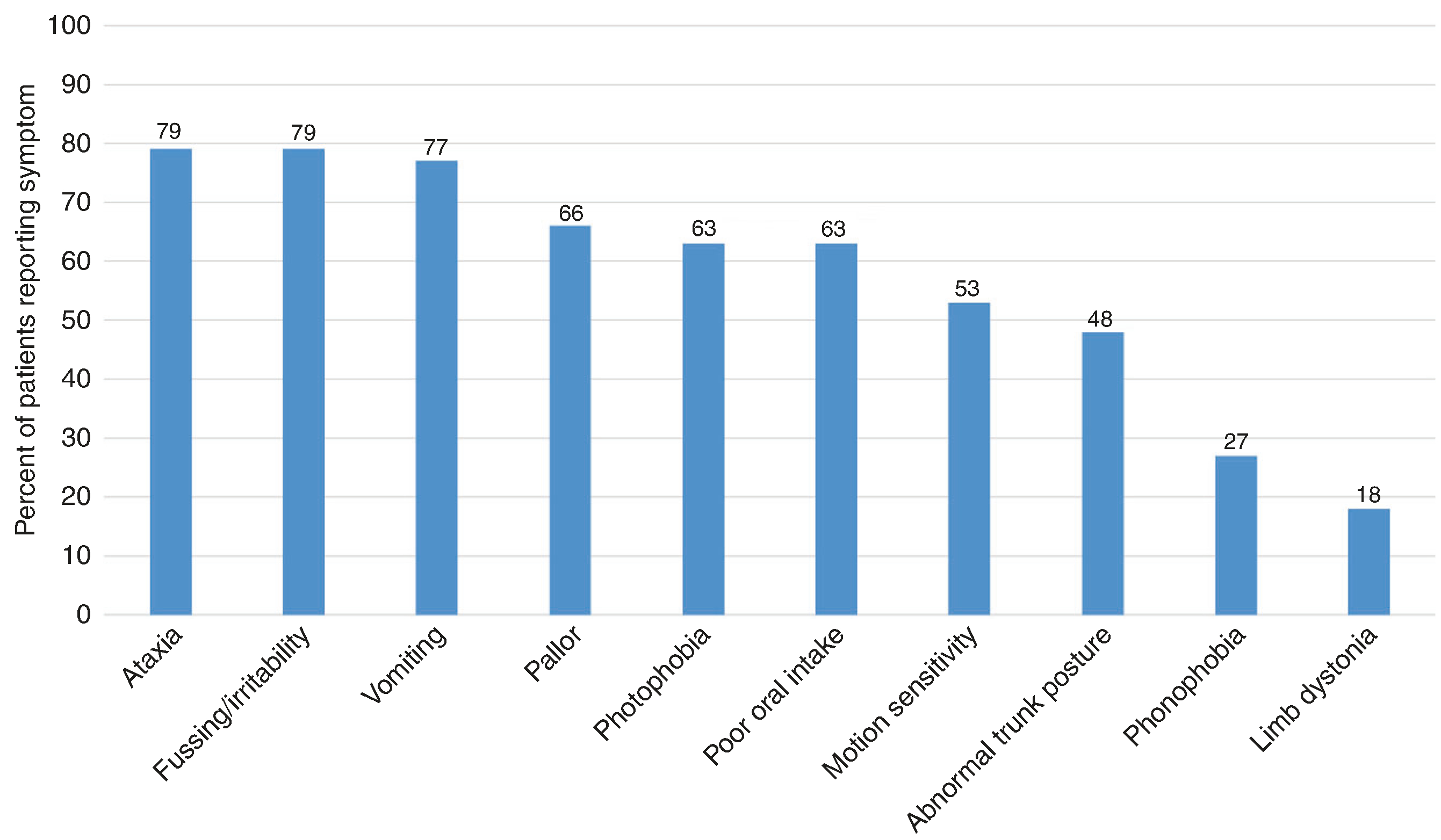
Associated symptoms occurring during benign paroxysmal torticollis (BPT) attacks.
The most bothersome symptom during an attack was vomiting in 26% (n = 19), followed by ataxia (n = 13, 18%), fussiness/ irritability (n = 10, 14%), discomfort (n = 9, 12%), head tilt itself (n = 8, 11%), abnormal trunk posture (“C-shape” or “banana shape”; n = 7, 10%), dizziness (n = 4, 5%), sleepiness or lethargy (n = 3, 4%), impact on motor skills or development (n = 2, 3%), decreased oral intake (n = 2, 3%), or motion sensitivity (n = 1, 2%).
Diagnostic testing
Diagnostic evaluations and testing results as reported by parents are shown in Supplemental Table S2. Of the 40 patients who had brain magnetic resonance imaging (MRI), 6 (15%) had reportedly abnormal results; in each case, the parents/caregivers reported that the imaging findings were felt not to be the cause of BPT by the diagnosing provider. Among those who had genetic testing (n = 8), 2 specifically reported testing for the CACNA1A mutation and both were negative.
Developmental impact
Fifty-two families (71%) reported concerns about development. The most common area of concern was gross motor delay (n = 50, 68%), followed by language (n = 13, 18%), fine motor (n = 8, 11%), and social (n = 2, 3%). Among those with gross motor concerns, 43 (86%) reported when their child first walked: the mean was 17.4 months (range 11–25.5 months), with 20 of the 43 (46%) walking before 18 months. Eighteen of the parents with developmental concerns (36%) spontaneously commented that their child eventually “caught up” with milestones.
Treatment
Forty-seven (64%) had used medication for acute treatment of BPT episodes. The most commonly used acute medications were ibuprofen (n = 30, 41%), acetaminophen (n = 30, 41%), ondansetron (n = 9, 12%), and diphenhydramine (n = 7, 10%). Additional acute treatments included caffeine (n = 3; two being siblings), cyproheptadine (n = 2), prochlorperazine (n = 2), metoclopramide (n = 1), lorazepam (n = 1), clonidine (n = 1), cannabidiol injections (n = 1), and a non-specified anti-emetic (n = 1). The perceived efficacy of these treatments is shown in Fig. 4.
Fig. 4. Perceived efficacy of most commonly used acute medications.
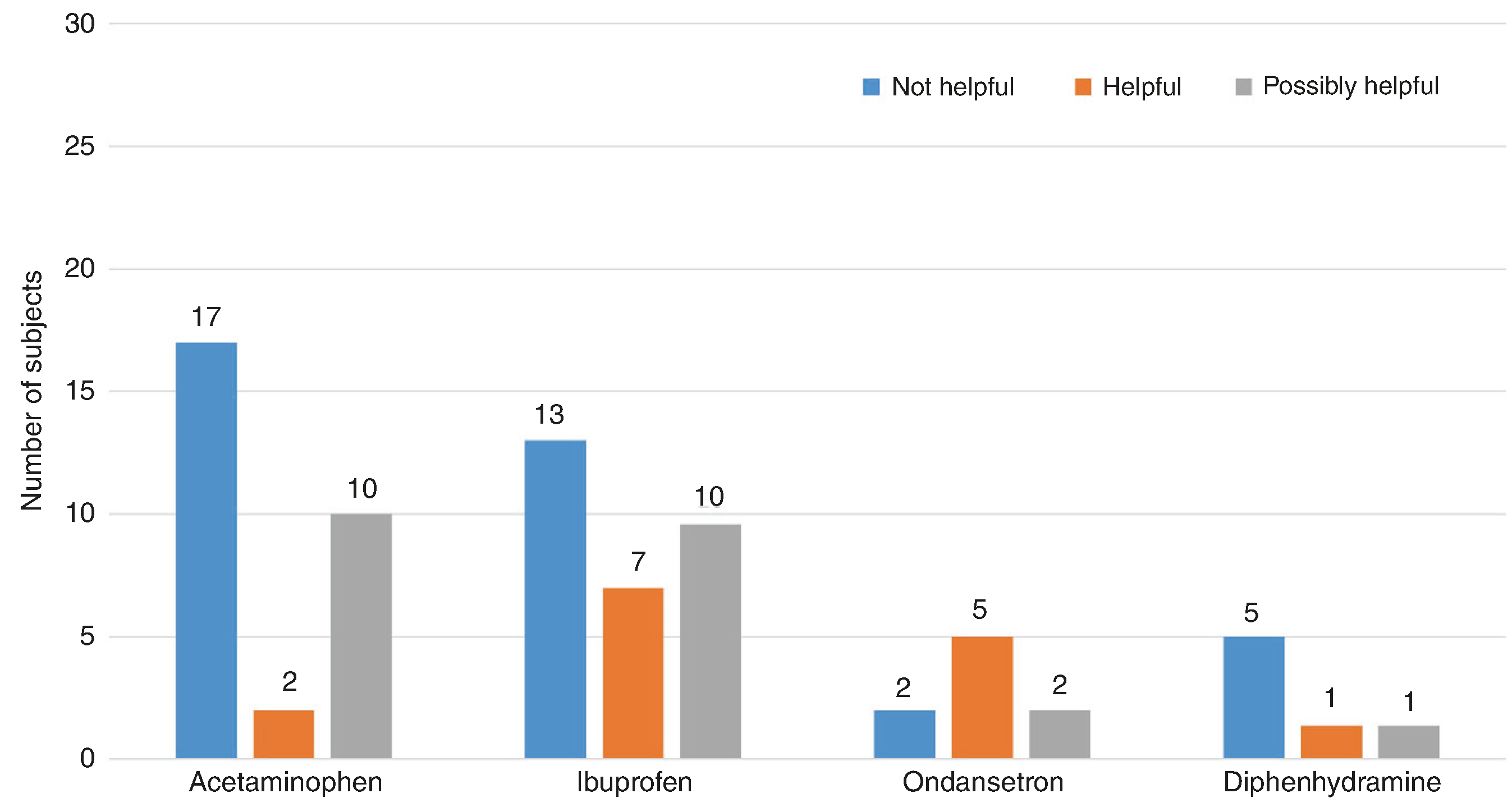
“Helpful” indicates definite benefit for benign paroxysmal torticollis (BPT) episodes, “Possibly helpful” indicates uncertain benefit or inability to recall benefit, and “Not helpful” indicates definitive lack of benefit for BPT episodes as reported by families.
Sixteen parents (22%) reported use of non-pharmacologic acute interventions; those reported by more than one family included maintaining a dark, quiet environment (n = 16), stretching (n = 7), holding the child (n = 7), encouraging sleep (n = 7), being still (n = 5), massage (n = 4), hydration (n = 2), and breastfeeding (n = 2).
Twelve parents (16%) reported using preventive medication. Seven children took cyproheptadine (n = 7). Among these, 3 reported benefit (duration of treatment: 3 months, 6–8 months and not reported), 2 did not take it long enough to determine benefit (duration of treatment: 4 days and “not long enough” per parent), 2 found it definitively unhelpful (duration of treatment: 3 months in both cases); 3 families reported problematic side effects. Two children took topiramate; one found it unhelpful without side effects (duration of treatment: 3 months) and the other reported potential benefit but problematic side effects (duration of treatment not reported). Two children took acetazolamide; one found it very helpful and did not report side effects (duration of treatment not reported), and the other had started it the day prior to the interview. One child took diphenhydramine nightly and one took riboflavin daily, each for an unspecified duration; neither treatment was perceived as helpful and side effects were not reported.
Non-pharmacologic preventive measures were taken by 50 parents (68%). Forty-three families (59%) tried physical therapy (PT). Of these, 36 (84%) commented on efficacy and none found PT helpful for BPT. Other non-pharmacologic preventive treatments included dietary changes (n = 8), chiropractic treatment (n = 7), osteopathic treatment (n = 4), acupuncture (n = 3), environmental measure (n = 2), craniosacral therapy (n = 2), occupational therapy (n = 2), and massage (n = 2).
Family history
Sixty-three parents (90%) reported that their child had a family history of migraine and 2 subjects (3%) reported a family history of hemiplegic migraine. Five (7%) had a family history of BPT and an additional 5 (7%) reported possible history of BPT. Thirty-three families (45%) reported history of infantile colic. Cyclic vomiting and abdominal migraine were each reported by one family. Thirteen families (19%) reported history of an unspecified episodic syndrome characterized by episodic abdominal pain, vomiting, and/or vertigo. Personal history of migraine and/or other episodic syndrome Fourteen (19%) reported their child had migraine; the median age of those who reported migraine was 9.3 years (range 2.5–23 years). Forty-six (63%) reported history of another episodic syndrome including infantile colic, BPV, cyclic vomiting syndrome, abdominal migraine, and episodic syndromes NOS. Figure 5 shows the percentage of patients with ongoing or resolved BPT who reported each of these syndromes.
Fig. 5. Percent of subjects who reported having each of the episodic syndromes associated with migraine, migraine, or other episodic syndrome not otherwise specified.
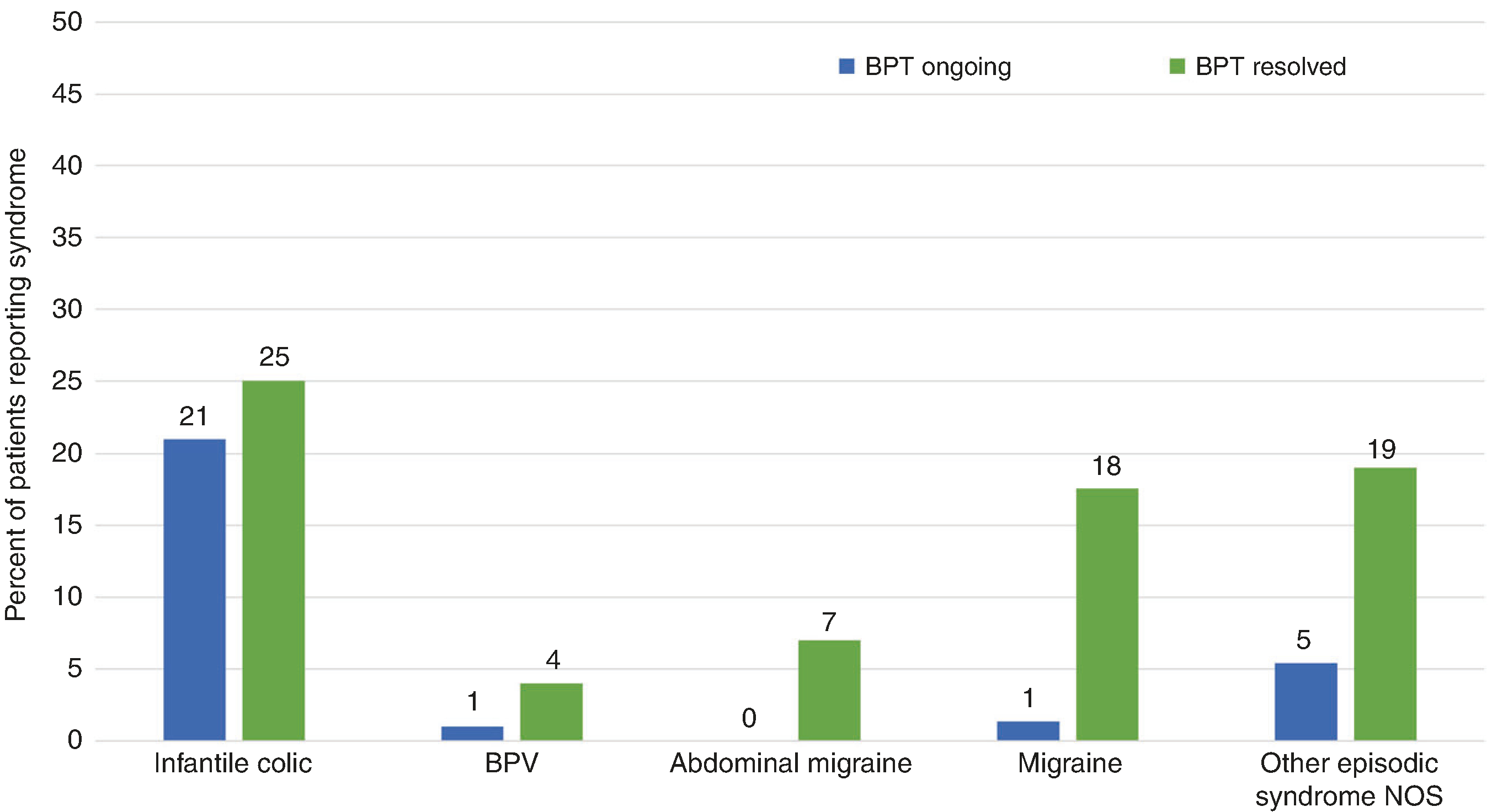
For “BPT ongoing,” median age was 2 years (range 3 months–4.5 years). For “BPT resolved,” median age was 5 years (range 4.5–23 years). No children reported definite history of cyclic vomiting syndrome.
Likelihood of developing migraine based on the presence of migrainous features (i.e., photophobia, phonophobia, vomiting, and/or motion sensitivity) or family history of migraine are shown in Table 2. Proportion of patients who developed migraine was overall higher among those with migrainous symptoms present during BPT attacks; findings reached statistical significance among those with phonophobia (58% with vs. 22% without, p = 0.02), photophobia and phonophobia (55% with vs. 23% without, p = 0.05), and photophobia, phonophobia, and motion sensitivity (60% with vs. 22% without, p = 0.02).
Table 2.
Proportion of patients ≥29 months of age who developed migraine if migrainous features were or were not present during benign paroxysmal torticollis (BPT) episodes.
| Symptom | Proportion of patients who developed migraine |
||
|---|---|---|---|
| Symptom present | Symptom absent | p value (Chi-squared) | |
| Photophobia | 10/28 (36%) | 4/18 (22%) | 0.33 |
| Phonophobia | 7/12 (58%) | 7/34 (21%) | 0.02 |
| Vomiting | 13/36 (36%) | 1/10 (10%) | 0.11 |
| Motion sensitivity | 9/24 (38%) | 5/22 (23%) | 0.28 |
| Photophobia and phonophobia | 6/11 (55%) | 8/35 (23%) | 0.05 |
| Photophobia, phonophobia, and motion sensitivity | 6/10 (60%) | 8/35 (22%) | 0.02 |
| Any migrainous symptom | 13/38 (34%) | 1/8 (13%) | 0.23 |
| Family history of migraine | 14/42 (33%) | 0/4 (0%) | 0.17 |
Analysis was restricted to children ≥29 months of age as this was the earliest age when migraine was reported.
ITQoL questionnaire
Results of the ITQoL are shown in Fig. 6. Compared to the reference population, patients with BPT had significantly lower scores, indicating worse health-related quality of life, in most areas.
Fig. 6. Mean score on each category of the Infant Toddler Quality of Life (ITQoL) for those with ongoing BPT (orange) vs. the ITQoL reference population (blue).20.
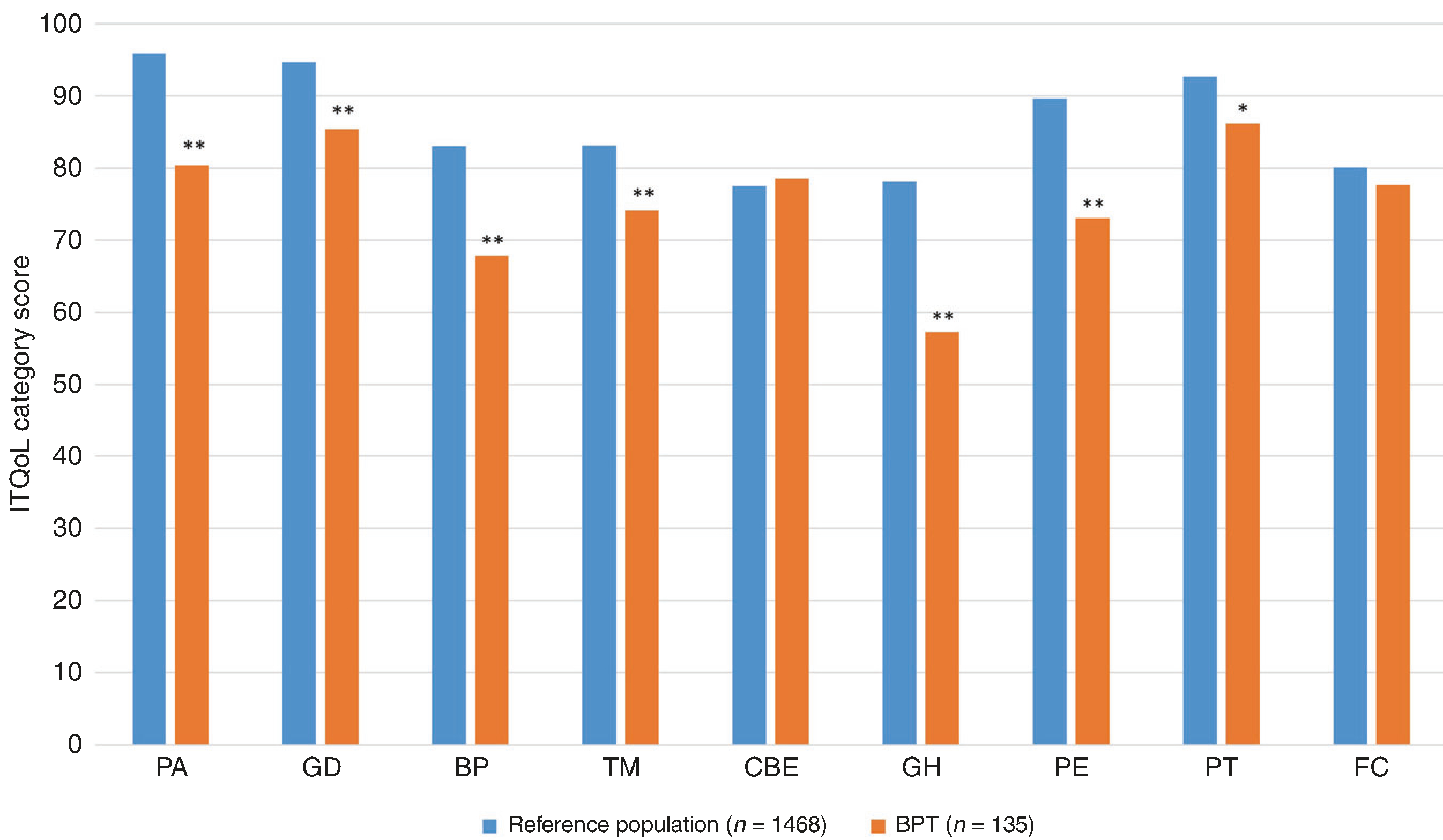
Values for the reference population are reprinted with permission from HealthActCHQ Inc. Lower scores indicate greater impact on quality of life. PA physical abilities, GD satisfaction with growth and development, BP bodily pain, TM temperament and moods, CBE combined behavior, getting along, GH general health perceptions, PE parent impact emotional, PT parent impact time, FC family cohesion. **p < 0.001; *p < 0.05.
DISCUSSION
This study expands the phenotype of BPT in a relatively large cohort. The age of onset of BPT in this study (median 3.5 months, range 2–48 months) is consistent with other studies,5,7,12,15 but the age of offset in our study was later. While prior studies have reported median age at BPT resolution of 18–36 months (range 3.5 months to almost 5 years),5,7 multiple families in our study reported ongoing episodes of head tilt with associated features into later childhood and even adolescence, suggesting that BPT may last longer in some children than previously appreciated.
The proportion of participants experiencing the cardinal “associated features” of BPT as delineated in ICHD-3 (i.e., pallor, irritability, malaise, vomiting, and ataxia) is variable across studies. While one study reported that almost half of the subjects had no associated symptoms during head tilt episodes,7 we found that almost 80% experienced ataxia, irritability, and vomiting while 60% reported pallor, similar to findings from another study.12 Interestingly, while “malaise” is one of the diagnostic criteria for BPT in ICHD-3, few studies including ours have specifically investigated presence of this vague symptom. Additionally, several symptoms that are not included in the ICHD-3 diagnostic criteria appear to be relatively common among children with BPT. These include abnormal posture (i.e., “c-shape” or “banana shape”) of the trunk, reported by 48% of subjects in this study and 24–35% in other series,5,12 and gaze abnormalities, described in early case series8 and reported by 21% in our study.
The likelihood of developing migraine was lower in our cohort (19%) than in prior studies (24–45%),7,12,15 which may reflect the younger age of children with BPT in this study (median of 2.9 years vs. 6.5 to 13.8 years in prior studies), as the prevalence of migraine is relatively low in preschool-aged children and increases throughout childhood and adolescence.21 Nonetheless, given that the U.S. population prevalence of migraine by age 10 years is approximately 5%,21 the frequency of early childhood migraine observed in our study population is higher than what would be expected based on background prevalence alone.
With regards to episodic syndromes that may be associated with migraine, 45% of parents reported that their child was colicky as an infant, which is notably greater than the population estimate for prevalence of colic of 5–19%.22–24 Although there is some evidence that children with BPT may be more likely to go on to develop BPV later in childhood,15 the frequency of BPV was relatively low in this study; this may reflect difficulty ascertaining a history of BPV that was distinct from BPT or migraine, or it may be that some in the category of “other episodic syndrome NOS” may have BPV.
Despite the association between BPT and migraine, prior studies have not systematically reported on presence of “migrainous symptoms,” such as photophobia and phonophobia, during BPT attacks. These sensitivity symptoms were common in our study, with two thirds experiencing vomiting and photophobia and just over half reporting motion sensitivity and phonophobia during BPT attacks. Interestingly, in a post hoc analysis, the proportion of children who developed migraine was significantly higher among those with certain migrainous symptoms. These data are hypothesis generating and should be examined a priori in a larger sample of patients in future settings. In the meantime, asking about these symptoms may help providers to provide families with better anticipatory guidance about their child’s likelihood of developing migraine in early childhood.
Though labeled “benign,” our findings from the ITQoL demonstrate that BPT has significant impact on children and their families. Compared to a reference population, families of children with BPT had significantly lower scores (indicating greater negative impact) in nearly all subscores of the ITQoL. To our knowledge, this is the first study to use a validated instrument to assess the impact of a migraine-related episodic syndrome in young children. Additionally, parent-reported developmental concerns in our study provide support for the hypothesis that BPT can be associated with developmental delay, particularly motor delay.5,12 Notably, 36% of families with developmental concerns commented that their child seemed to “catch up,” suggesting that delays may be transient.7
As data on BPT treatment are limited, we queried parental experience with acute and preventive treatments. Acutely, over-the-counter analgesics were used most commonly, but anti-emetics were perceived as helpful by a higher percentage of families who tried them. As vomiting was the most common “most bothersome symptom,” providers may wish to focus on management of nausea/vomiting when developing an acute treatment plan for BPT. In addition, many families were adept at utilizing non-pharmacologic therapies during acute episodes, including optimizing positioning and minimizing light and sound stimuli; it may be useful for providers to discuss this approach with families given how common sensory sensitivities are in BPT and the relative safety of such interventions.
The best options for preventive management remain unclear as few families utilized preventive medications in this study and data on duration of use is limited. Notably, it was common for families who tried preventive treatments to discontinue medications due to side effects, highlighting the importance of selecting treatments with the lowest side effect profile. Families also noted that physical therapy was unhelpful for treatment of BPT. The recommendation to participate in PT may be based on the presumed diagnosis of congenital muscular torticollis, for which PT is a mainstay of treatment.25
It is important to recognize and differentiate BPT from both muscular torticollis and other causes of head tilt in infancy, particularly after the first attack of BPT in which the characteristic alternating nature of head tilt may not yet be appreciated. As noted in the ICHD-3, the differential diagnosis for BPT includes gastrointestinal reflux, torsional dystonia, complex partial seizures, and congenital and acquired lesions of the posterior fossa and craniocervical junction,3 in addition to ocular misalignment or disorders of eye motility. It is therefore important to consider additional evaluation including MRI of the brain and/or spine, electroencephalogram (EEG), and ophthalmologic evaluation if there are abnormalities in the ophthalmologic and/or neurologic exam or if episodes are atypical of BPT in semiology. It is worth noting, however, that only 5/52 subjects who underwent MRI of the brain or spine and 1/39 who underwent EEG had abnormal findings, and none of these were felt to be the cause of torticollis according to parental report (Supplemental Table S2).
Given the rarity of BPT,1 the relatively large size of this study cohort is a strength, as is our inclusion of participants from around the globe. Rarity also makes it unlikely that randomized, controlled treatment trials for BPT will be conducted, therefore “crowd sourcing” parents’ perceptions about which treatments are or are not effective for BPT is the most practical way to advance the field in the near term. Another strength of this study is that interviews were conducted by child neurologists who have special expertise in pediatric migraine and episodic syndromes associated with migraine. Systematic querying about the presence or absence of symptoms and use of a validated instrument (the ITQoL) for measuring health-related quality of life in infants and toddlers are additional methodological strengths.
Limitations of this study include reliance on parental/caregiver report, which precluded confirmation of formal diagnosis of BPT or other episodic syndromes as it was not possible to perform a neurological exam or directly confirm exclusion of other diagnoses. It is possible that children with reported BPT may have had other neurologic conditions including epilepsy or other movement disorders; though work-up for these conditions was largely unrevealing based on parental report, this was not directly confirmed through review of medical records or neurologic exam. Additionally, while inclusion of subjects with resolved BPT allowed for a broader picture of BPT across childhood and adolescence, in some cases the BPT episodes had resolved over 10 years prior which made recall of specific phenotypic aspects of the episodes more difficult. Recruitment predominantly from a Facebook site for families of children with BPT may have led to selection bias as those whose children have more severe BPT may be more likely to join such a group; however, given the rarity of BPT this was felt to be the most feasible way to gather a cohort. Finally, there may have been overlap between ongoing BPT and emergence of other episodic syndromes associated with migraine, as well as migraine itself, as these syndromes have many shared features; this may lead to over- or under-estimation of the frequency of these conditions.
CONCLUSION
This article provides insight into the broad phenotypic spectrum of BPT in a large cohort of children. BPT may have a developmental impact and can have an impact on quality of life for children and families. The risk of developing migraine may be increased if migrainous features (e.g., phonophobia, photophobia, motion sensitivity) are present during BPT attacks. Optimal preventive treatment for BPT remains unknown, but parental reports suggest that acute treatment should focus on managing nausea and minimizing environmental stimuli.
Supplementary Material
IMPACT:
Benign paroxysmal torticollis (BPT) is a rare condition of early childhood characterized by episodes of head tilt associated with vomiting, irritability, ataxia, pallor, and/or malaise.
This cohort study describes the phenotypic spectrum of BPT, variable treatment, natural history and association with migraine, and impact on development and quality of life.
Children with BPT may go on to develop migraine or episodic syndromes that may be associated with migraine; presence of migrainous features during attacks may increase odds of developing migraine.
BPT can have significant impact on quality of life, demonstrated by findings from the Infant Toddler Quality of Life questionnaire.
Acknowledgments
Competing interests: M.S.L. has received consulting fees from Boston Scientific in 2019; receives grant support from NIH (NINDS, K23NS0099441-O1A), and receives personal compensation for medico-legal consulting. A.A.G. has received consulting fees from Theranica, Advanced Clinical, Biohaven, Impel Neuropharma, and Satsuma; has received honoraria from UpToDate (for authorship) and JAMA Neurology (as an associate editor); receives grant support from Amgen and the Duke Clinical Research Institute; has received personal compensation for medical—legal consulting (last in Jan 2019); her spouse reports research support (to UCSF) from Genentech for a clinical trial, honoraria for editorial work from Dynamed Plus, and personal compensation for medical—legal consulting. S.L.I. receives honoraria for authoring a chapter for the Canadian Pharmacy Association (CPhA) and research support from the Duke Clinical Research Institute. The remaining authors (K.A.G., V.L., W.Q., B.G.) have nothing to disclose.
Footnotes
Informed consent: All subjects provided written informed consent. Children aged 7–17 years provided assent, and those who had reached the age of 18 years provided consent for their parent/caregiver to be interviewed about their history of BPT. Families provided consent for use of photographs/videos for publication.
ADDITIONAL INFORMATION
The online version of this article (https://doi.org/10.1038/s41390-020-01309-1) contains supplementary material, which is available to authorized users.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
REFERENCES
- 1.Tarantino S et al. Migraine equivalents as part of migraine syndrome in childhood. Pediatr. Neurol. 51, 645–649 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Hadjipanayis A, Efstathiou E & Neubauer D Benign paroxysmal torticollis of infancy: an underdiagnosed condition. J. Paediatr. Child Health 10.1111/jpc.12841 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 38, 1–211 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Kimura S & Nezu A Electromyographic study in an infant with benign paroxysmal torticollis. Pediatr. Neurol. 19, 236–238 (1998). [DOI] [PubMed] [Google Scholar]
- 5.Rosman NP, Douglass LM, Sharif UM & Paolini J The neurology of benign paroxysmal torticollis of infancy: report of 10 new cases and review of the literature. J. Child Neurol. 24, 155–160 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Yaghini O, Badihian N & Badihian S The efficacy of topiramate in benign paroxysmal torticollis of infancy: report of four cases. Pediatrics 10.1542/peds.2015-0868 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Danielsson A et al. Benign paroxysmal torticollis of infancy does not lead to neurological sequelae. Dev. Med. Child Neurol. 10.1111/dmcn.13939 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Deonna T & Martin D Benign paroxysmal torticollis in infancy. Arch. Dis. Child. 56, 956–959, 10.1136/adc.56.12.956 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giffin NJ, Benton S & Goadsby PJ Benign paroxysmal torticollis of infancy: four new cases and linkage to CACNA1A mutation. Dev. Med. Child Neurol. 44, 490–493 (2002). [DOI] [PubMed] [Google Scholar]
- 10.Cuenca-Leon E et al. Genetic analysis of 27 Spanish patients with hemiplegic migraine, basilar-type migraine and childhood periodic syndromes. Cephalalgia 28, 1039–1047 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Vila-Pueyo M et al. A loss-of-function CACNA1A mutation causing benign paroxysmal torticollis of infancy. Eur. J. Paediatr. Neurol. 18, 430–433 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Humbertclaude V et al. Benign paroxysmal torticollis, benign paroxysmal vertigo, and benign tonic upward gaze are not benign disorders. Dev. Med. Child Neurol. 10.1111/dmcn.13935 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Roubertie A et al. Benign paroxysmal tonic upgaze, benign paroxysmal torticollis, episodic ataxia and CACNA1A mutation in a family. J. Neurol. 255, 1600–1602 (2008). [DOI] [PubMed] [Google Scholar]
- 14.Dale RC, Gardiner A, Antony J & Houlden H Familial PRRT2 mutation with heterogeneous paroxysmal disorders including paroxysmal torticollis and hemiplegic migraine. Dev. Med. Child Neurol. 54, 958–960 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Moavero R et al. Cyclic vomiting syndrome and benign paroxysmal torticollis are associated with a high risk of developing primary headache: a longitudinal study. Cephalalgia 10.1177/0333102419844542 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Harris PA et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inf. 42, 377–381 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dodick DW et al. Use of most bothersome symptom as a coprimary endpoint in migraine clinical trials: a post-hoc analysis of the pivotal ZOTRIP randomized, controlled trial. Headache 58, 986–992 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landgraf JM, Vogel I, Oostenbrink R, van Baar ME & Raat H Parent-reported health outcomes in infants/toddlers: measurement properties and clinical validity of the ITQOL-SF47. Qual. Life Res. 22, 635–646 (2013). [DOI] [PubMed] [Google Scholar]
- 19.HealthActCHQ Inc. Confidential Scoring Rules Infant and Toddler Quality of Life Questionnaire — 47 (ITQOL-SF47) (HealthActCHQ, Boston, MA, 2015). [Google Scholar]
- 20.HealthActCHQ Inc. ITQOL-SF47 US Norms (HealthActCHQ, Boston, MA, 2017). [Google Scholar]
- 21.Victor TW, Hu X, Campbell JC, Buse DC & Lipton RB Migraine prevalence by age and sex in the United States: a life-span study. Cephalalgia 30, 1065–1072 (2010). [DOI] [PubMed] [Google Scholar]
- 22.Castro-Rodriguez JA et al. Relation between infantile colic and asthma/atopy: a prospective study in an unselected population. Pediatrics 108, 878–882 (2001). [DOI] [PubMed] [Google Scholar]
- 23.Gelfand AA, Thomas KC & Goadsby PJ Before the headache: infant colic as an early life expression of migraine. Neurology 79, 1392–1396 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lucassen PL et al. Systematic review of the occurrence of infantile colic in the community. Arch. Dis. Child. 84, 398–403 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sargent B, Kaplan SL, Coulter C & Baker C Congenital muscular torticollis: bridging the gap between research and clinical practice. Pediatrics 10.1542/peds.2019-0582 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


