Abstract
BACKGROUND:
Currently there is limited knowledge of what imaging assessments of asthma are associated with accelerated longitudinal lung function decline.
OBJECTIVES:
We aimed to assess whether quantitative computerized tomography (qCT) metrics are associated with longitudinal lung function decline and morbidity in asthma.
METHODS:
We analyzed 205 qCT scans of adult asthma patients, and calculated baseline markers of airway remodeling, lung density, and pointwise regional change in lung volume (Jacobian measures) for each participant. Using multivariable regression models, we then assessed the association of qCT measurements with the outcomes of future lung function change, future exacerbation rate, and changes in validated measurements of morbidity.
RESULTS:
Greater baseline wall area percent (WA%) (β=−0.15, 95% CI −0.26 to −0.05, P<0.01), hyperinflation% (β=−0.25, 95% CI −0.41 to −0.09, P<0.01), and Jacobian gradient measurements (cranial-caudal β=10.64, CI 3.79 to 17.49, P<0.01; posterior-anterior β=−9.14, CI −15.49 to −2.78, P<0.01) were associated with more severe future lung function decline. Additionally, greater WA% (rate ratio=1.06, CI 1.01 to 1.10, P=0.02), air-trapping% (rate ratio=1.01, CI 1.00 to 1.02, P=0.03), and lower Jacobian determinant mean (rate ratio=0.58, CI 0.41 to 0.82, P<0.01) and Jacobian determinant standard deviation (rate ratio=0.52, CI 0.32 to 0.85, P=0.01) were associated with a greater rate of future exacerbations. However, imaging metrics were not associated with clinically meaningful changes in scores on validated asthma morbidity questionnaires.
CONCLUSIONS:
Baseline qCT measures of more severe airway remodeling, more small airways disease and hyperinflation, and less pointwise regional change in lung volumes were associated with future lung function decline and asthma exacerbations.
Keywords: Asthma, severe asthma, CT imaging, asthma morbidity, asthma exacerbations, longitudinal, lung function
CAPSULE SUMMARY:
Currently if imaging characteristics are associated with asthma outcomes is unknown. In this study, asthmatics with more airway remodeling and less regional change in lung expansion had more lung function decline and exacerbations.
INTRODUCTION
Asthma is a heterogeneous disorder that is characterized by chronic airway inflammation, airway hyperresponsiveness, and variable airway obstruction. Asthma is estimated to affect approximately 300 million people worldwide and is expected to only increase in prevalence in the future.1, 2 Prior studies have shown that when compared to healthy controls, a subset of adults with asthma experience an accelerated rate of the longitudinal decline in lung function.3–6 For example, in one large epidemiologic study of 17,506 participants, adults with asthma experienced an average decline in forced expiratory volume in 1 second (FEV1) of 38mL per year as compared to 22mL per year in adults without asthma.7 In a subset of patients with asthma, this accelerated rate of lung function decline can lead to irreversible, fixed airway obstruction and marked morbidity.8
Despite recent advances in the understanding and treatment of asthma, novel biomarkers that can reliably predict which patients with asthma will experience an accelerated decline in lung function have not yet been identified. Prior studies have shown that a more rapid decline in lung function in asthma is associated with prior tobacco abuse, a history of frequent asthma exacerbations, a low baseline FEV1, exposure to ambient air pollution, low serum 25-hydroxyvitamin D levels, and an elevated fractional exhaled nitric oxide (FeNO).7, 9–17 In addition, recent work from our NHLBI-sponsored Severe Asthma Research Program (SARP) group demonstrated that, in moderate-to-severe asthma, insensitivity to systemic steroids is associated with greater longitudinal loss of lung function.18 However, whether or not objective measures of airway remodeling are associated with future lung function changes remains unknown.
Results of quantitative analysis of multidetector computerized tomography (qCT) scans have previously been proposed as potential biomarkers that could prognosticate outcomes in chronic obstructive pulmonary disease (COPD),19 asthma,20 and interstitial lung disease.21–23 qCT analysis objectively quantifies airway, parenchymal, and pulmonary vascular characteristics,20, 24 and its use has previously led to the successful identification of novel phenotypes in both COPD and asthma.19, 25–29 Parametric response mapping (PRM), a relatively new qCT methodology that uses voxel-by-voxel image co-registration of inspiratory and expiratory scans, was recently shown to be a novel biomarker in post-lung transplant recipients.30 In that study, small airways disease, characterized by PRM analysis, was associated with future spirometric lung function decline post-transplant and was the single greatest predictor of post-transplant survival in multivariable modeling. Similarly, in COPD, the degree of small airways disease and emphysema defined using PRM analysis was found to be predictive of future lung function decline.31 To date no one has examined if qCT measurements could be used to predict future asthma outcomes.
In this study, we examined a well-characterized multicenter longitudinal cohort of adult asthma patients (SARP-3) in an attempt to determine if qCT metrics are associated with future asthma outcomes including changes in lung function. We first determined baseline markers of airway remodeling, small airways disease, and regional changes in lung expansion using qCT analyses in adults with asthma. We then determined longitudinal changes in both lung function and future asthma morbidity for each participant. We hypothesized that baseline markers of airway remodeling as determined by qCT would be associated with future lung function decline and greater subsequent asthma morbidity.
METHODS
Included Participants
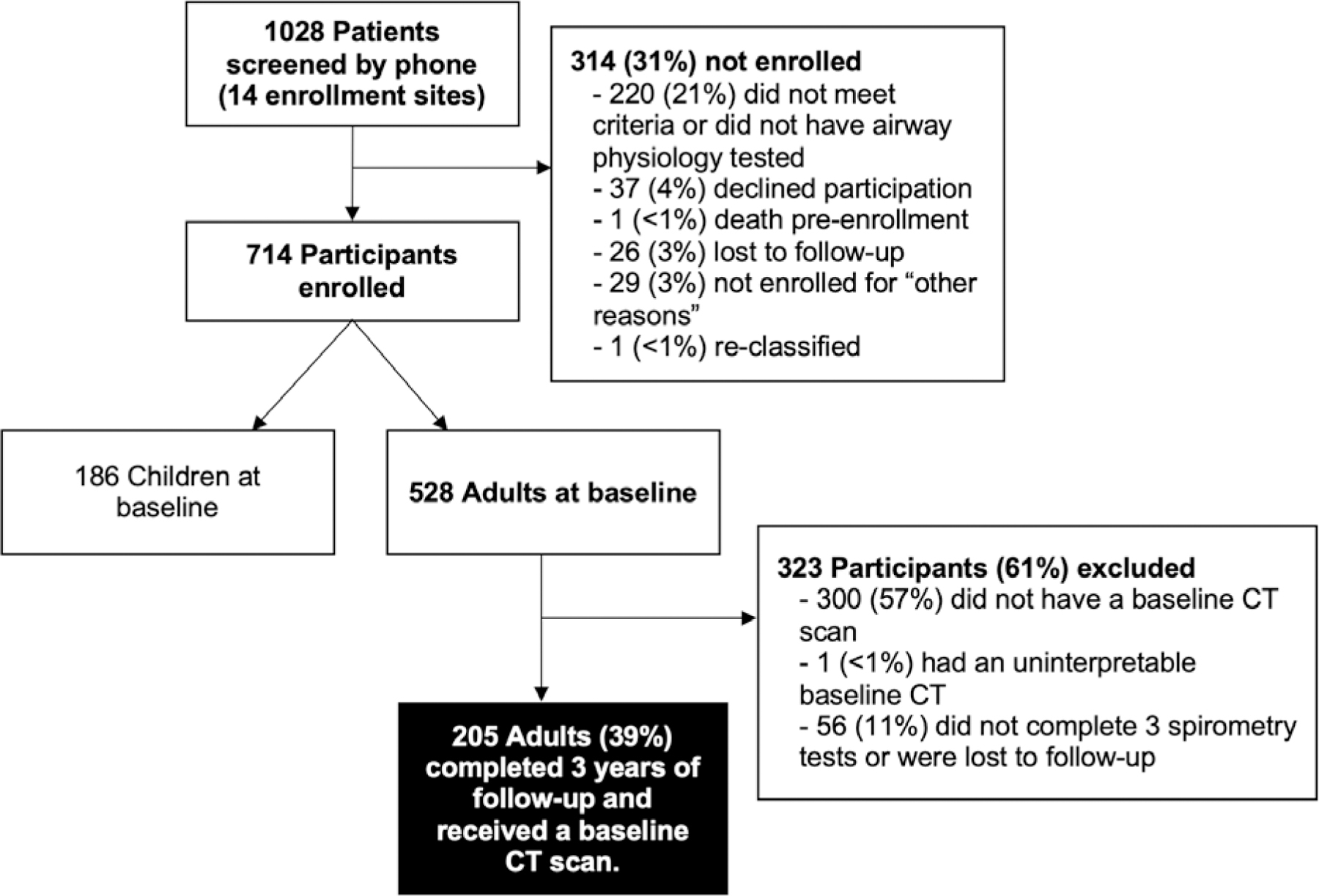
SARP-3 participants were enrolled and characterized from 2012–2015 as previously published32 and included in this study if they were age ≥18 at the time of initial spirometry, had a baseline interpretable CT scan, and had ≥3 visits with spirometry in order to assess for post-CT longitudinal lung function changes. A total of 205 adult participants met our study inclusion criteria (Figure 1). All SARP-3 participants provided written informed consent and the institutional review boards from each of the 14 enrollment sites approved of this study.
Figure 1.
Study flow diagram.
CT Image Acquisition and Analyses
SARP-3 used a qCT analysis protocol similar to other large multicenter studies, which has been described in detail by the SPIROMICS group.24, 25, 33, 34 Analyses were done with Apollo 2.0 software (VIDA Diagnostics, Inc., Coralville, IA, USA). CT integrity and all automated airway segmentations were manually confirmed by two trained analysts. Our primary predictor variables were wall area % (WA%) and wall thickness percent (WT%) defined as ([wall area/total area]*100%) and ([wall thickness/outer diameter]*100%) respectively. These measurements were calculated at the third generation, or segmental airways, along five airway pathways (RB1, RB4, RB10, LB1, and LB10) as previously described.35–37 Pi10 (airway wall area associated with a hypothetical inner perimeter of 10mm) and lumen area were also explored as predictor variables in secondary analyses.38
The effects of lung density measurements on asthma outcomes were evaluated using the following lung density measurements: hyperinflation% (% voxels ≤−950 Hounsfield units [HU] at total lung capacity [TLC]), air-trapping% (% voxels ≤−856HU at functional residual capacity [FRC]), and PRM and disease probability measures (DPM). PRM and DPM are measures that use image co-registration to quantify small airway disease as previously described.27, 31, 39–41 Finally, the Jacobian determinant and Jacobian determinant gradient measurements were explored to describe pointwise changes in lung volume characteristics.42, 43
Assessment of Longitudinal Lung Function Change and Asthma Morbidity
All SARP-3 participants were followed longitudinally and, in order to be included in this study, ≥3 annual follow-up visits with valid spirometry were required. The primary outcome variable was the per year (slope) change in post-bronchodilator FEV1 percent predicted (FEV1%), which was calculated using individual linear regression models for each participant. Changes in asthma morbidity were assessed as the annual rate of asthma exacerbations adjusted for time on study, the yearly change (slope) in asthma control test (ACT) scores, and the yearly change (slope) in asthma quality of life questionnaire (AQLQ) scores.
Statistical Analyses
Study participants were categorized based on their per year rate of lung function change with: severe decline defined as FEV1% loss of >2% per year; mild decline, 0.5 to 2% loss/year; no change, 0.5% loss/year to 1% gain/year; and improvement, >1% gain/year.18 Characteristics of each lung function change group were described and compared using analysis of variance (ANOVA), Kruskal-Wallis, chi-square, or Fisher’s exact tests as appropriate. Univariable regression models were then constructed to determine the association between qCT measurements and the individual outcomes of slope change in post-bronchodilator FEV1% predicted, ACT, AQLQ, and annual exacerbation rate (with each outcome treated as a continuous variable). All univariable and multivariable models controlled for biologic use, baseline post-bronchodilator FEV1%, as well as baseline ACT score, baseline AQLQ score, or 12-month prior-to-enrollment exacerbation rate as appropriate. Multivariable regression models were constructed separately for each qCT predictor variable and were conducted using a backward elimination model building process. All potential covariates with P-values less than 0.2 on univariate analysis were assessed for inclusion in the multivariate models. Only covariates with P-values < 0.05 in the multivariate analysis were maintained in the final models. In addition, the variables of baseline FEV1% response to triamcinolone, body mass index (BMI), exacerbation frequency in the year prior to SARP-3 enrollment, and blood eosinophil count were forced into all multivariable models used to predict longitudinal lung function change.
For exacerbations, a negative binomial distribution was assumed. Time on study was included as an offset term to account for the fact that not all participants had the same number of follow-up visits. All other models assumed a normal distribution and were weighted by the inverse of the mean-squared error of the individual-level linear regression models. Sensitivity analyses were conducted wherein we repeated the models with specific exclusion of those on biologics. We also assessed whether the association between the outcomes and qCT measures varied according to total time that a participant was on study (i.e., a qCT x total follow-up time interaction). Results from these analyses are included in the electronic supplement. A two-sided P-value of <0.05 was used to denote statistical significance. All statistical analyses were performed using SAS version 9.4 (Cary, NC, USA).
RESULTS
Demographics and Baseline Asthma and Imaging Characteristics
The demographic and clinical characteristics of the 205 participants who had a baseline CT scan and longitudinal follow-up are described and compared by subsequent change in lung function grouping in Table 1. SARP participants in this study were overall comparable to excluded adult SARP participants; however, included participants did have more severe disease (see Electronic Supplemental Table E1). The overall cohort represented a demographically diverse sample but consisted primarily of participants with ATS/ERS-defined severe asthma (n=138; 67%).44 In this study, 75 participants (36.6%) experienced future mild or severe lung function decline. The median length of follow-up in this study was 4.3 years (interquartile range [IQR] 3.9 to 5.1 years). Most baseline demographic and clinical characteristics were similar across future lung function decline group. However, biologic use did vary across change in lung function groups (P = 0.04) wherein participants with more severe future lung function decline were more likely to be on a biologic therapy. In addition, the exacerbation rate in the year prior to SARP-3 enrollment (P < 0.01) and exacerbation rate during the duration of the SARP-3 study (P = 0.049) varied across groups with a higher rate of exacerbations found in the severe lung function decline group. Baseline quantitative imaging results are described and compared by lung function groups in Table 2.
Table 1.
Characteristics of adult SARP-3 participants who had a baseline qCT and longitudinal follow-up categorized by future change in lung function.
| Improvement in Lung Function (N=58) | No Change in Lung Function (N=72) | Mild Lung Function Decline (N=52) | Severe Lung Function Decline (N=23) | P-value | |
|---|---|---|---|---|---|
| Baseline clinical characteristics: | |||||
| Age, year, mean ± SD | 46.6 ± 14.6 | 46.2 ± 13.8 | 48.5 ± 14.1 | 51.7 ± 12.2 | 0.36 |
| Female, n (%) | 36 (62%) | 48 (67%) | 35 (67%) | 15 (65%) | 0.94 |
| Non-white race, n (%) | 20 (34%) | 23 (32%) | 21 (40%) | 11 (48%) | 0.50 |
| BMI, kg/m2, median (IQR) | 30.3 (26 to −38.7) | 30.5 (26.1 to 35.8) | 30.0 (27.3 to 38.3) | 34.6 (29.8 to 39.4) | 0.09 |
| History of nasal polyps, n (%) | 15 (26%) | 10 (14%) | 10 (19%) | 9 (39%) | 0.06 |
| Baseline asthma characteristics: | |||||
| Age of asthma onset, years, median (IQR) | 14 (4 to 32) | 11 (3 to 27) | 11 (5 to 29) | 12 (3 to 30) | 0.90 |
| Duration of asthma symptoms, year, mean ± SD | 28.0 ± 15.6 | 29.5 ± 14.0 | 31.3 ± 15.9 | 35.0 ± 17.7 | 0.28 |
| Exacerbations in the prior 12 months, median (IQR) | 1 (0 to 2) | 0 (0 to 2) | 1 (0 to 3) | 2 (0 to 5) | <0.01 † |
| ATS/ERS severe classification, n (%) | 41 (71%) | 43 (60%) | 38 (73%) | 16 (70%) | 0.39 |
| On high dose ICS + 2nd controller, n (%) | 42 (72%) | 45 (63%) | 37 (71%) | 16 (70%) | 0.62 |
| On biologics in the last 12 months, n (%) | 6 (10%) | 9 (13%) | 9 (17%) | 8 (35%) | 0.04 * |
| Received OCS in the last 12 months, n (%) | 34 (59%) | 36 (50%) | 32 (62%) | 16 (70%) | 0.33 |
| ACT score, median (IQR) | 19 (13 to 21) | 18 (14 to 21) | 16 (13 to 20) | 14 (10 to 20) | 0.08 |
| AQLQ score, median (IQR) | 5.1 (4.2 to 5.9) | 5.1 (4.3 to 6.3) | 5.2 (4.1 to 5.9) | 4.8 (4.1 to 6.1) | 0.65 |
| Blood eosinophil, %, median (IQR) | 3 (2 to 5) | 3.2 (2 to 5) | 2.9 (1.7 to 6.9) | 2.9 (1.5 to 6.4) | 0.83 |
| Sputum eosinophil, %, median (IQR) | 0.8 (0 to 4.3) | 0.5 (0.2 to 3.5) | 0.3 (0 to 1.1) | 1 (0.7 to 1.4) | 0.18 |
| FeNO, ppb, median (IQR) | 25.5 (13 to 39) | 22 (13.5 to 36) | 17 (10 to 33) | 18 (12 to 42) | 0.32 |
| Serum IgE, kU/L, median (IQR) | 174 (67 to 362) | 150 (43 to 303) | 154 (46 to 345) | 139 (17 to 640) | 0.91 |
| Baseline spirometry characteristics: | |||||
| Pre-bronchodilator FEV1% predicted, mean ± SD | 70.4 ± 20.4 | 76.6 ± 17.9 | 68.9 ± 19.7 | 72.5 ± 23.7 | 0.15 |
| Pre-bronchodilator FVC% predicted, mean ± SD | 82.5 ± 18.8 | 87.3 ± 15.1 | 83.3 ± 16.8 | 84.3 ± 18.1 | 0.38 |
| Pre-bronchodilator FEV1/FVC ratio (%predicted), mean ± SD | 84.3 ± 12.1 | 86.8 ± 11.1 | 81.3 ± 12.6 | 84.4 ± 14.1 | 0.10 |
| Triamcinolone-induced difference from baseline in FEV1% predicted, mean ± SD | 5.0 ± 9.2 | 2.6 ± 6.2 | 1.6 ± 8.6 | 6.8 ± 11.2 | 0.04 * |
| Longitudinal characteristics: | |||||
| Length of follow-up, years, median (IQR) | 4.0 (3.1 to 5.0) | 4.4 (4.0 to 5.1) | 4.9 (4.0 to 5.1) | 4.3 (4.1 to 5.0) | 0.03 * |
| Slope of yearly change in post-bronchodilator FEV1% predicted, median (IQR) | 2.2 (1.8 to 3.0) | 0.1 (−0.2 to 0.5) | −1.1 (−1.5 to −0.8) | −2.9 (−4.0 to −2.2) | <0.0001 ‡ |
| Slope of yearly change in ACT score, median (IQR) | 0.5 (0.1 to 1.1) | 0.3 (−0.1 to 0.8) | 0.1 (−0.6 to 0.9) | 0.2 (−0.2 to 0.9) | 0.08 |
| Slope of yearly change in AQLQ score, median (IQR) | 0.1 (0 to 0.3) | 0.1 (−0.1 to 0.2) | 0.1 (−0.1 to 0.2) | 0 (0 to 0.3) | 0.06 |
| Exacerbations per year of follow-up, median (IQR) | 0.6 (0 to 1.4) | 0.3 (0 to 1) | 0.5 (0 to 1.3) | 1 (0 to 2) | 0.049 * |
Lung function groups were classified based on their rate (slope) of lung function change with severe decline defined as FEV1% loss of >2% per year; mild decline, 0.5 to 2% loss/year; no change 0.5% loss/year to 1% gain/year; and improvement, more than 1% gain/year. Lung function groups were compared using chi-square, Fisher’s exact test, ANOVA, or Kruskal-Wallis tests as appropriate. SD: standard deviation; BMI: body mass index; IQR: interquartile range; ICS: inhaled corticosteroids; OCS: oral corticosteroids; ACT asthma control test; AQLQ: asthma quality of life questionnaire; and FeNO: fractional exhaled nitric oxide.
P < 0.05
P < 0.01
P < 0.0001.
Table 2.
Quantitative computerized tomography (qCT) characteristics of SARP-3 participants categorized by future change in lung function.
| Baseline qCT characteristics | Improvement in Lung Function | No Change in Lung Function | Mild Lung Function Decline | Severe Lung Function Decline | P-value |
|---|---|---|---|---|---|
| Airway Measurements | |||||
| WA%, mean ± SD | 61.3 ± 3.2 | 60.9 ± 2.9 | 61.6 ± 2.9 | 63.4 ± 3.5 | <0.01 † |
| WT%, mean ± SD | 16.5 ± 1.3 | 16.3 ± 1.2 | 16.6 ± 1.1 | 17.4 ± 1.6 | <0.01 † |
| Lumen area, mm2, mean ± SD | 20.0 ± 6.1 | 19.4 ± 4.6 | 19.2 ± 5.4 | 17.0 ± 4.6 | 0.15 |
| Pi10, mm, mean ± SD | 3.8 ± 0.1 | 3.7 ± 0.1 | 3.7 ± 0.1 | 3.8 ± 0.1 | 0.46 |
| Lung Density Measurements | |||||
| Air trapping, %, median (IQR) | 5.8 (2.3 to 12.7) | 4.0 (2.1 to 11.7) | 8.7 (4.3 to 16.0) | 10.9 (3.6 to 15.4) | 0.05 |
| PRMSAD, %, median (IQR) | 5.8 (2.3 to 12.7) | 4.0 (2.0 to 11.0) | 8.8 (4.3 to 16.2) | 10.2 (3.8 to 15.3) | 0.05 * |
| DPMSAD, %, median (IQR) | 22.0 (14.3 to 36.1) | 19.2 (9.9 to 27.3) | 24.4 (17.3 to 38.2) | 27.9 (17.6 to 33.7) | 0.02 * |
| Hyperinflation, %, median (IQR) | 1.7 (0.8 to 2.7) | 1.6 (0.6 to 3.1) | 1.9 (0.8 to 3.4) | 1.7 (0.8 to 3.6) | 0.84 |
| PRMhyperinflation, %, median (IQR) | 0.2 (0 to 0.6) | 0.1 (0 to 0.4) | 0.2 (0 to 0.8) | 0.2 (0.1 to 0.8) | 0.32 |
| DPMhyperinflation, %, median (IQR) | 0.8 (0.1 to 2.8) | 0.4 (0.1 to 1.9) | 1.2 (0.3 to 3.6) | 1.1 (0.4 to 3.4) | 0.28 |
| Lung Deformation (Jacobian) Measurements | |||||
| Mean ± SD | 2.0 ± 0.5 | 2.1 ± 0.4 | 1.8 ± 0.3 | 1.9 ± 0.4 | <0.01 † |
| SD, median (IQR) | 0.6 (0.4 to 0.9) | 0.7 (0.5 to 0.9) | 0.6 (0.4 to 0.7) | 0.6 (0.4 to 0.8) | 0.03* |
| GradientCC, mean ± SD | 0 ± 0.05 | −0.02 ± 0.05 | −0.01 ± 0.04 | −0.01 ± 0.06 | 0.15 |
| GradientPA | 0.05 ± 0.05 | 0.06 ± 0.04 | 0.05 ± 0.03 | 0.05 ± 0.04 | 0.26 |
Lung function groups were classified based on their rate (slope) of lung function change with: severe decline defined as FEV1% loss of >2% per year; mild decline, 0.5 to 2% loss/year; no change 0.5% loss/year to 1% gain/year; and improvement, more than 1% gain/year. Lung function groups were compared using ANOVA or Kruskal-Wallis tests as appropriate. WA%: wall area percent; WT%: wall thickness percent; Pi10: inner perimeter of 10 mm; PRM: parametric response mapping; SAD: functional small airway disease; DPM: disease probability measure; PA: posterior – anterior; CC: cranial – caudal.
P < 0.05
P < 0.01.
Imaging Predictors of Longitudinal Lung Function Decline
In this study, we found that participants with more severe airway remodeling, more hyperinflation, and certain lung volume expansion gradation patterns on qCT were more likely to experience future lung function decline. The consolidated results of the effect of qCT variables on the outcome of lung function decline are presented in Table 3 and Figure 2 (as well as Electronic Supplemental Table E2 with use of standardized beta coefficients and Electronic Supplemental Tables E3–E16 showing results of all covariates included in individual models). On multivariable analyses, which included adjustment for covariates including baseline post-bronchodilator FEV1% predicted, biologic use, body mass index (BMI), FEV1% response to triamcinolone, exacerbation frequency in the year prior to SARP-3 enrollment, and baseline blood eosinophil count, greater WA% (β=−0.15, 95% CI −0.26 to −0.05, P < 0.01) was associated with more severe future lung function decline. However, WT% (β=−0.12, 95% CI −0.37 to 0.12, P = 0.32) did not have an association with lung function decline. Our findings indicate that a participant who had a 5% greater WA% at baseline would be expected to have an absolute FEV1% predicted decline of 0.75% more each year.
Table 3.
Univariable and multivariable predictors of lung function change over time.
| Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| Imaging characteristic | Parameter Estimate (95% CI) | P-Value | Parameter Estimate (95% CI) | P-value |
| Airway Measurements | ||||
| WA% | −0.13 (−0.23 to −0.04) | <0.01 † | −0.15 (−0.26 to −0.05) | <0.01 † |
| WT% | −0.09 (−0.32 to 0.14) | 0.45 | −0.12 (−0.37 to 0.12) | 0.32 |
| Lumen area, mm2 | 0.09 (0.04 to 0.15) | <0.01 † | 0.10 (0.04 to 0.15) | <0.001 ‡ |
| Pi10, mm | −0.54 (−3.27 to 2.19) | 0.70 | 0.11 (−3.33 to 3.55) | 0.95 |
| Lung Density Measurements | ||||
| Air trapping, % voxels ≤−856HU at FRC | 0.001 (−0.03 to 0.03) | 0.97 | −0.01 (−0.04 to 0.02) | 0.55 |
| PRMSAD, % | 0.002 (−0.03 to 0.03) | 0.89 | −0.01 (−0.04 to 0.02) | 0.58 |
| DPMSAD, % | 0.004 (−0.02 to 0.02) | 0.71 | 0 (−0.02, 0.02) | 0.71 |
| Hyperinflation, % voxels ≤−950HU at TLC | −0.29 (−0.46 to −0.13) | <0.001 ‡ | −0.25 (−0.41 to −0.09) | <0.01 † |
| PRMHyperinflation, % | −0.21 (−0.52 to 0.1) | 0.19 | −0.13 (−0.40 to 0.15) | 0.38 |
| DPMHyperinflation, % | −0.03 (−0.15 to 0.08) | 0.56 | 0 (−0.11, 0.10) | 0.94 |
| Lung Deformation (Jacobian) Measurements | ||||
| Mean | 0.05 (−0.76 to 0.86) | 0.90 | 0 (−0.74 to 0.74) | 0.99 |
| SD | −0.55 (−1.77 to 0.68) | 0.38 | −0.26 (−1.41 to 0.89) | 0.66 |
| GradientCC | 10.4 (3.32 to 17.49) | <0.01 † | 10.64 (3.79 to 17.49) | <0.01 † |
| GradientPA | −8.12 (−14.75 to −1.49) | 0.02 * | −9.14 (−15.49 to −2.78) | <0.01 † |
Univariable comparisons were adjusted for baseline post-bronchodilator FEV1% predicted and use of biologics. Multivariable models were adjusted for baseline post-bronchodilator FEV1% predicted, biologic use, body mass index (BMI), FEV1% response to triamcinolone, exacerbation frequency in the year prior to SARP-3 enrollment, baseline blood eosinophil count, and other significant covariates. WA%: wall area percent; WT%: wall thickness percent; Pi10: inner perimeter of 10 mm; FRC: functional residual capacity; PRM: parametric response mapping; SAD: functional small airway disease; TLC: total lung capacity; DPM: disease probability measure; PA: posterior – anterior; CC: cranial – caudal.
P < 0.05
P < 0.01
P < 0.001.
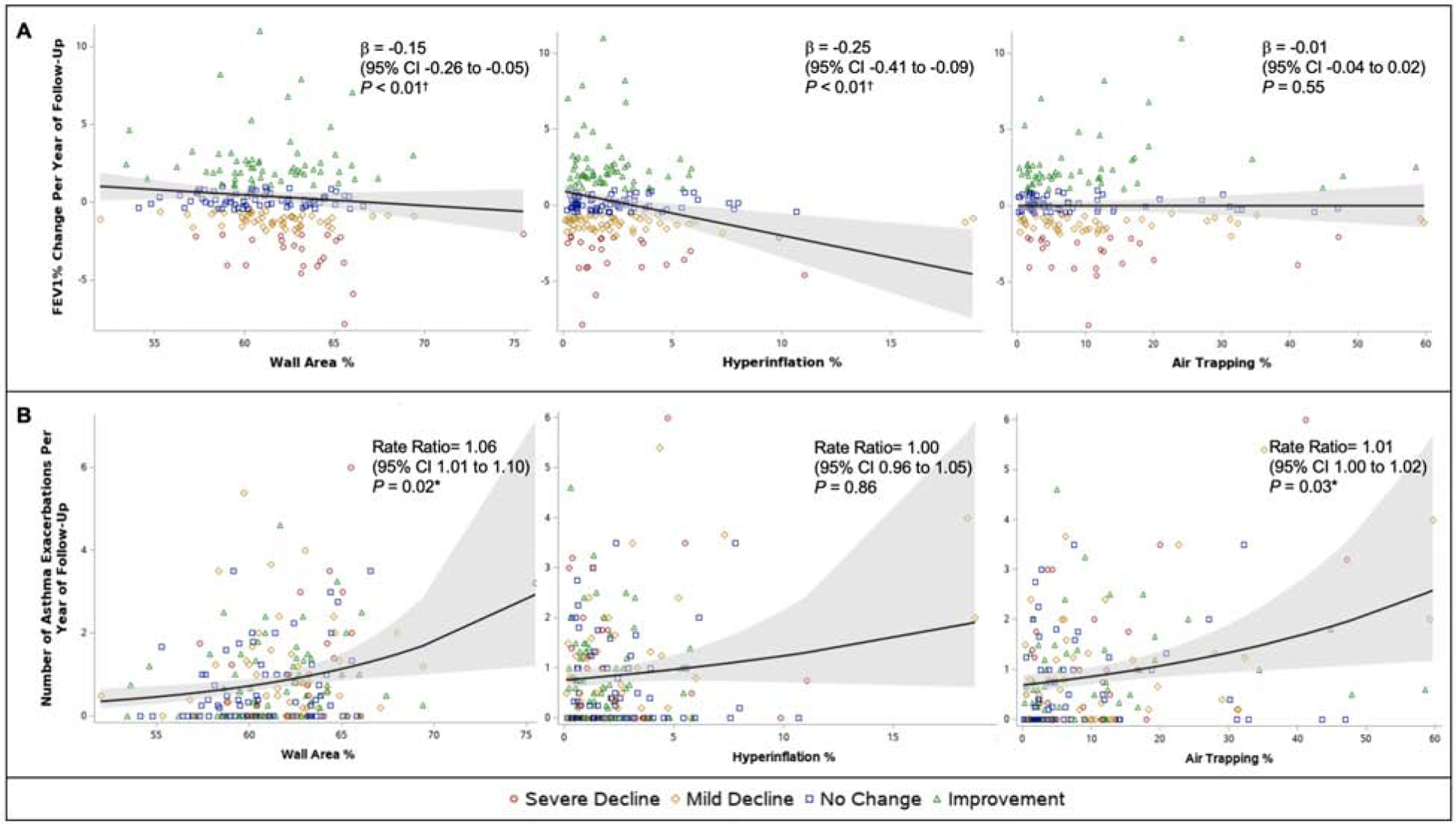
Figure 2. Predicted (A) annual change in post-bronchodilator FEV1% and (B) predicted annual asthma exacerbation rate according to baseline wall area % (WA%), hyperinflation %, and air-trapping % (AT%).
Solid black lines indicate the predicted annual change (unadjusted for covariates) in FEV1% and the predicted rate of asthma exacerbations (unadjusted for covariates) based on the baseline qCT variable with gray shaded areas representing 95% confidence intervals around the regression lines. Parameter estimates reported in the inset are from the multivariable models. Individual adult SARP participants are color coded according to the classification of their lung function change grouping with severe decline (red circle) defined as an FEV1% loss of >2% per year; mild decline (gold diamond), 0.5 to 2% loss/year; no change (blue square), 0.5% loss/year to 1% gain/year; and improvement (green triangle), more than 1% gain/year. After adjustment for covariates, baseline WA% and hyperinflation% were interpedently associated with future lung function decline, while baseline WA% and AT% were independently associated with a greater rate of future asthma exacerbations. *P < 0.05; †P < 0.01.
When looking at lung density measurements, only hyperinflation% was predictive of longitudinal lung function decline on multivariable analysis (β=−0.25, 95% CI −0.41 to −0.09, P < 0.01). Most lung deformation metrics were not associated with lung function decline on primary analysis; however, Jacobian determinant gradient patterns (cranial-caudal β=10.64, 95% CI 3.79 to 17.49, P < 0.01; posterior-anterior β=−9.14, CI −15.49 to −2.78, P < 0.01) were strongly associated with lung function changes. However, when patients on biologics were excluded, greater amounts of small airway disease (whether measured as AT%, PRMSAD, or DPMSAD) and less lung deformation (Jacobian determinant mean) were also associated with longitudinal lung function decline (Electronic Supplemental Table E17). Interestingly, the effect of several qCT variables on future lung function changes did vary based on the length of longitudinal follow-up. In cases with comparatively longer longitudinal follow-up, participants who had a greater Pi10 or lower Jacobian determinant mean had greater future lung function decline (Electronic Supplemental Table E18).
Imaging Predictors of Asthma Morbidity
The association of baseline qCT metrics with future asthma morbidity outcomes is presented in Table 4 (and Electronic Supplemental Table E19 with use of standardized beta coefficients and Electronic Supplemental Tables E20–E33 showing results of all covariates included in individual models). While airway measurements were not associated with ACT scores, several lung density measurements including hyperinflation% (β=−0.01, 95% CI 0 to 0.02, < 0.01) and air-trapping% (β=−0.004, 95% CI 0 to 0.01, P < 0.01) were significantly associated with AQLQ changes, but markedly below the minimal clinically important difference of 0.5 45. The Jacobian cranial-caudal gradient was also associated with changes in AQLQ scores (β=1.28, 95% CI 0.58 to 1.98, P < 0.001).
Table 4.
Multivariable predictors of future asthma morbidity.
| Exacerbation Rate | Slope ACT | Slope AQLQ | ||||
|---|---|---|---|---|---|---|
| Imaging characteristic | Rate Ratio (95% CI) | P-Value | Parameter Estimate (95% CI) | P-value | Parameter Estimate (95% CI) | P-value |
| Airway Measurements | ||||||
| WA% | 1.06 (1.01 to 1.10) | 0.02 * | −0.02 (−0.06 to 0.02) | 0.32 | 0.004 (−0.01 to 0.01) | 0.39 |
| WT% | 1.09 (0.97 to 1.23) | 0.13 | −0.07 (−0.17 to 0.03) | 0.19 | 0.001 (−0.02 to 0.02) | 0.96 |
| Lumen area, mm2 | 0.98 (0.96 to 1.01) | 0.17 | 0.01 (−0.02 to 0.03) | 0.56 | −0.002 (−0.01 to 0) | 0.28 |
| Pi10, mm | 2.78 (0.84 to 9.26) | 0.10 | −0.39 (−1.53 to 0.75) | 0.50 | −0.003 (−0.18 to 0.18) | 0.98 |
| Lung Density Measurements | ||||||
| Air trapping, % voxels ≤−856HU at FRC | 1.01 (1.00 to 1.02) | 0.03 * | −0.01 (−0.02 to 0) | 0.11 | 0.004 (0 to 0.01) | <0.01 † |
| PRMSAD, % | 1.01 (1.00 to 1.03) | 0.02 * | −0.01 (−0.02 to 0) | 0.11 | 0.01 (0 to 0.01) | <0.01 † |
| DPMSAD, % | 1.01 (1.00 to 1.02) | 0.01 * | −0.01 (−0.02 to 0) | 0.16 | 0.002 (0 to 0.004) | 0.13 |
| Hyperinflation, % voxels ≤−950HU at TLC | 1.00 (0.96 to 1.05) | 0.86 | −0.04 (−0.09 to 0.01) | 0.16 | 0.01 (0 to 0.02) | <0.01 † |
| PRMHyperinflation, % | 1.04 (0.97 to 1.12) | 0.25 | −0.04 (−0.12 to 0.04) | 0.35 | 0.02 (0 to 0.05) | 0.07 |
| DPMHyperinflation, % | 1.02 (0.99 to 1.05) | 0.16 | −0.02 (−0.05 to 0.01) | 0.25 | 0.01 (0 to 0.02) | 0.02 * |
| Lung Deformation (Jacobian) Measurements | ||||||
| Mean | 0.58 (0.41 to 0.82) | <0.01 † | 0.25 (−0.10 to 0.59) | 0.16 | 0.04 (−0.03 to 0.11) | 0.21 |
| SD | 0.52 (0.32 to 0.85) | 0.01 * | 0.30 (−0.19 to 0.79) | 0.23 | 0.06 (−0.03 to 0.15) | 0.17 |
| GradientCC | 1.13 (0.04 to 30.57) | 0.94 | −0.03 (−3.05 to 2.99) | 0.98 | 1.28 (0.58 to 1.98) | <0.001 ‡ |
| GradientPA | 0.27 (0.01 to 11.13) | 0.49 | −0.18 (−3.66 to 3.30) | 0.92 | −0.5 (−1.13 to 0.13) | 0.12 |
All multivariable models controlled for biologic use as well as baseline number of exacerbations in the year prior to SARP-3 enrollment, baseline ACT score, and baseline AQLQ score respectively. Other significant covariates were entered into the model, which was formed using a backwards elimination model selection process. ACT: asthma control test; AQLQ: asthma quality of life questionnaire; WA%: wall area percent; WT%: wall thickness percent; Pi10: inner perimeter of 10 mm; PRM: parametric response mapping; SAD: functional small airway disease; DPM: disease probability measure; PA: posterior – anterior; CC: cranial – caudal
P < 0.05
P < 0.01
P < 0.001.
Finally, in this study, imaging metrics that showed more severe airway remodeling, more small airways disease, and less lung deformation were associated with a greater future rate of asthma exacerbations (Table 4 and Figure 2). Even after adjustment for covariates including frequency of prior exacerbations and baseline eosinophil count, participants that had a greater baseline WA% (rate ratio=1.06, 95% CI 1.01 to 1.10, P = 0.02) had a higher future rate of asthma exacerbations. In addition, participants that had more small airways disease, assessed via simple voxel thresholding (air-trapping%, rate ratio=1.01, 95% CI 1.00 to 1.02, P = 0.03) or either image co-registration method (PRMSAD, rate ratio 1.01, 95% CI 1.00 to 1.03, P = 0.02; DPMSAD, rate ratio=1.01, 95% CI 1.00 to 1.02, P = 0.01) were more likely to experience exacerbations in the future even after controlling for prior exacerbations. Lastly, participants that had less lung deformation (Jacobian determinant mean, rate ratio=0.58, 95% CI 0.41 to 0.82, P < 0.01) and less heterogeneity in lung deformation (Jacobian determinant standard deviation, rate ratio=0.52, 95% CI 0.32 to 0.85, P = 0.01) had a greater number of future exacerbations.
DISCUSSION
This study showed that certain quantitative imaging metrics were associated with future lung function decline and asthma exacerbations. Specifically, adults with asthma who had more severe baseline airway remodeling, more hyperinflation, and certain lung deformation patterns were more likely to experience accelerated lung function decline. Similarly, participants with more severe airway remodeling, more air trapping, and less lung deformation were more likely to have future asthma exacerbations. However, qCT metrics were generally not associated with clinically meaningful changes in asthma control or quality of life.
Although investigators have known for decades that a subset of asthma patients have an accelerated decline in lung function, little progress has been made at predicting which patients with asthma will experience a more rapid lung function decline.7, 46 The current inability to predict future lung function decline in asthma limits our ability to research treatment paradigms specifically aimed at minimizing the risk of future lung function decline.47 If lung function decline and asthma morbidity could be more accurately predicted, it is possible that treatments (such as biologic therapies) could be explored at an earlier timepoint in “high risk” patients to potentially prevent or impede accelerated lung function loss. In this study, several qCT characteristics were predictive of future lung function decline. However, we should point out that these results represent prediction of average outcomes for the entire cohort. Based on these results, qCT metrics alone could not predict individual-level outcomes with enough accuracy to select patients for a personalized medicine treatment approach. Possibly, in the future, qCT metrics in combination with other biomarkers could predict future adverse outcomes in asthma with enough accuracy to guide individualized therapies however.
Illustrated in Figure 3 are representative coronal and axial CT images from a SARP-3 participant who suffered severe lung function decline and had numerous exacerbations during the duration of the study, which is directly contrasted with a CT from another participant who had improvement in lung function and had no exacerbations. These contrasting images show the differences in qCT metrics between these two participants who were otherwise clinically similar at baseline, but experienced drastically different subsequent clinical courses. Currently, qCT analyses are primarily used in research studies and require human verification and adjustment. However, complete automation and reporting of qCT values is likely on the horizon. Furthermore, with improved image reconstruction methods that reduce a patient’s radiation exposure and with further standardization of imaging acquisition protocols, qCT has the potential to have an increasingly important role in routine clinical care in the future.48
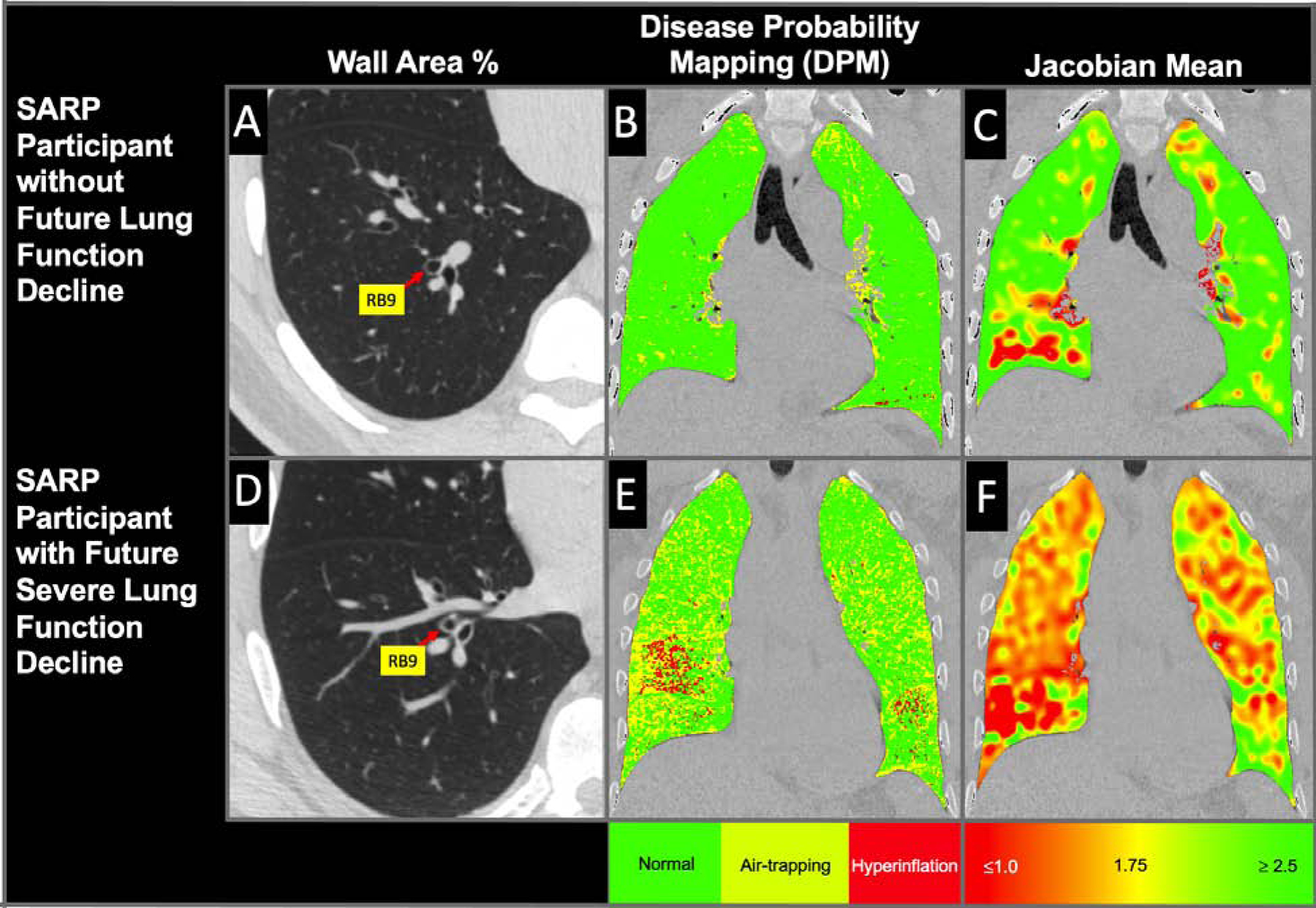
Figure 3. Coronal and axial views of (A-C) a SARP-3 participant who did not suffer lung function decline (post-BD FEV1% = 2.7% per year improvement) and had no exacerbations during the duration of SARP-3 as compared to (D-F) a participant who suffered severe lung function decline (post-BD FEV1% = 3.8% per year decline ) and had 7 exacerbations (1.75 exacerbations per year) during the duration of SARP-3.
A representative axial image from these two participants (A and D) is shown focused on RB9, which demonstrates an increased WA% in the participant who suffered greater lung function decline and asthma morbidity. In addition, coronal sections for the same two participants are shown demonstrating more small airways disease (B and E) and less lung deformation (C and F) in the participant who had more lung function decline and asthma exacerbations during the study period.
In the SARP-3 cohort, more severe airway remodeling, as assessed by WA%, was associated with an increased rate of future lung function decline and a greater frequency of asthma exacerbations after controlling for relavent confounders including baseline FEV1%, prior exacerbations, and eosinophil count. These results confirmed our primary hypothesis, which predicted that more severe airway remodeling, as reflected by a greater baseline WA%, would be predictive of future lung function decline and asthma morbidity. Prior studies have shown that patients with severe asthma, when compared to those with mild disease, have an increased WA% and WT% on qCT.13 Furthermore, both WA% and WT% have been shown to be directly correlated with response to bronchodilators as well as epithelial thickness from endobronchial biopsies.49–51 Our finding that an increased WA% is associated with future accelerated longitudinal lung function decline supports the pathobiological concept that underlying airway remodeling has future detrimental consequences including long-term loss of lung function and increased risk of exacerbations. While an association between increased airway remodeling and future loss of lung function has been previously hypothesized, prior studies have never clearly demonstrated this association.52, 53
In addition, this study demonstrated that greater hyperinflation, as assessed by qCT, was associated with greater future loss of lung function. Of note, in this study, hyperinflation% was used to describe the radiologic findings of hyperinflation (% voxels ≤−950 HU at TLC) rather than the often-described clinical phenomenon of “dynamic hyperinflation” in asthma. While this finding has not been reported before in asthma, prior studies have demonstrated that the equivalent imaging metric (termed emphysema-like lung) is associated with future lung function decline in moderate-to-severe COPD.54, 55 Prior studies in COPD and post lung transplantation have demonstrated that PRM measurements were predictive of future lung function decline.30, 31 Surprisingly in the current study, small airways disease measurements obtained using PRM and DPM analysis were not predictive of lung function decline. However, when patients on biologics were excluded from the analysis, baseline qCT markers of small airway disease were associated with greater future lung function decline.
Finally, in this study we demonstrated that lung deformation measurements (Jacobian determinant values), as well as lung deformation spatial heterogeneity (Jacobian gradient values), were independently associated with several asthma-related outcomes. The Jacobian determinant is a biomechanical measurement calculated from image co-registration and reflects the local volume change that occurs at a point from expiration to inspiration.56–58 While lung deformation measurements are less intuitive, Jacobian measurements have previously been found to be of importance in both COPD and asthma.56, 57, 59–62 In asthma, low Jacobian determinant values are associated with disease severity and believed to reflect less regional lung expansion, possibly secondary to air trapping and hyperinflation.56, 59 In COPD, lower Jacobian determiant values in the penumbra surrounding emphysematous areas of the lung have been found to be prognostic of future lung function decline.61 In asthma we found that less lung deformation (Jacobian determinant mean) and less lung deformation heterogeneity (Jacobian determinant standard deviation) were independently predictive of future exacerbations, but were not associated with lung function changes. However, as shown in the electronic supplement, in patients not on biologics or who had comparatively longer longitudinal follow-up, a lower baseline Jacobian determinant value was associated with greater longitudinal lung function decline. This finding indicates that with longer longitudinal follow-up, associations between baseline qCT variables and asthma outcomes may become even more apparent. Furthermore, in this study Jacobian spatial heterogeneity predicted lung function decline, with relatively greater caudal and relatively greater posterior Jacobian determinant values having associations with future lung function decline. To our knowledge Jacobian gradients have not been explored in predicting disease outcomes, and this finding should be viewed as hypothesis generating.
We believe this study has a number of strengths. To our knowledge, this study represents the first time qCT measurements have been evaluated in a longitudinal asthma cohort and shown to predict clinically meaningful asthma outcomes. Furthermore, all data in this study were collected from a well-characterized cohort of asthma patients from diverse racial and ethnic backgrounds and with a broad range of disease severity. However, limitations of this study must be considered. First, in this study airway remodeling was assessed via qCT analysis. Although qCT metrics of airway remodeling are correlated with endobronchial measurements, limitations of measuring airway remodeling in this way exist. For example, wall area cannot be normalized by basal membrane length wherein an increase in baseline airway smooth muscle tone could be incorrectly interperted for airway remodeling using qCT. Additionally, the findings of air-trapping were postulated to primarily reprsent underlying small airways disease. However, the exact contribution that larger airways or other abnormalities have on the finding of air-trapping cannot be certain. In this study, our only primary hypothesis was that qCT markers of airway remodeling, specifically an elevated WA% and WT%, would be predictive of the individual outcomes of lung function decline and asthma morbidity. As such, all further exploration of associations between other imaging metrics and study outcomes should be considered exploratory, and were not corrected for multiple comparisons. Finally, a subset of participants in this study were on biologic therapies, which have been shown to improve lung function.63, 64 However, all univariable and multivariable models in this study corrected for biologic use, and repeat exploratory analyses excluding these 24 participants on biologics were performed and showed similar or stronger associations.
CONCLUSIONS
In conclusion, in this study we found that adult asthma patients with more severe airway remodeling, more hyperinflation, and certain lung deformation gradation patterns, discerned from qCT analyses, were more likely to experience future lung function decline. While qCT measurements were generally not associated with clinically meaningful changes in asthma morbidity on questionnaires, more severe airway remodeling, more small airways disease, and less lung deformation were each associated with a greater future rate of asthma exacerbations. Based on results from this study, qCT metrics may be useful to prognosticate clinical outcomes in adult asthma.
Supplementary Material
CLINICAL IMPLICATIONS:
Quantitative imaging metrics were associated with important asthma outcomes including longitudinal lung function decline and future exacerbations. Quantitative imaging may be one tool that helps prognosticate future outcomes in asthma.
ACKNOWLEDGMENTS
The authors acknowledge and thank the SARP Data Coordinating Center, study participants, and clinical research coordinators. This study was supported by grants from the NHLBI and NCATS. The SARP study was supported beyond the third year with support from AstraZeneca, Boehringer-Ingelheim, Genentech, GSK, Sanofi-Genzyme-Regeneron, and TEVA. Spirometers in this study were provided by nSpire Health Inc (Longmont, Colorado).
Funding:
This study was funded by grants from the National Heart Lung and Blood Institute (NHLBI) to the Severe Asthma Research Program’s Principal (SARP) Investigators, Clinical Centers, and Data Coordinating Center including: U10 HL109086–04 (D.T.M.), U10 HL109146 (J.V.F.), U10 HL109168 (L.C.D.), U10 HL109172 (G.W.), U10 HL109152 (S.E.W.), and U10 HL109257 (M.C.). Support for SARP visits occurring beyond the third year of follow up was supported in part by SARP’s partnerships with AstraZeneca, Boehringer-Ingelheim, Genentech, GSK, Sanofi-Genzyme-Regeneron, and TEVA. In addition, this study was supported by Washington University’s institutional training award (2T32HL007317-41) from the NHLBI as well as support from National Center for Advancing Translational Science (NCATS) awards: UL1 TR000427 (University of Wisconsin), UL1 TR001102 (Harvard University), UL1TR002345 (Washington University), and UL1TR002366 (Frontiers: University of Kansas Clinical and Translational Science Institute).
ABBREVIATIONS USED:
- ACT
asthma control test
- ATS
American Thoracic Society
- AQLQ
asthma quality of life questionnaire
- BMI
body mass index
- CI 95%
confidence interval
- COPD
chronic obstructive pulmonary disease
- DPM
disease probability mapping
- ERS
European Respiratory Society
- FeNO
fractional exhaled nitric oxide
- FEV1
forced expiratory volume in 1 second
- FEV1%
forced expiratory volume in 1 second % predicted
- FRC
functional residual capacity
- GINA
Global Initiative for Asthma
- NHLBI
National Heart Lung and Blood Institute
- PRM
parametric response mapping
- qCT
quantitative analyses of multidetector computerized tomography scan
- SAD
small airway disease
- SARP-3
Severe Asthma Research Program-3
- SPIROMICS
Subpopulations of Intermediate Outcome Measures in Chronic Obstructive Pulmonary Disease
- TLC
total lung capacity
- WA%
wall area percent
- WT%
wall thickness percent
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study was registered with www.clinicaltrials.gov (NCT01716494).
Conflict of Interest
J.K., C.W.G., D.L., M.S., M.C.M., J.B., A.S., C.H., J.B., K.B.S., S.P., and S.M. have no conflicts of interest. L.B.B. reports grants from the NIH/NHLBI, during the conduct of the study; personal fees from GlaxoSmithKline, personal fees from Genentech/Novartis, personal fees and non-financial support from Merck, personal fees from DBV Technologies, personal fees and non-financial support from Teva, personal fees and non-financial support from Boehringer Ingelheim, personal fees from AstraZeneca, personal fees from WebMD/Medscape, personal fees from Sanofi/Regeneron, personal fees from Vectura, personal fees from Circassia, outside the submitted work. D.T.M. reports grants from the NIH, grants from Boehringer-Ingelheim, grants from TEVA, grants from AstraZeneca, grants from GlaxoSmithKline, grants from Sanofi, grants from Genentech, during the conduct of the study; non-financial support from Vifor-Pharma, non-financial support from Merck, outside the submitted work. J.V.H. reports grants from NIH/NHLBI, grants from Boehringer Ingelheim, during the conduct of the study; personal fees from Boehringer Ingelheim, personal fees from Pieris, personal fees from Arrowhead Pharmaceuticals, personal fees from Gossamer, outside the submitted work; In addition, Dr. Fahy has a patent US20110123530A1 - “Compositions and methods for treating and diagnosing asthma” issued, a patent WO2014153009A2 - Thiosaccharide mucolytic agents issued, and a patent WO2017197360 - “CT Mucus Score” - A new scoring system that quantifies airway mucus impaction using CT lung scans. S.B.F. reports grants from NIH/NHLBI, during the conduct of the study; personal fees from COPD Gene Foundation, personal fees from Sanofi/Regeneron, grants from GE Healthcare, outside the submitted work; and Serves as the Physics Chair of the CT lung density biomarker committee within the Quantitative Imaging Biomarker Alliance (QIBA). L.C.D. reports grants from NHLBI, during the conduct of the study; grants and personal fees from AstraZeneca, personal fees from Sanofi, grants from TEVA, outside the submitted work. G.W. reports grants from NIH, grants and other from Boehringer Ingelheim, other from Quantitative Imaging Solutions, other from PulmonX, grants from BTG Interventional Medicine, grants and other from Janssen Pharmaceuticals, other from GlaxoSmithKline, other from Novartis, other from Vertex, outside the submitted work; and G.W.’s spouse works for Biogen. E.I. reports grants from AstraZeneca, non-financial support from GlaxoSmithKline, during the conduct of the study; personal fees from AB Science, grants and personal fees from Amgen, grants and personal fees from AstraZeneca, grants and personal fees from Avillion, personal fees from Biometry, personal fees from Equillium, personal fees from Merck, grants and personal fees from Novartis, personal fees from 4D Pharma, personal fees from Pneuma Respiratory, personal fees from PPS Health, personal fees from Regeneron, personal fees from Sanofi Genzyme, personal fees from Sienna Biopharmaceutical, other from Vorso Corp, grants, personal fees and non-financial support from Genentech, personal fees and non-financial support from GlaxoSmithKline, personal fees and non-financial support from TEVA, grants from Gossamer Bio, grants and non-financial support from Circassia, non-financial support from Boehringer Ingelheim, outside the submitted work; E.H. is a founder and shareholder of VIDA Diagnostics, a company commercializing lung image analysis software developed, in part, at the University of Iowa. S.E.W. reports grants from Boerhringer-Ingelheim to support SARP3 visits, during the conduct of the study; grants and personal fees from AstraZeneca, grants and personal fees from GSK, grants and personal fees from Sanofi, grants from Novartis, personal fees from Pieris, outside the submitted work. M.C. receives University Grant Funding from NIH, American Lung Association, PCORI; receives Pharmaceutical Grant Funding from AstraZeneca, GSK, Novartis, Pulmatrix, Sanofi-Aventis, Shionogi; is a consultant for Genentech, Teva, Sanofi-Aventis, Novartis; he is a speaker for AstraZeneca, Genentech, GSK, Regeneron, Sanofi, Teva; and receives Royalties from Elsevier.
References:
- 1.To T, Stanojevic S, Moores G, Gershon AS, Bateman ED, Cruz AA, et al. Global asthma prevalence in adults: findings from the cross-sectional world health survey. BMC Public Health 2012; 12:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masoli M, Fabian D, Holt S, Beasley R, Global Initiative for Asthma P. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy 2004; 59:469–78. [DOI] [PubMed] [Google Scholar]
- 3.Matsunaga K, Akamatsu K, Miyatake A, Ichinose M. Natural history and risk factors of obstructive changes over a 10-year period in severe asthma. Respir Med 2013; 107:355–60. [DOI] [PubMed] [Google Scholar]
- 4.ten Brinke A, Zwinderman AH, Sterk PJ, Rabe KF, Bel EH. Factors associated with persistent airflow limitation in severe asthma. Am J Respir Crit Care Med 2001; 164:744–8. [DOI] [PubMed] [Google Scholar]
- 5.Covar RA, Spahn JD, Murphy JR, Szefler SJ, Childhood Asthma Management Program Research G. Progression of asthma measured by lung function in the childhood asthma management program. Am J Respir Crit Care Med 2004; 170:234–41. [DOI] [PubMed] [Google Scholar]
- 6.Peat JK, Woolcock AJ, Cullen K. Rate of decline of lung function in subjects with asthma. Eur J Respir Dis 1987; 70:171–9. [PubMed] [Google Scholar]
- 7.Lange P, Parner J, Vestbo J, Schnohr P, Jensen G. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med 1998; 339:1194–200. [DOI] [PubMed] [Google Scholar]
- 8.Boulet LP. Irreversible airway obstruction in asthma. Curr Allergy Asthma Rep 2009; 9:168–73. [DOI] [PubMed] [Google Scholar]
- 9.Bai TR, Vonk JM, Postma DS, Boezen HM. Severe exacerbations predict excess lung function decline in asthma. Eur Respir J 2007; 30:452–6. [DOI] [PubMed] [Google Scholar]
- 10.Matsunaga K, Hirano T, Oka A, Tanaka A, Kanai K, Kikuchi T, et al. Progression of Irreversible Airflow Limitation in Asthma: Correlation with Severe Exacerbations. J Allergy Clin Immunol Pract 2015; 3:759–64e1. [DOI] [PubMed] [Google Scholar]
- 11.Ortega H, Yancey SW, Keene ON, Gunsoy NB, Albers FC, Howarth PH. Asthma Exacerbations Associated with Lung Function Decline in Patients with Severe Eosinophilic Asthma. J Allergy Clin Immunol Pract 2018; 6:980–6e1. [DOI] [PubMed] [Google Scholar]
- 12.Vonk JM, Jongepier H, Panhuysen CI, Schouten JP, Bleecker ER, Postma DS. Risk factors associated with the presence of irreversible airflow limitation and reduced transfer coefficient in patients with asthma after 26 years of follow up. Thorax 2003; 58:322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Witt CA, Sheshadri A, Carlstrom L, Tarsi J, Kozlowski J, Wilson B, et al. Longitudinal changes in airway remodeling and air trapping in severe asthma. Acad Radiol 2014; 21:986–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coumou H, Westerhof GA, de Nijs SB, Zwinderman AH, Bel EH. Predictors of accelerated decline in lung function in adult-onset asthma. Eur Respir J 2018; 51. [DOI] [PubMed] [Google Scholar]
- 15.Brumpton BM, Langhammer A, Henriksen AH, Camargo CA Jr., Chen Y, Romundstad PR, et al. Vitamin D and Lung Function Decline in Adults With Asthma: The HUNT Study. Am J Epidemiol 2016; 183:739–46. [DOI] [PubMed] [Google Scholar]
- 16.Bui DS, Perret JL, Walters EH, Abramson MJ, Burgess JA, Bui MQ, et al. Lifetime Risk Factors for Pre- and Post-Bronchodilator Lung Function Decline: A Population-based Study. Ann Am Thorac Soc 2019. [DOI] [PubMed] [Google Scholar]
- 17.Wang M, Aaron CP, Madrigano J, Hoffman EA, Angelini E, Yang J, et al. Association Between Long-term Exposure to Ambient Air Pollution and Change in Quantitatively Assessed Emphysema and Lung Function. JAMA 2019; 322:546–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denlinger LC, Phillips BR, Sorkness RL, Bleecker ER, Castro M, Fitzpatrick AM, et al. Insensitivity to Corticosteroids Predicts Longitudinal Loss of Lung Function in the NHLBI Severe Asthma Research Program (SARP III) American Thoracic Society. Dallas, TX, 2019. [Google Scholar]
- 19.Washko GR, Parraga G. COPD biomarkers and phenotypes: opportunities for better outcomes with precision imaging. Eur Respir J 2018; 52. [DOI] [PubMed] [Google Scholar]
- 20.Trivedi A, Hall C, Hoffman EA, Woods JC, Gierada DS, Castro M. Using imaging as a biomarker for asthma. J Allergy Clin Immunol 2017; 139:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacob J, Bartholmai BJ, Rajagopalan S, van Moorsel CHM, van Es HW, van Beek FT, et al. Predicting Outcomes in Idiopathic Pulmonary Fibrosis Using Automated Computed Tomographic Analysis. Am J Respir Crit Care Med 2018; 198:767–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacob J, Bartholmai BJ, Rajagopalan S, Brun AL, Egashira R, Karwoski R, et al. Evaluation of computer-based computer tomography stratification against outcome models in connective tissue disease-related interstitial lung disease: a patient outcome study. BMC Med 2016; 14:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacob J, Hirani N, van Moorsel CHM, Rajagopalan S, Murchison JT, van Es HW, et al. Predicting outcomes in rheumatoid arthritis related interstitial lung disease. Eur Respir J 2019; 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sieren JP, Newell JD Jr., Barr RG, Bleecker ER, Burnette N, Carretta EE, et al. SPIROMICS Protocol for Multicenter Quantitative Computed Tomography to Phenotype the Lungs. Am J Respir Crit Care Med 2016; 194:794–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi S, Hoffman EA, Wenzel SE, Castro M, Fain S, Jarjour N, et al. Quantitative computed tomographic imaging-based clustering differentiates asthmatic subgroups with distinctive clinical phenotypes. J Allergy Clin Immunol 2017; 140:690–700e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta S, Hartley R, Khan UT, Singapuri A, Hargadon B, Monteiro W, et al. Quantitative computed tomography-derived clusters: redefining airway remodeling in asthmatic patients. J Allergy Clin Immunol 2014; 133:729–38e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galban CJ, Han MK, Boes JL, Chughtai KA, Meyer CR, Johnson TD, et al. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat Med 2012; 18:1711–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi S, Haghighi B, Choi J, Hoffman EA, Comellas AP, Newell JD, et al. Differentiation of quantitative CT imaging phenotypes in asthma versus COPD. BMJ Open Respir Res 2017; 4:e000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Subramanian DR, Gupta S, Burggraf D, Vom Silberberg SJ, Heimbeck I, Heiss-Neumann MS, et al. Emphysema- and airway-dominant COPD phenotypes defined by standardised quantitative computed tomography. Eur Respir J 2016; 48:92–103. [DOI] [PubMed] [Google Scholar]
- 30.Belloli EA, Degtiar I, Wang X, Yanik GA, Stuckey LJ, Verleden SE, et al. Parametric Response Mapping as an Imaging Biomarker in Lung Transplant Recipients. Am J Respir Crit Care Med 2017; 195:942–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhatt SP, Soler X, Wang X, Murray S, Anzueto AR, Beaty TH, et al. Association between Functional Small Airway Disease and FEV1 Decline in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 2016; 194:178–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teague WG, Phillips BR, Fahy JV, Wenzel SE, Fitzpatrick AM, Moore WC, et al. Baseline Features of the Severe Asthma Research Program (SARP III) Cohort: Differences with Age. J Allergy Clin Immunol Pract 2018; 6:545–54e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newell JD Jr., Sieren J, Hoffman EA. Development of quantitative computed tomography lung protocols. J Thorac Imaging 2013; 28:266–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD 2010; 7:32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niimi A, Matsumoto H, Amitani R, Nakano Y, Mishima M, Minakuchi M, et al. Airway wall thickness in asthma assessed by computed tomography. Relation to clinical indices. Am J Respir Crit Care Med 2000; 162:1518–23. [DOI] [PubMed] [Google Scholar]
- 36.Nakano Y, Muro S, Sakai H, Hirai T, Chin K, Tsukino M, et al. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am J Respir Crit Care Med 2000; 162:1102–8. [DOI] [PubMed] [Google Scholar]
- 37.Castro M, Fain SB, Hoffman EA, Gierada DS, Erzurum SC, Wenzel S, et al. Lung imaging in asthmatic patients: the picture is clearer. J Allergy Clin Immunol 2011; 128:467–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel BD, Coxson HO, Pillai SG, Agusti AG, Calverley PM, Donner CF, et al. Airway wall thickening and emphysema show independent familial aggregation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008; 178:500–5. [DOI] [PubMed] [Google Scholar]
- 39.Galban CJ, Boes JL, Bule M, Kitko CL, Couriel DR, Johnson TD, et al. Parametric response mapping as an indicator of bronchiolitis obliterans syndrome after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2014; 20:1592–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ostridge K, Gove K, Paas KHW, Burke H, Freeman A, Harden S, et al. Using Novel Computed Tomography Analysis to Describe the Contribution and Distribution of Emphysema and Small Airways Disease in Chronic Obstructive Pulmonary Disease. Ann Am Thorac Soc 2019; 16:990–7. [DOI] [PubMed] [Google Scholar]
- 41.Kirby M, Yin Y, Tschirren J, Tan WC, Leipsic J, Hague CJ, et al. A Novel Method of Estimating Small Airway Disease Using Inspiratory-to-Expiratory Computed Tomography. Respiration 2017; 94:336–45. [DOI] [PubMed] [Google Scholar]
- 42.Bell AJ, Foy BH, Richardson M, Singapuri A, Mirkes E, van den Berge M, et al. Functional CT imaging for identification of the spatial determinants of small-airways disease in adults with asthma. J Allergy Clin Immunol 2019; 144:83–93. [DOI] [PubMed] [Google Scholar]
- 43.Amelon R, Cao K, Ding K, Christensen GE, Reinhardt JM, Raghavan ML. Three-dimensional characterization of regional lung deformation. J Biomech 2011; 44:2489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43:343–73. [DOI] [PubMed] [Google Scholar]
- 45.Juniper EF, Svensson K, Mork AC, Stahl E. Modification of the asthma quality of life questionnaire (standardised) for patients 12 years and older. Health Qual Life Outcomes 2005; 3:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shaw D Putting the brake on accelerated lung function decline in asthma. Eur Respir J 2018; 51. [DOI] [PubMed] [Google Scholar]
- 47.Boulet LP, Reddel HK, Bateman E, Pedersen S, FitzGerald JM, O’Byrne PM. The Global Initiative for Asthma (GINA): 25 years later. Eur Respir J 2019; 54. [DOI] [PubMed] [Google Scholar]
- 48.Herth FJF, Kirby M, Sieren J, Herth J, Schirm J, Wood S, et al. The Modern Art of Reading Computed Tomography Images of the Lungs: Quantitative CT. Respiration 2018; 95:8–17. [DOI] [PubMed] [Google Scholar]
- 49.Aysola RS, Hoffman EA, Gierada D, Wenzel S, Cook-Granroth J, Tarsi J, et al. Airway remodeling measured by multidetector CT is increased in severe asthma and correlates with pathology. Chest 2008; 134:1183–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gono H, Fujimoto K, Kawakami S, Kubo K. Evaluation of airway wall thickness and air trapping by HRCT in asymptomatic asthma. Eur Respir J 2003; 22:965–71. [DOI] [PubMed] [Google Scholar]
- 51.Harmanci E, Kebapci M, Metintas M, Ozkan R. High-resolution computed tomography findings are correlated with disease severity in asthma. Respiration 2002; 69:420–6. [DOI] [PubMed] [Google Scholar]
- 52.Shifren A, Witt C, Christie C, Castro M. Mechanisms of remodeling in asthmatic airways. J Allergy (Cairo) 2012; 2012:316049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bai TR, Knight DA. Structural changes in the airways in asthma: observations and consequences. Clin Sci (Lond) 2005; 108:463–77. [DOI] [PubMed] [Google Scholar]
- 54.Mohamed Hoesein FA, de Hoop B, Zanen P, Gietema H, Kruitwagen CL, van Ginneken B, et al. CT-quantified emphysema in male heavy smokers: association with lung function decline. Thorax 2011; 66:782–7. [DOI] [PubMed] [Google Scholar]
- 55.Vestbo J, Edwards LD, Scanlon PD, Yates JC, Agusti A, Bakke P, et al. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med 2011; 365:1184–92. [DOI] [PubMed] [Google Scholar]
- 56.Young HM, Eddy RL, Parraga G. MRI and CT lung biomarkers: Towards an in vivo understanding of lung biomechanics. Clin Biomech (Bristol, Avon) 2019; 66:107–22. [DOI] [PubMed] [Google Scholar]
- 57.Bodduluri S, Newell JD Jr., Hoffman EA, Reinhardt JM. Registration-based lung mechanical analysis of chronic obstructive pulmonary disease (COPD) using a supervised machine learning framework. Acad Radiol 2013; 20:527–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jahani N, Yin Y, Hoffman EA, Lin CL. Assessment of regional non-linear tissue deformation and air volume change of human lungs via image registration. J Biomech 2014; 47:1626–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choi S, Hoffman EA, Wenzel SE, Tawhai MH, Yin Y, Castro M, et al. Registration-based assessment of regional lung function via volumetric CT images of normal subjects vs. severe asthmatics. J Appl Physiol (1985) 2013; 115:730–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jahani N, Choi S, Choi J, Haghighi B, Hoffman EA, Comellas AP, et al. A four-dimensional computed tomography comparison of healthy and asthmatic human lungs. J Biomech 2017; 56:102–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bhatt SP, Bodduluri S, Hoffman EA, Newell JD Jr., Sieren JC, Dransfield MT, et al. Computed Tomography Measure of Lung at Risk and Lung Function Decline in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 2017; 196:569–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bodduluri S, Bhatt SP, Hoffman EA, Newell JD Jr., Martinez CH, Dransfield MT, et al. Biomechanical CT metrics are associated with patient outcomes in COPD. Thorax 2017; 72:409–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McGregor MC, Krings JG, Nair P, Castro M. Role of Biologics in Asthma. Am J Respir Crit Care Med 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krings JG, McGregor MC, Bacharier LB, Castro M. Biologics for Severe Asthma: Treatment-Specific Effects Are Important in Choosing a Specific Agent. J Allergy Clin Immunol Pract 2019; 7:1379–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


