Abstract
Background
Plasma ferritin is a widely used indicator to detect iron deficiency, but the threshold ferritin that defines iron deficiency remains uncertain. Our aim was to define the ferritin concentration at which the body begins to upregulate iron absorption from the diet; this could provide a functionally-defined threshold of incipient iron deficiency. We hypothesized this threshold ferritin concentration would correspond to the threshold hepcidin concentration at which iron absorption begins to increase.
Methods
We performed a pooled analysis of our stable iron isotope studies (n = 1058) conducted from 2006 to 2019 in healthy women (age 18–50 years; mean±SD ferritin 33.7 ± 27.1 μg/L) that measured iron absorption from labeled test meals providing physiological amounts of iron. To fit relationships between iron absorption, ferritin and hepcidin, we used generalized additive modeling, and to identify thresholds, we estimated the first derivatives of the fitted trend to assess inflection points in these relationships.
Findings
Hepcidin increased linearly with increasing ferritin over the entire range of ferritin values. Iron absorption began to increase below a threshold hepcidin value of 3.09 (95%CI: 2.80, 3.38) nmol/l, above which iron absorption remained stable. Iron absorption began to increase below a threshold ferritin value of 51.1 (95%CI: 49.1, 53.1) µg/l, above which iron absorption remained stable. The latter two findings were internally consistent in that, in the relationship between hepcidin and ferritin, a hepcidin of ~3 nmol/l corresponded to a ferritin of ~51 µg/l.
Interpretation
Based on physiological upregulation of iron absorption, a threshold ferritin of <50 µg/L, corresponding to a threshold hepcidin of <3 nmol/l, indicates incipient iron deficiency in young women.
Research in context.
Evidence before this study
We initially searched PubMed using the search terms “ferritin” AND “iron deficiency” OR “ferritin” AND “iron absorption” OR “ferritin cut-off” OR “ferritin threshold” with no language or date restrictions; the date of our first search was July 10, 2020, and our last search was on April 21, 2021. Two recent systematic reviews concluded that the threshold ferritin concentration that defines iron deficiency remains unclear and diagnostic ferritin cutoffs from expert groups vary widely. A recent WHO systematic review emphasized the need for additional research to establish a ferritin cutoff for iron deficiency, and suggested upregulation of iron absorption based on isotopic studies could be useful for this purpose.
Added value of this study
To our knowledge, this is the first study to use the upregulation of iron absorption measured using stable isotopes to propose a ferritin threshold indicating early iron deficiency. Our data suggest a threshold ferritin of <50 µg/L indicates incipient iron deficiency in young women based on upregulation of iron absorption, and this threshold is coherent with relationships between plasma hepcidin and iron absorption in this population.
Implications of all the available evidence
Our proposed threshold is generally consistent with a recent systematic review and clinical practice guideline that suggests a ferritin threshold of <45 μg/L maximizes sensitivity to identify iron deficiency in young women with an acceptable number of false-positive diagnoses. In future research, the methods described here could be used in other populations, including pregnant women, children and infants, to define the ferritin threshold indicating incipient iron deficiency in those groups.
Alt-text: Unlabelled box
1. Introduction
Iron deficiency is estimated to affect two billion people and is the leading cause of anemia worldwide [1]. The serum or plasma ferritin concentration (referred to hereafter as ferritin) is the most widely used indicator to detect iron deficiency and a low ferritin indicates depleted iron stores [2]. However, the threshold ferritin that defines iron deficiency remains unclear [3] and diagnostic ferritin cutoffs from expert groups vary widely [4–6]. WHO proposes that iron deficiency is present in adults when ferritin is <15 µg/L 2. This threshold was derived from studies examining the highest ferritin among adults with iron-deficiency anemia who showed a therapeutic response to iron or who had absent bone marrow iron [3]. Considering this definition, it is not unexpected that a ferritin <15 µg/L is a specific but insensitive threshold for iron deficiency, which precedes iron-deficiency anemia. To increase the sensitivity of ferritin to detect iron deficiency, other experts recommend using higher cut-offs to avoid false-negative tests that would leave iron deficiency untreated [3,5–7]. However, there is little evidence available to justify higher ferritin thresholds. Moreover, many studies that compared ferritin to absent bone marrow iron were done in hospitalized patients and were likely biased by inflammation, that increases ferritin independent of iron stores [3].
A largely unexplored reference method for defining iron deficiency is based on the fact that low iron stores trigger an increase in dietary iron absorption [8]. In otherwise healthy individuals, low hepatic iron stores attenuate the bone morphogenetic protein–SMAD (BMP-SMAD) pathway, which reduces hepatic hepcidin synthesis, and low circulating hepcidin increases dietary iron absorption [9,10]. Importantly, dietary iron absorption is already increased when a reduction in liver iron stores has not yet reduced serum iron or transferrin saturation [8]. Iron absorption measured via erythrocyte iron incorporation of isotopic tracers has long been regarded as the most sensitive indirect measure of body iron stores [8,11,12].
A recent WHO review emphasized the need for additional research to establish a ferritin cutoff for iron deficiency [3]. Using standardized methods, our laboratory has performed multiple stable iron isotope studies in healthy young women with varying iron status to measure iron absorption from meals extrinsically-labeled with ferrous sulfate. Pooling these data, our study aim was to define the ferritin concentration in young women at which the body senses iron depletion and begins to upregulate iron absorption from the diet; this approach could provide a functionally-defined threshold of incipient iron deficiency. At the same time, we hypothesized that this threshold ferritin value would correspond to the hepcidin threshold at which iron absorption begins to increase.
2. Participants and methods
2.1. Studies and participants
We extracted the study design and participant characteristics from iron absorption studies performed by the ETH Zurich between 1990 and 2020. For this analysis, we included only meal-based studies that provided iron at levels found in usual mixed diets; supplementation studies with high doses of iron were excluded. We included studies if the extrinsic stable iron isotopic label was given as ferrous sulfate (FeSO4) in a test meal containing less than 16 mg total iron (as added label plus native iron content). From these studies, we included only data from healthy young women free of inflammation. Inclusion criteria were: (1) female; (2) premenopausal and non-pregnant/non-lactating; (3) C-reactive protein <5 mg/l (because of the known effect of inflammation on ferritin and iron absorption); (4) normal weight, defined as a BMI <25 kg/m2 in non-Asian women and <23 kg/m2 for Asian women (because of the effect of overweight on inflammation and iron absorption [13]; (5) non-smokers; (6) no disorders of iron metabolism, or chronic digestive, malabsorptive, renal or metabolic disease; (7) no chronic medications (except for oral contraceptives); (8) no recent use of iron supplements; and (9) no recent blood transfusion or blood donation or significant blood loss or surgery.
2.2. Ethical approval
All studies received ethical approval from a competent authority and informed written consent was obtained from all subjects. The Ethics Committee of the Canton Zurich approved the present pooled data analysis (2020–02,399). According to the requirements of the Ethics Committee, we excluded from the analysis women (n = 12) from studies performed in Switzerland since 2018 who did not specifically provide written informed consent for the further use of data.
2.3. Determination of iron absorption
In all studies, we measured iron absorption after an overnight fast (no food intake after 8PM, no drink intake after 12AM) by a standardized stable-isotope technique in which the incorporation of oral 57Fe-, 58Fe- or 54Fe into erythrocytes was measured 14 days after administration [14]. We measured height and weight using standardized methods. We collected an overnight fasting venous blood sample in the morning to measure ferritin, hepcidin and C-reactive protein either at baseline screening (8 studies) or just before meal administration (16 studies). The test meals (see Supplementary Material) were administered between 7 and 9AM under standardized conditions and close supervision. All meals were extrinsically-labelled with ferrous sulfate, added just before feeding. No intake of food and fluids was allowed for at least 3 h after test meal intake. Fourteen days after test meal administration, we collected another fasting morning venous blood sample for isotopic measurements.
2.4. Blood analyses
All hematological and biochemical measurements were performed at the time of each study. We measured hemoglobin on the day of venipuncture by using a Coulter Counter (Beckman Coulter Life Sciences, Indianapolis, USA) or Hemocue (HemoCue AB, Ängelholm, Sweden). Plasma was separated by centrifugation and stored at −20 °C until analysis. We measured hepcidin by using an immunoassay (DRG GmbH, Marburg, Germany). We measured ferritin (n = 368 observations) and C-reactive protein (n = 322 observations) concentrations by using a multiplex sandwich ELISA at the VitMin Laboratory [15]. For the remaining observations, we measured ferritin (n = 690) by using an immunoassay (IMMULITE automatic system, Siemens; Cobas 8000 analyzer, Roche Diagnostics; Spectro Ferritin MTs, Ramco Laboratories)) and C-reactive protein (n = 736) by using a high sensitivity immunoassay (IMMULITE automatic system, Siemens; Cobas 8000 analyzer, Roche Diagnostics; BN ProSpec System, Dade Behring). We pooled data from these assays because comparison between the VitMin Laboratory ELISA assay and commercially available immunoassays for ferritin and C-reactive protein has shown high specificity and sensitivity and low intra-assay and inter-assay variability [15]. Using the ELISA, a C-reactive protein concentration >5 mg/L indicates the presence of inflammation [15]. Anemia was defined as Hb <120 g/L and iron-deficiency anemia was defined as anemia and ferritin <15 μg/L 16.
2.5. Preparation of isotopically labeled iron and isotopic analyses
Isotopically labeled 54Fe, 58Fe or 57FeSO4 was prepared from isotopically enriched elemental iron (see Supplemental Material) and for the isotopic analysis, iron was separated from whole blood as previously described [14,16] and analyzed by multi-collector inductively coupled plasma mass spectrometry or negative thermal ionization mass spectrometry (see Supplemental Material).
2.6. Statistical analyses
We conducted data analyses using R statistical programming environment (v3.6.3, R Core Team 2020, R Foundation for Statistical Computing, Vienna, Austria) (see Supplemental Material). To visualize the relationship between iron absorption and ferritin (and any other dependent versus independent crude variables), we used generalized additive modeling (see Supplemental Material), a modeling alternative to polynomial regression in clinical research [17]. To identify thresholds, we then used the method of finite differences to estimate the first derivatives (the instantaneous rate of change) of the fitted trend spline from the generalized additive modeling smoother. A similar mathematical approach has been recently applied in environmental and clinical research [18,19].
To determine an algorithm for adjustment of fractional iron absorption from absorption studies to a common ferritin to allow for between-subject comparisons [20], we transformed variables using natural logarithm and investigated the effect of the ferritin variable on fractional iron absorption by using linear mixed effects model analysis (see Supplemental Material). Significance level was set to <0.05.
2.7. Role of the funding source
The study sponsor had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
3. Results
3.1. Subject and meal characteristics
We included in the analysis a total of 1058 observations from 624 women from 24 studies. Research was performed in 5 countries: Switzerland (20 studies in 530 women providing 887 observations), Taiwan (1 study in 26 women providing 52 observations), Thailand (1 study in 18 women providing 18 observations), Mexico (1 study in 17 women providing 68 observations), and Lebanon (1 study in 33 women providing 33 observations) (studies are shown in Supplemental Table 1). Characteristics of the women are shown in Table 1. Variables that scored a Shapiro–Wilk W < 97 were ferritin, hepcidin, C-reactive protein and fractional iron absorption. Women were aged from 18.4 to 50.0 years. Mean ± SD ferritin was 33.7 ± 27.1 μg/L and 27.5% of women were iron deficient (as defined by a ferritin <15 μg/L); mean ± SD hemoglobin was 133 ± 9 g/L and 5.7% of the women were anemic.
Table 1.
Subject and meal characteristics.
| N | Mean ± SD | Median (IQR) | Prevalence (CI) | |
|---|---|---|---|---|
| Subjects | ||||
| Age, y | 911 | 24.9 ± 5.7 | 23.2 (21, 26.6) | |
| BMI, kg/m2 | 1058 | 20.9 ± 1.7 | 20.8 (19.6, 22) | |
| Ferritin, μg/L | 1058 | 33.7 ± 27.1 | 24.8 (13.8, 47.7) | |
| % <15 μg/L | 291 | 27.5 (24.8, 30.3) | ||
| % <15 μg/L, but no anemia | 244 | |||
| % <30 μg/L | 603 | 57.0 (53.9, 60.0) | ||
| % <30 μg/L, but no anemia | 546 | |||
| Hemoglobin, g/L | 1058 | 133 ± 9 | 134 (128, 139) | |
| Anemia,% | 60 | 5.7 (4.4, 7.2) | ||
| IDA%, based on ferritin <15 μg/L | 47 | 4.4 (3.3, 5.9) | ||
| IDA%, based on ferritin <30 μg/L | 57 | 5.4 (4.1, 6.9) | ||
| Plasma hepcidin, nmol/L | 228 | 2.37 ± 2.24 | 1.67 (0.84, 2.95) | |
| C-reactive protein, mg/L | 1058 | 1.01 ± 1.14 | 0.50 (0.22, 1.40) | |
| Meals | ||||
| Iron, mg | 1058 | 7.1 ± 3.7 | 6.0 (4.4, 8.3) | |
| Phytic acid, mg | 1006 | 146.3 ± 189.1 | 74 (46, 119) | |
| PA:Fe molar ratio | 1006 | 1.81 ± 2.82 | 1.01 (0.62, 1.88) | |
| Ascorbic acid, mg | 652 | 11.7 ± 17.4 | 0.3 (0, 30.2) | |
| AA:Fe molar ratio | 652 | 0.55 ± 0.97 | 0.02 (0, 0.97) | |
| Fractional iron absorption,% | 1058 | 11.7 ± 12.3 | 7.1 (3.3, 15.8) |
Data presented as mean ± SD, median (IQR), or prevalence (CI). CI, 95% confidence interval calculated using the Clopper–Pearson method for binomial proportions. AA, ascorbic acid; Fe, iron; IDA, iron-deficiency anemia; PA, phytic acid.
Characteristics of the test meals are shown in Table 1. The total iron (native plus added iron) content in the test meals varied between 2.5 and 15.7 mg (mean ± SD of 7.1 ± 3.7). Mean ± SD phytic acid content was 146.3 ± 189.1 mg (yielding a phytic acid: iron molar ratio of 1.81 ± 2.82) and ascorbic acid content was 11.7 ± 17.4 mg (ascorbic acid: iron molar ratio of 0.55 ± 0.97); thus, the meal matrices were mostly non-inhibitory to iron absorption . The mean ± SD fractional iron absorption was 11.7 ± 12.3% (Table 1), indicating meal matrices with moderate iron bioavailability [21], suggesting the fractional iron absorption values are physiological.
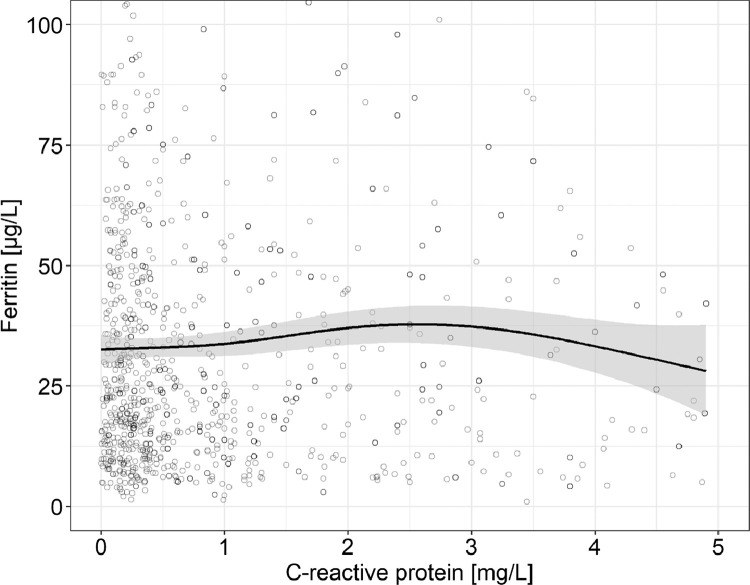
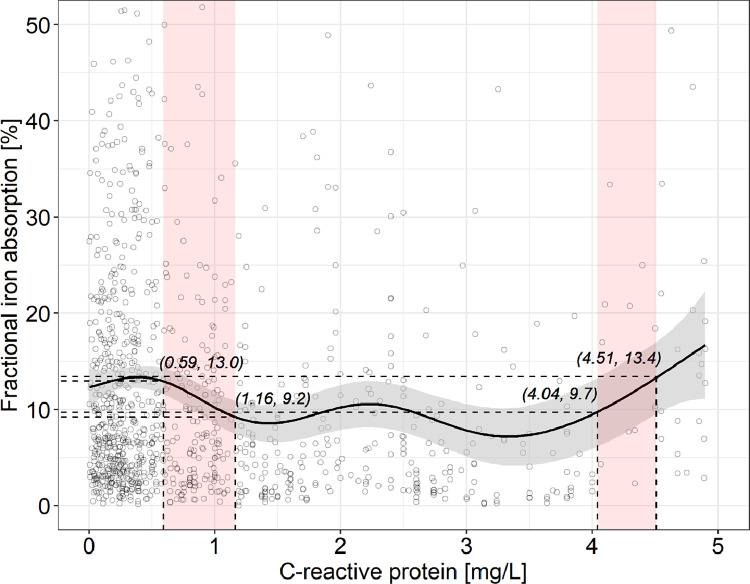
3.2. Relationships of C-reactive protein and ferritin, and C-reactive protein and fractional iron absorption
The fitted generalized additive modeling trend of C-reactive protein versus ferritin showed no clear effects of C-reactive protein on ferritin over the C-reactive protein range of 0 to 5 mg/L (R2 = 0.006) (Figs. 1 and 1S). The fitted generalized additive modeling trend of C-reactive protein versus fractional iron absorption showed no clear effect over the C-reactive protein range of 0 – 5 mg/L, with two exceptions (Figs. 2 and 2S): within the C-reactive protein range 0.59–1.16 mg/L, with a significantly negative slope, fractional iron absorption decreased from 13.0 to 9.2%; within the C-reactive protein range 4.04–4.51 mg/L, with a significantly positive slope, fractional iron absorption increased from 9.7 to 13.4%. However, overall, the variation in C-reactive protein explained only 2.8% of the variation in FIA.
Fig. 1.
C-reactive protein versus ferritin in healthy adult women with a C-reactive protein <5 mg/L (n = 1058). Observations with a ferritin >105 µg/L (n = 27) are not shown. The solid line shows the fitted values using a generalized additive model and the gray-shaded area shows the upper and lower 95% confidence intervals around the estimates. There are no clear patterns on the effect of C-reactive protein on ferritin (adjusted R2 = 0.006), as over the C-reactive protein range 0–5 mg/L, the model's slope never significantly differs from zero according to the estimation of the model's first derivative (Fig. S1).
Fig. 2.
C-reactive protein versus fractional iron absorption in healthy adult women with a C-reactive protein <5 mg/L (n = 1058). For greater clarity, observations with a fractional iron absorption >52.5% (n = 14) are not shown. The solid line shows the fitted values using a generalized additive model and the gray-shaded area shows the upper and lower 95% confidence intervals around the estimates. There are no clear patterns on the effect of C-reactive protein on fractional iron absoprtion (adjusted R2 = 0.028). Over the C-reactive protein range 0–5 mg/L, the model's slope oscillates periodically and in two ranges differs significantly from zero: fractional iron absorption decreases significantly from 13.0 to 9.2% with increasing C-reactive protein from 0.59 to 1.16 mg/l, and increases significantly from 9.7 to 13.4% with increasing C-reactive protein from 4.04 to 4.51 mg/l. The red-shaded areas show where the model's slope significantly differs from zero, according to the estimation of the model's first derivative (Fig. S2). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
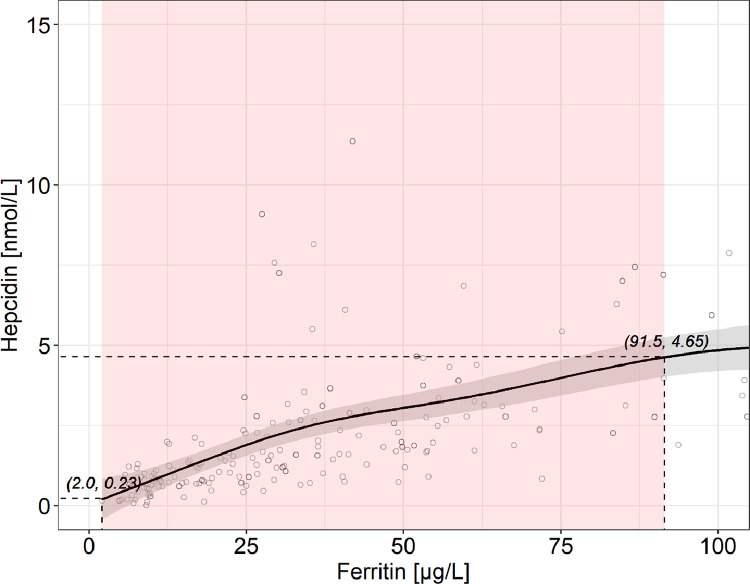
3.3. Relationship of hepcidin and ferritin
The fitted generalized additive modeling trend showed a near-linear relationship between ferritin and hepcidin, with a significantly positive slope over almost the entire ferritin range (until a ferritin of 91.5 µg/L) (Figs. 3 and 3S). In the relationship, a hepcidin of 3.09 nmol/L corresponded to a ferritin of 51.1 µg/L. The variability in ferritin explained 34.9% of the variability in hepcidin.
Fig. 3.
Ferritin versus hepcidin in healthy adult women with a C-reactive protein <5 mg/L (n = 228). For greater clarity, observations with a ferritin >105 µg/L (n = 10) are not shown. The solid line shows the fitted values using a generalized additive model and the gray-shaded area shows the upper and lower 95% confidence intervals around the estimates. Hepcidin increases significantly from 0.23 to 4.65 nmol/L with increasing ferritin over almost the entire range from 2.0 to 91.5 µg/L (adjusted R2 = 0.349). The red-shaded area shows where the model's slope significantly differs from zero, according to the estimation of the model's first derivative (Fig. S3). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
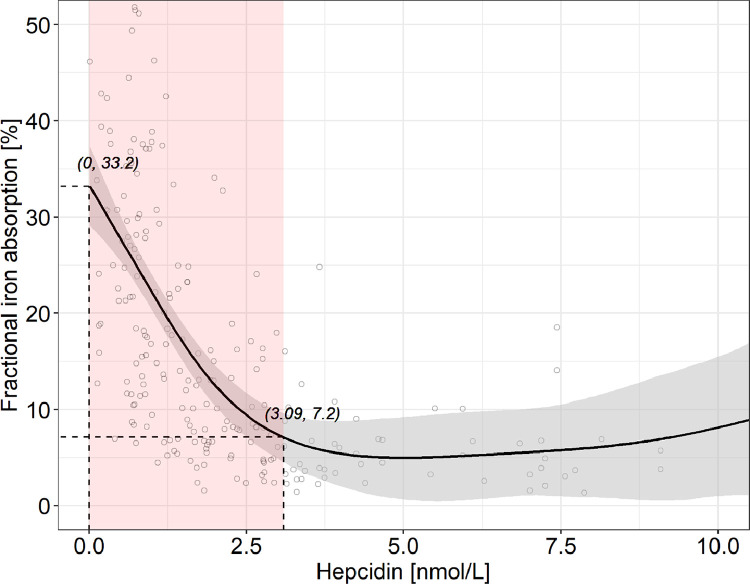
3.4. Relationship of hepcidin and fractional iron absorption
The fitted generalized additive modeling trend of hepcidin versus fractional iron absorption showed a significantly negative slope until a hepcidin of 3.09 (95% CI: 2.80, 3.38) nmol/L, which corresponded to a fractional iron absorption decrease from 33.2 to 7.2% (Figs. 4 and 4S). Above this hepcidin value, fractional iron absorption remained stable. Overall, the variation in hepcidin explained 41.8% of the variation in fractional iron absorption.
Fig. 4.
Hepcidin versus fractional iron absorption in healthy adult women with a C-reactive protein <5 mg/L (n = 228). For greater clarity, observations with a fractional iron absorption >50% (n = 3) or a hepcidin >10.5 nmol/L (n = 4) are not shown. The solid line shows the fitted values using a generalized additive model and the gray-shaded area shows the upper and lower 95% confidence intervals around the estimates. Fractional iron absorption decreases significantly from 33.2 to 7.2% with increasing hepcidin from 0 to 3.09 (95% CI: 2.80, 3.38) nmol/L, beyond which fractional iron absorption remains stable (adjusted R2 = 0.418). The red-shaded area shows where the model's slope significantly differs from zero, according to the estimation of the model's first derivative (Fig. S4). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.5. Relationship of fractional iron absorption and ferritin
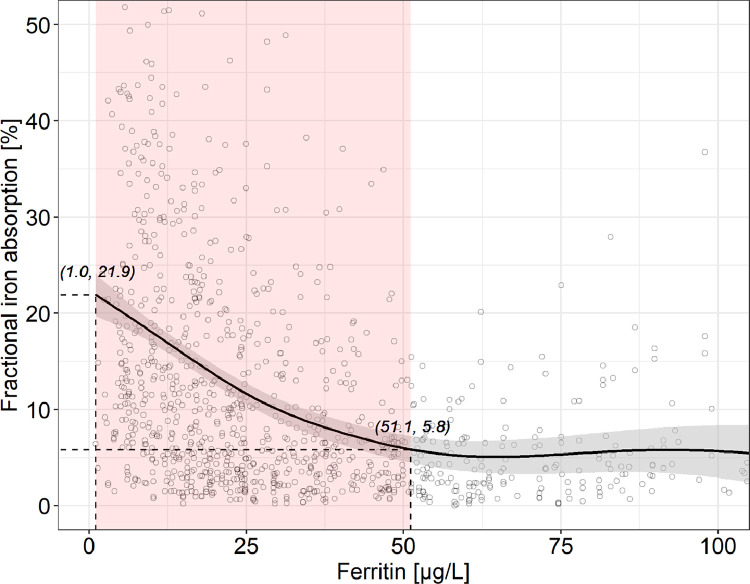
The fitted generalized additive modeling trend of ferritin versus fractional iron absorption showed a significantly negative slope until a ferritin of 51.1 (95% CI: 49.1, 53.1) µg/L, corresponding to a fractional iron absorption decrease from 21.9 to 5.8% (Figs. 5 and 5S). Above this ferritin value, fractional iron absorption remained stable. Overall, the variation in ferritin explained 18.1% of the variation in fractional iron absorption. Ferritin values of 15, 20, 30 and 50 µg/L correspond to a fractional iron absorption of 15.8, 13.6, 10.0% and 5.8%, respectively. When we repeated the analysis only in women with a CRP<1 mg/L (n = 710), there was a significantly negative slope until a ferritin of 48.7 (95% CI: 46.8, 50.6) µg/L, fractional iron absorption decreased from 23.7 to 6.7% and above this ferritin value, fractional iron absorption remained stable. Overall, the variation in ferritin explained 20.2% of the variation in fractional iron absorption.
Fig. 5.
Ferritin versus fractional iron absorption in healthy adult women with a C-reactive protein <5 mg/L (n = 1058). For greater clarity, observations with a fractional iron absorption >52.5% (n = 14) or a ferritin >105 µg/L (n = 27) are not shown. The solid line shows the fitted values using a generalized additive model and the gray-shaded area shows the upper and lower 95% confidence intervals around the estimates. Fractional iron absorption decreases significantly from 21.9 to 5.8% with increasing ferritin from 1.0 to 51.1 (95% CI: 49.1, 53.1) µg/L, beyond which fractional iron absorption remains stable (adjusted R2 = 0.181, effective degrees of freedom = 4.2, p < 0.001). The red-shaded area shows where the model's slope significantly differs from zero, according to the estimation of the model's first derivative (Fig. S5). Ferritin values of 15, 20, 30 and 50 µg/L correspond to a fractional iron absorption of 15.8, 13.6, 10.0% and 5.8%, respectively. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.6. Correction of individual iron absorption values to a common ferritin reference point
Because of the marked influence of iron status on iron absorption, comparisons of absorption between individuals requires a technique for correcting individual absorption values to a common reference point [20]. We used the linear mixed model shown in Table 2 to derive an algorithm which can be used to correct individual absorption values to a common ferritin reference value: Ln Ac = Ln Ao + 0.51 Ln (SFo / 46.5).
Table 2.
Linear mixed effects model analysis on the effect of ferritin on fractional iron absorption in healthy adult women with a C-reactive protein <5 mg/l (n = 1058).
| LN(fractional iron absorption) | |||
|---|---|---|---|
| Predictors | Estimates | CI | P |
| (Intercept) | 3.84 | 3.61 – 4.08 | <0.001 |
| LN(ferritin) | −0.51 | −0.58 – −0.44 | <0.001 |
| Random Effects | |||
| σ2 | 0.18 | ||
| τ00 Subject | 0.45 | ||
| N Subject | 624 | ||
| Observations | 1058 | ||
| Marginal R2 / Conditional R2 | 0.211 / 0.779 |
Data was analysed using linear mixed effects model analysis. CI, 95% confidence interval; LN, natural logarithm; τ00, variance of the random effect at the intercept; σ2, residual variance. Significance level was set to <0.05.
4. Discussion
The main findings of this study are, in healthy women without inflammation: (1) hepcidin increased linearly with increasing ferritin over nearly the entire range of ferritin values; (2) iron absorption began to increase below a threshold hepcidin value of 3.09 nmol/l, above which iron absorption remained stable; (3) iron absorption began to increase below a threshold ferritin value of 51.1 µg/l, above which iron absorption remained stable; and (4) the latter two findings were internally consistent in that, in the relationship between hepcidin and ferritin, a hepcidin of 3.09 nmol/l corresponded to a ferritin of 51.1 µg/l.
Ferritin is a positive acute phase protein [2] and is increased during inflammation independent of iron stores [22]. Most previous studies in hospital populations that compared ferritin to bone marrow iron to identify iron deficiency were likely biased by a high prevalence of inflammation [3]. In our analysis, we used C-reactive protein to assess the presence of inflammation, as recommended by the WHO [2] and we included only healthy women with a C-reactive protein <5 mg/L, the threshold for inflammation for our assay. In our data, there was no significant relationship between ferritin and C-reactive protein <5 mg/L (Fig. 1). This contrasts with the BRINDA study [22] that reported ferritin was increased in women even at a C-reactive protein value <5 mg/L, but that study included women from low- and middle-income countries with much higher rates of inflammation and the coefficients of determination (R2) for regressions between C-reactive protein and ferritin were low, at 0.06–0.19. When we repeated our ferritin versus iron absorption analysis including only women with a CRP <1 mg/L (n = 710), we derived a threshold ferritin that was comparable to the threshold derived including women with a CRP <5 mg/L. Therefore, we feel it is very unlikely that inflammation biased our ferritin measurements.
Classically, the reference definition of iron deficiency is the absence of stainable reticular iron in a bone marrow smear [3]. A recent technical review [6] comparing ferritin to absent bone marrow iron reported a ferritin threshold value of <15 μg/L had a sensitivity of 59% (95% CI: 55–62%) and specificity of 99% (95% CI: 89–99%) for iron deficiency, while a ferritin threshold value of <45 μg/L had a sensitivity of 85% (95% CI: 82–87%) with a specificity of 92% (95% CI: 91–94%). However, this technical review included data from studies that likely included participants with inflammation [23]. In healthy young adults (n = 20), a ferritin threshold of <40 μg/L had a sensitivity of 100% and specificity of 92% [24]. Another study in healthy young adults (n = 53), a ferritin threshold of <30 μg/L had a sensitivity of 100% and specificity of 89% [25]. In a study in healthy women, mean ferritin was 13 μg/L when bone marrow iron was absent (n = 69) and 54 μg/L when bone marrow iron was present (n = 105), and many women with ferritin in the range of 15–40 µg/L already had signs of iron deficient erythropoiesis [26]. Thus, our data suggesting a ferritin threshold of <50 μg/L identifies iron deficiency based on upregulation of iron absorption appears generally consistent with previous studies based on absent bone marrow iron.
An early study using radioiron [8] compared ferritin and iron absorption from ferrous sulfate reference dose in healthy non-anemic women (n = 83) without iron-deficient erythropoiesis, defined as a transferrin saturation >20%, and who had a mean ± SD ferritin of 36 ± 27 μg/L. There was an inverse relationship between ferritin and mean quintiles of iron absorption (r= −0.58, P < 0.001) and a progressive increase in iron absorption over a range of ferritin from 45 to 5 μg/L: absorption was less than 10% at a mean ferritin of 37 μg/L and 40% at a ferritin of 5 μg/L 8. A study that correlated iron absorption from a radiolabeled ferrous sulfate to ferritin in n = 870 adults, iron absorption correlated well with ferritin even in those with normal transferrin saturation [27]. Our findings are consistent with these studies, and others [28] that demonstrate that iron absorption is increased in ‘latent’ iron deficiency, a condition in which a reduction in iron stores has not yet reduced plasma iron and transferrin saturation [8,11,12]. Thus, upregulation of iron absorption appears to be a sensitive and very early indicator of depleted iron stores.
In healthy individuals, in the absence of recent iron supplementation (an exclusion criterion in our study), ferritin is an indirect measure of body iron stores [3]. The ferritin concentration is directly proportional to the amount of iron stored, with 1 µg/L ferritin corresponding to 8–10 mg storage iron [29]. Therefore, our threshold ferritin of 50 µg/L suggests iron sufficiency in young women may be the presence of at least 400–500 mg storage iron. Notably, this value was previously estimated to be the minimal iron store required in women for an iron-sufficient pregnancy, provided that the diet contained ample amounts of bioavailable iron [30]. Our data suggest that, as body iron stores fall below 400–500 mg, before complete depletion of stores lowers circulating iron and impairs erythropoiesis [8], there is a signal to decrease hepatic hepcidin synthesis, and circulating hepcidin falls. Although there was a linear relationship between hepcidin and ferritin in our data, our data suggest when hepcidin falls below ~3 nmol/L using our assay (corresponding to a ferritin of ~50 µg/L), decreased hepcidin signaling to ferroportin on enterocytes [9] allows upregulation of iron absorption. Thus, our data suggest a threshold hepcidin concentration (assay specific) that identifies incipient iron deficiency is 3.09 nmol/L.
Previous methods used to define ferritin thresholds to identify iron deficiency include receiver operating characteristic curves (versus absent bone marrow iron) and the evaluation of population reference ranges [3]. In this study, we used the non-parametric regression method of generalized additive modeling to fit the relationship between variables [19]. This approach is increasingly applied in biology and medicine[18,19], and we feel it has several advantages. First, it can be used when other predefined parametric forms do not perform well or cannot be effectively applied. Second, its flexibility to fit spline models with an automated selection of knots allows it, better than other models, to near the LOESS fit, a moving regression to localized subsets of data that describes the deterministic part of the variation in the data, point by point. Finally, this approach has advantages over previous methods because it uses non-transformed continuous variables and observes their (almost) true relationship, and derives at which x the slope between the fitted y and x begins to be significantly positive or negative by examining the first derivative.
Our study has other strengths. First, we studied young women with a broad range of iron status, and minimized potential bias on ferritin by excluding subjects with inflammation or recent iron supplementation. We examined the relationship between ferritin and iron absorption using physiological doses of non-heme iron in several test meals; this minimized potential bias that higher supplemental doses may have on iron absorption. We used the reference method for assessing iron absorption and included >1000 measurements allowing for high precision in the estimation of the ferritin threshold. Finally, the internal consistency of the proposed hepcidin and ferritin thresholds strengthens their validity. Our study also has limitations. Our proposed hepcidin threshold was derived from fewer measurements and is assay-specific, so should not be applied to data from other hepcidin assays. We only studied women with ferritin values up to 154 μg/L. Although there is no good evidence that ferritin thresholds differ for adult men and women [3], our proposed ferritin threshold should only be applied to young women and may not be applicable to other age groups.
To our knowledge, this is the first study to use the upregulation of iron absorption measured using stable isotopes to propose a ferritin threshold indicating incipient iron deficiency. Our findings imply early iron deficiency may be more common in young women than previously appreciated; e.g., just over half of US females aged 12 to 40 years have ferritin levels below 45 µg/L [31]. Our proposed threshold is consistent with a new clinical practice guideline [7] that defined iron deficiency according to absent bone marrow iron stores (not adjusted for inflammation) and recommends a ferritin threshold of <45 μg/L maximizes sensitivity to identify iron deficiency with an acceptable number of false-positive diagnoses. In future research, the methods described here could be used in other populations, including pregnant women, children and infants, to define the ferritin threshold indicating iron deficiency in those groups.
4.1. Data sharing statement
Individual participant data and study protocols are available to be shared. Data will be available immediately following publication and ending 5 years following publication. Data will be shared with researchers who provide a methodologically sound proposal. Proposals should be directed to michael.zimmermann@hest.ethz.ch; to gain access, data requestors will need to sign a data access agreement.
Authorship contributions
V.G., D.M. and M.B.Z. designed research; V.G., N.U.S., C.S., C.Z., D.M. and M.B.Z. conducted research; V.G., C.S. and M.B.Z. had access to the raw data; V.G. and C.S. analyzed data; V.G. and M.B.Z. wrote the first draft of the paper; all authors edited the paper; V.G. and M.B.Z. had primary responsibility for final content. All authors approved the final version of the paper.
Declaration of Competing Interest
None of the authors had a conflict of interest with regard to this manuscript.
Acknowledgments
Acknowledgments
We thank the participants and the nursing and laboratory staff at the Human Nutrition Laboratory at the ETH Zurich, Switzerland.
Funding
Funding for this study was provided by the ETH Zurich.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.101052.
Appendix. Supplementary materials
References
- 1.Kassebaum N.J., Jasrasaria R., Naghavi M. A systematic analysis of global anemia burden from 1990 to 2010. Blood. 2014;123(5):615–624. doi: 10.1182/blood-2013-06-508325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . World Health Organization; Geneva: 2020. Guideline on use of Ferritin Concentrations to Assess Iron Status in Individuals and Populations. [PubMed] [Google Scholar]
- 3.Garcia-Casal M.N., Pasricha S.R., Martinez R.X., Lopez-Perez L., Pena-Rosas J.P. Are current serum and plasma ferritin cut-offs for iron deficiency and overload accurate and reflecting iron status? A systematic review. Arch Med Res. 2018;49(6):405–417. doi: 10.1016/j.arcmed.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Peyrin-Biroulet L., Williet N., Cacoub P. Guidelines on the diagnosis and treatment of iron deficiency across indications: a systematic review. Am J Clin Nutr. 2015;102(6):1585–1594. doi: 10.3945/ajcn.114.103366. [DOI] [PubMed] [Google Scholar]
- 5.Daru J., Colman K., Stanworth S.J., De la Salle B., Wood E.M., Pasricha S.R. Serum ferritin as an indicator of iron status: what do we need to know? Am J Clin Nutr. 2017;106(6):1634s–1639s. doi: 10.3945/ajcn.117.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rockey D.C., Altayar O., Falck-Ytter Y., Kalmaz D. AGA technical review on gastrointestinal evaluation of iron deficiency anemia. Gastroenterology. 2020;159(3):1097–1119. doi: 10.1053/j.gastro.2020.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ko C.W., Siddique S.M., Patel A. AGA Clinical practice guidelines on the gastrointestinal evaluation of iron deficiency anemia. Gastroenterology. 2020;159(3):1085–1094. doi: 10.1053/j.gastro.2020.06.046. [DOI] [PubMed] [Google Scholar]
- 8.Cook J.D., Lipschit.Da M.L.E.M., Finch C.A. Serum ferritin as a measure of iron stores in normal subjects. Am J Clin Nutr. 1974;27(7):681–687. doi: 10.1093/ajcn/27.7.681. [DOI] [PubMed] [Google Scholar]
- 9.Sangkhae V., Nemeth E. Regulation of the iron homeostatic hormone hepcidin. Adv Nutr. 2017;8(1):126–136. doi: 10.3945/an.116.013961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Y., Alfaro-Magallanes V.M., Babitt J.L. Physiological and pathophysiological mechanisms of hepcidin regulation: clinical implications for iron disorders. Br J Haematol. 2020 doi: 10.1111/bjh.17252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finch C.A. Diagnostic value of different methods to detect iron deficiency. In: Hallberg L, Harwerth H-G, Vannotti A, editors. Iron Deficiency Pathogenesis, Clinical Aspects, Therapy. Academic; New York: 1970. p. 409. [Google Scholar]
- 12.Bothwell T.H. Iron deficiency. Med J Aust. 1972;2(8):433–438. [PubMed] [Google Scholar]
- 13.Stoffel N.U., El-Mallah C., Herter-Aeberli I. The effect of central obesity on inflammation, hepcidin, and iron metabolism in young women. Int J Obes. 2020;44(6):1291–1300. doi: 10.1038/s41366-020-0522-x. [DOI] [PubMed] [Google Scholar]
- 14.Walczyk T., Davidsson L., Zavaleta N., Hurrell R.F. Stable isotope labels as a tool to determine the iron absorption by Peruvian school children from a breakfast meal. Fresenius J Anal Chem. 1997;359(4–5):445–449. [Google Scholar]
- 15.Erhardt J.G., Estes J.E., Pfeiffer C.M., Biesalski H.K., Craft N.E. Combined measurement of ferritin, soluble transferrin receptor, retinol binding protein, and C-reactive protein by an inexpensive, sensitive, and simple sandwich enzyme-linked immunosorbent assay technique. J Nutr. 2004;134(11):3127–3132. doi: 10.1093/jn/134.11.3127. [DOI] [PubMed] [Google Scholar]
- 16.Hotz K., Walczyk T. Natural iron isotopic composition of blood is an indicator of dietary iron absorption efficiency in humans. J Biol Inorg Chem. 2013;18(1):1–7. doi: 10.1007/s00775-012-0943-7. [DOI] [PubMed] [Google Scholar]
- 17.Hastie T., Tibshirani R. Generalized additive models for medical research. 1995. (0962-2802 (Print)). [DOI] [PubMed]
- 18.Mullah M.A.S., Hanley J.A., Benedetti A. Modeling perinatal mortality in twins via generalized additive mixed models: a comparison of estimation approaches. BMC Med Res Methodol. 2019;19(1):209. doi: 10.1186/s12874-019-0861-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wand H., Dassaye R., Reddy T., Yssel J., Ramjee G. Geographical-level contributions of risk factors for HIV infections using generalized additive models: results from a cohort of South African women. AIDS Care. 2019;31(6):714–722. doi: 10.1080/09540121.2018.1556382. [DOI] [PubMed] [Google Scholar]
- 20.Cook J.D., Dassenko S.A., Lynch S.R. Assessment of the role of nonheme-iron availability in iron balance. Am J Clin Nutr. 1991;54(4):717–722. doi: 10.1093/ajcn/54.4.717. [DOI] [PubMed] [Google Scholar]
- 21.Allen L., De Benoist B., Hurrell R., Dary O. World Health Organization; Geneva: 2006. Guidelines for Food Fortification with Micronutrients. Rome: Food and Agriculture Organization. [Google Scholar]
- 22.Namaste S.M.L., Rohner F., Huang J. Adjusting ferritin concentrations for inflammation: biomarkers reflecting inflammation and nutritional determinants of anemia (BRINDA) project. Am J Clin Nutr. 2017;106(1):359s–371s. doi: 10.3945/ajcn.116.141762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guyatt G.H., Oxman A.D., Ali M., Willan A., McIlroy W., Patterson C. Laboratory diagnosis of iron-deficiency anemia: an overview. J Gen Intern Med. 1992;7(2):145–153. doi: 10.1007/BF02598003. [DOI] [PubMed] [Google Scholar]
- 24.Sorbie J., Valberg L.S., Corbett W.E., Ludwig J. Serum ferritin, cobalt excretion and body iron status. Can Med Assoc J. 1975;112(10):1173–1178. [PMC free article] [PubMed] [Google Scholar]
- 25.Milman N., Bangsboll S., Pedersen N.S., Visfeldt J. Serum ferritin in non-dialysis patients with chronic-renal-failure - relation to bone-marrow iron stores. Scand J Haematol. 1983;30(4):337–344. doi: 10.1111/j.1600-0609.1983.tb01502.x. [DOI] [PubMed] [Google Scholar]
- 26.Hallberg L., Bengtsson C., Lapidus L., Lindstedt G., Lundberg P.A., Hulten L. Screening for iron deficiency: an analysis based on bone-marrow examinations and serum ferritin determinations in a population sample of women. Br J Haematol. 1993;85(4):787–798. doi: 10.1111/j.1365-2141.1993.tb03225.x. [DOI] [PubMed] [Google Scholar]
- 27.Taylor P., Martineztorres C., Leets I., Ramirez J., Garciacasal M.N., Layrisse M. Relationships among iron-absorption, percent saturation of plasma transferrin and serum ferritin concentration in humans. J Nutr. 1988;118(9):1110–1115. doi: 10.1093/jn/118.9.1110. [DOI] [PubMed] [Google Scholar]
- 28.Cook J.D., Layrisse M., Finch C.A. The measurement of iron absorption. Blood. 1969;33(3):421–429. [PubMed] [Google Scholar]
- 29.Lynch S., Pfeiffer C.M., Georgieff M.K. Biomarkers of nutrition for development (BOND)-iron review. J Nutr. 2018;148:1001s–1067s. doi: 10.1093/jn/nxx036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bothwell T.H. Iron requirements in pregnancy and strategies to meet them. Am J Clin Nutr. 2000;72(1):257S–264S. doi: 10.1093/ajcn/72.1.257S. Suppl. [DOI] [PubMed] [Google Scholar]
- 31.U.S. Centers for Disease Control and Prevention. Second National report on biochemical indicators of diet and nutrition in the U.S. Population. Atlanta, Ga.: National Center for Environmental health, the centers for disease control and prevention, division of laboratory sciences; 2012.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.