Abstract
Serotonin signaling is ubiquitous in the gastrointestinal (GI) system, where it acts as a neurotransmitter in the enteric nervous system (ENS) and influences intestinal motility and inflammation. Since its discovery, serotonin has been linked to cellular proliferation in several types of tissues, including vascular smooth muscle, neurons, and hepatocytes. Activation of serotonin receptors on distinct cell types has been shown to induce well-known intracellular proliferation pathways. In the GI tract, potentiation of serotonin signaling results in enhanced intestinal epithelial proliferation, and decreased injury from intestinal inflammation. Furthermore, activation of the type 4 serotonin receptor on enteric neurons leads to neurogenesis and neuroprotection in the setting of intestinal injury. It is not surprising that the mitogenic properties of serotonin are pronounced within the GI tract, where enterochromaffin cells in the intestinal epithelium produce 90% of the body’s serotonin; however, these proliferative effects are attributed to increased serotonin signaling within the ENS compartment as opposed to the intestinal mucosa, which are functionally and chemically separate by virtue of the distinct tryptophan hydroxylase enzyme isoforms involved in serotonin synthesis. The exact mechanism by which serotonergic neurons in the ENS lead to intestinal proliferation are not known, but the activation of muscarinic receptors on intestinal crypt cells indicate that cholinergic signaling is essential to this signaling pathway. Further understanding of serotonin’s role in mucosal and enteric nervous system mitogenesis may aid in harnessing serotonin signaling for therapeutic benefit in many GI diseases, including inflammatory bowel disease, malabsorptive conditions, and cancer.
Keywords: Mitogenesis, Intestinal Epithelium, Enteric Nervous System
Abbreviations used in this paper: 5-HT, 5-hydroxytryptamine (serotonin); 5-HTR, serotonin receptor; CNS, central nervous system; EC, enterochromaffin; ENS, enteric nervous system; GI, gastrointestinal; MAPK, mitogen-activated protein kinase; SERT, serotonin transporter; SSRI, serotonin reuptake inhibitor; TPH, tryptophan hydroxylase
Summary.
Binding of serotonin receptors on intestinal epithelial cells and enteric neurons activates intracellular proliferative pathways. Potentiation of serotonin signaling has been linked to mucosal proliferation, enteric neurogenesis in the setting of intestinal injury, and the pathogenesis of colon cancer.
Serotonin has long been viewed as a critical signaling molecule for gastrointestinal (GI) health, with a well-established role in GI motility and inflammation.1,2 More recently, serotonin has come into focus for its mitogenic properties in the intestine, both physiologic and pathologic. Cellular proliferation has been linked to serotonin signaling in myriad cell types and organ systems.3, 4, 5 This effect is illustrated uniquely in the intestine as a result of the juxtaposition between serotonin signaling in the gut epithelium, which produces a majority of the body’s serotonin supply, and within the enteric nervous system.
In this review, we synthesize contemporary understanding of serotonin signaling as it relates to cellular proliferation in the intestine. Specifically, we describe the serotonin receptor–initiated pathways involved in intestinal epithelial proliferation, enteric neurogenesis, and intestinal neoplasia. Although further research is necessary to harness the translational potential of serotonin-mediated proliferation in the intestinal mucosa and enteric nervous system, the therapeutic promise of this effect is substantial. Chronic conditions of intestinal injury and insufficiency, for example, in patients with inflammatory bowel disease or short-bowel syndrome, are costly and difficult to treat because of the limitations of intestinal replacement therapies.6 Induction of intestinal mucosal proliferation through the modulation of serotonin signaling is already within the means of medicine, given the established arsenal of serotonin transporter (SERT) inhibitors and serotonin-receptor antagonists that exist, and offers a new therapeutic prospect for this clinical problem.
Historical Perspective
Serotonin, named for the location in which it was first discovered (Latin serum) and one of its earliest known functions (Greek tonic), has a storied scientific history. Initial reports of the substance later identified as serotonin date back to 1868, when Ludwig and Schmidt7 observed that perfusion of dog muscle with defibrinogenated blood resulted in vasoconstriction. In the following decades, other investigators proposed that epinephrine was the responsible vasoactive chemical.8 It was not until O’Connor9 observed that a substance stored in platelets exerted a vasoconstrictive effect on the intestine that a molecule distinct from epinephrine, which relaxes intestinal smooth muscle, was hypothesized to exist.10 The third and fourth decades of the 20th century saw substantial advancements in the understanding of serotonin physiology. Rapport et al11 are credited with isolating serotonin from blood and describing its structure as 5-hydroxytryptamine (5-HT) in 1948. However, it subsequently became clear that 5-HT was identical to the substance enteramine, which the Italian scientists Erspamer, Aspero, and Vialli12,13 had identified a decade earlier. Erspamer had selected this term to reflect the molecule’s site of production, in enterochromaffin (EC) cells in the gut mucosa, and contractile effect on intestinal smooth muscle.12 Discovery of serotonin’s excitatory neurotransmitter function originated in the hard-shell clam in 1953, which led soon thereafter to the identification of serotonergic cells in the vertebrate brain.14,15 Although the name serotonin has endured, the historical significance of enteramine has been realized as subsequent research showed the varied role of 5-HT in GI function, which we describe later.
Overview of Serotonin Physiology
Serotonin, or 5-HT, is a ubiquitous signaling molecule that has been ascribed diverse functions, including neurotransmission, vascular tone, hemostasis, bone resorption, GI function, and cellular proliferation.16, 17, 18, 19 5-HT is derived from the amino acid tryptophan in a 2-step enzymatic process involving tryptophan hydroxylase (TPH), followed by L-amino acid decarboxylase.20 TPH exists in 2 distinct isotypes in vertebrates: TPH1 is expressed in several non-neuronal cell types, most abundantly in the intestine, pineal gland, and pituitary gland, whereas TPH2 is expressed in serotonergic neurons in the central and enteric nervous systems (Figure 1).21, 22, 23, 24 L-amino acid decarboxylase is an abundant and fast-acting enzyme that also is involved in catecholamine synthesis. As such, TPH is the rate-limiting step for 5-HT synthesis in peripheral tissues where tryptophan is readily available; in the central nervous system (CNS), 5-HT synthesis is rate-limited by transport of tryptophan across the blood-brain barrier.20 5-HT also is unable to cross the blood-brain barrier, thus lending spatial and functional separation based on where biosynthesis occurs.
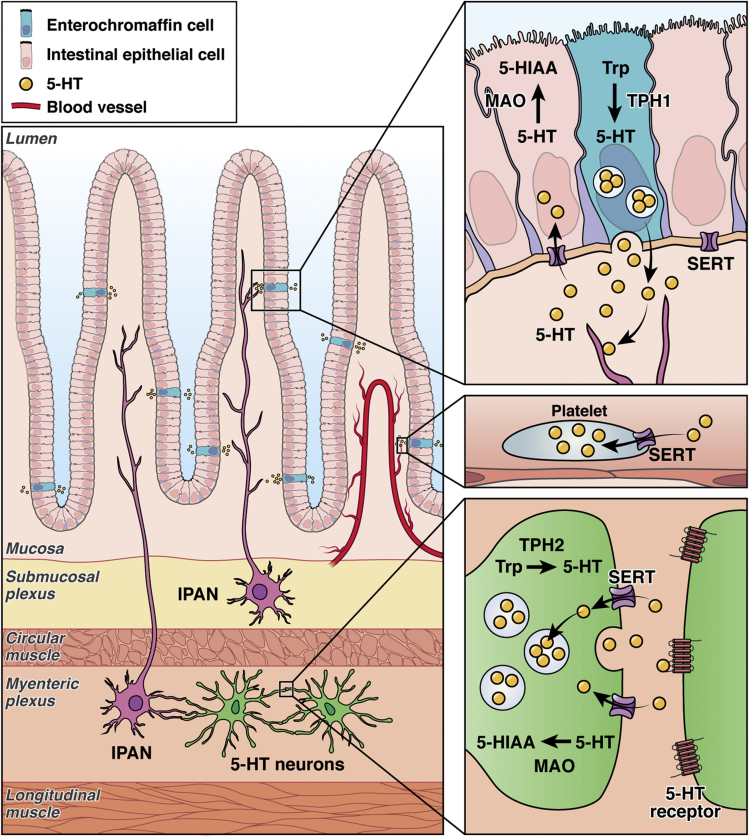
Figure 1.
Life cycle of serotonin in the intestinal epithelium and ENS. 5-HT is synthesized from tryptophan in enterochromaffin cells and in enteric neurons by distinct isoforms of TPH, respectively. TPH1-derived 5-HT in the mucosa enters the portal circulation, where it is stored in platelets that enter the systemic circulation. Serotonergic neurons in the ENS produce TPH2 to synthesize 5-HT, which is stored in synaptic vesicles. Upon release from intracellular vesicles or platelets, 5-HT binds to 5-HTRs on cell membranes. The majority of 5-HTRs are G-protein–coupled receptors, with the exception of the 5-HT3–receptor family, which are ligand-gated ion channels. The SERT is important in inactivating 5-HT by relocating the molecule intracellularly, where it subsequently is degraded by monoamine oxidase into 5-hydroxyindoleacetic acid (5-HIAA). IPAN, intrinsic primary afferent neuron; MAO, monoamine oxidase; Trp, tryptophan.
Irrespective of where it is synthesized, serotonin exerts biologic action by binding to cell membrane–bound serotonin receptors (5-HTR). Numerous 5-HTR genes have been identified, each with the potential to produce distinct splice variants and isoforms of the final receptor protein. To date, 18 unique serotonin receptors have been identified and grouped into 7 families (5-HTR1 through 5-HTR7) on the basis of genetic homology and associated second messenger systems (Table 1).25 All 5-HTR subtypes are G-protein–coupled receptors with the exception of the 5-HTR3 family, which are ligand-gated ion channels.26
Table 1.
Distribution of Serotonin Receptors on Cells Showing Serotonin-Induced Proliferation
| Receptor | Cell type – signaling pathway (if known) | Reference |
|---|---|---|
| 5-HT1A | Neurons Prostate cancer: MAPK/ERK, PI3K/Akt |
Segi-Nishida, 201792 Dizeyi et al, 2011116 |
| 5-HT1B | Pulmonary artery smooth muscle cells: ERK1/2, PDK | Liu et al, 201385 |
| 5-HT1D | Intestinal epithelium: WNT/β-catenin | Sui et al, 2015108 |
| 5-HT2A | Pulmonary artery fibroblasts: p38 MAPK Hepatocytes: protein kinase C Mouse fibroblast tumor: ERK1/2 |
Welsh et al, 200481 Balasubramanian and Paulose,88 1998; Lesurtel et al, 20064 Launay et al, 199680 |
| 5-HT2B | Neural crest cells Myocardial precursor cells Fibroblast: MAPK/cyclin D1, MAPK/cyclin E |
Choi et al, 199798 Choi et al, 199798 Nebigil et al, 200079 |
| 5-HTR2C | Hepatocytes: protein kinase C | Balasubramanian and Paulose,88 1998; Lesurtel et al, 20064 |
| 5-HT3 | Intestinal epithelium Enteric neurons |
Bertrand et al, 200053 Cunningham et al, 198755 |
| 5-HT4 | Neurons in hippocampus Intestinal epithelium Enteric neurons Mesangial kidney cells: protein kinase A, ERK1/2 Ovarian tumor cells |
Segi-Nishida 201792 Pauwelyn and Lefebvre, 201770 Liu et al, 200967 Norum et al, 200378 Henriksen et al, 201277 |
| 5-HT7 | Osteosarcoma: p38 MAPK, ERK2 | Ballou et al, 2018114 |
NOTE. Italics indicate neoplastic cell types.
ERK, extracellular-signal regulated kinase; PDK, pyruvate dehydrogenase kinase; PI3K, phosphoinositide-3 kinase.
Tight regulation of serotonin signaling is achieved by rapid intracellular reuptake of 5-HT by SERT on cell membranes. SERT is a sodium-dependent monoamine transporter protein responsible for removing free serotonin from the extracellular space, thus terminating the downstream effects of 5-HTR activation. Once within the cell, 5-HT can be degraded by a number of mechanisms. The most common pathway for serotonin catabolism involves the activity of monoamine oxidases A and B that produce the renally excreted metabolite 5-hydroxyindoleacetic acid.27
Serotonin Production and Receptor Activation in the Intestine
More than 90% of the body’s serotonin is produced by TPH1 in EC cells in the mucosa of the small intestine. From here, it either acts in a paracrine fashion on the intestinal epithelium or enters portal circulation where it subsequently is stored in circulating platelets.28, 29, 30 Enteroendocrine cells are encountered throughout the epithelium of the GI tract from the stomach to the rectum, and produce hormones involved in several GI functions including digestion and motility.31 5-HT–producing EC cells comprise the largest subset of enteroendocrine cells, and are present along the length of the crypt–villus axis.32 Recent profiling of enteroendocrine cells in the gut have shown significant overlap between hormones secreted by cells that once were thought to be distinct subtypes.33 EC cells, for example, produce substance P and tachykinin in addition to 5-HT.32 The nearby enteric nervous system (ENS), termed the local nervous system for its ability to function without CNS input, contains serotonin-producing neurons in both a submucosal plexus that controls mucosal secretions and blood flow and a myenteric plexus that regulates motility. Similar to serotonergic neurons in the CNS, ENS neurons produce serotonin through the TPH2 enzymatic pathway.28,34
The specific paracrine effects of 5-HT within the gastrointestinal tract are determined by the distribution and location of various 5-HTRs. Receptors from the 5-HT1, 5-HT2, 5-HT3, 5-HT4, and 5-HT7 receptor families have been identified in the intestine, with variable expression in the epithelium, ENS, and intestinal smooth muscle (reviewed by Mawe and Hoffman35). The 5-HTR4 and 5-HTR2A have been localized to the intestinal epithelium.36,37 Although the exact position of the 5-HT4 receptor along the crypt–villus axis has not been described definitively, there is convincing evidence that proliferative cells within the intestinal crypt are the targets of 5-HTR4 signaling effects.38 5-HTR2A expression is most prominent in Paneth cells within the intestinal crypt.
Although our focus is on the mitogenic properties of serotonin, 5-HTR binding is essential for multiple intestinal functions. First, 5-HT mediates the inflammatory response in the intestinal mucosa. Animal models have shown that potentiation of serotonin signaling, either through pharmacologic inhibition or genetic modification, results in more severe colitis and increased levels of proinflammatory cytokines in the intestinal mucosa.39 Proliferation of EC cells from intestinal epithelial progenitors is under immunologic control; specifically, an intact mucosal immune response, including the recruitment of CD3+ and CD4+ T lymphocytes and production of interleukin 13, is necessary for EC cell hyperplasia and consequent enhanced mucosal 5-HT production seen in states of enteric infection and inflammation.40, 41, 42, 43 Inflammatory states also result in down-regulation of SERT, further potentiating 5-HT signals.44 Conversely, depletion of serotonin, modeled with TPH1 knockout mice, is protective against mucosal inflammation.45 Interestingly, TPH2 knockout mice showed more severe colitis than wild-type littermates, suggesting an anti-inflammatory role of serotonin signaling in the ENS.39,46 5-HTR7 on enteric neurons and intestinal myeloid cells and 5-HTR1B on lymphocytes are the specific serotonin receptors activated during intestinal inflammation; although contrary to what one would expect, 5-HTR7 binding appears to confer an anti-inflammatory effect.2,47, 48, 49
Second, the peristaltic reflex, or the intrinsic propulsive motility of the intestine, is regulated by mucosal serotonin.50,51 Animal studies have shown prolonged gastric transit in Tph1-/- mice and mice treated with 5-HTR antagonists.19,50 This prokinetic effect is apparent in carcinoid syndrome, in which EC cell proliferation and the consequent increase in serotonin production manifests with symptoms of hypermotility and diarrhea.52 Neuronal 5-HT produced in the ENS has an apparent inhibitory effect on gastric emptying, but its role is more subtle than that of mucosal 5-HT in overall gut motility.50
The 5-HTR3 subtype warrants particular attention for its role in gut motility. In the myenteric plexus, 5-HTR3 activation is involved in initiating the peristaltic reflex53 and antagonism of this receptor opposes carcinoid-associated diarrhea.54 Inhibition of 5-HTR3 also has a potent anti-emetic effect, as shown by the 5-HTR3 antagonist ondansetron, which is used to treat chemotherapy-induced nausea and vomiting. This pharmacologic effect is mediated by competitive inhibition of 5-HTR3 on visceral afferent neurons and neurons in the vomiting center of the brainstem, as well as by inhibition of 5-HT production by EC cells.55,56
Serotonin-Mediated Growth of the Intestinal Epithelium
Evidence linking intestinal epithelial proliferation to serotonergic regulation was presented by Tutton and Barkla57 in the 1970s, who showed that serotonin blockade was associated with inhibition of colonic adenocarcinoma cell division in rats. Later, Gross et al5 showed that serotonin signaling enhances proliferation of non-neoplastic epithelial cells. Maintenance of the intestinal mucosal epithelium is achieved via a balance between cell production and cell death. Multipotent intestinal stem cells at the base of epithelial crypts proliferate and give rise to all epithelial cell types, including absorptive enterocytes, Paneth cells, goblet cells, and EC cells. Epithelial cell apoptosis and extrusion into the intestinal lumen, a process known as shedding, occurs at the tips of intestinal villi.58 Alterations in the fine regulation of these processes underlie phenotypic changes seen in the mucosal response to injury, adaptation after bowel resection, and the development of intestinal neoplasia.59 The findings by Gross,5 and others subsequently, showed that serotonin potentiation in mice modulated this intestinal homeostasis, leading to increased crypt cell proliferation, elongation of intestinal villi and crypts, and overall expansion of mucosal surface area in the small intestine.60 Interestingly, epithelial proliferation is linked specifically to the activity of neuronal serotonin, or 5-HT synthesized by TPH2; in contrast, the depletion of EC cell–produced 5-HT in experiments with TPH1 knockout mice did not alter mucosal proliferation.5
Furthermore, the absorptive function of the intestine is enhanced in animals with potentiated serotonin signaling, correlating with the observed increase in mucosal surface area.61, 62, 63 This change in absorptive capacity does not reflect an increased proportion of absorptive enterocytes in villi. The fact that the cellular composition of the epithelium is not altered in these animals suggests that cellular division is induced in stem cells at the base of the intestinal crypt, or in the adjacent undifferentiated transit-amplifying cells.61
5-HT–mediated control over crypt cell division also has been observed in studies of intestinal injury. In animal models of intestinal ischemia and reperfusion, mice with enhanced serotonin signaling showed less severe mucosal injury and increased enterocyte proliferation after injury compared with control animals.45,64 In addition, patients with inflammatory bowel disease show deficiencies in mucosal 5-HT signaling, including decreased EC cell populations in the intestinal epithelium.65 Together, these findings have therapeutic implications for malabsorptive conditions such as short-bowel syndrome, and bowel injury more broadly, which warrants further investigation.
Serotonin Receptors in the Intestinal Epithelium
Compelling evidence that serotonin stimulates mitogenic pathways in the intestinal epithelium has led to efforts to characterize the signaling pathways responsible for these effects, starting with identification of the specific 5-HT receptors present on intestinal villi and on proliferative cells within the intestinal crypt. The 5-HTR4 subtype has been localized to the intestinal epithelium throughout the small intestine and colon, and also is expressed in the ENS and on intestinal smooth muscle cells. The prolific expression of 5-HTR4 in different cellular compartments contributes to its role in enteric neuroprotection, intestinal inflammation, and motility.36,66, 67, 68, 69
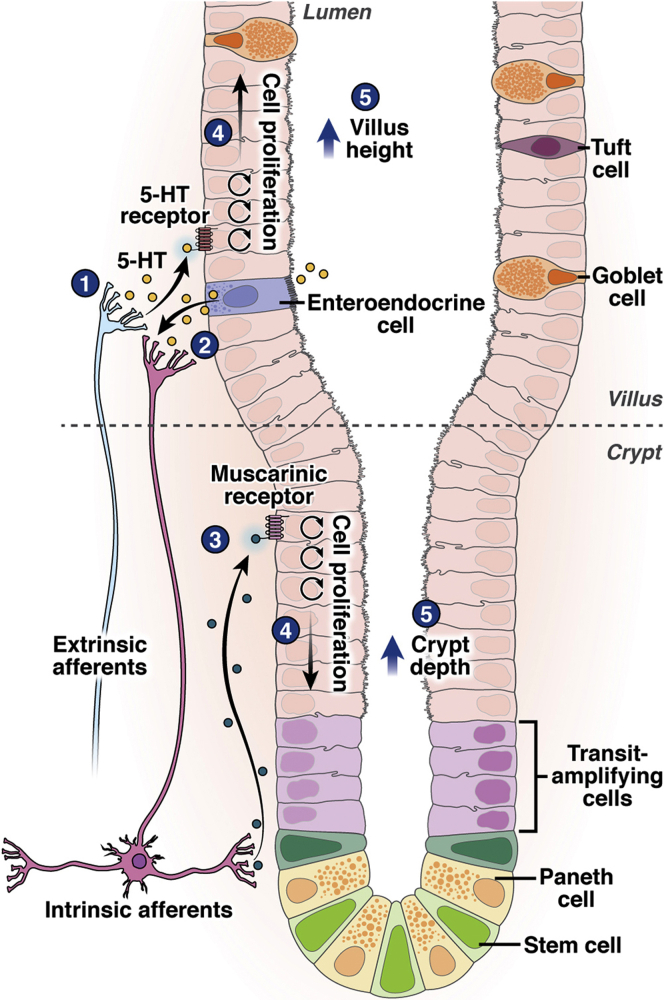
There is substantial evidence of the intersection between serotonergic and cholinergic signaling pathways in the intestine, for example, in the regulation of intestinal motility through binding of 5-HTR4 on myenteric cholinergic neurons (Figure 2).70 Cholinergic regulation of intestinal epithelial cell proliferation is an active area of investigation, with data linking muscarinic acetylcholine receptor activation to colon cancer proliferation and intestinal stem cell division.71, 72, 73 Recent work by Greig et al74,75 showed that stimulation of the muscarinic acetylcholine receptor M1 subtype leads to increased mucosal surface area and enterocyte proliferation in mice, similar to the effects seen with serotonin potentiation. Furthermore, the M1 receptor is localized to the stem cell niche in the intestinal crypt base. These findings, together with evidence that treatment with prucalopride, a pharmacologic 5-HTR4 agonist, increased small intestine morphometric and proliferative markers in mice, suggest that serotonin signaling causes intestinal epithelial cell proliferation both directly and via a cholinergic pathway.38
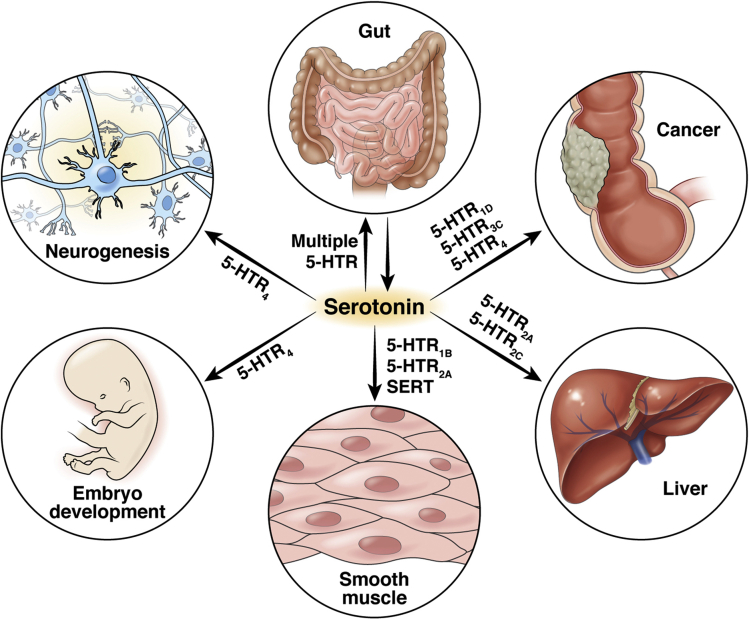
Figure 2.
Diagram of physiologic and pathologic processes in which serotonin exerts mitogenic effects. Potentiation of serotonin signaling leads to activation of cellular proliferation signaling pathways in diverse cell types. 5-HT receptors, namely 5-HTR1B and 5-HTR2A, and SERT play a role in smooth muscle proliferation seen in idiopathic pulmonary hypertension. 5-HTR2A and 5-HTR2C on hepatocytes are involved in liver regeneration after partial hepatectomy. 5-HT–receptor binding leads to uncontrolled cellular division and metastatic potential in multiple cancers. Intact 5-HT signaling is necessary for normal embryonic development, and 5-HT–receptor binding is central to neurogenesis.
The activity of 5-HTR4 on other cell types supports its role in intestinal epithelial proliferation. For example, 5-HTR4 on enteric neurons is implicated in neurogenesis through a pathway by which intracellular cyclic adenosine monophosphate production activates proteins involved in cellular proliferation—namely protein kinase A and the extracellular-signal regulated kinase pathway.67,76 These same pathways are activated by 5-HTR4 activation in non-neuronal cell types including human mesangial kidney cells and ovarian tissue.77,78
There is evidence that the 5-HTR2 family, which has been linked to mitogen-activated protein kinase (MAPK)-mediated cellular proliferation in mouse fibroblasts, also participates in mitogenic signaling in the intestinal epithelium.79,80 5-HTR2A is expressed in the intestinal mucosa and submucosal plexus,37 and the proliferative effects of serotonin potentiation are absent when SERT knock-out mice are treated concomitantly with ketanserin, a selective 5-HT2A antagonist.5
Serotonin-Mediated Mitogenic Pathways in Extraintestinal Tissues
To understand the potentially important intracellular pathways in serotonin-induced mitogenesis, it is useful to examine the role of 5-HT in pathologic cellular proliferation and induction of cellular division in nonproliferating cells (Figure 3). Nemecek et al3 described evidence of serotonin acting as a mitogen in their study of vascular remodeling. They determined that cellular division of bovine smooth muscle cells was enhanced by exposure to serotonin and platelet-derived growth factor stored within platelets. Further research linked idiopathic pulmonary hypertension to abnormal serotonin release from platelets, whereby 5-HT was shown to enhance pulmonary vascular remodeling. 5-HT–induced mitogenesis occurred both by stimulating the proliferation of and decreasing the apoptosis of pulmonary artery fibroblasts and vascular endothelial cells.81, 82, 83, 84, 85, 86 In addition, 5-HTR and SERT activity have been implicated in the up-regulation of mitogenic pathways and DNA replication in pulmonary artery smooth muscle cells.81,85,87
Figure 3.
Schematic of serotonergic and cholinergic receptors in the intestinal epithelium. The intestinal crypt is composed of intestinal stem cells and undifferentiated transit-amplifying cells, whose division underlies epithelial proliferation. Neuronal serotonin, synthesized by TPH2 in the enteric nervous system, leads to epithelial proliferation both through direct binding of 5-HTR on epithelial cells, and indirectly via a cholinergic pathway that leads to muscarinic-receptor activation of intestinal crypt cells.
Later, Balasubramanian and Palouse88 shed light on how serotonin induces differentiated hepatocytes to re-enter the cell cycle. Unlike fibroblasts in vascular endothelium and smooth muscle, hepatocytes typically are nonproliferating cells that can be induced to undergo cell division under specific conditions—namely, after a partial liver resection. Thus, the investigation of 5-HT–mediated hepatocyte division paved the way for understanding how serotonin signaling facilitates bypass of cell-cycle checkpoints. Balasubramanian and Palouse88 observed increased binding of 5-HTRs during hepatocyte regeneration, and found that 5-HTR2 activation was associated specifically with downstream activation of protein kinase C, an established intracellular messenger for cell growth and division. The 5-HTR2A subtype subsequently was identified as the specific receptor activated during liver regeneration, drawing a parallel between what is known about 5-HT signaling during proliferation of hepatocytes and intestinal epithelial cells.4
TPH1 knockout mice show impaired liver regeneration after hepatectomy, further supporting the mitogenic role of 5-HT, and implicating non-neuronal sources of serotonin in this process.4,89 However, CNS regulation also appears to play a role in this process, with data showing that autonomic nervous system activation of EC cells after partial hepatectomy increases peripheral 5-HT production and corresponding 5-HTR activation in regenerating hepatocytes.90
Serotonin-Mediated Neurogenesis and Neuroprotection in the ENS
Serotonin reuptake inhibitor (SSRI)-mediated neurogenesis underlies the effectiveness of this drug class as an antidepressant, and has contributed to understanding of 5-HT as a regulator of cellular proliferation in both the CNS and ENS.91, 92, 93
The ENS is derived from vagal- and sacral-level neural crest cells that migrate to and within the gut of the developing embryo, as well as from Schwann cells, which adopt a neuronal fate in the postnatal period.94,95 5-HT signaling contributes to the development of the ENS, as evidenced by decreased myenteric neuronal density in TPH1 knockout mice compared with wild type.50 Furthermore, the specific development of neurons responsive to the neurotransmitters dopamine and γ-aminobutyric acid in the ENS, is sensitive to serotonin signaling.50
Serotonin signaling is critical during embryonic development outside of the nervous system as well. Various 5-HTR subtypes are expressed differentially throughout mouse embryogenesis, and appear to regulate the rate of cellular division and cleavage of the early embryo. Specifically, 5-HTR2B binding and downstream signaling have been studied in detail and are necessary for normal myocardial development and neural crest differentiation.96, 97, 98 The role of serotonin signaling in intestinal epithelial development has not been well studied, although EC cells and 5-HT production are detected within the mouse intestinal epithelium as early as embryonic day 16.99
Although the ganglia that comprise the ENS are present and functional at birth, the intricate enteric neural circuitry continues to develop and mature throughout adult life.100, 101, 102, 103 Serotonergic signaling plays an important role in this maturation. Much of what we know about 5-HT–mediated cellular proliferation in the ENS comes from work by Liu et al,67 who studied neurogenesis in 5-HTR4 knockout mice. As discussed earlier, 5-HTR4 has an established role in gut motility and neurogenesis. Liu et al67 found that wild-type mice treated with 5-HTR4 agonists formed new neurons in the muscular layers of the small intestine that proceeded to migrate into the myenteric plexus; conversely, these newly generated neurons were absent when 5-HTR4 knockout mice were treated with 5-HTR4 agonists. Subsequent studies have recapitulated these findings and showed that 5-HT plays a neuroprotective role, with the capacity for 5-HTR4 activation to induce enteric neurogenesis after insults to the intestine.104, 105, 106, 107
Serotonin and Proliferation of Cancer Cells
Serotonin-mediated induction of the cell cycle regulates physiologic cell division in the setting of the intestinal crypt cell, and a beneficial adaptive response in the setting of hepatocyte regeneration after liver resection. At the molecular level, these intracellular pathways are not far removed from those activated in cancer cells to facilitate evasion of cell-cycle checkpoints. Since Tutton and Barkla’s57 discovery of serotonergic regulation of colorectal adenocarcinoma, our understanding of the role of 5-HT in intestinal stem cell proliferation and the pathogenesis of colonic neoplasia has grown substantially. Colorectal adenocarcinoma tumor specimens show 5-HTR overexpression when compared with normal colon tissue, specifically of the 5-HT1D, 5-HTR3C, and 5-HTR4–receptor subtypes.108 Serotonin-receptor binding has been linked to colorectal tumor angiogenesis, invasion, and migration in experimental models, correlating with data that circulating plasma serotonin levels are higher in colon cancer patients, and are associated with worse cancer prognosis.108, 109, 110 Recent research by Sakita et al111 further elucidated the role of 5-HT signaling in colorectal carcinogenesis by showing that colonocytes in TPH1 knockout animals show more DNA damage and worse intestinal inflammation than wild-type animals—both of which are etiologic precursors to colon cancer.
Despite this evidence linking 5-HTR activation to cancer progression, SSRI use has been associated with a decreased risk of colorectal cancer, both in a large database analysis of human beings and in rodent models.112,113 In the face of these seemingly contradictory data, study of serotonin’s role in the pathogenesis of other tumors supports the link between serotonin signaling and neoplasia. In a review of genomic data from multiple cancer cell lines, Ballou et al114 found that cancer cells showed clear gene overexpression of specific 5-HTR subtypes, with similarities seen among diverse cell lines that shared a tumor origin. Furthermore, they found that inhibition of serotonin signaling may have therapeutic benefit. In experimental models of breast cancer and sarcoma, pharmacologic inhibition with a variety of 5-HTR antagonists, with the exception of antagonists to the 5-HTR3 family, resulted in reduced phosphorylation of cancer signaling molecules including Protein Kinase B, MAP kinases, and cyclin-dependent kinases, along with a reduction in tumor cell viability.114
Importantly, serotonin potentiation has been linked to downstream activation of proliferative cellular pathways in non–colorectal cancer types. In human osteosarcoma cells, 5-HT exposure was associated with increased phosphorylation of p38 and p42 MAP kinases, which regulates expression of a number of transcription factors involved in cellular proliferation.114,115 In prostate cancer cells, the MAP kinase/extracellular-signal regulated kinase and phosphoinositide-3 kinase/Protein Kinase B cellular pathways are up-regulated by activation of the 5-HT1A receptor, facilitating cancer cell proliferation and migration.116
Therapeutic Considerations
Intestinal injury from inflammatory and ischemic disease and malabsorptive states in the setting of short-bowel syndrome pose a challenge to clinicians given the chronic nature of these conditions and the limitations of intestinal replacement therapies.6 Exploiting the mitogenic properties of serotonin offers a promising therapeutic approach to these difficult clinical problems. Fortunately, the agents that have enabled detailed study of the downstream effects of 5-HT signaling, namely SSRIs and 5-HTR agonists and antagonists, also are viable pharmacologic therapies. Citalopram, an SSRI, and prucalopride, a 5-HTR4 agonist, have been shown to increase mucosal surface area and the absorptive capacity of the intestinal epithelium in mouse models.38,60,62 In addition, citalopram treatment confers a protective effect to the intestinal epithelium in a mouse model of intestinal ischemia, with enhanced enterocyte renewal.64
As mentioned previously, SSRI use has not been associated with increased rates of intestinal neoplasia, despite the evidence linking serotonin potentiation to enterocyte proliferation and colorectal cancer pathogenesis, and has in fact been shown to have therapeutic potential in reducing tumor viability in other malignancies.109,110,114 This is reassuring for the therapeutic application of SSRIs to conditions of intestinal insufficiency, particularly when glucagon-like peptide 2 agonists, an alternate investigational treatment for this indication, carry risks of carcinogenesis.117
Indeed, as a class of drugs, SSRIs show a relatively benign side-effect and risk profile, despite the ubiquity of 5-HT and SERT in the body. When used for CNS indications, common off-target effects include GI symptoms and sexual dysfunction.118,119 Although further research is needed to determine optimal dosing and administration of SSRIs for therapeutic use in intestinal insufficiency, and to characterize adverse off-target effects, current evidence suggests that the harm of long-term SSRI use is minimal.
Conclusions
A critical element of 5-HT signaling is its ability to exert long-term changes on cell fate and gene expression, as evidenced by its mitogenic effect on the intestinal mucosa and enteric nervous system. 5-HTR binding on diverse cell types, including intestinal stem cells, activates intracellular pathways that lead to cellular proliferation and mitogenesis. Division of undifferentiated stem cells and transit-amplifying cells in the intestinal crypt is mediated by serotonergic signaling in the ENS. This effect is observed in normal, injured, and neoplastic cells, and evidently is mediated through cholinergic pathways, as well as through direct binding of 5-HTRs on mucosal cells. Furthermore, serotonin is essential for neurogenesis and continued regeneration of the enteric neurons throughout life, underscoring the importance of serotonergic neurotransmission in GI function. SSRIs and 5-HTR binding agents hold promise as therapies to harness the translational potential of serotonin-mediated proliferation for conditions of intestinal injury or insufficiency. Modification of 5-HT signaling is already within the means of medicine, given the established arsenal of SERT inhibitors and 5-HTR antagonists that exist, but additional research is warranted to characterize the risks and benefits to patients with intestinal insufficiency in the clinical setting.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding The work was funded by the Department of Surgery, Yale School of Medicine.
References
- 1.Tsukamoto K., Ariga H., Mantyh C., Pappas T.N., Yanagi H., Yamamura T., Takahashi T. Luminally released serotonin stimulates colonic motility and accelerates colonic transit in rats. Am J Physiol Regul Integr Comp Physiol. 2007;293:64–69. doi: 10.1152/ajpregu.00856.2006. [DOI] [PubMed] [Google Scholar]
- 2.Guseva D., Holst K., Kaune B., Meier M., Keubler L., Glage S., Buettner M., Bleich A., Pabst O., Bachmann O., Ponimaskin E.G. Serotonin 5-HT7 receptor is critically involved in acute and chronic inflammation of the gastrointestinal tract. Inflamm Bowel Dis. 2014;20:1516–1529. doi: 10.1097/MIB.0000000000000150. [DOI] [PubMed] [Google Scholar]
- 3.Nemecek G.M., Coughlin S.R., Handley D.A., Moskowitz M.A. Stimulation of aortic smooth muscle cell mitogenesis by serotonin. Proc Natl Acad Sci U S A. 1986;83:674–678. doi: 10.1073/pnas.83.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lesurtel M., Graf R., Aleil B., Walther D.J., Tian Y., Jochum W., Gachet C., Bader M., Clavien P.A. Platelet-derived serotonin mediates liver regeneration. Science. 2006;312:104–107. doi: 10.1126/science.1123842. [DOI] [PubMed] [Google Scholar]
- 5.Gross E.R., Gershon M.D., Margolis K.G., Gertsberg Z.V., Li Z., Cowles R.A. Neuronal serotonin regulates growth of the intestinal mucosa in mice. Gastroenterology. 2012;143:408–417.e2. doi: 10.1053/j.gastro.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fishbein T.M., Matsumoto C.S. Intestinal replacement therapy: timing and indications for referral of patients to an intestinal rehabilitation and transplant program. Gastroenterology. 2006;130:S147–S151. doi: 10.1053/j.gastro.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Ludwig C., Schmidt A. Das Verhalten der Gase, welche mit dem Blutdurch den reizbaren Säugethiermuskel strömen. Berichte über die Verhandlungen der Königlich Sächsischen Gesellschaft der Wissen-schaften zu Leipzig. 1868;20:12–72. [Google Scholar]
- 8.Trendelenburg P. Bestimmung des Adrenalingehaltes im normalen Blut sowie beim Abklingen der Wirkung einer einmaligen intravenösen Adrenalininjektion mittels physiologischer Messmethode. Arch Exp Pathol Pharmakol. 1910;63:161–176. [Google Scholar]
- 9.O’Connor J.M. Über den Adrenalingehalt des Blutes. Arch Exp Pathol Pharmakol. 1912;67:195–232. [Google Scholar]
- 10.Janeway T.C., Richardson H.B., Park E.A. Experiments on the vasoconstrictor action of blood serum. Arch Intern Med. 1918;21:565–603. [Google Scholar]
- 11.Rapport M.M., Green A.A., Page I.H. Serum vasoconstrictor (serotonin) J Biol Chem. 1949;176:1243–1251. [PubMed] [Google Scholar]
- 12.Erspamer V., Asero B. Identification of enteramine, the specific hormone of the enterochromaffin cell system, as 5-hydroxytryptamine. Nature. 1952;169:800–801. doi: 10.1038/169800b0. [DOI] [PubMed] [Google Scholar]
- 13.Vialli M., Erspamer V. Caratteristiche istochimiche delle cellule enterocromaffini. Boll Soc Ital Biol Sper. 1933;8:885–887. [Google Scholar]
- 14.Welsh J.H. Excitation of the heart of Venus mercenaria. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1953;219:23–29. doi: 10.1007/BF00246246. [DOI] [PubMed] [Google Scholar]
- 15.Twarog B.M., Page I.H. Serotonin content of some mammalian tissues and urine and a method for its determination. Am J Physiol. 1953;175:157–161. doi: 10.1152/ajplegacy.1953.175.1.157. [DOI] [PubMed] [Google Scholar]
- 16.Carneiro A.M.D., Cook E.H., Murphy D.L., Blakely R.D. Interactions between integrin alphaIIbbeta3 and the serotonin transporter regulate serotonin transport and platelet aggregation in mice and humans. J Clin Invest. 2008;118:1544–1552. doi: 10.1172/JCI33374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaumann A.J., Levy F.O. 5-hydroxytryptamine receptors in the human cardiovascular system. Pharmacol Ther. 2006;111:674–706. doi: 10.1016/j.pharmthera.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Warden S.J., Robling A.G., Sanders M.S., Bliziotes M.M., Turner C.H. Inhibition of the serotonin (5-hydroxytryptamine) transporter reduces bone accrual during growth. Endocrinology. 2005;146:685–693. doi: 10.1210/en.2004-1259. [DOI] [PubMed] [Google Scholar]
- 19.Gershon M.D., Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Ichiyama A., Nakamura S., Nishizuka Y., Hayaishi O. Enzymic studies on the biosynthesis of serotonin in mammalian brain. J Biol Chem. 1970;245:1699–1709. [PubMed] [Google Scholar]
- 21.Walther D.J., Peter J.U., Bashammakh S., Hörtnagl H., Voits M., Fink H., Bader M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- 22.Reiter R.J. Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr Rev. 1991;12:151–180. doi: 10.1210/edrv-12-2-151. [DOI] [PubMed] [Google Scholar]
- 23.Pelosi B., Pratelli M., Migliarini S., Pacini G., Pasqualetti M. Generation of a Tph2 conditional knockout mouse line for time- and tissue-specific depletion of brain serotonin. PLoS One. 2015;10 doi: 10.1371/journal.pone.0136422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Côté F., Thévenot E., Fligny C., Fromes Y., Darmon M., Ripoche M.A., Bayard E., Hanoun N., Saurini F., Lechat P., Dandolo L., Hamon M., Mallet J., Vodjdani G. Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proc Natl Acad Sci U S A. 2003;100:13525–13530. doi: 10.1073/pnas.2233056100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoyer D., Clarke D.E., Fozard J.R., Hartig P.R., Martin G.R., Mylecharane E.J., Saxena P.R., Humphrey P.P. International union of pharmacology classification of receptors for 5-hydroxytryptamine (serotonin) Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- 26.Filip M., Bader M. Overview on 5-HT receptors and their role in physiology and pathology of the central nervous system. Pharmacol Rep. 2009;61:761–777. doi: 10.1016/s1734-1140(09)70132-x. [DOI] [PubMed] [Google Scholar]
- 27.Ramsay R.R., Albreht A. Kinetics, mechanism, and inhibition of monoamine oxidase. J Neural Transm. 2018;125:1659–1683. doi: 10.1007/s00702-018-1861-9. [DOI] [PubMed] [Google Scholar]
- 28.Gershon M.D., Drakontides A.B., Ross L.L. Serotonin: synthesis and release from the myenteric plexus of the mouse intestine. Science. 1965;149:197–200. doi: 10.1126/science.149.3680.197. [DOI] [PubMed] [Google Scholar]
- 29.Gershon M.D. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes. 2013;20:14–21. doi: 10.1097/MED.0b013e32835bc703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keszthelyi D., Troost F.J., Masclee A.A.M. Understanding the role of tryptophan and serotonin metabolism in gastrointestinal function. Neurogastroenterol Motil. 2009;21:1239–1249. doi: 10.1111/j.1365-2982.2009.01370.x. [DOI] [PubMed] [Google Scholar]
- 31.Sjölund K., Sandén G., Håkanson R., Sundler F. Endocrine cells in human intestine: an immunocytochemical study. Gastroenterology. 1983;85:1120–1130. [PubMed] [Google Scholar]
- 32.Beumer J., Artegiani B., Post Y., Reimann F., Gribble F., Nguyen T.N., Zeng H., Van den Born M., Van Es J.H., Clevers H. Enteroendocrine cells switch hormone expression along the crypt-to-villus BMP signalling gradient. Nat Cell Biol. 2018;20:909–916. doi: 10.1038/s41556-018-0143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Habib A.M., Richards P., Cairns L.S., Rogers G.J., Bannon C.A.M., Parker H.E., Morley T.C.E., Yeo G.S.H., Reimann F., Gribble F.M. Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology. 2012;153:3054–3065. doi: 10.1210/en.2011-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wade P.R., Tamir H., Kirchgessner A.L., Gershon M.D. Analysis of the role of 5-HT in the enteric nervous system using anti- idiotopic antibodies to 5-HT receptors. Am J Physiol Gastrointest Liver Physiol. 1994;266:G403–G416. doi: 10.1152/ajpgi.1994.266.3.G403. [DOI] [PubMed] [Google Scholar]
- 35.Mawe G.M., Hoffman J.M. Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10:473–486. doi: 10.1038/nrgastro.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoffman J.M., Tyler K., Maceachern S.J., Balemba O.B., Johnson A.C., Brooks E.M., Zhao H., Swain G.M., Moses P.L., Galligan J.J., Sharkey K.A., Meerveld B.G., Mawe G.M. Activation of colonic mucosal 5-HT 4 receptors accelerates propulsive. Gastroenterology. 2012;142:844–854.e4. doi: 10.1053/j.gastro.2011.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fiorica-Howells E., Hen R., Gingrich J., Li Z., Gershon M.D. 5-HT(2A) receptors: location and functional analysis in intestines of wild-type and 5-HT(2A) knockout mice. Am J Physiol Gastrointest Liver Physiol. 2002;282:G877–G893. doi: 10.1152/ajpgi.00435.2001. [DOI] [PubMed] [Google Scholar]
- 38.Park C.J., Armenia S.J., Zhang L., Cowles R.A. The 5-HT4 receptor agonist prucalopride stimulates mucosal growth and enhances carbohydrate absorption in the ileum of the mouse. J Gastrointest Surg. 2019;23:1198–1205. doi: 10.1007/s11605-018-3907-6. [DOI] [PubMed] [Google Scholar]
- 39.Bischoff S.C., Mailer R., Pabst O., Weier G., Sedlik W., Li Z., Chen J.J., Murphy D.L., Gershon M.D. Role of serotonin in intestinal inflammation: knockout of serotonin reuptake transporter exacerbates 2,4,6-trinitrobenzene sulfonic acid colitis in mice. Am J Physiol Gastrointest Liver Physiol. 2009;296:G685–695. doi: 10.1152/ajpgi.90685.2008. [DOI] [PubMed] [Google Scholar]
- 40.Wheatcroft J., Wakelin D., Smith A., Mahoney C.R., Mawe G., Spiller R. Enterochromaffin cell hyperplasia and decreased serotonin transporter in a mouse model of postinfectious bowel dysfunction. Neurogastroenterol Motil. 2005;17:863–870. doi: 10.1111/j.1365-2982.2005.00719.x. [DOI] [PubMed] [Google Scholar]
- 41.Manocha M., Shajib M.S., Rahman M.M., Wang H., Rengasamy P., Bogunovic M., Jordana M., Mayer L., Khan W.I. IL-13-mediated immunological control of enterochromaffin cell hyperplasia and serotonin production in the gut. Mucosal Immunol. 2013;6:146–155. doi: 10.1038/mi.2012.58. [DOI] [PubMed] [Google Scholar]
- 42.Wang H., Steeds J., Motomura Y., Deng Y., Verma-Gandhu M., El-Sharkawy R.T., McLaughlin J.T., Grencis R.K., Khan W.I. CD4+ T cell-mediated immunological control of enterochromaffin cell hyperplasia and 5-hydroxytryptamine production in enteric infection. Gut. 2007;56:949–957. doi: 10.1136/gut.2006.103226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spiller R.C., Jenkins D., Thornley J.P., Hebden J.M., Wright T., Skinner M., Neal K.R. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804–811. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Linden D.R., Foley K.F., McQuoid C., Simpson J., Sharkey K.A., Mawe G.M. Serotonin transporter function and expression are reduced in mice with TNBS-induced colitis. Neurogastroenterol Motil. 2005;17:565–574. doi: 10.1111/j.1365-2982.2005.00673.x. [DOI] [PubMed] [Google Scholar]
- 45.Margolis K.G., Stevanovic K., Li Z., Yang Q.M., Oravecz T., Zambrowicz B., Jhaver K.G., Diacou A., Gershon M.D. Pharmacological reduction of mucosal but not neuronal serotonin opposes inflammation in mouse intestine. Gut. 2014;63:928–937. doi: 10.1136/gutjnl-2013-304901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghia J.E., Li N., Wang H., Collins M., Deng Y., El-Sharkawy R.T., Côté F., Mallet J., Khan W.I. Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology. 2009;137:1649–1660. doi: 10.1053/j.gastro.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 47.Shajib M.S., Wang H., Kim J.J., Sunjic I., Ghia J.E., Denou E., Collins M., Denburg J.A., Khan W.I. Interleukin 13 and serotonin: linking the immune and endocrine systems in murine models of intestinal inflammation. PLoS One. 2013;8 doi: 10.1371/journal.pone.0072774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim J.J., Bridle B.W., Ghia J.E., Wang H., Syed S.N., Manocha M.M., Rengasamy P., Shajib M.S., Wan Y., Hedlund P.B., Khan W.I. Targeted inhibition of serotonin type 7 (5-HT7) receptor function modulates immune responses and reduces the severity of intestinal inflammation. J Immunol. 2013;190:4795–4804. doi: 10.4049/jimmunol.1201887. [DOI] [PubMed] [Google Scholar]
- 49.Yin J., Albert R.H., Tretiakova A.P., Jameson B.A. 5-HT(1B) receptors play a prominent role in the proliferation of T-lymphocytes. J Neuroimmunol. 2006;181:68–81. doi: 10.1016/j.jneuroim.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 50.Li Z., Chalazonitis A., Huang Y.Y., Mann J.J., Margolis K.G., Yang Q.M., Kim D.O., Côté F., Mallet J., Gershon M.D. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J Neurosci. 2011;31:8998–9009. doi: 10.1523/JNEUROSCI.6684-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen J.J., Li Z., Pan H., Murphy D.L., Tamir H., Koepsell H., Gershon M.D. Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: abnormal intestinal motility and the expression of cation transporters. J Neurosci. 2001;21:6348–6361. doi: 10.1523/JNEUROSCI.21-16-06348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kulke M.H., Dorisio T.O., Phan A., Bergsland E., Law L., Banks P., Freiman J., Frazier K., Jackson J., Yao J.C., Lapuerta P., Zambrowicz B., Fleming D., Sands A. Telotristat etiprate, a novel serotonin synthesis inhibitor, in patients with carcinoid syndrome and diarrhea not adequately controlled by octreotide. Endocr Relat Cancer. 2015;21:705–714. doi: 10.1530/ERC-14-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bertrand P.P., Kunze W.A.A., Furness J.B., Bornstein J.C. The terminals of myenteric intrinsic primary afferent neurons of the guinea-pig ileum are excited by 5-hydroxytryptamine acting at 5-hydroxytryptamine-3 receptors. Neuroscience. 2000;101:459–469. doi: 10.1016/s0306-4522(00)00363-8. [DOI] [PubMed] [Google Scholar]
- 54.Terry N., Margolis K.G. Serotonergic mechanisms regulating the GI tract: experimental evidence and therapeutic relevance. Handb Exp Pharmacol. 2017;239:319–342. doi: 10.1007/164_2016_103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cunningham D., Hawthorn J., Pople A. Prevention of emesis in patients receiving cytotoxic drugs by GR38032F, a selective 5HT3 receptor antagonist. Lancet. 1987;1:461–1463. doi: 10.1016/s0140-6736(87)92208-2. [DOI] [PubMed] [Google Scholar]
- 56.Gan T.J. Selective serotonin 5-HT 3 receptor antagonists for postoperative nausea and vomiting are they all the same? 2005;19:225–238. doi: 10.2165/00023210-200519030-00004. [DOI] [PubMed] [Google Scholar]
- 57.Tutton P.J.M., Barkla D.H. The influence of serotonin on the mitotic rate in the colonic crypt epithelium and in colonic adenocarcinoma in rats. Clin Exp Pharmacol Physiol. 1978;5:91–94. doi: 10.1111/j.1440-1681.1978.tb00657.x. [DOI] [PubMed] [Google Scholar]
- 58.Bullen T.F., Forrest S., Campbell F., Dodson A.R., Hershman M.J., Pritchard D.M., Turner J.R., Montrose M.H., Watson A.J.M. Characterization of epithelial cell shedding from human small intestine. Lab Invest. 2006;86:1052–1063. doi: 10.1038/labinvest.3700464. [DOI] [PubMed] [Google Scholar]
- 59.Tappenden K.A. Pathophysiology of short bowel syndrome: considerations of resected and residual anatomy. JPEN J Parenter Enteral Nutr. 2014;38:14S–22S. doi: 10.1177/0148607113520005. [DOI] [PubMed] [Google Scholar]
- 60.Zhang L., Greig C.J., Cowles R.A. Orally dosed citalopram stimulates small intestinal mucosal growth. J Surg Res. 2019;236:326–331. doi: 10.1016/j.jss.2018.11.033. [DOI] [PubMed] [Google Scholar]
- 61.Tackett J.J., Gandotra N., Bamdad M.C., Muise E.D., Cowles R.A. Enhanced serotonin signaling stimulates ordered intestinal mucosal growth. J Surg Res. 2016;208:198–203. doi: 10.1016/j.jss.2016.09.036. [DOI] [PubMed] [Google Scholar]
- 62.Park C.J., Armenia S.J., Shaughnessy M.P., Greig C.J., Cowles R.A. Potentiation of serotonin signaling leads to increased carbohydrate and lipid absorption in the murine small intestine. J Pediatr Surg. 2019;54:1245–1249. doi: 10.1016/j.jpedsurg.2019.02.027. [DOI] [PubMed] [Google Scholar]
- 63.Greig C.J., Zhang L., Cowles R.A. Potentiated serotonin signaling in serotonin re-uptake transporter knockout mice increases enterocyte mass and small intestinal absorptive function. Physiol Rep. 2019;7 doi: 10.14814/phy2.14278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tackett J.J., Gandotra N., Bamdad M.C., Muise E.D., Cowles R.A. Potentiation of serotonin signaling protects against intestinal ischemia and reperfusion injury in mice. Neurogastroenterol Motil. 2019;31 doi: 10.1111/nmo.13498. [DOI] [PubMed] [Google Scholar]
- 65.Coates M.D., Mahoney C.R., Linden D.R., Sampson J.E., Chen J., Blaszyk H., Crowell M.D., Sharkey K.A., Gershon M.D., Mawe G.M., Moses P.L. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657–1664. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 66.Gershon M.D. 5-HT 4-mediated neuroprotection: a new therapeutic modality on the way? Am J Physiol Liver Physiol. 2020;10032:766–767. doi: 10.1152/ajpgi.00120.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu M.T., Kuan Y.H., Wang J., Hen R., Gershon M.D. 5-HT4 receptor-mediated neuroprotection and neurogenesis in the enteric nervous system of adult mice. J Neurosci. 2009;29:9683–9699. doi: 10.1523/JNEUROSCI.1145-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takaki M., Goto K., Kawahara I. The 5-hydroxytryptamine 4 receptor agonist-induced actions and enteric neurogenesis in the gut. J Neurogastroenterol Motil. 2014;20:17–30. doi: 10.5056/jnm.2014.20.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stakenborg N., Labeeuw E., Gomez-Pinilla P.J., Schepper S.D., Aerts R., Goverse G., Farro G., Appeltans I., Meroni E., Stakenborg M., Viola M.F., Gonzalez-Dominguez E., Bosmans G., Alpizar Y.A., Wolthuis A., Hoore A.D., Van Beek K., Verheijden S., Verhaegen M., Derua R., Waelkens E., Moretti M., Gotti C., Augustijns P., Talavera K., Berghe P.V., Matteoli G., Boeckxstaens G.E. Preoperative administration of the 5-HT4 receptor agonist prucalopride reduces intestinal inflammation and shortens postoperative ileus via cholinergic enteric neurons. Gut. 2019;68:1406–1416. doi: 10.1136/gutjnl-2018-317263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pauwelyn V., Lefebvre R.A. 5-HT(4) receptors facilitate cholinergic neurotransmission throughout the murine gastrointestinal tract. Neurogastroenterol Motil. 2017;29 doi: 10.1111/nmo.13064. [DOI] [PubMed] [Google Scholar]
- 71.Frucht H., Jensen R.T., Dexter D., Yang W. Human colon cancer cell proliferation mediated by the M 3 muscarinic cholinergic receptor. Clin Cancer Res. 1999;5:2532–2539. [PubMed] [Google Scholar]
- 72.Lundgren O., Jodal M., Jansson M., Ryberg A.T., Svensson L. Intestinal epithelial stem/progenitor cells are controlled by mucosal afferent nerves. PLoS One. 2011;6 doi: 10.1371/journal.pone.0016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ukegawa J., Takeuchi Y., Kusayanagi S., Mitamura K. Growth-promoting effect of muscarinic acetylcholine receptors in colon cancer cells. J Cancer Res Clin Oncol. 2003;129:272–278. doi: 10.1007/s00432-003-0433-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Greig C.J., Cowles R.A. Muscarinic acetylcholine receptors participate in small intestinal mucosal homeostasis. J Pediatr Surg. 2017;52:1031–1034. doi: 10.1016/j.jpedsurg.2017.03.037. [DOI] [PubMed] [Google Scholar]
- 75.Greig C.J., Armenia S.J., Cowles R.A. The M1 muscarinic acetylcholine receptor in the crypt stem cell compartment mediates intestinal mucosal growth. Exp Biol Med (Maywood) 2020;245:1194–1199. doi: 10.1177/1535370220938375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barthet G., Framery B., Gaven F., Pellissier L., Reiter E., Claeysen S., Dumuis A. 5-Hydroxytryptamine 4 receptor activation of the extracellular signal-regulated kinase pathway depends on src activation but not on G protein or beta-arrestin signaling. Mol Biol Cell. 2007;18:1979–1991. doi: 10.1091/mbc.E06-12-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Henriksen R., Dizeyi N., Abrahamsson P.A. Expression of serotonin receptors 5-HT1A, 5-HT1B, 5-HT2B and 5-HT4 in ovary and in ovarian tumours. Anticancer Res. 2012;32:1361–1366. [PubMed] [Google Scholar]
- 78.Norum J.H., Hart K., Levy F.O. Ras-dependent ERK activation by the human Gs-coupled serotonin receptors 5-HT4(b) and 5-HT7(a) J Biol Chem. 2003;278:3098–3104. doi: 10.1074/jbc.M206237200. [DOI] [PubMed] [Google Scholar]
- 79.Nebigil C.G., Launay J.M., Hickel P., Tournois C., Maroteaux L. 5-hydroxytryptamine 2B receptor regulates cell-cycle progression: cross-talk with tyrosine kinase pathways. Proc Natl Acad Sci U S A. 2000;97:2591–2596. doi: 10.1073/pnas.050282397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Launay J.M., Birraux G., Bondoux D., Callebert J., Choi D.S., Loric S., Maroteauxi L. Ras involvement in signal transduction by the serotonin 5-HT2B receptor. J Biol Chem. 1996;271:3141–3147. doi: 10.1074/jbc.271.6.3141. [DOI] [PubMed] [Google Scholar]
- 81.Welsh D.J., Harnett M., MacLean M., Peacock A.J. Proliferation and signaling in fibroblasts: role of 5- hydroxytryptamine2a receptor and transporter. Am J Respir Crit Care Med. 2004;170:252–259. doi: 10.1164/rccm.200302-264OC. [DOI] [PubMed] [Google Scholar]
- 82.Abenhaim L., Moride Y., Brenot F., Rich S., Benichou J., Kurz X., Higenbottam T., Oakley C., Wouters E., Aubier M., Simonneau G., Bégaud B. Appetite-suppressant drugs and the risk of primary pulmonary hypertension. N Engl J Med. 1996;335:609–616. doi: 10.1056/NEJM199608293350901. [DOI] [PubMed] [Google Scholar]
- 83.Chen C., Han X., Fan F., Liu Y., Wang T., Wang J., Hu P., Ma A., Tian H. Serotonin drives the activation of pulmonary artery adventitial fibroblasts and TGF-β1/Smad3-mediated fibrotic responses through 5-HT2A receptors. Mol Cell Biochem. 2014;397:267–276. doi: 10.1007/s11010-014-2194-0. [DOI] [PubMed] [Google Scholar]
- 84.Morecroft I., Dempsie Y., Bader M., Walther D.J., Kotnik K., Loughlin L., Nilsen M., MacLean M.R. Effect of tryptophan hydroxylase 1 deficiency on the development of hypoxia-induced pulmonary hypertension. Hypertension. 2007;49:232–236. doi: 10.1161/01.HYP.0000252210.58849.78. [DOI] [PubMed] [Google Scholar]
- 85.Liu Y., Tian H.Y., Yan X.L., Fan F.L., Wang W.P., Han J.L., Zhang J.B., Ma Q., Meng Y., Wei F. Serotonin inhibits apoptosis of pulmonary artery smooth muscle cell by pERK1/2 and PDK through 5-HT1B receptors and 5-HT transporters. Cardiovasc Pathol. 2013;22:451–457. doi: 10.1016/j.carpath.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 86.Pakala R., Willerson J.T., Benedict C.R. Mitogenic effect of serotonin on vascular endothelial cells. Circulation. 1994;90:1919–1926. doi: 10.1161/01.cir.90.4.1919. [DOI] [PubMed] [Google Scholar]
- 87.Eddahibi S., Humbert M., Fadel E., Raffestin B., Darmon M., Capron F., Simonneau G., Dartevelle P., Hamon M., Adnot S. Serotonin transporter overexpression is responsible for pulmonary artery smooth muscle hyperplasia in primary pulmonary hypertension. J Clin Invest. 2001;108:1141–1150. doi: 10.1172/JCI12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Balasubramanian S., Paulose C.S. Induction of DNA synthesis in primary cultures of rat hepatocytes by serotonin: possible involvement of serotonin S2 receptor. Hepatology. 1998;27:62–66. doi: 10.1002/hep.510270111. [DOI] [PubMed] [Google Scholar]
- 89.Nocito A., Georgiev P., Dahm F., Jochum W., Bader M., Graf R., Clavien P.A. Platelets and platelet-derived serotonin promote tissue repair after normothermic hepatic ischemia in mice. Hepatology. 2007;45:369–376. doi: 10.1002/hep.21516. [DOI] [PubMed] [Google Scholar]
- 90.Inoue R., Kamimura K., Nagoya T., Sakai N., Yokoo T., Goto R., Ogawa K., Shinagawa-Kobayashi Y., Watanabe-Mori Y., Sakamaki A., Abe S., Kamimura H., Miyamura N., Nishina H., Terai S. Effect of a neural relay on liver regeneration in mice: activation of serotonin release from the gastrointestinal tract. FEBS Open Bio. 2018;8:449–460. doi: 10.1002/2211-5463.12382. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 91.Samuels B.A., Mendez-David I., Faye C., David S.A., Pierz K.A., Gardier A.M., Hen R., David D.J. Serotonin 1A and serotonin 4 receptors: essential mediators of the neurogenic and behavioral actions of antidepressants. Neuroscientist. 2016;22:26–45. doi: 10.1177/1073858414561303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Segi-Nishida E. The effect of serotonin-targeting antidepressants on neurogenesis and neuronal maturation of the hippocampus mediated via 5-HT1A and 5-HT4 receptors. Front Cell Neurosci. 2017;11:142. doi: 10.3389/fncel.2017.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang J.-W., David D.J., Monckton J.E., Battaglia F., Hen R. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci. 2008;28:1374–1384. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Young H.M., Hearn C.J., Newgreen D.F. Embryology and development of the enteric nervous system. Gut. 2000;47:12–14. doi: 10.1136/gut.47.suppl_4.iv12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Uesaka T., Nagashimada M., Enomoto H. Neuronal differentiation in Schwann cell lineage underlies postnatal neurogenesis in the enteric nervous system. J Neurosci. 2015;35:9879–9888. doi: 10.1523/JNEUROSCI.1239-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lauder J.M., Wilkie M.B., Wu C., Singh S. Expression of 5-HT(2A), 5-HT(2B) and 5-HT(2C) receptors in the mouse embryo. Int J Dev Neurosci. 2000;18:653–662. doi: 10.1016/s0736-5748(00)00032-0. [DOI] [PubMed] [Google Scholar]
- 97.Buznikov G.A., Lambert W.H., Lauder J.M. Serotonin and serotonin-like substances as regulators of early embryogenesis and morphogenesis. Cell Tissue Res. 2001;305:177–186. doi: 10.1007/s004410100408. [DOI] [PubMed] [Google Scholar]
- 98.Choi D.S., Ward S.J., Messaddeq N., Launay J.M., Maroteaux L. 5-HT2B receptor-mediated serotonin morphogenetic functions in mouse cranial neural crest and myocardiac cells. Development. 1997;124:1745–1755. doi: 10.1242/dev.124.9.1745. [DOI] [PubMed] [Google Scholar]
- 99.Branchek T.A., Gershon M.D. Time course of expression of neuropeptide Y, calcitonin gene-related peptide, and NADPH diaphorase activity in neurons of the developing murine bowel and the appearance of 5-hydroxytryptamine in mucosal enterochromaffin cells. J Comp Neurol. 1989;285:262–273. doi: 10.1002/cne.902850208. [DOI] [PubMed] [Google Scholar]
- 100.Pham T.D., Gershon M.D., Rothman T.P. Time of origin of neurons in the murine enteric nervous system: sequence in relation to phenotype. J Comp Neurol. 1991;314 doi: 10.1002/cne.903140411. 798–798. [DOI] [PubMed] [Google Scholar]
- 101.Fiorica-Howells E., Maroteaux L., Gershon M.D. Serotonin and the 5-HT 2B receptor in the development of enteric neurons. J Neurosci. 2000;20:294–305. doi: 10.1523/JNEUROSCI.20-01-00294.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kruger G.M., Mosher J.T., Bixby S., Joseph N., Iwashita T., Morrison S.J. Neural crest stem cells persist in the adult gut but undergo changes in self-renewal, neuronal subtype potential, and factor responsiveness. Neuron. 2002;35:657–669. doi: 10.1016/s0896-6273(02)00827-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kulkarni S., Micci M.A., Leser J., Shin C., Tang S.C., Fu Y.Y., Liu L., Li Q., Saha M., Li C., Enikolopov G., Becker L., Rakhilin N., Anderson M., Shen X., Dong X., Butte M.J., Song H., Southard-Smith E.M., Kapur R.P., Bogunovic M., Pasricha P.J. Adult enteric nervous system in health is maintained by a dynamic balance between neuronal apoptosis and neurogenesis. Proc Natl Acad Sci U S A. 2017;114:E3709–E3718. doi: 10.1073/pnas.1619406114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gershon M.D. Serotonin is a sword and a shield of the bowel: serotonin plays offense and defense. Trans Am Clin Climatol Assoc. 2012;123:268–280. [PMC free article] [PubMed] [Google Scholar]
- 105.Bianco F., Bonora E., Natarajan D., Vargiolu M., Thapar N., Torresan F., Giancola F., Boschetti E., Volta U., Bazzoli F., Mazzoni M., Seri M., Clavenzani P., Stanghellini V., Sternini C., De Giorgio R. Prucalopride exerts neuroprotection in human enteric neurons. Am J Physiol Gastrointest Liver Physiol. 2016;310:G768–G775. doi: 10.1152/ajpgi.00036.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Takaki M., Goto K., Kawahara I., Nabekura J. Activation of 5-HT4 receptors facilitates neurogenesis of injured enteric neurons at an anastomosis in the lower gut. J Smooth Muscle Res. 2015;51:82–94. doi: 10.1540/jsmr.51.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Matsuyoshi H., Kuniyasu H., Okumura M., Misawa H., Katsui R., Obata K., Takaki M. A 5-HT 4-receptor activation-induced neural plasticity enhances in vivo reconstructs of enteric nerve circuit insult. Neurogastroenterol Motil. 2010;22:806–814. doi: 10.1111/j.1365-2982.2010.01474.x. [DOI] [PubMed] [Google Scholar]
- 108.Sui H., Xu H., Ji Q., Liu X., Zhou L., Song H., Zhou X., Xu Y., Chen Z., Cai J., Ji G., Li Q. 5-hydroxytryptamine receptor (5-HT1DR) promotes colorectal cancer metastasis by regulating axin 1/β-catenin/MMP-7 signaling pathway. Oncotarget. 2015;6:25975–25987. doi: 10.18632/oncotarget.4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xia Y., Wang D., Zhang N., Wang Z., Pang L. Plasma serotonin level is a predictor for recurrence and poor prognosis in colorectal cancer patients. J Clin Lab Anal. 2018;32:1–8. doi: 10.1002/jcla.22263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nocito A., Dahm F., Jochum W., Jae H.J., Georgiev P., Bader M., Graf R., Clavien P.A. Serotonin regulates macrophage-mediated angiogenesis in a mouse model of colon cancer allografts. Cancer Res. 2008;68:5152–5158. doi: 10.1158/0008-5472.CAN-08-0202. [DOI] [PubMed] [Google Scholar]
- 111.Sakita J.Y., Bader M., Santos E.S., Garcia S.B., Minto S.B., Alenina N., Brunaldi M.O., Carvalho M.C., Vidotto T., Gasparotto B., Martins R.B., Silva W.A., Brandão M.L., Leite C.A., Cunha F.Q., Karsenty G., Squire J.A., Uyemura S.A., Kannen V. Serotonin synthesis protects the mouse colonic crypt from DNA damage and colorectal tumorigenesis. J Pathol. 2019;249:102–113. doi: 10.1002/path.5285. [DOI] [PubMed] [Google Scholar]
- 112.Chubak J., Boudreau D.M., Rulyak S.J., Mandelson M.T. Colorectal cancer risk in relation to antidepressant medication use. Int J Cancer. 2011;128:227–232. doi: 10.1002/ijc.25322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kannen V., Marini T., Turatti A., Carvalho M.C., Brandão M.L., Jabor V.A.P., Bonato P.S., Ferreira F.R., Zanette D.L., Silva W.A., Garcia S.B. Fluoxetine induces preventive and complex effects against colon cancer development in epithelial and stromal areas in rats. Toxicol Lett. 2011;204:134–140. doi: 10.1016/j.toxlet.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 114.Ballou Y., Rivas A., Belmont A., Patel L., Amaya C., Lipson S., Khayou T., Dickerson E., Nahleh Z., Bryan B. 5-HT serotonin receptors modulate mitogenic signaling and impact tumor cell viability. Mol Clin Oncol. 2018:243–254. doi: 10.3892/mco.2018.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang W., Liu H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12:9–18. doi: 10.1038/sj.cr.7290105. [DOI] [PubMed] [Google Scholar]
- 116.Dizeyi N., Hedlund P., Bjartell A., Tinzl M., Austild-Taskén K., Abrahamsson P.A. Serotonin activates MAP kinase and PI3K/Akt signaling pathways in prostate cancer cell lines. Urol Oncol Semin Orig Investig. 2011;29:436–445. doi: 10.1016/j.urolonc.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 117.Thulesen J., Hartmann B., Hare K.J., Kissow H., Ørskov C., Holst J.J., Poulsen S.S. Glucagon-like peptide 2 (GLP-2) accelerates the growth of colonic neoplasms in mice. Gut. 2004;53:1145–1150. doi: 10.1136/gut.2003.035212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.James A., Ryan J., Parkman H. Effects of the selective serotonin reuptake inhibitor, fluoxetine, on regional gastric contractility. Neurogastroenterol Motil. 2005;17:76–82. doi: 10.1111/j.1365-2982.2004.00613.x. [DOI] [PubMed] [Google Scholar]
- 119.Keller Ashton A., Hamer R., Rosen R.C. Serotonin reuptake inhibitor-induced sexual dysfunction and its treatment: a large-scale retrospective study of 596 psychiatric outpatients. J Sex Marital Ther. 1997;23:165–175. doi: 10.1080/00926239708403922. [DOI] [PubMed] [Google Scholar]