Abstract
Background
Chemotherapy plus immune-checkpoint inhibitor (CTx+ICI) therapy has become the preferred 1st line treatment in patients with metastatic NSCLC without oncogenic driven mutations. However, the optimal subsequent 2nd line treatment is not defined and several alternatives exist. The purpose of this analysis was to evaluate the efficacy of 2nd line docetaxel plus ramucirumab (D+R) initiated after failure of 1st line CTx+ICI.
Methods
Retrospective data were collected during routine care from German thoracic oncology centers. Only patients who had received at least one course of 2nd line D+R were included. ORR, PFS, OS and numbers of courses of D+R were investigated with PFS after initiation of D+R being the primary endpoint.
Results
Seventy-seven patients met the inclusion criteria. 2nd line treatment with D+R achieved an ORR and DCR of 32.5% and 62.4%, respectively. Median PFS for 2nd line therapy was 3.9 months with a DOR of 6.4 months. Median OS of 15.5 and 7.5 months were observed from the start of 1st line therapy and 2nd line treatment, respectively. No unexpected toxicities occurred. Presence of KRAS mutations was associated with significantly worse median PFS to D+R (2.8 vs. 4.5 months in wild-type cases; P=0.021) and was an independent predictor of inferior PFS in multivariate analysis.
Conclusions
D+R is an effective and safe 2nd line treatment after failure of 1st line CTx+ICI irrespective of NSCLC histology. However, patients with a KRAS mutation did not benefit from D+R in terms of PFS and will require further investigations.
Keywords: Lung cancer, palliative treatment, angiogenesis inhibitor, ramucirumab, immune-checkpoint inhibitor (ICI)
Introduction
In recent years, ICI directed against PD-1 or PD-L1 have expanded the treatment options for advanced NSCLC. Initially, ICI were approved for palliative 2nd and 3rd line treatment of metastatic NSCLC in patients without druggable driver mutations (1-6). Up to 16% of NSCLC patients receiving the PD-1 inhibitor nivolumab as palliative 2nd line treatment survive for more than five years (7). ICI quickly became established in the 1st line setting. The PD-1 ICI pembrolizumab is approved in Europe as a 1st line monotherapy for patients with an immunohistochemically based tumor proportion score (TPS) for PD-L1 ≥50% or in combination with a platinum based combination chemotherapy regardless of the TPS score (8-10). Currently approved 1st line therapies also include the PD-L1 ICI atezolizumab either in combination with chemotherapy or even as a four-drug regimen in combination with the VEGF antibody bevacizumab (11,12). In addition, the PD-1 antibody nivolumab is approved in combination with the CTLA-4 ICI ipilimumab and platinum-based combination therapy (13). These ICI combination strategies have resulted in a median PFS rates of 7 to 9 months and median OS rates of 16 to 22 months. Most patients will sooner or later experience progression of their tumor disease and require 2nd line treatment. However, there is a lack of knowledge about the efficacy of drugs being considered for 2nd line therapy following CTx+ICI, since the current options were all tested and approved before the widespread use of immunotherapy. It is currently unclear to what extent the results of former trials can be extrapolated to inform the choice of 2nd line therapy after immunotherapy (14).
So far, no studies have prospectively compared different therapy strategies in 2nd line. Thus, real-world data from high-volume centers with well-defined treatment sequences could help physicians to make treatment decisions in individual NSCLC patients. Recently we could show excellent efficacy of 3rd line docetaxel plus ramucirumab (D+R) combination therapy after failure of both 1st line chemotherapy and 2nd line ICI monotherapy (15). In this retrospective analysis we now report on D+R in the palliative 2nd line treatment after progression on the current standard 1st line treatment comprising of platinum based combination chemotherapy plus ICI (CTx+ICI). We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/tlcr-21-197).
Methods
Design and participating centers
This retrospective analysis studies the clinical effects of a palliative 2nd line treatment with D+R in patients with metastatic NSCLC directly after progression on a 1st line combination therapy with platinum based chemotherapy and an anti-PD-1 or PD-L1 ICI agent (CTx+ICI). Patients had a histologically or cytologically proven NSCLC UICC stage IV according to the eight edition of the UICC TNM classification. Patients with ICI monotherapy as 1st line therapy were excluded. Patients with a history of adjuvant chemotherapy before 1st line palliative therapy could be included, if there was an interval of more than 12 months between the adjuvant treatment and the first systemic treatment for stage IV disease. Patients with a history of palliative radiation therapy could also be enrolled into the study. NSCLC with a non-squamous histology were tested for EGFR mutations and ALK translocations and in case of a positive result excluded.
Data were collected from 9 high-volume German centers with specialization in thoracic oncology and strong experience in chemo- and immune-oncology therapy. The centers included three university hospitals, three community-based hospitals, two private hospitals and one outpatient clinic. Patients were treated with CTx+ICI between February 2016 and May 2020 and started 2nd line D+R no later than August, 1st 2020. This allowed for a follow-up of at least five months prior to the data cut-off of December 31, 2020. Data were recorded by each center in a standardized manner with the following data being collected for each patient: age, sex, smoking habits, tumor stage, histology, PD-L1 expression determined by immunohistochemistry, mutational status, height and weight at diagnosis, date of NSCLC diagnosis, survival status at time point of documentation (alive or deceased) and date of last contact/death. For each treatment line the following details of therapy were documented: date of start, number of treatment cycles, cycles for combination and monotherapy, best response, date of progression and reason for treatment stop. In addition, for 2nd line therapy the following data were documented: weight at start of therapy and side effects [according to common toxicity criteria (CTC) grades 3 and 4].
Data were anonymized before being sent to the organizing center. Inclusion and exclusion criteria were verified centrally, and data were checked for completeness and plausibility. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of Paracelsus Medical University Nuremberg (IRB-2019-014). Due to the retrospective nature of the study and the strict anonymization of individual data, written informed consent was not required.
Measurement of immunohistochemical and molecular factors
PD-L1 expression was assessed with the following antibodies SP263 (6 centers), ZR3 (1 center), 22C3 (1 center) and QR001 (1 center). Tumor proportional scores (TPS) were classified into three groups (<1%, 1–49% and ≥50%). The genetic make-up of tumors was analyzed using tissue-based targeted next-generation-sequencing (NGS).
Evaluation of tumor response and efficacy of treatment
The primary endpoint was efficacy of the D+R 2nd line therapy in terms of progression-free survival (PFS). Secondary endpoints were duration of response (DOR) in patients responding to D+R as well as OS from start of 2nd line therapy. In addition, response to 1st and 2nd line treatment, time interval between both lines of treatment and the relationship between tumor response to CTx+ICI and D+R were evaluated.
Tumor responses were assessed by each center by chest computer tomography (CT) and abdominal ultrasound or other clinically appropriate abdominal imaging at least every 3 months or upon clinical deterioration. Tumor responses were evaluated according to the RECIST (Response Evaluation Criteria in Solid Tumors) version 1.1. (16). Complete response (CR) was defined as disappearance of all target and non-target lesions. Partial response (PR) was defined as ≥30% reduction in size in target lesions or disappearance of ≥1 non-target lesions. Stable disease (SD) was defined as <30% decrease or <20% increase in size of target lesions or the persistence of ≥1 non-target lesions. Progressive disease (PD) was defined as ≥20% increase in size or the appearance of new non-target lesions and/or progression of existing non-target lesions. The ORR was defined as the best response recorded from the start of treatment until disease progression or recurrence, confirmed by repeat assessments performed no less than four weeks after the first time criteria for response had been reached. Disease control rate was defined as CR plus PR plus SD.
Overall survival was recorded from the first day of 1st line palliative treatment with CTx+ICI and from the first day of 2nd line treatment with D+R to the date of death or last follow-up. PFS was defined for 2nd line therapy as the interval from the first day of 2nd line D+R to the first sign of disease progression or death whichever occurred first. For 1st line therapy, time on treatment was defined as the interval starting from the first day on drug until the date when the progression was documented.
Statistical analysis
Descriptive data were presented as mean and 95% confidence interval, categorical variables were presented using numbers and frequencies. A tornado diagram was implemented to visualize the relative importance of cycle length in 1st and 2nd line treatment. To analyze PFS and OS data were presented as median and 95% confidence interval (CI), and times to events were determined using the Kaplan-Meier method and compared with the log-rank test. Cox proportional hazards models were used to evaluate the influence of different patient factors. Statistical results were calculated using SPSS (version 23). A P value <0.05 was considered statistically significant.
Results
Patient population
After excluding 10 patients (5 patients with ICI monotherapy in 1st line; 3 patients with D+R in the 3rd line, 2 patients with insufficient follow-up data), 77 patients from 9 centers met the inclusion criteria. The baseline demographic data and tumor characteristics are listed in Table 1. The median age was 63 years (range, 41–83), and 68.8% of the patients were male. More than half of the patients had an ECOG-PS (Eastern Cooperative Oncology Group performance status) of 1 (54.8%). PD-L1 data were available for 70 patients (91%); of those 47.1%, 42.9% and 10% exhibited a tumor proportion score (TPS) of 0%, 1–49% and ≥50%, respectively. The KRAS mutational status was available from 48 tumors (68.6%); of those a KRAS mutation was detected in 17 (35.4%) of the cases. A G12C mutation was identified in 5 cases (29.4%). KRAS G12V, G12D, G12A, G12S and a codon 13 mutation were observed in 5, 3, 2,1 and 1 cases, respectively. All patients had received a platinum based combination CTx+ICI as palliative 1st line therapy with carboplatin being the backbone in 83.1%. ICI included pembrolizumab in 80.5%, atezolizumab in 13% and durvalumab (due to a clinical study) in 6.5%. In 20 patients a 3rd line therapy was initiated following D+R. 3rd line therapy consisted of combination CTx, CTx monotherapy, ICI monotherapy and a tyrosine kinase inhibitor (TKI) were given in 8, 3, 6 and 3 cases, respectively. Details of agents and combinations are reported in Table 2.
Table 1. Patients’ demographics.
| Parameters | N | (%) |
|---|---|---|
| Age | ||
| Median age 63 years (range, 41–83) | ||
| <65 years | 41 | (53.2) |
| ≥65 years | 36 | (46.8) |
| Gender | ||
| Male | 53 | (68.8) |
| Female | 24 | (31.2) |
| ECOG PS | ||
| 0 | 28 | (38.4) |
| 1 | 40 | (54.8) |
| 2 | 5 | (6.8) |
| n.r. | 4 | |
| Stage at start of 1st line chemotherapy | ||
| IVa | 20 | (26.0) |
| IVb | 57 | (74.0) |
| Histology | ||
| Adenocarcinoma | 55 | (71.4) |
| Squamous cell carcinoma | 16 | (20.8) |
| NOS or other type | 6 | (7.8) |
| PDL-1 expression status | ||
| Negative | 33 | (47.1) |
| 1–49% | 30 | (42.9) |
| ≥50% | 7 | (10.0) |
| n.r. | 7 | |
| Kras mutational status | ||
| Wild-type | 31 | (64.6) |
| Mutation | 17 | (35.4) |
| n.r. | 29 | |
| BMI at start of 2nd line | ||
| <25 | 44 | (57.1) |
| ≥25 | 33 | (42.9) |
| Palliative radiation therapy | ||
| No radiation | 37 | (48.1) |
| One site | ||
| Brain | 10 | (13.0) |
| Bone | 7 | (9.1) |
| Lung/mediastinum | 9 | (11.7) |
| Multiple sites | 4 | (5.2) |
| n.r. | 10 | (13.0) |
n.r., not reported; BMI, body mass index.
Table 2. Drugs and drug combinations used in different lines of treatment.
| 1st line | 2nd line | 3rd line therapy | |||||
|---|---|---|---|---|---|---|---|
| Chemotherapy + ICI (N=77) | N (%) | R+D (N=77) | N (%) | (N=20) | N (%) | ||
| Platinum + pemetrexed + pembrolizumab | 50 (64.9) | ||||||
| Platinum + paclitaxel/nab-paclitaxel + pembrolizumab | 9 (11.7) | Docetaxel + ramucirumab | 77 [100] | Chemotherapy mono | 8 [40] | ||
| Platinum + paclitaxel/nab-paclitaxel + atezolizumab | 8 (10.4) | Chemotherapy combination | 3 [15] | ||||
| Platinum + gemcitabine/vinorelbine + durvalumab + tremelimumab | 3 (3.9) | ICI | 6 [30] | ||||
| Platinum + pemetrexed + durvalumab +tremelimumab | 2 (2.6) | TKI | 3 [15] | ||||
| Carboplatin + paclitaxel + bevacizumab + atezolizumab | 2 (2.6) | ||||||
| Platinum + gemcitabine/vinorelbine + pembrolizumab | 2 (2.6) | ||||||
| Platinum + gemcitabine/vinorelbine + pembrolizumab | 1 (1.3) | ||||||
ICI, immune-checkpoint-inhibitor; TKI, tyrosine kinase inhibitor.
Efficacy and safety of D+R
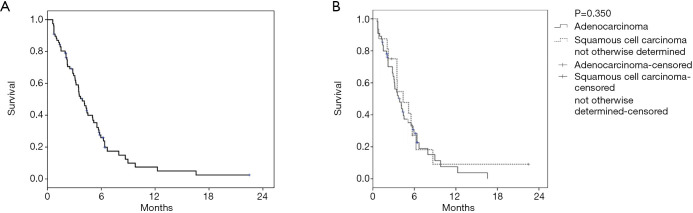
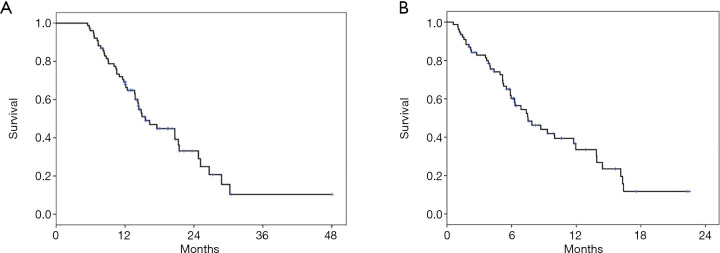
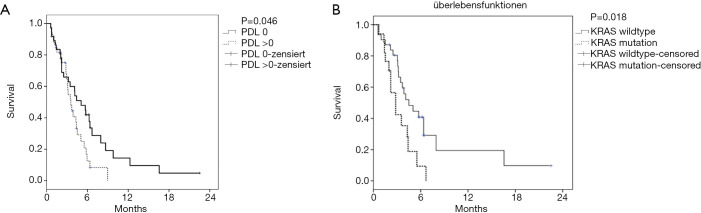
The mean number of 2nd line combination treatment cycles was 6.0 (95% CI, 5.1–7.0) with a mean number of 2.1 cycles given as a ramucirumab mono therapy. Fifteen patients (19.5%) were still on treatment with D+R at data cut-off. Overall, this treatment led to an ORR of 32.5% and a DCR of 62.4%. The median PFS for 2nd line therapy was 3.9 months (95% CI, 3.1–4.6) with a DOR of 6.4 months (95% CI, 5.4–7.4) (Table 3 and Figure 1A). There was no significant difference in PFS between patients with squamous or non-squamous tumor histology receiving D+R (Table 3, Figure 1B). The median OS from first dose of 2nd line therapy was 7.5 months (95% CI, 5.1–10.0). A median OS of 15.5 months (95% CI, 12.2–18.9) was observed from the start of 1st-line palliative treatment (Figure 2). Cox regression analyses were carried out to analyze potential prognostic effects on PFS including gender, age, stage, BMI, histology, response to 1st line treatment, PD-L1 TPS and KRAS mutational status. PD-L1 TPS and KRAS mutational status were prognostic in the univariate analysis. Only KRAS wild-type status turned out to be of independent positive prognostic value (Figure 3A,B, Table 4). There was no difference in terms of efficacy between patients with KRAS G12C and other KRAS mutations (Figure S1).
Table 3. Efficacy data in different lines of treatment.
| Parameters | 1st line CTx+ICI | 2nd line R+D | 3rd line | ||
| N=77 | N=77 | N=20 | |||
| Number of cycles; mean (95% CI) | 9.0 (7.4–10.6) | 6.0 (5.1–7.0) | – | ||
| Of those | |||||
| Maintenance CTx+ICI | 2.0 (1.2–2.8) | 2.1 (1.3–3.0) | |||
| Maintenance ICI mono | 3.5 (2.0–5.0) | ||||
| Ramucirumab mono | – | ||||
| ORR; N (%) | |||||
| CR | 3 (3.9) | ||||
| PR | 36 (46.8) | 25 (32.5) | 2 (2.6) | ||
| SD | 23 (29.9) | 23 (29.9) | 4 (5.2) | ||
| PD | 15 (19.5) | 23 (29.9) | 8 (10.4) | ||
| n.r. | – | 6 (7.8) | 6 (7.8) | ||
| 2nd line ongoing 15 (19.5); no further therapy 42 (54.5) |
|||||
| DOR; median (95% CI) | 8.7 (6.3–11.0) | 6.4 (5.4–7.4) | – | ||
| PFS; median (95% CI) (months) | 5.8 (5.0–6.6) | 3.9 (3.1–4.6) | – | ||
| PFS according to subgroups | |||||
| Histology | |||||
| Adenocarcinoma | 6.0 (5.0–7.0) | 3.9 (3.0–4.8) | – | ||
| Squamous cell carcinoma | 5.6 (5.0–6.2) | 4.4 (2.3–6.4) | – | ||
| PD-L1 | |||||
| TPS <1% | 4.8 (3.7–6.0) | 3.5 (2.6–4.5) | – | ||
| TPS 1–49% | 6.0 (5.6–6.4) | 5.1 (3.1–7.1) | – | ||
| TPS ≥50% | 7.2 (7.1–7.3) | 12.3 (2.5–22.0) | – | ||
| KRAS | |||||
| Wild-type | 6.0 (4.8–7.2) | 4.5 (2.6–6.4) | – | ||
| Mutated | 4.8 (3.2–6.3) | 2.8 (1.7–3.9) | – | ||
| OS from line of thx; median (95%CI) (months) | 15.5 (12.2–18.9) | 7.5 (5.1–10.0) | 3.4 (2.6–4.2) |
TPS, tumor proportional score on immunohistochemistry; DOR, duration of response.
Figure 1.
Kaplan-Meier curve for PFS. (A) PFS Kaplan Meier curves of the whole collective, (B) PFS due to different histologies, ADC, adeno carcinoma; SCC, squamous cell carcinoma.
Figure 2.
Kaplan-Meier curves for OS due to start of (A) 1st line and (B) 2nd line treatment, respectively.
Figure 3.
Kaplan-Meier curves for molecular subgroups. PFS. (A) PFS due to PD-L1 TPS scores; (B) PFS due to KRAS mutational status. mut, KRAS mutated; wt, KRAS wild-type.
Table 4. Univariate and multivariate analysis of different parameters of the whole cohort regarding PFS due to D+R treatment.
| Parameters | N | mPFS | 95% CI | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |||||
| Gender | ||||||||||
| Male | 53 | 3.7 | 2.6–4.7 | |||||||
| Female | 24 | 5.0 | 1.7–8.3 | 0.682 | 0.392–1.186 | 0.175 | – | |||
| Age (years) | ||||||||||
| <65 | 41 | 4.5 | 2.8–6.2 | |||||||
| ≥65 | 36 | 3.5 | 3.0–4.0 | 1.545 | 0.910–2.598 | 0.101 | – | |||
| Stage | ||||||||||
| IV A | 20 | 5.0 | 2.6–7.5 | |||||||
| IV B | 57 | 3.5 | 2.9–4.1 | 1.617 | 0.909–2.875 | 0.102 | – | |||
| BMI (kg/m2) | ||||||||||
| <25 | 44 | 3.7 | 1.9–5.6 | |||||||
| ≥25 | 33 | 4.3 | 2.1–6.5 | 0.702 | 0.417–1.179 | 0.181 | – | |||
| Histology | ||||||||||
| AC | 55 | 3.9 | 2.8–4.9 | |||||||
| SCC | 16 | 4.4 | 2.3–6.4 | |||||||
| NOS | 6 | 2.5 | 0–5.5 | 1.064 | 0.850–1.318 | 0.568 | – | |||
| Response 1st line | ||||||||||
| Response | 39 | 4.5 | 2.8–4.1 | |||||||
| No response | 38 | 3.5 | 3.3–5.7 | 0.786 | 0.427–1.310 | 0.356 | – | |||
| PD-L1 (TPS score) | ||||||||||
| <1 | 33 | 3.5 | 2.8–4.3 | |||||||
| ≥1 | 37 | 5.1 | 2.9–7.3 | 0.569 | 0.325–0.997 | 0.049 | 0.546 | 0.260–1.148 | 0.110 | |
| KRAS | ||||||||||
| Wild-type | 31 | 4.5 | 2.6–6.4 | |||||||
| Mutation | 17 | 2.8 | 1.7–3.9 | 2.293 | 1.130–4.652 | 0.021 | 2.229 | 1.077–4.605 | 0.030 | |
During D+R treatment no unexpected toxicity was documented. Neutropenia was the most frequent side effect with CTC grades 3 and 4 documented in 7 and 5 patients, respectively. Febrile neutropenia was reported in 3 patients, 2 of them suffering from CTC grade 4 leading to discontinuation of the D+R treatment. Prophylactic G-CSF was not routinely administered in any of the centers. However, in patients with grade 3 or 4 neutropenia prophylactic G-CSF was given for subsequent courses to prevent further hematologic adverse events. Alternatively, in some cases docetaxel was discontinued and ramucirumab was given as a mono-therapy. Further commonly documented grade 3-4 CTC toxicities included fatigue, dysparonychia, mucositis, stomatitis, and ileus in 5, 4, 3, 1 and 1 cases, respectively. No pulmonary toxicities, such as interstitial pneumonitis, or treatment-related deaths were observed. The adverse events associated with 2nd line therapy are listed in Table 5.
Table 5. Hematological and non-hematological side effects of D+R therapy.
| Adverse events | CTC grade ≥3*, N (%) |
|---|---|
| Neutropenia | 12 (15.6) |
| Febrile neutropenia | 3 (3.9) |
| Fatigue | 5 (6.5) |
| Dysparonychia | 4 (5.2) |
| Mucositis | 3 (3.9) |
| Stomatitis | 1 (1.3) |
| Ileus | 1 (1.3) |
*, there were no toxicities CTC grade 5.
Outcome of 1st line treatment
A response to 1st line therapy was observed in 50.7% of patients. The mean time on 1st line treatment was 5.8 months (95% CI, 5.0–6.6). After an average of 4 cycles of platinum-based CTX+ICI, 37.8% received a maintenance therapy with CT+ICI and 41% received ICI monotherapy (Table 3). There were no significant differences in OS between the different platinum agents. Regarding PD-L1 TPS and duration of treatment with ICI there was a trend toward longer duration of therapy in patients with a high expression (≥50%). The median time interval from end of 1st line to start of 2nd line treatment was 23 days (95% CI, 19.6–26.4). There was no statistically significant effect of this interval on the efficacy of 2nd line D+R. Radiation therapy had no significant effect on 1st or 2nd line response or outcome. However, BMI appeared to be of prognostic value in terms of OS. From the start of 1st line median OS was 21.4 months in patients with initial BMI ≥25 and 13.8 months in patients with BMI <25 (P=0.018). BMI did not affect clinical response or PFS in either treatment line.
Subgroup analysis of patients treated with platinum + pemetrexed + pembrolizumab in the 1st line
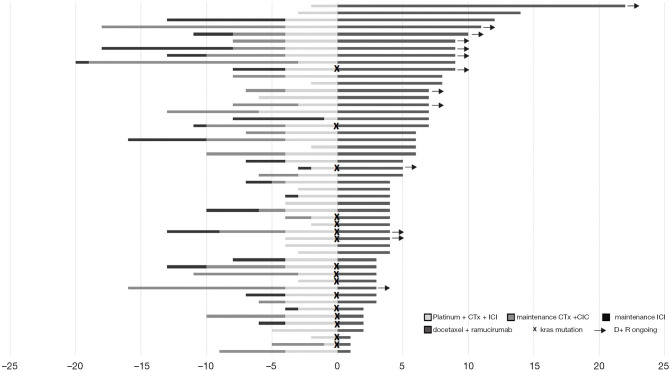
Nearly two-thirds of the patients (N=50; 64.9%) had been treated with CTx+ICI consisting of platinum + pemetrexed + pembrolizumab in 1st line therapy. All of these patients had a non-squamous tumor and in 84% the mutational status had been established. Thus, these patients represent a large, homogeneous, and well-characterized subgroup and were therefore analyzed in more detail in a subsequent post-hoc analysis. A tornado-plot representing the number of cycles in 1st and 2nd line treatment is given in Figure 4.
Figure 4.
Treatment duration in cycles of therapy for 1st and 2nd line treatment in the subgroup of patients treated by platinum + pemetrexed + pembrolizumab 1st line (N=50). 1st line treatment left side is divided into platinum based CTx+ICI, maintenance therapy with CTx+ICI and maintenance therapy with ICI monotherapy. D+R treatment is presented at the right side. Arrows define patients with D+R ongoing, X defines patients with an underlying KRAS mutation.
The platinum backbone was carboplatin and cisplatin in 78% and 22%, respectively, with no differences in duration of treatment in 1st line or 2nd line. PD-L1 TPS was 0%, 1–49% and ≥50% in 48%, 46% and 6% of the tumors, respectively, with no differences in outcomes. 2nd line treatment with D+R resulted in an ORR of 34% and a DCR of 68%, with a DOR of 6.4 months (95% CI, 5.6–7.2 months). The mPFS for D+R was 3.9 months (2.7–5.1 months) and the mOS from the start of 1st and 2nd line was 16.3 and 8.7 months, respectively. The KRAS status of the tumor was reported for 42 patients and a KRAS mutation was detected in 16 cases (38.1%) (Figure 4). KRAS mutations were significantly associated with poor median PFS for D+R in contrast to the wild type status (2.8 vs. 5.0 months; P=0.023). As described for the entire cohort, there was no difference between KRAS G12C and other KRAS mutations when comparing PFS in this subgroup. The duration of treatment in 1st line or overall survival (either from the start of 1st line or 2nd line) were not significantly affected by a KRAS mutation. Other clinical parameters including age, gender, BMI, histology (adeno-carcinoma or NOS), as well as response to 1st line and length of ICI maintenance therapy were not associated with significantly different effects on efficacy parameters for D+R treatment.
Discussion
The combination of chemotherapy plus ICI (CTx+ICI) has become the standard palliative 1st line treatment for driver negative metastatic NSCLC with more than four phase III trials demonstrating superiority of efficacy endpoints compared to chemotherapy alone (9-11,13). However, progression remains the leading clinical problem and there is an urgent medical need for efficacious 2nd line strategies to overcome resistance. As none of the pivotal trials had a predefined 2nd line therapy, no prospective data are available for specific treatment sequences. Second line phase III trials evaluating docetaxel with an anti-angiogenetic substance, e.g., nintedanib (D+N) or ramucirumab (D+R) have each shown superiority to the comparator docetaxel monotherapy and led to approval of both combinations (17,18). However, those trials were designed in the era before ICI were used in 1st line treatment. For D+N a prospective German non-interventional trial (VARGADO) is underway and will provide insights to the efficacy of this sequence (19).
No prospective studies have been published to date for D+R in this sequence. Thus, retrospective clinical data may provide at least some evidence for this therapeutic sequence. To the best of our knowledge, this cohort is the largest studied so far with a specific sequence of 1st line CTX+ICI followed by D+R in 2nd line. We showed that this sequence is feasible and effective in clinical routine. These results are consistent with the prospective data of the REVEL study on D+R from the pre-ICI era and our efficacy and safety data are highly comparable with response rates being even higher (17). As long as no other trial data are available for 2nd line therapy after CTx+ICI, our data provide a solid basis for the use of D+R in 2nd line after CTx+ICI in all histologies.
Nearly two-thirds of the patients in this study received platinum plus pemetrexed plus pembrolizumab in the 1st line setting. These patients represent a large, homogeneous and well-characterized subgroup, allowing for further in-depth analyses. The data for clinical efficacy and safety were very favorable in this subgroup, although OS and PFS remained slightly inferior to the corresponding arm of the Keynote 189 trial, while response rates were comparable (8,9). Patients in our analysis were treated in daily clinical practice and so were older and had more co-morbidities than those in the Keynote 189 trial. In addition, there may have been a negative selection bias in our patient population, since patients with exceptionally good response to 1st line CTx+ICI may not have yet required 2nd line treatment. Furthermore, there were less tumors with a PD-L1 TPS of ≥50% in our cohort than in the phase III trial with 6% versus 32%, respectively.
We and others recently reported excellent PFS and OS data for D+R given in 3rd line immediately after ICI monotherapy (15,20-24). We have hypothesized that anti-angiogenic agents not only act directly through VEGF-mediated effects but also produce synergistic effects with chemotherapeutic agents and ICI agents for instance by contributing to better drug penetration through their action on tumor vessels (15,25-27). Unfortunately, no clear evidence of such a synergistic effect could be observed in our analyses of the D+R second line cohort. Especially, no superior efficacy could be proven in patients with an objective response to 1st line therapy. However, there was at least a trend toward longer PFS with D+R in patients with moderate (1–49%) and high (>50%) PD-L1 TPS scores versus tumors negative for PD-L1. Knowing that ICI bind to the PD-1 receptor up to two months after the last infusion of the drug, this could be a hint for the synergistic effect described above at least in PD-L1 high expressing tumors (28).
Most patients in the platinum plus pemetrexed plus pembrolizumab sub-cohort had available KRAS mutation data. In line with results from the KEYNOTE-189 trial, KRAS status had no impact on 1st-line therapy (29). However, a positive KRAS status was significantly associated with worse median PFS in 2nd-line D+R and was also an independent predictor of worse PFS in multivariate Cox regression analysis. To date, there is little evidence on the role of KRAS status in D+R therapy (30). Although KRAS mutations might increase the expression of VEGF, VEGFR1 and VEGFR2 in various tumors including NSCLC, the underlying mechanisms is still unclear (31-33). In vitro data of ramucirumab showed inferior tumor regression in a KRAS mutated xenograft tumor (NCI-H2122) which could be significantly increased by the combination of ramucirumab with a pan-RAF inhibitor (34). In addition, a KRAS mutation was a significantly negative predictor for response and time on treatment when bevacizumab, a VEGF antibody, was given in combination with carboplatin and docetaxel in NSCLC (35). These observations support our findings. Nevertheless, the significance of KRAS status in this situation has not yet been conclusively determined, and KRAS status should be analyzed systematically in future studies investigating antiangiogenic combination therapies after Ctx+ICI (36).
Some potential limitations of our study must be addressed. This was a retrospective study and some underreporting of potential side effects may have occurred. Since severe side effects are usually well documented during clinical routine, underreporting may have mainly affected the documentation of those side effects not immediately apparent in routine care. Furthermore, there may have been some negative selection bias since patients with limited effect of 1st line treatment may have been over-represented in our cohort. Accordingly, there remains some uncertainty whether D+R is an effective 2nd line therapy for patients with a particularly good response to CTx+ICI 1st line therapy. In this study, patient response to treatment was assessed by criteria used during routine care at participating centers and was not collected in a predefined, standardized manner by independent investigators. Therefore, some variability between centers may be present for PFS, but this would not have affected survival data. KRAS mutation status was not reported for about one third of the patients. However, of those 20.4% presented with a squamous cell carcinoma. We do not have evidence that there was a systematic error in this regard, so the patients with unknown KRAS status should be a representative sample from the overall cohort.
Overall, our results clearly demonstrate a superb OS and PFS from 2nd line D+R therapy after 1st-line therapy with CTx+ICI for metastatic NSCLC of all histologies. This effect was even slightly more pronounced in a large subgroup treated with platinum plus pemetrexed plus pembrolizumab in 1st line. Some early evidence was found that patients with a KRAS mutation may have significantly less response to D+R after CTx+ICI. Further systematic studies are needed to identify robust criteria for patient selection for 2nd line therapy with D+R after CTs+ICI.
Supplementary
The article’s supplementary files as
Acknowledgments
We thank all the patients who participated in this study.
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work ensuing that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of Paracelsus Medical University Nuremberg (IRB-2019-014). Individual consent for this retrospective analysis was waived.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/tlcr-21-197
Data Sharing Statement: Available at https://dx.doi.org/10.21037/tlcr-21-197
Peer Review File: Available at https://dx.doi.org/10.21037/tlcr-21-197
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tlcr-21-197). Albrecht Stenzinger serves as an unpaid editorial board member of Translational Lung Cancer Research from Sep 2019 to Sep 2021. The following authors received honoraria for lectures, presentation, speakers bureaus, manuscript writing or educational events: WMB from AstraZeneca, Boehringer, Novartis, MSD, BMS, Roche and Lilly; MR from Amgen, AstraZeneca, BMS, Boehringer, Lilly, Minde, Merck, MSD, Novartis, Pfizer and Roche; AR from AbbVIe, AstraZeneca, BMS, Boehringer, Lilly, MSD, Novartis, Pfizer and Roche; CW from Boehringer, Lilly, MSD, Roche, AstraZeneca, BMS, Sanofi Aventis and Takeda; GHW from Boehringer, Lilly, MSD, Roche, AstraZeneca, BMS, Sanofi Aventis, Takeda, Glaxo and Berlin Chemie; PC from Roche, Takeda, AstraZeneca and Novartis; AS from Aignostics, Bayer, Thermo Fisher, Illumina, AstraZeneca, Novartis, Pfizer, Roche, Seattle Genetics, MSD, BMS, Takeda and Lilly; AT from GSK, Novartis, Amgen, BMS, MSD, Lilly, Pfizer, Boehringer, Roche, Takeda and Celgene; JHF from AstraZeneca, Boehringer, Novartis, MSD, BMS and Roche. The following authors received support for attending meetings and/or travel: WMB from Boehringer, AstraZeneca and Roche; CW from Boehringer, MSD, Roche, AstraZeneca and BMS; GW from Boehringer, MSD, Roche, AstraZeneca and Berlin Chemie; PC from AstraZeneca, Takeda, Novartis and Lilly; AT from GSK, Novartis, Amgen, MSD, BMS, Lilly, Pfizer, Boehringer, Roche, Takeda and Celgene; JHF from Boehringer and AstraZeneca. The following authors participated on a data safety monitoring board or an advisory board: WMB on AstraZeneca, Boehringer, Novartis, MSD, Lilly, BMS and Roche; JK on Roche, Boehringer, BMS, Merck, Amgen, Takeda and Lilly (all outside the submitted work and all without receiving any personal fees; CW on Boehringer, MSD, Roche, AstraZeneca and MBS; GHW on Boehringer, MSD, Roche, AstraZeneca and BMS; PC on Pfizer, Chugai, Boehringer and Roche; AT on GSK, Novartis, Amgen, MSD, BMS, Lilly, Pfizer, Boehringer, Roche, Takeda, Celgene and AIO Leadership Group; PH on Lilly, MSD, BMS, AstraZeneca, Takeda, Roche, Boehringer and Pfizer; JHF on AstraZenca. The following authors receipt equipment, materials, drugs, medical writing, gifts or other services: WMB from Boehringer (for medical writing); JHF from Novartis (for medical writing); the following authors received consulting fees: AR from AbbVie, AstraZeneca, BMS, Boehringer, Lilly, MSD, Novartis, Pfizer and Roche. The following authors received grants or contracts form any entity: PC from Roche, Takeda, AstraZeneca and Novartis (all research grants to the institution; AT from AstraZeneca (research grant). The other authors have no conflicts of interest to declare.
References
- 1.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. 10.1016/S0140-6736(16)00587-0 [DOI] [PubMed] [Google Scholar]
- 3.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 4.Horn L, Spigel DR, Vokes EE, et al. Nivolumab Versus Docetaxel in Previously Treated Patients With Advanced Non-Small-Cell Lung Cancer: Two-Year Outcomes From Two Randomized, Open-Label, Phase III Trials (CheckMate 017 and CheckMate 057). J Clin Oncol 2017;35:3924-33. 10.1200/JCO.2017.74.3062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 6.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. 10.1016/S0140-6736(16)32517-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gettinger S, Horn L, Jackman D, et al. Five-Year Follow-Up of Nivolumab in Previously Treated Advanced Non-Small-Cell Lung Cancer: Results From the CA209-003 Study. J Clin Oncol 2018;36:1675-84. 10.1200/JCO.2017.77.0412 [DOI] [PubMed] [Google Scholar]
- 8.Gadgeel S, Rodriguez-Abreu D, Speranza G, et al. Updated Analysis From KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol 2020;38:1505-17. 10.1200/JCO.19.03136 [DOI] [PubMed] [Google Scholar]
- 9.Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. 10.1056/NEJMoa1801005 [DOI] [PubMed] [Google Scholar]
- 10.Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2040-51. 10.1056/NEJMoa1810865 [DOI] [PubMed] [Google Scholar]
- 11.Reck M, Mok TSK, Nishio M, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med 2019;7:387-401. 10.1016/S2213-2600(19)30084-0 [DOI] [PubMed] [Google Scholar]
- 12.Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. 10.1056/NEJMoa1716948 [DOI] [PubMed] [Google Scholar]
- 13.Paz-Ares L, Ciuleanu TE, Cobo M, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:198-211. 10.1016/S1470-2045(20)30641-0 [DOI] [PubMed] [Google Scholar]
- 14.Santos ES. Treatment options after first-line immunotherapy in metastatic NSCLC. Expert Rev Anticancer Ther 2020;20:221-8. 10.1080/14737140.2020.1738930 [DOI] [PubMed] [Google Scholar]
- 15.Brueckl WM, Reck M, Rittmeyer A, et al. Efficacy of Docetaxel Plus Ramucirumab as Palliative Third-Line Therapy Following Second-Line Immune-Checkpoint-Inhibitor Treatment in Patients With Non-Small-Cell Lung Cancer Stage IV. Clin Med Insights Oncol 2020;14:1179554920951358. 10.1177/1179554920951358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 17.Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 2014;384:665-73. 10.1016/S0140-6736(14)60845-X [DOI] [PubMed] [Google Scholar]
- 18.Reck M, Kaiser R, Mellemgaard A, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol 2014;15:143-55. 10.1016/S1470-2045(13)70586-2 [DOI] [PubMed] [Google Scholar]
- 19.Grohe C, Gleiber W, Krüger S, et al. Efficacy and safety of nintedanib plus docetaxel in lung adenocarcinoma patients following treatment with immune checkpoint inhibitors: updated results of the ongoing NIS VARGADO (NCT02392455). Ann Oncol 2019;30:mdz449.020.
- 20.Harada D, Takata K, Mori S, et al. Previous Immune Checkpoint Inhibitor Treatment to Increase the Efficacy of Docetaxel and Ramucirumab Combination Chemotherapy. Anticancer Res 2019;39:4987-93. 10.21873/anticanres.13688 [DOI] [PubMed] [Google Scholar]
- 21.Kato R, Hayashi H, Chiba Y, et al. Propensity score-weighted analysis of chemotherapy after PD-1 inhibitors versus chemotherapy alone in patients with non-small cell lung cancer (WJOG10217L). J Immunother Cancer 2020;8:e000350. 10.1136/jitc-2019-000350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shiono A, Kaira K, Mouri A, et al. Improved efficacy of ramucirumab plus docetaxel after nivolumab failure in previously treated non-small cell lung cancer patients. Thorac Cancer 2019;10:775-81. 10.1111/1759-7714.12998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tozuka T, Kitazono S, Sakamoto H, et al. Addition of ramucirumab enhances docetaxel efficacy in patients who had received anti-PD-1/PD-L1 treatment. Lung Cancer 2020;144:71-5. 10.1016/j.lungcan.2020.04.021 [DOI] [PubMed] [Google Scholar]
- 24.Yoshimura A, Yamada T, Okuma Y, et al. Retrospective analysis of docetaxel in combination with ramucirumab for previously treated non-small cell lung cancer patients. Transl Lung Cancer Res 2019;8:450-60. 10.21037/tlcr.2019.08.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukumura D, Jain RK. Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization. Microvasc Res 2007;74:72-84. 10.1016/j.mvr.2007.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang H, Wang M. Prospect of immunotherapy combined with anti-angiogenic agents in patients with advanced non-small cell lung cancer. Cancer Manag Res 2019;11:7707-19. 10.2147/CMAR.S212238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tong RT, Boucher Y, Kozin SV, et al. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res 2004;64:3731-6. 10.1158/0008-5472.CAN-04-0074 [DOI] [PubMed] [Google Scholar]
- 28.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010;28:3167-75. 10.1200/JCO.2009.26.7609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gadgeel S, Rodrigues-Abreu D, Felip E, et al. KRAS mutational status and efficacy in KEYNOTE-189: pembrolizumab (pembro) plus chemotherapy (chemo) vs placebo plus chemo as first-line therapy for metastatic non-squamous NSCLC. Ann Oncol 2019;30:xi64. 10.1093/annonc/mdz453.002 [DOI] [Google Scholar]
- 30.Brueckl WM, Ficker JH, Zeitler G. Clinically relevant prognostic and predictive markers for immune-checkpoint-inhibitor (ICI) therapy in non-small cell lung cancer (NSCLC). BMC Cancer 2020;20:1185. 10.1186/s12885-020-07690-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mancikova V, Inglada-Perez L, Curras-Freixes M, et al. VEGF, VEGFR3, and PDGFRB protein expression is influenced by RAS mutations in medullary thyroid carcinoma. Thyroid 2014;24:1251-5. 10.1089/thy.2013.0579 [DOI] [PubMed] [Google Scholar]
- 32.Schimanski CC, Zimmermann T, Schmidtmann I, et al. K-ras mutation status correlates with the expression of VEGFR1, VEGFR2, and PDGFRalpha in colorectal cancer. Int J Colorectal Dis 2010;25:181-6. 10.1007/s00384-009-0843-7 [DOI] [PubMed] [Google Scholar]
- 33.Yuan XH, Yang J, Wang XY, et al. Association between EGFR/KRAS mutation and expression of VEGFA, VEGFR and VEGFR2 in lung adenocarcinoma. Oncol Lett 2018;16:2105-12. 10.3892/ol.2018.8901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu W, Stewart J, King C, et al. Combined inhibition of pan-RAF and VEGFR-2 mediates antitumor activity in KRAS mutant NSCLC through enhanced inhibition of tumor angiogenesis and growth. Am Assoc Cancer Res (AACR) 2106;76:76.
- 35.Domine M, Rojo F, Izarzugaza Y, et al. Kras status as predictive marker of response and time to progression in EGFR wild-type stage IV NSCLC patients treated with platin-docetaxel-bevacizumab. Ann Oncol 2021;23:134. [Google Scholar]
- 36.Chen H, Zhao J. KRAS oncogene may be another target conquered in non-small cell lung cancer (NSCLC). Thorac Cancer 2020;11:3425-35. 10.1111/1759-7714.13538 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as