Abstract
Objective
Update the last known review, and summarize the definitions, diagnostic criteria, reported risk factors, possible mechanisms and potential biomarkers of hyperprogressive disease (HPD) under immunotherapy.
Background
Immunotherapy is a relatively new systemic therapy adding a new method of treatment of especially advanced cancer patients. In a variety of immunotherapies, however, an unexpected acceleration of tumor growth, known as HPD, is observed in approximately 30% of patients after immune checkpoint inhibitor (ICI) treatment. HPD has a deleterious survival effect on patients and represents an urgent issue for both clinicians and patients. Existing literature has reviewed and summarized the definition, diagnostic criteria, reported risk factors and possible mechanisms of hyperprogression. However, with the gradual deepening of the exploration of HPD, researchers have made significant breakthroughs in elucidating the mechanism and mechanism of HPD and exploring biomarkers.
Methods
The search was conducted on Google Scholar and PubMed in January and May of 2021. We searched among English papers with no limitation on the publication year. We have included retrospective studies, case reports and basic researches related to HPD in the collection, we also referred to some review articles on HPD in recent years. A qualitative-interpretive approach was used for data extraction.
Conclusions
HPD is considered to be an acceleration of tumor growth after ICI treatment that is not only due to immune infiltration but also due to real disease progression, with an incidence of about 4–30% in all retrospective published studies to date. Currently, the most widely used criteria of HPD contain Response Evaluation Criteria in Solid Tumors (RECIST) and tumor growth rate (TGR) or tumor growth kinetics. The common risk factors and underlying mechanisms of HPD have not yet been fully elucidated. However, based on the poor prognosis of HPD, there have been many advances in the exploration of biomarkers in recent years, like the prediction of HPD, such as LDH levels of peripheral blood, liquid biopsy, and radiomics, etc.
Keywords: Immunotherapy, hyperprogressive disease (HPD), hyperprogression (HP)
Introduction
The treatment of especially metastatic cancer patients has been dramatically improved by the introduction of immunotherapy. There are already 3 immune checkpoint inhibitors (ICIs) that target cytotoxic T lymphocyte-associated molecule-4 (CTLA-4), programmed cell death receptor-1 (PD-1), and programmed cell death ligand-1 (PD-L1) are the most widely studied and recognized. It has been proven that ICIs can increase the overall survival (OS) in variety of malignancies, such as melanoma, non-small cell lung cancer (NSCLC), renal cancer, head and neck squamous cell carcinoma (HNSCC), urothelial bladder cancer, and endometrial stromal sarcoma (1-3). However, a small subset of patients on immunotherapy may not benefit at all from immunotherapy, instead, they experience a faster and more aggressive progression of the tumor than expected, with a dramatic acceleration of the disease, which is referred to as hyperprogression (HP) or hyperprogressive disease (HPD). Patients with HPD could suffer a deleterious survival effect and significantly shorter OS, suggesting that HP should be managed as fulminant toxicity and needs to be considered before immunotherapy is initiated. Existing literature has reviewed and summarized the definition, diagnostic criteria, reported risk factors and possible mechanisms of HP. However, with the gradual deepening of the exploration of HPD, researchers have made significant breakthroughs in elucidating the mechanism and mechanism of HPD and exploring biomarkers. On this basis, we reviewed and summarized the definition, incidence, diagnostic criteria, reported risk factor and possible mechanisms of HPD in recent years, and briefly introduced a novel perspective that few people mentioned: the potential biomarkers of HPD. We present the following article in accordance with the Narrative Review reporting checklist (available at https://dx.doi.org/10.21037/tlcr-21-575).
Methods and results
The search was conducted on google scholar and PubMed in January and May of 2021. We searched among English papers with no limitation on the publication year. We have included retrospective studies, case reports, basic research articles related to HPD in the collection, we also refer to some review articles, commentary articles on HPD in recent years. A qualitative-interpretive approach was used for data extraction.
We have collected 61 references in total, 24 of which are clinical articles, including retrospective studies, cohort studies, case reports etc., 15 of which are basic research articles, 9 of which are review articles or commentary articles, and the rest of which are some guidelines and consensus in the oncology field, and reference articles that needed to explain definitions.
Discussion
Appearance, definitions, and diagnosis of HPD
HPD is considered to be an acceleration of tumor growth after ICI treatment that is not only due to immune infiltration but also due to real disease progression (4,5). It was first reported in a retrospective study published by Lahmar et al. in 2016. They performed a retrospective study of 89 NSCLC patients treated by ICIs, calculated the tumor growth rate [TGR: the log-scale calibrated change in the sum of the volumes of the target lesions according to Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria per month] before and after ICIs treatment, and found that ΔTGR (difference between TGR during ICIs and TGR at baseline) was <0 in 79 patients and >0 in 20 patients; and among these 20 patients, 9 had a ΔTGR >50%, meaning that tumor volume increased at least 50% during immunotherapy (6). In the same year, Chubachi et al. also reported a case of rapid lung cancer progression after 3 cycles of nivolumab treatment and described it as a “disease flare” (7). Later, in 2017, Champiat and his colleagues defined HPD as patients who have been assessed with disease progression by the RECIST for the first evaluation with at least a 2-fold increase in TGR before and after receiving immunotherapy. Subsequently, an increasing number of studies have confirmed the existence of the HPD phenomenon in many types of tumors, like NSCLC, esophageal squamous carcinoma, melanoma, soft tissue sarcoma, etc. (8-11). HP has become an increasingly recognized phenomenon.
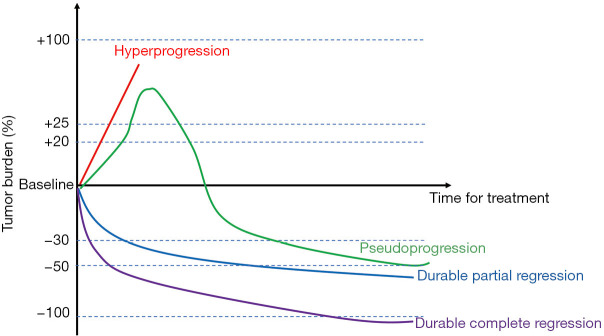
Despite its growing incidence, the diagnostic criteria for HPD have not yet been standardized. Clinicians have previously evaluated the activity and efficacy of new cancer therapeutics in solid tumors by immune-related response criteria (irRC), RECIST, immune RECIST (iRECIST), immune-related RECIST (irRECIST), or immune-modified RECIST (imRECIST) (12-14). We have compiled these criteria in Table 1, all of these criteria divide efficacy into 4 levels (Figure 1): complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). In PD, several subclassifications are not mentioned in detail in RECIST: confirmed PD; HP, and pseudoprogression.
Table 1. Overview of radiological criteria in immuno-oncology.
| Variables | RECIST1.1 (15) | irRC (13) | irRECIST (16) | iRECIST (12) | imRECIST (17) |
|---|---|---|---|---|---|
| Lesion measurement | Unidimensional | Bidimensional | Unidimensional | Unidimensional | Unidimensional |
| Measurable lesion size (CT) (mm) | ≥10 | 5×5 | ≥10 | ≥10 | ≥10 |
| Baseline lesion number | 5 in total, 2 per organ | 10 in total, 5 per organ | 5 in total, 2 per organ | 5 in total, 2 per organ | 5 in total, 2 per organ |
| CR | All lesions disappeared | All lesions disappeared | All lesions disappeared | All lesions disappeared | All lesions disappeared |
| PR | Decline ≥30% from baseline | Decline ≥50% from baseline | Decline ≥30% from baseline | Decline ≥30% from baseline | Decline ≥30% from baseline |
| SD | Neither PR nor PD | Neither PR nor PD | Neither PR nor PD | Neither PR nor PD | Neither PR nor PD |
| PD | ≥20% increase from nadir (≥5 mm); appearance of new lesions | ≥25% increase from nadir | ≥20% increase from nadir | ≥20% increase from nadir | ≥20% increase from nadir |
| PD-confirmed | No applicable | ≥4 weeks | 4 to 12 weeks | 4 to 8 weeks | ≥4 weeks |
RECIST, Response Evaluation Criteria in Solid Tumors; irRC, immune-related response criteria; iRECIST, immune RECIST; irRECIST, immune-related RECIST; imRECIST, immune-modified RECIST; CT, computed tomography; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Figure 1.
Patterns of response to immunotherapy.
Currently, the most widely used criteria contain RECIST and TGR or tumor growth kinetics [TGK: the change in the sum of the longest diameters (SLD) of the target lesions according to RECIST 1.1 criteria per month](Figure 2). For example, in the study of Kanjanapan et al., HPD was considered to be RECIST-defined PD at first assessment and a more than 2-time TGR increase after the initiation of immunotherapy (18). Meanwhile, Saâda-Bouzid et al. defined HP as TGK ratio (TGKR: TGK post-immunotherapy/TGK pre-immunotherapy) ≥2, and these authors reported a 29% incidence of HPD (19). The common requirement of TGR and TGK measurement is a comparison of the variation of target lesions before and after immunotherapy is initiated, which mainly depends on computed tomography (CT) scan. As Ferrara et al., they defined HPD as disease progression at the first evaluation with ΔTGR exceeding 50% (20). However, the exclusive use of a radiological definition of HPD could easily misdiagnose pseudoprogression as HP (21), which could lead to an overestimate. Moreover, in some cases of rapid clinical decline, performing a confirmatory CT scan may not be feasible. Therefore, some authors propose adding other clinical criteria to the diagnostic criteria for HPD. For instance, Kato et al. suggest 3 criteria to define HPD: time to treatment failure (TTF) <2 months; and a tumor burden increase of 50% with a progression rate increase of at least 2 times (22). In addition to these, Lo Russo et al. also proposed the following diagnostic criteria: ≥2 new lesions appearing in 1 already-involved organ or new involved organs appearing at the first radiological evaluation after the initiation of immunotherapy, and an Eastern Cooperative Oncology Group (ECOG) score decrease of 2 points or more within the first 2 months of treatment (23). However, too great a consideration for clinical factors can confer disadvantages, as progressive PD may be mistaken for HPD; this contradiction may be part of the reason why there is still no consensus on the diagnostic criteria for HPD.
Figure 2.
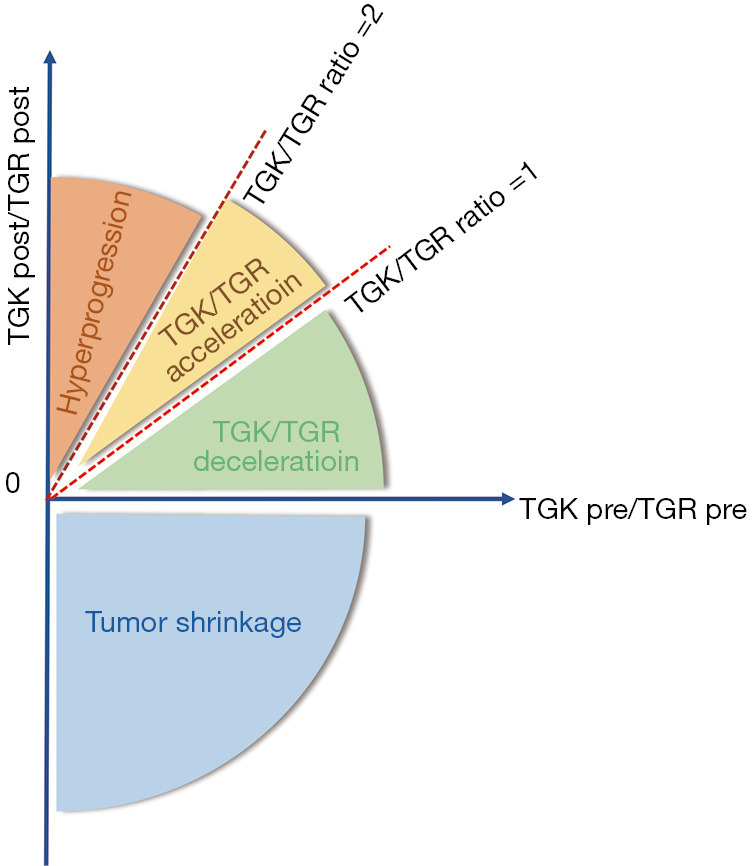
TGK/TGR criteria in defining HPD. TGK, tumor growth kinetics; TGR tumor growth rate; HPD, hyperprogressive disease.
Incidence, risk factors, prognosis, and potential biomarkers
Incidence
HPD is not only caused by immunotherapy. One post hoc analysis from the OAK trial suggested that rapid progression is a common phenomenon that co-occurs with chemotherapy and ICIs. Likewise, Gandara et al. found in their study that the atezolizumab and docetaxel groups had a similar proportion of patients with rapid progression, meaning that the rapid progression after baseline was not specific to PD-L1 inhibitor therapy (24). For radiotherapy, the correlation between HPD and radiotherapy is more ambiguous, Saâda-Bouzid et al. noticed that almost all cases of HPD during immunotherapy in their study occurred in patients who had at least one locoregional recurrence in an irradiated field, suggesting that radiotherapy might play a role in the process of HPD (19). And a recent case report also reported a 42-year-old woman with stage IV renal clear cell carcinoma, experienced HPD during immunotherapy after receiving stereotactic body radiation therapy (SBRT) (25). However, there are no reports of HPD cases during pure radiotherapy.
Overall, HPD is not a unique phenomenon of immunotherapy, while in immunotherapy, the incidence of HPD is higher, especially when PD-1 or PD-L1 inhibitor is used. According to published relevant research data, the prevalence of immunotherapy-related HPD ranges from a few percent to about 30% (5,26). Champiat et al. reported an incidence of HPD of 9% (12/131), and this ratio may be underestimated, as some patients could not be evaluated due to clinical progression; thus, the true incidence of HPD might be higher than that reported (4). Additionally, the incidence of HPD may also be related to the type of tumor. Saâda-Bouzid et al. reported a 29% incidence of HPD in hypopharyngeal squamous cell carcinoma (19); the incidence of HPD in other cancers has been reported, with gastric cancer having an HPD incidence of 11% (27),NSCLC a range from 13.8% to 25.7% (20,23,28), melanoma 6–34%, gynecological cancer 16%, cutaneous squamous cell carcinoma 9%, renal cancer 5–7%, colorectal cancer 6%, and urothelial cancer 6% (29).
Risk factor
HPD has been putatively associated with several risk factors, including age (>65syears), genomic alterations, and metastasis burden (the number of sites of metastatic disease), or locoregional recurrence (4,19,23,24,29,30).
Age (>65 years)
Many studies have shown that older patients have a higher chance of developing HPD. Champiat et al. observed that patients with HPD were older than those without HPD (P=0.07), and suspected that old age might be related to a different immunological background or higher concentrations of inflammatory cytokines (4). Meanwhile, Refae et al. also reported that HPD is significantly associated with age ≥70 years (25% versus 6%; P=0.025) (31).This may be related to immune cells, chemokines, phagocytosis, and the weakened ability of intracellular antigens in elderly patients, but the specific mechanism is unknown.
Genomic alterations
Murine double minute 2/4 proto-oncogene (MDM2/4) family amplification and epidermal growth factor receptor (EGFR) aberrations are the most widely studied alterations in HPD. One study that analyzed 11 patients with multiple tumors who experienced HP in a multi tumor cohort demonstrated that rs2282055 (PD-L1) and rs1870377 [vascular endothelial growth factor receptor 2 (VEGFR2)] variations have a significant and independent influence on the occurrence of HPD (31). Another preliminary report by Tawbi and colleagues suggested that liposarcoma patients, who commonly harbor MDM2 amplification, may benefit from immunotherapy (PR rate of 11%) (32). Meanwhile, Kato et al. found that 6 patients with MDM2/MDM4 expansion had TTF in less than 2 months; with 4 of them meeting the definition of HP; the tumor volume increased by 55% to 258%, and the rate of progression increased by 2.3, 7.1, 7.2, and 42.3 times from the baseline, respectively. Patients with MDM2/MDM4 amplification showed significantly accelerated tumor growth compared with that observed before treatment (30). In the study by Ye et al. (33), even though no statistically significant association was observed between MDM2, MDM4, or EGFR amplification and the efficacy of checkpoint inhibitors, it was nonetheless observed that patients with MDM2/4 or EGFR amplification were more prone to developing HP and had a lower chance of disease control.
Metastasis burden and locoregional recurrence
A retrospective study published by Ferrara et al. found that NSCLC patients with multiple metastases at baseline tended to experience HP. They found in their study that patients with HP were significantly more likely to have had 2 or more metastases before receiving PD-1/PD-L1 therapy (among patients with ≥2 metastases, 62.5% developed HP, P=0.006) (20). Saâda-Bouzid et al. also found in their study that the incidence of HP was 42% in patients with metastatic cervical lymph nodes and only 26% in patients without cervical lymph nodes metastasis and that regional recurrences occurred in 90% of patients with HP, but only in 37% of patients without progression, indicating that HP was significantly associated with region recurrence (P=0.008), but not with region recurrence (19).
Potential biomarkers
Peripheral blood biomarkers
For years, scientists have been looking for a non-invasive cancer biomarker, and the marker in the peripheral blood is undoubtedly one of the most convenient. Peripheral white blood cell (WBC) counts such as neutrophil count (NC), lymphocyte count (LC), the neutrophil to lymphocyte ratio (NLR), and the derived neutrophil to lymphocyte ratio [dNLR: absolute NC/(WBC − absolute NC)] have been demonstrated that can provide some clue to HPD. Kim et al. obeserved 19 patients treated with nivolumab, and found that NLR is rising from baseline in 5 out of 7 patients with PD and patients with an >30% increase in NLR were associated with a significantly shorter TTF compared with those with stable or decrease in NLR both after first cycle (P=0.014) and second cycle (P=0.001) (34). Another popular biomarker is serum lactate dehydrogenase (LDH), Sasaki et al. indicated that a high level of LDH was associated with HPD (18,35). In a recent study, the investigator demonstrated that the risk of HPD in LDH > upper limit of normal (ULN) was 2.32 times higher than that in LDH ≤ ULN (P=0.0001) (36). However, some researchers hold different opinions, like Champiat et al. and Ferrara et al., they didn’t find the association in find any significant differences in dNLR, WBC, NC, LC, or LDH (4,20).
Liquid biopsy
As a branch of in vitro diagnosis, liquid biopsy is a non-invasive blood test to monitor circulating tumor cells (CTCs), circulating tumor DNA (ctDNA) and cell-free DNA (cfDNA) fragments released into the blood by tumors or metastatic lesions. ctDNA and cfDNA can reflect blood-based tumor mutational burden (bTMB) and chromosomal instability of cancer cells, which has been linked to poor prognosis and resistance to treatment in several malignancies. By analyzing the mutations detected in ctDNA, bTMB can be calculated to evaluate tumor response to immunotherapy, and increased TMB has been associated with a higher likelihood of immunotherapy response (37-39). More importantly, the dynamics of ctDNA may be more sensitive than radiological tests. In a study involving 125 melanoma patients, the researchers found that none of the patients (n=9) who were finally diagnosed with pseudoprogression did not observe a significant increase in ctDNA (even it is detectable at baseline, subsequent ctDNA levels will be reduced by >10 timesdue to treatment), despite the significant increase in tumor size on imaging (40). And Weiss et al. used chromosomal number instability (CNI) score to evaluate 56 patients treated with immunotherapy across multiple tumor types, six of whom had HP, and in 5 of these 6 cases, the CNI score also predicted progression at an earlier time (∼6–9 weeks)compared with routine imaging (41). This shows that cfDNA is expected to become a potential biomarker for predicting HPD.
Radiomics
Radiomics is an emerging field that translates medical images into quantitative data and enables these data to be extracted and applied in clinical decision-making, thereby improving diagnostic, prognostic, and predictive accuracy. In cancer research, radiomics has also shown promise in tumor diagnosis, clinical staging, response evaluation, recurrence, distant metastasis, and prognosis prediction (42,43). The image information extracted by Radiomic includes but is not limited to size/shape, heterogeneity/texture, relationship with surrounding tissues, which also provides a basis for evaluating the efficacy of immunotherapy, because tumor size changes caused by tumor cell necrosis may not necessarily occur in the course of immunotherapy. Besides, Sun et al. observed a significant correlation between the genomic and radiomic characteristics (P<0.0001) and the abundance of CD8 cells as well as tumor-associated neutrophils (P=0.0079), which are associated with poor response to immunotherapy (44). The study published by Ji et al. also validated that radiomics could separate patients who did and did not benefit from immunotherapy, they constructed 4 radiomics models to explore that whether radiomics can use in predicting the effectiveness of immunotherapy. And the sensitivity, specificity, and area under the curve (AUC) of the best model can reach 83.3%, 88.9%, and 0.806 respectively (42). From this point of view, radiomics may be used as one of the promising markers, through which, it may be possible to stratify people undergoing immunotherapy and screen out potential populations that may develop HPD. While more research is still needed to support this.
Prognosis of HPD
Patients with HPD may have a significantly shorter OS than other patients (Table 2). A study by Kurman et al. found that the median OS of non-HPD patients was 7.6 months, while that of HPD patients was only 4.6 months (21). Another meta-analysis reported that HPD patients were associated with the worse OS when compared to non-HPD individuals (3). However, no such difference in survival was found in patients with HPD after receiving chemotherapy, thus implicating immunotherapy as a possible factor in HPD emergence.
Table 2. Characteristics of the included studies in immunotherapy-related HPD.
| Authors | Publication year |
Study design | Tumor type | Treatment | Incidence of HPD | Definition of HPD | Predictive factor of HPD | Survival outcome for the HPD |
|---|---|---|---|---|---|---|---|---|
| Lahmar et al. (6) | 2016 | Retrospective | NSCLC | Nivolumab, pembrolizumab, atezolizumab | 10.1% (9/89) | TGR >50% | – | – |
| Champiat et al. (4) | 2017 | Retrospective | Melanoma, lung cancer, renal cancer, colorectal cancer, urothelial cancer, and others | Anti-PD-1/PD-L1 monotherapy | 9.2% (12/131) | RECIST progression disease at first evaluation | Age | HPD patients: median OS 4.6 months (95% CI, 2.0–NA) |
| TGRR ≥2 | Non-HPD patients: median OS 7.6 months (95% CI, 5.9–16.0) | |||||||
| Saâda-Bouzid et al. (19) | 2017 | Retrospective | Head and neck squamous cell carcinoma (HNSCC) | Anti-PD-1/PD-L1 mAbs | 29.4% (10/34) | TGKR ≥2 | Metastatic cervical nodes | HPD patients: PFS according to RECIST and irRECIST: 2.5, 2.9 months (P=0.003); OS 6.1 months (P=0.77) |
| The presence of a regional recurrence | Non-HPD patients: PFS according to RECIST and irRECIST: 3.4, 5.1 months (P=0.003); OS 8.1 months (P=0.77) | |||||||
| Kato et al. (30) | 2017 | Genomic analysis | Bladder cancer, squamous cell carcinoma, breast cancer, endometrial stromal, sarcoma, lung cancer | CTLA-4, PD-1/PD-L1 inhibitors, or other agents | 3.9% (6/155) | TTF <2 months | MDM2/4 family amplification | – |
| Tumor burden increased by 50% | Epidermal growth factor receptor mutations | |||||||
| Progression rate increase >2-fold | ||||||||
| Ferrara et al. (20) | 2018 | Retrospective | NSCLC | Anti-PD-1/PD-L1 mAbs vs. single-agent chemotherapy | Immunotherapy cohort:13.8% (56/406) | ΔTGR exceeding 50% | More than 2 metastatic sites before PD-1/PD-L1 |
HPD patients: median OS 3.4 months (95% CI, 2.8–7.5 months) |
| Non-HPD patients: median OS 6.2 months (95% CI, 5.3–7.9 months) | ||||||||
| Kanjanapan et al. (18) | 2019 | Retrospective | Head and neck, gynecological, lung, gastrointestinal, genitourinary, melanoma, endocrine, breast cancer, and sarcoma | PD-1/PD-L1 inhibitors or other checkpoint inhibitors | – | RECIST1.1 progression at the first on treatment | Age | HPD patients: PFS 1.6 months (HR: 3.7; 95% CI, 2.0–7.1; P<0.001); OS 5.9 months (HR: 1.7; 95% CI, 0.9–3.3; P=0.11) |
| ≥2-fold increase in TGR | Sex | Non-HPD patients: PFS 2.8 months (HR: 3.7; 95% CI, 2.0–7.1; P<0.001); OS 14.3 months (HR: 1.7; 95% CI, 0.9–3.3; P=0.11) | ||||||
| Immunotherapy toxicity | ||||||||
| Lo Russo et al. (23) | 2019 | Prospective | NSCLC | Anti-PD-1/PD-L1 mAbs | 25.7% (39/152) | TTF <2 months | NA | HPD patients: median OS 4.4 months (95% CI, 3.4–5.4) |
| The total major diameter of the target lesion increased by ≥50% | Non-HPD patients: median OS 17.7 months (95% CI, 13.4–24.1) | |||||||
| At least 2 new lesions in an already involved organ | ||||||||
| Appearance of new involved organ | ||||||||
| Decrease of ECOG scores ≥2 during the first 2 months of treatment | ||||||||
| Gandara et al. (45) | 2018 | Prospective | NSCLC | Atezolizumab vs. docetaxel | Atezolizumab: 10.4% (44/425) | Sum of longest diameters (per investigator) increased by 50% from baseline to first assessment or death due to PD | NA | – |
| Kim et al. (28) | 2019 | Retrospective | NSCLC | Ant-PD-1/PD-L1 mAbs | 20.5% (54/263) | RECIST 1.1 progression at the first response evaluation | >2 metastatic sites | HPD patients: PFS 19 days (HR: 4.619; 95% CI, 2.868–7.440); median OS 50 days (HR: 5.079; 95% CI, 3.136–8.226) |
| Two-fold increase of TGK or TGR between the experimental period and reference period | Liver metastases | Non-HPD patients: PFS 48 days (HR: 4.619; 95% CI, 2.868–7.440); median OS 205 days (HR: 5.079; 95% CI, 3.136–8.226) | ||||||
| TTF <2 months | Higher serum lactate dehydrogenases level | |||||||
| RMH score >2 | ||||||||
| Sasaki et al. (35) | 2019 | Retrospective | NSCLC | Anti-PD-1/PD-L1 mAbs | 21.0% (13/62) | TGKR ≥2 and >50% increase in tumor burden | Advanced gastric cancer patients with poor performance status | HPD patients: median PFS 0.7 months (P<0.001); median OS 2.3months (P<0.001) |
| Liver metastases | Non-HPD patients: median PFS 2.4 months (P<0.001); median OS not reach (P<0.001) | |||||||
| Large tumor size | ||||||||
| Higher neutrophil-to-lymphocyte ratio | ||||||||
| Higher C-reactive protein level | ||||||||
| Higher serum LDH | ||||||||
| Tunali et al. (46) | 2019 | Retrospective | Gastric cancer | CTLA-4, PD-1/PD-L1 inhibitors or other agents | 21.0% (13/62) | RECIST-defined PD at first evaluation and TGRR ≥2, and TTF <2 months | RMH prognostic score ≥2 | – |
| Higher serum LDH | ||||||||
| Ji et al. (47) | 2019 | Retrospective | Stomach, esophagus, colorectal, liver, pancreas, and ampulla cancer | PD-1/PD-L1 inhibitor alone or combined with the CTLA-4 inhibitor | 20.0% (5/25) | The TGKR ≥2 | Age | – |
| Neuroendocrine cancer | ||||||||
| Refae et al. (31) | 2020 | Retrospective | NSCLC, HNSCC, melanoma, renal cell carcinoma (RCC) | Anti-PD-1/PD-L1 monotherapy | 11 (14%) | TGKR ≥2 | Age ≥70 years | Median follow-up was 13.3 months (95% CI, 10.6–15.4); median irPFS 16.8 months (95% CI, 10.2–NA); median OS: not reached |
| Immune-relate toxicity grade ≥3 | ||||||||
| VEGFR 2 rs1870377A/T or A/A | ||||||||
| PD-L1 rs2282055 G/T or G/G | ||||||||
| PD-L1 rs2227981 G/G | ||||||||
| Matos et al. (48) | 2020 | Retrospective | Melanoma, NSCLC, xcolorectal cancer, triple-negative breast cancer, head and neck cancer, cervical cancer, bladder, others | Anti-PD-1/PD-L1 mAbs | RECIST cohort: 10.7% (29/270); TGR cohort: 6.3% (14/221) | RECIST cohort: RECIST-defined PD in the 8 weeks after treatment initiation; HPD =1.2× baseline sum target lesions + new lesions in at least 2 different organs | NA | HPD patients: median OS according to TGR criteria: 4.2 months (95% CI, 2.07–6.33); median OS according to RECIST: 5.23 months (95% CI, 3.97–6.45) |
| TGR cohort: TGRR ≥2 | Non-HPD patients: median OS according to TGR criteria: 6.27 months (95% CI, 3.88–8.67); median OS 7.33 months (95% CI, 4.53–10.12) |
TGK, tumor growth kinetics [the change in the sum of the longest diameters (SLD) of the target lesions according to RECIST1.1 criteria per month]; TGR, tumor growth rate (the difference between TGR during ICIs and TGR at baseline); TGKR, TGK post-immunotherapy/TGK pre-immunotherapy; TGRR, TGR post-immunotherapy/TGR pre-immunotherapy; TTF, time to treatment failure (the time from the initiation of ICIs to the discontinuation of ICIs for any reason, including progression, patient preference, toxicity, or death); ECOG, Eastern Cooperative Oncology Group; RMH prognostic score, Royal Marsden Hospital prognostic score; RECIST, Response Evaluation Criteria in Solid Tumors; irRECIST, immune-related RECIST; irPFS, immune-related PFS; HPD, hyperprogressive disease; NSCLC, non-small cell lung cancer; VEGFR, vascular endothelial growth factor receptor; OS, overall survival; PFS, progression-free survival; HR, hazard ratio; CI, confidence interval; mAbs, monoclonal antibodies; ICI, immune checkpoint inhibitor; NA, not available.
Potential mechanisms
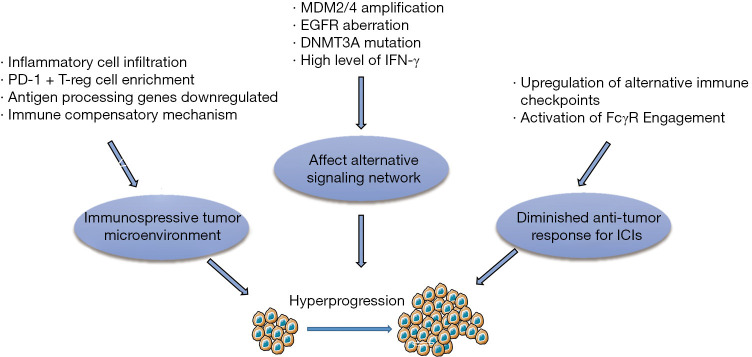
Multiple mechanisms have been proposed to participate in the development of HP. We here summarize the most commonly discussed mechanisms in the recent literature (Figure 3).
Figure 3.
Potential mechanisms of HPD. T-reg cell, regulatory T cell; MDM2/4, murine double minute 2/4 proto-oncogene; EGFR, epidermal growth factor receptor; DNMT3A, DNA methyltransferase 3 alpha; IFN-γ, interferon-γ; FcγR, Fcγ receptor; HPD, hyperprogressive disease; ICIs, immune checkpoint inhibitors.
Massive expansion of PD-1 and T regulatory cells
Regulatory T (T-reg) cells are a kind of immunosuppressive cell that exist in the immunosuppressive tumor microenvironment (TME) (49). ICIs such as CTLA-4 and PD-1, along with stimulating receptors like tumor necrosis factor receptor superfamily member 4 (OX40) and glucocorticoid-induced TNFR-related protein (GITR), are also expressed by T-reg cells. PD-1 works by blocking TCR and CD28 signals to inhibit the excessive activation of effector T cells (49). PD-L1 can increase the possibility of mutual PD-1 and PD-L1 signaling, allowing delicate control of immune cell homeostasis (50,51). Meanwhile, the survival and function of T-reg cells in TME are dependent on T cell receptor (TCR) and CD28 signals (49),and the blockade of PD-1/PD-L1 may activate the immune-suppressive function of T-reg cells, promoting the proliferation of T-reg cells, resulting in further suppression of immunity and rapid tumor progression (49). In line with this hypothesis, Kamada et al. found that non-HPD patients exhibited a drastically reduced ratio of effector regulatory T (eT-reg) cells to CD8+ T cells and the ratio of Ki67+ eT-reg cells to Ki67+CD8+ T cells (mainly with Ki67+ eT-reg cells decreases) in tumor Infiltrating lymphocytes; meanwhile, HPD patients showed no significant change or just a slight increase. This clinical result was validated through in vitro and in vivo mouse models, providing strong support for the role of T reg cells in HP (27).
TME
Research shows that in HPD tumors, an immunosuppressive environment forms after ICI treatment. The presence of inflammatory cells in TME can lead to tumor escape by triggering local inflammation, remodeling matrix tissue, modifying metabolism, or promoting angiogenesis (46,52). Moreover, Xiong et al. also identified that the activities of immune cell populations were significantly decreased while other immune cell populations that attenuate immune responses in the TME were upregulated in the HPD tumors after anti-PD-1 treatment (52). Another study by Lo Russo et al. retrospectively analyzed 152 patientswith NSCLC who received immunotherapy and found an enrichment of tumor-associated macrophages in patients with HP. By using immunofluorescence staining, immunophenotypic macrophages were observed in patients with HP; CD163, CD33, and PD-L1 were all positive and co-expressed, and statistically showed different expression compared with non-HPD patients (P<0.0001) (23). In addition, vascular endothelial growth factor (VEGF) and high LDH concentration in serum may also play a part in affecting the TME. VEGF can influence the immune regulatory environment of tumors by inhibiting the maturation of myeloid and dendritic precursors and the recruiting immunosuppressive M2 macrophages which may be involved in HPD (30). Finally, elevated levels of LDH promote hypoxia in the tumor and acidification of the extracellular environment, which may reduce ICI efficacy, leading to tumor cell proliferation, angiogenesis, and anti-apoptosis (53).
Specific genomic alterations
Particular mutation could cause HPD (30). Xiong et al.analyzed post-therapy HPD tumors with mutation analysis and identified 11 genes with deleterious mutations, including TRPC4, POTEE, FBN2, KMT2C, FUT10, PQBP1, TSC2, etc. (52). Kato et al. found that patients with MDM2/MDM4 amplification or DNMT3A gene mutation were more likely to develop HP (30). Ye et al. found that patients with MDM2/4 or EGFR amplification are more likely to developed HP. MDM2 can directly interact with p53, induce its degradation and limit its function (33),JAK-STAT signaling activated by ICIs can also increase interferon regulatory factor (IRF)-8, which can bind to MDM2 promoter and induce MDM2 expression (54).Moreover, MDM2 expression can regulate VEGF expression in some cancers, and this may contribute to a variety of MDM2 functions in promoting cancer growth and metastasis (55). EGFR activation has also been associated with the upregulation of the checkpoints CTLA-1, PD-1, and PD-L1, which can drive immune escape (56). Epidermal growth factor (EGF) signal transduction has also been shown to induce posttranslational modification of PD-L1, which might be associated with poor prognosis and metastasis (29).
Activation of Fcγ receptor (FcγR) engagement
ICIs could likely promote tumor growth through stimulating PD-1 on myeloid cells. Du et al. found that tumors treated with PD-1 inhibitor displayed elevated Ki-67 expression, reduced cleaved caspase-3 expression, increased proliferation, and decreased apoptosis, suggesting that PD-1 blockade may enhance cancer viability and contribute to the procession of HPD (57). However, this hypothesis is still in its infancy, and more cases are needed to support it. In another study by Knorr and colleagues (58), infiltrating leukocytes were evaluated, and the analysis showed that inhibitors of FcγR IIB (FcγRIIB) in tumor draining lymph node (TDLN) and the TME, FcγR expression within tumors, and FcγRIIA and FcγRIIIA in the TDLN were significantly increased, indicating that the mechanism of HP may be Fc-receptor dependent. Indeed, in 2015, Dahan et al. had already demonstrated that activation of FcγR involvement could reduce the antitumor response of anti-PD-1 antibodies (59). In 2018, Zhang et al. designed 2 anti-PD-1 monoclonal antibody with the same specificity, one which bound to FcR and the other which did not, and the result showed that the latter does prevent tumor growth, while the former can cause macrophage PD-1-T cell cross-link and phagocytosis of PD-1 and T-cells (60).
In this mechanism, FcyRIIB might be the key receptor, as 2141-V11 and FcγRIIB have the strongest binding, while FcyRIIB cross-linking can strengthen myeloid cell signal PD-1 (58,61). In addition, the repolarization of tumor-associated macrophages is dependent on FcyRIIB, and FcR on specific M2-like intratumoral macrophages may result in these cells being reprogrammed to promote tumorigenesis, the discovery of which may provide insight into the mechanism of HP (1,61).
Conclusions
HP is a distinct response pattern in immunotherapy, with an incidence of about 4–30% in all retrospective published studies to date, but with no consensus concepts, predictors, diagnosis criteria, or clear mechanisms related to this condition. Now we looking back at these published studies, there are still limitations in the exploration of HPD, such as the limitations in cohort size, lack of control, and different evaluation methods so on. Besides, there are also many confusions about HPD: why does HPD perform prominently in immunotherapy? Is immunotherapy really completely contraindicated for patients with HPD? Is it possible to reverse HPD?
For now, HP is as much of a challenge as it is an opportunityin immunotherapy, as and the molecular and clinical understanding of HPD is still in its preliminary stages, and it has been reported that HP under immunotherapy may be deleterious to survival, for patients who already experience HP occurs, the current immunotherapy regimen should be stopped first, relieve life threats for patients and choose a treatment-sensitive treatment plan for the patient at the appropriate time. But for patients who have already experienced HPD, the follow-up treatment is not conclusive. Therefore, before exploring what to do after it has happened, stratify the expected benefits of patients, thereby improving the accuracy of predicting individual response to treatment, and identify especially the patients who are at risk for HPD, and select them for an alternative approach is more important in current status. Exciting thing is there have been many promising markers for the prediction of HPD, such as LDH levels of peripheral blood, liquid biopsy, and radiomics, I believe in the future there will be more studies appear jointly to open the veil of HPD under immunotherapy.
Supplementary
The article’s supplementary files as
Acknowledgments
The authors appreciate the academic support from AME Cancer Immunotherapy Collaborative Group.
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://dx.doi.org/10.21037/tlcr-21-575
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tlcr-21-575). The authors have no conflicts of interest to declare.
References
- 1.Frelaut M, Le Tourneau C, Borcoman E. Hyperprogression under Immunotherapy. Int J Mol Sci 2019;20:2674. 10.3390/ijms20112674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denis M, Duruisseaux M, Brevet M, et al. How Can Immune Checkpoint Inhibitors Cause Hyperprogression in Solid Tumors? Front Immunol 2020;11:492. 10.3389/fimmu.2020.00492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim JY, Lee KH, Kang J, et al. Hyperprogressive Disease during Anti-PD-1 (PDCD1) / PD-L1 (CD274) Therapy: A Systematic Review and Meta-Analysis. Cancers (Basel) 2019;11:1699. 10.3390/cancers11111699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Champiat S, Dercle L, Ammari S, et al. Hyperprogressive Disease Is a New Pattern of Progression in Cancer Patients Treated by Anti-PD-1/PD-L1. Clin Cancer Res 2017;23:1920-8. 10.1158/1078-0432.CCR-16-1741 [DOI] [PubMed] [Google Scholar]
- 5.Sabio E, Chan TA. The good, the bad, and the ugly: hyperprogression in cancer patients following immune checkpoint therapy. Genome Med 2019;11:43. 10.1186/s13073-019-0661-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lahmar J, Mezquita L, Koscielny S, et al. Immune checkpoint inhibitors (IC) induce paradoxical progression in a subset of non-small cell lung cancer (NSCLC). Ann Oncol 2016;27:vi416–vi454. 10.1093/annonc/mdw383.22 [DOI] [Google Scholar]
- 7.Chubachi S, Yasuda H, Irie H, et al. A Case of Non-Small Cell Lung Cancer with Possible "Disease Flare" on Nivolumab Treatment. Case Rep Oncol Med 2016;2016:1075641. 10.1155/2016/1075641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernal Vaca L, Mendoza SD, Vergel JC, et al. Hyperprogression in Pediatric Melanoma Metastatic to the Breast Treated with a Checkpoint Inhibitor. Cureus 2019;11:e3859. 10.7759/cureus.3859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan AS, Ng VY, Snider J, et al. Hyperprogression of Liver Metastasis With Neoadjuvant Immunotherapy for Soft Tissue Sarcoma. Cureus 2020;12:e8575. 10.7759/cureus.8575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fricke J, Mambetsariev I, Pharaon R, et al. Hyperprogression on immunotherapy with complete response to chemotherapy in a NSCLC patient with high PD-L1 and STK11: A case report. Medicine (Baltimore) 2020;99:e22323. 10.1097/MD.0000000000022323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang W, Wu M, Liu M, et al. Hyperprogression to camrelizumab in a patient with esophageal squamous cell carcinoma harboring EGFR kinase domain duplication. J Immunother Cancer 2020;8:e000793. 10.1136/jitc-2020-000793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 2017;18:e143-52. 10.1016/S1470-2045(17)30074-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009;15:7412-20. 10.1158/1078-0432.CCR-09-1624 [DOI] [PubMed] [Google Scholar]
- 14.Zang H, Peng J, Zheng H, et al. Hyperprogression After Immune-Checkpoint Inhibitor Treatment: Characteristics and Hypotheses. Front Oncol 2020;10:515. 10.3389/fonc.2020.00515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 16.Nishino M, Giobbie-Hurder A, Gargano M, et al. Developing a common language for tumor response to immunotherapy: immune-related response criteria using unidimensional measurements. Clin Cancer Res 2013;19:3936-43. 10.1158/1078-0432.CCR-13-0895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hodi FS, Ballinger M, Lyons B, et al. Immune-Modified Response Evaluation Criteria In Solid Tumors (imRECIST): Refining Guidelines to Assess the Clinical Benefit of Cancer Immunotherapy. J Clin Oncol 2018;36:850-8. 10.1200/JCO.2017.75.1644 [DOI] [PubMed] [Google Scholar]
- 18.Kanjanapan Y, Day D, Wang L, et al. Hyperprogressive disease in early-phase immunotherapy trials: Clinical predictors and association with immune-related toxicities. Cancer 2019;125:1341-9. 10.1002/cncr.31999 [DOI] [PubMed] [Google Scholar]
- 19.Saâda-Bouzid E, Defaucheux C, Karabajakian A, et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol 2017;28:1605-11. 10.1093/annonc/mdx178 [DOI] [PubMed] [Google Scholar]
- 20.Ferrara R, Mezquita L, Texier M, et al. Hyperprogressive Disease in Patients With Advanced Non-Small Cell Lung Cancer Treated With PD-1/PD-L1 Inhibitors or With Single-Agent Chemotherapy. JAMA Oncol 2018;4:1543-52. 10.1001/jamaoncol.2018.3676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurman JS, Murgu SD. Hyperprogressive disease in patients with non-small cell lung cancer on immunotherapy. J Thorac Dis 2018;10:1124-8. 10.21037/jtd.2018.01.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kato S, Kurzrock R. Genomics of Immunotherapy-Associated Hyperprogressors-Response. Clin Cancer Res 2017;23:6376. 10.1158/1078-0432.CCR-17-1990 [DOI] [PubMed] [Google Scholar]
- 23.Lo Russo G, Moro M, Sommariva M, et al. Antibody-Fc/FcR Interaction on Macrophages as a Mechanism for Hyperprogressive Disease in Non-small Cell Lung Cancer Subsequent to PD-1/PD-L1 Blockade. Clin Cancer Res 2019;25:989-99. 10.1158/1078-0432.CCR-18-1390 [DOI] [PubMed] [Google Scholar]
- 24.Gandara DR, Reck M, Morris S, et al. Fast progression in patients treated with a checkpoint inhibitor (cpi) vs chemotherapy in OAK, a phase III trial of atezolizumab (atezo) vs docetaxel (doc) in 2L+ NSCLC. Ann Oncol 2018;29:x39-x43. 10.1093/annonc/mdy511 [DOI] [Google Scholar]
- 25.Liu C, Piao J, Shang Z. Hyperprogressive disease after radiotherapy combined with anti-PD-1 therapy in renal cell carcinoma: a case report and review of the literature. BMC Urol 2021;21:42. 10.1186/s12894-021-00813-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Popat S. Hyperprogression with immunotherapy: Is it real? Cancer 2019;125:1218-20. 10.1002/cncr.31997 [DOI] [PubMed] [Google Scholar]
- 27.Kamada T, Togashi Y, Tay C, et al. PD-1+ regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc Natl Acad Sci U S A 2019;116:9999-10008. 10.1073/pnas.1822001116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim CG, Kim KH, Pyo KH, et al. Hyperprogressive disease during PD-1/PD-L1 blockade in patients with non-small-cell lung cancer. Ann Oncol 2019;30:1104-13. 10.1093/annonc/mdz123 [DOI] [PubMed] [Google Scholar]
- 29.Kocikowski M, Dziubek K, Parys M. Hyperprogression Under Immune Checkpoint-Based Immunotherapy-Current Understanding, The Role of PD-1/PD-L1 Tumour-Intrinsic Signalling, Future Directions and a Potential Large Animal Model. Cancers (Basel) 2020;12:804. 10.3390/cancers12040804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kato S, Goodman A, Walavalkar V, et al. Hyperprogressors after Immunotherapy: Analysis of Genomic Alterations Associated with Accelerated Growth Rate. Clin Cancer Res 2017;23:4242-50. 10.1158/1078-0432.CCR-16-3133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Refae S, Gal J, Brest P, et al. Hyperprogression under Immune Checkpoint Inhibitor: a potential role for germinal immunogenetics. Sci Rep 2020;10:3565. 10.1038/s41598-020-60437-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tawbi AHH, Burgess MA, Crowley J, et al. Safety and efficacy of PD-1 blockade using pembrolizumab in patients with advanced soft tissue (STS) and bone sarcomas (BS): Results of SARC028—A multicenter phase II study. J Clin Oncol 2016;34:abstr 11006.
- 33.Matos I, Martin-Liberal J, Hierro C, et al. Incidence and clinical implications of a new definition of hyperprogression (HPD) with immune checkpoint inhibitors (ICIs) in patients treated in phase 1 (Ph1) trials. J Clin Oncol 2018;36:abstr 3032.
- 34.Kiriu T, Yamamoto M, Nagano T, et al. The time-series behavior of neutrophil-to-lymphocyte ratio is useful as a predictive marker in non-small cell lung cancer. PLoS One 2018;13:e0193018. 10.1371/journal.pone.0193018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu J, Wu Q, Wu S, et al. Investigation on potential biomarkers of hyperprogressive disease (HPD) triggered by immune checkpoint inhibitors (ICIs). Clin Transl Oncol 2021;23:1782-93. 10.1007/s12094-021-02579-9 [DOI] [PubMed] [Google Scholar]
- 36.Araujo DV, Wang A, Torti D, et al. Applications of Circulating Tumor DNA in a Cohort of Phase I Solid Tumor Patients Treated With Immunotherapy. JNCI Cancer Spectr 2021;5:pkaa122. [DOI] [PMC free article] [PubMed]
- 37.Wang Z, Duan J, Cai S, et al. Assessment of Blood Tumor Mutational Burden as a Potential Biomarker for Immunotherapy in Patients With Non-Small Cell Lung Cancer With Use of a Next-Generation Sequencing Cancer Gene Panel. JAMA Oncol 2019;5:696-702. 10.1001/jamaoncol.2018.7098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pessoa LS, Heringer M, Ferrer VP. ctDNA as a cancer biomarker: A broad overview. Crit Rev Oncol Hematol 2020;155:103109. 10.1016/j.critrevonc.2020.103109 [DOI] [PubMed] [Google Scholar]
- 39.Lee JH, Long GV, Menzies AM, et al. Association Between Circulating Tumor DNA and Pseudoprogression in Patients With Metastatic Melanoma Treated With Anti-Programmed Cell Death 1 Antibodies. JAMA Oncol 2018;4:717-21. 10.1001/jamaoncol.2017.5332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weiss GJ, Beck J, Braun DP, et al. Tumor Cell-Free DNA Copy Number Instability Predicts Therapeutic Response to Immunotherapy. Clin Cancer Res 2017;23:5074-81. 10.1158/1078-0432.CCR-17-0231 [DOI] [PubMed] [Google Scholar]
- 41.Ji Z, Cui Y, Peng Z, et al. Use of Radiomics to Predict Response to Immunotherapy of Malignant Tumors of the Digestive System. Med Sci Monit 2020;26:e924671. 10.12659/MSM.924671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lambin P, Leijenaar RTH, Deist TM, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 2017;14:749-62. 10.1038/nrclinonc.2017.141 [DOI] [PubMed] [Google Scholar]
- 43.Sun R, Limkin EJ, Vakalopoulou M, et al. A radiomics approach to assess tumour-infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy: an imaging biomarker, retrospective multicohort study. Lancet Oncol 2018;19:1180-91. 10.1016/S1470-2045(18)30413-3 [DOI] [PubMed] [Google Scholar]
- 44.Gandara DR, von Pawel J, Mazieres J, et al. Atezolizumab Treatment Beyond Progression in Advanced NSCLC: Results From the Randomized, Phase III OAK Study. J Thorac Oncol 2018;13:1906-18. 10.1016/j.jtho.2018.08.2027 [DOI] [PubMed] [Google Scholar]
- 45.Tunali I, Gray JE, Qi J, et al. Novel clinical and radiomic predictors of rapid disease progression phenotypes among lung cancer patients treated with immunotherapy: An early report. Lung Cancer 2019;129:75-9. 10.1016/j.lungcan.2019.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ji Z, Peng Z, Gong J, et al. Hyperprogression after immunotherapy in patients with malignant tumors of digestive system. BMC Cancer 2019;19:705. 10.1186/s12885-019-5921-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matos I, Martin-Liberal J, García-Ruiz A, et al. Capturing Hyperprogressive Disease with Immune-Checkpoint Inhibitors Using RECIST 1.1 Criteria. Clin Cancer Res 2020;26:1846-55. 10.1158/1078-0432.CCR-19-2226 [DOI] [PubMed] [Google Scholar]
- 48.Ohue Y, Nishikawa H., Regulatory T. (Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer Sci 2019;110:2080-9. 10.1111/cas.14069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Y, Carlsson R, Comabella M, et al. FoxA1 directs the lineage and immunosuppressive properties of a novel regulatory T cell population in EAE and MS. Nat Med 2014;20:272-82. 10.1038/nm.3485 [DOI] [PubMed] [Google Scholar]
- 50.Chevalier MF, Schneider AK, Cesson V, et al. Conventional and PD-L1-expressing Regulatory T Cells are Enriched During BCG Therapy and may Limit its Efficacy. Eur Urol 2018;74:540-4. 10.1016/j.eururo.2018.06.045 [DOI] [PubMed] [Google Scholar]
- 51.Xiong D, Wang Y, Singavi AK, et al. Immunogenomic Landscape Contributes to Hyperprogressive Disease after Anti-PD-1 Immunotherapy for Cancer. iScience 2018;9:258-77. 10.1016/j.isci.2018.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koukourakis MI, Giatromanolaki A, Sivridis E, et al. Lactate dehydrogenase-5 (LDH-5) overexpression in non-small-cell lung cancer tissues is linked to tumour hypoxia, angiogenic factor production and poor prognosis. Br J Cancer 2003;89:877-85. 10.1038/sj.bjc.6601205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ye F, Tang C, Shi W, et al. A MDM2-dependent positive-feedback loop is involved in inhibition of miR-375 and miR-106b induced by Helicobacter pylori lipopolysaccharide. Int J Cancer 2015;136:2120-31. 10.1002/ijc.29268 [DOI] [PubMed] [Google Scholar]
- 54.Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines. J Biol Chem 2007;282:20059-63. 10.1074/jbc.R700016200 [DOI] [PubMed] [Google Scholar]
- 55.Zhou S, Gu L, He J, et al. MDM2 regulates vascular endothelial growth factor mRNA stabilization in hypoxia. Mol Cell Biol 2011;31:4928-37. 10.1128/MCB.06085-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Akbay EA, Koyama S, Carretero J, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov 2013;3:1355-63. 10.1158/2159-8290.CD-13-0310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Du S, McCall N, Park K, et al. Blockade of Tumor-Expressed PD-1 promotes lung cancer growth. Oncoimmunology 2018;7:e1408747. 10.1080/2162402X.2017.1408747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Knorr DA, Dahan R, Ravetch JV. Toxicity of an Fc-engineered anti-CD40 antibody is abrogated by intratumoral injection and results in durable antitumor immunity. Proc Natl Acad Sci U S A 2018;115:11048-53. 10.1073/pnas.1810566115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dahan R, Sega E, Engelhardt J, et al. FcγRs Modulate the Anti-tumor Activity of Antibodies Targeting the PD-1/PD-L1 Axis. Cancer Cell 2015;28:285-95. 10.1016/j.ccell.2015.08.004 [DOI] [PubMed] [Google Scholar]
- 60.Zhang T, Song X, Xu L, et al. The binding of an anti-PD-1 antibody to FcγRI has a profound impact on its biological functions. Cancer Immunol Immunother 2018;67:1079-90. 10.1007/s00262-018-2160-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Knorr DA, Ravetch JV. Immunotherapy and Hyperprogression: Unwanted Outcomes, Unclear Mechanism. Clin Cancer Res 2019;25:904-6. 10.1158/1078-0432.CCR-18-3144 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as