Abstract
Anti-Ma2 encephalitis is a rare neurological disorder with a predominant involvement of brainstem, limbic and diencephalic structures. Although an unspecific encephalopathy is the usual form of presentation, acute-onset neurologic symptoms and other atypical manifestations have been described and account for the challenging diagnosis of this entity. Despite being usually detected as a paraneoplastic syndrome in patients with early-stage tumors or without a previous history of malignancy, a growing concern has arisen from several cases reported in metastatic patients under treatment with immune checkpoint inhibitors. We report what to our knowledge is the first known case of anti-Ma2 encephalitis associated to pembrolizumab and presenting as an acute-onset focal neurological syndrome, consisting on acute global aphasia, right upper limb paresia, hypoacusia, sleep disorder, decreased conscious level and a motor focal status that was refractory to anticonvulsant therapy. A brain MRI scan showed a focal alteration of the cortical-subcortical signal on the left parietal lobe. CSF study found a significant hyperproteinorrhachia and electroencephalography showed lateralized periodic discharges (LPDs), suggestive of a diffuse encephalopathy. A positive result for anti-Ma2 antibodies was obtained both in blood and CSF samples through indirect immune-fluorescence (IFI) and later confirmed by western-blot technique. Our patient obtained a mild response to steroid therapy and a significant improvement after the administration of intravenous immunoglobulins. The hypothesis that checkpoint inhibitors may trigger the expression of previously subclinical paraneoplastic events, through the strengthening of cytotoxic T cells-mediated immune response, is supported by our finding of preexisting anti-Ma2 antibodies in preserved blood samples obtained before the initiation of pembrolizumab in our patient. Further research is needed to reveal if the detection of onconeural antibodies prior to a treatment with checkpoint inhibitors may be used as a predictive biomarker of neurologic immune-related high-grade toxicity.
Keywords: Anti-Ma2, paraneoplastic, encephalitis, checkpoint inhibitors, case report
Introduction
Being the most frequent histological subtype of lung malignant tumors, non-small cell lung cancer (NSCLC) remains a major cause of cancer-related death around the world. The spreading use of immune checkpoint inhibitors (ICIs) and successful therapies against several molecular targets have recently transformed the therapeutic management of metastatic NSCLC.
In the study KEYNOTE-024, first-line pembrolizumab significantly improved progression-free survival (PFS) and overall survival (OS) compared with platinum-based chemotherapy in patients with advanced NSCLC without sensitizing EGFR mutations, ALK rearrangements and PDL1 tumor proportion score (TPS) ≥50% (1). With 5 years follow-up, pembrolizumab continued to provide durable OS benefit compared with chemotherapy, confirming its role in monotherapy as a standard first-line treatment for PDL1-positive advanced NSCLC (2).
Through the blockade of the interaction between programmed cell death protein-1 (PD-1) and its ligand 1 (PD-L1), pembrolizumab is able to suppress the tumoral inhibition of cytotoxic T-cell mediated response, causing a systemic immunogenic activity that may lead to a wide spectrum of immune-related side effects (3,4). Although less frequent than gastrointestinal, hepatic or lung toxicity, different forms of autoimmune encephalitis have been associated with ICIs (5).
In some cases, this neurological toxicity has been linked to an increased incidence of paraneoplastic syndromes associated with onconeural antibodies (Abs) (6). Since their clinical manifestations are usually unspecific, sometimes misleading and appear in a context where brain metastasis, meningeal carcinomatosis, infectious diseases, neuroischemic pathology, psychiatric disorders and multifactorial clinical deterioration are much more frequent causes of neurological symptoms, it is of vital importance to consider paraneoplastic syndromes linked to immune-mediated toxicity in the differential diagnosis for an early recognition and management.
Up to date, the physiopathology of ICIs side effects remains to be fully understood, and no scientific data are available about the pathogenic role of preexisting onconeural antibodies and their possible role as predictive biomarkers of neurologic toxicity.
We present the following article in accordance with the provided CARE reporting checklist (available at https://dx.doi.org/10.21037/tlcr-21-222).
Case presentation
Our patient is a 72-year-old non-smoker woman with a history of lung adenocarcinoma with a PD-L1 TPS >90%, stage IV (T4N3M1c) at diagnosis, with distant lymphatic dissemination, bilateral lung and adrenal metastasis. No targetable alterations were found in the OncomineTM next generation study (NGS) performed.
First-line treatment with pembrolizumab was initiated (200 mg each 3 weeks) and stopped after two cycles due to grade 3 immune-related hepatitis. After initially requiring prednisone at 1.5 mg/kg/day (90 mg) for the normalization of liver enzymes levels, steroid therapy was progressively tapered and the patient reached a stable daily dose of prednisone 5 mg. A body-CT scan revealed no brain metastases and stable disease 2 months after pembrolizumab discontinuation, so the patient received no further treatment.
Three months after the second cycle of pembrolizumab, the patient was admitted at the emergency department due to mild fever without apparent origin, so fluid therapy and empiric antibiotics were initiated. First night after admission, she showed an acute-onset global aphasia, right-sided hearing loss and right upper limb complete paresia (1/5), followed by a decreased conscious level and a focal motor status epilepticus that was refractory to various anticonvulsant drugs.
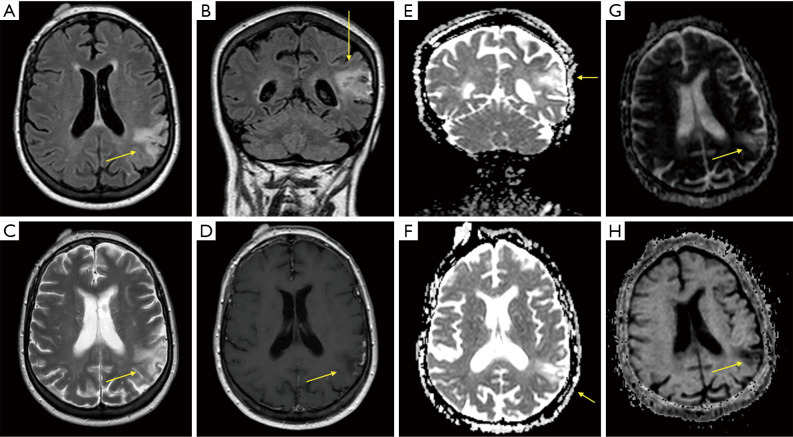
With an initial suspected diagnosis of an epileptic status secondary to an acute ischemic stroke, a multimodal cranial CT scan was performed, without detecting any contrast filling defects or any brain area with a reduced blood supply, so an acute ischemic event was discarded. An alteration of the cortical-subcortical signal in the left parietal lobe was observed in an MRI scan, with no signs of brain metastasis, meningeal carcinomatosis or any other structural findings (Figure 1).
Figure 1.
Images from brain MRI at diagnosis. Arrows point at a triangular focal alteration of the cortical-subcortical signal that can be observed on the left parietal lobe, associated to a slimming of the cortex and a mild ampliation of the adjacent subarachnoid space, hyperintense in FLAIR (A,B) and T2 (C) sequences, and hypointense in T1 sequence (D). The diffusion-weighted magnetic resonance imaging (DWI) technique shows a cortical restricted diffusion in this same area (E,F). Correlation with apparent diffusion coefficient (ADC) and exponential apparent diffusion coefficient (eADC) maps is shown in (G) and (H) respectively.
A cerebrospinal fluid (CSF) study revealed a significant hyperproteinorrhachia (147 mg/dL), without hypo-glycorrhachia (86 mg/dL) or cell count alterations (3 leukocytes/mm3, 6 RBC/mm3). Negative results were obtained from cytological and microbiological studies. An electroencephalography was performed, showing 1.5 Hz-frequency lateralized periodic discharges (LPDs) which were suggestive of a diffuse encephalopathy. Blood viral serologies were negative.
Although acute-onset symptoms are not suggestive of immune-related encephalitis, considering the lack of clinical improvement, empirical treatment with high-dose methylprednisolone (1 g/day, 5 days) was initiated.
A determination of onconeural Abs was performed through indirect immune-fluorescence (IFI), with a positive result for anti-Ma2 Abs both in blood and CSF samples. This was later confirmed by western-blot technique, with a serum quantitative expression of anti-Ma2 Abs of 95 UA/100 (Figure 2).
Figure 2.
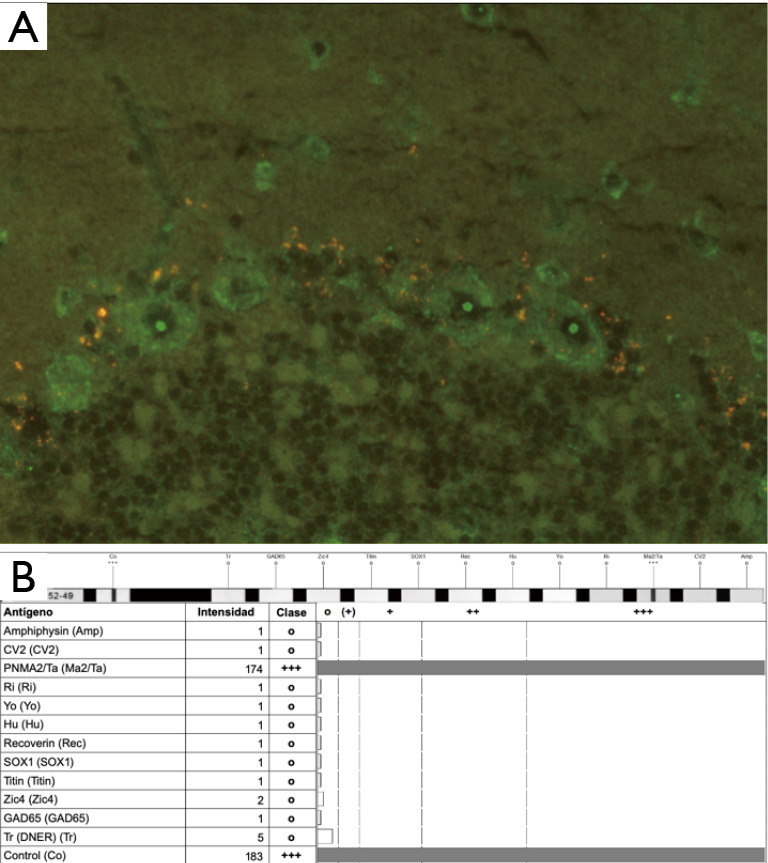
Detection of anti-Ma2 antibodies. (A) Indirect immunofluorescence demonstrating presence of anti-Ma2 Abs from the peripheral blood sample of our patient. Anti-Ma2 Abs are shown in cerebellar dentate nucleus Purkinje cells, with a high uptake in the neuronal nucleolus. (Magnification times: 40×/0.75). (B) Western blot analysis revealing a high intensity band corresponding to the anti-Ma2 antibody, with a quantitative value of expression of 95 UA/100.
Clinical response to steroid therapy was favorable, with a mild improvement of the aphasia and the upper limb paresia. The patient slowly recovered the ability to speak, presenting frequent paraphasia and significant fatigability, with initial appropriate answers but quick worsening of neurological performance after a simple conversation. Despite the moderate clinical improvement, strong drowsiness, right hypoacusia and altered sleep behavior persisted for several days.
Following the discovery of anti-Ma2 Abs, intravenous immunoglobulins (iv Igs) were administered for 5 days, with a rapid neurological improvement that allowed a progressive decrease of steroids dosage, initially to methylprednisolone 50 mg/day and then prednisone at descending doses. Nearly complete recovery of limb paresia (4/5) and a significant improvement of aphasia (from total loss of verbal expression to a mild-moderate language disability) were achieved in two weeks, with no adverse events, allowing the patient to be discharged with prednisone 10 mg/day and continue out-patient follow-up, with adequate adherence and tolerability to oral steroid treatment.
A second CSF study performed two days after the last dose of iv Igs showed a normalization of protein levels (53.7 mg/dL). A control determination of anti-Ma2 Abs gave a negative result by IFI but was weakly positive when performed with western-blot technique, with a quantitative value of 74 UA/100, confirming the persistence of the Abs at a lower titer.
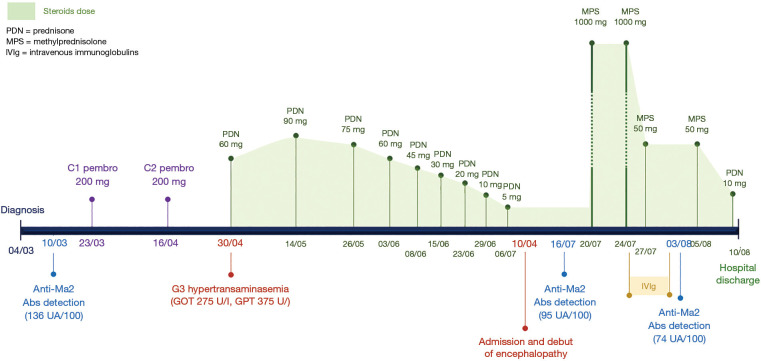
A preserved serum sample obtained five months before, just after lung cancer diagnosis and before administering the first cycle of pembrolizumab, was retrospectively used to perform a determination of anti-Ma2 Abs. Interestingly, an intensely positive result was obtained both by IFI and Western-blot, with much higher quantitative titers than those obtained in the subsequent samples (136 UA/100). A graphical timeline of the case evolution is presented in Figure 3.
Figure 3.
Chronological timeline. The figure shows the changes in steroid therapy dosage and the most important events of the case. PDN, prednisone; MPS, methylprednisolone; IVIg, intravenous immunoglobulins; Abs, antibodies.
A control MRI scan was performed 6 weeks after hospital discharge, showing no radiological changes despite the good clinical evolution, with persistence of similar cortical-subcortical alterations in the left parietal lobe.
A full-body CT scan showed disease progression one month after hospital discharge, with an increase of lung and adrenal lesions and new bone and lymph node metastases, so second line treatment with platinum-based chemotherapy was initiated.
Steroids daily dose was reduced to prednisone 5 mg two months after discharge and two weeks later to 2.5 mg, which the patient maintains at the moment of writing this article, three months after the initiation of chemotherapy.
The patient has achieved almost complete recovery from neurological symptoms except for the persistence of mild paraphasia.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report.
Discussion
PD-1/PD-L1 inhibitors have shown remarkable efficacy in the treatment of advanced NSCLC but often cause a variety of immune-related side effects (irAEs) that have become more relevant with the increasing use of ICIs in daily practice.
A recent meta-analysis including 10 trials with PD-1 inhibitors nivolumab and pembrolizumab (3,734 patients) and 6 trials with PD-L1 inhibitors atezolizumab, durvalumab and avelumab (2,474 patients) has shown an overall incidence of irAEs of 22% (95% CI, 17–28) for all grades and 4% (95% CI, 2–6) for high-grade events (7). Most frequent organ-specific irAEs were observed, in decreasing order, in the endocrine system, skin, pulmonary tract and gastrointestinal tract, whereas neurological irAEs were extremely rare, including two cases of myasthenia gravis and just one case of cerebral paraneoplastic vasculitis/encephalitis (<0.001%).
Paraneoplastic neurological syndromes (PNS) are rare disorders in which the ectopic expression of neural antigens by tumor cells triggers the production of onconeural Abs. Although onconeural Abs have not been demonstrated to be pathogenic, their expression is often associated to a neurological impairment due to an autoimmune or inflammatory response (8). Several Abs have been identified, being anti-Hu and anti-Yo the most frequent. Except for anti-Hu Abs, that are typically related to peripheral sensory neuropathy, most of them are associated with central nervous system disorders (9).
Anti-Ma2 are rare onconeural Abs that target paraneoplastic antigen Ma2, an intracellular protein encoded by the gene PNMA2, usually in patients with testicular cancer (40%), lung cancer (22%) or gastro-intestinal tumors (15%) (10). Ma2 is expressed in all neurons of the human brain, particularly in the brainstem, amygdala, hippocampus, thalamus and hypothalamus. In these areas, pathology exams of patients with anti-Ma2 Abs demonstrate neuronal loss, gliosis and inflammatory infiltrates (11).
Although limbic and temporal encephalitis account for the most prevalent clinical form of presentation of anti-Ma2 encephalopathy, atypical manifestations such as motor-neuron syndromes, autonomic dysfunction and cerebellum impairment have been described. The acute appearance of focal symptoms related to brain cortex dysfunction, such as aphasia, paresia and vision or hearing loss, may give rise to a misleading clinical onset suggestive of an acute ischemic stroke, without any sign of brain hypoperfusion (12).
Neurological fatigability, with myotonic patterns that resemble myasthenia-like syndromes, as well as mild non-infectious fever and narcoleptic features secondary to REM sleep disorders have also been observed in previously reported cases of anti-Ma2 encephalitis (13).
Additionally, as it happened in our case, nearly 15% of the patients present at debut epileptic crisis that are typically refractory to anticonvulsant drugs (14).
In the classic form of anti-Ma2 encephalitis, since symptoms precede tumor detection in around 70% of patients, the finding of circulating Abs may be the key to the diagnosis of a limited-stage underlying malignancy.
However, there is a growing concern about several reported cases of metastatic patients without previous neurologic symptoms that developed an anti-Ma2 encephalitis after initiating a treatment with ICIs (15). To our knowledge, 16 previous cases of anti-Ma2 encephalitis have been reported so far in patients with advanced-stage malignancies during a treatment with immune checkpoint inhibitors, with just 3 previous cases related to pembrolizumab, all of them in patients with advanced NSCLC and fulfilling the criteria for definite PNS (16). Most of them appeared between 4 and 8 weeks after the initiation of immunotherapy, although there were 3 cases where the first symptoms occurred beyond five months from the start of ICIs. Their clinical characteristics are synthetized in Table 1.
Table 1. Reported cases of anti-Ma2 encephalopathy associated with immune checkpoint inhibitors.
| Gender, age | Oncological disease | Type of ICI | Clinical presentation |
|---|---|---|---|
| F, 72 | Stage IV NSCLC | Pembrolizumab | Acute-onset global aphasia, right upper limb paresia, hypoacusia, sleep disorder, decreased conscious level and refractory focal status |
| M, 79 (17) | Stage IV NSCLC | Pembrolizumab | Altered behavior, impulsivity, disinhibition, confusion, and decreased consciousness |
| M, 71 (17) | Stage IV pleural mesothelioma | Nivolumab + ipilimumab | Narcolepsy-cataplexy, hyperphagia and weight gain |
| F, 57 (17) | Stage IV NSCLC | Nivolumab | Memory deficits, new-onset epilepsy and psychomotor retardation |
| M, 47 (17) | Stage IV NSCLC | Pembrolizumab | Diplopia |
| M, 55 (17) | Stage IV renal cancer | Nivolumab | Abrupt onset of right ear hearing loss, ataxia, vertigo and memory deficits |
| M, 69 (17) | Stage IV NSCLC | Nivolumab | Confusion and focal seizures |
| M, 56 (18) | Stage IV renal cancer | Nivolumab | Altered behavior, memory deficits and worsening of previous epilepsy |
| M, 63 (19) | Stage IV renal cancer | Nivolumab | Chorea-like dyskinesia |
| F, 26 (20) | Stage IV Hodgkin lymphoma | Nivolumab | Seizures and limbic encephalopathy |
| M, 78 (21) | Unresectable malignant pleural mesothelioma | Nivolumab | Fever and somnolence syndrome |
| Non-specified (22) | Stage IV NSCLC | Anti-PD1 (non-specified) | Non-specified |
| Non-specified (22) | Stage IV NSCLC | Anti-PD1 (non-specified) | Non-specified |
| Non-specified (22) | Stage IV NSCLC | Anti-PD1 (non-specified) | Non-specified |
| Non-specified (22) | Stage IV NSCLC | Anti-PD1 (non-specified) | Non-specified |
| M, 69 (23) | Stage IV NSCLC | Pembrolizumab | Rhombencephalitis (subacute cerebellar ataxia with left gaze oculomotor palsy and vertical nystagmus) |
| M, 70 (24) | Unresectable malignant pleural mesothelioma | Nivolumab + ipilimumab | Excessive daytime sleepiness and depressive disorder followed by narcolepsy-cataplexy |
Numbers in brackets indicate the references in bibliography. First cell regards our clinical case; the following cells regard the remaining ICI-induced anti-Ma2 encephalitis cases reported in literature. F, female; M, male; NSCLC, non-small cell lung cancer.
Despite the low number of reported cases, according to a study from the French National Reference Center for Paraneoplastic Neurological Syndromes (17), they account for a 112% increase of anti-Ma2 Abs detection since the spread of ICIs in clinical practice, in contrast with a much lower increase (30–50%) in the detection of other onconeural Abs (anti-Hu, anti-Yo, anti-GAD, anti-NMDA, anti-LGI1, anti-CASPR2, anti-GABABr, anti-AMPAr). Although anti-Ma2 Abs were sometimes found incidentally in asymptomatic patients, this correlation may suggest a particularly strong association between ICIs and anti-Ma2 encephalopathy.
As it happened in our patient, partial response or stable disease were observed in 15 out of 16 previously reported cases, and only 1 of them (case 4) was in a situation of progressive disease at the moment of encephalitis diagnosis.
Although the pathogenesis of neurologic irAEs due to ICIs remains to be elucidated, it has been shown that post-ICI encephalitis is characterized by prominent CD8 lymphocytic perivascular infiltration (25). Since ICIs act by blocking the signaling of molecules that inhibit T-cell activation, such as CTLA-4 and PD-1, the antitumor immune response might cross-react with CNS auto-antigens and activate CD8 T-cells, which have been found to play a major effector role in neuronal death. This has been shown to trigger the clinical manifestations of an encephalitis in preclinical models (26).
Interestingly, anti-Ma2 Abs have been retrospectively detected in blood samples obtained before the initiation of immunotherapy, both in our patient and in at least two others amongst the previously reported cases (17,21). These findings demonstrate that onconeural antibodies are present before initiation of ICI-therapy in some, if not all, patients who develop ICI-associated paraneoplastic neurologic syndromes.
It has been hypothesized that the Ma2 protein and other antigens targeted by onconeural Abs could be aberrantly expressed by NSCLC cells, leading to the early presence of Abs whose titers might correlate with the degree of neoantigens expression, and therefore the burden of malignant cells. In our patient, this may explain why the highest titers were obtained before the initiation of pembrolizumab, although they were per se not enough to elicit a clinical PNS in a situation of a compromised anti-tumor response.
The loss of self-tolerance induced by the initiation of an ICI may be the unleashing event that leads to a massive activation of CD8 T-cells and the emergence of neurologic symptoms. Therefore, the pathogenic role of ICIs may be facilitating the symptomatic expression of a previously subclinical paraneoplastic phenomenon.
For several years, the possible association between the development of irAEs and the disease response to ICIs has been a matter of debate. A recent meta-analysis of a total of 30 studies including 4,324 patients treated with ICIs showed that patients who developed irAEs presented a reduced risk of death (HR 0.49, 95% CI, 0.38–0.62, P<0.001) and a reduced risk of disease progression (HR 0.51, 95% CI, 0.42–0.64, P<0.001), concluding that irAEs predict survival and response possibly due to a strongest activation of the T-cells-mediated response (27). The benefit from ICIs associated with the occurrence of irAEs was similar for NSCLC and melanoma, which accounted for the 90% of the included cases. This is consistent with the concept that irAEs are predictive of efficacy independently of histology (28).
Although the correlation with high grades (G3–G4) toxicity was established in 4 of the studies, further evidence is needed to confirm if this OS and PFS benefit is also present in patients who require discontinuation of immunotherapy due to toxicity, as it happened in this case.
It has been clearly established that a high tumor mutational burden (TMB), which is inherently associated with a high expression of neoantigens and aberrant proteins, is a predictive biomarker for response to ICIs in NSCLC, among other malignancies (29). This may entail that precisely those patients who benefit from immunotherapy could be at a higher risk of developing immune-related toxicities, although this hypothesis has not been validated by prospective studies so far.
In the opinion of the authors, the performance of prospective studies would be of particular interest to evaluate the correlation between pre-existing onconeural Abs and the development of clinical encephalitis after the initiation of immunotherapy, as well as the monitorization of Abs titers both after the discontinuation of ICIs and the administration of steroids or intravenous immunoglobulins. Although the low incidence of paraneoplastic neurologic disorders is a clear limitation, this line of research might be useful to clarify if the determination of onconeural Abs prior to the initiation of a treatment with ICIs is helpful to predict a higher risk of immune-related encephalitis, what might facilitate an earlier detection and better therapeutic management.
In previous retrospective series of anti-Ma2 encephalitis (usually as a paraneoplastic syndrome preceding cancer diagnosis) MRI study showed alterations in around 78% of patients, predominantly a T2 and FLAIR hyperintense signal on diencephalon, mesencephalon and temporal lobes. FLAIR hyperintensity involving the mesial temporal lobes was found in 4 cases, including 1 with coexisting hyperintensity of the periventricular regions of the third ventricle and hypothalamus. Contrast enhancement was not detected in any patient. In addition, CSF was altered in around 52% of cases, being a mild or moderate hyperproteinorrhachia the most frequent finding (30). Our patient results were in accordance with this pattern.
Current recommendations for ICIs-related neurotoxicity include immunotherapy discontinuation for grade 3-4 events and administration of high-dose corticosteroids, considering intravenous immunoglobulins, plasma-exchange therapy or other immunosuppressant drugs in refractory cases (31,32).
Nevertheless, since these recommendations are extrapolated from general guidelines regarding autoimmune encephalopathies, with just a limited number of ICIs-induced anti-Ma2 encephalitis cases reported up to date, it is difficult to derive specific evidence-based recommendations until further structured and standardized research regarding neurologic toxicity of ICI-therapy has been developed.
Given the spreading use of immunotherapy in patients with increasing survival rates, the diagnostic challenge of neurological symptoms in advanced-stage oncological diseases, as well as the high impact that neurologic deterioration may have on quality of life, it is mandatory to intensify scientific research regarding a better clinical definition and therapeutic management of paraneoplastic and immune-related neurological syndromes.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report.
Footnotes
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dx.doi.org/10.21037/tlcr-21-222
Peer Review File: Available at https://dx.doi.org/10.21037/tlcr-21-222
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tlcr-21-222). PG declares personal financial interests as advisor for Abbvie, AstraZeneca, Blueprint Medicines, Boehringer Ingelheim, Bristol, Gilead, Guardant Health, Janssen, Lilly, MSD, Novartis, Pfizer, Roche, Rovi, Sysmex and Takeda. AS reports personal fees from Merck, Bristol and Roche. The other authors have no conflicts of interest to declare.
References
- 1.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 2.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J Clin Oncol 2019;37:537-46. 10.1200/JCO.18.00149 [DOI] [PubMed] [Google Scholar]
- 3.Tartarone A, Roviello G, Lerose R, et al. Anti-PD-1 versus anti-PD-L1 therapy in patients with pretreated advanced non-small-cell lung cancer: a meta-analysis. Future Oncol 2019;15:2423-33. 10.2217/fon-2018-0868 [DOI] [PubMed] [Google Scholar]
- 4.Mohsenzadegan M, Peng RW, Roudi R. Dendritic cell/cytokine-induced killer cell-based immunotherapy in lung cancer: What we know and future landscape. J Cell Physiol 2020;235:74-86. 10.1002/jcp.28977 [DOI] [PubMed] [Google Scholar]
- 5.Peters S, Kerr KM, Stahel R. PD-1 blockade in advanced NSCLC: A focus on pembrolizumab. Cancer Treat Rev 2018;62:39-49. 10.1016/j.ctrv.2017.10.002 [DOI] [PubMed] [Google Scholar]
- 6.Williams TJ, Benavides DR, Patrice KA, et al. Association of Autoimmune Encephalitis With Combined Immune Checkpoint Inhibitor Treatment for Metastatic Cancer. JAMA Neurol 2016;73:928-33. 10.1001/jamaneurol.2016.1399 [DOI] [PubMed] [Google Scholar]
- 7.Sun X, Roudi R, Dai T, et al. Immune-related adverse events associated with programmed cell death protein-1 and programmed cell death ligand 1 inhibitors for non-small cell lung cancer: a PRISMA systematic review and meta-analysis. BMC Cancer 2019;19:558. 10.1186/s12885-019-5701-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siles AM, Martínez-Hernández E, Araque J, et al. Antibodies against cell adhesion molecules and neural structures in paraneoplastic neuropathies. Ann Clin Transl Neurol 2018;5:559-69. 10.1002/acn3.554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giometto B, Grisold W, Vitaliani R, et al. Paraneoplastic neurologic syndrome in the PNS Euronetwork database: a European study from 20 centers. Arch Neurol 2010;67:330-5. 10.1001/archneurol.2009.341 [DOI] [PubMed] [Google Scholar]
- 10.Dalmau J, Graus F, Villarejo A, et al. Clinical analysis of anti-Ma2-associated encephalitis. Brain 2004;127:1831-44. 10.1093/brain/awh203 [DOI] [PubMed] [Google Scholar]
- 11.Compta Y, Iranzo A, Santamaría J, et al. REM sleep behavior disorder and narcoleptic features in anti-Ma2-associated encephalitis. Sleep 2007;30:767-9. 10.1093/sleep/30.6.767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vogrig A, Joubert B, Maureille A, et al. Motor neuron involvement in anti-Ma2-associated paraneoplastic neurological syndrome. J Neurol 2019;266:398-410. 10.1007/s00415-018-9143-x [DOI] [PubMed] [Google Scholar]
- 13.Compta Y, Iranzo A, Santamaría J, et al. REM sleep behavior disorder and narcoleptic features in anti-Ma2-associated encephalitis. Sleep 2007;30:767-9. 10.1093/sleep/30.6.767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waragai M, Chiba A, Uchibori A, et al. Anti-Ma2 associated paraneoplastic neurological syndrome presenting as encephalitis and progressive muscular atrophy. J Neurol Neurosurg Psychiatry 2006;77:111-3. 10.1136/jnnp.2005.068775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darnell RB, Posner JB. Paraneoplastic syndromes affecting the nervous system. Semin Oncol 2006;33:270-98. 10.1053/j.seminoncol.2006.03.008 [DOI] [PubMed] [Google Scholar]
- 16.Graus F, Delattre JY, Antoine JC, et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry 2004;75:1135-40. 10.1136/jnnp.2003.034447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vogrig A, Fouret M, Joubert B, et al. Increased frequency of anti-Ma2 encephalitis associated with immune checkpoint inhibitors. Neurol Neuroimmunol Neuroinflamm 2019;6:e604. 10.1212/NXI.0000000000000604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lyons S, Joyce R, Moynagh P, et al. Autoimmune encephalitis associated with Ma2 Abs and immune checkpoint inhibitor therapy. Pract Neurol 2020;20:256-9. 10.1136/practneurol-2019-002464 [DOI] [PubMed] [Google Scholar]
- 19.Kopecký J, Kubeček O, Geryk T, et al. Nivolumab induced encephalopathy in a man with metastatic renal cell cancer: a case report. J Med Case Rep 2018;12:262. 10.1186/s13256-018-1786-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fellner A, Makranz C, Lotem M, et al. Neurologic complications of immune checkpoint inhibitors. J Neurooncol 2018;137:601-9. 10.1007/s11060-018-2752-5 [DOI] [PubMed] [Google Scholar]
- 21.Shibaki R, Murakami S, Oki K, et al. Nivolumab-induced autoimmune encephalitis in an anti-neuronal autoantibody-positive patient. Jpn J Clin Oncol 2019;49:793-4. 10.1093/jjco/hyz087 [DOI] [PubMed] [Google Scholar]
- 22.Manson G, Maria ATJ, Poizeau F, et al. Worsening and newly diagnosed paraneoplastic syndromes following anti-PD-1 or anti-PD-L1 immunotherapies, a descriptive study. J Immunother Cancer 2019;7:337. 10.1186/s40425-019-0821-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leempoel J, Ruyssen A, Kessler R, et al. Anti-Ma2/Ta paraneoplastic rhombencephalitis in a patient with lung cancer responsive to anti-PD1 therapy. Acta Neurol Belg 2020;120:451-2. 10.1007/s13760-019-01179-3 [DOI] [PubMed] [Google Scholar]
- 24.Du Rusquec P, Peyre A, Toulgoat F, et al. Fatal Anti-Ma2 Encephalitis Related to Treatment of Malignant Pleural Mesothelioma With a Combination of Anti-Programmed Death 1 and Anti-Cytotoxic T-Lymphocyte Associated Protein 4 Antibodies. J Thorac Oncol 2019;14:e174-6. 10.1016/j.jtho.2019.03.017 [DOI] [PubMed] [Google Scholar]
- 25.Kopecky ´J, Kubecˇek O, Geryk T, et al. Nivolumab induced encephalopathy in a man with metastatic renal cell cancer: a case report. J Med Case Rep 2018;12:262. [DOI] [PMC free article] [PubMed]
- 26.Yshii LM, Gebauer CM, Pignolet B, et al. CTLA4 blockade elicits paraneoplastic neurological disease in a mouse model. Brain 2016;139:2923-34. 10.1093/brain/aww225 [DOI] [PubMed] [Google Scholar]
- 27.Petrelli F, Grizzi G, Ghidini M, et al. Immune-related Adverse Events and Survival in Solid Tumors Treated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. J Immunother 2020;43:1-7. 10.1097/CJI.0000000000000300 [DOI] [PubMed] [Google Scholar]
- 28.Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med 2018;378:158-68. 10.1056/NEJMra1703481 [DOI] [PubMed] [Google Scholar]
- 29.Alborelli I, Leonards K, Rothschild SI, et al. Tumor mutational burden assessed by targeted NGS predicts clinical benefit from immune checkpoint inhibitors in non-small cell lung cancer. J Pathol 2020;250:19-29. 10.1002/path.5344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ortega Suero G, Sola-Valls N, Escudero D, et al. Anti-Ma and anti-Ma2-associated paraneoplastic neurological syndromes. Neurologia 2018;33:18-27. 10.1016/j.nrl.2016.05.010 [DOI] [PubMed] [Google Scholar]
- 31.Möhn N, Beutel G, Gutzmer R, et al. Neurological Immune Related Adverse Events Associated with Nivolumab, Ipilimumab, and Pembrolizumab Therapy-Review of the Literature and Future Outlook. J Clin Med 2019;8:E1777. 10.3390/jcm8111777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shin YW, Lee ST, Park KI, et al. Treatment strategies for autoimmune encephalitis. Ther Adv Neurol Disord 2017;11:1756285617722347. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as