Abstract
Levosimendan is a distinctive inodilator combing calcium sensitization, phosphodiesterase inhibition and vasodilating properties through the opening of adenosine triphosphate-dependent potassium channels. It was first approved in Sweden in 2000 for the short-term treatment of acutely decompensated severe chronic heart failure when conventional therapy is not sufficient, and in cases where inotropic support is considered appropriate. After more than 20 years, clinical applications have considerably expanded across critical care and emergency medicine, and levosimendan is now under investigation in different cardiac settings (eg, septic shock, pulmonary hypertension) and for non-cardiac applications (eg, amyotrophic lateral sclerosis). This narrative review outlines key milestones in levosimendan history, by addressing regulatory issues, pharmacological peculiarities and clinical aspects (efficacy and safety) of a drug that did not receive great attention in the heart failure guidelines. A brief outlook to the ongoing clinical trials is also offered.
Keywords: levosimendan, heart failure, cardiogenic shock, cardiac surgery, amyotrophic lateral sclerosis
Introduction and Regulatory Aspects
Levosimendan is an inodilator combing calcium sensitization, phosphodiesterase inhibition and vasodilating properties through the opening of adenosine triphosphate-dependent potassium channels. It was first approved in Sweden in 2000, receiving subsequent final marketing authorization in other European countries (including Italy) through the Mutual Recognition Process,1 while it is still in active clinical evaluation by the Food and Drug Administration (FDA). It is indicated for the short-term treatment of acutely decompensated severe chronic heart failure (HF) when conventional therapy is not sufficient, and in cases where inotropic support is considered appropriate. Furthermore, levosimendan can also be used in paediatric populations.
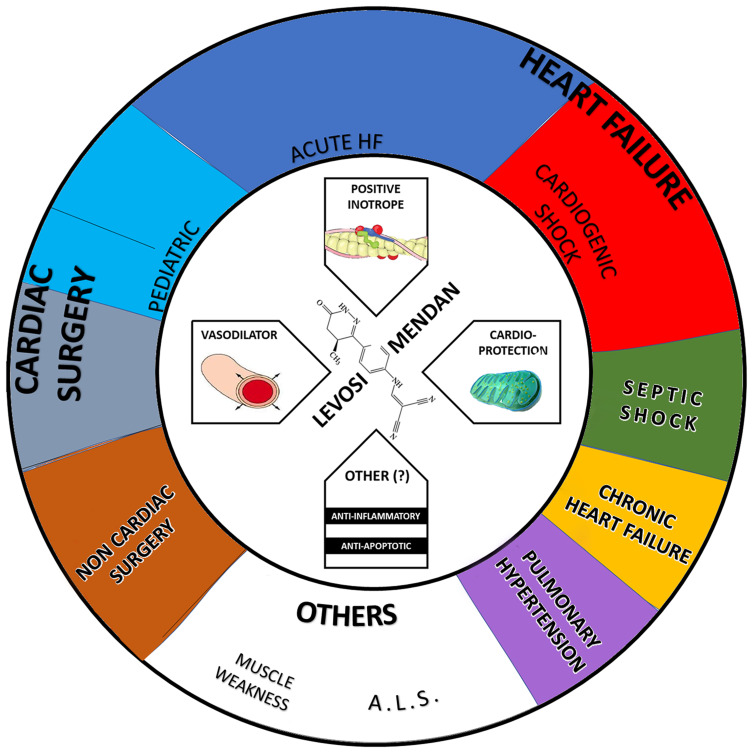
Regardless of the approved indications, the settings of levosimendan use are under active investigation: we retrieved 86 studies on ClinicalTrials.gov (search performed on 29th March 2021; Table 1, Figure 1). Notably, several levosimendan off-label indications are under investigation (eg, septic shock, pulmonary hypertension, muscle weakness conditions). In this context, the Levosimendan for the Prevention of Acute Organ Dysfunction in Sepsis (LeoPARDS) trial failed to demonstrate a beneficial effect of levosimendan on top of standard therapy in reducing severe organ dysfunction, with a higher reported rate of supraventricular arrhythmias.2 Of note, lack of effect was confirmed in the subgroup of patients with elevated cardiac troponin I (cTnI) or N-terminal prohormone of brain natriuretic peptide (NT-proBNP).3 On February 22nd 2018, the European Commission granted orphan designation (EU/3/18/1980) to levosimendan for the treatment of amyotrophic lateral sclerosis (AML).4 Levosimendan was able to enhance in vitro the contractility of the diaphragm muscle fibres of non-ALS patients and to improve in vivo diaphragm neuromuscular efficiency in healthy subjects, thus providing the rationale basis for the use in ALS patients.5 Currently, three studies in which oral levosimendan formulation (1–2 mg/day for two weeks) was administered in patients affected by AML have been completed, without published results (Table 1).
Table 1.
Established and Emerging Therapeutic Indications for Levosimendan Assessed in Ongoing or Completed Studies Retrieved from Clinicaltrials.gov (as of 09th March 2021)
| Therapeutic Indications | No. of Retrieved Studies | Dose | Setting | Comparator | Status | ||||
|---|---|---|---|---|---|---|---|---|---|
| Acute | Inpatients | Outpatients | None (Single Arm) | Placebo | Active | ||||
| Cardiogenic shock/Acute heart failure | 42 | 0.05–0.4 µg/kg/min for 24 hours | 32 | 5 | 5 | 13 | 21 | 2 Dobutamine 2 Milrinone 2 Usual care 1 Inotropes 1 Intra-aortic balloon pump |
16 Completed 9 Unknown 8 Recruiting 4 Not yet recruiting 4 Terminated 1 Enrolling by invitation |
| Pre-emptive administration in non-cardiac surgery | 6 | 0.1–0.2 µg/kg/min for 24 hours | 6 | - | - | 1 | 3 | 2 Dobutamine | 2 Completed 2 Unknown 1 Recruiting 1 Withdrawn |
| Valve surgery | 6 | 0.1–0.2 µg/kg/min for 24 hours | 6 | - | - | 1 | 4 | 1 Dobutamine | 3 Completed 2 Terminated 1 Recruiting |
| Septic shock | 5 | 0.2 µg/kg/min for 24 hours | 5 | - | - | 1 | - | 3 Dobutamine 1 Norepinephrine + Milrinone |
3 Unknown 1 Completed 1 Recruiting |
| Pulmonary hypertension | 4 | 3–12 µg/kg | 2 | - | 2 | 2 | 1 | 1 Milrinone | 2 Recruiting 1 Completed 1 Active not recruiting |
| Amyotrophic lateral sclerosis | 3 | 1–2 mg/day | - | - | 3 (oral) | 1 | 2 | - | 3 Completed |
| Low cardiac output syndrome in paediatric setting | 3 | 0.1–0.2 µg/kg/min for 24 hours | 3 | - | - | - | 2 | 1 Milrinone | 3 Completed |
| Acute kidney injury after cardiac surgery | 3 | LD 12 µg/kg in 30 minutes MD 0.1–0.2 µg/kg/min for 24 hours |
3 | - | - | - | 1 | 1 Milrinone 1 Conventional therapy |
2 Completed 1 Unknown |
| Cardiac surgery in paediatric setting | 3 | 0.1 µg/kg/min for 24 hours | 3 | - | - | - | - | 2 Milrinone 1 Magnesium sulphate |
1 Completed 1 Unknown |
| Muscle weakness conditions | 2 | 0.2 µg/kg/min for 7 hours | - | 2 | - | 1 | 1 | - | 2 Unknown |
| Cardiorenal syndrome in heart failure | 2 | LD 12 µg/kg over 10 minutes MD 0.1 µg/kg/min over 65 minutes |
2 | - | - | 1 | - | 1 Dobutamine | 1 Completed 1 Unknown |
| Chronic heart failure | 2 | 0.2 µg/kg/min for 6 hours NA (oral dosage) |
- | 1 | 1 (oral) | 1 | 1 | - | 2 Completed |
| Pharmacokinetics in special populations | 2 | NA | 2 | - | - | 2 | - | - | 1 Recruiting 1 Unknown |
| ECG variables | 1 | 0.125–2 mg | - | - | 1 (oral) | - | 1 | - | 1 Completed |
| Respiratory muscle function in healthy subjects | 1 | NA | - | - | 1 | 1 | - | - | 1 Completed |
| Acute respiratory distress syndrome | 1 | 0.1–0.2 µg/kg/min for 24 hours | 1 | - | - | 1 | - | - | 1 Recruiting |
Abbreviations: ECG, electrocardiogram; LD, loading dose; MD, maintenance dose; NA, not available.
Figure 1.
Levosimendan mechanism of action and principal settings/applications according to available evidence (see Table 1 for details).
Abbreviations: A.L.S., amyotrophic lateral sclerosis; HF, heart failure; PULM. HYPERT., pulmonary hypertension.
Pharmacokinetic and Pharmacodynamic Peculiarities
Levosimendan is available as parenteral formulation, exhibiting linear kinetic in healthy subjects.6 Main pharmacokinetic/pharmacodynamic (PK/PD) properties of levosimendan and comparator inotropic agents are reported in Table 2. Levosimendan exhibits high oral bioavailability (85%), limited volume of distribution (0.2 L/Kg), and high binding protein (97–98%). Levosimendan is extensively metabolised before excretion into urine (54%) and faeces (44%), mainly via conjugation with glutathione to form inactive metabolites. The minor pathway (≈6% of the total levosimendan dose) is reduction in the intestine to an intermediate metabolite (OR-1855), which is further acetylated to OR-1896, the active metabolite having haemodynamic and pharmacologic properties similar to those of the parent drug. The terminal elimination half-life (t1/2) of levosimendan is about 1 hour both in healthy volunteers and in patients with HF, with relevant rapid disappearance from the circulation after stopping the infusion. On the other hand, the mean elimination t1/2 for metabolites OR-1855 and OR-1896 are approximately 80 hours, with plasma protein binding of ≈40%.7 These PK features of levosimendan metabolites are associated with two important clinical implications: a) the long t1/2 exhibited by OR-1896 allows to use a levosimendan 6-hours instead of 24-hours infusion with similar PD activity; b) the use of levosimendan in renal population. Particularly, PK behavior was similar in patients affected by mild or moderate renal impairment compared to healthy subjects. Conversely, in patients with severe renal dysfunction or required intermittent haemodialysis, the t1/2 of the levosimendan metabolites was prolonged 1.5-fold and their area under the concentration–time curve and peak concentrations were 2-fold higher compared to healthy subjects. These findings suggest that, although levosimendan should not be used in patients affected by severe renal impairment or requiring intermittent haemodialysis according to summary of product characteristics, a dose reduction should be implemented when levosimendan is used for the treatment of congestive HF in patients with severe renal insufficiency.8 Notably, the remarkable difference in plasma-binding protein between levosimendan and its metabolites (97–98% vs 30–37%) accounts for the fact that the metabolites are dialyzable in contrast to parent drug.
Table 2.
Pharmacokinetic/Pharmacodynamic Properties and Therapeutic Indications of Available Inotropes Compared to Levosimendan
| Inotropic Agents (Administration) | PK Features | PD Features/Mechanism of Action | Dose | Approved Therapeutic Indications# | Emerging Indications |
|---|---|---|---|---|---|
| Levosimendan (IV) | Vss: 0.2 L/Kg Protein binding: 97–98% Hepatic metabolism t1/2: 1 h Urinary (54%)/Biliary (44%) elimination |
Increase sensitivity of troponin C to intracellular calcium (inotropic effect) Activity on ATP-dependent potassium channels (coronary and peripheral vasodilation) |
0.05–0.6 µg/kg/min CI (6–24 hours) No loading dose* 1–2 mg/day (oral) |
- Short-term therapy of acutely decompensated severe chronic heart failure - Including paediatric population |
- Amyotrophic lateral sclerosis (included in orphan drug list of EMA) - Muscular weakness conditions - Pulmonary hypertension - Septic shock (metabolic/microcellular alterations) |
| Adrenaline (IV, IM) | Rapid onset and short duration t1/2: 5–10 minutes Protein binding: 50% Hepatic/tissue metabolism by COMT >90% urinary excretion as metabolites in urine |
α1 +++ β1: +++ β2: ++ D: - Inotropy, chronotropy, dromotropy and vasoconstriction Lower doses: β1 and β2-effects Higher doses: α1-effects |
2 to 10 μg/min infusion (IV) 0.5 mg (IM) |
- Adjunctive use in the management of cardiac arrest - Anaphylactic shock - Including paediatric population |
- Chronic sinusitis/rhinitis - Asthma/bronchospasm (inhaled) |
| Dobutamine (IV) | Onset of action: 2 minutes Tmax: 10 minutes t1/2: 2 minutes Hepatic/tissue metabolism by COMT >90% urinary excretion as metabolites in urine |
α1 + β1: +++ β2: ++ D: - Lower doses: mild vasodilation Higher doses: inotropy, vasoconstriction |
Start with 2 μg/kg/min up to a maximum dose of 40 μg/kg/min |
- Inotropic support in the treatment of low output cardiac failure associated with myocardial infarction, open heart surgery, cardiomyopathies, septic shock and cardiogenic shock - Increase or maintain cardiac output during positive end expiratory pressure ventilation - Stress echocardiography - Including paediatric population |
- Chronic fatigue - Post-traumatic stress disorder |
| Dopamine (IV) | t1/2: 2 minutes Hepatic/tissue metabolism by COMT urinary excretion as metabolites in urine |
α1 ++ β1: +++ β2: ++ D: +++ Lower doses: dopaminergic-effect promoting vasodilation and increased blood flow Intermediate doses: β1-effects promoting inotropic activity Higher doses α1-effects promoting vasoconstriction |
Low dose: 2 to 5 μg/kg/min Intermediate dose: 5 to 10 μg/kg/min High dose: 10 to 20 μg/kg/min |
- For the correction of haemodynamic imbalance such as is seen in circulatory decompensation accompanying myocardial infarction, trauma, endotoxic septicaemia, renal failure, congestive cardiac failure and open-heart surgery | |
| Isoproterenol (IV, IM, SC) | 25–35% metabolized in the liver and other tissues by COMT to inactive metabolite 50% excreted unchanged in urine | β1: +++ β2: ++ Inotropy, chronotropy, dromotropy and bronchodilation |
IM-SC: 0.2 mg followed by 0.02–1 mg IV: 0.5–20 µg/min |
- Treatment for total AV block (including the Stokes-Adams syndrome) and cardiac arrest - Treatment for bronchospasm during anaesthesia - In addition to a treatment for cardiogenic shock. |
- Treatment of lipomas - Anorexia nervosa - Decrease the intrarenal pressure in the kidney during endoscopic management of kidney stone (topical administration) |
| Milrinone (IV) | Vss: 0.38 L/Kg t1/2: 2.3 h Renal excretion (up to 60% in 2 h and 90% in 8 h) |
PDE-3 inhibitor Inotropic effect coupled with peripheral vasodilation |
Loading dose: 50 µg/kg (over 10 minutes) Maintenance dose: 0.375 to 0.75 µg/kg/min (up to a maximum of 1.13 mg/kg/day) |
- Short-term intravenous therapy of severe congestive heart failure irresponsive to conventional maintenance therapy - Acute heart failure including low output states following cardiac surgery - Including paediatric population |
- Pulmonary hypertension - Vasospasm treatment in subarachnoid haemorrhage - Septic shock |
Notes: *The Italian SPC says “6–12 mcg/kg over 10 minutes, followed by infusion of 0.1 mcg/kg/min, for 24 h”. - no receptor affinity; +Low binding affinity; ++Moderate binding affinity; +++High binding affinity. #Levosimendan is approved overall in 58 countries worldwide, including Europe, South East Asia, Middle East, and New Zealand. This group of countries does not include US, Canada, Australia and UK.
Abbreviations: AV, atrioventricular; CI, continuous infusion; COMT, catechol-O-methyltransferase; EMA, European Medicines Agency; IM, intramuscular; IV, intravenous; PDE-3, phosphodiesterase type-3; SC, subcutaneous; t1/2, half-life; Tmax, time to achieve peak concentration; Vss, volume of distribution at steady state.
Given that steady state is achieved within 4–8 hours of constant infusion, a loading dose may be administered to achieve a more rapid effect. Levosimendan as continuous infusion (0.05–0.2 μg/kg/min for 24 hours) preceded by a loading dose (6–12 μg/kg in 10 minutes) was used in the active controlled pivotal studies LIDO and SURVIVE,9,10 in which dobutamine served as comparator. Given that the elimination half-life of dobutamine is a few minutes, the haemodynamic effects of dobutamine were seen immediately after the infusion, whereas a bolus of levosimendan was required to achieve immediate action. However, in the case of hypovolemia or initial low blood pressure, the use of an initial bolus of levosimendan is not generally recommended to avoid hypotension or arrhythmias.1
From a PD standpoint, levosimendan possesses a triple mechanism of action: 1) calcium sensitisation by selective binding to calcium-saturated cardiac troponin C; 2) opening of KATP channels in vascular smooth muscle cells; 3) opening of KATP channels in the mitochondria of cardiomyocytes.11 Through calcium sensitisation, levosimendan increases cardiac contractility in HF without affecting muscle electrophysiology and relaxation. Through the opening of KATP channels in vascular smooth muscle cells, levosimendan improves oxygen supply to the myocardium without causing increased myocardial oxygen demand, ischaemia, or tolerance. Furthermore, this mechanism promotes arterial and venous vasodilation, improving coronary artery circulation. Finally, the opening of KATP channels in cardiomyocytes mitochondria leads to cardioprotective effect in ischaemic settings.7 Pleiotropic effects have also been demonstrated, including anti-inflammatory (reduction of the pro-inflammatory interleukin-6 for at least 1 month after infusion) and antiapoptotic effects (reduction of soluble apoptosis mediators), although clinical relevance is uncertain as compared to main cardiac mechanisms.12
Levosimendan offers a predictable safety profile. The most commonly reported adverse events are hypotension, headache and atrial arrhythmias, whose risk was increased as compared with reference therapy comprising placebo or dobutamine in a meta-analysis of 25 randomized controlled trials (RCTs) by Gong et al.13 Considering this, 2016 European Society of Cardiology (ESC) guidelines on chronic and acute HF advised against levosimendan use for treatment of patients with systolic blood pressure <85 mmHg or cardiogenic shock (CS) unless in combination with other inotropes or vasopressors.14
Although levosimendan use is contraindicated in severe renal failure, the estimated glomerular filtration rate (eGFR) improves in levosimendan treated patients compared to dobutamine or placebo. In addition to beneficial effects on hemodynamics, levosimendan has direct effects on renal circulation and induces renal arterial vasodilation, thus improving renal blood flow without compromising renal oxygen demand/supply relationship.15–17
Levosimendan in Acute Heart Failure
A wide range of RCTs analyzed the effect of levosimendan in acute HF setting (Table 3).9,10,18–21 While significant, dose-dependent increase in cardiac output and stroke volume, and decrease in pulmonary capillary wedge pressure, mean blood pressure, mean pulmonary artery pressure, mean right atrial pressure, and total peripheral resistance are obtained, clinical advantage versus placebo or dobutamine was restricted to self-reported symptom improvement and to a beneficial effect on neurohormone levels. Thus, in the context of acutely decompensated HF requiring inotropes or intravenous diuretics, levosimendan is well tolerated and may improve functional outcomes, but data supporting potential benefits on hard endpoints such hospitalizations and mortality are somehow contradictory.
Table 3.
Main Randomized Controlled Trials on the Use of Levosimendan in Acute Heart Failure
| Study | n (Total/L) | Loading Dose (ug/kg) | Dose Range (ug/kg/min) | Duration (h) | Comparator | Diagnosis | Ischemic Etiology (%) | Setting | Primary Endpoint | P value |
|---|---|---|---|---|---|---|---|---|---|---|
| Nieminen et al,18 | 151/95 | 3–36 | 0.05–0.6 | 24 | Placebo/ Dobutamine | Chronic HF (NYHA II–IV) | 100 | Hospital | Increase in CO and SV, reduced PCWP | <0.001 (favors levosimendan) |
| Slawsky et al,19 | 146/98 | 6 | 0.1–0.4 | 6 | Placebo | Acute HF | 62 | ICU | Increase in CO and SV, reduced PCWP | <0.001 (favors levosimendan) |
| LIDO9 | 203/103 | 24 | 0.1–0.2 | 24 | Dobutamine | Acute HF | 45 | ICU | 30% increase in CO and 25% decrease in PCWP | HR 1.9 (95% CI 1.1–3.3); p= 0.022 (favors levosimendan) |
| RUSSLAN20 | 504/402 | 6–24 | 0.1–0.4 | 6 | Placebo | Post-AMI HF | 100 | ICU | Safety | 0.319 |
| REVIVEI21 | 100/51 | 6–12 | 0.1–0.2 | 24 | Placebo | Acute HF | 49 | ICU | Clinical composite (short term clinical course measures) | 0.029 (favors levosimendan) |
| REVIVE II21 | 600/299 | 6–12 | 0.1–0.2 | 24 | Placebo | Acute HF | 55 | ICU | Clinical composite (short term clinical course measures) | 0.015 (favors levosimendan) |
| SURVIVE10 | 1327/664 | 12 | 0.1–0.2 | 24 | Dobutamine | Acute HF | 76 | ICU | Mortality | HR 0.91 (95% CI 0.74–1.13); p= 0.40 (favors levosimendan) |
Abbreviations: AMI, acute myocardial infarction; ICU, intensive care unit; NYHA, New York Heart Association; HR, hazard ratio; CI, confidence interval; CO, cardiac output; SV, stroke volume, PCWP, pulmonary capillary wedge pressure.
Duration of hospitalization and hospital readmissions are proxies accounting for the severity of HF. In REVIVE II study, the mean duration of the initial hospitalization was almost 2 days shorter for patients in the levosimendan group than for those in the placebo group (7 vs 8.9 days) and in RUSSLAN, the combined risk of death and worsening HF was significantly lower in patients treated with levosimendan than in the control group during the infusion period (2% vs 6%; p = 0.033) and at 24 h (4% vs 9%; p = 0.044). In a retrospective 180-day follow-up analysis of the LIDO study, levosimendan-treated patients spent significantly more days alive and out of hospital (median 157 vs 133 days for levosimendan and dobutamine, respectively; p = 0.027). Conversely, in SURVIVE trial, the mean number of days alive and out of hospital during the 189 days of follow-up was similar between the two groups (120.2 in the levosimendan group vs 116.6 in the dobutamine group; p = 0.3). A large meta-analysis by Landoni et al22 showed a reduction of length of hospital stay in the levosimendan group compared to both dobutamine and placebo (weighted mean difference = −1.31 [−1.95; −0.31], p for effect = 0.007, with 17 studies included).
Mortality endpoint was well heterogeneous in the available randomized studies. In REVIVE I and II studies, a 24-h infusion with levosimendan was associated with numerically higher mortality at 90 days as compared to placebo in patients with acute heart failure remaining symptomatic despite intravenous diuretics. Of note, excess mortality was driven by patients with blood pressure below 100/60 mmHg. On the other hand, in the setting of acute HF complicating myocardial infarction analyzed in RUSSLAN study, levosimendan showed lower early mortality than placebo (11.7% vs 19.6% at 14 days, p = 0.031), but the difference was only numerically higher at 180 days (22.6% vs 31.4%, hazard ratio 0.67 [95% CI 0.45-1.00], p = 0.053). As compared to dobutamine, levosimendan was associated with lower mortality at both one month (8% vs 17%) and six months (26% vs 38%, p = 0.029) in the LIDO study, but not in the SURVIVE trial, where a potential benefit of levosimendan was seen only in patients with previously diagnosed HF and with previous treatment with beta-blockers.23 Despite these partial contradictory results, data from the meta-analysis by Landoni et al22 showed a significant reduced mortality in patients treated with levosimendan over both dobutamine and placebo (risk ratio 0.80 [0.72; 0.89], p for effect <0.001, number needed to treat = 17 with 45 studies included).
Levosimendan in Patients Previously Receiving Beta-Blockers
Beta-adrenergic receptor function is modified in subjects receiving beta-blockers in an acute or chronic setting. Unlike catecholamines, levosimendan mechanism of action is independent of beta-adrenergic receptor and may be advantageous in this subgroup of patients.
This hypothesis is supported by a few small clinical studies.24 In addition, subgroup analyses of the LIDO and SURVIVE reported consistent exploratory findings favoring levosimendan in terms of hemodynamic advantage and mortality, respectively, in patients who were on beta-blockers before the acute decompensation. Based on these possible beneficial hemodynamic effects, levosimendan may be considered a first-choice in patients with acute decompensated HF on beta-blocker therapy, if beta-blockade is likely contributing to hypotension with relevant hypoperfusion (class IIb, level of evidence C).14 In addition, in the specific context of HF complicating acute coronary syndromes (ACS), levosimendan is proposed as first-line therapy over catecholamines in all patients in chronic beta-blocker therapy or with insufficient urinary output after diuretics.25
Levosimendan in Patients with HF Complicating Acute Coronary Syndrome
Acute HF as a complication of ACS is frequent and associated with worse outcomes.
In RUSSLAN study, decreased incidence of worsening HF and improvements in short- and long-term mortality were recognized. In the 13.4% of patients in the SURVIVE study cohort who had an acute myocardial infarction (AMI), 31-day mortality was 4% lower in the levosimendan arm vs dobutamine, whereas in the non-AMI subgroup no difference in death rates was noted.
A few smaller studies have investigated the use levosimendan in this setting and suggested potential benefits on hemodynamics and left ventricular function.26–29
Levosimendan in Right HF and Pulmonary Hypertension
The effect of levosimendan on patients with right HF with or without pulmonary hypertension remains unclear. Conclusive randomized trials in this field are lacking. Several small studies have examined this issue, but the population was heterogeneous, sample sizes were small, and the results were inconclusive or even conflicting.30–33 Most of the evidence relates to patients with group 2 pulmonary hypertension and consequent right ventricular failure. In this setting, the observed benefits may either be due to the effect of levosimendan on right ventricular contractility and on the dilatation of pulmonary vessels, or to the improvement in left ventricular function and consequent reduction of pulmonary congestion. Fewer data are available on the other groups of pulmonary hypertension. A meta-nalysis by Qiu et al34 including 10 trials shows that a 24-h infusion of levosimendan is safe and effective for the short-term treatment of right HF in patients with a variety of heart and lung diseases. In particular, they observed a significant increase in tricuspid annular plane systolic excursion and right ventricular ejection fraction, as well as a significant reduction in systolic pulmonary artery pressure and pulmonary vascular resistance, whereas changes in mean pulmonary pressure were nonsignificant.
A Phase II trial (HELP, NCT03541603) will evaluate the efficacy and safety of repetitive use of levosimendan in patients with pulmonary hypertension and HF with preserved ejection fraction.
Current Real-Life Use of Levosimendan in Acute Decompensated HF
In ALARM-HF (Acute Heart Failure Global Survey of Standard Treatment) registry study,35 which reviewed in-hospital treatments of patients with acute HF in eight countries, levosimendan was administered in 14.5% of patients, while the most used inotropes were dobutamine (57.3%) and dopamine (33.5%). A significant in-hospital mortality reduction was found through a propensity-based analysis between levosimendan with catecholamines in the first 24 hours after therapy initiation (hazard ratio 0.25, 95% CI 0.07–0.85).
The ESC HF Long-Term registry36 included patients with unscheduled hospitalizations for acute HF in 21 countries. Dobutamine was the most used agent (43%), followed by dopamine (25%) and levosimendan (13%). This analysis confirmed that the use of inotropes and/or vasopressors in acute HF was consistently associated with in-hospital death and with long-term all-cause and cardiovascular mortality. However, no association was found with long-term mortality in hospital survivors, suggesting an immediate rather than prolonged detrimental effect of inotropes and/or vasopressors on clinical outcome. Dopamine was associated with an excess mortality compared to dobutamine and levosimendan, but the study was not adequately powered to analyze the differences between individual drugs.
In summary, levosimendan, through a unique pharmacodynamics, is a valuable approach in the early treatment of acute HF, especially in the presence of beta-blockers or ischemic cardiomyopathy, provided that hypovolemia and hypokalemia are avoided or corrected, also considering the potential protective effect on cardio-renal syndrome.
Future Perspectives
At present, more than 20 clinical trials are ongoing to determine other possible therapeutic benefits of levosimendan in acute HF. Among these, we believe that some may definitely lead to changes in clinical practice. In particular, with a randomized placebo-controlled design, these studies will investigate levosimendan effect on NT-proBNP levels in new settings, such as in patients with symptoms of HF undergoing transcatheter aortic valve replacement (NCT04573049) or with non ST-elevation myocardial infarction (EMSAHF, NCT03189901), while novel administration schedules, such as repetitive use of levosimendan, will be investigated in chronic HF to prevent hospitalizations (NCT03764722) and to improve exercise capacity and exercise hemodynamics (NCT03576677).
Levosimendan in Cardiogenic Shock
Compared with standard adrenergic therapy, levosimendan does not increase myocardial oxygen consumption, has a lusitropic effect, with less direct pro-arrhythmic effects. Moreover, levosimendan is not sensitive to the action of beta-blockers. On the other side, the use of levosimendan in cardiogenic shock faces potential difficulties. First of all, levosimendan is also a potent vasodilator and could precipitate hypotension in vasoplegic patients or in vasopressor-dependent patients. Another limit is the delayed action (2–5 h) to achieve an increase in stroke volume without a loading bolus. Furthermore, given its long half-life, rapid reversal of the vasodilation may be difficult after drug administration.
Currently, there are no high-quality RCTs on the use of levosimendan in this setting. From small low-quality RCTs emerged that levosimendan is generally well tolerated, improves multiple indices of cardiac function and reduces systemic vascular resistance but is often responsible for systemic arterial hypotension resulting in increased need of vasopressors (Table 4).37–43 A meta-analysis44 performed on 13 RCTs in patients with myocardial infarction, HF or cardiac surgery complicated by cardiac shock (CS) reported that levosimendan may reduce short-term mortality compared with dobutamine, but this initial survival benefit was not confirmed on long-term follow-up. Levosimendan did not affect ischemic events, acute kidney injury, dysrhythmias, or hospital length of stay. Due to lack of statistical power, uncertainty remained regarding the effect of levosimendan compared with placebo or enoximone.
Table 4.
Main Studies on the Use of Levosimendan in Cardiogenic Shock
| Study | Design | n (Total/L) | Loading Dose (ug/kg) | Dose Range (ug/kg/min) | Duration (h) | Comparator | Diagnosis | Setting | Primary Endpoint | P value |
|---|---|---|---|---|---|---|---|---|---|---|
| Garcia-Gonzalez et al,38 | RCT | 22/11 | 24 | 0.1 | 24 | Dobutamine | CS after PCI in AMI | ICU | 30% increase in CPO | <0.05 (favors levosimendan) |
| Samimi-Fard et al,40 | RCT | 22/11 | 24 | 0.1 | 24 | Dobutamine | CS after PCI in AMI | ICU | Cardiac death at 12 months | 0.24 |
| Russ et al,43 | PO | 56/24 | 12 | 0.05–0.2 | 24 | Catecholamine | Persisting CS 24h after AMI | ICU | Hemodynamic improvement | <0.01 for CIn increase at 3/24/48h (favors levosimendan) |
| Fuhrmann et al,37 | RCT | 32/16 | 12 | 0.1–0.2 | 24 | Enoximone | Refractory CS 2h after AMI | ICU | Survival at 30 days | 0.023 (favors levosimendan) |
| Christoph et al,41 | PO | 22/10 | 12 | 0.1 | 24 | IABP | Refractory CS after PCI in AMI | ICU | Hemodynamic improvement | 0.076 for CIn increase at 3h |
| Omerovic et al,42 | PO | 94/46 | 12 | 0.1 | 24–48 | Standard inotropes | CS complicating AMI | ICU | Mortality at 30 days and 1 year | HR 0.97 (95% CI 0.53–1.78); p= 0.93 and HR 1.05 (95% CI 0.57–1.92); p= 0.87 |
Abbreviations: RCT, randomized controlled trial; PO, prospective observational; PCI, percutaneous coronary intervention; AMI, acute myocardial infarction; IABP, intra-aortic balloon pump; ICU, intensive care unit; CIn, cardiac index; HR, hazard ratio; CI, confidence interval; CPO, cardiac power output.
Another meta-analysis by Fang et al45 including 13 randomized and non-randomized clinical trials comparing levosimendan to standard therapy or placebo in adult patients with CS complicating myocardial infarction found no evidence of benefit in terms of survival, even if a non-significant trend was noted. However, an improvement in hemodynamic parameters and cardiac function (pulmonary arterial pressure and end-systolic volume were significantly reduced, while cardiac index, cardiac power index, ejection fraction, mean blood pressure and mixed venous oxygen saturation were significantly increased) emerged in the levosimendan group.
A recent position statement from the HF Association of the ESC46 suggests that levosimendan may be used in particular CS subgroups, like patients on chronic beta-blocker therapy, concomitant acute right ventricular failure or pulmonary hypertension, knowing its favorable effects on pulmonary vascular resistance.
Levosimendan in CS Complicating Takotsubo Syndrome
To date, there have been no large RCTs to define the optimal management of patients with Takotsubo syndrome. As catecholamines appear to have a central role in its pathophysiology, levosimendan has been advocated as the first-choice inotropic support when mechanical circulatory assist devices are not available, due to the fact that it does not increase oxygen consumption.47 The safety and efficacy of levosimendan use in this setting has been explored in encouraging case-series and is supported by the pathophysiological rationale.48,49 Indeed, the use of adrenergic inotropes or phosphodiesterase inhibitors is generally contraindicated to avoid further activation of catecholamine receptors or their downstream pathways.
Levosimendan in CS Treated with Veno-Arterial Extra-Corporeal Membrane Oxygenation (VA ECMO)
VA ECMO is used to restore and maintain adequate end-organ perfusion in patients with refractory CS.50 However, the retrograde flow from femoral artery cannulation leads to increased left ventricular afterload, with left ventricular dilatation and pulmonary edema. To avoid this complication, low doses of inotropes like dobutamine are commonly administered to maintain left ventricular ejection and facilitate the weaning of the device, since the duration of VA ECMO support is directly correlated to complications. However, many studies have suggested an increase in mortality due to myocardial ischemia and arrhythmias with standard inotropes. Considering the pharmacologic properties of levosimendan, a few preliminary studies have been conducted and together support the effectiveness of levosimendan in facilitating the weaning of VA ECMO with a survival benefit, but no specific evidence is available regarding the ideal dosing and timing to initiate levosimendan infusion in these patients.51–55 On the other hand, two recent retrospective studies found no difference in successful weaning rate from VA ECMO in patients treated with levosimendan, with no effect on mortality.56,57 A multicenter randomized controlled trial (LEVOECMO, NCT04728932) will test the hypothesis that levosimendan in addition to standard care facilitates the weaning from VA ECMO.
Current Real-Life Use of Levosimendan in CS
Data on real-world use of inotropes are scattered.
A large retrospective analysis of three observational cohorts of patients with acute HF (ALARM-HF, EFICA and AHEAD)58 suggested that combining a vasopressor with an inodilator (ie, dobutamine, levosimendan, or PDE3i) may improve short-term mortality of patients with CS compared to vasopressors alone. In this registry, levosimendan was administered in 10% of the overall population.
In the CardShock prospective observational study,59 vasopressors and inotropes were administered in 94% of patients with CS. Dobutamine (49%) and levosimendan (24%) were the most commonly used inotropes. In this setting, the combination of levosimendan with noradrenaline, similarly to the combination of dobutamine with noradrenaline, was associated with improved survival at 90-day when compared with other vasopressors or inotropes.
Future Perspectives
The LevoHeartShock (NCT04020263) is testing the hypothesis that an early use of levosimendan, by permitting dobutamine discontinuation, would accelerate the resolution of signs of low cardiac output and facilitate myocardial recovery. It will compare levosimendan versus placebo on top of conventional vasopressor therapy on a combined morbidity-mortality endpoint in patients with CS.
Levosimendan in Advanced HF
A series of small-scale preliminary studies have suggested the benefit of repeated infusions of levosimendan in an ambulatory setting to ameliorate symptoms and prevent recurrent hospitalizations in patients with advanced HF or terminal HF not eligible for heart transplantation (HTx) or left ventricular assist device (LVAD) implantation.60 However, the optimal posology had not been identified. Three larger RCTs have then been developed (Table 5). 61–63 Overall, these larger studies demonstrated a significant reduction in NT-proBNP levels and in some cases in HF readmissions. It must be noted, however, that hospitalizations were not always the primary endpoint of these studies. Intermittent levosimendan therapy was well tolerated, with comparable frequencies of adverse events between the groups, despite in LevoRep trial significantly reduced systolic blood pressure as compared to placebo (p = 0.01), but without an increased need for active measures for symptomatic hypotension and, in LION-HEART, a greater proportion of patients in the levosimendan group needed dose reduction or interruption due to clinically important hypotension, although this difference was not statistically significant (levosimendan, 15%; placebo, 9%).
Table 5.
Main Randomized Controlled Trials on the Planned Repetitive Use of Levosimendan in Advanced Chronic Heart Failure
| Study | n | Loading Dose (ug/kg) | Dose (ug/kg/min) | Duration (h) | Interval/ Cycles (n) | Comparator | Diagnosis | Ischemic Etiology (%) | Setting | Primary Endpoint | P value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LevoRep61 | 120 | No | 0.2 | 6 | Every 2 weeks/4 | Placebo | AdHF | 61.9 | Ambulatory | Clinical composite (functional capacity and QoLimprovement) | OR 1.25 (95% CI 0.44–3.59); p= 0.810 |
| LIONHEART62 | 69 | No | 0.2 | 6 | Every 2 weeks/6 | Placebo | AdHF | 60 | Ambulatory | NT-proBNP levels | 0.003 (favors levosimendan) |
| LAICA63 | 97 | NO | 0.1 | 24 | Every 30 days/6–13 | Placebo | AdHF | n.a. | Ambulatory | Clinical composite (admissions for ADHF or HF worsening) | Not significant difference |
Abbreviations: RCT, randomized controlled trial; AdHF, advanced heart failure; NT-proBNP, N-terminal pro-brain natriuretic peptide; QoL, quality of life; OR, odds ratio; CI, confidence interval, ADHF, acute decompensated heart failure.
Taken together, these trials support the use of repeated levosimendan in ambulatory patients with refractory chronic HF to improve symptoms and, likely, to reduce the recurrence of HF hospitalization. This approach may have an impact in particular in stabilizing severe patients during the waitlist time for HTx, as well as a palliative approach in patients with no access to transplant or LVAD.
Current Real-Life Use of Levosimendan in AdHF
Real-life data for the use of levosimendan has been reported in a multicenter retrospective observational study from the Swedish Heart Failure Registry,64 including 22 Swedish hospitals. The analysis involved 87 patients (1.8% of those included in the Registry) with chronic HF who had been treated in an outpatient setting with repetitive levosimendan and showed lower mortality compared to subjects receiving levosimendan during an acute HF admission and controls who never received inotropes (one-year survival was 81% for planned repetitive levosimendan, 62% for acute levosimendan and 66% for controls). Patients who received planned repetitive levosimendan, however, were younger, predominantly male, had reduced left ventricular ejection fraction (LVEF) and lower NT-proBNP levels and were mostly on optimal HF therapy, with a 41% prevalence of cardiac resynchronization therapy.
RELEVANT-HF multicenter registry65 was then designed to assess the efficacy and safety of scheduled repetitive levosimendan infusions in a cohort of real-world advanced HF patients. A total of 185 ambulatory advanced HF patients in NYHA class III–IV, with ≥2 HF hospitalizations/unplanned visits in the previous 6 months and systolic dysfunction were enrolled. The indication to this treatment was palliation in 63% of patients and bridge in 47%; in this latter group, 48% of patients were bridged to HTx, 41% to candidacy to HTx or LVAD and 12% to decision on further options. Patients were treated in a hospital or outpatient setting with specifically tailored intermittent levosimendan doses (0.05–0.2 μg/kg/min) without preliminary bolus every 3–4 weeks. Repetitive treatment with levosimendan was well tolerated and associated with lower number and duration of hospitalizations from the 6 months before to the 6 months after treatment start. Infusion-related adverse events occurred 12.4% patients and the most common was ventricular arrhythmias (8.6%). During follow-up, 20% of patients required treatment adjustments for clinical instability. One-year survival was 86% overall and 78% free from death/LVAD/urgent transplant.
Despite potential selection biases, and a very limited use of this approach in clinical practice, the evidence available from real-world data support the findings of randomized studies showing a benefit over hospitalization, and potentially over survival of scheduled levosimendan and infusions in patients with advanced heart failure in ambulatory setting.
Future Perspectives
The LeoDOR RCT (NCT03437226)66 is underway to confirm the efficacy and safety of repeated infusions of levosimendan in this population. The primary efficacy assessment will use a hierarchical composite clinical endpoint comprising (i) time to death or urgent HTx or implantation of a LVAD; (ii) time to non-fatal HF hospitalization requiring i.v. vasoactive therapy; and (iii) time-averaged proportional change in NT-proBNP. The use of this endpoint should increase statistical power to assess whether, compared with placebo, repetitive administration of levosimendan early after a hospitalization for worsening HF increases clinical stability over subsequent weeks.
Another multicenter, randomized, double-blind, placebo-controlled study (LEIA-HF, NCT04705337) is about to start to investigate the efficacy of repeated infusions of levosimendan in outpatients with advanced systolic HF on preventing unplanned hospitalizations and death. This study will also evaluate the safety of treatment discontinuation in a second phase.
Levosimendan in Cardiac Surgery
Acute perioperative left ventricular dysfunction is a major complication of cardiac surgery affecting up to 20% of patients and is associated with increased mortality. Preexisting left ventricular dysfunction predisposes to low cardiac output syndrome (LCOS).67 Several preliminary studies have investigated the role of levosimendan in the prevention of LCOS following cardiac surgery in patients with impaired ejection fraction,68 suggesting a benefit on mortality and adverse postoperative outcomes such as the need for renal replacement therapy.69 Three large RCTs (Table 6),70–72 however, did not show a reduction in any of the composite endpoint reflecting LCOS and mortality in a mixed population of coronary artery bypass graft (CABG), valvular, or combined surgery with impaired LVEF. Moreover, a pooled analysis of high-quality RCTs on perioperative levosimendan therapy in cardiac surgery found an increased rate of supraventricular arrhythmias and hypotension with neutral effect on postoperative outcomes.73 In light of the available evidence, in 2017 an international consensus document concluded that levosimendan could not be routinely recommended in all cardiac surgery settings.74 Conversely, 2018 German guidelines on intensive care unit (ICU) therapy after cardiac surgery states that levosimendan should be used to prevent hemodynamic complications in patients with severely impaired LVEF and in patients with LCOS as the only positive inotropic agent for which a lethality benefit has been described.75
Table 6.
Main Randomized Controlled Trials on Levosimendan Use in Cardiac Surgery
| Study | n (Total/L) | Loading Dose (ug/kg) | Dose Range (ug/kg/min) | Duration (h) | Comparator | Diagnosis | CABG (%) | Timing | Primary Endpoint | P value |
|---|---|---|---|---|---|---|---|---|---|---|
| LEVO-CTS71 | 882/849 | No | 0.1–0.2 | 24 | Placebo | LVEF < 35% | 88.5 | Immediately before surgery | Two clinical composites (mortality and morbidity) | OR 1.00 (99% CI 0.66–1.54); p = 0.98 and OR 1.18 (96% CI 0.76–1.82); p= 0.45 |
| CHEETAH72 | 506/248 | No | 0.025–0.2 | Up to 48 | Placebo | Perioperative cardiac dysfunction | 45.9 | Briefly before surgery or in OpR | 30-day mortality | ARD 0.1% (95% CI 5.7–5.9); p= 0.97 |
| LICORN70 | 336/167 | No | 0.1 | 24 | Placebo | LVEF < 40% | 100 | After anesthetic induction | Clinical composite (LCOS or RRT) | ARD −7% (95% CI −17%-3%; p= 0.15 |
Abbreviations: RCT, randomized controlled trial; CABG, coronary artery bypass graft; LVEF, left ventricular ejection fraction; OpR, operating room; OR, odds ratio; CI, confidence interval; ARD, absolute risk difference; LCOS, low cardiac output syndrome; RRT, renal replacement therapy.
More recently, further data led to the hypothesis of a potential mortality benefit in specific subgroups of patients.
First, a meta-analysis by Wang et al,76 found that prophylactic use of levosimendan in the isolated CABG population was associated with reduced mortality and postoperative atrial fibrillation, but a higher incidence of hypotension. This finding was corroborated by a subgroup analysis of the LEVO-CTS trial77 that found that levosimendan was associated with lower 90-day mortality and LCOS in patients undergoing isolated CABG, but not in those undergoing isolated valve or combined CABG/valve procedures.
Furthermore, it has been shown in preliminary studies78 that levosimendan infusion 24–48 hours before LVAD implantation can improve short- and long-term outcomes. Despite Kocabeyoglu et al,79 failed to confirm a reduction in early right ventricular failure and mortality rates in the post-operative LVAD setting, the authors claimed levosimendan as a preferable alternative to conventional adrenergic inotropes in these patients due to its hemodynamic effects of reduction in pulmonary arterial pressure and improvement in right ventricular function.
Future Perspectives
A multicenter RCT (SPARTANS, NCT04179604) is currently testing the hypothesis that an earlier preoperative infusion of levosimendan reduces perioperative LCOS in patients with compromised left ventricular function undergoing cardiac surgery. The main difference of this study compared to the previous RCTs discussed above is that levosimendan will be started 24 hours before surgery. This is supported by some retrospective studies suggesting favorable effects on mortality, LCOS incidence and renal function with an early perioperative administration of levosimendan.80,81
Conclusion
Levosimendan, through a unique pharmacodynamics, is an effective therapeutic option to treat severely decompensated HF, in the context of both acute onset and progressive worsening of chronic HF. Its predictable safety profile requires to appropriately manage hypovolemia and hypokalemia to reduce the risk of arrhythmias and hypotension.82
After its first approval over 20 years ago levosimendan underwent a profound and diverse development that have brought the drug from the management of acute HF in ICUs to the treatment of chronic heart insufficiency in ambulatory setting, with additional emerging evidence in the neuromuscular disease area such as ALS. Most of this development has been linked to investigator-driven studies, which did not lead to any significant change of the original 20y-old label of the drug. This process is not only an example on how the independent development of a drug can expand and improve its utilization, but it is also a marker of the unmet need for the clinical community for effective drugs acting on cardiac muscular function. We believe that current evidence supports the use of levosimendan to partially fill this need.
Acknowledgments
Authors at the University of Bologna are supported by institutional research funds (Ricerca Fondamentale Orientata). No sources of funding were received for the preparation of this article.
Disclosure
Professor Emanuel Raschi reports personal fees from Novartis for Consultancy, outside the submitted work. Prof. Dr. Luciano Potena reports personal fees from Novartis, personal fees from Biotest, personal fees from AstraZeneca, personal fees from Abbott, personal fees from Sandoz, outside the submitted work. The author reports no other conflicts of interest relevant to this work.
References
- 1.Papp Z, Agostoni P, Alvarez J, et al. Levosimendan efficacy and safety: 20 years of SIMDAX in clinical use. Card Fail Rev. 2020;6:e19. doi: 10.15420/cfr.2020.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gordon AC, Perkins GD, Singer M, et al. Levosimendan for the prevention of acute organ dysfunction in sepsis. N Engl J Med. 2016;375(17):1638–1648. doi: 10.1056/NEJMoa1609409 [DOI] [PubMed] [Google Scholar]
- 3.Antcliffe DB, Santhakumaran S, Orme RML, et al. Levosimendan in septic shock in patients with biochemical evidence of cardiac dysfunction: a subgroup analysis of the LeoPARDS randomised trial. Intensive Care Med. 2019;45:1392–1400. doi: 10.1007/s00134-019-05731-w [DOI] [PubMed] [Google Scholar]
- 4.European Medicines Agency. EU/3/18/1980. Available from: https://www.ema.europa.eu/en/medicines/human/orphan-designations/eu3181980. Accessed March29, 2021.
- 5.Al-Chalabi A, Heunks LMA, Papp Z, Pollesello P. Potential of the cardiovascular drug levosimendan in the management of amyotrophic lateral sclerosis: an overview of a working hypothesis. J Cardiovasc Pharmacol. 2019;74:389–399. doi: 10.1097/FJC.0000000000000728 [DOI] [PubMed] [Google Scholar]
- 6.Antila S, Sundberg S, Lehtonen LA. Clinical pharmacology of levosimendan. Clin Pharmacokinet. 2007;46(7):535–552. doi: 10.2165/00003088-200746070-00001 [DOI] [PubMed] [Google Scholar]
- 7.Cleland JGF, Nikitin N, James M. Levosimendan: first in a new class of inodilator for acute and chronic severe heart failure. Expert Rev Cardiovasc Ther. 2004;2:9–19. doi: 10.1586/14779072.2.1.9 [DOI] [PubMed] [Google Scholar]
- 8.Puttonen J, Kantele S, Kivikko M, et al. Effect of severe renal failure and haemodialysis on the pharmacokinetics of levosimendan and its metabolites. Clin Pharmacokinet. 2007;46(3):235–246. doi: 10.2165/00003088-200746030-00004 [DOI] [PubMed] [Google Scholar]
- 9.Follath F, Cleland JG, Just H, et al. Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the LIDO study): a randomised double-blind trial. Lancet. 2002;360(9328):196–202. doi: 10.1016/S0140-6736(02)09455-2 [DOI] [PubMed] [Google Scholar]
- 10.Mebazaa A, Nieminen MS, Packer M, et al. Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE randomized trial. JAMA. 2007;297(17):1883–1891. doi: 10.1001/jama.297.17.1883 [DOI] [PubMed] [Google Scholar]
- 11.Figgitt DP, Gillies PS, Goa KL. Levosimendan. Drugs. 2001;61:613–627; discussion 628–9. doi: 10.2165/00003495-200161050-00006 [DOI] [PubMed] [Google Scholar]
- 12.Antoniades C, Tousoulis D, Koumallos N, Marinou K, Stefanadis C. Levosimendan: beyond its simple inotropic effect in heart failure. Pharmacol Ther. 2007;114(2):184–197. doi: 10.1016/j.pharmthera.2007.01.008 [DOI] [PubMed] [Google Scholar]
- 13.Gong B, Li Z, Yat Wong PC. Levosimendan treatment for heart failure: a systematic review and meta-analysis. J Cardiothorac Vasc Anesth. 2015;29(6):1415–1425. doi: 10.1053/j.jvca.2015.03.023 [DOI] [PubMed] [Google Scholar]
- 14.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 15.Yilmaz MB, Grossini E, Silva Cardoso JC, et al. Renal effects of levosimendan: a consensus report. Cardiovasc Drugs Ther. 2013;27(6):581–590. doi: 10.1007/s10557-013-6485-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fedele F, Bruno N, Brasolin B, Caira C, D’Ambrosi A, Mancone M. Levosimendan improves renal function in acute decompensated heart failure: possible underlying mechanisms. Eur J Heart Fail. 2014;16(3):281–288. doi: 10.1002/ejhf.9 [DOI] [PubMed] [Google Scholar]
- 17.Lannemyr L, Ricksten S-E, Rundqvist B, et al. Differential effects of levosimendan and dobutamine on glomerular filtration rate in patients with heart failure and renal impairment: a randomized double-blind controlled trial. J Am Heart Assoc. 2018;7:e008455. doi: 10.1161/JAHA.117.008455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nieminen MS, Akkila J, Hasenfuss G, et al. Hemodynamic and neurohumoral effects of continuous infusion of levosimendan in patients with congestive heart failure. J Am Coll Cardiol. 2000;36(6):1903–1912. doi: 10.1016/S0735-1097(00)00961-X [DOI] [PubMed] [Google Scholar]
- 19.Slawsky MT, Colucci WS, Gottlieb SS, et al. Acute hemodynamic and clinical effects of levosimendan in patients with severe heart failure. Circulation. 2000;102(18):2222–2227. doi: 10.1161/01.CIR.102.18.2222 [DOI] [PubMed] [Google Scholar]
- 20.Moiseyev VS, Põder P, Andrejevs N, et al. Safety and efficacy of a novel calcium sensitiser, levosimendan, in patients with left ventricular failure due to an acute myocardial infarction: a randomized, placebo-controlled, double-blind study (RUSSLAN). Eur Heart J. 2002;23:1422–1432. doi: 10.1053/euhj.2001.3158 [DOI] [PubMed] [Google Scholar]
- 21.Packer M, Colucci W, Fisher L, et al. Effect of levosimendan on the short-term clinical course of patients with acutely decompensated heart failure. JACC Heart Fail. 2013;1:103–111. PMID: 12133653. doi: 10.1016/s0140-6736(02)09455-2 [DOI] [PubMed] [Google Scholar]
- 22.Landoni G, Biondi-Zoccai G, Greco M, et al. Effects of levosimendan on mortality and hospitalization. A metaanalysis of randomized controlled studies. Crit Care Med. 2012;40:634–646. doi: 10.1097/CCM.0b013e318232962a [DOI] [PubMed] [Google Scholar]
- 23.Mebazaa A, Nieminen MS, Filippatos GS, et al. Levosimendan vs. dobutamine: outcomes for acute heart failure patients on beta-blockers in SURVIVE. Eur J Heart Fail. 2009;11:304–311. doi: 10.1093/eurjhf/hfn045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bergh CH, Andersson B, Dahlstrom U, et al. Intravenous levosimendan vs. dobutamine in acute decompensated heart failure patients on beta-blockers. Eur J Heart Fail. 2010;12:404–410. doi: 10.1093/eurjhf/hfq032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nieminen MS, Buerke M, Cohen-Solál A, et al. The role of levosimendan in acute heart failure complicating acute coronary syndrome: a review and expert consensus opinion. Int J Cardiol. 2016;218:150–157. doi: 10.1016/j.ijcard.2016.05.009 [DOI] [PubMed] [Google Scholar]
- 26.Sonntag S, Sundberg S, Lehtonen LA, Kleber FX. The calcium sensitizer levosimendan improves the function of stunned myocardium after percutaneous transluminal coronary angioplasty in acute myocardial ischemia. J Am Coll Cardiol. 2004;43(12):2177–2182. doi: 10.1016/j.jacc.2004.02.052 [DOI] [PubMed] [Google Scholar]
- 27.De Luca L, Proietti P, Celotto A, et al. Levosimendan improves hemodynamics and coronary flow reserve after percutaneous coronary intervention in patients with acute myocardial infarction and left ventricular dysfunction. Am Heart J. 2005;150(3):563–568. doi: 10.1016/j.ahj.2004.10.013 [DOI] [PubMed] [Google Scholar]
- 28.Jia Z, Guo M, Zhang YQ, Liang HQ, Zhang LY, Song Y. Efficacy of intravenous levosimendan in patients with heart failure complicated by acute myocardial infarction. Cardiology. 2014;128:195–201. doi: 10.1159/000357864 [DOI] [PubMed] [Google Scholar]
- 29.Husebye T, Eritsland J, Müller C, et al. Levosimendan in acute heart failure following primary percutaneous coronary intervention-treated acute ST-elevation myocardial infarction. Results from the LEAF trial: a randomized, placebo-controlled study. Eur J Heart Fail. 2013;15(5):565–572. doi: 10.1093/eurjhf/hfs215 [DOI] [PubMed] [Google Scholar]
- 30.Hansen MS, Andersen A, Nielsen-Kudsk JE. Levosimendan in pulmonary hypertension and right heart failure. Pulm Circ. 2018;8(3):2045894018790905. doi: 10.1177/2045894018790905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parissis JT, Paraskevaidis I, Bistola V, et al. Effects of levosimendan on right ventricular function in patients with advanced heart failure. Am J Cardiol. 2006;98(11):1489–1492. doi: 10.1016/j.amjcard.2006.06.052 [DOI] [PubMed] [Google Scholar]
- 32.Russ MA, Prondzinsky R, Carter JM, et al. Right ventricular function in myocardial infarction complicated by cardiogenic shock: improvement with levosimendan. Crit Care Med. 2009;37(12):3017–3023. doi: 10.1097/CCM.0b013e3181b0314a [DOI] [PubMed] [Google Scholar]
- 33.Poelzl G, Zwick RH, Grander W, et al. Safety and effectiveness of levosimendan in patients with predominant right heart failure. Herz. 2008;33:368–373. doi: 10.1007/s00059-008-3051-2 [DOI] [PubMed] [Google Scholar]
- 34.Qiu J, Jia L, Hao Y, et al. Efficacy and safety of levosimendan in patients with acute right heart failure: a meta-analysis. Life Sci. 2017;184:30–36. doi: 10.1016/j.lfs.2017.07.001 [DOI] [PubMed] [Google Scholar]
- 35.Mebazaa A, Parissis J, Porcher R, et al. Short-term survival by treatment among patients hospitalized with acute heart failure: the global ALARM-HF registry using propensity scoring methods. Intensive Care Med. 2011;37:290–301. doi: 10.1007/s00134-010-2073-4 [DOI] [PubMed] [Google Scholar]
- 36.Mebazaa A, Motiejunaite J, Gayat E, et al; Long-Term Registry Investigators. Long-term safety of intravenous cardiovascular agents in acute heart failure: results from the European society of cardiology heart failure long-term registry. Eur J Heart Fail. 2018;20:332–341. doi: 10.1002/ejhf.991 [DOI] [PubMed] [Google Scholar]
- 37.Fuhrmann JT, Schmeisser A, Schulze MR, et al. Levosimendan is superior to enoximone in refractory cardiogenic shock complicating acute myocardial infarction. Crit Care Med. 2008;36(8):2257–2266. doi: 10.1097/CCM.0b013e3181809846 [DOI] [PubMed] [Google Scholar]
- 38.García-González MJ, Domínguez-Rodríguez A, Ferrer-Hita JJ, et al. Cardiogenic shock after primary percutaneous coronary intervention: effects of levosimendan compared with dobutamine on haemodynamics. Eur J Heart Fail. 2006;8(7):723–728. doi: 10.1016/j.ejheart.2006.01.007 [DOI] [PubMed] [Google Scholar]
- 39.Dominguez-Rodriguez A, Samimi-Fard S, Garcia-Gonzalez MJ, et al. Effects of levosimendan versus dobutamine on left ventricular diastolic function in patients with cardiogenic shock after primary angioplasty. Int J Cardiol. 2008;128(2):214–217. doi: 10.1016/j.ijcard.2007.05.018 [DOI] [PubMed] [Google Scholar]
- 40.Samimi-Fard S, García-González MJ, Domínguez-Rodríguez A, et al. Effects of levosimendan versus dobutamine on longterm survival of patients with cardiogenic shock after primary coronary angioplasty. Int J Cardiol. 2008;127:284–287. doi: 10.1016/j.ijcard.2007.04.143 [DOI] [PubMed] [Google Scholar]
- 41.Christoph A, Prondzinsky R, Russ M, et al. Early and sustained haemodynamic improvement with levosimendan compared to intraaortic balloon counterpulsation (IABP) in cardiogenic shock complicating acute myocardial infarction. Acute Card Care. 2008;10:49–57. doi: 10.1080/17482940701358564 [DOI] [PubMed] [Google Scholar]
- 42.Omerovic E, Råmunddal T, Albertsson P, et al. Levosimendan neither improves nor worsens mortality in patients with cardiogenic shock due to ST-elevation myocardial infarction. Vasc Health Risk Manag. 2010;6:657–663. doi: 10.2147/VHRM.S8856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Russ MA, Prondzinsky R, Christoph A, et al. Hemodynamic improvement following levosimendan treatment in patients with acute myocardial infarction and cardiogenic shock. Crit Care Med. 2007;35:2732–2739. [DOI] [PubMed] [Google Scholar]
- 44.Schumann J, Henrich EC, Strobl H, et al. Inotropic agents and vasodilator strategies for the treatment of cardiogenic shock or low cardiac output syndrome. Cochrane Database Syst Rev. 2018;1:CD009669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fang M, Cao H, Wang Z. Levosimendan in patients with cardiogenic shock complicating myocardial infarction: a meta-analysis. Med Int. 2018;42:409–415. [DOI] [PubMed] [Google Scholar]
- 46.Chioncel O, Parissis J, Mebazaa A, et al. Epidemiology, pathophysiology and contemporary management of cardiogenic shock - a position statement from the Heart Failure Association of the European society of cardiology. Eur J Heart Fail. 2020;22:1315–1341. doi: 10.1002/ejhf.1922 [DOI] [PubMed] [Google Scholar]
- 47.Lyon AR, Bossone E, Schneider B, et al. Current state of knowledge on takotsubo syndrome: a position statement from the taskforce on takotsubo syndrome of the Heart Failure Association of the European society of cardiology. Eur J Heart Fail. 2016;18:8–27. doi: 10.1002/ejhf.424 [DOI] [PubMed] [Google Scholar]
- 48.Hering D, Jaguszewski M. Levosimendan: new hope therapy for takotsubo syndrome. Cardiol J. 2016;23(6):616–617. doi: 10.5603/CJ.2016.0101 [DOI] [PubMed] [Google Scholar]
- 49.De Santis V, Vitale D, Tritapepe L, et al. Use of levosimendan for cardiogenic shock in a patient with the apical ballooning syndrome. Ann Intern Med. 2008;149(5):365–367. doi: 10.7326/0003-4819-149-5-200809020-00028 [DOI] [PubMed] [Google Scholar]
- 50.Abrams D, Combes A, Brodie D. Extracorporeal membrane oxygenation in cardiopulmonary disease in adults. J Am Coll Cardiol. 2014;63(25):2769–2778. doi: 10.1016/j.jacc.2014.03.046 [DOI] [PubMed] [Google Scholar]
- 51.Affronti A, Di Bella I, Carino D, Ragni T. Levosimendan may improve weaning outcomes in venoarterial ECMO patients. ASAIO J. 2013;59:554–557. doi: 10.1097/MAT.0b013e3182a4b32e [DOI] [PubMed] [Google Scholar]
- 52.Sangalli F, Avalli L, Laratta M, et al. Effects of levosimendan on endothelial function and hemodynamics during weaning from veno-arterial extracorporeal life support. J Cardiothorac Vasc Anesth. 2016;30(6):1449–1453. doi: 10.1053/j.jvca.2016.03.139 [DOI] [PubMed] [Google Scholar]
- 53.Jacky A, Rudiger A, Krüger B, et al. Comparison of levosimendan and milrinone for ECLS weaning in patients after cardiac surgery – a retrospective before and after study. J Cardiothorac Vasc Anesth. 2018;32:2112–2119. doi: 10.1053/j.jvca.2018.04.019 [DOI] [PubMed] [Google Scholar]
- 54.Distelmaier K, Roth C, Schrutka L, et al. Beneficial effects of levosimendan on survival in patients undergoing extracorporeal membrane oxygenation after cardiovascular surgery. Br J Anaesth. 2016;117:52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vally S, Ferdynus C, Persichini R, et al. Impact of levosimendan on weaning from peripheral venoarterial extracorporeal membrane oxygenation in intensive care unit. Ann Intensive Care. 2019;9(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guilherme E, Jacquet-Lagrèze M, Pozzi M, Achana F, Armoiry X, Fellahi JL. Can levosimendan reduce ECMO weaning failure in cardiogenic shock?: a cohort study with propensity score analysis. Crit Care. 2020;24:442. doi: 10.1186/s13054-020-03122-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alonso-Fernandez-Gatta M, Merchan-Gomez S, Gonzalez-Cebrian M, et al. Levosimendan in veno-arterial extracorporeal membrane oxygenator supported patients: impact on the success of weaning and survival. Artif Organs. 2021;45:717–725. doi:10.1111/aor.13899 [DOI] [PubMed] [Google Scholar]
- 58.Pirracchio R, Parenica J, Resche Rigon M, et al; GREAT network. The effectiveness of inodilators in reducing short term mortality among patient with severe cardiogenic shock: a propensity-based analysis. PLoS One. 2013;8(8):e71659. Erratum in: PLoS One. 2013; 8(12). doi: 10.1371/journal.pone.0071659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tarvasmäki T, Lassus J, Varpula M, et al; CardShock study investigators. Current real-life use of vasopressors and inotropes in cardiogenic shock - adrenaline use is associated with excess organ injury and mortality. Crit Care. 2016;20(1):208. doi: 10.1186/s13054-016-1387-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pölzl G, Altenberger J, Baholli L, et al. Repetitive use of levosimendan in advanced heart failure: need for stronger evidence in a field in dire need of a useful therapy. Int J Cardiol. 2017;243:389–395. doi: 10.1016/j.ijcard.2017.05.081 [DOI] [PubMed] [Google Scholar]
- 61.Altenberger J, Parissis JT, Costard-Jaeckle A, et al. Efficacy and safety of the pulsed infusions of levosimendan in outpatients with advanced heart failure (LevoRep) study: a multicentre randomized trial. Eur J Heart Fail. 2014;16:898–906. doi: 10.1002/ejhf.118 [DOI] [PubMed] [Google Scholar]
- 62.Comín-Colet J, Manito N, Segovia-Cubero J, et al. Efficacy and safety of intermittent intravenous outpatient administration of levosimendan in patients with advanced heart failure: the LION-HEART multicentre randomised trial. Eur J Heart Fail. 2018;20(7):1128–1136. doi: 10.1002/ejhf.1145 [DOI] [PubMed] [Google Scholar]
- 63.LAICA Study Investigators. Efficacy and security of intermittent repeated levosimendan administration in patients with advanced heart failure: a randomized, double-blind, placebo controlled multicenter trial: LAICA study. Presented at the European Society of Cardiology–Heart Failure Association Congress; May 21; 2016; Florence, Italy. [Google Scholar]
- 64.Thorvaldsen T, Benson L, Hagerman I, Dahlström U, Edner M, Lund LH. Planned repetitive use of levosimendan for heart failure in cardiology and internal medicine in Sweden. Int J Cardiol. 2014;175:55–61. [DOI] [PubMed] [Google Scholar]
- 65.Oliva F, Perna E, Marini M, et al; RELEVANT-HF study group. Scheduled intermittent inotropes for ambulatory advanced heart failure. The RELEVANT-HF multicentre collaboration. Int J Cardiol. 2018;272:255–259. [DOI] [PubMed] [Google Scholar]
- 66.Pölzl G, Allipour Birgani S, Comín-Colet J, et al. Repetitive levosimendan infusions for patients with advanced chronic heart failure in the vulnerable post-discharge period. ESC Heart Fail. 2019;6:174–181. doi: 10.1002/ehf2.12366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mebazaa A, Pitsis AA, Rudiger A, et al. Clinical review: practical recommendations on the management of perioperative heart failure in cardiac surgery. Crit Care. 2010;14(2):201. doi: 10.1186/cc8153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harrison RW, Hasselblad V, Mehta RH, et al. Effect of levosimendan on survival and adverse events after cardiac surgery: a meta-analysis. J Cardiothorac Vasc Anesth. 2013;27(6):1224–1232. doi: 10.1053/j.jvca.2013.03.027 [DOI] [PubMed] [Google Scholar]
- 69.Sanfilippo F, Knight JB, Scolletta S, et al. Levosimendan for patients with severely reduced left ventricular systolic function and/or low cardiac output syndrome undergoing cardiac surgery: a systematic review and meta-analysis. Crit Care. 2017;21(1):252. doi: 10.1186/s13054-017-1849-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cholley B, Caruba T, Grosjean S, et al. Effect of levosimendan on low cardiac output syndrome in patients with low ejection fraction undergoing coronary artery bypass grafting with cardiopulmonary bypass: the LICORN randomized clinical trial. JAMA. 2017;318(6):548–556. doi: 10.1001/jama.2017.9973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mehta RH, Leimberger JD, van Diepen S, et al; LEVO-CTS Investigators. Levosimendan in patients with left ventricular dysfunction undergoing cardiac surgery. N Engl J Med. 2017;376(21):2032–2042. doi: 10.1056/NEJMoa1616218 [DOI] [PubMed] [Google Scholar]
- 72.Landoni G, Lomivorotov VV, Alvaro G, et al. Levosimendan for hemodynamic support after cardiac surgery. N Engl J Med. 2017;376:2021–2031. [DOI] [PubMed] [Google Scholar]
- 73.Putzu A, Clivio S, Belletti A, Cassina T. Perioperative levosimendan in cardiac surgery: a systematic review with meta-analysis and trial sequential analysis. Int J Cardiol. 2018;251:22–31. doi: 10.1016/j.ijcard.2017.10.077 [DOI] [PubMed] [Google Scholar]
- 74.Guarracino F, Heringlake M, Cholley B, et al. Use of levosimendan in cardiac surgery: an update after the LEVOCTS, CHEETAH, and LICORN trials in the light of clinical practice. J Cardiovasc Pharmacol. 2018;71:1–9. doi: 10.1097/FJC.0000000000000551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Habicher M, Zajonz T, Heringlake M, et al. S3-Leitlinie zur intensivmedizinischen Versorgung herzchirurgischer Patienten: Hämodynamisches Monitoring und Herz-Kreislauf – ein Update [S3 guidelines on intensive medical care of cardiac surgery patients: hemodynamic monitoring and cardiovascular system-an update]. Anaesthesist. 2018;67:375–379. German. [DOI] [PubMed] [Google Scholar]
- 76.Wang W, Zhou X, Liao X, Liu B, Yu H. The efficacy and safety of prophylactic use of levosimendan on patients undergoing coronary artery bypass graft: a systematic review and meta-analysis. J Anesth. 2019;33:543–550. doi: 10.1007/s00540-019-02643-3 [DOI] [PubMed] [Google Scholar]
- 77.van Diepen S, Mehta RH, Leimberger JD, et al. Levosimendan in patients with reduced left ventricular function undergoing isolated coronary or valve surgery. J Thorac Cardiovasc Surg. 2020;159(6):2302–2309.e6. doi: 10.1016/j.jtcvs.2019.06.020 [DOI] [PubMed] [Google Scholar]
- 78.Theiss HD, Grabmaier U, Kreissl N, et al. Preconditioning with levosimendan before implantation of left ventricular assist devices. Artif Organs. 2014;38(3):231–234. doi: 10.1111/aor.12150 [DOI] [PubMed] [Google Scholar]
- 79.Kocabeyoglu SS, Kervan U, Sert DE, et al. Optimization with levosimendan improves outcomes after left ventricular assist device implantation. Eur J Cardiothorac Surg. 2020;57(1):176–182. doi: 10.1093/ejcts/ezz159 [DOI] [PubMed] [Google Scholar]
- 80.Treskatsch S, Balzer F, Geyer T, et al. Early levosimendan administration is associated with decreased mortality after cardiac surgery. J Crit Care. 2015;30(4):859.e1–6. doi: 10.1016/j.jcrc.2015.03.008 [DOI] [PubMed] [Google Scholar]
- 81.Ávalos R, MartinezSanz R, Jiménez J, et al. Levosimendan preconditioning in patients undergoing elective cardiac surgery with poor ejection fraction. preliminary results. J Cardiothorac Surg. 2015;10:A310. doi: 10.1186/1749-8090-10-S1-A310 [DOI] [Google Scholar]
- 82.Heringlake M, Alvarez J, Bettex D, et al. An update on levosimendan in acute cardiac care: applications and recommendations for optimal efficacy and safety. Expert Rev Cardiovasc Ther. 2021;19(4):325–335. doi: 10.1080/14779072.2021.1905520 [DOI] [PubMed] [Google Scholar]