Abstract
Background
Evidence from single and multicenter phase II trials have suggested diffusion MRI is a predictive imaging biomarker for survival benefit in recurrent glioblastoma (rGBM) treated with anti-VEGF therapy. The current study confirms these findings in a large, randomized phase III clinical trial.
Methods
Patients with rGBM were enrolled in a phase III randomized (1:1), controlled trial (NCT02511405) to compare the efficacy and safety of bevacizumab (BV) versus BV in combination with ofranergene obadenovec (BV+VB-111), an anti-cancer viral therapy. In 170 patients with diffusion MRI available, pretreatment enhancing tumor volume and ADC histogram analysis were used to phenotype patients as having high (>1.24 µm2/ms) or low (<1.24 µm2/ms) ADCL, the mean value of the lower peak of the ADC histogram, within the contrast enhancing tumor.
Results
Baseline tumor volume (P = .3460) and ADCL (P = .2143) did not differ between treatment arms. Univariate analysis showed patients with high ADCL had a significant survival advantage in all patients (P = .0006), as well as BV (P = .0159) and BV+VB-111 individually (P = .0262). Multivariable Cox regression accounting for treatment arm, age, baseline tumor volume, and ADCL identified continuous measures of tumor volume (P < .0001; HR = 1.0212) and ADCL phenotypes (P = .0012; HR = 0.5574) as independent predictors of OS.
Conclusion
Baseline diffusion MRI and tumor volume are independent imaging biomarkers of OS in rGBM treated with BV or BV+VB-111.
Keywords: anti-VEGF therapy, bevacizumab, diffusion MRI, imaging biomarker, recurrent GBM, VB-111
Key Points.
Pretreatment volume and ADC were significant predictors of overall survival in rGBM treated with anti-VEGF treatment.
Patients with a high ADC prior to bevacizumab +/- VB-111 have 2–3 months additional median survival (HR = 0.55).
Importance of the Study.
Bevacizumab, an antiangiogenic drug and the U.S. standard of care for rGBM, has demonstrated a PFS benefit, but has not shown an advantage in OS. Overwhelming evidence from single center and multicenter phase II data suggests patients with a higher apparent diffusion coefficient (ADC) within their tumors have a significant OS benefit when treated with anti-VEGF therapy, including bevacizumab. The current study validates this observation in a randomized, controlled phase III trial of rGBM treated with bevacizumab with or without VB-111, a novel antiangiogenic treatment. Results suggest diffusion MRI, which is recommended as part of the standardized brain tumor imaging protocol, may be a valuable tool for determining survival benefit in rGBM patients considering bevacizumab or other antiangiogenic therapies.
Despite promising initial data and widespread exploration of anti-VEGF therapies in recurrent GBM, randomized phase II trials have not demonstrated an overall survival (OS) benefit for all patients with recurrent GBM.1–3 We have demonstrated retrospectively in both single center4–7 and multicenter phase II trials8–10 that recurrent GBM patients with distinct diffusion MR imaging features have a significant survival benefit when treated with bevacizumab. We have demonstrated that measurements of the apparent diffusion coefficient (ADC) within the area of contrast enhancement on T1-weighted images can be modeled using a double Gaussian mixed model where ADCL is the mean value of the lower Gaussian distribution, reflecting the more densely cellular component of the tumor, and ADCH is the mean value of the higher Gaussian distribution, more representative of tumor tissue containing more necrosis, edema, or cerebrospinal contamination (Figure 1B–G). Data in rGBM treated with a wide range of anti-VEGF therapies (eg, cediranib [NCT00035656]; bevacizumab (BRAIN Trial, AVF3708g; NCT00345163); cabozantinib (XL184-201; NCT00704288); and aflibercept (VEGF Trap; NCT00369590) suggests patients with tumors exhibiting an ADCL higher than 1.24 µm2/ms on pretreatment diffusion MR images have a significant survival benefit compared with patients who have a lower ADCL, even after controlling for known prognostic variables including age and baseline tumor size.8 This was independently confirmed8 using data from BELOB,3 which showed ADCL and tumor volume to be predictive for patients treated with bevacizumab monotherapy, but not single agent lomustine. Furthermore, a recent post-hoc analysis of the EORTC-26101 trial by Schell et al.11 confirmed our findings, showing that ADCL using a threshold of 1.24 µm2/ms is prognostic for both progression-free survival (PFS) and OS in rGBM patients treated with bevacizumab (10.4 vs 8.1 months median OS, P = .0004).
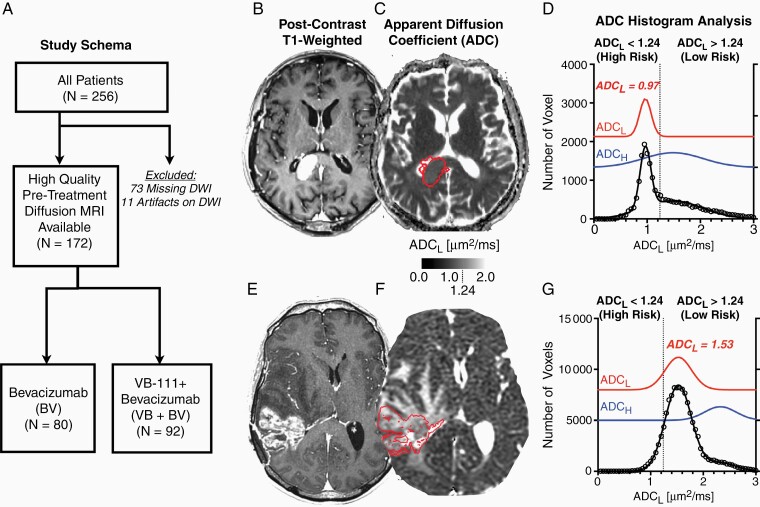
Figure 1.
Study schema and ADC histogram analyses for representative rGBM patients. (A) Study schema showing initial study size (N = 256), for which 84 patients were excluded for lack of high-quality pretreatment diffusion MRI data (73 missing data and 11 with significant artifacts), leaving N = 172 patients for this imaging sub study. Of these 172 patients, N = 80 were randomized to the bevacizumab monotherapy arm (BV) and 92 were randomized to the combination of VB-111 and bevacizumab (VB+BV). (B) Post-contrast T1-weighted, (C) apparent diffusion coefficient (ADC) map, and (D) ADC histograms for a patient with low ADCL, or high risk (ADCL = 0.97 µm2/ms). (E) Post-contrast T1-weighted, (F) ADC map, and (G) ADC histograms for a patient with high ADCL, or low risk (ADCL = 1.53 µm2/ms).
Ofranergene obadenovec (VB-111),12,13 a viral gene therapy that triggers apoptosis of tumor vascular endothelium, is a novel antiangiogenic therapy and an alternative to traditional anti-VEGF therapies. Phase II studies of VB-111 have shown to increase survival in rGBM when used prior to treatment with bevacizumab.13 In the current study, we examine whether diffusion MR imaging phenotypes in tumors included in the GLOBE trial,12 a randomized controlled phase III study of VB-111 combined with bevacizumab versus bevacizumab monotherapy in patients with recurrent glioblastoma.12 Using this study, we aim to further validate the ability of diffusion MR phenotypes to predict survival in patients treated with bevacizumab as well as combined antiangiogenic therapy consisting of bevacizumab and VB-111.
Methods
Study Objectives
This study was approved by UCLA institutional review board (#10-000655; #14-001261). We retrospectively reviewed clinical, imaging, and survival data from a phase III randomized (1:1), controlled trial (NCT02511405) aimed to compare efficacy of bevacizumab alone versus bevacizumab with ofranergene obadenovec (VB-111) in recurrent glioblastoma to determine whether diffusion MRI and other factors were independent prognostic factors for OS.
Patient Eligibility
Eligible participants were adults aged >18 years with first or second progression of histologically confirmed GBM, who had received previous treatment with standard of care radiotherapy and temozolomide. Additionally, patients needed to have a KPS of at least 70%, life expectancy of at least 3 months, an interval of at least 12 weeks since the cessation of radiotherapy, and measurable lesion by RANO.28 Exclusion criteria included prior antiangiogenic therapy, history of recent grade 2 or higher CNS hemorrhage, gastrointestinal bleeding or pulmonary hemorrhage/hemoptysis, inherited bleeding diathesis or significant coagulopathy at risk of bleeding, surgical treatment or significant trauma within 4 weeks, active vascular disease, proliferative and/or vascular retinopathy, inadequately controlled hypertension, history of gastrointestinal perforation or abscess.
Study Design
This was a phase III multisite, international, randomized, open-labeled, controlled trial. Study design and treatment regimens were determined in agreement with an FDA Special Protocol Assessment (SPA). Eligible patients with rGBM were randomized 1:1 to receive either VB-111 1 × 1013 VPs every 8 weeks in combination with bevacizumab 10 mg/kg every 2 weeks (VB+BV) or bevacizumab monotherapy 10 mg/kg every 2 weeks (BV). Treatment assignment was determined by central randomization, and was stratified by age, KPS and first or second progression. Disease characteristics, including local assessment of the prognostic factors MGMT Methylation, EGFRvIII and IDH-1 mutation, were collected from patients’ medical history (if available). Primary endpoint was OS, defined as the time from randomization until death from any cause.
Upon evidence of PD by RANO (defined as ≥25% increase in the sum of enhancing lesions diameters) continuation or discontinuation of study therapy was decided per physician’s discretion, as long as the patient did not have increase in tumor measurements >50% or any confirmed T2/FLAIR and/or clinical deterioration. All patients who discontinued study drug were treated according to standard of care, and there was no cross-over from bevacizumab monotherapy to VB-111. All efforts were made to collect post-study MRIs, health related quality of life measures, follow-up of anti-cancer treatments and survival data every 2–3 months until the patient expired. Dose reductions of VB-111 and bevacizumab were not allowed. Repeat VB-111 dosing was delayed for patients who experienced a drug related AE, until the severity of the event was no more than CTCAE Grade 1. All patients received concomitant pre-dose acetaminophen to mitigate post-treatment fever, and pre-dose dexamethasone (10 mg) followed by 4 mg twice daily for 3 days post-dosing to prevent potential cerebral edema.
Magnetic Resonance Imaging
For the current study, only baseline (pretreatment) MRI scans were used. All MRI scans were obtained on a 1.5T or 3T MR scanner and were compliant with the standardized brain tumor imaging protocol,14 including 1–1.5 mm isotropic pre- and post-contrast T1-weighted images, T2-weighted turbo spin echo and T2-weighted FLAIR images, and diffusion-weighted images with at least b = 0 and 1000 s/mm2 and slice thickness ≤4 mm.
T1 Subtraction Maps
Contrast enhanced T1-weighted digital subtraction maps were used to insulate areas of contrast enhancement and exclude blood products and necrotic regions within the tumor using previously described techniques.15–17 Briefly, T1-weighted images with and without contrast were co-registered, intensity normalized, then subtracted from each other on a voxel-wise basis to highlight the change in signal intensity due to contrast administration. Regions with positive values were segmented and used for ADC histogram analysis.
ADC Histogram Analysis
ADC histogram analysis was applied using standard techniques described elsewhere.4,6,8,9,18 Simply, histograms were generated from ADC values extracted from contrast enhancing regions from T1 subtraction maps were generated, with average histogram bin size of 0.05 µm2/ms. Then, nonlinear regression was performed using a double Gaussian model defined as: , where p(ADC) is the probability of a specific ADC value, f is the proportion of voxels in the histogram, N(μ,σ) is a normal Gaussian distribution with mean = μ and standard deviation = σ, and ADCL is mean of the lower Gaussian distribution and ADCH is mean of the higher Gaussian distribution. From this, ADCL was used for further analyses.
Statistical Analysis
All analysis was carried out in MATLAB or using GraphPad Prism v9.0.1 (GraphPad Software). Baseline volume and ADCL were compared using unpaired t-tests. Overall survival between different diffusion phenotypes as well as between different treatment groups were evaluated using cox regression survival analysis. Multivariate cox regression analysis including age, pretreatment tumor volume, ADCL, and treatment arm was carried out to evaluate independent predictors of overall survival. P values of < 0.05 were considered significant.
Results
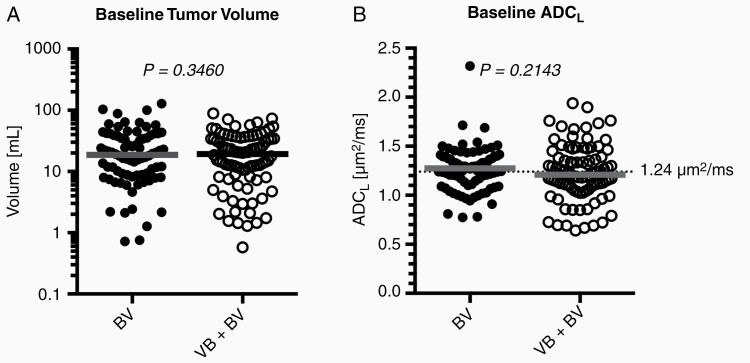
A total of 172 of the original 256 patients with recurrent glioblastoma enrolled in the phase III randomized controlled trial (NCT02511405) had available pretreatment diffusion imaging and were included in this study (Figure 1A). Of these patients, 92 of original 128 patients were randomized to VB+BV and had adequate diffusion imaging available (32 missing diffusion data and 4 with significant artifacts by quality control procedures described previously19), while 80 of the original 128 patients were randomized to BV and had adequate imaging available (41 missing diffusion data and 7 with significant artifacts). Table 1 highlights the patient characteristics for patients included in the current study. Figure 1B–G illustrates representative patients with low and high ADCL. There was no significant difference in mean pretreatment contrast enhancing tumor volume between the 2 groups (Figure 2A; t-test, P = .3460; BV, mean volume = 26.2 mL; range 18.7–128.1 mL; VB+BV, mean volume = 23.0 mL; range 19.3–88.5 mL) Similarly, pretreatment ADCL values were not significantly different between the 2 groups (Figure 2B; t-test, P = 0.2143; BV, mean ADCL = 1.28 µm2/ms; range 0.78–3.03 µm2/ms; VB+BV, mean ADCL = 1.23 µm2/ms; range 0.64–1.94 µm2/ms). MGMT promoter methylation status was evenly split between ADCL high and low (20 vs 17 methylated, 34 vs 46 unmethylated, and 28 vs 27 missing or unknown). Similarly, IDH mutation status was also evenly distributed between ADCL high and low phenotypes (10 vs 12 mutated, 56 vs 58 wild type, 16 vs 20 missing or unknown.
Table 1.
Patient Characteristics for Imaging Sub Study of GLOBE Trial
| VB-111 + Bevacizumab | Bevacizumab | ||
|---|---|---|---|
| Characteristics | (VB+BV) (N = 92) | (BV) (N = 80) | P-Value |
| Mean age, y (SD) | 55.1 (11.3) | 54.4 (13.1) | .7072 |
| Sex | |||
| Male (%) | 60 (65%) | 55 (69%) | |
| Female (%) | 32 (35%) | 25 (31%) | |
| Baseline KPS (SD) | 83 (9.5) | 84 (9.3) | .4878 |
| Median Survival (Months) | 6.4 | 7.9 | .3471 |
Figure 2.
Baseline tumor volume and diffusion MRI measurements. (A) Comparison of pretreatment, baseline enhancing tumor volume (P = .3460) and (B) ADCL (P = .2143) between BV and VB+BV treatment arms showing no significant differences.
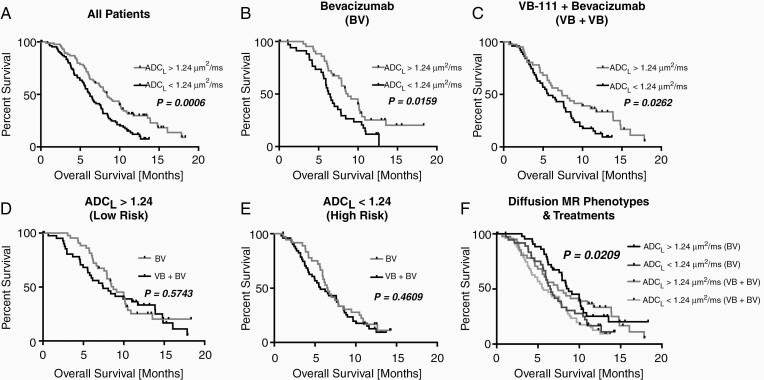
Pretreatment diffusion phenotypes as stratified by ADCL greater than or less than 1.24 µm2/ms were significantly associated with survival. Specifically, high ADCL (ADCL > 1.24 µm2/ms) was associated with increased overall survival relative to patients with low pretreatment ADCL when pooling all patients (Figure 3A; Log-rank, P = .0006; HR = 0.5726; median OS = 8.4 vs 6.2 months). The association of increased overall survival with high ADCL held true when examining patients within the BV (Figure 3B; Log-rank, P = .0159; HR = 0.5513; median OS = 8.7 vs 6.4 months) and VB+BV combination cohorts (Figure 3C; Log-rank, P = .0263; HR = 0.6175; median OS = 7.4 vs 5.7 months).
Figure 3.
Overall survival comparisons between treatment arms and diffusion characteristics. Kaplan–Meier plots showing higher overall survival in rGBM with ADCL > 1.24 µm2/ms compared with ADCL < 1.24 µm2/ms when examining (A) all patients (Log-rank, P = .0006), (B) patients in the BV arm (Log-rank, P = .0159), and (C) patients in the VB+BV arm (Log-rank, P = .0262). No significant difference between treatment groups was observed when looking within tumors exhibiting (D) ADCL > 1.24 µm2/ms (Low Risk; Log-rank, P = .5743) or (E) ADC < 1.24 µm2/ms (High Risk; Log-rank, P = .4609). (F) Comparison of survival between diffusion MR phenotypes and treatment groups, showing a significant trend (Log-rank test for trends, P = .0209).
In patients with high ADCL, which has been associated with higher overall survival in anti-VEGF treatment, there was no difference in overall survival by treatment strategy (Figure 3D; Log-rank, P = .5743; BV median OS = 8.7 months vs VB+BV median OS = 7.4 months). Similarly, in patients with low ADCL, which is associated with lower overall survival when treated with anti-VEGF therapies, there was no difference in overall survival by treatment strategy (Figure 3E; Log-rank, P = .4609; BV median OS = 6.5 months vs VB+BV median OS = 5.7 months). Overall, when comparing 4 distinct groups: (1) high ADCL & BV, (2) low ADCL & BV, (3) high ADCL & VB+BV, and (4) low ADCL & VB+BV there was a significant trend in survival outcomes (Figure 3F; Log-rank test for trends, P = .0209), likely dominated by diffusion phenotypes rather than treatment strategy. A multivariable cox regression accounting for treatment arm, age, baseline tumor volume, and ADCL identified continuous measures of tumor volume (Table 2; P < .0001; HR = 1.0212) and ADCL phenotypes (Table 2; P = .0012; HR = 0.5574) as independent predictors of overall survival.
Table 2.
Cox Multivariate Regression Results for Age, Treatment, Tumor Volume, and ADCL
| Variable | Coefficient | Hazard Ratio (HR) [95% CI] | P-Value |
|---|---|---|---|
| Age (years, continuous) | −0.0086 | 0.9914 [0.9765–1.0066] | .2660 |
| Treatment (BV vs VB+BV) | 0.1271 | 1.1356 [0.8042–1.6036] | .4702 |
| Tumor Volume (mL, continuous) | 0.0209 | 1.0212 [1.0122–1.0302] | <.0001 |
| ADCL Phenotype (High/Low) | −0.5844 | 0.5574 [0.3913–0.7940] | .0012 |
Discussion
Identifying effective therapies in recurrent GBM remains one of the most difficult challenges in neuro-oncology. Given that these patients may often not be eligible for repeat debulking surgery, personalized treatment strategies in the recurrent setting are significantly limited because of lack of tissue biomarkers to inform decision-making. While some molecular markers such as MGMT methylation status and IDH mutational harbor prognostic implications, these have a limited role in the setting of recurrent GBM and specifically in the setting of salvage therapies like anti-VEGF therapies. Thus, a pretreatment imaging biomarker that can be used to guide treatment decisions in rGBM is greatly desired. Results from the current study strongly support previous single institution4–7 and multicenter phase II trials8–11 showing that ADCL, using a threshold of 1.24 µm2/ms, within contrast enhancing tumor is an independent imaging biomarker for predicting overall survival benefit from anti-VEGF treatments, including combination therapies.
Whether diffusion MR phenotypes are predictive for anti-VEGF treatment or merely prognostic for all treatments in rGBM is still open to considerable debate. Previous single institution studies have suggested that diffusion MR phenotypes are predictive for survival benefit in anti-VEGF monotherapy, but not cytotoxic chemotherapies4 or surgical resection.7 This was independently confirmed8 in a separate cohort using data from BELOB,3 which showed ADCL to be predictive for patients treated with bevacizumab monotherapy, but not single agent lomustine. When this was tested post-hoc using data from EORTC-26101, Schell et al.11 found that ADCL using a similar threshold was prognostic for both bevacizumab and non-bevacizumab treated patients. However, unlike previous studies,4,8 35% of these “non-bevacizumab” patients crossed over and received bevacizumab treatment after disease progression, suggesting diffusion MR phenotypes may still be predictive to anti-VEGF treatment. Regardless, all these studies harmonize and together strongly support the hypothesis that pretreatment ADCL is a powerful tool for identifying patients that will have a favorable outcome to anti-VEGF treatment, including bevacizumab.
The biological basis of these diffusion MR phenotypes has also been under recent investigation.5,20 Specifically, we have noted that ADCL is significantly correlated with increased DNA, RNA, and protein expression of decorin, a small proteoglycan that both modulates angiogenesis and alters viscosity of the extracellular matrix, and this relationship is preserved in patient derived xenograft (PDX) models.5 Data suggest decorin may be a multifaceted antiangiogenic agent,21–24 as it interferes with thrombospondin-1,25 suppress endogenous tumor cell production of VEGF22,26 and VEGF-A,27 the therapeutic targets of bevacizumab, and binds with a high affinity to VEGFR1/2.28 Decorin also concurrently modulates the stiffness of the extracellular matrix by binding with various macromolecules and activating specific matrix metalloproteinases (MMPs),22 which may explain the relationship between increased decorin expression and increased ADCL, a measure of water mobility (or viscosity) within the tumor tissue. Studies aimed at identifying any causal associations between decorin expression, diffusion MR phenotypes, and anti-VEGF efficacy are yet to be conducted.
In addition, the molecular status of the tumor may also contribute to the specific diffusion MR phenotypes. For example, a TCGA study by Wu et al.29 suggested that newly diagnosed IDH mutant gliomas have significantly higher average ADC values within areas of bulk tumor compared with newly diagnosed IDH wild-type gliomas, irrespective of tumor grade. Also, a study by Han et al.30 suggests newly diagnosed MGMT methylated tumors may have a higher ADC measurement compared to unmethylated tumors; however, a larger and more comprehensive study with more than twice the patients suggested the opposite, with newly diagnosed MGMT methylated tumors exhibiting significantly lower ADC.31 While these other factors may have contributed a part in the underlying diffusion phenotype, these previous studies were in newly diagnosed gliomas and not recurrent disease. Additionally, the current study did not require IDH and MGMT status be obtained for all patients. Approximately 30% of patients in the VB+BV cohort and 25% of patients in the BV cohort did not have IDH status available, and 18% of patients on both arms did not have MGMT status available.12 Despite this, MGMT methylation status and IDH mutation status appeared evenly distributed across ADCL phenotypes. However, a proper accountability of these variables cannot be considered in the current study and is a potential limitation.
Conclusions
Results from the current phase III trial confirms that pretreatment contrast enhancing tumor volume and diffusion MR imaging phenotypes are independent predictors of overall survival in rGBM treated with bevacizumab with or without VB-111. Additionally, results suggest clinicians should consider using diffusion measurements within the enhancing tumor prior to bevacizumab treatment in order to estimate potential patient benefits.
Funding
This study was supported by VBL Therapeutics (Ellingson, Cloughesy); American Cancer Society (ACS) Research Scholar Grant [RSG-15-003-01-CCE] (Ellingson); American Brain Tumor Association (ABTA) Research Collaborators Grant [ARC1700002] (Ellingson); UCLA SPORE in Brain Cancer [NIH/NCI 1P50CA211015-01A1] (Ellingson, Liau, Nghiemphu, Lai, Pope, Cloughesy); NIH/NCI 1R21CA223757-01 (Ellingson).
Conflict of interest statement. Y.C., T.R.M., S.F.S., and N.L.S. are/were employees of VBL Therapeutics. B.M.E. is an advisor and paid consultant for Medicenna, MedQIA, Imaging Endpoints, VBL, Agios Pharmaceuticals, BBI, Siemens, Janssen and Neosoma. B.M.E. received grant funding from Siemens, Agios, VBL, and Janssen. T.F.C. is on the advisory board for Roche/Genentech, Amgen, Tocagen, NewGen, LPath, Proximagen, Celgene, Vascular Biogenics Ltd, Insys, Agios, Cortice Bioscience, Pfizer, Human Longevity, BMS, Merck, Notable Lab, and MedQIA. J.L.C. received research funding from NeoImmuneTech, Incyte and Merck. J.Fd.G. is a paid consultant for Del Mar Pharmaceuticals, Samus Therapeutics, Insigtec, Bioasis Technologies, Magnolia Innovation, Karyopharm Therapeutics, GlaxoSmithKline, ResTORbio, Roche, GenomiCare, and Merck. J.Fd.G. is on the advisory board for Prelude Therapeutics, Cure Brain Cancer Foundation, Head for the Cure, Merck, Sapience Therapeutics, Janssen, Kiyatec, and Novartis. P.Y.W. is on the advisory board of Agios, Astra Zeneca, Bayer, Boston Pharmaceuticals, CNS Pharmaceuticals, Elevate Bio Immunomic Therapeutics, Imvax, Karyopharm, Merck, Novartis, Nuvation Bio, Vascular Biogenics, VBI Vaccines, Voyager, QED, Celularity, and Sapience.
References
- 1. Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. [DOI] [PubMed] [Google Scholar]
- 2. Gilbert MR, Pugh SL, Aldape K, et al. NRG oncology RTOG 0625: a randomized phase II trial of bevacizumab with either irinotecan or dose-dense temozolomide in recurrent glioblastoma. J Neurooncol. 2017;131(1):193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Taal W, Oosterkamp HM, Walenkamp AM, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15(9):943–953. [DOI] [PubMed] [Google Scholar]
- 4. Ellingson BM, Sahebjam S, Kim HJ, et al. Pretreatment ADC histogram analysis is a predictive imaging biomarker for bevacizumab treatment but not chemotherapy in recurrent glioblastoma. AJNR Am J Neuroradiol. 2014;35(4):673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patel KS, Yao J, Raymond C, et al. Decorin expression is associated with predictive diffusion MR phenotypes of anti-VEGF efficacy in glioblastoma. Sci Rep. 2020;10(1):14819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pope WB, Kim HJ, Huo J, et al. Recurrent glioblastoma multiforme: ADC histogram analysis predicts response to bevacizumab treatment. Radiology. 2009;252(1):182–189. [DOI] [PubMed] [Google Scholar]
- 7. Patel KS, Everson RG, Yao J, et al. Diffusion magnetic resonance imaging phenotypes predict overall survival benefit from bevacizumab or surgery in recurrent glioblastoma with large tumor burden. Neurosurgery. 2020;87(5):931–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ellingson BM, Gerstner ER, Smits M, et al. Diffusion MRI phenotypes predict overall survival benefit from anti-VEGF monotherapy in recurrent glioblastoma: converging evidence from phase II trials. Clin Cancer Res. 2017;23(19):5745–5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pope WB, Qiao XJ, Kim HJ, et al. Apparent diffusion coefficient histogram analysis stratifies progression-free and overall survival in patients with recurrent GBM treated with bevacizumab: a multi-center study. J Neurooncol. 2012;108(3):491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ellingson BM, Kim HJ, Woodworth DC, Cloughesy TF. Contrast-enhanced T1-weighted digital subtraction maps combined with diffusion MRI to identify recurrent glioblastoma patients that benefit from bevacizumab therapy. J Clin Oncol. 2014;32(15):5s. [Google Scholar]
- 11. Schell M, Pflüger I, Brugnara G, et al. Validation of diffusion MRI phenotypes for predicting response to bevacizumab in recurrent glioblastoma: post-hoc analysis of the EORTC-26101 trial. Neuro Oncol. 2020;22(11):1667–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cloughesy TF, Brenner A, de Groot JF, et al. ; GLOBE Study Investigators . A randomized controlled phase III study of VB-111 combined with bevacizumab vs bevacizumab monotherapy in patients with recurrent glioblastoma (GLOBE). Neuro Oncol. 2020;22(5):705–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brenner AJ, Peters KB, Vredenburgh J, et al. Safety and efficacy of VB-111, an anticancer gene therapy, in patients with recurrent glioblastoma: results of a phase I/II study. Neuro Oncol. 2020;22(5):694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ellingson BM, Bendszus M, Boxerman J, et al. ; Jumpstarting Brain Tumor Drug Development Coalition Imaging Standardization Steering Committee . Consensus recommendations for a standardized Brain Tumor Imaging Protocol in clinical trials. Neuro Oncol. 2015;17(9):1188–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ellingson BM, Kim HJ, Woodworth DC, et al. Recurrent glioblastoma treated with bevacizumab: contrast-enhanced T1-weighted subtraction maps improve tumor delineation and aid prediction of survival in a multicenter clinical trial. Radiology. 2014;271(1):200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ellingson BM, Aftab DT, Schwab GM, et al. Volumetric response quantified using T1 subtraction predicts long-term survival benefit from cabozantinib monotherapy in recurrent glioblastoma. Neuro Oncol. 2018;20(10):1411–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ellingson BM, Abrey LE, Nelson SJ, et al. Validation of postoperative residual contrast-enhancing tumor volume as an independent prognostic factor for overall survival in newly diagnosed glioblastoma. Neuro Oncol. 2018;20(9):1240–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Woodworth DC, Pope WB, Liau LM, et al. Nonlinear distortion correction of diffusion MR images improves quantitative DTI measurements in glioblastoma. J Neurooncol. 2014;116(3):551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ellingson BM, Kim E, Woodworth DC, et al. Diffusion MRI quality control and functional diffusion map results in ACRIN 6677/RTOG 0625: a multicenter, randomized, phase II trial of bevacizumab and chemotherapy in recurrent glioblastoma. Int J Oncol. 2015;46(5):1883–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pope WB, Mirsadraei L, Lai A, et al. Differential gene expression in glioblastoma defined by ADC histogram analysis: relationship to extracellular matrix molecules and survival. AJNR Am J Neuroradiol. 2012;33(6):1059–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Neill T, Schaefer L, Iozzo RV. Decorin: a guardian from the matrix. Am J Pathol. 2012;181(2):380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Järveläinen H, Sainio A, Wight TN. Pivotal role for decorin in angiogenesis. Matrix Biol. 2015;43:15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sofeu Feugaing DD, Götte M, Viola M. More than matrix: the multifaceted role of decorin in cancer. Eur J Cell Biol. 2013;92(1):1–11. [DOI] [PubMed] [Google Scholar]
- 24. Rosca EV, Koskimaki JE, Rivera CG, Pandey NB, Tamiz AP, Popel AS. Anti-angiogenic peptides for cancer therapeutics. Curr Pharm Biotechnol. 2011;12(8):1101–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Davies Cde L, Melder RJ, Munn LL, Mouta-Carreira C, Jain RK, Boucher Y. Decorin inhibits endothelial migration and tube-like structure formation: role of thrombospondin-1. Microvasc Res. 2001;62(1):26–42. [DOI] [PubMed] [Google Scholar]
- 26. Grant DS, Yenisey C, Rose RW, Tootell M, Santra M, Iozzo RV. Decorin suppresses tumor cell-mediated angiogenesis. Oncogene. 2002;21(31):4765–4777. [DOI] [PubMed] [Google Scholar]
- 27. Chui A, Murthi P, Gunatillake T, et al. Altered decorin leads to disrupted endothelial cell function: a possible mechanism in the pathogenesis of fetal growth restriction? Placenta. 2014;35(8):596–605. [DOI] [PubMed] [Google Scholar]
- 28. Khan GA, Girish GV, Lala N, Di Guglielmo GM, Lala PK. Decorin is a novel VEGFR-2-binding antagonist for the human extravillous trophoblast. Mol Endocrinol. 2011;25(8):1431–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wu CC, Jain R, Radmanesh A, et al. Predicting genotype and survival in glioma using standard clinical MR imaging apparent diffusion coefficient images: a pilot study from The Cancer Genome Atlas. AJNR Am J Neuroradiol. 2018;39(10):1814–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Han Y, Yan LF, Wang XB, et al. Structural and advanced imaging in predicting MGMT promoter methylation of primary glioblastoma: a region of interest based analysis. BMC Cancer. 2018;18(1):215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ellingson BM. Radiogenomics and imaging phenotypes in glioblastoma: novel observations and correlation with molecular characteristics. Curr Neurol Neurosci Rep. 2015;15(1):506. [DOI] [PubMed] [Google Scholar]