Abstract
Introduction
Breast Conserving Surgery (BCS) with whole breast radiation is now standard of care as a safer alternative to Mastectomy in terms of loco-regional recurrence and long-term survival. Despite this, a frequent pitfall of conventional BCS is positive surgical margins and need for second surgery with a reported frequency of 12–59 % in literature. Oncoplastic Surgery can be a safer, more cost effective alternate to conventional BCS owing to its higher rate of negative surgical margins (4–6% vs 12–59 %) and better cosmetic results. We aim to prove utility of Oncoplastic surgery for Low-Middle income countries.
Objective
The aim of this study was to determine Oncoplastic Surgery as a more appropriate alternative to Conventional Breast Conserving Surgery for Low-Middle Income countries in terms of its lower positive margins and re-excision rates.
Methodology
A retrospective comparative single center study by reviewing patient's medical records from August 2016 to June 2020 was conducted. Rate of positive margins and re-excisions along with mean volume of resection specimen, mean tumor size and quadrant dealt by both surgical procedures were compared.
Results
Out of 421 patients 249 patients underwent oncoplastic surgery and were compared with 173 patients who had conventional breast conserving surgery. Positive margins were seen in 5 patients (2 %) in OPS group whereas in 31 (17.9 %) patients in BCS group (p value < 0.001). Therefore, 2 from OPS group and 17 from BCS group underwent re-excision (p value < 0.002).None in OPS group while 7 out of 17 patients in BCS group underwent mastectomy as second procedure. Mean tumor size in OPS group was 2.26 cm ± SD 1.66 and in BCS group was 1.94 cm ± SD 1.28. Majority of Lobular carcinoma and Ductal carcinoma in-situ, multifocal, upper inner and central quadrant tumors and those unresponsive to neo-adjuvant therapy were treated by Oncoplastic techniques.
Conclusion
Oncoplastic surgery has shown promising results as a safer tool to deal with large, complex tumors, lesions in difficult anatomical locations, multifocal or progressing on neo-adjuvant therapy. With its low Re-excision rates, it is a better alternative to traditional Breast Conserving approach for overburdened and resource limited health care system of Low-Middle Income countries. Multi-center, prospective trials are needed to determine its feasibility.
Keywords: Oncoplastic surgery, Breast conserving surgery, Re-excision, Positive margins
Highlights
-
•
Oncoplastic surgery has proven to be oncologically safe when compared to conventional breast conserving surgery.
-
•
Oncoplastic procedures results in lower rate of positive surgical margins and re-excision rates.
-
•
Difficult anatomic quadrants, multi-focal and tumors not responding to neo-adjuvant therapy can be dealt with oncoplastic techniques.
-
•
These advanteges makes it a better alternative to conventional breast conserving procedure in resource limited countries.
-
•
The concept of oncoplastic breast surgery is still contemporary to developing world there is a need to bridge gap in training.
1. Introduction
Breast Conserving Surgery (BCS) followed by whole breast radiation has been established as standard of care after publication of several trials like NSABP 06 and MILAN trials validating it as a safer alternative to Mastectomy in terms of loco-regional recurrence and long-term survival [1]. It gained popularity for offering higher patients satisfaction without compromising survival outcomes and is now routinely offered to patients with early breast cancer [2,3]. Despite this, a frequent pitfall of conventional BCS is positive surgical margins and need for second surgery with a reported frequency of 12–59 % in literature [[4], [5], [6]]. Revision surgeries results in poor cosmesis in 25–30 % of women, morbidity, texture change and additional expenses which becomes economically challenging in an already overburdened health care system of most middle- and low-income countries with limited resources and healthcare budget [[7], [8], [9]]. Moreover, many patients end up having mastectomy owing to difficult anatomical location or multi-focality of the tumor, or as a second surgery to avoid multiple re-excisions and poor cosmetic results [10].
Oncoplastic surgery (OPS), first introduced by Werner Audretsch in 1980s, is an ingenious approach with initial emphasis on refinement of BCS procedure and incorporating plastic surgery techniques to maintain natural shape of breast [10]. It is defined as breast-conservation surgery incorporating an oncologic partial mastectomy with ipsilateral defect repair using volume displacement or volume replacement techniques with contralateral symmetry surgery as appropriate [11]. Although in its early days it evolved as a procedure more inclined towards aesthetic outcome, within the past three decades of its global establishment OPS has proven to be oncologically safer than BCS for tumor excision with negative surgical margins (4–6% vs 12–59 %), which has always been the prime focus of breast cancer surgery [[12], [13], [14], [15]]. OPS allows the surgeon to remove substantial volume of breast tissue without compromising cosmetic outcome which is a limitation in conventional Breast conserving surgery and is of key importance in developing countries where a large number of patients present late with larger tumor size or locally advanced disease [16]. Furthermore, it extends the indications of breast conservation to multifocal disease, high tumor-to-breast ratio, very complex breast lesions with unfavorable anatomical locations like Upper inner, central quadrants in which simple BCS techniques can result in poor cosmetic outcome with less patient satisfaction having a greater psychological impact and therefore, many of these patients until recently were subjected to mastectomy in fear of inadequate tumor excision a common practice in many countries [17]. Therefore, oncoplastic techniques makes it possible to resect larger tumors with adequate margins with lower rate of re-excisions and conversion mastectomies.
The concept of oncoplastic breast surgery is still contemporary to developing countries. The disparity from western world stretches beyond economic factors and owe to limited number of surgeons adequately trained to perform oncoplastic procedures. Therefore, data on the efficacy of OPS in breast cancer treatment from low and middle socioeconomic country is not substantial [18,[19], [20]]. With the advent of neo-adjuvant therapy allowing more and more breast conserving procedures to be performed and declining mortality of breast cancer patients due to advances in adjuvant treatments, more women will live with the surgical treatment decided for them. Although cosmetic and oncological safety of Oncoplastic has been reported in various studies, its usefulness as a cost effective procedure in developing countries has not been affirmed.
We sought to determine that oncoplastic surgery can be a safer tool to deal with complex tumors with low positive margins and re-excision rates as compare to conventional breast surgery in low-middle income countries, where cancer treatment is associated with financial constraints having limited resources and struggling to find a way in optimizing care of breast cancer patients without compromising the cosmesis.
2. Materials and methods
This was a single center, retrospective study conducted on cohort of breast cancer patients. All patients who underwent oncoplastic or breast conserving surgery from August 1, 2016 to December 31, 2020 were recruited through consecutive sampling technique. Females above 18 years who were diagnosed with breast cancer on core biopsy (early stage or locally advanced) and underwent either upfront surgery or after receiving neo-adjuvant systemic therapy were included. Pregnant females, those who had mastectomy, and whose histopathology were sent outside our institute were excluded. Two group of patients (OPS and BCS) based on the type of procedure were formed. Oncoplastic surgery included volume displacement techniques by mobilization of adjacent glandular breast tissue in filling the defect through Inverted T, lateral mammoplasty, Benelli, Grisotti, Matrix rotation, lazy ‘S’ and volume replacement techniques by using local flaps through Quadrantectomy + Latissimus Dorsi flap, Lateral thoracic artery perforator flap and Lateral intercostal artery perforator flap techniques. Choice of procedure was subjective to Breast surgeon's decision depending upon breast to tumor size ratio, post neoadjuvant downgrading of tumor, patients general condition and wish and commitment to receive radiation after surgery. Oncoplastic procedures performed solely by breast surgeons were included, plastic surgeon was not part of team in these procedures. The breast surgeons are formally trained in oncoplastic procedures.
After obtaining approval from Ethical Review Committee 433 medical files were reviewed. 12 patients were excluded from study due to lost to follow-up. Data from patients medical records included demographics; age, size of tumor at presentation, neo-adjuvant systemic therapy, post neo-adjuvant size of tumor, tumor size and volume, type of surgical procedure performed. Information of margin status was collected from final histopathology report, number of re-excisions and need for conversion mastectomies were also reported.
Analyzation of data was performed using descriptive analysis; continuous variables were reported as mean±SD and categorical variables as median, comparison between two groups was done using Student-t test for continuous variable and Chi-square test for categorical variables. A p-value of less than 0.05 was considered significant. SPSS version 26 was used to perform statistical analysis. The work has been reported in line with STROCSS criteria [[26], [29]].
3. Results
A total of 422 patients were included in the study. Of these 249 (57.1 %) patients underwent an oncoplastic procedure and 173 (39.7 %) had conventional breast conserving surgery. Mean age in years was 50.07 ± 13.21 SD, mean age in Oncoplastic group was 49.9 years and 51.1 years in BCS group. Patient's demographics are presented in Table 1.
Table 1.
Patients and tumor characteristics.
| Variable | Total pts | Oncoplastic group | Breast Conserving surgery group | |
|---|---|---|---|---|
| No of patients | n = 422 | 249 (59.7 %) | 173(39.7 %) | |
| Age in years | 50.07 ± 13.3 | 49.9 | 51.1 | p = 2.43 |
| Mean tumor size(cm) | 5.85 ± 2.6 | 2.26 cm ± 1.66 | 1.94 cm +/1.28 | P = 1.54 |
| Mean volume of specimen | 8.75 cm | 6.03 cm±2.8 | 5.76 cm ± 2.4 | p = 1.05 |
| Neoadjuvant therapy | 98 (22.5 %) | 53(21.3 %) | 45(23.3 %) | p = 1.09 |
Mean tumor size in OPS group was 2.26 cm ± SD 1.66 and in BCS group was 1.94 cm ± SD 1.28. Majority of patients (61.6 % in OPS and 38.3 % in BCS) had invasive ductal carcinoma followed by ductal carcinoma in-situ 12.4 %, malignant phyllodes 2.6 %, metaplastic 2.1 % and invasive lobular carcinoma 1.6 %. It was interesting to note that most patients who had DCIS and Invasive Lobular Carcinoma underwent BCS and had positive margins on excision, likely because the tumor size was underestimated due to vague tumor margins clinically, thus suggesting OPS as a better option when the tumor margins are ill defined. 21.8 % in OPS group and 25.4% patients in BCS group received Neoadjuvant systemic therapy. In our group 3.2 % patients who were partially or completely unresponsive to Neo adjuvant chemotherapy were treated by Oncoplastic techniques. Individuals in OPS group mostly had T2 tumor size (48.8 %) while those in BCS group mostly had T1 tumor size (50.3 %) Table 1. 20.2 % multifocal lesions were treated by OPS while 17 % were excised by BCS. OPS was performed in 9.5 % upper inner and 12.5 % central tumors. Positive margins were seen in 5 patients (2 %) in OPS group whereas 31 (17.9 %) patients in BCS group (p value < 0.001). Therefore, 2 from OPS group and 17 from BCS group underwent re-excision (p value < 0.002) while remaining patients declined a second procedure. None in OPS group while 7 out of 17 patients in BCS group underwent mastectomy as second procedure (Table 2). Adjuvant treatment received by both groups were same.
Table 2.
Characteristics of resected specimen.
| Histological types | |||||
|---|---|---|---|---|---|
| IDC | DCIS | Malignant Phyllodes | Metaplastic | ILC | |
| OPS | 193(84.3 %) | 23(10 %) | 6(2.6 %) | 5(2.2 %) | 2(0.9 %) |
|
BCS |
105(76.1 %) |
22(15.9 %) |
3(2.2 %) |
6(4.3 %) |
2(1.4 %) |
| Quadrants Involved | |||||
|
Upper Outer |
Upper Inner |
Lower Inner |
Lower Outer |
Central |
|
| OPS | 121(48.8 %) | 27(10.9 %) | 23(9.3 %) | 46(18.5 %) | 31(12.5 %) |
| BCS | 90(50.2 %) | 27(15.6 %) | 17(9.8 %) | 28(16.2 %) | 11(6.4 %) |
IDC= Invasive Ductal Carcinoma, DCIS = Ductal Carcinoma in Situ, ILC= Invasive Lobular Carcinoma.
% = within group.
4. Discussion
Oncoplastic surgery is a third pathway between mastectomy and conventional breast conservation incorporating plastic surgery techniques into cancer surgery for treating breast cancer while maintaining the natural shape of the breast [24]. While it initially emerged as “an aesthetic cancer cure”, oncological safety of OPS has now been proven in growing evidence of publications [21]. Clough bi-level classification was one of the selected classification system in First international consensus conference on standardization of oncoplastic breast conserving surgery in 2015, according to which oncoplastic procedures can be divided into volume displacement technique/level-I surgery where less than 20 % of breast tissue is excised and defect is closed by adjacent glandular mobilization, and volume replacement/level-II surgery when 20–50 % of breast tissue is excised with repairing the defect by using extra mammary local flaps such as latissimus dorsi flap [22].
Breast conserving surgery, although widely accepted, have limitations in certain situations like upper inner or central quadrant tumors, large tumors to small breast size ratio, multi-focal tumors, lobular carcinoma and extensive ductal carcinoma in-situ where wider margins are required. Traditional wide local excision in these scenarios may results in poor cosmetic outcomes in up to 30 % of cases and more importantly positive margins resulting in increased number of re-excision surgeries [25]. Many patients opt for mastectomy when informed about the high risk of re-excision as they have limited access to medical facilities in low income countries or simply in fear of undergoing a second procedure. A higher re-excision rate and poor cosmetic outcome translates into substandard quality of life an additional economic burden to resource limited health sectors of developing world. Oncoplastic surgery, thus helped in expanding the indications of breast conservations without fear of compromising oncological safety [26].
In our comparative analysis between two procedures, we were able to demonstrate that OPS has clear advantage over BCS in reducing the number of re-excision surgeries i.e., 9.8 % in BCS group vs 1.6% in OPS group. This was in line with the data published by Benjamin et al. in their single institutional experience and literature review which demonstrated the re-excision rate of 17.2 % in BCS group and 4.0 % in OPS group [11]. In a meta-analysis 18,103 patients Kosaish and colleges also proved oncoplastic surgery as an oncologically safe procedure [[25], [27], [28], [29]]. Although critics may argue that oncoplastic procedures may cost more in terms of surgical expertise and time for surgery required. But when compared to a second procedure with its associated morbidity and a second hospital admission which is more often required in conventional breast conserving procedures the overall cost is actually less which is a major advantage in low resource countries. Also, OPS has extended the indications of breast conservation and has proven to be oncological safe in patients who were considered to undergo mastectomy in the past to avoid risk of recurrence such as those with multi-focal lesions [21], with DCIS and lobular carcinoma, tumors which are unresponsive to chemotherapy and where tumor is in a difficult quadrant e.g., upper inner quadrant and central quadrant tumors.
The flexibility of oncoplastic techniques permits surgeon to excise tumors from any quadrant without compromising cosmesis or oncological safety, similarly patients with multifocal lesions are observed to benefit from advanced surgical options like chest wall perforator flaps. We also observed advantage of OPS in tumor that did not downsize response to Neoadjuvant systemic therapy.
5. Case discussion
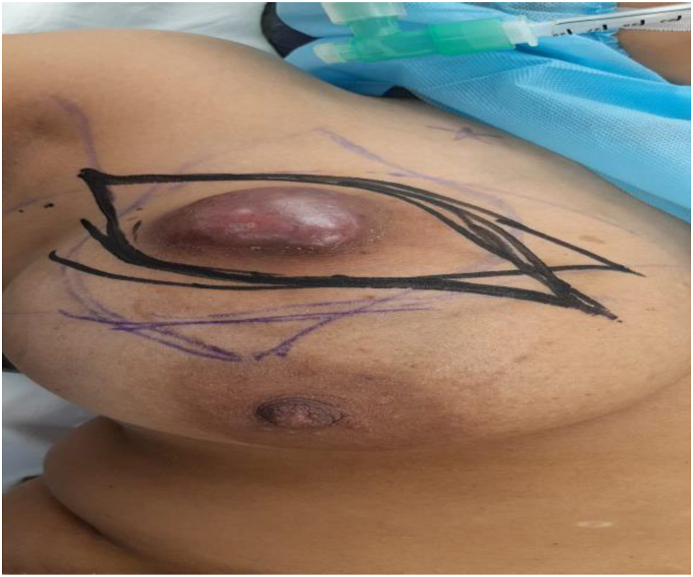
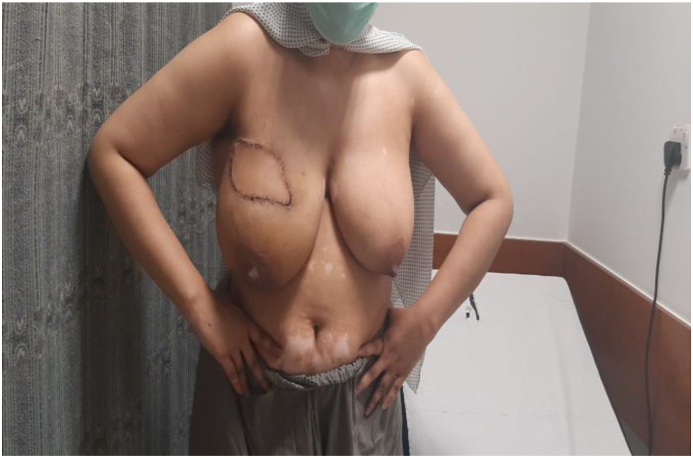
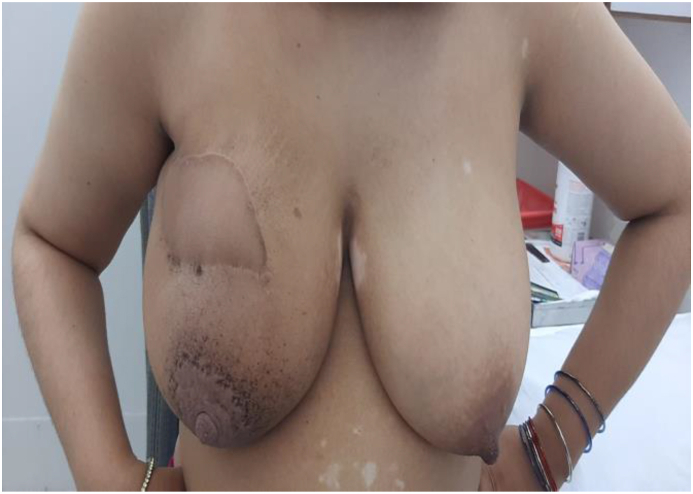
A 32 year female presented to us with a lump in upper outer quadrant of Right breast 9 × 7 cm in size with clinically negative axilla. She was diagnosed with Invasive ductal carcinoma; Triple negative on core biopsy. Metastatic workup was negative and patient was started on Neo-adjuvant chemotherapy to downsize the tumor with intention to conserve breast. After four cycles of receiving Adriamycin and cyclophosphamide, she had partial response and the tumor downsized to 4 × 4 cm but unfortunately her Liver function tests got deranged and she could not continue with systemic therapy. Breast was conserved by performing Oncoplastic local Latissimus dorsi flap reconstruction. Tumor margins were negative and patient completed adjuvant chemotherapy. She has recurrence free survival two years postoperatively with excellent cosmetic outcome.(see Table 3, Table 4, Fig. 1, Fig. 2, Fig. 3)
Table 3.
1: Rate of Positive margins and Re-excisions.
| Positive Margins | |||
|---|---|---|---|
| OPS | 1(0.4 %) | Odds Ratio 0.41(0.006,0.305) 95 % C.I | (p = 0.001) |
|
BCS |
17(9.8 %) |
1.105(1.051,1.161) 95 % C.I |
|
|
Re-Excision | |||
| OPS | 2 (1.8 %) |
Odds Ratio 0.480(0.256.0.901) 95 % C.I |
(p = 0.002) |
|
BCS |
17(9.8 %) |
1.803(1.356,2.395) 95 % C.I |
|
|
Mastectomy | |||
| OPS | 0 |
Odds Ratio - |
(p = 0.001) |
| BCS | 7(63.6 %) | 1.143(0.77,16.947) 95 % C.I | |
Table 4.
Type of Oncoplastic techniques used.
| Volume Displacement | |
|---|---|
| Benelli | 76 (17.4 %) |
| Inverted-T | 43(9.9 %) |
| Lateral Mammoplasty | 30(6.9 %) |
| Matrix Rotation | 20(4.6 %) |
| Reduction Mammoplasty | 14(3.2 %) |
| Lazy’S | 31(7.1 %) |
| Grisotti | 6(1.4 %) |
| Lateral Pouch | 2(0.5 %) |
| Batwing |
3(0.9 %) |
| Volume Replacement | |
| Quadrantectomy + Latissimus Dorsi flap | 10(2.3 %) |
| Lateral Intercostal artery/Thoracic artery perforator Flap | 13(3.0 %) |
Fig. 1.
Pre-operative; partial response to chemotherapy which was interrupted due to deranged Liver function tests.
Fig. 2.
One month post-operative Oncoplastic breast conservation by volume replacement with local Latissimus Dorsi flap technique.
Fig. 3.
Two years post-operative; Recurrence free survival with excellent cosmetic outcome.
Our data demonstrated that out 57 % patients who underwent oncoplastic surgery 10.9 % had upper inner, 9.8 % lower inner and 12.5 % had central tumors, 20.2 % patients had multi focal lesions, 10 % had extensive DCIS and 1.6 % had lobular carcinoma. We had 3.2 % cases who had who progressed on Neo adjuvant chemotherapy but were able to conserve breast using volume replacement oncoplastic techniques. Therefore, as reported by Koppikar and associates in their paper ‘Extreme oncoplastic surgery for multifocal/multi-centric and locally advanced breast cancer’ we were also able to demonstrate that OPS gives a clear advantage for the surgeon to resect larger volume of specimen in patients with DCIS and lobular carcinoma, multifocal and locally advanced disease that often lacks defined margins [16]. However, as the techniques and implications of this procedure gains popularity there is a need for careful patient selection keeping in mind the realistic goals that can be achieved and risk of complications like fat necrosis and lack of symmetry that might affect cosmetic outcomes, standardization of practices and prospective assessment of patient reported outcomes [[23], [30]].
The study has limitations of being a retrospective review from a single center. Technical expertise unique to individual surgeon's skill level was also a confounding factor. Data on oncoplastic surgery from developing world is sparse, its utility and challenges in low resource countries needs to be assessed through prospective trials.
6. Conclusion
Oncoplastic surgery can offer breast conservation to tumors which would otherwise have been des tined to mastectomy. Our analysis indicate that patients who underwent oncoplastic surgery had less positive margins and re-excisions as compared to those who underwent traditional breast conserving procedures (1.8 % vs 9.8 %). We postulate that Oncoplastic techniques can bring favorable change to breast cancer management in Low-Middle income countries in terms of cost effectiveness and decreased work load by reducing the numbers of re-operations in already overburdened healthcare system. It is a paradigm shift from traditional breast conservation without compromising cosmetic and oncologic safety of tumor surgery. However larger prospective studies need to be conducted to prove its utility in developing world. This will help bridge the gap of difference in practice from western world, and highlight the need to train surgeons from developing countries.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Declaration of competing interest
I have no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2021.102618.
Contributor Information
Syeda Sakina Abidi, Email: sakina.abidi@aku.edu.
Lubna Mushtaque Vohra, Email: lubna.vohra@aku.edu.
Muhammad Rizwan Javed, Email: rizwan.javed@aku.edu.
Nargis Khan, Email: nargis.khan76@gmail.com.
Ethical approval
Ethics Review Committee Aga Khan Hospital.
Reference Number: 2020-5418-14043.
Sources of funding
Aga Khan Hospital (No funding was required).
Author contributions
Dr. Syeda Sakina Abidi; Conceptualization, Methodology, visualization original draft writing.
Dr.Lubna Vohra; supervision, investigation, editing.
Dr.Muhammad Rizwan Javed; data curation.
Nargis Khan; software, data analysis.
Research registration Unique Identifying number (UIN)
-
1.
Name of the registry: DRKS - German Clinical Trials Register.
-
2.
Unique Identifying number or registration ID: DRKS00025511.
-
3.
Hyperlink to your specific registration (must be publicly accessible and will be checked): https://www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00025511.
Guarantor
Dr. Syeda Sakina Abidi
Fellow
Breast Surgery
Aga Khan Hospital. Karachi, Pakistan
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Wrubel E., Natwick R., Wright G.P. Breast-conserving therapy is associated with improved survival compared with mastectomy for early-stage breast cancer: a propensity score matched comparison using the national cancer database. Ann. Surg Oncol. 2021;28:914–919. doi: 10.1245/s10434-020-08829-. [DOI] [PubMed] [Google Scholar]
- 2.Margenthaler J.A., Dietz J.R., Chatterjee A. The landmark series: breast conservation trials (including oncoplastic breast surgery) Ann. Surg Oncol. 2021;28:2120–2127. doi: 10.1245/s10434-020-09534-y. [DOI] [PubMed] [Google Scholar]
- 3.Johns N., Dixon J.M. Should patients with early breast cancer still be offered the choice of breast conserving surgery or mastectomy? EJSO. 2016 Nov 1;42(11):1636–1641. doi: 10.1016/j.ejso.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 4.Coopey S., Smith B.L., Hanson S. The safety of multiple Re-excisions after lumpectomy for breast cancer. Ann. Surg Oncol. 2011;18:3797–3801. doi: 10.1245/s10434-011-1802-4. [DOI] [PubMed] [Google Scholar]
- 5.Catsman C.J., Beek M.A., Voogd A.C., Mulder P.G., Luiten E.J. The COSMAM TRIAL a prospective cohort study of quality of life and cosmetic outcome in patients undergoing breast conserving surgery. BMC Canc. 2018 Dec;18(1):1–7. doi: 10.1186/s12885-018-4368-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chauhan A., Sharma M.M. Evaluation of surgical outcomes following oncoplastic breast surgery in early breast cancer and comparison with conventional breast conservation surgery. Med. J. Armed Forces India. 2016 Jan 1;72(1):12–18. doi: 10.1016/j.mjafi.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Talsma A.K., Reedijk A.M., Damhuis R.A., Westenend P.J., Vles W.J. Re-resection rates after breast-conserving surgery as a performance indicator: introduction of a case-mix model to allow comparison between Dutch hospitals. Eur. J. Surg. Oncol. 2011 Apr;37(4):357–363. doi: 10.1016/j.ejso.2011.01.008. Epub 2011 Feb 2. PMID: 21292434. [DOI] [PubMed] [Google Scholar]
- 8.Pataky R.E., Baliski C.R. Reoperation costs in attempted breast-conserving surgery: a decision analysis. Curr. Oncol. 2016 Oct;23(5):314–321. doi: 10.3747/co.23.2989. Epub 2016 Oct 25. PMID: 27803595; PMCID: PMC5081007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Morais TB, Juliano Y, Veiga DF, Ferreira LM. Oncoplastic approach in the conservative treatment of breast cancer. Analysis of costs. Acta Cir. Bras.;27(5):2012-2311. [DOI] [PubMed]
- 10.Kelemen P., Pukancsik D., Újhelyi M., Sávolt Á., Kovács E., Ivády G., Kenessey I., Kovács T., Stamatiou A., Smanykó V., Mátrai Z. Comparison of clinicopathologic, cosmetic and quality of life outcomes in 700 oncoplastic and conventional breast-conserving surgery cases: a single-centre retrospective study. Eur. J. Surg. Oncol. 2019 Feb 1;45(2):118–124. doi: 10.1016/j.ejso.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Chatterjee A., Gass J., Patel K., Holmes D., Kopkash K., Peiris L., Peled A., Ryan J., El-Tamer M., Reiland J. A consensus definition and classification system of oncoplastic surgery developed by the American society of breast surgeons. Ann. Surg Oncol. 2019 Oct;26(11):3436–3444. doi: 10.1245/s10434-019-07345-4. Epub 2019 Apr 11. PMID: 30977016. [DOI] [PubMed] [Google Scholar]
- 12.Benjamin M.A., Sinnott C., Bawa S., Kaufman D.I., Guarino K., Addona T. Re-excision rate after partial mastectomy in oncoplastic breast-conserving surgery: a single-institutional experience and review of the literature. Ann. Plast. Surg. 2019 Apr 1;82(4S):S170–S172. doi: 10.1097/SAP.0000000000001874. [DOI] [PubMed] [Google Scholar]
- 13.Mansell J., Weiler-Mithoff E., Stallard S., Doughty J.C., Mallon E., Romics L. Oncoplastic breast conservation surgery is oncologically safe when compared to wide local excision and mastectomy. Breast. 2017 Apr 1;32:179–185. doi: 10.1016/j.breast.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Chauhan A., Sharma M.M. Evaluation of surgical outcomes following oncoplastic breast surgery in early breast cancer and comparison with conventional breast conservation surgery. Med. J. Armed Forces India. 2016 Jan 1;72(1):12–18. doi: 10.1016/j.mjafi.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clough Krishna B., van la Parra, Raquel F.D., Thygesen Helene H., Levy Eric, Russ Elisabeth, Halabi Najeeb M., Sarfati Isabelle, Nos Claude. Long-term results after oncoplastic surgery for breast cancer. Ann. Surg. July 2018;268(1):165–171. doi: 10.1097/SLA.0000000000002255. [DOI] [PubMed] [Google Scholar]
- 16.De La Cruz L., Blankenship S.A., Chatterjee A., Geha R., Nocera N., Czerniecki B.J., Tchou J., Fisher C.S. Outcomes after oncoplastic breast-conserving surgery in breast cancer patients: a systematic literature review. Ann. Surg Oncol. 2016 Oct;23(10):3247–3258. doi: 10.1245/s10434-016-5313-1. [DOI] [PubMed] [Google Scholar]
- 17.Carrara G.F., Scapulatempo-Neto C., Abrahão-Machado L.F., Brentani M.M., Nunes J.S., Folgueira M.A., Vieira R.A. Breast-conserving surgery in locally advanced breast cancer submitted to neoadjuvant chemotherapy. Safety and effectiveness based on ipsilateral breast tumor recurrence and long-term follow-up. Clinics. 2017 Mar;72(3):134–142. doi: 10.6061/clinics/2017(03)02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koppiker C.B., Noor A.U., Dixit S., Busheri L., Sharan G., Dhar U., Allampati H.K., Nare S. Extreme oncoplastic surgery for multifocal/multicentric and locally advanced breast cancer. International journal of breast cancer. 2019 Feb 20:2019. doi: 10.1155/2019/4262589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koppiker C.B., Chintamani, Dixit S. Oncoplastic breast surgery in India: thinking globally, acting locally. Indian J. Surg. 2019;81:103–110. doi: 10.1007/s12262-019-01890-8. [DOI] [Google Scholar]
- 20.Freitas-Junior R., Ferreira-Filho D.L., Soares L.R. Oncoplastic breast-conserving surgery in low- and middle-income countries: training surgeons and bridging the gap. Curr Breast Cancer Rep. 2019;11:136–142. doi: 10.1007/s12609-019-00317-3. [DOI] [Google Scholar]
- 21.Kaufman C.S. Increasing role of oncoplastic surgery for breast cancer. Curr. Oncol. Rep. 2019 Dec;21(12) doi: 10.1007/s11912-019-0860-9. 1-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weber W.P., Soysal S.D., El-Tamer M. First international consensus conference on standardization of oncoplastic breast conserving surgery. Breast Canc. Res. Treat. 2017;165:139–149. doi: 10.1007/s10549-017-4314-5. [DOI] [PubMed] [Google Scholar]
- 23.Crown A., Scovel L.G., Rocha F.G., Scott E.J., Wechter D.G., Grumley J.W. Oncoplastic breast conserving surgery is associated with a lower rate of surgical site complications compared to standard breast conserving surgery. Am. J. Surg. 2019 Jan 1;217(1):138–141. doi: 10.1016/j.amjsurg.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 24.Masannat Y.A., Agrawal A., Maraqa L., Fuller M., Down S.K., Tang S.S., Pang D., Kontos M., Romics L., Heys S.D. Multifocal and multicentric breast cancer, is it time to think again? Ann. R. Coll. Surg. Engl. 2020 Jan;102(1):62–66. doi: 10.1308/rcsann.2019.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Audretsch W.P., Rezai M., Kolotas C., Zamboglou N., Schnabel T., Bojar H. Tumor-specific immediate reconstruction in breast cancer patients. Perspect. Plast. Surg. 1998;11(1):71–100. [Google Scholar]
- 26.Clough K.B., Gouveia P.F., Benyahi D. Positive margins after oncoplastic surgery for breast cancer. Ann. Surg Oncol. 2015;22:4247–4253. doi: 10.1245/s10434-015-4514-3. [DOI] [PubMed] [Google Scholar]
- 27.Chauhan A., Sharma M.M. Evaluation of surgical outcomes following oncoplastic breast surgery in early breast cancer and comparison with conventional breast conservation surgery. Med. J. Armed Forces India. 2016 Jan 1;72(1):12–18. doi: 10.1016/j.mjafi.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kosasih S., Tayeh S., Mokbel K., Kasem A. Is oncoplastic breast conserving surgery oncologically safe? A meta-analysis of 18,103 patients. Am. J. Surg. 2020 Aug 1;220(2):385–392. doi: 10.1016/j.amjsurg.2019.12.019. [DOI] [PubMed] [Google Scholar]
- 29.Agha R., Abdall-Razak A., Crossley E., Dowlut N., Iosifidis C., Mathew G., for the STROCSS Group The STROCSS 2019 guideline: strengthening the reporting of cohort studies in surgery. Int. J. Surg. 2019;72:156–165. doi: 10.1016/j.ijsu.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Acea‐Nebril B., García‐Novoa A., Cereijo‐Garea C. Cosmetic sequelae after oncoplastic breast surgery: long‐term results of a prospective study. Breast J. 2021 Jan;27(1):35–43. doi: 10.1111/tbj.14142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.