Abstract
Natural deep eutectic solvent (NADES) is an alternative approach in natural product extraction with various advantages, including low toxicity, biodegradable, and suitable phytochemical compounds in a wide range of polarity. Chlorogenic acid (CGA) and caffeine, a well-known compound in the coffee bean, have various potential health benefits. This study aims to optimize the betaine–sorbitol NADES-based ultrasound-assisted extraction (UAE) method of CGA and caffeine from Robusta green coffee beans and determine the inhibitory activity of robusta green coffee beans extract of the betaine-sorbitol NADES-UAE from the optimum condition on pancreatic lipase in vitro and in silico. The betaine-sorbitol NADES-UAE factors as experimental design variable parameters include betaine-sorbitol ratio (0.5:1.2, 1.25:1.2, and 2:1.2 mol), extraction time (10, 35, and 60 min), and solid-liquid ratio (1:10, 1:20, and 1:30 g/mL). Response surface methodology and Box-Behnken Design were used to optimize the extraction process. The response surface was calculated by using CGA and caffeine content as response values. CGA and caffeine content was determined by High-Performance Liquid Chromatography. Whereas in vitro lipase inhibitory activity assay examined by spectrophotometric measurement and in silico molecular docking analysis on PDB ID: 1LPB. According to the results, the optimum conditions of the betaine-sorbitol NADES-UAE have obtained the betaine-sorbitol ratio of 1.25: 1.2 mol, solid-liquid ratio of 1:30 mg/mL, and 60 min extraction time. Furthermore, obtained Robusta green coffee extract from the optimum condition of the betaine-sorbitol NADES-UAE showed high potential to inhibit lipase activity with IC50 of 18.02 μg/ml, comparable with IC50 of standard CGA (11.90 μg/ml) and caffeine (15.59 μg/ml), where potential interaction of both standards was confirmed using molecular docking analysis. Our finding demonstrated the optimum condition of the betaine-sorbitol NADES-UAE method for CGA and caffeine extraction and the potential pancreatic lipase inhibition activity from the Robusta green coffee bean.
Keywords: Caffeine, Chlorogenic acid, Natural deep eutectic solvent, Pancreatic lipase, Robusta green coffee beans, Ultrasonic-assisted extraction
Caffeine, Chlorogenic acid, Natural deep eutectic solvent, Pancreatic lipase, Robusta green coffee beans, Ultrasonic-assisted extraction
1. Introduction
The extraction of natural products by natural deep eutectic solvent (NADES) has several advantages compared to conventional organic solvent. It is environmentally safe, low toxicity, biodegradable, and the obtained extraction can be used directly as a finished product (Espino et al., 2018; Leng and Suyin, 2019). In addition, the NADES mixture forms a homogenous liquid with a lower melting point than its single constituents. Therefore, it can be used as a greener alternative solvent in natural product extraction. Several studies reported that NADES has a high ability to extract phenolic compounds because of a wide range of polarity, adjustable viscosity, and the interaction of hydrogen bonds formed between substitution groups (such as hydroxyl, carboxylic, amino groups) and NADES itself (Paiva et al., 2014; Boyko et al., 2020; da Silva et al., 2020; Dai, 2013; Dai et al., 2013; Rodríguez-Juan et al., 2021).
Some studies report the application of NADES as a solvent to extract bioactive substances from coffee. For example, Paiva et al. (2014) reported that NADES has successfully extracted gallic acid from green coffee beans better than organic solvents (Paiva et al., 2014). Furthermore, lactic-sucrose, citric acid-sucrose, and choline chloride-sorbitol based NADES also yields higher caffeine and polyphenol level from green coffee bean (C. canephora) (Ahmad et al., 2018; Yuniarti et al., 2019a, 2019b). Our previous studies have also demonstrated that NADES better extract efficiency of bioactive substances from green coffee beans, which yielded a higher phenolic compound than conventional organic solvent (Syakfanaya et al., 2019). However, further study is needed to obtain the optimum extraction conditions to extract CGA and caffeine using betaine-sorbitol-based NADES.
Both CGA and caffeine have been shown to reduce body weight, visceral fat mass, and triglyceride (Turnbull et al., 2017; Naveed et al., 2018). CGA is likely to exhibit its anti-obesity properties by inhibiting fatty acid synthase, whereas caffeine by pancreatic lipase inhibition and triggering lipolysis in tissue (Lunagariya et al., 2014). Today one-third of the world population is affected by obesity, and 38% of the adult population will have an overweight burden if the trend does not change. Obesity is closely associated with type 2 diabetes, cardiovascular diseases, cancer, disability, and mortality (WHO, 2014). A cup of coffee will approximately have 15–325 mg of CGA content that would deliver a health benefit of a reduced risk of diabetes (Choi et al., 2016; Naveed et al., 2018). In this regard, green beans of C. canephora or C. robusta might better protect against diabetes risk due to higher CGA and caffeine content.
Robusta green coffee variant has higher CGA and caffeine contents than the Arabica variant. Both CGA and caffeine have anti-obesity properties (Turnbull et al., 2017; Naveed et al., 2018). CGA is a polyphenolic compound with an ester backbone bound together with (-)-quinic acid and hydroxycinnamic acid, belongs to polyphenol esters family group such as tamarind, ferulic and p-coumaric acids (Choi et al., 2016). The other compound, Caffeine, has anti-obesity effects through the inhibitory activity of pancreatic lipase (Lunagariya et al., 2014).
Pancreatic lipase is an enzyme secreted by the pancreas. This enzyme has a major function in hydrolyzing dietary fat molecules in the human digestive system. Pancreatic lipase hydrolyzes triglycerides in the presence of nonspecific colipases, phospholipases, and esterases which defend monoglycerides, cholesterol esters, and many other substrates (Kimura et al., 1982). In drug discovery research, mainly natural products, pancreatic lipase is a valid target for finding potential natural drugs (Seyedan et al., 2015). Inhibition of this enzyme is one approach used to find effective natural products as anti-obesity agents (Alias et al., 2017).
Response surface methodology (RSM) in the Design-Expert v12 software was used to study the reciprocal influence between extraction factors used in research and determine the optimal combination of process parameters that vary at three levels. The RSM method was chosen because it was a design that can be used for multi-factor tests in a mathematically and statistically adequate time (Meeker et al., 2017). In addition, RSM design reduces the number of samples in experiments and can also help determine the process factors' interactions (Dahmoune et al., 2014).
RSM with Box-Behnken design (BBD) was used to analyze the association between several extraction factors as independent variables to the CGA and caffeine content as dependent variables. We considered that betaine-sorbitol ratio, sample-NADES ratio, and extraction time significantly affected the extraction process, determining CGA and caffeine content in the obtained extract. Thus, RSM and BBD are beneficial and efficient in optimizing complex extraction variables and obtaining a predictive model (Piepel et al., 2002; Brusco et al., 2009).
This study aims to obtain the optimal betaine-sorbitol NADES-UAE method to extract CGA and caffeine content from Robusta green coffee beans and determine the inhibitory activity of Robusta green coffee beans extract-based NADES-UAE from the optimum condition on pancreatic lipase in vitro and in silico.
2. Materials and methods
2.1. Materials
The sample used and preparation was similar to (Syakfanaya et al., 2019). Standard caffeine (CSPC, China), chlorogenic acid (CGA) standard (Wako Pure Chemical Industries, Japan), anhydrous betaine (Shandong, China), sorbitol (Roquette Freres, France), demineralized water, and ethanol (PT. Brataco, Indonesia). Acetonitrile, methanol for HPLC, and glacial acetic acid (Merck, Germany). Aqua Pro Injection/API (PT. Ikapharmindo Putramas, Indonesia), porcine pancreas lipase (PPL, Sigma Aldrich, USA), morpholine acid propane sulfonate, ethylene acid diamine tetraacetate, Tris HCl, CaCl2, p-nitrophenyl butyrate (Sigma Aldrich, USA). The equipment used is a magnetic stirrer, hotplate stirrer (IKA® C-MAG HS 4), analytical scales (VIBRA HT), High-Performance Liquid Chromatography (Shimadzu LC 20 AD, Sil-20A-HT, CTO-20A, Kyoto, Japan), ultrasonicator (Krisbow, China), HPLC column (Insertsil ODS-3 5 μm 4.6 × 150 mm C18, GI Science, Tokyo, Japan), centrifugator (Universal 320, Germany), 1–10, 10–100, 100–1000 μl micropipettes and Finpipette multichannel (Thermo Scientific, USA), microtube, refrigerator (Philips), vacuum drying oven (Yamato ADP200C, USA), rotary vacuum evaporator (Buchi, Switzerland), pH meter (Eutech Instruments), a microfilter (Whatman 0.45 μm), alcohol meter, microplate reader (Glomax, USA), the licensed Design Expert v12 software (Stat-Ease, Minneapolis, MN, USA), and other supporting glass tools. Moreover, for in silico molecular docking, was used AutoDock4.2.6 (The Scripps Research Institute, La Jolla CA, USA), MarvinSketch 20.18., MGLTools 1.5.6 package, and Biova Discovery Studio v20.1.0.19295, copyright ©2019 (Dassault Systèmes Biovia Corp, VélizyVillacoublay, France).
2.2. Extraction process
2.2.1. Conventional reflux extraction method
The sample of green coffee powder (25 g) was refluxed with 250 ml of 70% ethanol at 80 °C for 1 h (starting from the first drop that fell from the mouth of the reflux device). Furthermore, the extract solution was separated from the residue using filter paper. Next, the extraction solution was evaporated using a rotary vacuum evaporator, and then the thick extract was dried at 40 °C using a vacuum oven until the dry extract was obtained.
2.2.2. Nonconventional betaine-sorbitol based NADES-UAE method
2.2.2.1. Betaine-sorbitol NADES preparation
NADES was made with a composition in the form of a mixture of betaine and sorbitol with some variations (include 0.5:1.2, 1.25:1.2, and 2:1.2 g/g) put into a Beaker glass. The composition of betaine-sorbitol was chosen and optimized according to a previous study (Syakfanaya et al., 2019). Furthermore, the mixture was stirred constantly using a magnetic stirrer and placed on a hotplate stirrer with a temperature of 80 °C accompanied by agitation at a speed of 3000 rpm until a clear solution was formed. Then, water was added (with the ratio of 1:1 v/v) to reduce the viscosity of the formed NADES solution (Syakfanaya et al., 2019; Dai et al., 2013).
2.2.2.2. Extraction process by betaine-sorbitol based NADES-UAE method
According to a previous study, the extraction process using the betaine-sorbitol-based NADES-UAE method was performed (Syakfanaya et al., 2019). Briefly, 1 g of green coffee bean powder was put into the closed glass bottle, NADES solutions were added and sonicated (with some different extraction conditions, as shown in Table 1). After sonication, NADES extraction liquid was transferred into the tube and centrifuged (4500 rpm for 17 min), then solid and liquid parts were separated by filtration using 0.45 μm cellulose acetate membrane. Filtrated liquid portions were then diluted for CGA and caffeine determination by HPLC. Thus, each extraction condition was done in three replications.
Table 1.
The factor of extraction and its level used in BBD for optimization.
| Factors | Symbol | Range and Level |
||
|---|---|---|---|---|
| Low (-1) | Medium (0) | High (1) | ||
| Betaine–sorbitol ratio (mol) | X1 | 0.5:1.2 | 1.25:1.2 | 2:1.2 |
| Extraction time (min) | X2 | 10 | 35 | 60 |
| Solid–Liquid ratio (g/mL) | X3 | 1:10 | 1:20 | 1:30 |
2.3. Determination of caffeine and CGA contents
Determination of caffeine and CGA contents was performed using a High-Performance Liquid Chromatography (HPLC) based on a previous study (Syakfanaya et al., 2019), with 272 nm (caffeine) and 326 nm (CGA) UV-Vis detection. Briefly, the sample (100 μL) was dissolved with ethanol (80% v/v) and homogenized with a shaker, then filtered by a syringe filter (0.45 μm). Next, each 20 μL of standard or sample solution was injected. Finally, the mobile phase was pumped at a 1 mL/min rate using a gradient profile from 90% solvent A and 10% solvent B for 20 min. Then converted to 80% solvent A and 20% solvent B for 10 min isocratic, then returned to the original state (90% solvent A and 10% solvent B) for 5 min (Navarra et al., 2017; Yuniarti et al., 2019a). Caffeine and CGA (with each multilevel series concentration from 0.1 to 100 μg/mL, respectively) were used as standards.
The CGA and caffeine contents in the extract were determined by comparing the area under curve (AUC) with standards. The linear regression equations were obtained and used to calculate CGA and caffeine in each extract, as follows:
| For CGA content: Y = 46057X – 26606 (with R2 = 0.999) | (1) |
| For caffeine content: Y = 79580X – 24933 (with R2 = 0.999) | (2) |
2.4. Experimental design for optimization using Response Surface Methodology
Extraction of the green coffee bean was carried out in three different factors, betaine-sorbitol ratio (X1), solid – NADES ratio (X2), extraction time (X3), and three levels (Table 1). Those factors and levels were considered significant to achieve optimum extraction conditions characterized by CGA and caffeine content as dependent variables. The variations of extraction were then simulated by the Response Surface Methodology (RSM) with Box-Behnken Design (BBD) method to obtain 17 research samples run. The second-order polynomial model was used in the response surface analysis to predict the dependent variables and evaluate each extraction factor's effect. The model is explained by the following equation (Peng et al., 2020):
| (3) |
Experimental analysis and calculation of predicted extraction values were performed using Design Expert 12.0 software (Stat-Ease, Minneapolis, MN, USA). The significance of the model was determined by analysis of variance (ANOVA), and R2 values expressed the fitness of the model.
2.5. Inhibition of lipase activity assay
2.5.1. In vitro activity assay
Dechakhamphu and Wongchum, 2015 used the inhibition test of lipase activity. The test was carried out using p-Nitrophenyl Butyrate (p-NPB) as a substrate and Porcine Pancreas Lipase (PPL) as an enzyme (Dechakhamphu and Wongchum, 2015). Briefly, a total of 20 μL of each sample and standard in 70% ethanol (with some different concentration of 6.25, 12.5, 25, 50, 100, and 200 μg/mL), 6 μL of lipase enzyme, and 170 μL of Tris buffer were put in every 96 well microplates, incubated for 15 min at 37 °C. Then, 2 μL p-NPB substrate (10 mmol/L) was added and incubated again for 30 min at 37 °C. Next, a blank of each concentration was made without the addition of the enzyme solution. The lipase inhibition test was determined by measuring absorption using the spectrophotometric principle on the microplate reader Glomax at a wavelength of 405 nm. Measured uptake was absorption from hydrolysis of p-nitrophenyl butyrate to p-nitrophenol, which is characterized by discoloration. Next, a calculation of the inhibitory percentage and inhibition concentration 50 (IC50) value was carried out (Dechakhamphu and Wongchum, 2015). The percentage inhibitory activity was examined by equation formula, as follows:
| (4) |
2.5.2. In silico molecular docking analysis
Molecular docking study of compound 1 methoxy undecyl phosphinic acid (MUP), 2 caffeine, 3 CGA, 4 caffeic acids, 5 quinic acids, and 6 pyrocatechol were performed using AutoDock4.2.6. Software (The Scripps Research Institute, La Jolla, CA, USA). The 3D structure of pancreatic lipase with PDB code: 1LPB (Egloff et al., 1995) was obtained from the RCSB Protein Data Bank (www.rcsb.org/structure/1LPB). The 3D structure energy of all compounds was minimized using the MMFF94 force field on MarvinSketch 20.18. The docking input file was generated using the MGLTools 1.5.6 package. The binding site of MUP was defined using a dimension size of x: 20; y: 32; z: 22 with a spacing of 0.375 Å centered on x: 8.692; y: 22.943; z: 52.624. Each compound's best-scoring poses were carried out by 100 runs of the Lamarckian genetic algorithm on AutoDock4. The protein-ligand complexes were visually analyzed using Biovia Discovery Studio v20.1.0.19295, copyright ©2019 (Dassault Systèmes Biovia Corp, VélizyVillacoublay, France).
3. Results
3.1. Extraction process and determination of CGA and caffeine contents
In this study, the design and optimization of the extraction method were conducted based on the green extraction principles approach to obtain efficient extraction conditions with optimum target compounds from natural products. The extraction conditions refer to previous studies (Syakfanaya et al., 2019). Different contents of CGA and caffeine were obtained according to HPLC analysis from the sample obtained by reflux and betaine-sorbitol NADES-UAE methods. The results obtained using both Eqs. (1) and (2), respectively, were 1.0997 mg/g CGA and 1.2974 mg/g caffeine contents in green coffee bean extract with reflux methods. Whereas, in the betaine-sorbitol NADES-UAE method with various conditions, CGA and caffeine contents were obtained with the range of 3.08–10.65 mg/g and 1.70–4.98 mg/g, respectively.
3.2. Optimization of betaine-sorbitol NADES-UAE method
This study used the betaine-sorbitol NADES-UAE method to extract green coffee beans with BBD approach (with three factors and three levels) was chosen as process parameters, which included betaine-sorbitol or NADES ratio, extraction time, and comparison of sample and solvent or solid-liquid ratio. Determination of process parameters is something that influences the efficiency of extraction performance.
In Table 2, the extraction condition of green coffee beans with NADES betaine-sorbitol solvent showed that the maximum contents were obtained from run 10th with a CGA yield of 10.65 mg/g dried sample and caffeine content 4.98 mg/g dried sample. Moreover, the minimum contents (CGA and caffeine) were obtained from run 6th and 14th for CGA and run 2nd for caffeine content. Furthermore, the data was analyzed to determine the optimum condition of the betaine-sorbitol NADES-UAE method using licensed Design-Expert v12 software with multilinear regression. The best models of multilinear regression equation were given based on the results of analysis using the Eq. (3), as follow:
| Y1 = 6.89 – 0.2975X1 + 2.17X2 + 1.29X3 – 0.9675X1X3 – 2.01X12 – 3.47X12X2 – 2.02X12X3 + 1.14X1X22, with R2 value of 0.78 | (5) |
| Y2 = 3.10 + 0.035X1 + 0.812X2 + 0.828X3 – 0.44X1X3 – 0.812X12 – 1.28X12X2 – 0.71X12X3, with R2 value = 0.75 | (6) |
Where Y1 is the yield of CGA (mg/g), Y2 is the yield of Caffeine (mg/g), X1 is betaine: sorbitol or NADES ratio, X2 is extraction time, X3 is the solid-liquid ratio, and R2 = the correlation coefficient.
Table 2.
Experimental design of CGA and caffeine content extraction in green coffee beans.
| Run | X1: NADES Ratio (mol) | X2: Extraction Time (min) | X3: Solid-Liquid Ratio (g/mL) | Y1: CGA Content (mg/g) | Y2: Caffeine Content (mg/g) |
|---|---|---|---|---|---|
| 1 | 1.25:1.2 | 10 | 1:30 | 5.50 + 1.82 | 2.97 + 0.70 |
| 2 | 1.25:1.2 | 10 | 1:10 | 3.74 + 3.62 | 1.70 + 1.34 |
| 3 | 1.25:1.2 | 35 | 1:20 | 8.41 + 6.85 | 3.70 + 2.57 |
| 4 | 1.25:1.2 | 60 | 1:10 | 7.26 + 4.15 | 2.94 + 1.38 |
| 5 | 2:1.2 | 10 | 1:20 | 7.37 + 0.68 | 3.27 + 0.22 |
| 6 | 0.5:1.2 | 60 | 1:20 | 3.08 + 2.30 | 1.74 + 0.93 |
| 7 | 1.25:1.2 | 35 | 1:20 | 7.86 + 3.72 | 3.42 + 1,33 |
| 8 | 2:1.2 | 60 | 1:20 | 3.66 + 3.43 | 1.85 + 1.31 |
| 9 | 1.25:1.2 | 35 | 1:20 | 8.16 + 3.29 | 3.56 + 1.20 |
| 10 | 1.25:1.2 | 60 | 1:30 | 10.65 + 0.32 | 4.98 + 0.13 |
| 11 | 0.5:1.2 | 35 | 1:30 | 5.61 + 2.58 | 3.11 + 0.98 |
| 12 | 0.5:1.2 | 35 | 1:10 | 5.14 + 5.19 | 1.99 + 1.69 |
| 13 | 0.5:1.2 | 10 | 1:20 | 4.58 + 1.34 | 2.16 + 0.45 |
| 14 | 2:1.2 | 35 | 1:30 | 3.08 + 2.25 | 1.77 + 0.83 |
| 15 | 1.25:1.2 | 35 | 1:20 | 3.78 + 1.32 | 1.83 + 0.53 |
| 16 | 1.25:1.2 | 35 | 1:20 | 6.62 + 2.28 | 2.77 + 0.87 |
| 17 | 2:1.2 | 35 | 1:10 | 6.48 + 2.56 | 2.41 + 0.76 |
Based on analysis of variance (ANOVA) from CGA content, as shown in Table 3. The Model F-value was 3.46, which implies the Model was significant (p-value >0.005). Moreover, the F-value significance of “Lack of Fit” was 0.1567 (p-value > 0.005), which shows not significant or 94.99% occur due to extraction condition factors used. In this case, X2, X12, X12X2 were significant model terms.
Table 3.
Analysis of Variance for CGA content as a response.
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 58.43 | 8 | 7.30 | 3.46 | 0.0490 |
| X1 | 0.354 | 1 | 0.354 | 0.1679 | 0.6927 |
| X2 | 18.79 | 1 | 18.79 | 8.91 | 0.0174 |
| X3 | 6.63 | 1 | 6.63 | 3.15 | 0.1141 |
| X1X3 | 3.74 | 1 | 3.74 | 1.78 | 0.2193 |
| X12 | 17.14 | 1 | 17.14 | 8.13 | 0.0214 |
| X12X2 | 24.08 | 1 | 24.08 | 11.42 | 0.0096 |
| X12X3 | 8.16 | 1 | 8.16 | 3.87 | 0.0847 |
| X1X22 | 2.60 | 1 | 2.60 | 1.23 | 0.2991 |
| Residual | 16.86 | 8 | 2.11 | ||
| Lack of Fit | 2.28 | 4 | 0.5710 | 0.1567 | 0.9499 |
| Pure Error |
14.58 |
4 |
3.65 |
||
| Cor Total | 73.30 | 16 |
Table 4 demonstrated the results of an analysis of variance (ANOVA) from the caffeine content as a response. The Model F-value was 3.75, which indicates the Model was significant (refers to p-value >0.005). On the other hand, the “Lack of Fit” F-value was 0.3348, which indicated that p-value >0.005 was not significant or 86.94% come to pass because the effect of the extraction condition was used. In this case, X2, X3, X12, X12X2 were significant model terms.
Table 4.
Analysis of Variance for caffeine content as a response.
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 9.88 | 7 | 1.41 | 3.75 | 0.0347 |
| X1 | 0.0098 | 1 | 0.0098 | 0.1679 | 0.6927 |
| X2 | 2.64 | 1 | 2.64 | 8.91 | 0.0174 |
| X3 | 2.74 | 1 | 2.74 | 3.15 | 0.1141 |
| X1X3 | 0.7744 | 1 | 0.7744 | 1.78 | 0.2193 |
| X12 | 2.79 | 1 | 2.79 | 8.13 | 0.0214 |
| X12X2 | 3.26 | 1 | 3.26 | 11.42 | 0.0096 |
| X12X3 | 1.00 | 1 | 1.00 | 3.87 | 0.0847 |
| Residual | 3.38 | 9 | 0.3761 | ||
| Lack of Fit | 0.9985 | 5 | 0.1997 | 0.3348 | 0.8694 |
| Pure Error |
2.39 |
4 |
0.5965 |
||
| Cor Total | 13.26 | 16 |
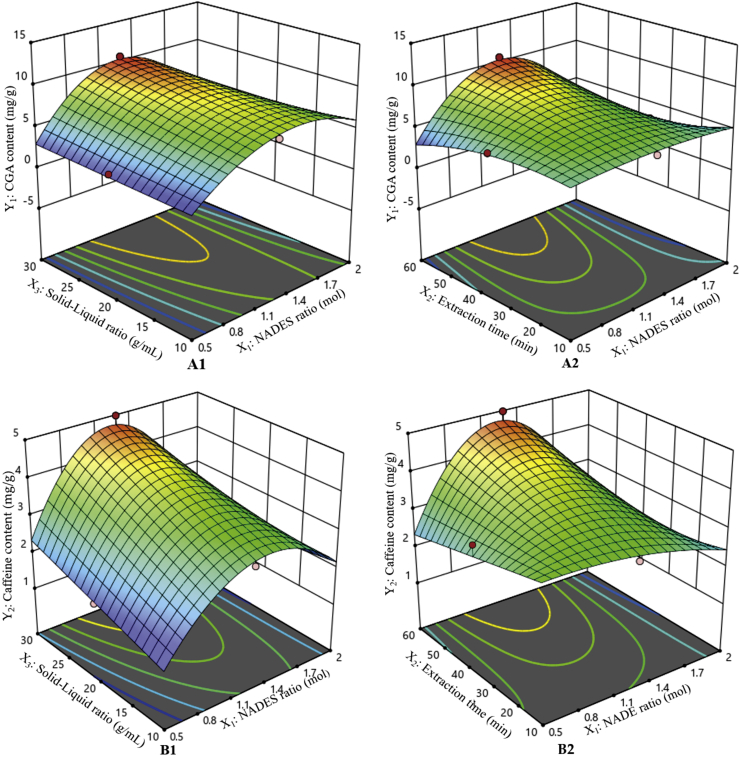
Based on the results obtained by the RSM (in Figure 1), it shows the three dimensions (3D) of response surface plots for mutual interaction between responses (CGA and caffeine content) and factors. Figure 1 represented the 3D-surface plots of factors (NADES ratio with solid-liquid ratio and NADES ratio with extraction time) on CGA and caffeine content as responses.
Figure 1.
Response surface plots representing the effect of solid-liquid ratio and NADES ratio on the responses of CGA content (A1) and Caffeine content (B1); and the effect of extraction time and NADES ratio on the responses of (A2) CGA content and (B2) Caffeine content.
3.3. Inhibition of lipase activity assay
3.3.1. In vitro assay results
Table 5 shows the percentage inhibition of each sample (calculating using eq. (4)) and demonstrated that orlistat had an inhibition concentration (IC50) value of 8.86 μg/mL. The percentage of inhibition by the test sample in green coffee bean extract with NADES betaine-sorbitol insignificantly different from the inhibition of activity produced by positive control of orlistat and both standards. The percentage inhibition of green coffee bean extract produced at a test concentration of 25 ppm has exceeded 50%, equal to 50.79%. Thus, the inhibition of the lipase activity in green coffee bean extract with NADES betaine-sorbitol has an IC50 value of 18.02 μg/mL. In contrast, the extract of green coffee beans with reflux cannot be calculated because the percentage of inhibition obtained up to 200 ppm does not reach 50%. Therefore, it can be interpreted that the contents of CGA and caffeine in green coffee beans affect the ability to inhibit lipase activity.
Table 5.
Percentage of porcine pancreas lipase enzyme inhibition and IC50 value.
| Inhibitor | Concentration (μg/mL) | Inhibition Percentage (%) | IC50 Value (μg/mL) |
|---|---|---|---|
| Robusta Green Coffee Beans Extract of NADES-UAE from Optimum condition | 200 | 79.63 + 8.03 | 18.02 |
| 100 | 76.09 + 0.90 | ||
| 50 | 66.25 + 6.36 | ||
| 25 | 50.79 + 6.34 | ||
| 12.5 | 43.52 + 11.22 | ||
| 6.25 | 37.60 + 6.45 | ||
| Robusta Green Coffee Beans Extract of Reflux Method | 200 | 39.57 + 2.51 | >200 |
| 100 | 32.05 + 7.81 | ||
| 50 | 28.53 + 7.94 | ||
| 25 | 22.30 + 8.50 | ||
| 12.5 | 18.91 + 1.37 | ||
| 6.25 | 14.59 + 10.43 | ||
| Orlistat | 200 | 93.29 + 4.60 | 8.86 |
| 100 | 89.41 + 10.27 | ||
| 50 | 77.31 + 7.20 | ||
| 25 | 55.57 + 9.95 | ||
| 12.5 | 52.38 + 2.33 | ||
| 6.25 | 50.60 + 11.39 | ||
| Caffeine Standard | 200 | 83.88 + 2.08 | 15.59 |
| 100 | 81.44 + 2.08 | ||
| 50 | 76.19 + 1.78 | ||
| 25 | 64.60 + 6.59 | ||
| 12.5 | 38.64 + 0.88 | ||
| 6.25 | 34.04 + 3.18 | ||
| CGA Standard | 200 | 89.63 + 0.44 | 11.90 |
| 100 | 86.74 + 2.75 | ||
| 50 | 77.57 + 1.82 | ||
| 25 | 64.23 + 0.02 | ||
| 12.5 | 59.23 + 0.40 | ||
| 6.25 | 29.43 + 2.72 | ||
| NADES Solution | Pure | -29.40 + 8.84 | - |
| 100 | -30.14 + 2.11 |
3.3.2. In silico molecular docking
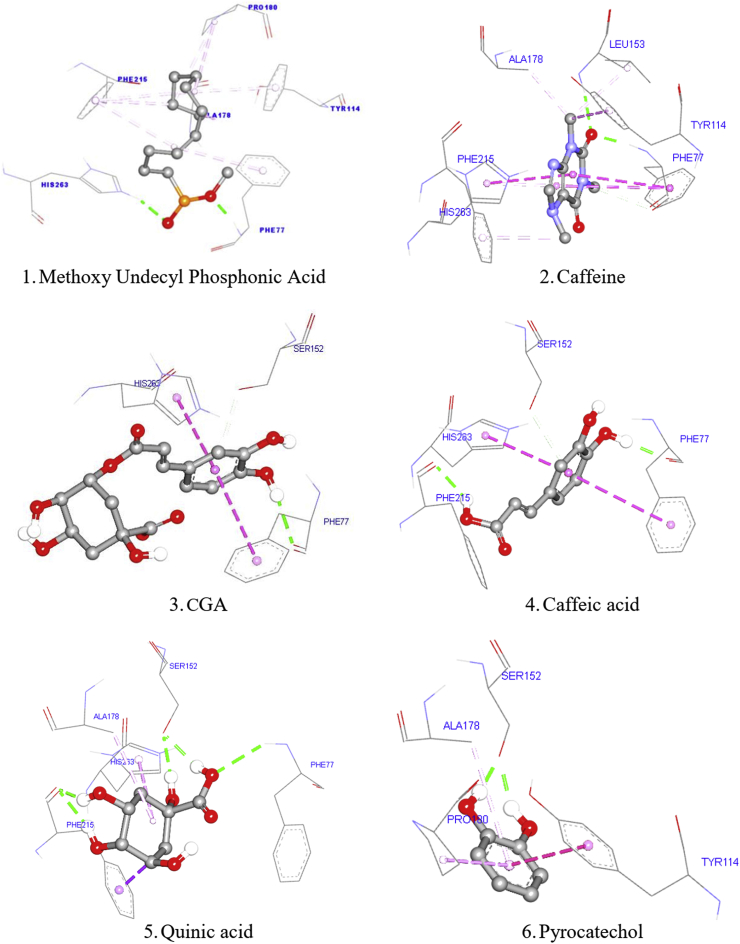
Molecular docking studies were performed to predict the free binding energy (ΔG) and interaction of ligand test (with compound 1 methoxy undecyl phosphinic acid (MUP) as a native ligand, 2 caffeine, 3 CGA, 4 caffeic acid, 5 quinic acid, and 6 pyrocatechols as ligand-target) in the active site of protein receptors as can be seen in Table 6. The docking of the native ligand (methoxy undecyl phosphonic acid = MUP) gave an RMSD value of 1.940 Å (<2 Å). Furthermore, the docking results indicated that the grid box and grid size were valid with a docking score (ΔG) value of -5.3 kcal/mol and a cluster of 54% with 100 times running. The binding interactions of native ligand (MUP), caffeine, and CGA on the pancreatic lipase colipase complex inhibited (PDB code: 1LPB) were shown in Figure 2.
Table 6.
The binding interactions of methoxy undecyl phosphonic acid (mup = native ligand), caffeine, CGA, caffeic acid, quinic acid, and pyrocatechol with 1LPB.
| Compound | Docking Score of ΔG (kcal mol-1) | Interaction | |
|---|---|---|---|
| 1 | Native Ligand (Methoxy Undecyl Phosphonic Acid) | -5.3 | Phe77, Tyr114, Ala178, Pro180, Phe215, His263 |
| 2 | Caffeine | -6.73 | Phe77, Tyr114, Leu153, Ala178, Phe215, His263 |
| 3 | CGA | -5.54 | Phe77, Ser152, Phe215, Ala259, His263 |
| 4 | Caffeic acid | -6.0 | Phe77, Ser125, Phe215, His263 |
| 5 | Quinic acid | -4.49 | Phe77, Ser125, Ala178, Phe215, His263 |
| 6 | Pyrocatechol | -3.9 | Tyr114, Ser152, Ala178 |
Figure 2.
Binding interaction of methoxy undecyl phosphonic Acid (1), caffeine (2), CGA (3), caffeic acid (4), quinic acid (5), and pyrocatechol (6) in the binding site of the pancreatic lipase colipase (PDB code:1LPB).
4. Discussion
This research shows that betaine-sorbitol NADES-UAE methods can extract target secondary metabolites (CGA and caffeine) in Robusta green coffee beans. This method was chosen because it has some advantages: time and cost efficiency, environmentally friendly, low toxicity and edible, biodegradability, comfortable for use (Paiva et al., 2014; Espino et al., 2018; Ahmad et al., 2020), and availability in the laboratory. By comparing the results of green coffee bean extraction between conventional reflux and non-conventional betaine-sorbitol NADES-UAE methods, the betaine-sorbitol NADES-UAE method showed 7.5 times higher contents CGA and four times higher contents of caffeine than the 70% ethanol with reflux method.
Some variable factors of extraction conditions should be considered in the target secondary metabolite extraction using the betaine-sorbitol NADES-UAE method, including betaine-sorbitol ratio, extraction time, and solid-liquid ratio (Bubalo et al., 2016; de los Angeles Fernandez et al., 2017). These three factors affect the extraction efficiency of the target secondary metabolite (CGA and caffeine) from the sample (Syakfanaya et al., 2019; Yuniarti et al., 2019a).
The optimum condition of betaine-sorbitol NADES-UAE method was recommended related to RSM analysis results using both Eqs. (5) and (6) from Design-Expert v12 application as follow: the NADES (betaine-sorbitol) ratio of 1.25:1.2 mol and the solid-liquid ratio of 1:30 g/mL for 60 min extraction time with the prediction of CGA and caffeine of 10.34 ± 1.14 and 4.74 ± 0.48 mg/g, respectively. Furthermore, a confirmation test was carried out on the optimum extraction conditions obtained and the scale-up of the extract. As a result, a 10 g of sample was extracted using the optimum betaine-sorbitol NADES-UAE method and were obtained CGA contents of 10.98 mg/g sample and caffeine contents of 4.98 mg/g sample.
The effect of the betaine-sorbitol ratio was in the range from 0.5:1.2 to 2:1.2 mol. A ratio of 1.25:1.2 mol showed the highest CGA and caffeine contents result according to a study has previously reported (Syakfanaya et al., 2019). NADES with the composition of betaine-sorbitol was a mixture of two solids, where betaine as a hydrogen bond acceptor (HBA) and sorbitol as a hydrogen bond donor (HBD) (Duan et al., 2016; Leng and Suyin, 2019). NADES with a specific ratio (HBA and HBD mixture) will make the deep eutectic system, were formed a liquid below 100 °C (Zhang et al., 2012; Vian et al., 2017).
Effect of extraction time on the range of 15–60 min was demonstrated the optimum extraction time (Syakfanaya et al., 2019). The extraction time was obtained at 60 min with the highest CGA and caffeine contents in this work. This moment indicated that the solute dissolution process from the matrix sample occurs in equilibrium (Sahin and Samli, 2013) with both target compound levels in the extraction process for 60 min.
Effect of solid-liquid ratio was evaluated using different solid-liquid ratio (1:10, 1:20, and 1:30 g/mL). In this study, 1:30 g/mL shows the maximum of the target compounds extraction. This indicated that an increase in the solid-liquid ratio causes full direct contact between the target compound and the solvent (Liu et al., 2019). The maximum target compound was obtained. However, if a huge solid-liquid ratio can cause the extraction process to be inefficient, a small NADES ratio could cause an incomplete extraction process (Ozturk et al., 2018).
Next, we investigated the inhibitory potential of Robusta green coffee beans extract with the optimum betaine-sorbitol NADES-UAE and reflux method, orlistat as positive control (Lunagariya et al., 2014), CGA and caffeine standard, and NADES solution. The pancreatic lipase activity was performed in vitro and in silico method.
In this study, orlistat standard was used as the standard because, to date, only orlistat has been considered and approved by the FDA as a pancreatic lipase inhibitor. Therefore, it is only available for the long-term treatment of obesity. Orlistat is a lipstatin derivative isolated from the Gram-positive bacterium Streptomyces toxytricini and is an inhibitor of gastrointestinal and pancreatic lipases capable of preventing the absorption of approximately 30% of dietary fat (Guercioloni, 1997; Bellinger and Peikin, 2002; Othman et al., 2021).
Based on the data results on the percentage of inhibition and the IC50 value of the test samples (extracts from the optimal method and extracts from reflux), the positive control of orlistat and standard of CGA and caffeine showed that each of the test samples had lipase inhibition activity. However, the percentage inhibition of green coffee bean extract with NADES betaine-sorbitol (with IC50 of 18.02 g/mL) did not significantly differ from the inhibitory activity produced by the positive control orlistat and CGA and standard caffeine. This is due to the high content of CGA, which can form covalent bonds in serine lipase (Lunagariya et al., 2014). These findings carry relevance to the research has reported by (Almoosawi et al., 2010), where green coffee beans extract with rich CGA produced a pancreatic lipase inhibition with the percentage inhibition ranging from 11.8 to 61.5%. In addition, another study stated that decaffeination of green coffee bean extract inhibits lipase activity by reducing fat emulsion surface area because lipase activity is regulated by the oil and water interface area (Narita et al., 2012).
Meanwhile, the NADES solution had no inhibitory activity on the pancreatic lipase enzyme, indicating that the content of CGA and caffeine compounds contained in the extract was responsible for inhibiting activity against pancreatic lipase. However, the mechanism of this inhibition was not yet known. The findings in this study indicated that the optimum condition of the betaine-sorbitol NADES-UAE method with increased levels of target compounds (CGA and caffeine) in the extract had a significant correlation with pancreatic lipase inhibitory activity. Thus, the anti-obesity effect (through pancreatic lipase inhibitory activity) from coffee drinking is highly dependent on the levels of CGA and caffeine in the drink.
In order to describe the prediction of the action mechanism, a molecular docking analysis was performed. Based on the docking results of the ligand test, the binding energy was better than the native ligand with a docking score (ΔG) value of -6.73 kcal/mol (caffeine) and -5.54 kcal/mol (for CGA). However, under certain heat conditions, the chlorogenic acid contained in green coffee beans is unstable to heat and is broken down by the roasting process. Therefore, the results of the formation of compounds (caffeic acid, quinic acid, and pyrocatechol) were analyzed for their interactions by in silico molecular docking, resulting in a docking score (ΔG) in the formation of caffeic acid (-6.0 kcal/mol), quinic acid (-4.49 kcal/mol), and pyrocatechol (-3.9 kcal/mol). Meanwhile, betaine with a docking score (ΔG) value of -4.05 kcal/mol and sorbitol with docking score (ΔG) value of -3.05 kcal/mol have weaker binding energy. The native ligand of methoxy undecyl phosphonic acid (MUP) formed two hydrogen bonding with Phe77, His263 residues (Menteşe et al., 2018; Noor et al., 2019). Compound 1 (MUP) was also found to form a hydrophobic interaction with Tyr114, Ala178, Pro180, Phe215 residues. Compound 2 (caffeine) showed two hydrogen bonding with Phe77, Leu153 residues and was also found to form a hydrophobic interaction with His263, Tyr114, Ala178, Phe215 residues. Compound 3 (CGA) formed four hydrogen bonds with Phe77, Ser152, Phe215, Ala259 residues, and other hydrophobic interactions with His263 residue. Compound 4 (caffeic acid) formed hydrogen bonds with Phe77, Ser152, Phe215 residues, and other hydrophobic interactions with His263, Phe77 residue. Compound 5 (quinic acid) formed hydrogen bonds with Phe77, Ser152, Phe215 residues, and other hydrophobic interactions with His263, Phe215, Ala178 residue, and Compound 6 (pyrocatechols) formed hydrogen bonds with Ser152, Ala178 residues, and other hydrophobic interactions with Tyr114, Ala178 residue. In this study, the pancreatic lipase colipase (His263 and Phe77) with ligand (caffeine, CGA, caffeic acid, and quinic acid) interactions have similarities between the native ligand (MUP) bound ligands. Based on the in silico molecular docking analysis, caffeic acid and quinic acid which are formed from changes in CGA under certain heat conditions, still have potential interactions with receptor of pancreatic lipase. The in vitro activity assay has been successfully confirmed by the in silico molecular docking analysis.
5. Conclusion
After a statistical optimization process, we showed that the betaine-sorbitol NADES-UAE method provides an excellent extraction efficiency. The optimized extraction conditions were as follows: the betaine-sorbitol ratio of 1.25:1.2 mol and the solid-liquid ratio of 1:30 g/mL for 60 min extraction time. While Robusta green coffee beans extract with rich CGA and caffeine were obtained using the optimum betaine-sorbitol NADES-UAE method, which has a potential inhibition activity on pancreatic lipase in vitro. Meanwhile, CGA and caffeine have a direct role in the inhibition of pancreatic lipase activity in silico.
Declarations
Author contribution statement
Islamudin Ahmad: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Adisya Miftah Syakfanaya: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Azminah Azminah: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Fadlina Chany Saputri: Analyzed and interpreted the data.
Abdul Mun'im: Conceived and designed the experiments; Wrote the paper.
Funding statement
This work was supported by Universitas Indonesia, Indonesia (NKB-1459/UN2.RST/HKP.05.00/2020).
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
This research was supported by the Ministry of Research, Technology and Higher Education, the Republic of Indonesia, and the Directorate of Research and Community Engagement, Universitas Indonesia.
References
- Ahmad I., Pertiwi A.S., Kembaren Y.H., Rahman A., Mun'im A. Application of natural deep eutectic solvent-based ultrasonic assisted extraction of total polyphenolic and caffeine content from Coffee Beans (Coffea Beans L.) for instant food products. J. Appl. Pharmaceut. Sci. 2018;8(8):138–143. [Google Scholar]
- Ahmad I., Arifianti A.E., Sakti A.S., Saputri F.C., Mun'im A. Simultaneous natural deep eutectic solvent-based compounds of cinnamon Bark and sappan wood as a dipeptidyl peptidase IV inhibitor. Molecules. 2020;25(17):3832–3843. doi: 10.3390/molecules25173832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alias N., Leow T.C., Mohammad Ali M.S., Tajudin A.A., Salleh A.B., Raja Abul Rahman R.N.Z. Anti-obesity potential of selected tropical plants via pancreatic lipase inhibition. Adv. Obes. Weight Manag. Cont. 2017;6(4):122–131. [Google Scholar]
- Almoosawi S., McDougall G.J., Fyfe L., Al-Dujaili E.A.S. Investigating the inhibitory activity of green coffee and cacao bean extracts on pancreatic lipase. Nutr. Bull. 2010;35(3):207–212. [Google Scholar]
- Bellinger A., Peikin S.R. Orlistat: its current status as an anti-obesity drug. Eur. J. Pharmacol. 2002;440(2–3):109–117. doi: 10.1016/s0014-2999(02)01422-x. [DOI] [PubMed] [Google Scholar]
- Boyko N., Zhilyakova E., Malyutina A., Novikov O., Pisarev D., Abramovich R., Potanina O., Lazar S., Mizina P., Sahaidak-Nikitiuk R. Studying and modeling of the extraction properties of the natural deep eutectic solvent and sorbitol-based solvents in regard to biologically active substances from glycyrrhizae roots. Molecules. 2020;25(7):1–10. doi: 10.3390/molecules25071482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusco M.J., Steinley D., Cradit J.D. An exact algorithm for hierarchically well-formulated subsets in second-order polynomial regression. Technometrics. 2009;51(3):306–315. [Google Scholar]
- Bubalo M., Curko N., Tomasevic M., Ganic K.K., Redovnikovic I.R. Green extraction of grape skin phenolics by using deep eutectic solvents. Food Chem. 2016;200:159–166. doi: 10.1016/j.foodchem.2016.01.040. [DOI] [PubMed] [Google Scholar]
- Choi B.K., Park S.B., Lee D.R., Lee H.J., Jin Y.Y., Yang S.H., Suh J.W. Green coffee bean extract improves obesity by decreasing body fat in high-fat diet-induced obese mice. Asian Pac. J. Trop. Med. 2016;9(7):635–643. doi: 10.1016/j.apjtm.2016.05.017. [DOI] [PubMed] [Google Scholar]
- da Silva D.T., Pauletto R., da Silva Cavalheiro S., Bochi V.C., Rodrigues E., Weber J., de Bona da Silva C., Morisso F.D.P., Barcia M.T. Natural deep eutectic solvents as a biocompatible tool for the extraction of blueberry anthocyanins. J. Food Compos. Anal. 2020;89:103470. [Google Scholar]
- Dahmoune F., Spigno G., Moussi K., Remini H., Cherbal A., Madani K. Pistacia lentiscus leaves as a source of phenolic compounds: microwave-assisted extraction optimized and compared with ultrasound-assisted and conventional solvent extraction. Ind. Crop. Prod. 2014;61:31–40. [Google Scholar]
- Dai Y. 2013. Natural Deep Eutectic Solvents and Their Application in Natural Product Research and Development.www.sps-print.eu Available at: [Google Scholar]
- Dai Y., Witkamp G.J., Verpoorte R., Choi Y.H. Natural deep eutectic solvents as a new extraction media for phenolic metabolites in Carthamus tinctorius L. Anal. Chem. 2013;85(13):6272–6278. doi: 10.1021/ac400432p. [DOI] [PubMed] [Google Scholar]
- Dechakhamphu A., Wongchum N. Screening for anti-pancreatic lipase properties of 28 traditional Thai medicinal herbs. Asian Pac. J. Trop. Biomed. 2015;5(12):1042–1045. [Google Scholar]
- de los Angeles Fernandez M., Espino M., Gomez F.J.V., Silva M.F. Novel approaches mediated by tailor-made green solvents for the extraction of phenolic compounds from agro-food industrial by-products. Food Chem. 2017;238:671–678. doi: 10.1016/j.foodchem.2017.06.150. [DOI] [PubMed] [Google Scholar]
- Duan L., Dou L., Guo L., Li P., Liu E. Comprehensive evaluation of deep eutectic solvents in extraction of bioactive natural products. ACS Sustain. Chem. Eng. 2016;4(4):2405–2411. [Google Scholar]
- Egloff M.-P., Marguet F., Buono G., Verger R., Cambillau C., van Tilbeurhg H. A resolution structure of the pancreatic lipase-colipase complex. Biochemistry. 1995;34:2751–2762. doi: 10.1021/bi00009a003. [DOI] [PubMed] [Google Scholar]
- Espino M., de los Angeles Fernandez M., Gomez F.J.V., Boiteux J., Silva M.F. Green analytical chemistry metrics: towards a sustainable phenolics extraction from medicinal plants. Microchem. J. 2018;141:438–443. [Google Scholar]
- Guercioloni R. Mode of action of orlistat. Int. J. Obes. Relat. Metabol. Dis. 1997;21(Suppl 3):S12–23. [PubMed] [Google Scholar]
- Kimura H., Futami Y., Tarui S.i., Shinomiya T. Activation of human pancreatic lipase activity by calcium and bile salts. J. Biochem. 1982;92(1):243–251. doi: 10.1093/oxfordjournals.jbchem.a133920. [DOI] [PubMed] [Google Scholar]
- Leng K.Y., Suyin G. Natural deep eutectic solvent (NADES) as a greener alternative for the extraction of hydrophilic (polar) and lipophilic (non-polar) phytonutrients. Key Eng. Mater. 2019;797(4):20–28. [Google Scholar]
- Liu Y., Li J., Fu R., Zhang L., Wang D., Wang S. Enhanced extraction of natural pigments from Curcuma longa L. using natural deep eutectic solvents. Ind. Crop. Prod. 2019;140:111620. [Google Scholar]
- Lunagariya N.A., Patel N.K., Jagtap S.C., Bhutani K.K. Inhibitors of pancreatic lipase: state of the art and clinical perspectives. Excli J. 2014;13:897–921. [PMC free article] [PubMed] [Google Scholar]
- Meeker W., Hahn G., Escobar L. second ed. John Wiley & Sons; New Jersey: 2017. Statistical Interval, A Guide for Practioner and Researcher. [Google Scholar]
- Menteşe E., Yilmaz F., Emirik M., Ulker S., Kahveci B. Synthesis, molecular docking and biological evaluation of some benzimidazole derivatives as potent pancreatic lipase inhibitors. Bioorg. Chem. 2018;76:478–486. doi: 10.1016/j.bioorg.2017.12.023. [DOI] [PubMed] [Google Scholar]
- Narita Y., Iwai K., Fukunaga T., Nakagiri O. Inhibitory activity of chlorogenic acids in decaffeinated green coffee beans against porcine pancreas lipase and effect of a decaffeinated green coffee bean extract on an emulsion of olive oil. Biosc. Biotech. Biochem. 2012;76(12):2329–2331. doi: 10.1271/bbb.120518. [DOI] [PubMed] [Google Scholar]
- Navarra G., Moschetti M., Guarrasi V., Mangione M.R., Militello V., Leone M. Simultaneous determination of caffeine and chlorogenic acids in green coffee by UV/Vis spectroscopy. J. Chem. 2017;2017:1–8. [Google Scholar]
- Naveed M., Hejazi V., Abbas M., Kamboh A.A., Jhan G.J., Shumzaid M., Ahmad F., Babazadah D., FangFang X., Modarresi-Ghazani F., WenHua L., XiaoHui Z. Chlorogenic acid (CGA): a pharmacological review and call for further research. Biomed. Pharmacother. 2018;97:67–74. doi: 10.1016/j.biopha.2017.10.064. [DOI] [PubMed] [Google Scholar]
- Noor Z.I., Ahmed B., Rehman H.M., Qamar M.T., Froeyen M., Ahmad S., Mirza M.U. In vitro antidiabetic, anti-obesity and antioxidant analysis of Ocimum basilicum aerial biomass and in silico molecular docking simulations with alpha-amylase and lipase enzymes. Biology. 2019;8(4):92–112. doi: 10.3390/biology8040092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Othman Z.A., Zakaria Z., Suleiman J.B., Wan Ghazali W.S., Mohamed M. Anti-atherogenic effects of orlistat on obesity-induced vascular oxidative stress rat model. Antioxidants. 2021;10(2):1–16. doi: 10.3390/antiox10020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozturk B., Parkinson C., Gonzalez-Miquel M. Extraction of polyphenolic antioxidants from orange peel waste using deep eutectic solvents. Separ. Purif. Technol. 2018;206:1–13. [Google Scholar]
- Paiva A., Creveiro R., Aroso I., Martins M., Reis R.L., Duarte A.R.C. Natural deep eutectic solvents−solvents for the 21st century. Am. Chem. Soc. Sustain. Chem. Eng. 2014;2:1063–1071. [Google Scholar]
- Peng Y., Khaled U., Al-Rashed A.A.A., Meer R., Goodarzi m., Sarafiraz M.M. Potential application of Response Surface Methodology (RSM) for the prediction and optimization of thermal conductivity of aqueous CuO (II) nanofluid: a statistical approach and experimental validation. Physic A: Stat. Mech. Appl. 2020;554:124353. [Google Scholar]
- Piepel G., Szychowski J., Leoppky J. Augmenting scheffe linear mixture models with squared and/or crossproduct terms. J. Qual. Technol. 2002;34(3):297–314. [Google Scholar]
- Rodríguez-Juan E., Rodrıguez-Romero C., Fernandez-Bolanos J., Florido M.C., Garcia-Borrego A. Phenolic compounds from virgin olive oil obtained by natural deep eutectic solvent (NADES): effect of the extraction and recovery conditions. J. Food Sci. Technol. 2021;58(2):552–561. doi: 10.1007/s13197-020-04567-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin S., Samli R. Optimization of olive leaf extract obtained by ultrasound-assisted extraction with response surface methodology. Ultrason. Sonochem. 2013;20(1):595–602. doi: 10.1016/j.ultsonch.2012.07.029. [DOI] [PubMed] [Google Scholar]
- Seyedan A., Alshawash M.A., Alshagga M.A., Koosha S., Mohamed Z. Medicinal plants and their inhibitory activities against pancreatic lipase: a review. Evid. base Compl. Alternative Med. 2015;2015:1–14. doi: 10.1155/2015/973143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syakfanaya A.M., Saputri F.C., Mun’im A. Simultaneously extraction of caffeine and chlorogenic acid from Coffea canephora bean using natural deep eutectic solvent-based ultrasonic assisted extraction. Phcog. J. 2019;11(2):267–271. [Google Scholar]
- Turnbull D., Rodricks J.v., Mariano G.F., Chowdhury F. Caffeine and cardiovascular health. Regul. Toxicol. Pharmacol. 2017;89:165–185. doi: 10.1016/j.yrtph.2017.07.025. [DOI] [PubMed] [Google Scholar]
- Vian M., Breil C., Vernes L., Chaabani E., Chemat F. Green solvents for sample preparation in analytical chemistry. Curr. Opin. Green Sustain. Chem. 2017;5:44–48. [Google Scholar]
- WHO . 2014. Obesity and Overweight. Fact Sheet No 311, Reviewed April 2020.http://www.who.int/mediacentre/factsheets/fs311/en/ [Google Scholar]
- Yuniarti E., Saputri F.C., Mun’im A. Application of the natural deep eutectic solvent choline chloridesorbitol to extract chlorogenic acid and caffeine from green coffee beans (Coffea canephora) J. Appl. Pharmaceut. Sci. 2019;9(3):82–90. [Google Scholar]
- Yuniarti E., Saputri F.C., Mun’im A. Natural deep eutectic solvent extraction and evaluation of caffeine and chlorogenic acid from green coffee beans of Coffea canephora. Indian J. Pharmaceut. Sci. 2019;81(6):1062–1069. [Google Scholar]
- Zhang Q., de Oliveira Vigier K., Royes S., Jerome F. Deep eutectic solvents: syntheses, properties and applications. Chem. Soc. Rev. 2012;41(21):7108–7146. doi: 10.1039/c2cs35178a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.