Graphical abstract
Keywords: Quercetin, Novel extraction, Noninvasive assessment, Bioavailability
Highlights
-
•
Potential sources and therapeutic properties of quercetin.
-
•
In recent years, the different novel extractions techniques for the extraction of quercetin.
-
•
Non-destructive assessment techniques for the identification of quercetin in recent years.
-
•
Innovative drug delivery strategies to improve the bioavailability and providing novel therapeutic approaches.
Abstract
Quercetin (QUR) have got the attention of scientific society frequently due to their wide range of potential applications. QUR has been the focal point for research in various fields, especially in food development. But, the QUR is highly unstable and can be interrupted by using conventional assessment methods. Therefore, researchers are focusing on novel extraction and non-invasive tools for the non-destructive assessment of QUR. The current review elaborates the different novel extraction (ultrasound-assisted extraction, microwave-assisted extraction, supercritical fluid extraction, and enzyme-assisted extraction) and non-destructive assessment techniques (fluorescence spectroscopy, terahertz spectroscopy, near-infrared spectroscopy, hyperspectral imaging, Raman spectroscopy, and surface-enhanced Raman spectroscopy) for the extraction and identification of QUR in agricultural products. The novel extraction approaches facilitate shorter extraction time, involve less organic solvent, and are environmentally friendly. While the non-destructive techniques are non-interruptive, label-free, reliable, accurate, and environmental friendly. The non-invasive spectroscopic and imaging methods are suitable for the sensitive detection of bioactive compounds than conventional techniques. QUR has potential therapeutic properties such as anti-obesity, anti-diabetes, antiallergic, antineoplastic agent, neuroprotector, antimicrobial, and antioxidant activities. Besides, due to the low bioavailability of QUR innovative drug delivery strategies (QUR loaded gel, QUR polymeric micelle, QUR nanoparticles, glucan-QUR conjugate, and QUR loaded mucoadhesive nanoemulsions) have been proposed to improve its bioavailability and providing novel therapeutic approaches.
1. Introduction
QUR (3, 5, 7, 3′, 4′-pentahydroxyflavone) is a dietary flavonoid, chemically comprised of three benzene rings and five hydroxyl groups, and abundantly present in flowers, stems, wine, tea, vegetables, bark roots, and vegetables (Table 1). QUR is a crystalline, yellow solid with a bitter taste, water-insoluble, and is an aglucone or aglycon which does not include any carbohydrate molecule in its structure and it imparts beautiful colors to a variety of flowers [1].
Table 1.
Concentrations of QUR in some selected plant sources and their therapeutic properties.
| Common name | Scientific name | Sources (mg/100 g) | Therapeutic properties |
|---|---|---|---|
| Raw Celery | Apium graveolens | 3.51 | Lowers blood pressure, Lowers glucose, Anti-inflammatory, Antibacterial, Antihypertensive, Wound healing, Antioxidant, Reduce depressive disorders, Neurological effects, Reduce the risk of stroke, Neuropathy, Reduce cholesterol, Reduce dyspepsia, Antineoplastic, Antiulcer, Anti-tussive, Iron deficiency anemia, Reduce osteoporosis, Anti-laxative, Antiviral, Antispasmodic, Antidiabetic, Disinfectants, Antiatherosclerotic agent, and Anti-cancer [2], [4], [5], [82], [1], [159] |
| Raw Kale | Brassica oleracea | 23.1 | |
| Raw Sweet Cherry | Prunus avium | 1.24 | |
| Welsh onion | Allium fistulosum | 5.20 | |
| Red onion | Allium cepa | 19.9 | |
| Black grapes | Vitis vinifera | 2.56 | |
| Broccoli | Brassica oleracea | 3.10 | |
| Tomato | Solanum Lycopersicum | 4.13 | |
| Coriander | Coriandrum Sativum | 5.01 | |
| Lettuce, raw | Lactuca sativa | 8.00 | |
| Raw Watercress | Nasturtium officinale | 30.0 | |
| Plums | Prunus domestica | 2.00 | |
| Apple | Malus domestica | 4.70 | |
| Cranberry | Vaccinium oxycoccus | 14.3 | |
| Okra | Abelmoschus esculentus | 21.0 |
Sources of QUR references: Source: http://www.swisstargetprediction.ch, [82], [1], [159].
Furthermore, various studies have proved QURs potent therapeutic potential due to their anti-inflammatory [2], antineoplastic [3], antioxidant [4], neuroprotective [2], antiallergic [5], and antimicrobial activities [6]. Due to these health-promoting attributes, QUR obtained extensive approval and application in the pharmaceutical industry. The antineoplastic consequences of QUR are achieved by the alteration of WNT1/β-catenin and PI3K/Akt/mTOR pathways to assist cell apoptosis [7]. Researchers have also established that QUR can overcome the extreme expression of inflammatory cytokines [8]. Hence, the flavonoid is deemed an encouraging competitor to be employed as a powerful pharmaceutical tool for illness suppressions (Table 1). Furthermore, it also has satisfactory therapeutic potential to act as anti-obesity [9], anti-diabetes [10], and to relieve Alzheimer’s disease [11]. However, QURs comparatively lower bioavailability due to their low aqueous solubility, high metabolic rate, inactive metabolic products and rapid clearance from body. Consequently, comprehensive attempts have been executed to reduce the toxicity and improve the bioavailability of the phytochemical by QUR encapsulation techniques. Currently, encapsulating delivery systems include lipid-based carriers [12], [13], polymer nanoparticles, inclusion complexes, micelles, and conjugated-based capsulations has been investigated [1]. Exceptional progress in bioavailability-related fields ensures improved therapeutics effectiveness to obtain more clinical advantages from QUR.
Keeping in view the health advantages, several novel extractions and assessment techniques have been adopted for the extraction and identification of QUR in agricultural products. Moreover, acknowledging the current high demand for food safety and quality assurance, researchers have performed experiments into exploring non-destructive, fast, and convenient novel approaches. Also, some scholars exerted their effort for QUR extraction through some emerging extraction modalities such as ultrasound-assisted extraction (UAE) [14], microwave-assisted extraction (MWAE) [15], supercritical fluid extraction (SFE) [16], enzymes extraction (EE), and [17], ultrasound-microwave extraction (UMWE) [18]. Nonetheless, scientists have also recently developed innovative non-destructive tools for QUR estimations including fluorescence spectroscopy, terahertz spectroscopy, near-infrared spectroscopy, hyperspectral imaging, Raman spectroscopy, and surface-enhanced Raman spectroscopy [19], [20].
In the last few years, some review articles have been published, among them, most of the papers focused only on different therapeutic properties, while some discussed the conventional extraction techniques for QUR from different agricultural products. However, so far no attempt has covered advances in novel extraction techniques for QUR, emerging non-invasive assessment, and no review has elaborated recent strategies for bioavailability enhancement for the phytochemical. Therefore, we tried to cover recent advances in the three aforementioned domains employed for QUR. The first part describes the novel extraction techniques for QUR, while the second section represents the applications of recent non-invasive methods for rapid assessment of the phytochemical and elaborates the strengths and weaknesses of the methods used in different studies. Furthermore, the last part highlights the bioavailability improvement approaches for QUR such as QUR loaded gel, QUR nanoparticles, QUR loaded polymeric micelle, glucan-QUR conjugate, and QUR loaded mucoadhesive nanoemulsions.
2. Novel techniques for the extraction of quercetin
Conventional extraction methods such as maceration, soxhlet, hydro distillation, and heat-reflux extraction have been performed for decades for the extraction of flavonoids. However, the techniques need long treatment times, high temperatures, involve more solvents, only trained personnel can operate the equipment, environmentally unfriendly and the systems are cost-ineffective. Therefore, to overcome these drawbacks, some alternative novel extraction techniques have been developed and successfully adopted for the extraction of QUR (Table 2). These methods include ultrasound-assisted extraction (UAE), microwave-assisted extraction (MWAE), supercritical fluid extraction (SFE), and enzyme-assisted extraction (EAE). Each technique has a unique mechanism to enhance extraction and principles and processing parameters. The novel extraction approaches facilitate shorter extraction time, involve less organic solvent, and are environmentally friendly.
Table 2.
Novel extraction of QUR from agricultural products.
| Technique | Matrix | Optimum conditions and extraction yield | References |
|---|---|---|---|
| UAE | Euonymus alatus (Thunb.) Sieb | Aqueous ethanol 70 %, extraction time 3 × 30 min, solvent: sample ratio 40:1 (v/w), and QUR extraction yield 0.29 mg/g | [160] |
| Raphanus sativus L. | Ultrasound intensity 50%, frequency 50/60 KHz, time 10 min, solvent methanol and QUR extraction yield 11.8% | [25] | |
| Onion solid wastes | Ethanol 59%, temperature of 49 °C, and total QUR yield 11.08 mg/g dry weight | [24] | |
| Dendrobium officinale | Ethanol 90%, Temperature 50 °C, Time 60 min, power 140 W, liquid ratio 60%, and QUR yield 4.3385 µg/g | [161] | |
| Apple Peels | Treatment time 15 min of ultrasound wavelengths of dehydrated apple peel powder in 80% to 100% (v/v) methanol in 1:50 (w: v) solid to solvent ratio provided the optimum extraction conditions for quercetin | [162] | |
| Onion skin | Treatment time 21.7 min, power 606.4 W with 43.8% ethanol and QUR yield 20.3% | [31] | |
| Allamanda cathartica | Total flavonoids yield was 172.90 mg QE/g and QUR yielded 51.39 mg/g | [163] | |
| Abies nephrolepis (Trautv.) | Ultrasound power of 160 W, frequency of 45 kHz, the temperature of 332.19 K, extraction time 39.25 min, the ethanol concentration of 50%, a liquid–solid ratio of 20 mL/g, and QUR yielded 69.59 ± 2.57 mg/g | [27] | |
| Cabbage | Sonication by 60:40 methanol/water (v/v), temperature 30 °C, time 40 min, and QUR yielded 1378. 9 μg/mL | [164] | |
| Hypericum perforatum L. | Extraction temperature 67 °C, time 67 min, HCl concentration 1.2 M, methanol concentration 77% (v/v) and QUR yielded 10.81 mg/g | [165] | |
| Flos populi | Temperature 70 °C, liquid–solid ratio 25 mL/g, particle diameter 0.18 mm, ethanol concentration 60%, ultrasonic time 35 min, ultrasonic intensity 3.3 W/cm2 and two experimental runs. Under these optimum conditions, approximately 16.26 mg of QUR was obtained from 1 g of Flos populi | [26] | |
| MWAE | Stalks of Euonymus alatus (Thunb.) Sieb | MW power 170 W, irradiation time 6 min, 50% ethanol (v/v) solution, extractant volume 40 mL | [166] |
| Solid Onion (Allium cepa L.) | MW irradiation for 150 sec at pH 6.25 yielded QUR 209 mg/ 100 gm fresh weight | [32] | |
| Red Kidney Bean | Solvent 60 w/w% acetone, solvent to solid ratio was 10:1 and the MW irradiation power 800 W, treatment time 1 min and QUR yielded 35.8 mg/g | [167] | |
| Carica papaya flower | Solid to liquid ratio (1:15), MW power 400 W, extraction time 4 min, and QUR yielded 0.214% | [33] | |
| Latinos edodes stem | MW radiation power 385 W, irradiation time 50 s, ethanol concentration 50%, liquid-to-solid ratio was 30:1 (mL: g) and QUR yielded 0.75–0.02) mg/g | [168] | |
| UMWAE | Red onion skin wastes | MW irradiation time 60 s followed by sonication time 15 min at 70 °C, 70% ethanol, solvent to solid ratio of 30 mL/g and yielded QUR 7.66% and total flavonoids10.18% | [169] |
| Iranian Propolis | MW power 300 W, irradiation time 1.5 min and ultrasound treatment time 10 min and temperature 40 °C. obtained QUR yield 44.53% | [170] | |
| SFE | Sumac (Rhus coriaria L.) | Temperature 40οC, pressure 250 bar, 6% ethanol content and yielded QUR 2196 μg/100 g | [171] |
| Onion skin | Extraction time 15 min, temperature 165 °C, the mixture ratio of 1.5:2.5 for onion skin and diatomaceous earth and yielded QUR 16.29 ± 0.75 mg/g | [172] | |
| Rosa damascene Mill | The temperature of 46.3 °C, Pressure 25.5 MPa, CO2 flow rate of 0.7 mL/min, extraction time of 120 min and yielded QUR 32.0% | [41] | |
| Phyllanthus niruri Callus Culture | Temperature 60 °C, pressure 200 bars, time 30 min and yielded QUR 1.72% | [173] | |
| Taxus chinensis | Temperature 46 °C, pressure 24 MPa, time 2.3 h, solvent 82% ethanol and yielded QUR 3.73 mg/g | [174] | |
| EAE | Medicage Stativa L. | Enzymolysis time 92 min, ethanol concentration 22%, liquid/solid ratio was 40: 1 (m L/g) and yielded QUR 12.56 μg/g | [175] |
| Onions | Ethanol concentration 5%, flow rate 3 mL/min, temperature 84 °C, pH 5.5, and yielded maximum QUR | [46] | |
| Onion peel waste | Pectinase 0.16 mg, cellulose 0.72 mg, xylanase 1.0 mg, and QUR yield was enhanced by 1.61 folds | [47] |
2.1. Ultrasound-assisted extraction (UAE)
UAE is an emerging extraction tool that requires a shorter reaction time, simple to operate, and minimum releasing of toxic solvents in the environment. During extraction ultrasound possibly increase the penetration of solvent, rupturing the cell walls and increase contact surface area, and encourages the soluble compound solvation [21], [22], [23]. In the UAE treatment temperature, time, power, and liquid–solid ratio affect the flavonoids extraction. In UAE, when the liquid–solid ratio is enhanced the extraction yield quickly improves. One limitation of the UAE is the decomposition of active ingredients by ultrasound waves. Jang, Asnin, Nile, Keum, Kim and Park [24] designed a method for QUR extraction from onion solid waste with an aqueous ethanol solution under ultrasonic treatment. The study concluded that ethanol concentration (40–80%, v/v) and extraction temperature (40–60 °C) were the most important parameters affecting the recovery rate. While the pH, liquid/solid extraction ratio, and mining time had no significant effect on the integrity of the extraction. The best extraction yield of QUR (11.08 mg/g dry weight) was obtained with 59% ethanol with 49 °C extraction temperature from onion dry waste. Similarly, another study was designed to compare various extraction methods for QUR from Raphanus sativus leaves. Results showed UAE as the best extraction approach for the flavonoid at 50% of ultrasound intensity for 10 min in methanol. Higher QUR content was detected with UAE as compared to the soxhlet, maceration, and digestion method Sharifi, Mahernia and Amanlou [25].
Moreover, Wang, Goldsmith, Zhao, Zhao, Sheng and Yu [26] conducted a study to optimize the five types of flavonoids (QUR, luteolin, apigenin, pine cortex, and chrysanthemum) extracted from Schisandra. A combination of the Plackett-Burman design and the Taguchi method was used to adjust the process parameters of the UAE. Based on the single-factor investigation, the Plackett-Burman tool was originally adopted and revealed that temperature, particle size, ultra-sonication time, and intensity greatly affect the extraction rate. Moreover, the Taguchi method was useful for further assessment of the best conditions. The best outcomes (16.26 mg/g of sample) were obtained with the temperature set at 70 °C, 60% ethanol, 0.18 mm particle size, 35 min ultrasonic time, 25 mL/g liquid–solid ratios, and with the adjustment of ultrasonic density to 3.3 W/cm2 respectively.
Also, Wei, Zhao, Peng, Feng, Gu and Yang [27] also studied the extraction behavior of three phytochemicals QUR, diosmin, and taxifolin from the leaves and bark of Abies nephrolepis. Kinetic harmony of extraction yield for 3 kinds of flavonoids was performed with a first-order kinetic model. From the thermodynamic analysis results, it was concluded that the UAE of the QUR, diosmin, and taxifolin from the samples leaves and bark is a spontaneous endothermic process in which the disorder is exacerbated. Also, Thuy, Tuyen, Cuong, Huyen, Phuong, Nguyen, Kim, Thu and Tai [14] carried out the extraction and identification of QUR from the skin and flesh of shallot. A higher yield was obtained with the UAE than the solvent extraction technique. UAE improved the extraction yield of QUR by 13.38–15.64% for shallot skin and 49.46–56.88 % for the flesh.
2.2. Microwave-assisted extraction (MWAE)
MWAE applies microwave energy to generate the rotation of dipole molecules. At the same time temperature of the solvent quickly rises, which breaks/ruptures the cell wall of the sample and stimulates the extraction of target compounds [23]. During MWAE operation, the power of microwave irradiation is an important factor affecting the extraction yield, powerful irradiation increases the temperature of the extraction solvent and, sample matrix and improving the rate of mass transfer as well. The microwave irradiation time is also an important factor for extraction yield, as increasing time at a specific limit the extraction yield distinctly improves. For solvent, energy, and time-saving, 1 to 3 extraction cycles are sufficient to obtain a higher yield [28], [29]. One limitation of MWAE is the selection of solvent. Only those solvents are suitable which can absorb microwaves such as ethanol and methanol [29]. Earlier, a study was reported by Zhang, Yang, Su and Guo [30] for QUR and rutin investigations extracted from Euonmus alatus stalks using the MWAE method. MWAE results were compared with UAE and soxhlet extraction. The finding showed a higher extraction yield of 0.016 mg/g when compared with other approaches.
Similarly, three extraction methods (solvent extraction, UAE, and MWAE) for QUR extraction from onion skin were also reported. The maximum extraction yield was 20.3–30.8 % obtained by MWAE, higher than other extraction protocols Jin, Lim, oh Kim, Park, Jang, Chung, Park, Shim and Choi [31]. Earlier, Kumar, Smita, Kumar, Cumbal and Rosero [32] developed a facile renewable energy-based process to attain the optimum conditions for UAE and MWAE. The effects of extraction time, pH, and other operating conditions for the QUR extraction rate were investigated. MWA ionic liquid-based silica adsorbents (ILSis) were developed by using synthetic ionic liquids to chemically modify the surface of commercial silica. The resulting particles acted as a special adsorbent to separate QUR and its glycosides from the solid onion. As compared to traditional C18 adsorbents the ionic liquid-based silica adsorbent showed higher selectivity.
Recently, Mukhaimin, Saraswati, Ajizah and Triyastuti [33] also extracted QUR from dried flowers of Carica papaya through MWAE with methanol (80%). Different microwave (MW) powers (120, 200, 280, and 400 W) and extraction time (1–5 min) were used in the study. The highest yield (0.214%) was obtained at 400 W MW power and 4 min of treatment time.
2.3. Ultrasound-microwave-assisted extraction (UMWAE)
UMWAE is another combination of novel techniques, used for the extraction of flavonoid compounds from plant sources [34]. During extraction, ultrasound possibly increases the penetration of solvents, rupturing the cell walls and increase contact surface area, and encouraging the soluble compound solvation [21], [22]. Moreover, microwave improves the rate of mass transfer and solute solubility by rapidly increasing the sample temperature, and improving extraction efficiency by stimulating the desorption of the targeted compound's action efficiency [29]. The irradiations of ultrasound and microwave simultaneously would add sufficient heat and energy to the extraction solvent, but it requires a specially designed system for the incorporation of both irradiations. Sun, Li, Ni, Yao, Jiang, Ren, Fu and Zhao [34] reported that the combination of microwave and ultrasound delivers high extraction yields and is valuable for heat-sensitive compounds.
A study was conducted by using the UMWAE technique for the extraction of QUR from A. roxburghii powder. For effective extraction of QUR, ethanol concentration, extraction time, temperature, and liquid to solid ratio were regarded as important parameters. Temperature range of 50 °C, ethanol with 50% concentration, extraction time of 15 min, and the liquid–solid ratio of 8:1 delivered maximum QUR yield [35]. In another study, outcomes demonstrated that the UMWAE is an effective tool for the extraction of QUR while high-speed counter-current chromatography was useful for QUR purification. This detachment strategy was more successful than some traditional procedures. The HSCCC test was performed with a two-phase solvent system made out of n-hexane, ethyl acetic acid derivation, methanol, and water (4:6:3:3, v/v/v/v). Every dissolvable blend was completely equilibrated in a separating funnel at room temperature [35].
2.4. Supercritical fluid extraction (SFE)
SFE is one of the separation methods developed in recent years. Supercritical fluid can be defined as any substance above its thermodynamic critical point which ultimately generates high diffusivity and low viscosity which helps to increase the transfer of matter [36]. Nowadays, SFE is widely employed not only in drugs and food but also in the fields of chemistry, toxicology, environment, petrochemical, textile, and polymers [37]. Notable executions of SFE in different research areas in the last three decades have promoted the extraction of bioactive compounds [38]. Chávez-González, Sepúlveda, Verma, Luna-García, Rodríguez-Durán, Ilina and Aguilar [39] reported that the SFE exhibited a higher extraction yield of flavonoids especially QUR.
Earlier, Lévai, Martín, de Paz, Rodríguez-Rojo and Cocero [40] produced submicron encapsulated QUR particles by extracting biological solvents through SFE. Due to the speedy exclusion of organic solvent, the dispersed organic phase was rapidly oversaturated, causing submicron QUR particles to precipitate and become entangled with surfactant material. In an experiment using Pluronic, needle-shaped QUR atoms with a particle size of about 1 µm were obtained after SFE processing and the encapsulation efficiency was poor. For soybean lecithin, QUR loaded multivesicular liposomes with an average particle size of about 100 nm, the encapsulation efficiency of QUR up to 70% was obtained with no isolated QUR crystals.
Ghoreishi, Hedayati and Mousavi [41] carried out QUR extraction from Rosa damascena Mill by soxhlet extraction and modified SFE with ethanol. The pressure was adjusted from 10 to 30 MPa, extraction temperature was 35 to 55 °C, a flow rate of CO2 0.3 to 1.5 mL/min, and extraction time varied between 40 min and 2 h in the study. Response Surface Methodology (RSM) analysis confirmed that the modified R2 and R2 of the model were 87.1% and 93.1%, respectively. RSM test predicted the optimum operating temperature of 46.3 °C, the pressure of 25.5 MPa, extraction time of 2 h, and flow rate of CO2 0.7 mL/min, which showed the highest QUR yield of 32.0% in the study.
2.5. Enzyme-assisted extraction (EAE)
According to environmental laws and regulations concerning the extraction of a bioactive compound at an industrial scale, EAE is expected to attain broader popularity than conventional extraction techniques. Because conventional methods are acknowledged causes of environmental hazards due to their unusable residues, flammable solvents, and industrial methods needing a large number of steps [42]. Some recent studies on EAE have conferred faster extraction, higher recovery, less energy usage, and lower solvent consumption when collating with other methods. Moreover, EAE also offers numerous benefits such as using the whole plant material, mild reaction conditions, needing fewer steps for processing, extract the bioactive compounds with high quality and bioavailability [42], [43]. The cell membrane micelles and cell wall structure are composed of macromolecules (polysaccharides and protein). So, during the extraction process, protein coagulation and denaturation are the principal barriers of extraction at high temperatures [44]. EAE improves the extraction yield and efficiency through enzymatic hydrolytic action on macromolecules, cell walls, and cell membranes inside the cell, which improves the extraction rate [45]. Normally, cellulose, α‐amylase, and pectinase enzymes are used in EAE.
Lindahl, Liu, Khan, Karlsson and Turner [46] proposed a novel continuous hot water extraction and enzymatic hydrolysis method for the determination of QUR in onions. The first step deals with the optimization of enzymatic hydrolysis of QUR-3, 4′-diglucoside to QUR using a three-level central composite design at 75–95 °C, pH varied from 3 to 6 along with 5–15 % concentration of ethanol. In the second step, the experiment was conducted at 2.6 and 5.5 pH, ethanol concentrations at 0 and 5%, and the flow rate was controlled to 1 and 3 mL/ min. The best condition for QUR extraction was observed as 5% ethanol, the flow rate was 3 mL/min, pH 5.5, and 84 °C temperature range. Earlier, Choi, Cho, Moon and Bae [47] also designed a study for QUR and bio-sugar were extraction from onion peel waste (OSW). In the study, optimal conditions were investigated by changing the enzyme mixtures for effective conversion. Results showed that by using 0.16 mg of pectinase, 0.72 mg of cellulose and 1.0 mg of xylanase per gram of dry OSW, the enzyme conversion ratio of OSW into biological sugar was 98.5%. Whereas, after the full enzymatic hydrolysis, the amount of extracted QUR was enhanced by 1.61 folds. Also, a newly developed nano matrix was used to separate QUR from OSW extracts. The absorption rate of QUR in the terpyridine-immobilized silica-coated magnetic nanoparticles-zinc (TSMNP-Zn) matrix was about 90% and after ethylenediaminetetraacetic acid treatment, the QUR recovery rate was around 75%. Moreover, to enhance the extraction yield of insoluble-bound phenolics, enzyme-assisted extraction methods including xylanase-assisted extraction, cellulase-assisted extraction, complex enzyme-assisted extraction, and β-glucosidase-assisted extraction were employed. Among all the tested methods, β-glucosidase-assisted extraction showed the maximum extraction yield of 123 mg/100 g of dry weight for QUR estimations Wang, Wu, Liu and Wu [48].
3. Non-invasive techniques for the measurement of QUR
With the rapid increase in population and awareness, the provision of good quality food is an emerging challenge globally. Therefore, researchers are focusing on establishing reliable approaches for authenticating the quality parameters of agricultural products including internal and external attributes. Non-destructive assessment techniques are the main part of high-quality control functions and they assist the different established techniques as well. Non-interruptive examination leads to the surface testing of agricultural products without any interfering technique concerning the food quality and appearance. The non-destructive evaluation techniques provide data on food properties such as mechanical, chemical-physical, and structural properties. A brief description of the strengths and weaknesses of nondestructive techniques is displayed in Table 3.
Table 3.
Strengths and weakness of non-destructive methods used in current work.
| Techniques | Type | Advantages | Limitations | References |
|---|---|---|---|---|
| FS | Spectroscopic | Good signal–noise ratio, an abundant fluorophore | Autofluorescence, limited to the samples exhibiting fluoresce | [20], [59], [60], [176], [177] |
| THzS | Spectroscopic | Use low energy and lower ionizing energy, Can generate frequency-domain and time-domain data from physical properties and chemical structure of the sample | Cannot penetrate in water and metals, scattering effect for irregular samples is also a weakness in THzS | |
| NIRS | Spectroscopic | The cost-effective tool can conduct qualitative and quantitative detections | Difficult to analyze samples containing water, can generate spectral data only | |
| HIS | Spectral imaging | provide spectral and spatial data, accurately differentiate the similar components of the sample even with similar color, can detect trace elements efficiently | Abundant redundant data, data processing needs a lot of time, adaptability of chemometric methods is another problem in HSI | |
| RS | Spectroscopic | No interference to water, provide rich molecular Raman signatures | Weak Raman scattering cost-ineffective | |
| SERS | Spectroscopic | An ultra-sensitive and specific tool, Direct/minor sample preparation needed, No interference to water and glass | Unstable hotspot regions in substrates, Sensitivity depends on the characteristics of employed nanoparticles |
Note: FS = fluorescence spectroscopy, THzS = terahertz spectroscopy, NIRS = near infrared spectroscopy, HSI = hyper spectral imaging, RS = Raman spectroscopy, SERS = surface enhanced Raman spectroscopy.
3.1. Fluorescence spectroscopy (FS)
FS is among fast spectroscopic methods that provide rapid spectral signatures (from samples) by estimating emission of light compares to absorbed UV or visible light. The samples absorb energy (from light) at a particular wavelength while releasing energy in form of emission with a higher wavelength [49]. However, the chemical, physical, and biological fluorescence spectra are typically overlapped. Moreover, the spectra seem very similar for different samples which make it difficult to differentiate the results. Thus chemometric models are needed to extract the target peaks from the overlapped data [50].
Owing to the non-destructiveness and rapidness attributes, FS has proved some promising results for QUR detection in different agricultural items (Table 4). For example, in a study, four medical plants were tested for quantification of QUR content using the FS method coupled with the partial least squares regression (PLSR) model. Among the tested samples R. himalense had the highest QUR values of 3.83% while, B. calliobotrys exhibited 1.91%, A. eruentus showed 1.83% and S. muricatum delivered 0.87% respectively [51]. Similarly, emission spectra from Ziziphus mucronata and Ziziphus Sativa (medicinal plants) for QUR were determined using FS with the PLSR approach. Higher content of QUR was recorded from Z. mucronata samples as 1.50%. While Z. Sativa delivered 1.21% results for the tested parameter. Moreover, the Folin-Ciocalteu colorimetric assessment of samples also revealed former with higher QUR levels as compare to later [52]. Besides, Alabri, Hussain, Mabood, Rehman, Ali, Al-Harrasi, Hamaed, Khan, Rizvi and Jabeen [53] also ascertained QUR in Euphorbia masirahensis plants with the FS-PLSR technique. The highest QUR content was determined from the samples with an n-butanol fraction (5.83%) with slope estimations of 0.997 and root mean square error (RMSE) of 0.355%.
Table 4.
Nondestructive detection of QUR in agricultural products.
| Technique | Matrix | Model | Results | References |
|---|---|---|---|---|
| FS | R. himalense, B. calliobotrys, A. eruentus, S. muricatum | PLSR | QUR yield in R. himalense = 3.83%, B. calliobotrys = 1.91%, A. eruentus = 1.83%, S. muricatum = 0.87% | [51] |
| Ziziphus mucronata, Ziziphus Sativa | PLSR | QUR yield in Z. mucronata 1.50%, and Z. Sativa delivered 1.21% | [52] | |
| THzS | QUR, myricetin, and kaempferol Discrimination | PLSR and LS-SVM | LS-SVM showed better results: RMSEP = 0.0039, 0.0044, and 0.0048 R2p = 0.9688, 0.9601, and 0.9359, RPD = 9.6333, 8.6272, and 7.9083 for QUR, myricetin, and kaempferol | [55] |
| NIRS | Grapes and red wine | PLSR | Determined QUR (0.124% and 0.078%), TR, (0.447% and 0.124%), TPC (12.82% and 0.168%) and antioxidant % value (1.279% and 0.014%) for grapes and red wine samples | [61] |
| Mung bean (Vigna radiata L.) | PLSR | For quercetin, catechin, chlorogenic acid, caffeic acid, p-coumaric acid, t-ferulic acid, vitexin, isovitexin, myricetin, and kaempferol, R2 more than 0.987 and RMSE<1.82% was recorded. For QUR: R2C = 0.982, RMSEC = 1.771, R2V = 0.969, RMSEV = 2.393 |
[178] | |
| Wine from DO Rías Baixas and DO Ribeira Sacra | PCR and PLS models | PLS showed good results as compare to PCR model. For DO Ribeira Sacra red wines: R2cal = 0.82, RMSEC = 0.15, RMSECV = 0.55, RPD = 1.51. For wines from mencía grapes: R2cal = 0.56, RMSEC = 0.24, RMSECV = 0.40, RPD = 1.12 | [179] | |
| Grapes and grapes wine | PLS | Tested trans-resveratrol; QUR; gallic acid equivalent; Trolox equivalent in samples For QUR in grapes: SEC = 0.124, Rcal = 0.973, SECV = 0.008, Rcv = 0.977, RPD = 4.40 In wine; SEC = 0.078, Rcal = 0.994, SECV = 0.073, Rcv = 0.995, RPD = 9.0 |
[61] | |
| HSI | white grape marc | PLSR | QUR = 1.6 ± 0.1 mg/100 g dry mass, 2.1 ± 0.4 mg/100 g dry mass, and 2.0 ± 0.3 mg/100 g dry mass in the seed, skin, and stem of the samples. | [66] |
| RS | dried onion | R2 = 0.9998 and 0.9998 for QUR in methanol and ethanol LOD = 5 × 10-5 mol/L | [75] |
TR = trans-resveratrol, TPC = total phenolic (TPC), R2 = coefficient of determination, PLSR: partial least squares regression, PCR: principal component regression, PLS: partial least squares, LS-SVM: least-squares support vector machine, RMSEP: root mean square error of prediction, RMSE = root-mean-square error, R2cal: coefficient of determination in calibration; RMSEC: root mean square error of calibration; RMSECV: root mean square error of cross-validation; RPD: residual predictive deviation, RPD-relationship between the standard deviation of the reference method and the standard error of cross-validation, R2CV: coefficient of determination of cross-validation, R2C: coefficient of determination of calibration, R2p: coefficient of determination for prediction.
3.2. Terahertz spectroscopy (THzS)
THzS is a fast spectral method with non-ionization features, extensively used for the discrimination of compounds with much similar molecular structures [54]. Terahertz is a small electromagnetic region between microwave (MW) and infrared (IR) spectrum, so the THzS generates an informative association between MW spectroscopy and IR spectroscopy. The noninvasive method works with frequencies ranging between 0.1 and 10 THz to determine torsional and rotational modes of vibrations from chemical compounds [19].
The rapid method was employed for the discrimination of three flavonoids (QUR, myricetin, and kaempferol) having similar structures. The k-nearest neighbor (KNN), random forest (RF), and extreme learning machine ELM approaches were established for the discrimination of phytochemicals. Among the applied models, RF showed the best differentiation results with a 100 % correct classification rate (CCR) in the prediction set. Moreover, PLSR and least-squares support vector machine (LS-SVM) methods were used for the quantitative assessment of the samples. Good results were obtained with LS-SVM models, showing low root mean square error of prediction (RMSEP) of 0.0039, 0.0044, and 0.0048 for QUR, myricetin, and kaempferol while, higher Rp (0.9688, 0.9601, and 0.9359) and, residual predictive deviation (RPD) values of 9.6333, 8.6272, and 7.9083 were estimated by using the model respectively [55].
3.3. Near-infrared spectroscopy (NIRS)
NIRS is another non-destructive spectroscopic approach, works best in spectra ranging from 780 to 2500 nm, detecting chemical bonds in a sample such as O–H, C–H, N–H, and S-H [56], [57]. During an examination of a sample, the chemical bonds (in a sample) absorb spectral energy while, remaining NIR frequencies either reflect or transmit with different wavelengths, which are calculated by the detectors [58]. However, the method still possesses some weaknesses which limit its wider research applications. For example, NIRS is not suitable for the detection of volatile compounds in fruits and other samples, effective chemometric models are needed to extract target peaks from non-targeted peaks and the calibration method in the system is a time-consuming and tiresome step [59], [60].
Most recently, the method was applied to examine the QUR, trans-resveratrol (TR), total phenolic (TPC) values, and the antioxidant properties of different grapes and red wine samples using the partial least squares (PLS) regression technique. In grapes and wine samples, the standard error of calibration (SEC) for QUR, TR, TPC, and antioxidant %value was 0.124% and 0.078%, 0.447% and 0.124%, 12.82% and 0.168% and, 1.279% and 0.014%. Moreover, standard errors of cross-validation (SECV) was 0.008 and 0.073, 0.424 and 0.113, 12.15 and 0.144 and, 0.991 and 0.998 in graph and wine respectively [61]. Similarly, the technique was also successfully applied for the evaluation of kombucha (k) prepared from black tea (BT) and green tea (GT) for bioactive compounds using a PCA chemometric model. QUR, chlorogenic acid, gallic acid, caffeine, catechin, and rutin were determined in the study. Results showed BTK with higher values for QUR, caffeine, and rutin as 1.22 mg/L, 177.37 mg/L, and 30.19 mg/L while, the GTK sample was dominant in chlorogenic acid (65.42 mg/L) respectively [62].
3.4. Hyperspectral imaging (HSI)
HSI is an optical sensing method that provides spatial and spectral information useful for the identification and quantifications of various components from a sample [59], [63]. The rapid method is a combination of spectroscopy with digital imaging to produce a spatial map of spectral variations. A 3D ‘hypercube’ of image data (x, y, and λ) can be achieved by capturing a series of 2D spatial images as a function of wavelength and by superimposing the obtained data. HSI can be obtained as a 3D hypercube I (x, y, λ), or 2D spatial image I (x, y), or a combination of spectra I (λ) at pixel positions (x, y) [64], [65]. However, the presence of many redundant data in hypercube needs extra computational work and time to extract target results, which limits HSI's broader applications.
In addition to various food applications, HSI is also suitable for the assessment of phytochemicals in different food items. Recently, the tool was successfully applied to analyze 27 phenolic compounds from white grape marc (Viti's vinifera cv. Zalema) with the PLSR model. Among them, QUR was recorded to be 1.6 ± 0.1 mg/100 g dry mass, 2.1 ± 0.4 mg/100 g dry mass, and 2.0 ± 0.3 mg/100 g dry mass in the seed, skin, and stem of the samples. Also, the results for root mean square error of calibration (RMSEC) (0.16), R2C (0.79), root mean square error of cross-validation (RMSECV) (0.19), and R2CV (0.72) were also satisfactory, establishing that the proposed method was ecofriendly and non-destructive for the assessment of phenolic compounds in the sample [66].
3.5. Raman spectroscopy (RS) and surface-enhanced Raman spectroscopy (SERS)
RS is a versatile vibrational spectroscopic method that works on the inelastic Raman scattering principle. It provides Raman signatures for the quantification of various components in a sample [20], [67]. The approach is preferred over other methods due to more sensitivity and specificity, direct analysis of a sample is possible, and has no interference to water in a sample. However, Raman scattering is a weak phenomenon, as only one photon out of one million photons follows the process [68]. Therefore, to enhance the weak Raman signals (in normal RS) researchers employed nanoparticles (NPs) near samples to enhance the peak intensities and the technique is called SERS [69]. By employing substrates, chemical and physical mechanisms can occur to boost the Raman signals. The chemical process occurs due to charge transfer between a sample and nano substrate while, amplification of signals due to optical attributes of NPs is a physical process [70], [71]. The stable nanomaterial makes SERS an ultra-sensitive tool for numerous food and non-food detections. Gold, silver, and copper are simple and easily available NPs that are further employed for designing other sensitive SERS substrates [72], [73], [74].
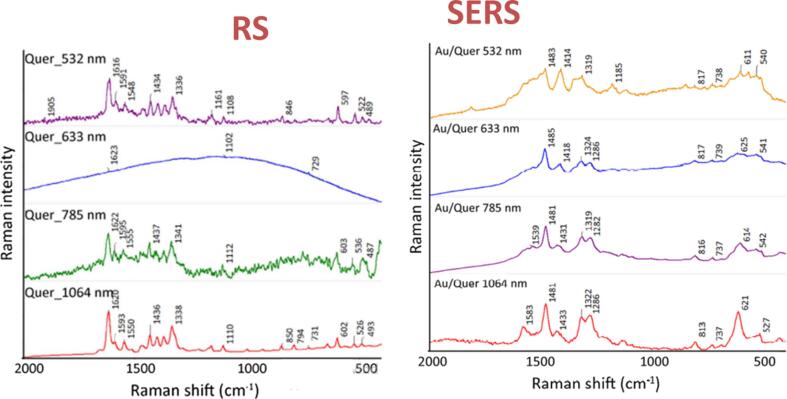
Both RS and SERS have been extensively applied for different research areas in recent years [69]. The techniques are also suitable for the non-interruptive assessment of phytochemicals in agriculture items. For instance, RS equipped with an Argon ion laser having an excitation wavelength of 488 nm was employed for label-free detection of QUR in dried onion samples. Results revealed a high coefficient of determination (R2) of 0.9998 and 0.9998 for QUR in methanol and ethanol solvent with a limit of detection up to 5 × 10−5 mol/L [75]. Moreover, four different lasers with 532, 633, 785, and 1064 nm excitation wavelengths were used for the detection of QUR, flavone, 3-hydroxyfavone, and chrysin using RS and SERS method. With normal RS, the spectral intensity was greatly influenced for QUR and chrysin by different laser wavelengths, attributed to their high fluorescence resonance signals. Whereas, SERS based on AuNPs delivered good results for all the phytochemicals (even for QUR and chrysin), which depicted the sensitivity and quenching ability of SERS for high fluorescent materials [76]. The normal RS and SERS peaks for QUR using 532, 633, 785, and 1064 nm excitation wavelengths lasers are presented in Fig. 1. In another study, ethylenediamine-β-cyclodextrin (EβC) immobilized silver nanoparticles (AgNPs) embedded Silica (SiO2) were used as SERS substrate for nondestructive detection of QUR, hesperetin, naringenin, and luteolin. The capturing ligand SiO2 @Ag@Et-β-CDNPs improved the sensitivity down to 10-7 M for flavonoids detections [77].
Fig. 1.
The normal RS and SERS peaks for QUR using 532, 633, 785, and 1064 nm excitation wavelengths lasers adopted from Dendisová, Palounek, Švecová and Prokopec [76].
4. Bioavailability enhancing strategies for QUR
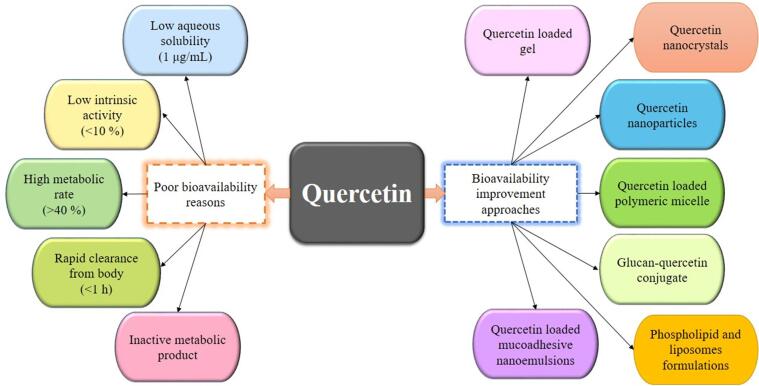
Bioavailability is regarded as the specific amount of substance reaching the targeted site of action. Moreover, it is also an estimation of the dosage present as per the urinary measurement of its constituents and metabolites whereas, in the case of polyphenol absorption, bioavailability is considered as the amount present in plasma. In the pharmaceutical industry, ADME (absorption, metabolism, disposition, and excretion) is used as its comparable term [78], [79], [80]. In recent years, the characterization of quercetin (QUR) dosage and bioavailability from various agricultural products have been reviewed thoroughly by [81], [82], [83], [84], [85]. In the human body, QUR can only get ingested around 20 mg/day [86], [87] while, the total plasma concentration of both free and conjugated QUR ranged from 72 to 193 nmol/L from QUR rich sources for short term intake. Whereas, long-term dietary usage does not result in plasma accumulation [88]. Due to the low solubility, it is also considered to have very low absorption in the gastrointestinal tract with oral bioavailability, calculated to be 1% in humans [89].
As per the biopharmaceutical classification system, QUR is classified in class IV with poor water solubility, low permeation, and a short biological half-life [90] (Fig. 2). Therefore, improvement in bioavailability is imperative for enhancing the application portfolio of the phytochemical [91]. Numerous delivery systems have been developed over the last decade to improve and modify the dispersed state of this bioactive constituent, which can enhance the chemical stability and increase the applications [92], [93]. Current delivery systems include lipid-based carriers [94], [95], polymer nanoparticles, inclusion complexes, micelles, and conjugated-based capsulations [88] (Table 5).
Fig. 2.
Quercetin poor bioavailability reasons and improvement approaches.
Table 5.
QUR delivery vehicles and in vitro therapeutic properties.
| System | Process detail | Therapeutic effect | References |
|---|---|---|---|
| Hydrogels | Chemical: Hydrogels WPI-QUR-LRA Size (d): 179.5 nm Entrapment capability: 92 % |
Increase drug loading and release | [180] |
| Metal and metal oxide nanoparticles | Chemical: Gold nanoparticles Size (d): 100 nm Entrapment capability: 79% |
Reduce the toxicity and improved the antioxidant activity | [181], [182] |
| Chitosan nanoparticle | Chemical: QUR-chitosan conjugate-loaded with paclitaxel Size (d): 185.8 nm Entrapment capability: 62 % |
Advanced aqueous solubility of novel anticancer | [183], [184] |
| PLGA/PLA nanoparticles | chemical: PLGA (coencapsulated QUR and tamoxifen) Size (d): 185.3 nm Entrapment capability: 68.60 % |
Enhance in cell cytotoxicity | [185], [186] |
| Solid lipid nanoparticles | Chemical: Trilaurin and phospholipid Size (d): 74.61 nm Entrapment capability: 83.27 % |
Improve aqueous solubility | [187] |
| Micelles | Chemical: Nano micelles Size (d): 15.4–18.0 nm Entrapment capability: ≥ 89 % |
Reduced cell viability at 72 h | [188], [146] |
| Liposome | Lecithin, Cholesterol, Polyethyleneglycol (PEG) 4000 and QUR | Decreased collagen deposition and lung fibrosis areas after two weeks | [151] |
| Liposome | QUR, PEG-4000 | Anti-angiogenesis and anti-tumor effects | [189] |
| Liposome | Cholesterol, egg sphingomyelin, QUR, PEG 2000-ceramide, and vincristine | No bodyweight loss and inhibited tumor xenograft | [190] |
| Lipid nanocapsules | Solutol, Lipophile WL 1349, QUR, and Phospholipon | Improve solubility (aqueous) by a factor of 100 | [156] |
| Nanostructured lipid carriers | Stearic acid, glyceryl monostearate, soya lecithin, media chain triglyceride, and QUR | Enhanced the QUR retention in dermis and epidermis by 3.03 and 1.52 times | [155] |
| Solid lipid nanoparticles | Soya lecithin, glyceryl monostearate, PEG 400, QUR, and Tween-80 |
Cmax increased up to12.22 μg/mL, AUC(048 h) increased up to 5.71-fold |
[191] |
| Complex | Phospholipid and QUR | Improve the anti-oxidant activity,Improve water solubility by 12 folds | [157] |
Note: PLA, poly (D, L-lactic acid); PLGA, poly(lactic-co-glycolic acid); WPI, whey protein isolate; QUR, quercetin; LRA, lotus root amylopectin.
4.1. QUR loaded gel
To improve the solubilization and encapsulation properties of QUR, various formulations such as micro emulsions, microspheres, nanostructured lipid carriers, solid lipid nanoparticles, polymer nanoparticles are proposed. Whereas, the colloidal delivery system includes nanoemulsions (NE) ranging from 50 to 100 nm in size with a larger surface area, high drug loading capacity, sustained release, protection of drug candidates, and excellent permeation. But owing to the low viscosity NE, a gel system is also employed [96], [97], [98], [99]. Such strategies improve the bioavailability of QUR and thus consequents in improved health maximum benefits. For example, administration of QUR incorporated with oleic: Arachis oil, tween 20, and PEG-400 (15:6:6) resulted in a successful carrier system with improved physicochemical stability, mechanical properties, skin permeability and exhibited good therapeutic effects for arthritis. The research concluded QUR-NE gel to have promising potential for complicated rheumatic disease [96].
In another research, NE prepared with QUR showed the highest solubility in cinnamon oil, followed by triacetin, castor oil, sesame oil, labrafac, palm oil, isopropyl myristate while, lowest in miglitol. Experiments indicated that cinnamon oil, tween 80, carbitol ®, poloxamer 407 with QUR improved the physical properties, gel stability, sol–gel transition, and syringe-ability. The administration among patients with periodontitis in in-vitro showed improvement in their condition [100].
In wound healing research, QUR loaded multiphase hydrogel was analyzed both in-vivo and in-vitro. The high entrapment efficiency of QUR and resveratrol was higher (92.85 & 89.85%) as compared to other liposomal nano-vector (71.20% QUR). These nano lipid carriers and both QUR and resveratrol had potential in healing skin cancer with the latter two acting synergistically. Further, future research can also be focused on the cytotoxic impact on numerous cancer types with dermatokinetic studies [101]. Furthermore, in another research QUR-loaded liposomes in hydrogels improved gastric release up to 40% with significantly better texture, and the structure leading to lipogels cargo release enhancing the potential applications of the lipogels as a bio-food [102].
4.2. QUR nanoparticles (NPs)
Other than physical, chemical, and physiological properties including temperature, oxygen, and enzymatic activity of QUR, chemical instability and high biodegradability at different pH of the stomach has encouraged the researchers to preserve it in the hydrophobic matrices as NPs [103], [104]. Among the natural polymer-based NPs, protein-based NPs are considered easy for the gastrointestinal tract [105]. QUR-NPs with bovine serum albumin (BSA) showed bioactivity in both the acidic and alkaline medium for longer durations [106]. Another study with QUR-loaded zein NPs by electro spraying was evaluated. The researcher determined in vitro gastrointestinal release of trapped QUR up to 79.1%, while, for free QUR it was 99.2%. The in vitro bioavailability was 5.9% for trapped while 1.9% for the free QUR, displaying better results with QUR zein NPs [103].
For polysaccharide-based NPs, QUR-loaded linoleic acid (LA) modified with chitosan oligosaccharise/β-lactoglobulin (CSO-LA/β-lg) NPs indicated higher cell permeation ability resulting in better quercetin accumulation in the skin (epidermis) [107]. Similarly, zein NPs were encapsulated with 2-hydroxypropyl-β-cyclodextrin with 300 nm diameter. The QUR release resulted in zero-order kinetic and oral bioavailability was calculated to be around 60% [108]. Moreover, improvement in solubility for QUR was also determined with the NPs prepared by using sodium alginate and chitosan polymeric microparticles through the ionic cross-linking approach [109], [110].
Also, lipid-based Nanocarriers can resolve the bioavailability issues for QUR [111]. For instance, in vitro tests conducted to access bio-accessibility in simulated intestinal medium (SIM) was 60% in lipid nano-emulsions (LNEs), 50% in solid lipid NPs (SLNs), 40% in nanostructured lipid carriers (NLCs), and only 10% in the purest form. Similarly, another study evaluated the water solubility of QUR-loaded lipid NPs designed by using polyvinylpyrrolidone (PVP), indicated an optimized formation of NPs enhancing 20,000-fold QUR solubility [112], [113].
Furthermore, to improve the solubility of QUR and enhance the drug release mechanism, pulsed laser ablation was employed to obtain QUR NPs. The experiments exhibited a better anti-oxidative effect as well as in modulating the amyloid fibrillation, revealing the therapeutic role of QUR NPs for amyloid-related diseases [114]. Moreover, super paramagnetic iron oxide NPs (SPIONs) in conjugation with QUR also showed a ten-fold increase in bioavailability in plasma and brain tissues, in Wistar male rats fed with QT-SPION at 50 and 100 mg/kg daily dosage for 7 days [115]. Also, chitosan-quinoline NPs were promising for QUR delivery as a pH-responsive anticancer drug, with 141–174.8 nm particle size, the zeta potential of −2.4 to 14.1 mV, and had a regular nanorod shape. The drug loading capacity was 4.8–9.6% while encapsulation efficiency of 65.8–77% was achieved. Efficacy of the QUR-loaded chitosan-quinoline NPs was also reported to exhibit a strong cytotoxic effect against the HeLa cell line, indicating a strong anticancer effect and potential for further research [116]. Some studies also indicated the effectiveness of QUR-loaded phytosome NPs in ovariectomized rats for hormone replacement therapy. The NPs enhanced the delivery, resulting in improving serum calcium, inorganic phosphorus, and glutathione content. It also significantly declined the serum alkaline phosphatase, acid phosphatase, malondialdehyde levels, tumor necrosis factor-alpha (TNF-α), lipid and glucose profile in the rats resulting in better outcomes for the therapy [117].
4.3. QUR nanocrystals
QUR nanocrystals have exhibited novel physiochemical characteristics in diverse disciplines. Some primary properties include the escalated ability to transport easily through a cell membrane. Different applications of nanocrystals are administrated via various routes, for instance peroral [118], parenteral [119], pulmonary, ocular [120], dermal [121]. Additionally, owing to the larger surface area, the higher dissolution rate of these drug nanocrystals is also improving their application spectrum, contributing positively to bioavailability [122]. Among these techniques used for production include both the “bottom-up” and “top-down” processes classified as per the principle employed in their production [123]. Commonly used “top-down” methods include bead milling and high-pressure homogenization. Higher energy input and lower power efficiency with no harsh solvents employed are the highlights of these methods [124]. Precipitation is the foremost technique of “bottom-up”. It is also coupled up with some “top-down” methods including homogenization primarily to prevent the growth of precipitated nanocrystals to microcrystals. This technique is known as “caviprecipitation” [125].
Caviprecipitation, bead milling, and homogenization have been used to prepare the QUR nanocrystals with bead milling manifesting the smallest particle size while high-pressure homogenization produced the lowest polydispersity index [126]. The caviprecipitated product is reported to have a larger particle size along with increased saturation solubility owing to the ethanol present inside. However, the zeta potential of all the other products showed stability other than the caviprecipitated one.
Previous research compared the high homogenization press process (HPH) and evaporative precipitation into the aqueous solution (EPAS) process to analyze the feasibility of each process in the formation of chemically stable QUR nanosuspension [127]. There was no alteration observed in the crystalline state of QUR with the HPH process while a crystalline to the amorphous transition phase was seen through the EPAS process. Additionally, a higher improvement was also observed in terms of solubility and dissolution rate as compared to HPH suspension owing to the higher inner energy linked to the amorphous phase. Tremendous improvement in both the chemical and photo-stability of nanosuspension of QUR molecules was observed when compared with the solution. Further research indicated that QUR-loaded nanosuspension (QUR-NS) was produced by the HPH method and a tandem of nano-precipitation (NP) [128]. The QUR solubility in suspension form was enhanced about 70 times as compared to the crude QUR. Similarly, the dissolution of QUR from QUR-NS also escalated as compared to the primary QUR powder. In plasmas form, QUR-NS showed a significant decrease in the clearance rate (2 ± 0.2 mL/min vs. 15 ± 3 mL/min) while an increase in the area under the curve, the plasma concentration–time (AUC) (53996 ± 4125 μg/mL·min versus 3471 ± 110.2 μg/mL· min) was also observed when compared with the control suspension.
4.4. QUR loaded polymeric micelle
Researchers also focused on polymeric micelles for injecting drug delivery in the systemic circulation [129]. This method is characterized by a longer retention time, high tissue permeability and is also associated with a focus on diseased tissue to enhance passive targeting [130], [131]. Owing to the solubility, biocompatibility, and stability properties, polymeric micelles are given significant importance in the oral administration of QUR
In polymeric micelles, the inner ‘core’ and outer ‘shell’ form the amphiphilic copolymers of hydrophilic and hydrophobic chains, capable of self-assembly in water present in critical micelle concentration (CMC). This micelle encapsulates the hydrophobic drug into its core, delivering it to the targeted site with higher bioavailability than the pure drug. It also prevents the degradation and metabolism of the drug in the GI tract [132], [133]. Therefore, better permeability and retention rates are expected with polymeric micelle NPs. These polymeric micelles are considered perfect candidates for anticancer drug delivery, mainly by amphiphilic micelles, with major applications for QUR formulations [134], [135], [136]. Moreover, QUR loaded into nano-sized polymeric micelles with amphiphilic polymers soluplus using a modified film dispersion model, exhibited sustained release for up to 10 days in vitro with high bioavailability [137].
Another study involved nano-polymeric microspheres formed with the spray-dried method using synthesized amphiphilic CTS were loaded with hydrophobic QUR and paclitaxel for pulmonary drug delivery. It exhibited sustained releasing effects along with higher retention of paclitaxel in vivo [138]. Furthermore, mixed micelle polymeric prepared with QUR via hydration method for breast, ovarian, and multidrug-resistant cancers, resulted in improving the drug delivery along with solubility of QUR for in vitro experiments [139]. Another study indicated better cytotoxicity of QUR-SPION loaded micelles by HepG2.2.15 cancer cells. For hepatocellular carcinoma, QUR-loaded SPION micelles exhibited better vehicle properties and cell cycle arrest at the G0/G1 phase [140].
In another study, lecithin-stabilized polymeric micelles were also designed for QUR delivery against tumor activities. The optimal combination resulted in lowering the IC50 values against MCF-7, SKBR-3, and MDA-MB-231 breast cancer cells (human). Similar results were also obtained for CT26 mouse colon cancer cells, administered intravenously for animal studies. The oral and intravenous administration of QUR showed 158 % and 360 % bioavailability as compared to the absolute bioavailability (5.13%). The study confirmed the properties of QUR loaded with stabilized lecithin nanocarrier for enhancing antitumor efficacy against chemotherapy and systemic toxicities [141]. Additionally, the QUR-chitosan conjugate was employed for the delivery of doxorubicin (anti-cancer drug) and determined that the conjugate micelles can improve the cellular uptake of the drug to 2.2 folds higher as compared to the free drug accumulation. Higher permeability co-efficient with better epithelial electrical resistance for Caco-2 cells was also obtained, indicating the conjugate as a promising carrier for oral delivery of anticancer drugs [142]. Moreover, QUR-cRGDfK-PM (QUR loaded in cRGDfK-PM) significantly optimized the therapeutic efficiency of Chinese medicinal herbs and reduces systemic and pulmonary toxicities [143]. Other antitumor activities of QUR with polymeric micelles are also an emerging research domain to further improve the bioavailability of the targeted drug. [144], [145], [146].
4.5. Glucan-QUR conjugate
Chitin glucan conjugate (ChGC) is a non-toxic, water-insoluble, and high-water absorption capacity containing polysaccharides found in yeasts and fungal cell walls. Due to its biocompatibility, it is used for various biomedical, pharmaceutical, and engineering applications. In a study, curcumin-loaded chitin-glucan QUR conjugate was used for anticancer activity. The entrapment efficiency of the conjugate was 77.32%, measured via pelletizing. Faster drug elution was estimated in an acidic environment, as the chitin-glucan polymer swelled up owing to the anime group protonation. Results also indicated a significant cytotoxic effect against the J774 cancer cell line. Nonetheless, the anticancer potential of ChGCQ and curcumin-loaded ChGC QUR conjugate needs further research in chemotherapy and pharmaceuticals [147].
Singh, Dutta, Kumar, Kureel and Rai [148] also synthesized chitin-glucan-aldehyde-QUR conjugate through the condensation reaction. This conjugate exhibited strong antioxidant properties with anticancer activities on macrophage cancer cell lines (J774). Strong biocompatibility was also ascertained in the peripheral blood mononuclear cells (PMBCs). The cytotoxicity was observed only against the J774 cell line but no activity for PMBCs was determined.
4.6. Phospholipid and liposomes formulations
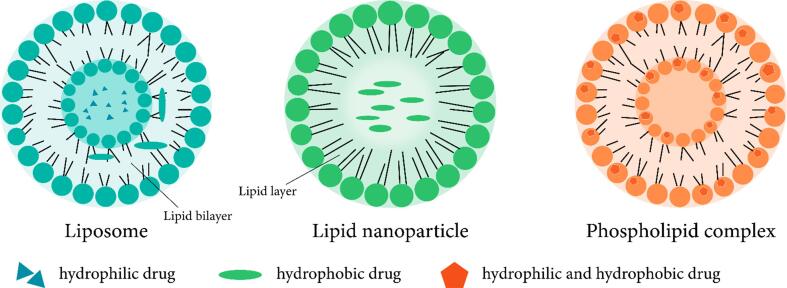
Active QUR is delivered by different formulations lipid-based including liposomes, lipid nanoparticles, phospholipid complex, etc. (Fig. 3). Liposomes are small spherical lipid bilayer vesicles enclosed in an aqueous compartment [149]. These liposomes shelter the drugs against external stimuli along with improving the water insolubility and enhancing the overall drug-delivering efficacy [150]. Anticancer drugs are also reported to have enhanced efficacy owing to the extended exposure of the drug to the tumor site during the prolonged circulation time. Additionally, enhanced permeability and retention effect also resulted in preferential accumulation of the anti-cancer drugs in the tumor. Liposomal QUR was prepared successfully against the in vivo bleomycin-induced pulmonary fibrosis [151]. This liposomal QUR decreased the increase of total cell counts along with macrophage counts in the bronchial veolar lavage fluid. The neutrophil and lymphocyte count decreased significantly on days 7 and 14 after the liposomal QUR injection (p < 0.05). This treatment also reduced the hydroxyproline content (approx. 35.8%) as compared to the bleomycininduced group (p < 0.05). Another research highlighted the QUR liposomes contribution in lessening the lung fibrosis areas and deposition of collagen along with decreasing the expression of transforming growth factor (TGF-1), as per the histopathological assessments. The therapeutic efficacy of QUR liposomes also contributes to combating the arsenic toxicity mediated oxidative damage reported in both hepatocytes and brain cells in rat models [152].
Fig. 3.
Different kind of lipid formulations and drug-loaded liposomes.
Liposomal QUR was found most potent for complete prevention of arsenite-induced reduction in antioxidant levels in the liver and brain of rats. Solid lipid nanoparticles (SLNs) are submicron’s type drug delivery system are gaining attention. Solid lipids employed are both from natural and synthetic sources with the main advantages include high biocompatibility, high bioavailability, controlled release mechanism, and using oral, intravenous, pulmonary, and transdermal routes of administration [153]. Recent studies indicate that SNLs with 13.2% QUR drug-loaded resulted in a slow release of over 48 h enhancing the oral bioavailability in the rat’s plasma. The maximum concentration (Cmax) value of QUR in SLNs (12.21 ± 2.14 μg/mL) was estimated to be higher than that obtained with QUR entrapped in sodium carboxymethyl cellulose suspension (5.91 ± 1.23 μg/mL) after a single dose of 50 mg/kg. After the oral provision of QUR-SLNs suspension for 48 h, the QUR plasma concentration was reported to be still more than 2 μg/mL, while it went unidentified at 16 h for QUR suspension. Dhawan, Kapil and Singh [154] formulated the SLNs of QUR using compritol as the lipid with Tween 80 being used as the surfactant. Overviewing the in vivo behavioral and biochemical analysis, better memory retention was observed in the rats treated with SLN-encapsulated QUR than pure QUR-treated rats.
Emulsion evaporation solidification was carried out at low temperatures to produce QUR-loaded nanostructured lipid carriers (QUR-NLCs) [155]. Studies conducted on skin permeation indicated that QUR-NLCs could also escalate the permeation of QUR, increase the concentration of QUR retention in both the epidermis and dermis, along with enhancing the effects of QUR’s anti-oxidation and anti-inflammatory functions. Skin histological studies revealed that the skin stratum corneum treated with QUR-NLCs was more scattered and loose as compared to that treated with QUR propylene glycol solution. Concluding that QUR-NLCs could also contribute to the weakening of the barrier function of stratum corneum along with promoting the drug permeation.
Flavonoid-loaded lipid nanocapsules (LNC) were designed and characterized with encapsulation of QUR in LNC resulting in increasing its apparent aqueous solubility by a factor of 100 [156]. QUR alignment at this LNC interface between oil and hydrophilic polyethylene glycol moieties of the surfactant with high stability of 10 weeks was reported as per the recent studies without any oxidation. Furthermore, to enhance the absorption of quercetin through the gastrointestinal tract a quercetin complex was prepared with phospholipid (QUR-PC) [157]. This complex has a fluffy, porous, and rough surface in SEM with 12 times higher water solubility reported in the complex from 3.43 μg/ mL to 36.80 μg/ mL. Analysis of in vitro antioxidation activity of crude quercetin and quercetin complex indicated that no statistical differences were observed highlighting the lack of the complexation process’s impact on the bioactive ingredient’s bioactivity.
4.7. QUR loaded mucoadhesive nanoemulsions
So far, limited research has been reported with QUR-loaded mucoadhesive nanoemulsions (QMNE). QMNE is considered a novel, effective, non-invasive, and safe delivery system for brain targeting for cerebral ischemia treatment [158]. In a study, intranasal delivery of QMNE was researched with a mean globule size of 91.63 ± 4.36 nm, the zeta potential of −17.26 mV, drug content 99.84, and viscosity of 121 cp. Enhanced bioavailability of QUR was found along with better neurobehavioral activity in terms of locomotion and grip strength, histopathology, and reduced infarction volume effects in middle cerebral artery occlusion (MCAO)-induced ischemic rat models. The research concluded high brain targeting potential, better formulation efficiency, and effective targeting capability of QMNE [158]. However, further clinical and pre-clinical trials are needed to prepare the formulations that can further strengthen the low risk and higher benefit ratio for the patients in different diseases
5. Conclusion and future prospect
QUR is a phytochemical with beneficial health benefits against different diseases. However, some studies proved its poor absorption in the human body. Recently innovative strategies have been proposed which enhanced QUR bioactivity in humans, lower costs, and are better described during clinical examinations. Researchers also designed novel extraction methods that provide a better extraction yield of QUR. Moreover, future work can be focused on minimizing the limitations of the aforementioned methods, and integration of one or more extraction methods is also an option to improve the results. In recent years, some non-invasive methods were also established for the evaluation of QUR from agricultural products. These methods are fast, non-destructive, environmentally friendly, and online detection is possible. However, improvement in the weaknesses of these techniques can expand their applications in more research domains. For example, strategies to deal with auto fluorescence issues in fluorescence spectroscopy, methods to resolve scattering phenomenon in terahertz spectroscopy, designing of new chemometric models, and improvement in near-infrared spectroscopy ability to detect volatile compounds in food can extend its applications. Also, algorithms for rapid processing of raw data and techniques to deal with data mining can make hyperspectral imaging a more efficient tool, while stable, reproducible, while sensitive nanoparticles can make surface-enhanced Raman spectroscopy a more sensitive and reliable sensing platform. Besides, integration of these non-interruptive methods can also make a more accurate, sensitive, and fast analytical tool for the detection of phytochemicals from different fruits and vegetables.
CRediT authorship contribution statement
Muhammad Faisal Manzoor: Conceptualization, Methodology, Writing – original draft, Writing - review & editing. Abid Hussain: Writing – original draft. Aysha Sameen: Data curation, Software. Amna Sahar: Data curation, Writing – original draft. Sipper Khan: Writing – original draft. Rabia Siddique: Data curation, Writing – original draft. Rana Muhammad Aadil: Data curation, Writing – original draft. Bin Xu: Supervision, Writing - review & editing, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The review was funded by Key Research & Development project of Jiangsu Province (Grant No. BE2020324), Key Research & Development Project of Yangzhou City (Grant No. YZ2020043) and Key Research & Development Project of Qinghai Province (Grant No. 2021-QY-214).
References
- 1.Batiha G.-S., Beshbishy A.M., Ikram M., Mulla Z.S., El-Hack M.E.A., Taha A.E., Algammal A.M., Elewa Y.H.A. The pharmacological activity, biochemical properties, and pharmacokinetics of the major natural polyphenolic flavonoid: quercetin. Foods. 2020;9(3):374. doi: 10.3390/foods9030374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bossolani G.D.P., Silva B.T., Perles J.V.C.M., Lima M.M., Frez F.C.V., de Souza S.R.G., Sehaber-Sierakowski C.C., Bersani-Amado C.A., Zanoni J.N. Rheumatoid arthritis induces enteric neurodegeneration and jejunal inflammation, and quercetin promotes neuroprotective and anti-inflammatory actions. Life Sci. 2019;238:116956. doi: 10.1016/j.lfs.2019.116956. [DOI] [PubMed] [Google Scholar]
- 3.Kundur S., Prayag A., Selvakumar P., Nguyen H., McKee L., Cruz C., Srinivasan A., Shoyele S., Lakshmikuttyamma A. Synergistic anticancer action of quercetin and curcumin against triple-negative breast cancer cell lines. J. Cell. Physiol. 2019;234:11103–11118. doi: 10.1002/jcp.27761. [DOI] [PubMed] [Google Scholar]
- 4.Caddeo C., Gabriele M., Fernàndez-Busquets X., Valenti D., Fadda A.M., Pucci L., Manconi M. Antioxidant activity of quercetin in Eudragit-coated liposomes for intestinal delivery. Int. J. Pharm. 2019;565:64–69. doi: 10.1016/j.ijpharm.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Ding Y., Li C., Zhang Y., Ma P., Zhao T., Che D., Cao J., Wang J., Liu R., Zhang T. Quercetin as a Lyn kinase inhibitor inhibits IgE-mediated allergic conjunctivitis. Food Chem. Toxicol. 2020;135:110924. doi: 10.1016/j.fct.2019.110924. [DOI] [PubMed] [Google Scholar]
- 6.Güran M., Şanlıtürk G., Kerküklü N.R., Altundağ E.M., Yalçın A.S. Combined effects of quercetin and curcumin on anti-inflammatory and antimicrobial parameters in vitro. Eur. J. Pharmacol. 2019;859:172486. doi: 10.1016/j.ejphar.2019.172486. [DOI] [PubMed] [Google Scholar]
- 7.Reyes-Farias M., Carrasco-Pozo C. The anti-cancer effect of quercetin: molecular implications in cancer metabolism. Int. J. Mol. Sci. 2019;20(13):3177. doi: 10.3390/ijms20133177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Škandík M., Mrvová N., Bezek Š., Račková L. Semisynthetic quercetin-quinone mitigates BV-2 microglia activation through modulation of Nrf2 pathway. Free Radic. Biol. Med. 2020;152:18–32. doi: 10.1016/j.freeradbiomed.2020.02.030. [DOI] [PubMed] [Google Scholar]
- 9.Perdicaro D.J., Lanzi C.R., Tudela J.G., Miatello R.M., Oteiza P.I., Prieto M.A.V. Quercetin attenuates adipose hypertrophy, in part through activation of adipogenesis in rats fed a high-fat diet. J. Nutr. Biochem. 2020;79:108352. doi: 10.1016/j.jnutbio.2020.108352. [DOI] [PubMed] [Google Scholar]
- 10.Eitah H.E., Maklad Y.A., Abdelkader N.F., el Din A.A.G., Badawi M.A., Kenawy S.A. Modulating impacts of quercetin/sitagliptin combination on streptozotocin-induced diabetes mellitus in rats. Toxicol. Appl. Pharmacol. 2019;365:30–40. doi: 10.1016/j.taap.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X.-W., Chen J.-Y., Ouyang D., Lu J.-H. Quercetin in Animal Models of Alzheimer’s Disease: a systematic review of preclinical studies. Int. J. Mol. Sci. 2020;21(2):493. doi: 10.3390/ijms21020493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng S.-C., Wu Y.-H., Huang W.-C., Pang J.-H., Huang T.-H., Cheng C.-Y. Anti-inflammatory property of quercetin through downregulation of ICAM-1 and MMP-9 in TNF-α-activated retinal pigment epithelial cells. Cytokine. 2019;116:48–60. doi: 10.1016/j.cyto.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Thanitwatthanasak S., Sagis L.M.C., Chitprasert P. Pluronic F127/Pluronic P123/vitamin E TPGS mixed micelles for oral delivery of mangiferin and quercetin: Mixture-design optimization, micellization, and solubilization behavior. J. Mol. Liq. 2019;274:223–238. [Google Scholar]
- 14.Thuy N.M., Thuy N.T.M., Cuong N.P., Huyen L.T.N., Phuong N.P., Nguyen L.T.T., Kim J.H., Thu N.T., Tai N.V. Identification and extraction method of quercetin from flesh and skin of shallot (Allium ascalonicum) cultivated in Soc Trang Province, Vietnam. Food Res. 2019;4(2):358–365. [Google Scholar]
- 15.Rahmi I.A., Jufri M., Mun’im A. Extraction of Quercetin from Nothopanax scutellarium Leaves via Ionic Liquid-based Microwave-assisted Extraction. Pharmacogn. J. 2020;12(6s):1512–1517. [Google Scholar]
- 16.Santiago B., Calvo A.A., Gullon B., Feijoo G., Moreira M.T., Gonzalez-Garcia S. Production of flavonol quercetin and fructooligosaccharides from onion (Allium cepa L.) waste: An environmental life cycle approach. Chem. Eng. J. 2020;392:123772. [Google Scholar]
- 17.Costa J.R., Tonon R.V., Cabral L., Gottschalk L., Pastrana L., Pintado M.E. Valorization of Agricultural Lignocellulosic Plant Byproducts through Enzymatic and Enzyme-Assisted Extraction of High-Value-Added Compounds: A Review. ACS Sustain. Chem. Eng. 2020;8(35):13112–13125. [Google Scholar]
- 18.Câmara J.S., Albuquerque B.R., Aguiar J., Corrêa R.C., Gonçalves J.L., Granato D., Pereira J.A., Barros L., Ferreira I.C. Food Bioactive Compounds and Emerging Techniques for Their Extraction: Polyphenols as a Case Study. Foods. 2021;10:37. doi: 10.3390/foods10010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Afsah-Hejri L., Hajeb P., Ara P., Ehsani R.J. A comprehensive review on food applications of terahertz spectroscopy and imaging. Comprehensive Rev. Food Sci. Food Safety. 2019;18:1563–1621. doi: 10.1111/1541-4337.12490. [DOI] [PubMed] [Google Scholar]
- 20.Abbas O., Pissard A., Baeten V. Near-infrared, mid-infrared, and Raman spectroscopy, in. Chem. Anal. Food, Elsevier. 2020:77–134. [Google Scholar]
- 21.Manzoor M.F., Zeng X.-A., Rahaman A., Siddeeg A., Aadil R.M., Ahmed Z., Li J., Niu D. Combined impact of pulsed electric field and ultrasound on bioactive compounds and FT-IR analysis of almond extract. J. Food Sci. Technol. 2019;56:2355–2364. doi: 10.1007/s13197-019-03627-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahaman A., Siddeeg A., Manzoor M.F., Zeng X.-A., Ali S., Baloch Z., Li J., Wen Q.-H. Impact of pulsed electric field treatment on drying kinetics, mass transfer, colour parameters and microstructure of plum. J. Food Sci. Technol. 2019;56(5):2670–2678. doi: 10.1007/s13197-019-03755-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manzoor M.F., Ahmad N., Ahmed Z., Siddique R., Zeng X.-A., Rahaman A., Muhammad Aadil R., Wahab A. Novel extraction techniques and pharmaceutical activities of luteolin and its derivatives. J. Food Biochem. 2019;43(9) doi: 10.1111/jfbc.v43.910.1111/jfbc.12974. [DOI] [PubMed] [Google Scholar]
- 24.Jang M., Asnin L., Nile S.H., Keum Y.S., Kim H.Y., Park S.W. Ultrasound-assisted extraction of quercetin from onion solid wastes. Int. J. Food Sci. Technol. 2013;48:246–252. [Google Scholar]
- 25.Sharifi N., Mahernia S., Amanlou M. Comparison of different methods in quercetin extraction from leaves of Raphanus sativus L. Pharmaceut. Sci. 2016;23:59–65. [Google Scholar]
- 26.Wang B., Goldsmith C.D., Zhao J., Zhao S., Sheng Z., Yu W. Optimization of ultrasound-assisted extraction of quercetin, luteolin, apigenin, pinocembrin and chrysin from Flos populi by Plackett-Burman design combined with Taguchi method. Chiang Mai J. Sci. 2018;45:427–439. [Google Scholar]
- 27.Wei M., Zhao R., Peng X., Feng C., Gu H., Yang L. Ultrasound-Assisted Extraction of Taxifolin, Diosmin, and Quercetin from Abies nephrolepis (Trautv.) Maxim: Kinetic and Thermodynamic Characteristics. Molecules. 2020;25:1401. doi: 10.3390/molecules25061401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orsat V., Routray W. Water Extraction of Bioactive Compounds. Elsevier; 2017. pp. 221–244. [DOI] [Google Scholar]
- 29.Vinatoru M., Mason T., Calinescu I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. TrAC, Trends Anal. Chem. 2017;97:159–178. [Google Scholar]
- 30.Zhang F., Yang Y., Su P., Guo Z. Microwave-assisted extraction of rutin and quercetin from the stalks of Euonymus alatus (Thunb.) Sieb. Phytochem. Anal. 2009;20:33–37. doi: 10.1002/pca.1088. [DOI] [PubMed] [Google Scholar]
- 31.Jin E.Y., Lim S., oh Kim S., Park Y.-S., Jang J.K., Chung M.-S., Park H., Shim K.-S., Choi Y.J. Optimization of various extraction methods for quercetin from onion skin using response surface methodology. Food Sci. Biotechnol. 2011;20:1727–1733. [Google Scholar]
- 32.Kumar B., Smita K., Kumar B., Cumbal L., Rosero G. Microwave-Assisted Extraction and Solid-Phase Separation of Quercetin from Solid Onion (Allium cepaL.) Sep. Sci. Technol. 2014;49:2502–2509. [Google Scholar]
- 33.Mukhaimin I., Saraswati E.A., Ajizah R., Triyastuti M.S. Product Quality of Quercetin Extract From Carica Papaya L Flower by Microwave-Assisted Extraction (MAE) Jurnal Rekayasa Kimia & Lingkungan. 2019;14:139–146. [Google Scholar]
- 34.Sun H., Li C., Ni Y., Yao L., Jiang H., Ren X., Fu Y., Zhao C. Ultrasonic/microwave-assisted extraction of polysaccharides from Camptotheca acuminata fruits and its antitumor activity. Carbohydr. Polym. 2019;206:557–564. doi: 10.1016/j.carbpol.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 35.Huang L., Cao Y., Chen G. Purification of quercetin in Anoectochilu roxburghii (wall) Lindl using UMAE by high-speed counter-current chromatography and subsequent structure identification. Sep. Purif. Technol. 2008;64:101–107. [Google Scholar]
- 36.Ahmadkelayeh S., Hawboldt K. Extraction of lipids and astaxanthin from crustacean by-products: A review on supercritical CO2 extraction. Trends Food Sci. Technol. 2020 [Google Scholar]
- 37.Gandhi K., Arora S., Kumar A. Industrial applications of supercritical fluid extraction: a review. Int. J. Chem. Stud. 2017;5:336–340. [Google Scholar]
- 38.Nikolai P., Rabiyat B., Aslan A., Ilmutdin A. Supercritical CO 2: Properties and Technological Applications-A Review. J. Therm. Sci. 2019;28:394–430. [Google Scholar]
- 39.Chávez-González M.L., Sepúlveda L., Verma D.K., Luna-García H.A., Rodríguez-Durán L.V., Ilina A., Aguilar C.N. Conventional and Emerging Extraction Processes of Flavonoids. Processes. 2020;8:434. [Google Scholar]
- 40.Lévai G., Martín Á., de Paz E., Rodríguez-Rojo S., Cocero M.J. Production of stabilized quercetin aqueous suspensions by supercritical fluid extraction of emulsions. J. Supercritical Fluids. 2015;100:34–45. [Google Scholar]
- 41.Ghoreishi S., Hedayati A., Mousavi S. Quercetin extraction from Rosa damascena Mill via supercritical CO2: Neural network and adaptive neuro fuzzy interface system modeling and response surface optimization. J. Supercritical Fluids. 2016;112:57–66. [Google Scholar]
- 42.Gligor O., Mocan A., Moldovan C., Locatelli M., Crișan G., Ferreira I.C. Enzyme-assisted extractions of polyphenols–A comprehensive review. Trends Food Sci. Technol. 2019;88:302–315. [Google Scholar]
- 43.Picot-Allain M.C.N., Ramasawmy B., Emmambux M.N. Extraction, characterisation, and application of pectin from tropical and sub-tropical fruits: a review. Food Rev. Int. 2020:1–31. [Google Scholar]
- 44.Zhang Q.-W., Lin L.-G., Ye W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018;13:1–26. doi: 10.1186/s13020-018-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu T., Sui X., Li L., Zhang J., Liang X., Li W., Zhang H., Fu S. Application of ionic liquids based enzyme-assisted extraction of chlorogenic acid from Eucommia ulmoides leaves. Anal. Chim. Acta. 2016;903:91–99. doi: 10.1016/j.aca.2015.11.029. [DOI] [PubMed] [Google Scholar]
- 46.Lindahl S., Liu J., Khan S., Karlsson E.N., Turner C. An on-line method for pressurized hot water extraction and enzymatic hydrolysis of quercetin glucosides from onions. Anal. Chim. Acta. 2013;785:50–59. doi: 10.1016/j.aca.2013.04.031. [DOI] [PubMed] [Google Scholar]
- 47.Choi I.S., Cho E.J., Moon J.-H., Bae H.-J. Onion skin waste as a valorization resource for the by-products quercetin and biosugar. Food Chem. 2015;188:537–542. doi: 10.1016/j.foodchem.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 48.Wang L., Wu Y., Liu Y., Wu Z. Complex enzyme-assisted extraction releases antioxidative phenolic compositions from guava leaves. Molecules. 2017;22:1648. doi: 10.3390/molecules22101648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lakowicz J.R. Springer Science & Business Media; 2013. Principles of fluorescence spectroscopy. [Google Scholar]
- 50.Valeur B., Brochon J.-C. Springer Science & Business Media; 2012. New trends in fluorescence spectroscopy: applications to chemical and life sciences. [Google Scholar]
- 51.Hussain Javid, Rehman Najeeb Ur, Mabood Fazal, Al-Harrasi Ahmed, Ali Liaqat, Rizvi Tania Shamim, Khan Ajmal, Rafiq Kashif, Al-Rabaani Hamida, Jabeen Farah. Application of fluorescence spectroscopy coupled with PLSR for the estimation of quercetin in four medicinal plants. Chem. Data Collect. 2019;21:100228. doi: 10.1016/j.cdc.2019.100228. [DOI] [Google Scholar]
- 52.Hussain Javid, Mabood Fazal, Al-Harrasi Ahmed, Ali Liaqat, Rizvi Tania Shamim, Jabeen Farah, Gilani Syed Abdullah, Shinwari Shehla, Ahmad Mushtaq, Alabri Zahra Khalfan, Al Ghawi Said Hamood Salim. New robust sensitive fluorescence spectroscopy coupled with PLSR for estimation of quercetin in Ziziphus mucronata and Ziziphus sativa. Spectrochim. Acta Part A: Mol. Biomol. Spectroscopy. 2018;194:152–157. doi: 10.1016/j.saa.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 53.Alabri Zahra K., Hussain Javid, Mabood Fazal, Rehman Najeeb Ur, Ali Liaqat, Al-Harrasi Ahmed, Hamaed Ahmed, Khan Abdul L., Rizvi Tania S., Jabeen Farah, Khan Ajmal, Naureen Zakira, Farooq Saima. Fluorescence spectroscopy-partial least square regression method for the quantification of quercetin in Euphorbia masirahensis. Measurement. 2018;121:355–359. [Google Scholar]
- 54.Jepsen P.U., Cooke D.G., Koch M. Terahertz spectroscopy and imaging–Modern techniques and applications. Laser Photon. Rev. 2011;5(1):124–166. [Google Scholar]
- 55.Yan Ling, Liu Changhong, Qu Hao, Liu Wei, Zhang Yan, Yang Jianbo, Zheng Lei. Discrimination and measurements of three flavonols with similar structure using terahertz spectroscopy and chemometrics. J. Infrared Millimeter Terahertz Waves. 2018;39(5):492–504. [Google Scholar]
- 56.Weeranantanaphan Jittima, Downey Gerard, Allen Paul, Sun Da-Wen. A review of near infrared spectroscopy in muscle food analysis: 2005–2010. J. Near Infrared Spectrosc. 2011;19(2):61–104. [Google Scholar]
- 57.Cen H., He Y. Theory and application of near infrared reflectance spectroscopy in determination of food quality. Trends Food Sci. Technol. 2007;18:72–83. [Google Scholar]
- 58.Silalahi D.D., Reaño C.E., Lansigan F.P., Panopio R.G., Bantayan N.C., Davrieux F., Caliman J., Yuan Y.Y., Sudarno Near infrared spectroscopy: a rapid and non-destructive technique to assess the ripeness of oil palm (Elaeis guineensis Jacq.) fresh fruit. J. Near Infrared Spectrosc. 2016;24:179–190. [Google Scholar]
- 59.Hussain A., Pu H., Sun D.-W. Innovative nondestructive imaging techniques for ripening and maturity of fruits–a review of recent applications. Trends Food Sci. Technol. 2018;72:144–152. [Google Scholar]
- 60.Hussain A., Pu H., Sun D.-W. Measurements of lycopene contents in fruit: A review of recent developments in conventional and novel techniques. Crit. Rev. Food Sci. Nutr. 2019;59:758–769. doi: 10.1080/10408398.2018.1518896. [DOI] [PubMed] [Google Scholar]
- 61.Tzanova M., Atanassova S., Atanasov V., Grozeva N. Content of Polyphenolic Compounds and Antioxidant Potential of Some Bulgarian Red Grape Varieties and Red Wines, Determined by HPLC, UV, and NIR Spectroscopy. Agriculture. 2020;10:193. [Google Scholar]
- 62.Barbosa C.D., Baqueta M.R., Santos W.C.R., Gomes D., Alvarenga V.O., Teixeira P., Albano H., Rosa C.A., Valderrama P., Lacerda I.C. Data fusion of UPLC data, NIR spectra and physicochemical parameters with chemometrics as an alternative to evaluating kombucha fermentation. LWT-Food Sci. Technol. 2020;133:109875. [Google Scholar]
- 63.Caporaso N., Whitworth M.B., Grebby S., Fisk I.D. Rapid prediction of single green coffee bean moisture and lipid content by hyperspectral imaging. J. Food Eng. 2018;227:18–29. doi: 10.1016/j.jfoodeng.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ravikanth L., Jayas D.S., White N.D., Fields P.G., Sun D.-W. Extraction of spectral information from hyperspectral data and application of hyperspectral imaging for food and agricultural products. Food Bioprocess Technol. 2017;10:1–33. [Google Scholar]
- 65.Orrillo I., Cruz-Tirado J., Cardenas A., Oruna M., Carnero A., Barbin D.F., Siche R. Hyperspectral imaging as a powerful tool for identification of papaya seeds in black pepper. Food Control. 2019;101:45–52. [Google Scholar]
- 66.Jara-Palacios M., Rodríguez-Pulido F.J., Hernanz D., Escudero-Gilete M.L., Heredia F. Determination of phenolic substances of seeds, skins and stems from white grape marc by near-infrared hyperspectral imaging. Aust. J. Grape Wine Res. 2016;22:11–15. [Google Scholar]
- 67.J. Qin, K. Chao, M.J.L.S.T.f.F.P. Kim, Quality, S. Assessment, R. Lu, Ed, Raman scattering for food quality and safety assessment, (2016) 387–428.
- 68.Jarvis R.M., Brooker A., Goodacre R. Surface-enhanced Raman scattering for the rapid discrimination of bacteria. Faraday Discuss. 2006;132:281–292. doi: 10.1039/b506413a. [DOI] [PubMed] [Google Scholar]
- 69.Hussain A., Sun D.-W., Pu H. Bimetallic core shelled nanoparticles (Au@ AgNPs) for rapid detection of thiram and dicyandiamide contaminants in liquid milk using SERS. Food Chem. 2020;317:126429. doi: 10.1016/j.foodchem.2020.126429. [DOI] [PubMed] [Google Scholar]
- 70.He X., Yang S., Xu T., Song Y., Zhang X. Microdroplet-captured tapes for rapid sampling and SERS detection of food contaminants. Biosens. Bioelectron. 2020;152:112013. doi: 10.1016/j.bios.2020.112013. [DOI] [PubMed] [Google Scholar]
- 71.Hussain A., Pu H., Hu B., Sun D.-W. Au@ Ag-TGANPs based SERS for facile screening of thiabendazole and ferbam in liquid milk. 2020;245:118908. doi: 10.1016/j.saa.2020.118908. [DOI] [PubMed] [Google Scholar]
- 72.Hussain Abid, Pu Hongbin, Sun Da-Wen. Cysteamine modified core-shell nanoparticles for rapid assessment of oxamyl and thiacloprid pesticides in milk using SERS. J. Food Measur. Charact. 2020;14(4):2021–2029. [Google Scholar]
- 73.Hussain Abid, Pu Hongbin, Sun Da-Wen. SERS detection of sodium thiocyanate and benzoic acid preservatives in liquid milk using cysteamine functionalized core-shelled nanoparticles. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2020;229:117994. doi: 10.1016/j.saa.2019.117994. [DOI] [PubMed] [Google Scholar]
- 74.Ahmed Zahoor, Faisal Manzoor Muhammad, Hussain Abid, Hanif Zia-ud-Din Muddasir, Zeng Xin-An. Study the impact of ultra-sonication and pulsed electric field on the quality of wheat plantlet juice through FTIR and SERS. Ultrason. Sonochem. 2021;76:105648. doi: 10.1016/j.ultsonch.2021.105648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Numata Y., Tanaka H. Quantitative analysis of quercetin using Raman spectroscopy. Food Chem. 2011;126:751–755. [Google Scholar]
- 76.Dendisová M., Palounek D., Švecová M., Prokopec V. SERS study of fluorescent and non-fluorescent flavonoids: what is the role of excitation wavelength on SERS optical response? Chem. Pap. 2019;73:2945–2953. [Google Scholar]
- 77.Choi J.M., Hahm E., Park K., Jeong D., Rho W.-Y., Kim J., Jeong D.H., Lee Y.-S., Jhang S.H., Chung H.J. Sers-based flavonoid detection using ethylenediamine-β-cyclodextrin as a capturing ligand. Nanomaterials. 2017;7:8. doi: 10.3390/nano7010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Almeida A.F., Borge G.I.A., Piskula M., Tudose A., Tudoreanu L., Valentová K., Williamson G., Santos C.N. Bioavailability of quercetin in humans with a focus on interindividual variation. Compr. Rev. Food Sci. Food Saf. 2018;17:714–731. doi: 10.1111/1541-4337.12342. [DOI] [PubMed] [Google Scholar]
- 79.Pérez-Jiménez J., Neveu V., Vos F., Scalbert A. Identification of the 100 richest dietary sources of polyphenols: an application of the Phenol-Explorer database. Eur. J. Clin. Nutr. 2010;64(S3):S112–S120. doi: 10.1038/ejcn.2010.221. [DOI] [PubMed] [Google Scholar]
- 80.Prot J.M., Maciel L., Bricks T., Merlier F., Cotton J., Paullier P., Bois F.Y., Leclerc E. First pass intestinal and liver metabolism of paracetamol in a microfluidic platform coupled with a mathematical modeling as a means of evaluating ADME processes in humans. Biotechnol. Bioeng. 2014;111:2027–2040. doi: 10.1002/bit.25232. [DOI] [PubMed] [Google Scholar]
- 81.Bentz A.B. A review of Quercetin: chemistry, Antioxident properties, and bioavailability. J. Young Investig. 2017 [Google Scholar]
- 82.Dabeek Wijdan M., Marra Melissa Ventura. Dietary quercetin and kaempferol: Bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients. 2019;11(10):2288. doi: 10.3390/nu11102288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kaşıkcı Müzeyyen, Bağdatlıoğlu Neriman. Bioavailability of quercetin. Curr. Res. Nutr. Food Sci. J. 2016;4(Special-Issue-October):146–151. [Google Scholar]
- 84.Akhlaghi M., Foshati S. Bioavailability and metabolism of flavonoids: A review. Int. J. Nutr. Sci. 2017;2:180–184. [Google Scholar]
- 85.Bhimanwar R., Kothapalli L., Khawshi A. Quercetin as Natural Bioavailability Modulator: An Overview. Res. J. Pharm. Technol. 2020;13:2045–2052. [Google Scholar]
- 86.Beking K., Vieira A. An assessment of dietary flavonoid intake in the UK and Ireland. Int. J. Food Sci. Nutr. 2011;62:17–19. doi: 10.3109/09637486.2010.511165. [DOI] [PubMed] [Google Scholar]
- 87.Dilis V., Trichopoulou A. Antioxidant intakes and food sources in Greek adults. J. Nutr. 2010;140:1274–1279. doi: 10.3945/jn.110.121848. [DOI] [PubMed] [Google Scholar]
- 88.Wang Weiyou, Sun Cuixia, Mao Like, Ma Peihua, Liu Fuguo, Yang Jie, Gao Yanxiang. The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends Food Sci. Technol. 2016;56:21–38. [Google Scholar]
- 89.Fujimori M., Kadota K., Shimono K., Shirakawa Y., Sato H., Tozuka Y. Enhanced solubility of quercetin by forming composite particles with transglycosylated materials. J. Food Eng. 2015;149:248–254. [Google Scholar]
- 90.H. Lennernäs, V.P. Shah, J.R. Crison, G.L. Amidon, A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability, (1995). [DOI] [PubMed]
- 91.McClements D.J., Decker E.A., Park Y., Weiss J. Structural design principles for delivery of bioactive components in nutraceuticals and functional foods. Crit. Rev. Food Sci. Nutr. 2009;49:577–606. doi: 10.1080/10408390902841529. [DOI] [PubMed] [Google Scholar]
- 92.Lu W., Kelly A.L., Miao S. Emulsion-based encapsulation and delivery systems for polyphenols. Trends Food Sci. Technol. 2016;47:1–9. [Google Scholar]
- 93.D.C. Ferraz da Costa, L. Pereira Rangel, J. Quarti, R.A. Santos, J.L. Silva, E. Fialho, Bioactive Compounds and Metabolites from Grapes and Red Wine in Breast Cancer Chemoprevention and Therapy, Molecules, 25 (2020) 3531. [DOI] [PMC free article] [PubMed]
- 94.Fathi M., Mozafari M.R., Mohebbi M. Nanoencapsulation of food ingredients using lipid based delivery systems. Trends Food Sci. Technol. 2012;23:13–27. [Google Scholar]
- 95.Yao M., Xiao H., McClements D.J. Delivery of lipophilic bioactives: assembly, disassembly, and reassembly of lipid nanoparticles. Ann. Rev. Food Sci. Technol. 2014;5:53–81. doi: 10.1146/annurev-food-072913-100350. [DOI] [PubMed] [Google Scholar]
- 96.Gokhale Jayanti P., Mahajan Hitendra S., Surana Sanjay J. Quercetin loaded nanoemulsion-based gel for rheumatoid arthritis: In vivo and in vitro studies. Biomed. Pharmacother. 2019;112:108622. doi: 10.1016/j.biopha.2019.108622. [DOI] [PubMed] [Google Scholar]
- 97.Lawrence M.J., Rees G.D. Microemulsion-based media as novel drug delivery systems. Adv. Drug Deliv. Rev. 2000;45:89–121. doi: 10.1016/s0169-409x(00)00103-4. [DOI] [PubMed] [Google Scholar]
- 98.Chen H., Chang X., Weng T., Zhao X., Gao Z., Yang Y., Xu H., Yang X. A study of microemulsion systems for transdermal delivery of triptolide. J. Control. Release. 2004;98:427–436. doi: 10.1016/j.jconrel.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 99.Gulsen D., Chauhan A. Dispersion of microemulsion drops in HEMA hydrogel: a potential ophthalmic drug delivery vehicle. Int. J. Pharm. 2005;292:95–117. doi: 10.1016/j.ijpharm.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 100.Aithal G.C., Nayak U.Y., Mehta C., Narayan R., Gopalkrishna P., Pandiyan S., Garg S. Localized in situ nanoemulgel drug delivery system of quercetin for periodontitis: development and computational simulations. Molecules. 2018;23:1363. doi: 10.3390/molecules23061363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jangde R., Srivastava S., Singh M.R., Singh D. In vitro and In vivo characterization of quercetin loaded multiphase hydrogel for wound healing application. Int. J. Biol. Macromol. 2018;115:1211–1217. doi: 10.1016/j.ijbiomac.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 102.Liu W., Kong Y., Ye A., Shen P., Dong L., Xu X., Hou Y., Wang Y., Jin Y., Han J. Preparation, formation mechanism and in vitro dynamic digestion behavior of quercetin-loaded liposomes in hydrogels. Food Hydrocolloids. 2020;104:105743. [Google Scholar]
- 103.Rodríguez-Félix F., Del-Toro-Sánchez C.L., Javier Cinco-Moroyoqui F., Juárez J., Ruiz-Cruz S., López-Ahumada G.A., Carvajal-Millan E., Castro-Enríquez D.D., Barreras-Urbina C.G., Tapia-Hernández J.A. Preparation and Characterization of Quercetin-Loaded Zein Nanoparticles by Electrospraying and Study of In Vitro Bioavailability. J. Food Sci. 2019;84:2883–2897. doi: 10.1111/1750-3841.14803. [DOI] [PubMed] [Google Scholar]
- 104.Ni Shilei, Hu Caibiao, Sun Rui, Zhao Guodong, Xia Qiang. Nanoemulsions-based delivery systems for encapsulation of quercetin: Preparation, characterization, and cytotoxicity studies. J. Food Process Eng. 2017;40(2):e12374. doi: 10.1111/jfpe.2017.40.issue-210.1111/jfpe.12374. [DOI] [Google Scholar]
- 105.Joye Iris Julie, McClements David Julian. Biopolymer-based nanoparticles and microparticles: Fabrication, characterization, and application. Curr. Opin. Colloid Interface Sci. 2014;19(5):417–427. [Google Scholar]
- 106.Fang R., Hao R., Wu X., Li Q., Leng X., Jing H. Bovine serum albumin nanoparticle promotes the stability of quercetin in simulated intestinal fluid. J. Agric. Food. Chem. 2011;59:6292–6298. doi: 10.1021/jf200718j. [DOI] [PubMed] [Google Scholar]
- 107.Xing J., Zhang D., Tan T. Studies on the oridonin-loaded poly (D, L-lactic acid) nanoparticles in vitro and in vivo. Int. J. Biol. Macromol. 2007;40:153–158. doi: 10.1016/j.ijbiomac.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 108.Penalva R., Gonzalez-Navarro C.J., Gamazo C., Esparza I., Irache J.M. Zein nanoparticles for oral delivery of quercetin: Pharmacokinetic studies and preventive anti-inflammatory effects in a mouse model of endotoxemia, Nanomedicine: Nanotechnology. Biol. Med. 2017;13:103–110. doi: 10.1016/j.nano.2016.08.033. [DOI] [PubMed] [Google Scholar]
- 109.Hazra M., Mandal D.D., Mandal T., Bhuniya S., Ghosh M. Designing polymeric microparticulate drug delivery system for hydrophobic drug quercetin. Saudi Pharmaceut. J. 2015;23:429–436. doi: 10.1016/j.jsps.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang Y., Yang Y., Tang K., Hu X., Zou G. Physicochemical characterization and antioxidant activity of quercetin-loaded chitosan nanoparticles. J. Appl. Polym. Sci. 2008;107:891–897. [Google Scholar]
- 111.Aditya N., Macedo A.S., Doktorovova S., Souto E.B., Kim S., Chang P.-S., Ko S. Development and evaluation of lipid nanocarriers for quercetin delivery: a comparative study of solid lipid nanoparticles (SLN), nanostructured lipid carriers (NLC), and lipid nanoemulsions (LNE) LWT-Food Sci. Technol. 2014;59:115–121. [Google Scholar]
- 112.Porcu Elena Piera, Cossu Massimo, Rassu Giovanna, Giunchedi Paolo, Cerri Guido, Pourová Jana, Najmanová Iveta, Migkos Thomas, Pilařová Veronika, Nováková Lucie, Mladěnka Přemysl, Gavini Elisabetta. Aqueous injection of quercetin: An approach for confirmation of its direct in vivo cardiovascular effects. Int. J. Pharm. 2018;541(1-2):224–233. doi: 10.1016/j.ijpharm.2018.02.036. [DOI] [PubMed] [Google Scholar]
- 113.Faridi Esfanjani Afshin, Assadpour Elham, Jafari Seid Mahdi. Improving the bioavailability of phenolic compounds by loading them within lipid-based nanocarriers. Trends Food Sci. Technol. 2018;76:56–66. [Google Scholar]
- 114.Han Qiusen, Wang Xinhuan, Cai Shuangfei, Liu Xueliang, Zhang Yufei, Yang Lin, Wang Chen, Yang Rong. Quercetin nanoparticles with enhanced bioavailability as multifunctional agents toward amyloid induced neurotoxicity. J. Mater. Chem. B. 2018;6(9):1387–1393. doi: 10.1039/c7tb03053c. [DOI] [PubMed] [Google Scholar]
- 115.Najafabadi R.E., Kazemipour N., Esmaeili A., Beheshti S., Nazifi S. Using superparamagnetic iron oxide nanoparticles to enhance bioavailability of quercetin in the intact rat brain. BMC Pharmacol. Toxicol. 2018;19:59. doi: 10.1186/s40360-018-0249-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rahimi S., Khoee S., Ghandi M. Preparation and characterization of rod-like chitosan–quinoline nanoparticles as pH-responsive nanocarriers for quercetin delivery. Int. J. Biol. Macromol. 2019;128:279–289. doi: 10.1016/j.ijbiomac.2019.01.137. [DOI] [PubMed] [Google Scholar]
- 117.Abd El-Fattah A.I., Fathy M.M., Ali Z.Y., El-Garawany A.E.-R.A., Mohamed E.K. Enhanced therapeutic benefit of quercetin-loaded phytosome nanoparticles in ovariectomized rats. Chem. Biol. Interact. 2017;271:30–38. doi: 10.1016/j.cbi.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 118.Cerdeira A.M., Mazzotti M., Gander B. Miconazole nanosuspensions: influence of formulation variables on particle size reduction and physical stability. Int. J. Pharm. 2010;396:210–218. doi: 10.1016/j.ijpharm.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 119.Neubert R.H. Potentials of new nanocarriers for dermal and transdermal drug delivery. Eur. J. Pharmaceut. Biopharmaceut. 2011;77:1–2. doi: 10.1016/j.ejpb.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 120.Roa W.H., Azarmi S., Al-Hallak M.K., Finlay W.H., Magliocco A.M., Löbenberg R. Inhalable nanoparticles, a non-invasive approach to treat lung cancer in a mouse model. J. Control. Release. 2011;150:49–55. doi: 10.1016/j.jconrel.2010.10.035. [DOI] [PubMed] [Google Scholar]
- 121.Kamel Hassan Amany O., Elshafeey Ahmed H. Nanosized particulate systems for dermal and transdermal delivery. J. Biomed. Nanotechnol. 2010;6(6):621–633. doi: 10.1166/jbn.2010.1141. [DOI] [PubMed] [Google Scholar]
- 122.Yallapu Murali M., Jaggi Meena, Chauhan Subhash C. Curcumin nanoformulations: a future nanomedicine for cancer. Drug Discov. Today. 2012;17(1-2):71–80. doi: 10.1016/j.drudis.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Keck C., Muller R. Drug nanocrystals of poorly soluble drugs produced by high pressure homogenisation. Eur. J. Pharmaceut. Biopharmaceut. 2006;62(1):3–16. doi: 10.1016/j.ejpb.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 124.Patravale V., Date A.A., Kulkarni R.J. Nanosuspensions: a promising drug delivery strategy. J. Pharm. Pharmacol. 2004;56:827–840. doi: 10.1211/0022357023691. [DOI] [PubMed] [Google Scholar]
- 125.Müller R.H., Möschwitzer J. Method and device for producing very fine particles and coating such particles. Google Patents. 2015 [Google Scholar]
- 126.Kakran Mitali, Shegokar Ranjita, Sahoo Nanda Gopal, Al Shaal Loaye, Li Lin, Müller Rainer H. Fabrication of quercetin nanocrystals: comparison of different methods. Eur. J. Pharmaceut. Biopharmaceut. 2012;80(1):113–121. doi: 10.1016/j.ejpb.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 127.Gao Lei, Liu Guiyang, Wang Xiaoqing, Liu Fei, Xu Yuefang, Ma Jing. Preparation of a chemically stable quercetin formulation using nanosuspension technology. Int. J. Pharm. 2011;404(1-2):231–237. doi: 10.1016/j.ijpharm.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 128.Sun Min, Gao Yan, Pei Yan, Guo Chenyu, Li Houli, Cao Fengliang, Yu Aihua, Zhai Guangxi. Development of nanosuspension formulation for oral delivery of quercetin. J. Biomed. Nanotechnol. 2010;6(4):325–332. doi: 10.1166/jbn.2010.1133. [DOI] [PubMed] [Google Scholar]
- 129.Blanchette James, Peppas Nicholas A. Oral chemotherapeutic delivery: design and cellular response. Ann. Biomed. Eng. 2005;33(2):142–149. doi: 10.1007/s10439-005-8973-8. [DOI] [PubMed] [Google Scholar]
- 130.Dufresne M.-H., Garrec D. Le, Sant V., Leroux J.-C., Ranger M. Preparation and characterization of water-soluble pH-sensitive nanocarriers for drug delivery. Int. J. Pharm. 2004;277(1-2):81–90. doi: 10.1016/j.ijpharm.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 131.Palle S., Neerati P. Enhancement of oral bioavailability of rivastigmine with quercetin nanoparticles by inhibiting CYP3A4 and esterases. Pharmacol. Rep. 2017;69:365–370. doi: 10.1016/j.pharep.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 132.Jin Y., Song Y., Zhu X., Zhou D., Chen C., Zhang Z., Huang Y. Goblet cell-targeting nanoparticles for oral insulin delivery and the influence of mucus on insulin transport. Biomaterials. 2012;33:1573–1582. doi: 10.1016/j.biomaterials.2011.10.075. [DOI] [PubMed] [Google Scholar]
- 133.Yao H.-J., Ju R.-J., Wang X.-X., Zhang Y., Li R.-J., Yu Y., Zhang L., Lu W.-L. The antitumor efficacy of functional paclitaxel nanomicelles in treating resistant breast cancers by oral delivery. Biomaterials. 2011;32:3285–3302. doi: 10.1016/j.biomaterials.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 134.Hamaguchi T., Matsumura Y., Suzuki M., Shimizu K., Goda R., Nakamura I., Nakatomi I., Yokoyama M., Kataoka K., Kakizoe T. NK105, a paclitaxel-incorporating micellar nanoparticle formulation, can extend in vivo antitumour activity and reduce the neurotoxicity of paclitaxel. Br. J. Cancer. 2005;92:1240–1246. doi: 10.1038/sj.bjc.6602479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nakanishi T., Fukushima S., Okamoto K., Suzuki M., Matsumura Y., Yokoyama M., Okano T., Sakurai Y., Kataoka K. Development of the polymer micelle carrier system for doxorubicin. J. Control. Release. 2001;74:295–302. doi: 10.1016/s0168-3659(01)00341-8. [DOI] [PubMed] [Google Scholar]
- 136.Gao Xiang, Wang BiLan, Wei XiaWei, Men Ke, Zheng Fengjin, Zhou Yingfeng, Zheng Yu, Gou MaLing, Huang Meijuan, Guo Gang, Huang Ning, Qian ZhiYong, Wei Yuquan. Anticancer effect and mechanism of polymer micelle-encapsulated quercetin on ovarian cancer. Nanoscale. 2012;4(22):7021. doi: 10.1039/c2nr32181e. [DOI] [PubMed] [Google Scholar]
- 137.Dian Linghui, Yu Enjiang, Chen Xiaona, Wen Xinguo, Zhang Zhengzan, Qin Lingzhen, Wang Qingqing, Li Ge, Wu Chuanbin. Enhancing oral bioavailability of quercetin using novel soluplus polymeric micelles. Nanoscale Res. Lett. 2014;9(1):684. doi: 10.1186/1556-276X-9-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liu K., Chen W., Yang T., Wen B., Ding D., Keidar M., Tang J., Zhang W. Paclitaxel and quercetin nanoparticles co-loaded in microspheres to prolong retention time for pulmonary drug delivery. Int. J. Nanomed. 2017;12:8239. doi: 10.2147/IJN.S147028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Patra A., Satpathy S., Shenoy A.K., Bush J.A., Kazi M., Hussain M.D. Formulation and evaluation of mixed polymeric micelles of quercetin for treatment of breast, ovarian, and multidrug resistant cancers. Int. J. Nanomed. 2018;13:2869. doi: 10.2147/IJN.S153094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Srisa-nga Korawith, Mankhetkorn Samlee, Okonogi Siriporn, Khonkarn Ruttiros. Delivery of superparamagnetic polymeric micelles loaded with quercetin to hepatocellular carcinoma cells. J. Pharm. Sci. 2019;108(2):996–1006. doi: 10.1016/j.xphs.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 141.Chang C.-E., Hsieh C.-M., Huang S.-C., Su C.-Y., Sheu M.-T., Ho H.-O. Lecithin-Stabilized Polymeric Micelles (L sb PMs) for Delivering Quercetin: Pharmacokinetic Studies and Therapeutic Effects of Quercetin Alone and in Combination with Doxorubicin. Sci. Rep. 2018;8:1–11. doi: 10.1038/s41598-018-36162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mu Yuzhi, Fu Yangmu, Li Jing, Yu Xiaoping, Li Yang, Wang Yanan, Wu Xuanjin, Zhang Kaichao, Kong Ming, Feng Chao, Chen Xiguang. Multifunctional quercetin conjugated chitosan nano-micelles with P-gp inhibition and permeation enhancement of anticancer drug. Carbohydr. Polym. 2019;203:10–18. doi: 10.1016/j.carbpol.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 143.Xu P., Wang H., Hu H., Ye Y., Dong Y., Li S., Mei D., Guo Z., Wang D., Sun Y. cRGDfK-Grafted Small-Size Quercetin Micelles For Enhancing Therapy Efficacy Of Active Ingredient From The Chinese Medicinal Herb. Int. J. Nanomed. 2019;14:9173. doi: 10.2147/IJN.S219578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nie Zhi-Yong, Zhang Ming-Zhi, Zheng Dong-Huan, Zhang Ming-Xing, Jia Hai-Quan, Zhang Xu-Dong, Yue Guang-Cheng, Li Xiao-Ming, Liu Peng, Zhang Yan-Feng, Liang Kai. Enhanced antitumoral activity of quercetin against lung cancer cells using biodegradable poly (lactic acid)-based polymeric nanoparticles. J. Biomater. Tissue Eng. 2017;7(4):269–274. [Google Scholar]
- 145.Singh Juhi, Mittal Pooja, Vasant Bonde Gunjan, Ajmal Gufran, Mishra Brahmeshwar. Design, optimization, characterization and in-vivo evaluation of Quercetin enveloped Soluplus®/P407 micelles in diabetes treatment. Artif. Cells Nanomed. Biotechnol. 2018;46(sup3):S546–S555. doi: 10.1080/21691401.2018.1501379. [DOI] [PubMed] [Google Scholar]
- 146.Fatease Adel Al, Shah Vidhi, Nguyen Duc X., Cote Brianna, LeBlanc Nicole, Rao Deepa A., Alani Adam W.G. Chemosensitization and mitigation of Adriamycin-induced cardiotoxicity using combinational polymeric micelles for co-delivery of quercetin/resveratrol and resveratrol/curcumin in ovarian cancer. Nanomed. Nanotechnol. Biol. Med. 2019;19:39–48. doi: 10.1016/j.nano.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 147.Singh A., Kureel A.K., Dutta P., Kumar S., Rai A.K. Curcumin loaded chitin-glucan quercetin conjugate: synthesis, characterization, antioxidant, in vitro release study, and anticancer activity. Int. J. Biol. Macromol. 2018;110:234–244. doi: 10.1016/j.ijbiomac.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 148.Singh A., Dutta P., Kumar H., Kureel A.K., Rai A.K. Synthesis of chitin-glucan-aldehyde-quercetin conjugate and evaluation of anticancer and antioxidant activities. Carbohydr. Polym. 2018;193:99–107. doi: 10.1016/j.carbpol.2018.03.092. [DOI] [PubMed] [Google Scholar]
- 149.Crommelin D.J., van Hoogevest P., Storm G. The role of liposomes in clinical nanomedicine development. What now? Now what? J. Control. Release. 2020;318:256–263. doi: 10.1016/j.jconrel.2019.12.023. [DOI] [PubMed] [Google Scholar]
- 150.Antimisiaris S.G., Marazioti A., Kannavou M., Natsaridis E., Gkartziou F., Kogkos G., Mourtas S. Overcoming barriers by local drug delivery with liposomes. Adv. Drug Deliv. Rev. 2021;174:53–86. doi: 10.1016/j.addr.2021.01.019. [DOI] [PubMed] [Google Scholar]
- 151.Baowen Q., Yulin Z., Xin W., Wenjing X., Hao Z., Zhizhi C., Xingmei D., Xia Z., Yuquan W., Lijuan C. A further investigation concerning correlation between anti-fibrotic effect of liposomal quercetin and inflammatory cytokines in pulmonary fibrosis. Eur. J. Pharmacol. 2010;642:134–139. doi: 10.1016/j.ejphar.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 152.Ghosh A., Mandal A.K., Sarkar S., Das N. Hepatoprotective and neuroprotective activity of liposomal quercetin in combating chronic arsenic induced oxidative damage in liver and brain of rats. Drug Deliv. 2011;18:451–459. doi: 10.3109/10717544.2011.577110. [DOI] [PubMed] [Google Scholar]
- 153.Chattopadhyay P., Shekunov B.Y., Yim D., Cipolla D., Boyd B., Farr S. Production of solid lipid nanoparticle suspensions using supercritical fluid extraction of emulsions (SFEE) for pulmonary delivery using the AERx system. Adv. Drug Deliv. Rev. 2007;59:444–453. doi: 10.1016/j.addr.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 154.Dhawan S., Kapil R., Singh B. Formulation development and systematic optimization of solid lipid nanoparticles of quercetin for improved brain delivery. J. Pharm. Pharmacol. 2011;63:342–351. doi: 10.1111/j.2042-7158.2010.01225.x. [DOI] [PubMed] [Google Scholar]
- 155.Chen-yu G., Chun-fen Y., Qi-lu L., Qi T., Yan-wei X., Wei-na L., Guang-Xi Z. Development of a quercetin-loaded nanostructured lipid carrier formulation for topical delivery. Int. J. Pharm. 2012;430:292–298. doi: 10.1016/j.ijpharm.2012.03.042. [DOI] [PubMed] [Google Scholar]
- 156.Barras A., Mezzetti A., Richard A., Lazzaroni S., Roux S., Melnyk P., Betbeder D., Monfilliette-Dupont N. Formulation and characterization of polyphenol-loaded lipid nanocapsules. Int. J. Pharm. 2009;379:270–277. doi: 10.1016/j.ijpharm.2009.05.054. [DOI] [PubMed] [Google Scholar]
- 157.Singh D., Rawat M.S.M., Semalty A., Semalty M. Quercetin-phospholipid complex: an amorphous pharmaceutical system in herbal drug delivery. Curr. Drug Discov. Technol. 2012;9:17–24. doi: 10.2174/157016312799304507. [DOI] [PubMed] [Google Scholar]
- 158.Ahmad N., Ahmad R., Naqvi A.A., Alam M.A., Ashafaq M., Abdur Rub R., Ahmad F.J. Intranasal delivery of quercetin-loaded mucoadhesive nanoemulsion for treatment of cerebral ischaemia. Artif. Cells Nanomed. Biotechnol. 2018;46:717–729. doi: 10.1080/21691401.2017.1337024. [DOI] [PubMed] [Google Scholar]
- 159.Deng Q., Li X.X., Fang Y., Chen X., Xue J. Therapeutic potential of quercetin as an antiatherosclerotic agent in atherosclerotic cardiovascular disease: A review. Evidence-Based Complementary Alternative Med. 2020;2020 doi: 10.1155/2020/5926381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yang Y., Zhang F. Ultrasound-assisted extraction of rutin and quercetin from Euonymus alatus (Thunb.) Sieb. Ultrason. Sonochem. 2008;15:308–313. doi: 10.1016/j.ultsonch.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 161.Zhu Yingpeng, Yu Jiangliu, Jiao Chunyan, Tong Jinfeng, Zhang Lei, Chang Yan, Sun Weina, Jin Qing, Cai Yongping. Optimization of quercetin extraction method in Dendrobium officinale by response surface methodology. Heliyon. 2019;5(9):e02374. doi: 10.1016/j.heliyon.2019.e02374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Vasantha Rupasinghe H., Kathirvel P., Huber G.M. Ultrasonication-assisted solvent extraction of quercetin glycosides from ‘Idared’apple peels. Molecules. 2011;16:9783–9791. doi: 10.3390/molecules16129783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Khanam M.N., Anis M. Organogenesis and efficient in vitro plantlet regeneration from nodal segments of Allamanda cathartica L. using TDZ and ultrasound assisted extraction of quercetin. Plant Cell, Tissue and Organ Culture (PCTOC) 2018;134:241–250. [Google Scholar]
- 164.Kumar B., Smita K., Kumar B. Ultrasound assisted extraction of quercetin from cabbage. Int. J. Pharmaceut. Sci. Res. 2014;5:3779. [Google Scholar]
- 165.Aybastıer Ö., Şahin S., Demir C. Response surface optimized ultrasonic-assisted extraction of quercetin and isolation of phenolic compounds from Hypericum perforatum L. by column chromatography. Sep. Sci. Technol. 2013;48:1665–1674. [Google Scholar]
- 166.Zhang F., Yang Y., Su P., Guo Z. Microwave-assisted extraction of rutin and quercetin from the stalks of Euonymus alatus (Thunb.) Sieb. Phytochem. Anal. 2011;20:33–37. doi: 10.1002/pca.1088. [DOI] [PubMed] [Google Scholar]
- 167.Aghajanian S., Kazemi S., Esmaeili S., Aghajanian S., Moghadamnia A.A. Sequential Microwave-assisted Extraction for Isolation of Quercetin from Red Kidney Bean. Int. J. Eng. 2020;33:12–17. [Google Scholar]
- 168.Sun X., Xu Y., Liu Y., Li L., Wang L., Song Y. Optimization of microwave-assisted extraction of quercetin from Letinous edodes stem by response surface methodology. J. Food Safety Qual. 2017;8:2098–2104. [Google Scholar]
- 169.Z.J. Velisdeh, G.D. Najafpour, M. Mohammadi, F. Poureini, Optimization of Sequential Microwave-Ultrasound-Assisted Extraction for Maximum Recovery of Quercetin and Total Flavonoids from Red Onion (Allium cepa L.) Skin Wastes, arXiv preprint arXiv:2104.06109, (2021).
- 170.Najafpour Darzi G., Heidari G., Mohammadi M., Moghadamnia A.A. Microwave Ultrasound Assisted Extraction: Determination of Quercetin for Antibacterial and Antioxidant Activities of Iranian Propolis. Int. J. Eng. 2019;32:1057–1064. [Google Scholar]
- 171.Ekinci M.S. Supercritical fluid extraction of quercetin from sumac (Rhus coriaria L.): effects of supercritical extraction parameters. Sep. Sci. Technol. 2021:1–7. [Google Scholar]
- 172.Ko Min-Jung, Cheigh Chan-Ick, Cho Sang-Woo, Chung Myong-Soo. Subcritical water extraction of flavonol quercetin from onion skin. J. Food Eng. 2011;102(4):327–333. [Google Scholar]
- 173.Anuar N., Markom M., Khairedin S., Johari N. Production and extraction of Quercetin and (+)-Chatechin from Phyllanthus niruri callus culture. Int. J. Biol. Sci. 2012;6:1240–1243. [Google Scholar]
- 174.Ruan Xiao, Yan Liu-Ye, Li Xian-Xian, Liu Ben, Zhang Huan, Wang Qiang. Optimization of process parameters of extraction of amentoflavone, quercetin and ginkgetin from Taxus chinensis using supercritical CO2 plus co-solvent. Molecules. 2014;19(11):17682–17696. doi: 10.3390/molecules191117682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Shi W.-M., Xie J., Wang Y. Determining the Content of Quercetin in Medicage Stativa L. with Enzyme Extraction Fluorescence Method. J. Gannan Med. Univ. 2017:02. [Google Scholar]
- 176.Zhou Lei, Zhang Chu, Qiu Zhengjun, He Yong. Information fusion of emerging non-destructive analytical techniques for food quality authentication: A survey. TrAC, Trends Anal. Chem. 2020;127:115901. doi: 10.1016/j.trac.2020.115901. [DOI] [Google Scholar]
- 177.Afsah-Hejri Leili, Akbari Elnaz, Toudeshki Arash, Homayouni Taymaz, Alizadeh Azar, Ehsani Reza. Terahertz spectroscopy and imaging: A review on agricultural applications. Comput. Electron. Agric. 2020;177:105628. doi: 10.1016/j.compag.2020.105628. [DOI] [Google Scholar]
- 178.Meenu M., Kamboj U., Sharma A., Guha P., Mishra S. Green method for determination of phenolic compounds in mung bean (Vigna radiata L.) based on near-infrared spectroscopy and chemometrics. Int. J. Food Sci. Technol. 2016;51:2520–2527. [Google Scholar]
- 179.Martelo-Vidal M., Vázquez M. Determination of polyphenolic compounds of red wines by UV–VIS–NIR spectroscopy and chemometrics tools. Food Chem. 2014;158:28–34. doi: 10.1016/j.foodchem.2014.02.080. [DOI] [PubMed] [Google Scholar]
- 180.Liu K., Zha X.-Q., Shen W.-D., Li Q.-M., Pan L.-H., Luo J.-P. The hydrogel of whey protein isolate coated by lotus root amylopectin enhance the stability and bioavailability of quercetin. Carbohydr. Polym. 2020;236:116009. doi: 10.1016/j.carbpol.2020.116009. [DOI] [PubMed] [Google Scholar]
- 181.Wang Ying, Zhao Qinfu, Han Ning, Bai Ling, Li Jia, Liu Jia, Che Erxi, Hu Liang, Zhang Qiang, Jiang Tongying, Wang Siling. Mesoporous silica nanoparticles in drug delivery and biomedical applications, Nanomedicine: Nanotechnology. Biol. Med. 2015;11(2):313–327. doi: 10.1016/j.nano.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 182.AbouAitah K., Swiderska-Sroda A., Farghali A.A., Wojnarowicz J., Stefanek A., Gierlotka S., Opalinska A., Allayeh A.K., Ciach T., Lojkowski W. Folic acid–conjugated mesoporous silica particles as nanocarriers of natural prodrugs for cancer targeting and antioxidant action. Oncotarget. 2018;9:26466. doi: 10.18632/oncotarget.25470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lozano Omar, Lázaro-Alfaro Anay, Silva-Platas Christian, Oropeza-Almazán Yuriana, Torres-Quintanilla Alejandro, Bernal-Ramírez Judith, Alves-Figueiredo Hugo, García-Rivas Gerardo. Nanoencapsulated Quercetin improves Cardioprotection during hypoxia-Reoxygenation injury through preservation of mitochondrial function. Oxidative Med. Cell. Longevity. 2019;2019:1–14. doi: 10.1155/2019/7683051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Islam S., Bhuiyan M.R., Islam M. Chitin and chitosan: structure, properties and applications in biomedical engineering. J. Polym. Environ. 2017;25:854–866. [Google Scholar]
- 185.Jain A.K., Thanki K., Jain S. Co-encapsulation of tamoxifen and quercetin in polymeric nanoparticles: implications on oral bioavailability, antitumor efficacy, and drug-induced toxicity. Mol. Pharm. 2013;10:3459–3474. doi: 10.1021/mp400311j. [DOI] [PubMed] [Google Scholar]
- 186.Pimple S., Manjappa A.S., Ukawala M., Murthy R. PLGA nanoparticles loaded with etoposide and quercetin dihydrate individually: in vitro cell line study to ensure advantage of combination therapy. Cancer Nanotechnol. 2012;3:25–36. doi: 10.1007/s12645-012-0027-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kumar P., Sharma G., Kumar R., Singh B., Malik R., Katare O.P., Raza K. Promises of a biocompatible nanocarrier in improved brain delivery of quercetin: Biochemical, pharmacokinetic and biodistribution evidences. Int. J. Pharm. 2016;515:307–314. doi: 10.1016/j.ijpharm.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 188.Tan B.-J., Liu Y., Chang K.-L., Lim B.K., Chiu G.N. Perorally active nanomicellar formulation of quercetin in the treatment of lung cancer. Int. J. Nanomed. 2012;7:651. doi: 10.2147/IJN.S26538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yuan Z.-P., Chen L.-J., Fan L.-Y., Tang M.-H., Yang G.-L., Yang H.-S., Du X.-B., Wang G.-Q., Yao W.-X., Zhao Q.-M. Liposomal quercetin efficiently suppresses growth of solid tumors in murine models. Clin. Cancer Res. 2006;12:3193–3199. doi: 10.1158/1078-0432.CCR-05-2365. [DOI] [PubMed] [Google Scholar]
- 190.Wong Man-Yi, Chiu Gigi N.C. Liposome formulation of co-encapsulated vincristine and quercetin enhanced antitumor activity in a trastuzumab-insensitive breast tumor xenograft model, Nanomedicine: Nanotechnology. Biol. Med. 2011;7(6):834–840. doi: 10.1016/j.nano.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 191.Li H., Zhao X., Ma Y., Zhai G., Li L., Lou H. Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J. Control. Release. 2009;133:238–244. doi: 10.1016/j.jconrel.2008.10.002. [DOI] [PubMed] [Google Scholar]