Abstract
Background
Identifying factors that contribute to the development of sarcopenia in older adults is a public health priority. Although several studies have examined the association between sleep duration and sarcopenia, additional evidence is needed to reveal the causality of this association, especially from a longitudinal study. The purpose of the present study was to examine whether sleep duration was associated with the progression to sarcopenia and its subcomponents among community‐dwelling older adults in Japan.
Methods
A total of 3918 older community‐dwelling people (mean age: 73.2 ± 6.0 years, 51.8% female) included in the National Center for Geriatrics and Gerontology Study of Geriatric Syndromes were analysed. Sleep duration was assessed using a self‐reported questionnaire. Logistic regression analysis was used to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) of progression of sarcopenia at Wave 2 (4 years later), according to the three categories of sleep duration [short: ≤6.0 h, medium: 6.1–8.9 h (control), & long: ≥9.0 h)] at Wave 1.
Results
The numbers in each group in the second wave among the total sample were as follows: short 403 (10.3%), medium 2877 (73.4%), and long 638 (16.3%). Significant associations with the progression of sarcopenia were found in the long sleep duration group compared with the medium one, even after adjustment for other covariates (OR 1.66, 95% CI: 1.02–2.69, P = 0.040). Long sleep duration was significantly associated with slow gait (OR: 1.55, 95% CI: 1.17–2.06, P = 0.002) and low grip strength (OR: 1.34, 95% CI: 1.00–1.78, P = 0.047) but was not associated with low muscle mass (OR: 1.33, 95% CI: 0.74–2.38, P = 0.343).
Conclusions
This study revealed that long sleep duration was associated with an increased risk of progression to sarcopenia among older adults.
Keywords: Sleep duration, Elderly, Sarcopenia, Slow gait, Low grip strength
Introduction
Sarcopenia, defined as the changes in body composition including a loss of skeletal muscle mass with advancing age, was proposed by Rosenberg. 1 Under normal conditions, after 30 years of age, muscle mass decreases by about 3% to 5% every 10 years. 2 , 3 Sarcopenia was also found to predispose affected older adults to consecutive functional impairment, disability, and frailty. 4 , 5 , 6 Therefore, identifying factors that may contribute to the development of sarcopenia in older adults is a public health priority.
An adequate amount of sleep plays an important role in physical health and quality of life among older adults. Many previous studies have established that both too short and too long sleep are related to all‐cause mortality, 7 cardiovascular disease, 8 , 9 diabetes, 10 and metabolic syndrome. 11 In addition, several previous studies demonstrated that sleep duration was associated with skeletal muscle loss or sarcopenia. 12 , 13 , 14 , 15 Although a systematic review showed the U‐shaped association of sleep duration and the risk of sarcopenia, 16 the studies used in this meta‐analysis were cross‐sectional in nature, not longitudinal. Therefore, additional evidence is needed to reveal the association between sleep duration and sarcopenia, especially from a longitudinal study. The purpose of the present study was to examine whether sleep duration was associated with the progression to sarcopenia and its subcomponents among community‐dwelling older adults in Japan.
Methods
Setting and participants
This study was part of a prospective cohort survey, the National Center for Geriatrics and Gerontology Study of Geriatric Syndromes, conducted in Obu, Japan. 17 , 18 Individuals selected for participation in the first wave of National Center for Geriatrics and Gerontology Study of Geriatric Syndromes were those eligible from among the 15 974 older people living in Obu, Japan, between August 2011 and February 2012. The survey recruited 5104 community‐dwelling older individuals aged ≥ 65 years at the time of the first wave, and the detailed protocol of which has been presented elsewhere. 19 The second wave (2015–2016) was carried out nearly 4 years after the first wave. The exclusion criteria were as follows: health problems such as Alzheimer's disease (n = 8), Parkinson's disease (n = 23), depression (n = 144), or stroke (n = 268); inability to perform basic daily living tasks such as eating, grooming, bathing, and climbing up and down stairs (n = 26); need for support or care as certified by the Japanese public long‐term care insurance due to disability (n = 96); global cognitive impairment as indicated by a Mini Mental State Examination (MMSE) score < 18 (n = 28); missing data on exclusion criteria (n = 274); and defined sarcopenia at the first wave (n = 168). Moreover, those who moved elsewhere (n = 45) or died (n = 106) during the follow‐up period were also excluded. Finally, 3918 participants were included in the analyses (Figure 1). All participants gave their written informed consent before they were included in the study. The study protocol was approved by the Ethics Committee of the National Center for Geriatrics and Gerontology.
Figure 1.
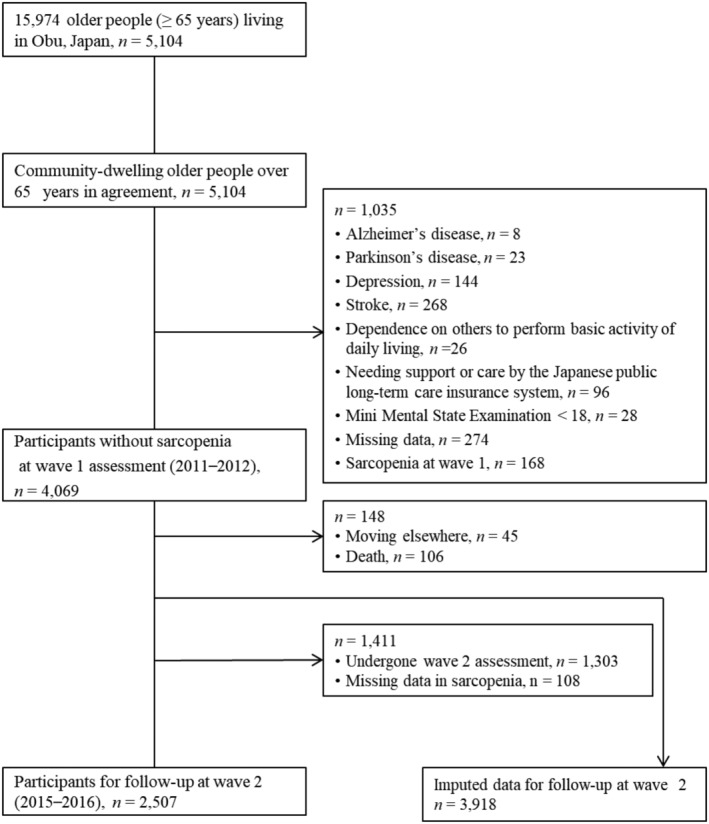
Participant flow in this study.
Assessment of sarcopenia
Sarcopenia was evaluated in accordance with the flowchart used in the 2019 revised recommendations of the EWGSOP (EWGSOP2). 20 We assessed sarcopenia indices including muscle mass, muscle strength, and physical performance.
As muscle mass, the appendicular skeletal muscle mass was evaluated using multi‐frequency bioelectrical impedance analysis (MC‐980A; TANITA, Tokyo, Japan). The surface of the hand electrode was placed in contact with each of the participants' five fingers, and their heels and forefeet were placed on the circular‐shaped foot electrode. The participants held out their arms and legs so that they would not contact any other body parts during the measurements. The appendicular skeletal muscle mass was calculated in accordance with a previous study 21 and was converted into the skeletal muscle mass index by dividing the muscle mass by the height in meters squared (kg/m2). Low muscle mass was defined using cut‐off values of skeletal muscle mass index (<7.0 kg/m2 in men and <5.7 kg/m2 in women). 22 Muscle strength was assessed using handgrip strength.
Handgrip strength was measured in kilograms on the dominant hand of each participant using a Smedley‐type handheld dynamometer (Takei Ltd., Niigata, Japan). Low muscle strength was defined by cut‐off values of <26 kg in men and <18 kg in women. 22
As physical performance, we measured walking speed under normal conditions. Walking time was measured over 2.4 m in the middle of a straight walkway of 6.4 m, between marks at 2.0 and 4.4 m from the start of the walkway. Low physical performance was defined by a cut‐off value of <1.0 m/s in both men and women. 19 Based on the results of these indices, participants were classified as being ‘robust’ or having ‘sarcopenia’ (‘sarcopenia confirmed’ and ‘sarcopenia severe’). 20 Sarcopenia confirmed involved having low muscle mass and low muscle strength, while in sarcopenia severe, there was the additional feature of low physical performance.
Assessment of sleep habits
At the first wave, participants were asked about their usual sleep and wake times, and the answers were used to calculate sleep duration, which was calculated as the difference between them. The participants were divided into three groups according to their self‐reported sleep duration [short: ≤6 h, medium: 6.1–8.9 h (control), & long: ≥9 h]. 23
Other measurements
Data on sociodemographic variables including sex, age, and educational level (years) were collected, along with medical history, weight (kg), and height (m). Body mass index (BMI) was derived as weight in kilograms divided by the square of height in meters. Participants were asked about medical diagnoses (heart disease, diabetes, respiratory disease, hypertension, and hyperlipidaemia) and medications in face‐to‐face interviews. Depressive symptoms were measured with the 15‐item Geriatric Depression Scale (GDS, range 0–15), with higher scores indicating more depressive symptoms. 24 Global cognitive function was measured using the MMSE. 25 We evaluated physical inactivity by asking the following questions about time spent engaged in sports and exercise: (i) ‘Do you engage in moderate levels of physical exercise or sports aimed at health?’ and (ii) ‘Do you engage in low levels of physical exercise aimed at health?’ Participants who answered ‘no’ to both of these questions were classified as having physical inactivity. 19 For smoking and alcohol drinking status, participants were categorized as current, past, or never smokers or alcohol drinkers, respectively.
Statistical analysis
All analyses were performed using SPSS v.25 (IBM Corp., Chicago, IL, USA). Statistical significance was set at P < 0.05. Sarcopenia status at the second wave was imputed for participants with missing data (n = 1411). Multiple imputation was used to adjust for selection bias and loss of information. Fifty imputed values were generated for each participant with missing data, yielding 50 complete data sets. Analysis of variance and χ 2 test were used to investigate the differences of demographic data among the sleep duration groups at the first wave. Univariate and multivariate logistic regression analyses were used to examine the associations of sleep duration with sarcopenia status at the second wave. In the multivariate models, adjustments were performed for age (<75 or ≥75 years), sex, BMI (<18.5, 18.5–24.9, or ≥25 kg/m2), education (<10 or ≥10 years), number of medications (<5 or ≥5), GDS score (<6 or ≥6), MMSE score (<24 and ≥24), medical history, current drinking habit, current smoking habit, and physical inactivity. Sleep duration was submitted as an independent variable to the crude and adjusted models. Sarcopenia status (confirmed and severe) at the second wave was submitted as a dependent variable. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. In addition, each subcomponent of sarcopenia at the second wave was individually submitted as a dependent variable to the same models. Each analysis was conducted after excluding participants with the component at the first wave (low muscle mass: n = 400; slow gait: n = 510; low grip strength: n = 498). Therefore, there were 3518 participants for analysis of low muscle mass, 3408 for analysis of slow gait, and 3420 for analysis of low grip strength.
Results
At the first wave, there were 403 participants (10.3%) classified as having short sleep duration, 2877 (73.4%) with medium sleep duration, and 638 (16.3%) with long sleep duration. The differences of characteristics among the sleep duration groups at the first wave are summarized in Table 1. During follow‐up (mean period 47.9 ± 1.8 months), 45 participants had moved out of the area and 106 died. There were 2507 participants who were in both the first and the second waves. In terms of their sleep duration, they were classified as follows at the first wave: short 266 (10.6%), medium 1896 (75.6%), and long 345 (13.8%).
Table 1.
Characteristics of participants stratified by sleep duration at first wave
| Short | Medium | Long | ||
|---|---|---|---|---|
| Characteristic | (n = 403) | (n = 2877) | (n = 638) | P |
| Age (year) | 70.43 ± 4.44 | 71.20 ± 4.98 | 73.65 ± 5.67 | <0.001 |
| Female, n (%) | 234 (58.1) | 1554 (54.0) | 243 (38.1) | <0.001 |
| Education level (year) | 11.75 ± 2.51 | 11.48 ± 2.48 | 11.02 ± 2.54 | <0.001 |
| Medications (number) | 1.86 ± 2.09 | 1.81 ± 1.92 | 2.17 ± 2.11 | <0.001 |
| Body mass index (kg/m2) | 24.10 ± 3.2 | 23.53 ± 2.97 | 23.46 ± 3.01 | <0.001 |
| Mini Mental State Examination (points) | 26.52 ± 2.52 | 26.56 ± 2.43 | 25.65 ± 2.8 | <0.001 |
| Geriatric Depression Scale (points) | 2.85 ± 2.57 | 2.49 ± 2.34 | 3.18 ± 2.71 | <0.001 |
| Current alcohol consumption, n (%) | 0.069 | |||
| Never | 193 (47.9) | 1361 (47.3) | 266 (41.7) | |
| Past | 27 (6.7) | 187 (6.5) | 55 (8.6) | |
| Current | 183 (45.4) | 1329 (46.2) | 317 (49.7) | |
| Current smoking habit, n (%) | <0.001 | |||
| Never | 249 (61.8) | 1800 (62.6) | 323 (60.5) | |
| Past | 106 (26.3) | 816 (28.4) | 253 (39.7) | |
| Current | 48 (11.9) | 261 (9.1) | 62 (9.7) | |
| Physical inactivity, n (%) | 121 (30.0) | 753 (26.2) | 224 (35.1) | <0.001 |
| Disease, n (%) | ||||
| Heart disease | 72 (17.9) | 416 (14.5) | 122 (19.1) | 0.005 |
| Diabetes | 60 (14.9) | 351 (12.2) | 100 (15.7) | 0.032 |
| Respiratory disease | 46 (11.4) | 293 (10.2) | 70 (11.0) | 0.669 |
| Hypertension | 175 (43.4) | 1262 (43.9) | 307 (48.1) | 0.133 |
| Hyperlipidaemia | 164 (40.7) | 1227 (42.6) | 241 (37.8) | 0.072 |
Values are presented as n (%) or mean ± standard deviation. Continuous variables were analysed by analysis of variance, and categorical variables were analysed by χ 2 tests.
In the univariate logistic regression analyses, long sleep duration had an impact on the progression of sarcopenia compared with medium sleep duration (Table 2, crude model). Significant association between long sleep duration and the progression of sarcopenia was found, even after adjustment for other covariates (Table 2, adjusted model). Among the covariates, age (≥75 years), male, and low BMI were significantly associated with the progression of sarcopenia (Table 2). Short sleep duration was not associated with the progression of sarcopenia in both univariate and multivariate models.
Table 2.
Associations between progression of sarcopenia and sleep duration by logistic regression models
| Variable | Crude model | Adjusted model | ||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Sleep duration | ||||
| Short | 0.77 (0.35–1.68) | 0.750 | 0.98 (0.44–2.21) | 0.965 |
| Medium | ref. | ref. | ||
| Long | 2.18 (1.40–3.41) | <0.001 | 1.66 (1.02–2.69) | 0.040 |
| Age (≥75 years) | 1.78 (1.11–2.85) | 0.017 | ||
| Sex (male) | 4.02 (2.13–7.58) | <0.001 | ||
| Education (≥10 years) | 0.97 (0.58–1.61) | 0.899 | ||
| Medications (≥5) | 1.06 (0.51–2.21) | 0.868 | ||
| MMSE ≥ 24 | 0.83 (0.46–1.48) | 0.521 | ||
| GDS (≥6 points) | 1.21 (0.68–2.17) | 0.513 | ||
| Low | 9.33 (4.93–17.67) | <0.001 | ||
| High | 0.00 (0.00–0.00) | 0.988 | ||
| Current alcohol consumption | ||||
| Past | 1.44 (0.67–3.10) | 0.346 | ||
| Current | 0.88 (0.52–1.47) | 0.610 | ||
| Current smoking habit | ||||
| Past | 0.57 (0.32–1.00) | 0.050 | ||
| Current | 0.91 (0.45–1.81) | 0.780 | ||
| Physical inactivity | 1.35 (0.84–2.14) | 0.212 | ||
| Heart disease | 1.17 (0.65–2.11) | 0.598 | ||
| Diabetes | 1.08 (0.55–2.14) | 0.823 | ||
| Respiratory disease | 1.70 (0.99–2.92) | 0.056 | ||
| Hypertension | 0.82 (0.51–1.32) | 0.405 | ||
| Hyperlipidaemia | 0.93 (0.58–1.50) | 0.769 | ||
BMI, body mass index; CI, confidence interval; GDS, geriatric depression scale; MMSE, Mini Mental State Examination; OR, odds ratio.
Regarding the progression of each subcomponent, long sleep duration was significantly associated with slow gait (OR: 1.82, 95% CI: 1.40–2.36, P = 0.002) and low grip strength (OR: 1.77, 95% CI: 1.36–2.31, P < 0.001) and was not associated with low muscle mass (OR: 1.37, 95% CI: 0.79–2.38, P = 0.257). Short sleep duration was not associated with all components (slow gait OR: 0.93, 95% CI: 0.65–1.31, P = 0.665; low grip strength OR: 0.85, 95% CI: 0.59–1.22, P = 0.376; low muscle mass OR: 0.90, 95% CI: 0.44–1.87, P = 0.783). The significance of results remained in logistic regression models (Table 3).
Table 3.
Associations between development of sarcopenia subcomponents and sleep duration at baseline by logistic regression models
| Variable | Lower muscle mass (n = 3518) | Slow gait (n = 3408) | Lower grip strength (n = 3420) | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Sleep duration | ||||||
| Short | 0.99 (0.45–2.14) | 0.970 | 0.89 (0.62–1.29) | 0.541 | 0.91 (0.62–1.32) | 0.616 |
| Medium | ref. | ref. | ref. | |||
| Long | 1.33 (0.74–2.38) | 0.343 | 1.55 (1.17–2.06) | 0.002 | 1.34 (1.00–1.78) | 0.047 |
Adjusted for age (<75 or ≥75 years), sex, BMI (<18.5, 18.5–24.9, or ≥25 kg/m2), education (<10 or ≥10 years), number of medications (<5 or ≥5), GDS score (<6 or ≥6), MMSE score (<24 and ≥24), medical history, current drinking habit, current smoking habit, and physical inactivity. Each analysis was conducted after excluding participants with the component of sarcopenia at the first wave.
CI, confidence interval; OR, odds ratio.
Discussion
Our study revealed that long sleep duration was associated with the risk of progression to sarcopenia among older adults. These results were sustained in multivariate analyses adjusted for age, sex, BMI, education, medication, medical history, current drinking habit, current smoking habit, physical inactivity, GDS score, and MMSE score. Furthermore, long sleep duration was significantly associated with slow gait and low grip strength and was not associated with low muscle mass in univariate and multivariate models.
In this study, long sleep duration was associated with progression to sarcopenia during 4 years, whereas short sleep duration was not. Several cross‐sectional studies revealed that older adults with short or long sleep duration were at an increased risk of having sarcopenia, 12 , 13 although the results of subanalyses according to sex were inconsistent; only long sleep duration 12 or long and short sleep duration 13 was associated with an increased risk of sarcopenia among women, and not among men. Other studies among middle‐aged and older adults showed that only long sleep duration was cross‐sectionally associated with sarcopenia. 14 , 15 Moreover, a meta‐analysis revealed a U‐shaped association between sleep duration and the risk of sarcopenia, although short sleep duration was not associated with it among men. 16 The results of that meta‐analysis were calculated from a small number of cross‐sectional studies, so the association between sleep duration and sarcopenia has remained controversial. This is the first study to examine the temporal association between long sleep duration and sarcopenia using longitudinal cohort data, although it was not possible to examine the association according to sex because of the small number of cases that progressed to sarcopenia during the follow‐up period.
Among each subcomponent of sarcopenia, long sleep duration was associated with slow gait and lower grip strength, but was not associated with lower muscle mass. Our previous study examined the cross‐sectional association between sleep duration and physical frailty and showed that long sleep duration was associated with higher rates of slowness and weakness. 26 Other studies also showed that long sleep duration was associated with lower muscle strength among older adults. 27 , 28 Our findings were in line with these studies and provided additional evidence on the temporal association of long sleep duration and physical performance. Conversely, sleep duration was not associated with muscle mass in our study. Differences between the sexes in the association between sleep duration and muscle mass or sarcopenia were also observed; there were significant associations among women, but not among men. 12 , 13 , 15 In our study, being male was highly significantly associated with the progression of sarcopenia in logistic regression analysis. Because we could not examine the association between sleep duration and progression of sarcopenia according to sex because of the small number of cases that progressed to sarcopenia during the follow‐up period, the discrepancy between previous studies and our study was fully confirmed. There is thus a need for additional studies considering the effects of sex while examining the relationship between sleep duration and muscle mass in a longer follow‐up period or with larger samples.
The biological mechanisms underlying the relationship between sleep and sarcopenia remain unclear, and further research is needed to investigate the related pathophysiologies. Several previous studies reported that poor sleep quality and long sleep duration are associated with low physical activity. 29 , 30 , 31 Indeed, low physical activity is considered an important risk factor for sarcopenia. 32 As a result, it is possible that reduced levels of physical activity resulting from long sleep duration can increase the risk for sarcopenia. 29 , 31 , 32 Regarding sleep‐related hormonal pathways, poor sleep quality has been associated with decreased secretion of growth hormone, insulin‐like growth factor 1, and testosterone, which results in the deterioration of muscle mass. 33 , 34 In addition, circadian rhythms and the molecular clock may be critical for both maintenance and adaptation of skeletal muscle. 35 , 36 These findings suggest that general physical disorders associated with long sleep duration may increase the risk of sarcopenia.
The strength of this study was its use of a prospective cohort design, which can address the causality between sleep duration and the progression of sarcopenia. However, there were also several limitations to this study. First, self‐reported measures of sleep duration based on the interval between the usual time of going to bed and the usual time of waking up were used, so we could not consider the daily variance of sleep duration or change of sleep habit. In addition, previous research suggested that self‐reported long sleepers, 37 as well as average sleepers, 38 tend to overestimate their total sleep time and instead consider it to be equivalent to their total time in bed. Mortality risks associated with self‐reported long sleep might be partly attributable to a long time spent in bed. However, given the high correlation of time in bed with total sleep time, reported long sleep is likely to be indicative of long physiological sleep, as confirmed recently. 39 Additionally, as sleep quality, such as prolonged sleep latency, waking prematurely after sleep onset, and a delay in getting up due to morning stiffness, was not assessed, we could not discuss whether the association of long sleep duration with frailty was mediated by prolonged sleep latency or just poor sleep quality. Second, there were other potential confounders that we could not assess, such as drug therapy and diet quality. Third, medical diagnoses were obtained by self‐report, not by consulting medical records. Furthermore, given the absence of random sampling methods, our participants were exclusively those who had the ability to access health check‐ups from their homes. Therefore, our results may not be directly applicable to all older adults in Japan, which is one of the limitations of this study. Finally, we failed to address other covariates related to sleep duration or sarcopenia, such as caffeine intake, daily living conditions, and other diseases. Thus, future studies must confirm the associations of sleep duration with sarcopenia using objective instruments, such as wrist actigraphy measurements, while considering other covariates.
In summary, this study revealed that long sleep duration was associated with an increased risk of progression to sarcopenia among older adults. Although further studies are required to confirm these findings, our results underscore the importance of assessing sleep characteristics to prevent sarcopenia in older adults.
Author contributions
SN designed and supervised all aspects of the study implementation and drafted the manuscript. TD and HS contributed to the conception and design of the study and provided feedback on the study implementation. KT contributed to the design of the study, and subject recruitment and screening. SK and IH contributed to subject recruitment and screening. All authors participated in drafting the manuscript and revising it critically for important intellectual content. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle.
Conflict of interest
SN, TD, KT, SK, IH, and HS declare that they have no conflicts of interest.
Funding
This work was supported by Health and Labour Sciences Research Grants (Comprehensive Research on Aging and Health); a Grant‐in‐Aid for Scientific Research (B) (23300205); a Grant‐in‐Aid for Young Scientists (A) (15H05369); and Research Funding for Longevity Sciences (22‐16) from the National Center for Geriatrics and Gerontology, Japan. The authors also received financial support through Grant‐in‐Aid for Scientific Research (C) (18K11122 and 21K11616).
Acknowledgements
The authors would like to thank Obu City Office for help with participant recruitment.
Nakakubo S., Doi T., Tsutsumimoto K., Kurita S., Ishii H., and Shimada H. (2021) Sleep duration and progression to sarcopenia in Japanese community‐dwelling older adults: a 4 year longitudinal study, Journal of Cachexia, Sarcopenia and Muscle, 12, 1034–1041, 10.1002/jcsm.12735
References
- 1. Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr 1997;127:990S–991S. [DOI] [PubMed] [Google Scholar]
- 2. Holloszy JO. The biology of aging. Mayo Clin Proc 2000;75:S3–S8, discussion S‐9. [PubMed] [Google Scholar]
- 3. Melton LJ 3rd, Khosla S, Crowson CS, O'Connor MK, O'Fallon WM, Riggs BL. Epidemiology of sarcopenia. J Am Geriatr Soc 2000;48:625–630. [PubMed] [Google Scholar]
- 4. Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998;147:755–763. [DOI] [PubMed] [Google Scholar]
- 5. Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 2002;50:889–896. [DOI] [PubMed] [Google Scholar]
- 6. Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol 2004;159:413–421. [DOI] [PubMed] [Google Scholar]
- 7. Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Sleep duration and all‐cause mortality: a systematic review and meta‐analysis of prospective studies. Sleep 2010;33:585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cappuccio FP, Cooper D, D'Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta‐analysis of prospective studies. Eur Heart J 2011;32:1484–1492. [DOI] [PubMed] [Google Scholar]
- 9. Niijima S, Nagai M, Hoshide S, Takahashi M, Shimpo M, Kario K, et al. Long sleep duration: a nonconventional indicator of arterial stiffness in Japanese at high risk of cardiovascular disease: the J‐HOP study. J Am Soc Hypertens 2016;10:429–437. [DOI] [PubMed] [Google Scholar]
- 10. Pyykkonen AJ, Isomaa B, Pesonen AK, Eriksson JG, Groop L, Tuomi T, et al. Sleep duration and insulin resistance in individuals without type 2 diabetes: the PPP‐Botnia study. Ann Med 2014;46:324–329. [DOI] [PubMed] [Google Scholar]
- 11. Ju SY, Choi WS. Sleep duration and metabolic syndrome in adult populations: a meta‐analysis of observational studies. Nutr Diabetes 2013;3:e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chien MY, Wang LY, Chen HC. The relationship of sleep duration with obesity and sarcopenia in community‐dwelling older adults. Gerontology 2015;61:399–406. [DOI] [PubMed] [Google Scholar]
- 13. Hu X, Jiang J, Wang H, Zhang L, Dong B, Yang M. Association between sleep duration and sarcopenia among community‐dwelling older adults: a cross‐sectional study. Medicine (Baltimore) 2017;96:e6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kwon YJ, Jang SY, Park EC, Cho AR, Shim JY, Linton JA. Long sleep duration is associated with sarcopenia in Korean adults based on data from the 2008‐2011 KNHANES. Journal of clinical sleep medicine: JCSM: official publication of the American Academy of Sleep Medicine 2017;13:1097–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim RH, Kim KI, Kim JH, Park YS. Association between sleep duration and body composition measures in Korean adults: the Korea National Health and Nutrition Examination Survey 2010. Korean journal of family medicine 2018;39:219–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pourmotabbed A, Ghaedi E, Babaei A, Mohammadi H, Khazaie H, Jalili C, et al. Sleep duration and sarcopenia risk: a systematic review and dose‐response meta‐analysis. Sleep & breathing = Schlaf & Atmung. 2019; [DOI] [PubMed]
- 17. Shimada H, Makizako H, Lee S, Doi T, Lee S, Tsutsumimoto K, et al. Impact of cognitive frailty on daily activities in older persons. J Nutr Health Aging 2016;20:729–735. [DOI] [PubMed] [Google Scholar]
- 18. Makizako H, Shimada H, Doi T, Tsutsumimoto K, Hotta R, Nakakubo S, et al. Social frailty leads to the development of physical frailty among physically non‐frail adults: a four‐year follow‐up longitudinal cohort study. Int J Environ Res Public Health 2018;15:e490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shimada H, Makizako H, Doi T, Yoshida D, Tsutsumimoto K, Anan Y, et al. Combined prevalence of frailty and mild cognitive impairment in a population of elderly Japanese people. J Am Med Dir Assoc 2013;14:518–524. [DOI] [PubMed] [Google Scholar]
- 20. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yoshida D, Suzuki T, Shimada H, Park H, Makizako H, Doi T, et al. Using two different algorithms to determine the prevalence of sarcopenia. Geriatr Gerontol Int 2014;14:46–51. [DOI] [PubMed] [Google Scholar]
- 22. Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014;15:95–101. [DOI] [PubMed] [Google Scholar]
- 23. Nakakubo S, Doi T, Makizako H, Tsutsumimoto K, Hotta R, Ono R, et al. Sleep duration and excessive daytime sleepiness are associated with incidence of disability in community‐dwelling older adults. J Am Med Directors Assoc 2016;17:768 e1–768 e5. [DOI] [PubMed] [Google Scholar]
- 24. Yesavage JA. Geriatric depression scale. Psychopharmacol Bull 1988;24:709–711. [PubMed] [Google Scholar]
- 25. Folstein MF, Robins LN, Helzer JE. The mini‐mental state examination. Arch Gen Psychiatry 1983;40:812. [DOI] [PubMed] [Google Scholar]
- 26. Nakakubo S, Makizako H, Doi T, Tsutsumimoto K, Hotta R, Lee S, et al. Long and short sleep duration and physical frailty in community‐dwelling older adults. J Nutr Health Aging 2018;22:1066–1071. [DOI] [PubMed] [Google Scholar]
- 27. Chen HC, Hsu NW, Chou P. The association between sleep duration and hand grip strength in community‐dwelling older adults: the Yilan study. Taiwan Sleep 2017;40. [DOI] [PubMed] [Google Scholar]
- 28. Auyeung TW, Kwok T, Leung J, Lee JS, Ohlsson C, Vandenput L, et al. Sleep duration and disturbances were associated with testosterone level, muscle mass, and muscle strength—a cross‐sectional study in 1274 older men. J Am Med Directors Assoc 2015;16:630 e1–630 e6. [DOI] [PubMed] [Google Scholar]
- 29. Bellavia A, Akerstedt T, Bottai M, Wolk A, Orsini N. Sleep duration and survival percentiles across categories of physical activity. Am J Epidemiol 2014;179:484–491. [DOI] [PubMed] [Google Scholar]
- 30. Chien MY, Chen HC. Poor sleep quality is independently associated with physical disability in older adults. J Clin Sleep Med 2015;11:225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Patel SR, Malhotra A, Gottlieb DJ, White DP, Hu FB. Correlates of long sleep duration. Sleep 2006;29:881–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia Journal of the American Medical Directors Association 2011;12:249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bhasin S, Storer TW, Berman N, Callegari C, Clevenger B, Phillips J, et al. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med 1996;335:1–7. [DOI] [PubMed] [Google Scholar]
- 34. Balbo M, Leproult R, Van Cauter E. Impact of sleep and its disturbances on hypothalamo‐pituitary‐adrenal axis activity. Int J Endocrinol 2010;2010:759234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lefta M, Wolff G, Esser KA. Circadian rhythms, the molecular clock, and skeletal muscle. Curr Top Dev Biol 2011;96:231–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Harfmann BD, Schroder EA, Esser KA. Circadian rhythms, the molecular clock, and skeletal muscle. J Biol Rhythms 2015;30:84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kline CE, Zielinski MR, Devlin TM, Kripke DF, Bogan RK, Youngstedt SD. Self‐reported long sleep in older adults is closely related to objective time in bed. Sleep Biol Rhythms 2010;8:42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self‐reported and measured sleep duration: how similar are they? Epidemiology 2008;19:838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Patel SR, Blackwell T, Ancoli‐Israel S, Stone KL, MrOS OFM. Sleep characteristics of self‐reported long sleepers. Sleep 2012;35:641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]


