Abstract
Background
Reference ranges for lean mass (LM) and fat mass (FM) are essential in identifying soft tissue disorders; however, no such reference ranges exist for the most commonly used Hologic dual‐energy X‐ray absorptiometry (DXA) machine in Australia.
Methods
Cross‐sectional study of community‐dwelling adults (aged 18–88 years) who underwent a Hologic DXA scan at one of three commercialized densitometry centres in Australia. Age‐specific and sex‐specific percentile curves were generated for LM [LM, appendicular lean mass (ALM), ALM adjusted for height squared (ALM/h2), and ALM adjusted for body mass index (ALM/BMI)] and FM [FM, FM adjusted for height squared (FM/h2), appendicular fat mass, and android and gynoid fat] parameters using the LMS statistical method. Cutpoints equivalent to T‐scores of −1, −2, and −2.5 standard deviations below the young mean reference group (20–29 years) were also generated for LM parameters.
Results
A total of 15 479 community‐dwelling adults (54% men) with a median age of 33 years (interquartile range: 28, 42) were included. LM, ALM, and ALM/h2 remained stable until age 50, after which these parameters started to decline in both sexes. Compared with age 50, median percentiles of LM, ALM, and ALM/h2 declined by −5.9 kg, −3.7 kg, and −0.86 kg/m2 in men and by −2.5 kg, −1.8 kg, and −0.10 kg/m2 in women at age 70, respectively. Adjusting ALM for BMI (rather than height squared) resulted in different trends, with ALM/BMI decreasing from as early as age 20. Compared with age 20, median percentiles of ALM/BMI at age 40 declined by −0.10 kg/kg/m2 in men and by −0.06 kg/kg/m2 in women; and at age 70, ALM/BMI declined by −0.25 kg/kg/m2 in men and by −0.20 kg/kg/m2 in women. Cutpoints equivalent to T‐scores of −1, −2, and −2.5 standard deviations for ALM/BMI were 1.01, 0.86, and 0.77 kg/kg/m2 in men and 0.70, 0.59, and 0.53 kg/kg/m2 in women, respectively. All FM parameters progressively increased from age 20 and continued up until age 70.
Conclusions
We developed reference ranges for LM and FM parameters from Hologic DXA machines in a large cohort of Australian adults, which will assist researchers and clinicians in identifying soft tissue disorders such as obesity, sarcopenia, and cachexia.
Keywords: Body composition, Dual‐energy X‐ray absorptiometry, Fat mass, Lean mass, Reference ranges
Introduction
Body composition plays a fundamental role in health and disease across the life cycle. Lean mass (LM) protects bone density and skeletal muscle mass and strength 1 and contributes to energy expenditure via basal metabolic rate, as well as facilitating whole‐body metabolism by partitioning nutrients into several tissues. 2 Age‐related declines in LM (or low muscle mass) are linked to mobility impairments, falls, fragility fractures, disability and reduced quality of life, 1 , 3 and several acute and chronic conditions such as cachexia, sarcopenia, sarcopenic obesity, and type 2 diabetes. 1 , 4 , 5 , 6
On the other hand, fat mass (FM) is the body's largest energy reservoir, and, when required, this organ provides a sustained release of energy via lipid metabolism. 2 While this tissue also supports processes such as hormone functioning and thermogenesis, 2 significant increases in FM (or adiposity) is detrimental to health. Indeed, obesity drives systemic low‐grade inflammation, 2 a key player in the pathology of several metabolic diseases such as cancer, diabetes, and cardiovascular diseases. 7 Obesity also exacerbates the risk of falls in older populations. 8 Thus, body composition assessments are valuable in research and clinical settings to identify those with, or at risk of, LM and FM abnormalities.
Body composition is commonly measured through non‐invasive imaging techniques such as computed tomography (CT), magnetic resonance imaging (MRI), and dual‐energy X‐ray absorptiometry (DXA). While CT and MRI offer the highest accuracy and precision, they are costly and inconvenient for clinical purposes. 9 DXA is a practical option that accurately quantifies LM and FM (as well as bone mineral content) and provides total and regional values for these parameters. 10 When compared with MRI and CT measured skeletal muscle mass, DXA‐derived LM demonstrates high reliability and offers shorter scan time, lower cost, and less radiation exposure. 9 However, a caveat with DXA technology is that LM and FM measures differ between manufactures due to the calibration methods and/or algorithms used to distinguish soft tissue compartments. 11 An experimental study showed that when comparing GE Healthcare Lunar and Hologic DXA scans in 199 healthy participants from the USA (n = 40, 6–16 years) and China (n = 159, 5–81 years), significant differences were found in total and regional LM and FM values. 11 Others have also reported differences in regional FM values when comparing DXA machines. 12 These factors can affect the use of LM and FM normative data in research settings (e.g. entry points onto clinical trials) and clinical practice (e.g. diagnosis).
To account for this, US cohort studies developed LM reference ranges from GE Healthcare Lunar 13 and Hologic 14 DXA machines, with reference equations for both machines derived from a diverse multinational population. 11 However, in Australia, LM reference ranges developed by the Geelong Osteoporosis Study (GOS) 15 , 16 were derived from GE Healthcare Lunar machines, and at present, no normative data exist for LM and FM parameters using Hologic machines in this region, despite Hologic being the most commonly employed imaging technique.
Conventional body composition parameters include total and regional appendicular lean mass (ALM) adjusted for height squared (ALM/h2) or body mass index (ALM/BMI) 17 and total (kg and %) FM, including adjustments for height squared (FM/h2). 10 Alternatively, regional body composition parameters may offer higher predictive value for health outcomes. For instance, appendicular (the sum of arms and legs) LM parameters (ALM, ALM/h2, and ALM/BMI) are preferred over total LM, as the latter is subject to higher confounding of organ masses in the trunk region. 17 ALM parameters are also more relevant to activities of daily living and thus are recommended in sarcopenia definitions. 17 Studies have also found that regional trunk adiposity is a better predictor of risk factors for cardiometabolic disease, as opposed to total FM or other conventional anthropometric measures (i.e. waist/hip/thigh circumference and BMI). 18 , 19 Moreover, adiposity of the lower limbs (i.e. intra/intermuscular fat) is linked to mobility impairment 20 and cardiometabolic disease 21 , 22 ; thus, appendicular fat mass (AFM) may offer a useful surrogate marker for this phenomenon. However, again, reference ranges for these parameters are yet to be developed using Hologic DXA technology in Australia. This is an important aspect as in addition to age, sex, and ethnicity, body composition normative values should be specific to the DXA system and representative of the underlying population. 16
Here, we developed reference ranges for total and regional LM (LM, ALM/h2, and ALM/BMI) and FM (FM, FM/h2, AFM, and android and gynoid fat) parameters directly from Hologic DXA machines in community‐dwelling Australian adults.
Methods
Participants
Deidentified whole‐body scans of 26 999 community‐dwelling adults were acquired from three Australian commercialized densitometry centres: Mobile DEXA Pty Ltd (n = 13 138), EC Group Pty Ltd (n = 7843), and DEXA Melbourne Pty Ltd (n = 6018). Participants attended the densitometry centres on their own accord between 2013 and 2020 to seek body composition evaluation for health and fitness purposes. The data were pooled, and the most recent scan for each individual was used (excluded scans: n = 10 782). This was carried out by using the unique scan identifier number as the authors were blind to participant names. Participants were excluded if younger than 18 years (n = 87), had missing height or weight (n = 17), taller than 192 cm (n = 623, maximum length of scan area of the machine), or shorter than 130 cm (n = 6, possible dwarfism). None of the participants had weight recorded above 200 kg (maximum capacity of Hologic machines is 206 kg), but those with a weight <20 kg were excluded (n = 1) as this was deemed to be a typographic error. Four (n = 4) participants whose ALM was 0 were also excluded. A total of n = 15 479 scans for total and regional LM and FM were included in the analysis. LM is described here as bone free.
Informed consent was waived as participants were not recruited for this study, with only pre‐existing data in a non‐identifiable format supplied by the densitometry centres. This study was approved by the Melbourne Health Human Research Ethics Committee (Reference Number: HREC/56560/MH‐2019).
Anthropometry and imaging
Machines in all three densitometry centres were Hologic™ (Mobile DEXA Pty Ltd: Hologic Horizon A, Software: Apex Versions 5.6.0.5 and 5.6.0.7; EC Group Pty Ltd: Hologic Discovery A, Software: Apex Version 4.5.0.3; DEXA Melbourne Pty Ltd: Hologic Horizon A, Software: Apex Version 5.6.0.4) and operated as per the guidelines of the manufacturer. Both models (Hologic Horizon A and Discovery A) have shown similar precision in densitometry. 23 Using spine phantom daily and whole‐body phantom at least once weekly, quality assurance was carried out on all machines. The intra‐machine coefficient of variation calculated from 30 phantom scans at each centre was within acceptable ranges (<5%) for body composition parameters: total LM (Mobile DEXA Pty Ltd: 0.96%; EC Group Pty Ltd: 0.60%; DEXA Melbourne Pty Ltd: 0.52%), total FM (Mobile DEXA Pty Ltd: 1.30%; EC Group Pty Ltd: 0.79%; DEXA Melbourne Pty Ltd: 0.59%), leg LM (Mobile DEXA Pty Ltd: 2.96%; EC Group Pty Ltd: 1.74%; DEXA Melbourne Pty Ltd: 2.11%), leg FM (Mobile DEXA Pty Ltd: 4.19%; EC Group Pty Ltd: 2.94%; DEXA Melbourne Pty Ltd: 2.97%), trunk LM (Mobile DEXA Pty Ltd: 2.06%; EC Group Pty Ltd: 0.70%; DEXA Melbourne Pty Ltd: 1.08%), and trunk FM (Mobile DEXA Pty Ltd: 1.52%; EC Group Pty Ltd: 0.67%; DEXA Melbourne Pty Ltd: 0.79%).
All centres measured participants' height to the nearest centimetre. However, one centre (Mobile DEXA Pty Ltd) did not measure the weight of the participants using a scale and instead used whole‐body mass estimates from the DXA machine. A correlation between scale and DXA‐derived weight (for all participants from EC Group Pty Ltd and DEXA Melbourne Pty Ltd centres, n = 7737) was excellent (r = 0.975). Therefore, for uniformity purposes, we used the weight estimates from the DXA machines for all centres.
Statistical analysis
Participant characteristics and descriptives on all outcomes are presented as median (interquartile range) or frequency (percentage).
Reference (centile) curves were generated using the LMS method developed by Cole 24 and Cole and Green. 25 This method fits a single explanatory variable (age in our study) to a response variable (body composition outcomes) to create centile curves. The outcome can follow skewed distribution; a Box‐Cox transformation is applied (with or without power transformation of age) to estimate three parameters representing skewness (lambda, L), median (M), and coefficient of variation (sigma, S). Z‐scores can be calculated from these parameters using the following formula:
Body composition outcome is represented by X, and σ stands for population standard deviation (SD) (calculated as M*S). 26 The model fit was evaluated using residuals inspection of Q statistics (values less than 2 considered good fit) and worm plots. 27
While Z‐scores represent the deviation of an individual from the average of age–sex‐matched population, T‐scores represent the deviation from the average of young adults of the same sex. In this study, young adults are participants aged 20–29 years. 28 T‐scores were calculated using the same formula as for Z‐score (above), but replacing parameters (M, L, and σ) with those of the young adults. Cutpoints for each outcome are presented using T‐scores of −1.0, −2.0, and −2.5 describing the number of SDs below the young adult reference mean. The number and proportion of participants within these cutpoints are shown for each 10 year age group. BMI was calculated as body weight divided by height squared (kg/m2) and followed the World Health Organization classifications (underweight: <18.5 kg/m2; normal weight: 18.5–24.9 kg/m2; overweight: 25–29.9 kg/m2; obese: ≥30 kg/m2) for reporting. 29
All analyses were performed separately for men and women. The LMS method was fitted using package ‘gamlss’ in R; other analyses were performed using Stata 16.1.
Results
Participants
Participant characteristics are presented in Table 1, and the number and proportion of men and women across age ranges are available in Supporting Information, Table S1. A total of 15 479 community‐dwelling adults (54% men) with a median age of 33 years (interquartile range: 28, 42) were included. Of these, 8678 (69%) were younger adults (18–40 years), 4259 (28%) were middle‐aged adults (40–60 years), and 458 (3%) and 78 (0.5%) were aged between 60–70 and 70–80 years, respectively. Six participants (<0.1%) were aged above 80 years. In all age groups, median height, weight, BMI, LM, ALM, ALM/height2, and ALM/BMI were higher in men compared with women, and conversely, FM (kg and %), FM/height2, AFM, and gynoid fat were higher in women compared with men. Aside from age 20, median gynoid fat was higher in men compared with women in all other age groups. According to BMI classifications, 99 (<1%) were underweight, 6296 (~41%) were normal weight, 6094 (~39%) were overweight, and 2990 (~19%) were obese.
Table 1.
Participant characteristics
| Parameter | Total (n = 15 479) | Men (n = 8381) | Women (n = 7098) |
|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | |
| Age (years) | 33.0 (28.0, 42.0) | 33.0 (28.0, 42.0) | 33.0 (28.0, 43.0) |
| Height (m) | 1.7 (1.6, 1.8) | 1.8 (1.7, 1.8) | 1.6 (1.6, 1.7) |
| Weight (kg) a | 78.0 (66.7, 89.2) | 85.0 (77.2, 94.5) | 67.1 (60.0, 77.2) |
| BMI (kg/m2) | 25.8 (23.5, 28.9) | 26.7 (24.6, 29.5) | 24.4 (22.2, 27.9) |
| LM (kg) | 56.3 (45.9, 66.4) | 65.5 (59.5, 71.3) | 45.3 (41.2, 50.0) |
| ALM (kg) | 25.1 (19.9, 30.4) | 29.9 (27.0, 33.0) | 19.6 (17.5, 21.9) |
| ALM/height2 (kg/m2) | 8.4 (7.2, 9.6) | 9.4 (8.7, 10.2) | 7.2 (6.5, 7.9) |
| ALM/BMI (kg/kg/m2) | 1.0 (0.8, 1.1) | 1.1 (1.0, 1.2) | 0.8 (0.7, 0.9) |
| Fat mass (kg) | 17.6 (13.3, 23.9) | 16.2 (12.3, 21.9) | 19.3 (14.7, 26.3) |
| Fat mass (%) | 23.8 (18.1, 30.3) | 19.5 (15.5, 24.4) | 29.5 (24.2, 35.3) |
| Fat mass/height2 (kg/m2) | 6.0 (4.4, 8.2) | 5.1 (3.9, 6.9) | 7.1 (5.4, 9.7) |
| AFM (kg) | 8.9 (6.7, 12.1) | 7.6 (5.9, 10.0) | 10.7 (8.4, 14.1) |
| Android fat (kg) | 1.3 (0.8, 2.0) | 1.3 (0.9, 2.0) | 1.2 (0.8, 1.9) |
| Gynoid fat (kg) | 3.3 (2.5, 4.4) | 2.9 (2.2, 3.7) | 3.9 (3.1, 5.1) |
AFM, appendicular fat mass; ALM, appendicular lean mass; BMI, body mass index; IQR, interquartile range; LM, lean mass.
Derived from dual‐energy X‐ray absorptiometry scan.
Lean mass parameters
Lean mass parameters are presented in Table 2. LM, ALM, and ALM/h2 remained stable until the fifth decade, after which these parameters started to decline in both sexes (Figures 1 and 2). Compared with age 50, median percentiles of LM, ALM, and ALM/h2 declined by −5.9 kg, −3.7 kg, and −0.86 kg/m2 in men and by −2.5 kg, −1.8 kg, and −0.10 kg/m2 in women at age 70, respectively. Adjusting ALM for BMI (rather than height squared) resulted in different trends, with ALM/BMI decreasing from age 20 and continuing to age 70 (Figure 2). Compared with age 20, median percentiles of ALM/BMI at age 40 declined by −0.10 kg/kg/m2 in men and by −0.06 kg/kg/m2 in women; and at age 70, ALM/BMI declined by −0.25 kg/kg/m2 in men and by −0.20 kg/kg/m2 in women.
Table 2.
Percentiles [median (3rd, 97th)] of lean mass parameters for men and women at specific ages
| Age | Men | Women | ||||||
|---|---|---|---|---|---|---|---|---|
| Lean mass (kg) | ALM (kg) | ALM/height2 (kg/m2) | ALM/BMI (kg/kg/m2) | Lean mass (kg) | ALM (kg) | ALM/height2 (kg/m2) | ALM/BMI (kg/kg/m2) | |
| 20 | 64.3 (48.9, 82.1) | 30.0 (22.2, 39.4) | 9.24 (7.30, 11.76) | 1.19 (0.91, 1.43) | 45.2 (34.4, 58.5) | 19.8 (14.2, 26.9) | 7.19 (5.46, 9.31) | 0.83 (0.61, 1.08) |
| 30 | 65.2 (49.5, 86.1) | 30.1 (22.1, 40.7) | 9.47 (7.40, 12.45) | 1.13 (0.85, 1.38) | 45.4 (34.6, 60.7) | 19.8 (14.3, 27.2) | 7.21 (5.51, 9.48) | 0.80 (0.58, 1.06) |
| 40 | 65.9 (50.9, 86.1) | 30.0 (22.6, 39.9) | 9.46 (7.49, 12.23) | 1.09 (0.83, 1.34) | 45.8 (35.1, 62.3) | 19.6 (14.4, 27.4) | 7.22 (5.54, 9.64) | 0.77 (0.55, 1.03) |
| 50 | 66.0 (50.8, 84.7) | 29.6 (22.2, 38.6) | 9.36 (7.38, 11.87) | 1.06 (0.80, 1.31) | 45.4 (34.8, 62.1) | 19.1 (14.0, 26.9) | 7.09 (5.45, 9.62) | 0.73 (0.52, 0.99) |
| 60 | 63.8 (48.4, 80.9) | 28.0 (20.7, 36.2) | 8.99 (6.98, 11.24) | 1.00 (0.75, 1.25) | 44.2 (33.5, 60.1) | 18.3 (13.2, 25.6) | 6.83 (5.19, 9.25) | 0.68 (0.48, 0.93) |
| 70 | 60.1 (45.0, 75.8) | 25.9 (18.7, 33.1) | 8.50 (6.47, 10.52) | 0.94 (0.69, 1.20) | 42.9 (31.8, 57.7) | 17.3 (12.1, 23.9) | 6.56 (4.88, 8.78) | 0.63 (0.45, 0.86) |
ALM, appendicular lean mass; BMI, body mass index.
Figure 1.
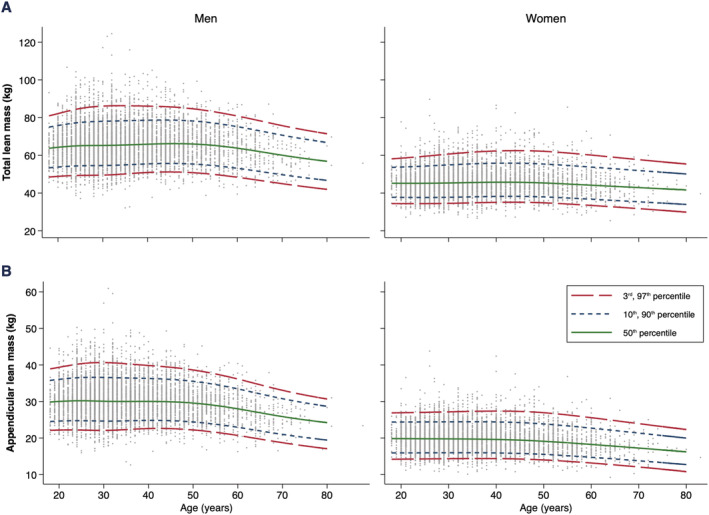
Age‐specific and sex‐specific percentile curves for (A) lean mass and (B) appendicular lean mass.
Figure 2.
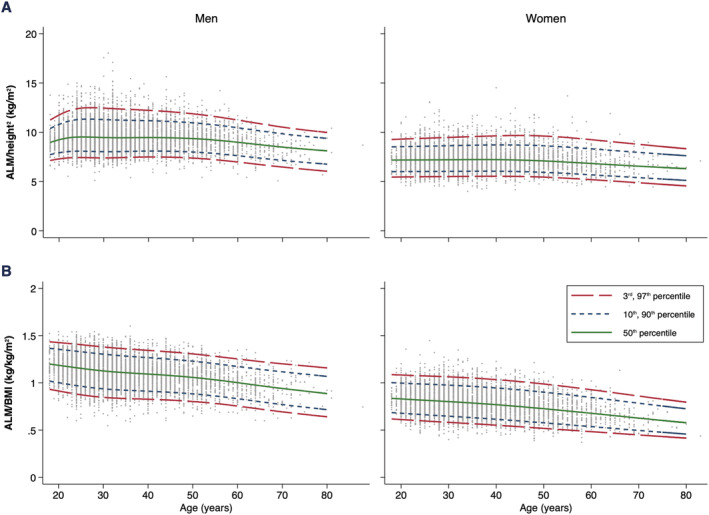
Age‐specific and sex‐specific percentile curves for (A) appendicular lean mass adjusted for height squared (ALM/height2) and (B) appendicular lean mass adjusted for body mass index (ALM/BMI).
Cutpoints equivalent to T‐scores of −1, −2, and −2.5 SDs below the mean reference group (20–29 years) for all LM parameters are presented in Table 3. The prevalence of LM parameters within defined T‐score categories for men and women is presented in Table 4.
Table 3.
Young adult (20–29 years) reference data for lean mass parameters and cutpoints equivalent to T‐scores of −1.0, −2.0, and −2.5 standard deviations
| T‐score category | Men (n = 2685) | Women (n = 2340) | ||||||
|---|---|---|---|---|---|---|---|---|
| Lean mass (kg) | ALM (kg) | ALM/height2 (kg/m2) | ALM/BMI (kg/kg/m2) | Lean mass (kg) | ALM (kg) | ALM/height2 (kg/m2) | ALM/BMI (kg/kg/m2) | |
| T‐score = −1.0 | 56.96 | 26.04 | 8.39 | 1.01 | 39.38 | 16.82 | 6.30 | 0.70 |
| T‐score = −2.0 | 49.79 | 22.38 | 7.47 | 0.86 | 34.30 | 14.22 | 5.50 | 0.59 |
| T‐score = −2.5 | 46.49 | 20.71 | 7.05 | 0.77 | 32.01 | 13.06 | 5.14 | 0.53 |
ALM, appendicular lean mass; BMI, body mass index; T‐score = −1.0, 1 standard deviation (SD) below the young adult reference mean; T‐score = −2.0, 2 SDs below the young adult reference mean; T‐score = −2.5, 2.5 SDs below the young adult reference mean.
Table 4.
Prevalence [n (%)] of lean mass parameters within defined T‐score categories for men and women
| T‐score category | Men (n = 8381) | Women (n = 7098) | ||||||
|---|---|---|---|---|---|---|---|---|
| Lean mass (kg) | ALM (kg) | ALM/height2 (kg/m2) | ALM/BMI (kg/kg/m2) | Lean mass (kg) | ALM (kg) | ALM/height2 (kg/m2) | ALM/BMI (kg/kg/m2) | |
| Less than −2.5 | 107 (1.3%) | 109 (1.3%) | 123 (1.5%) | 114 (1.4%) | 65 (0.9%) | 70 (1.0%) | 88 (1.2%) | 160 (2.3%) |
| −2.5 to −2.0 | 140 (1.7%) | 193 (2.3%) | 187 (2.2%) | 274 (3.3%) | 134 (1.9%) | 161 (2.3%) | 152 (2.1%) | 286 (4.0%) |
| −2.0 to −1.0 | 1110 (13.2%) | 1252 (14.9%) | 1203 (14.4%) | 1832 (21.9%) | 936 (13.2%) | 1070 (15.1%) | 1028 (14.5%) | 1415 (19.9%) |
| Greater than −1.0 | 7024 (83.8%) | 6827 (81.5%) | 6868 (81.9%) | 6161 (73.5%) | 5963 (84.0%) | 5797 (81.7%) | 5830 (82.1%) | 5237 (73.8%) |
ALM, appendicular lean mass; BMI, body mass index; less than −2.5, more than 2.5 standard deviations (SDs) below the young adult reference mean; −2.5 to −2.0, equal to or between 2.5 and 2 SDs below the young adult reference mean; −2.0 to −1.0, equal to or between 2.0 and 1 SDs below the young adult reference mean; greater than −1.0, less than 1 SD below the young adult reference mean.
All other LM reference range parameters (ALM/height, ALM/height3, ALM/total body fat, ALM/per cent body fat, ALM/weight, ALM/AFM, ALM/body surface area, ALM/whole‐body total area, and ALM/whole body subtotal) are available in Tables S2–S4. Reference charts for LM, ALM, ALM/h2, and ALM/BMI are available in Figures S2–S5.
Reference values to calculate T‐scores and Z‐scores for LM parameters (using the formulas outlined in the Methods section) are available in Files S1 and S2.
Fat mass parameters
Fat mass parameters are presented in Table 5. FM (kg and %) progressively increased from age 20 up until age 70 (Figure 3). Compared with age 20, median percentiles of FM (kg) at age 40 increased by 4.4 kg in men and 2.9 kg in women; and at age 70, FM (kg) increased by 7.3 kg in men and by 9.5 kg in women. Additionally, compared with age 20, median percentiles of FM proportion (%) at age 40 increased by 4.2% in men and 3.1% in women, while at age 70, it increased by 8.5% in men and by 9.0% in women.
Table 5.
Percentiles [median (3rd, 97th)] of fat mass parameters for men and women at specific ages
| Age | Men | |||||
|---|---|---|---|---|---|---|
| Fat mass (kg) | Fat mass (%) | Fat mass/height2 (kg/m2) | AFM (kg) | Android fat (kg) | Gynoid fat (kg) | |
| 20 | 13.2 (6.7, 28.4) | 16.5 (8.7, 28.9) | 4.1 (2.1, 9.0) | 6.6 (3.0, 15.9) | 0.9 (0.4, 2.5) | 2.5 (1.2, 5.2) |
| 30 | 15.5 (7.1, 37.6) | 18.9 (9.0, 33.7) | 4.9 (2.3, 11.7) | 7.5 (3.4, 17.2) | 1.2 (0.4, 3.8) | 2.8 (1.3, 6.4) |
| 40 | 17.5 (7.9, 40.9) | 20.6 (10.2, 34.7) | 5.5 (2.6, 12.7) | 8.0 (3.7, 17.9) | 1.5 (0.5, 4.5) | 3.0 (1.4, 6.5) |
| 50 | 18.7 (8.8, 40.4) | 21.6 (11.4, 34.6) | 5.9 (2.8, 12.7) | 8.2 (3.7, 17.6) | 1.7 (0.6, 4.6) | 3.0 (1.5, 6.1) |
| 60 | 19.6 (9.1, 41.3) | 23.1 (12.4, 36.0) | 6.3 (3.0, 13.0) | 8.2 (3.8, 17.3) | 1.9 (0.7, 4.8) | 3.0 (1.4, 5.9) |
| 70 | 20.5 (9.2, 43.3) | 25.0 (13.3, 38.8) | 6.7 (3.1, 13.9) | 8.4 (3.9, 17.2) | 2.1 (0.7, 5.0) | 3.0 (1.4, 6.0) |
| Women | ||||||
|---|---|---|---|---|---|---|
| 20 | 17.4 (8.9, 37.6) | 27.2 (15.7, 41.5) | 6.3 (3.4, 13.5) | 9.9 (5.1, 19.8) | 1.0 (0.4, 2.7) | 3.7 (2.0, 7.1) |
| 30 | 18.7 (8.8, 43.5) | 28.6 (15.3, 44.0) | 6.8 (3.2, 15.7) | 10.5 (4.9, 22.5) | 1.1 (0.4, 3.6) | 3.9 (1.9, 8.0) |
| 40 | 20.3 (8.8, 48.5) | 30.3 (15.7, 45.8) | 7.5 (3.3, 17.4) | 11.1 (4.9, 24.5) | 1.3 (0.4, 4.3) | 4.0 (1.9, 8.5) |
| 50 | 22.2 (9.2, 51.9) | 32.4 (16.6, 47.4) | 8.3 (3.4, 19.1) | 11.8 (5.1, 25.8) | 1.5 (0.4, 4.6) | 4.2 (1.9, 8.7) |
| 60 | 24.3 (10.9, 50.1) | 34.8 (19.7, 48.1) | 9.3 (4.1, 18.8) | 12.5 (5.9, 24.9) | 1.7 (0.5, 4.6) | 4.4 (2.2, 8.3) |
| 70 | 26.8 (12.4, 51.2) | 37.7 (23.1, 49.7) | 10.4 (4.9, 19.2) | 13.3 (6.8, 24.3) | 2.0 (0.6, 4.9) | 4.6 (2.4, 8.2) |
AFM, appendicular fat mass.
Figure 3.
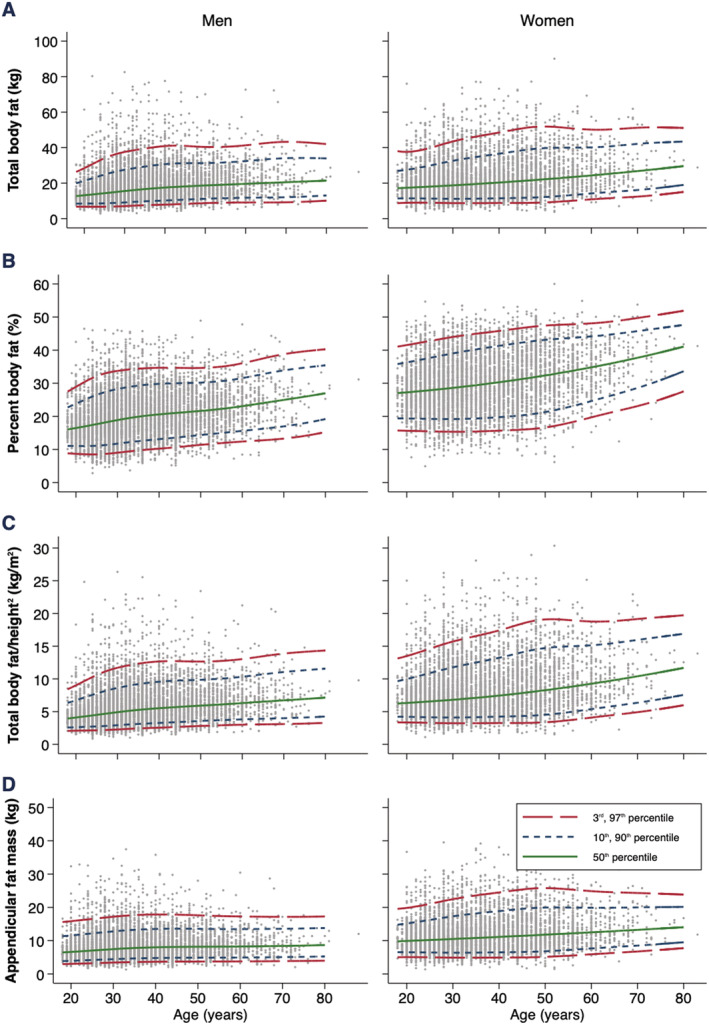
Age‐specific and sex‐specific percentile curves for (A) fat mass (kg), (B) fat mass (%), (C) fat mass/height2, and (D) appendicular fat mass.
Fat mass/height2, AFM, and android and gynoid fat increased from age 20 up until age 70 (Figures 3 and S1). Compared with age 20, median percentiles of FM/height2, AFM, and android and gynoid fat increased by 1.4 kg/m2, 1.4 kg, 0.6 kg, and 0.5 kg in men and by 1.2 kg/m2, 1.2 kg, 0.3 kg, and 0.3 kg in women at age 40, respectively. Compared with age 40, median percentiles of FM/height2, AFM, and android and gynoid fat increased by 1.2 kg/m2, 0.4 kg, 0.6 kg, and 0.0 kg in men and by 2.9 kg/m2, 2.2 kg, 0.7 kg, and 0.6 kg in women at age 70, respectively. Reference charts for all FM parameters are available in Figures S6–S11.
Reference values to calculate Z‐scores and T‐scores for FM parameters (using the formulas outlined in the Methods section) are available in Files S1 and S2.
Discussion
We have developed body composition reference ranges for total and regional LM and FM parameters using data from Hologic DXA machines in a large cohort of Australian community‐dwelling men and women. More specifically, we generated new percentiles and cutpoints for a range of LM (LM, ALM/h2, and ALM/BMI) and FM (FM, FM/h2, AFM, and android and gynoid fat) parameters (Figures [Link], [Link], [Link], [Link], [Link], [Link], [Link], [Link], [Link], [Link], [Link]) , which can be adopted by national and international working groups on sarcopenia, cachexia, and adiposity/obesity, as well as other muscle wasting and metabolic disorders. These data will help researchers and clinicians identify soft tissue disorders in adults using the most commercialized DXA system (Hologic) in Australia.
Lean mass parameters
Lean mass reference ranges have been developed in various regions worldwide, including Australia, 15 , 16 Europe, 30 and North America 13 , 14 , 31 ; however, reference equations are available for both Hologic and Lunar DXA systems only from a multinational study. 11 Our data provide an important extension to these findings by generating LM reference ranges directly from Hologic DXA systems among an Australian cohort that were previously only available from Lunar DXA machines in this region. 15 , 16
In our Australian population, peak LM, ALM, and ALM/h2 occurred at around age 50 in both sexes, which is consistent with findings from the US NHANES cohort of non‐Hispanic adults scanned on Hologic DXA systems. 14 Interestingly, in our study, adjusting ALM for BMI resulted in a different trend with this parameter peaking at around age 20 and declining thereafter in both sexes, a finding that is consistent with 2371 community‐dwelling Australians (99% Caucasian) scanned on Lunar DXA machines. 16 Aside from age and sex, several studies have shown that approximately 50% of the variance in ALM can be explained by body size (height and weight) 31 , 32 , 33 , 34 ; it is for this reason that ALM/BMI is considered a better index of muscle mass than ALM or ALM/h2 alone, in addition to its more consistent relationship with muscle weakness and mobility impairment. 35 , 36 , 37 , 38 Thus, we recommend using ALM/BMI in clinical practice to identify LM abnormalities wherever possible (Figure S5).
Following the established criteria in the osteoporosis field, we generated new cutpoints (equivalent to T‐scores of −1.0, −2.0, and −2.5 SDs) for a range of LM parameters using a significantly larger reference sample than previous cohort studies both nationally and internationally. 13 , 14 , 15 , 16 , 31 These aspects are important as the larger sample size increases our power and precision of results, and more conservative T‐scores (i.e. −2.5 SDs) have been recommended to identify low LM in the diagnosis of sarcopenia 39 but had not been developed previously. Our cutpoints (equivalent to T‐scores of −1.0 and −2.0 SDs) for LM, ALM, and ALM/h2 were higher than the GOS, which comprised 2371 community‐dwelling adults scanned on Lunar DXA machines. 15 Considering that population demographics were similar between studies and Hologic compared with Lunar DXA systems provides higher estimates of total and regional LM, 11 this finding was unsurprising.
We also developed cutpoints for clinically relevant LM parameters such as ALM/BMI from Hologic machines that were lacking in this region. When using the third percentile of ALM/BMI for an individual aged 60 years, our reference ranges (women: 0.48 kg/kg/m2, men: 0.75 kg/kg/m2) were similar to those developed by the Foundation for the National Institutes of Health (women: 0.51 kg/kg/m2, men: 0.79 kg/kg/m2) that were derived from nine US datasets of 26 625 community‐dwelling adults (≥65 years) using both Hologic and Lunar DXA systems. Our T‐scores of −2.5 SDs for ALM/BMI (women: 0.53 kg/kg/m2, men: 0.77 kg/kg/m2) were also close to the Foundation for the National Institutes of Health reference values. This provides us with confidence in the accuracy of our reference ranges, which could be easily implemented in research and clinical practice (Figure S5). In saying this, future studies should investigate the relationship between our LM reference ranges and adverse health outcomes (mobility impairments, falls, fractures, and mortality) in community‐dwelling adults and other clinical populations through cross‐sectional and longitudinal studies.
Fat mass parameters
Median values for all FM parameters (apart from android fat) were higher in women than men in our Australian population, a finding that corroborates previous cohort studies. 14 , 30 , 31 However, total FM (% and kg) and FM adjusted for height squared (FM/h2) increased monotonically from age 20 up until age 70 in both sexes. This contrasts with previous cohort studies. Among the NHANES dataset of US non‐Hispanic adults 14 scanned using Hologic DXA technology, total FM (%) and FM/h2 peaked at around age 80 in men and age 65 in women. Similarly, in a population of healthy Mexican adults scanned on Lunar DXA technology, 31 FM/h2 peaked at 60–69 years in men and 50–59 years in women. While genetic, epigenetic, and hormonal factors may, in part, account for the different peaks in adiposity between men and women, 31 other factors such as sample size and geographical region may explain the findings here. Irrespective of this, our data will facilitate researchers and clinicians in identifying individuals with excess FM (i.e. adiposity/obesity), a condition linked to several metabolic diseases 7 , 19 as well as falls in older populations. 8
Another novel aspect of our study is the introduction of reference ranges for AFM and android and gynoid fat. These three regional parameters progressively increased across all ages in our dataset. Considering the former, ectopic fat accumulation within the appendicular limbs is a hallmark of aging and degrades muscle and bone quality. 40 Greater intra/intermuscular fat is associated with mobility impairment 20 and metabolic risk factors, 21 , 22 potentially via the adverse effects of lipids on myofibres as well as glucose metabolism. However, AFM is a composite of not only intra/intermuscular fat but also fat located in subcutaneous and fascia regions. Studies have shown an inverse relationship of lower‐limb AFM with metabolic risk factors, 18 , 41 , 42 although a cross‐sectional study showed a positive association of total AFM with metabolic risk factors in adults with metabolic syndrome whereas the association was inverse in those without the condition. 43 Additional large, prospective studies are required to explore the relationship between AFM and metabolic risk factors in those with and without metabolic abnormalities, as this measure may be a practical surrogate marker for fat infiltration of the appendicular limbs.
Findings have been more consistent when examining the link between trunk adiposity and cardiometabolic risk factors, with numerous studies showing adverse effects of android/gynoid fat in community‐dwelling men and women. 18 , 19 , 43 Recent studies also suggest that regional trunk adiposity is a better predictor for risk factors of cardiometabolic disease, as opposed to total FM or other conventional anthropometric measures (i.e. waist/hip/thigh circumference and BMI). 18 , 19 Thus, the development of reference ranges for these parameters in our study may assist in assessing and monitoring cardiometabolic health, independent of total FM parameters, in both research and clinical settings. However, it should be highlighted that our reference ranges for total and regional FM parameters need to be validated in future clinical studies.
Limitations
Our reference ranges should be interpreted in the context of the study limitations. First, the authors were blind to metal artefacts (i.e. pacemakers and prosthetic limbs) and, as such, did not develop reference ranges for other body composition components such as bone mineral content/density. Second, the number of participants aged between 70 and 80 years was low (n = 78, 0.5%), which can affect the validity of the reference range in this age group. We did not develop reference ranges for those aged 80 years and above due to extremely low numbers (n = 6, <0.1%). Lastly, our reference ranges were developed from a cohort of men and women attending commercialized densitometry centres. This introduces the possibility of selection bias of individuals across all age ranges who are seeking body composition evaluation to support athletic/sporting endeavours. In saying this, our reference ranges were comparable with the NHANES 14 dataset scanned on Hologic DXA systems and slightly higher than the GOS 15 values using Lunar DXA systems, which was expected. 11
Conclusions
To conclude, we have developed reference ranges for total and regional LM and FM parameters using data from Hologic DXA machines in a large cohort of Australian community‐dwelling men and women, which will help researchers and clinicians identify soft tissue disorders. Future studies should investigate the relationship between our reference ranges and adverse outcomes (cardiometabolic disease, mobility impairments, falls, fractures, and mortality) in community‐dwelling adults and other clinical populations through cross‐sectional and longitudinal studies.
Ethical statement
The authors certify that they comply with the ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. 44
Author contributions
All authors contributed to the study design, interpretation of data, drafting, and critical appraisal of the manuscript and approved the final version.
Conflict of interest
B.K., E.B.H., S.B.O., S.V., S.B., J.Z., and S.P. have nothing to declare. J.D.M. is the director of Mobile DEXA Pty Ltd (Trading name: MeasureUp). S.B.H. was previously employed by Merck and Co., Inc and is a member of the Tanita Corporation Medical Advisory Board. G.D. has provided consultancy support to Hologic (Australia) Pty Ltd.
Funding
This study was funded by the Australian Institute for Musculoskeletal Science (AIMSS), Mobile DEXA Pty Ltd (Trading name: MeasureUp), and Hologic (Australia) Pty Ltd.
Supporting information
Data S1. Supporting information.
Data S2. Supporting information.
Figure S1. Age‐ and sex‐specific percentile curves for android and gynoid fat.
Figure S2. Age‐ and sex‐specific reference chart for total lean mass (kg).
Figure S3. Age‐ and sex‐specific reference chart for appendicular lean mass (kg).
Figure S4. Age‐ and sex‐specific reference chart for appendicular lean mass adjusted for height squared (ALM/height2).
Figure S5. Age‐ and sex‐specific reference chart for appendicular lean mass adjusted for body mass index (ALM/BMI).
Figure S6. Age‐ and sex‐specific reference chart for total body fat (kg).
Figure S7. Age‐ and sex‐specific reference chart for percent body fat (%).
Figure S8. Age‐ and sex‐specific reference chart for total body fat adjusted for height squared (kg/m2).
Figure S9. Age‐ and sex‐specific reference chart for appendicular fat mass (kg).
Figure S10. Age‐ and sex‐specific reference chart for android fat (kg).
Figure S11. Age‐ and sex‐specific reference chart for gynoid fat (kg).
Table S1. Distribution of men and women across age ranges.
Table S2. Participant characteristics.
Table S3. Percentiles [median (3rd, 97th)] of appendicular lean mass adjustments for men and women at specific ages.
Table S4. Percentiles [median (3rd, 97th)] of appendicular lean mass adjustments for men and women at specific ages.
Acknowledgement
The authors would like to thank Don Vicendese for his statistical support.
Kirk B., Bani Hassan E., Brennan‐Olsen S., Vogrin S., Bird S., Zanker J., Phu S., Meerkin J. D., Heymsfield S. B., and Duque G. (2021) Body composition reference ranges in community‐dwelling adults using dual‐energy X‐ray absorptiometry: the Australian Body Composition (ABC) Study, Journal of Cachexia, Sarcopenia and Muscle, 12, 880–890, 10.1002/jcsm.12712
References
- 1. Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International Working Group on Sarcopenia. J Am Med Dir Assoc 2011;12:249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kirk B, Feehan J, Lombardi G, Duque G. Muscle, bone, and fat crosstalk: the biological role of myokines, osteokines, and adipokines. Curr Osteoporos Rep 2020;18:388–400. [DOI] [PubMed] [Google Scholar]
- 3. Cawthon PM, Orwoll ES, Peters KE, Ensrud KE, Cauley JA, Kado DM, et al. Strong relation between muscle mass determined by D3‐creatine dilution, physical performance, and incidence of falls and mobility limitations in a prospective cohort of older men. J Gerontol Ser A 2019;74:844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr 2006;84:475–482. [DOI] [PubMed] [Google Scholar]
- 5. Gandham A, Mesinovic J, Jansons P, Zengin A, Bonham MP, Ebeling PR, et al. Falls, fractures, and areal bone mineral density in older adults with sarcopenic obesity: a systematic review and meta‐analysis [published online ahead of print]. Obes Rev 2021;22:e13187, 10.1111/obr.13187 [DOI] [PubMed] [Google Scholar]
- 6. Orwoll ES, Peters KE, Hellerstein M, Cummings SR, Evans WJ, Cawthon PM. The importance of muscle versus fat mass in sarcopenic obesity: a re‐evaluation using D3‐creatine muscle mass versus DXA lean mass measurements. J Gerontol Ser A 2020;75:1362–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006;444:860–867. [DOI] [PubMed] [Google Scholar]
- 8. Neri SGR, Oliveira JS, Dario AB, Lima RM, Tiedemann A. Does obesity increase the risk and severity of falls in people aged 60 years and older? A systematic review and meta‐analysis of observational studies. J Gerontol Ser A 2020;75:952–960. [DOI] [PubMed] [Google Scholar]
- 9. Buckinx F, Landi F, Cesari M, Fielding RA, Visser M, Engelke K, et al. Pitfalls in the measurement of muscle mass: a need for a reference standard. J Cachexia Sarcopenia Muscle 2018;9:269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shepherd JA, Ng BK, Sommer MJ, Heymsfield SB. Body composition by DXA. Bone 2017;104:101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shepherd JA, Fan B, Lu Y, Wu XP, Wacker WK, Ergun DL, et al. A multinational study to develop universal standardization of whole‐body bone density and composition using GE Healthcare Lunar and Hologic DXA systems. J Bone Miner Res 2012;27:2208–2216. [DOI] [PubMed] [Google Scholar]
- 12. Morrison SA, Petri RM, Hunter HL, Raju D, Gower B. Comparison of the Lunar Prodigy and iDXA dual‐energy X‐ray absorptiometers for assessing total and regional body composition. J Clin Densitom 2016;19:290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Imboden MT, Swartz AM, Finch HW, Harber MP, Kaminsky LA. Reference standards for lean mass measures using GE dual energy x‐ray absorptiometry in Caucasian adults. PLoS One 2017;12:e0176161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kelly TL, Wilson KE, Heymsfield SB. Dual energy X‐ray absorptiometry body composition reference values from NHANES. PLoS One 2009;4:e7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gould H, Brennan SL, Kotowicz MA, Nicholson GC, Pasco JA. Total and appendicular lean mass reference ranges for Australian men and women: the Geelong Osteoporosis Study. Calcif Tissue Int 2014;94:363–372. [DOI] [PubMed] [Google Scholar]
- 16. Pasco JA, Holloway‐Kew KL, Tembo MC, Sui SX, Anderson KB, Rufus‐Membere P, et al. Normative data for lean mass using FNIH criteria in an Australian setting. Calcif Tissue Int 2019;104:475–479. [DOI] [PubMed] [Google Scholar]
- 17. Cawthon PM. Assessment of lean mass and physical performance in sarcopenia. J Clin Densitom 2015;18:467–471. [DOI] [PubMed] [Google Scholar]
- 18. Vasan SK, Osmond C, Canoy D, Christodoulides C, Neville MJ, Di Gravio C, et al. Comparison of regional fat measurements by dual‐energy X‐ray absorptiometry and conventional anthropometry and their association with markers of diabetes and cardiovascular disease risk. Int J Obes (Lond) 2018;42:850–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Konieczna J, Abete I, Galmés AM, Babio N, Colom A, Zulet MA, et al. Body adiposity indicators and cardiometabolic risk: cross‐sectional analysis in participants from the PREDIMED‐Plus trial. Clin Nutr 2019;38:1883–1891. [DOI] [PubMed] [Google Scholar]
- 20. Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well‐functioning older persons. J Gerontol ‐ Ser A Biol Sci Med Sci 2005;60:324–333. [DOI] [PubMed] [Google Scholar]
- 21. Zoico E, Rossi A, Di Francesco V, Sepe A, Olioso D, Pizzini F, et al. Adipose tissue infiltration in skeletal muscle of healthy elderly men: relationships with body composition, insulin resistance, and inflammation at the systemic and tissue level. J Gerontol ‐ Ser A Biol Sci Med Sci 2010;65:295–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr 2000;71:885–892. [DOI] [PubMed] [Google Scholar]
- 23. Whittaker LTG, McNamara EA, Vath S, Shaw E, Malabanan AO, Parker RA, et al. Direct comparison of the precision of the new Hologic Horizon model with the old Discovery model. J Clin Densitom 2018;21:524–528. [DOI] [PubMed] [Google Scholar]
- 24. Cole TJ. Fitting smoothed centile curves to reference data. J R Stat Soc Ser A (Statistics Soc) 1988;151:418. [Google Scholar]
- 25. Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med 1992;11:1305–1319. [DOI] [PubMed] [Google Scholar]
- 26. Pan H, Cole TJ. A comparison of goodness of fit tests for age‐related reference ranges. Stat Med 2004;23:1749–1765. [DOI] [PubMed] [Google Scholar]
- 27. Metcalfe C. Goodness‐of‐fit statistics for age‐specific reference intervals. Stat Med 2002;21:3749–3750. [DOI] [PubMed] [Google Scholar]
- 28. Kanis JA, McCloskey EV, Johansson H, Oden A, Melton LJ, Khaltaev N. A reference standard for the description of osteoporosis. Bone 2008;42:467–475. [DOI] [PubMed] [Google Scholar]
- 29. World Health Organization technical report series . Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation. Geneva; 2000. doi: https://pubmed.ncbi.nlm.nih.gov/11234459/ [PubMed] [Google Scholar]
- 30. Ofenheimer A, Breyer‐Kohansal R, Hartl S, Burghuber OC, Krach F, Schrott A, et al. Reference values of body composition parameters and visceral adipose tissue (VAT) by DXA in adults aged 18–81 years—results from the LEAD cohort. Eur J Clin Nutr 2020;74:1181–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Clark P, Denova‐Gutiérrez E, Ambrosi R, Szulc P, Rivas‐Ruiz R, Salmerón J. Reference values of total lean mass, appendicular lean mass, and fat mass measured with dual‐energy X‐ray absorptiometry in a healthy Mexican population. Calcif Tissue Int 2016;99:462–471. [DOI] [PubMed] [Google Scholar]
- 32. Janssen I, Heymsfield SB, Wang Z, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol 2000;89:81–88. [DOI] [PubMed] [Google Scholar]
- 33. Iannuzzi‐Sucich M, Prestwood KM, Kenny AM. Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J Gerontol ‐ Ser A Biol Sci Med Sci 2002;57:772–777. [DOI] [PubMed] [Google Scholar]
- 34. Prado CMM, Siervo M, Mire E, Heymsfield SB, Stephan BCM, Broyles S, et al. A population‐based approach to define body‐composition phenotypes. Am J Clin Nutr 2014;99:1369–1377. [DOI] [PubMed] [Google Scholar]
- 35. McLean RR, Shardell MD, Alley DE, Cawthon PM, Fragala MS, Harris TB, et al. Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: the Foundation for the National Institutes of Health (FNIH) Sarcopenia Project. J Gerontol ‐ Ser A Biol Sci Med Sci 2014;69:576–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, et al. The FNIH Sarcopenia Project: rationale, study description, conference recommendations, and final estimates. J Gerontol Ser A 2014;69:547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cawthon PM, Peters KW, Shardell MD, McLean RR, Dam TTL, Kenny AM, et al. Cutpoints for low appendicular lean mass that identify older adults with clinically significant weakness. J Gerontol ‐ Ser A Biol Sci Med Sci 2014;69:567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bani Hassan E, Phu S, Vogrin S, Escobedo Terrones G, Pérez X, Rodriguez‐Sanchez I, et al. Diagnostic value of mid‐thigh and mid‐calf bone, muscle, and fat mass in osteosarcopenia: a pilot study. Calcif Tissue Int 2019;105:392–402. [DOI] [PubMed] [Google Scholar]
- 39. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wong AK, Chandrakumar A, Whyte R, Reitsma S, Gillick H, Pokhoy A, et al. Bone marrow and muscle fat infiltration are correlated among postmenopausal women with osteoporosis: the AMBERS Cohort Study. J Bone Miner Res 2020;35:516–527. [DOI] [PubMed] [Google Scholar]
- 41. Snijder MB, Visser M, Dekker JM, Goodpaster BH, Harris TB, Kritchevsky SB, et al. Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia 2005;48:301–308. [DOI] [PubMed] [Google Scholar]
- 42. Bos G, Snijder MB, Nijpels G, Dekker JM, Stehouwer CDA, Bouter LM, et al. Opposite contributions of trunk and leg fat mass with plasma lipase activities: the Hoorn study. Obes Res 2005;13:1817–1823. [DOI] [PubMed] [Google Scholar]
- 43. Park SY, Kwon KY, Kim JH, Choi HH, Han KH, Han JH. Association between appendicular fat mass and metabolic risk factors. Korean J Fam Med 2014;35:182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting information.
Data S2. Supporting information.
Figure S1. Age‐ and sex‐specific percentile curves for android and gynoid fat.
Figure S2. Age‐ and sex‐specific reference chart for total lean mass (kg).
Figure S3. Age‐ and sex‐specific reference chart for appendicular lean mass (kg).
Figure S4. Age‐ and sex‐specific reference chart for appendicular lean mass adjusted for height squared (ALM/height2).
Figure S5. Age‐ and sex‐specific reference chart for appendicular lean mass adjusted for body mass index (ALM/BMI).
Figure S6. Age‐ and sex‐specific reference chart for total body fat (kg).
Figure S7. Age‐ and sex‐specific reference chart for percent body fat (%).
Figure S8. Age‐ and sex‐specific reference chart for total body fat adjusted for height squared (kg/m2).
Figure S9. Age‐ and sex‐specific reference chart for appendicular fat mass (kg).
Figure S10. Age‐ and sex‐specific reference chart for android fat (kg).
Figure S11. Age‐ and sex‐specific reference chart for gynoid fat (kg).
Table S1. Distribution of men and women across age ranges.
Table S2. Participant characteristics.
Table S3. Percentiles [median (3rd, 97th)] of appendicular lean mass adjustments for men and women at specific ages.
Table S4. Percentiles [median (3rd, 97th)] of appendicular lean mass adjustments for men and women at specific ages.


