Abstract
Background
A validated, standardized, and feasible test to assess muscle power in older adults has recently been reported: the sit‐to‐stand (STS) muscle power test. This investigation aimed to assess the relationship between relative STS power and age and to provide normative data, cut‐off points, and minimal clinically important differences (MCID) for STS power measures in older women and men.
Methods
A total of 9320 older adults (6161 women and 3159 men) aged 60–103 years and 586 young and middle‐aged adults (318 women and 268 men) aged 20–60 years were included in this cross‐sectional study. Relative (normalized to body mass), allometric (normalized to height squared), and specific (normalized to legs muscle mass) muscle power values were assessed by the 30 s STS power test. Body composition was evaluated by dual energy X‐ray absorptiometry and bioelectrical impedance analysis, and legs skeletal muscle index (SMI; normalized to height squared) was calculated. Habitual and maximal gait speed, timed up‐and‐go test, and 6 min walking distance were collected as physical performance measures, and participants were classified into two groups: well‐functioning and mobility‐limited older adults.
Results
Relative STS power was found to decrease between 30–50 years (−0.05 W·kg−1·year−1; P > 0.05), 50–80 years (−0.10 to −0.13 W·kg−1·year−1; P < 0.001), and above 80 years (−0.07 to −0.08 W·kg−1·year−1; P < 0.001). A total of 1129 older women (18%) and 510 older men (16%) presented mobility limitations. Mobility‐limited older adults were older and exhibited lower relative, allometric, and specific power; higher body mass index (BMI) and legs SMI (both only in women); and lower legs SMI (only in men) than their well‐functioning counterparts (all P < 0.05). Normative data and cut‐off points for relative, allometric, and specific STS power and for BMI and legs SMI were reported. Low relative STS power occurred below 2.1 W·kg−1 in women (area under the curve, AUC, [95% confidence interval, CI] = 0.85 [0.84–0.87]) and below 2.6 W·kg−1 in men (AUC [95% CI] = 0.89 [0.87–0.91]). The age‐adjusted odds ratios [95% CI] for mobility limitations in older women and men with low relative STS power were 10.6 [9.0–12.6] and 14.1 [10.9–18.2], respectively. MCID values for relative STS power were 0.33 W·kg−1 in women and 0.42 W·kg−1 in men.
Conclusions
Relative STS power decreased significantly after the age of 50 years and was negatively and strongly associated with mobility limitations. Our study provides normative data, functionally relevant cut‐off points, and MCID values for STS power for their use in daily clinical practice.
Keywords: Chair stand, Mobility limitations, Muscle, Sarcopenia, Intrinsic capacity, Functional ability
Introduction
The maintenance and improvement of functional ability among older adults is currently one of the main health priorities established by the World Health Organization. 1 Functional ability results from the interaction between subject's intrinsic capacity—composite of all the physical and mental capacities—and his or her environment. Intrinsic capacity is severely affected by aging. For instance, maximal gait speed (GS) and chair stand ability are reduced by ~30% in people aged 50–70 years, and by ~50% in people over 70 years, compared with their younger counterparts. 2 Notably, impaired physical function in mid‐life predicts the incidence of disability at old age. 3
Thus, it is paramount to identify the risk factors associated to impaired intrinsic capacity in older people and to implement effective countermeasures to prevent or revert physical disability. It is accepted that a failure at the organism level (e.g. impaired physical performance) can occur as a consequence of a failure at the organ level (e.g. impaired muscle function: strength, power, and/or endurance). 4 , 5 In this regard, there are several reasons that make muscle power one of the most important outcomes to be monitored during aging: (i) muscle power declines with aging at an earlier and faster rate than muscle mass and strength (i.e. sarcopenia) 6 , 7 , 8 ; (ii) muscle power is more strongly related to mobility limitations than muscle mass, muscle strength, or maximal aerobic capacity 9 , 10 ; (iii) muscle power predicts 10 year cognitive decline and brain atrophy 11 ; and (iv) muscle power is more strongly related with mortality than muscle mass and strength. 12
Despite muscle power is recognized as one of the primary therapeutic targets for resistance training interventions in older adults, its measurement may be complicated by the lack of standardized and feasible protocols and the absence of normative data that allow the identification of low muscle power in daily practice. 13 For these reasons, the assessment of muscle power in daily clinical practice has not been encouraged. 5 However, a validated, standardized, and feasible muscle power test has recently been developed 14 , 15 : the sit‐to‐stand (STS) muscle power test. In brief, muscle power can be calculated from the STS test by implementing the subject's body mass and height and chair height in an equation. Notably, STS muscle power demonstrated a greater clinical relevance than probable (i.e. low handgrip strength) and confirmed (i.e. both low handgrip strength and low appendicular lean mass) sarcopenia in older adults according to their relationships with physical performance, frailty, disability, and poor quality of life. 16 Nevertheless, normative data of STS power and functionally relevant cut‐off points for the recognition of low muscle power have not been reported yet. Moreover, muscle power normalized to body mass (i.e. relative muscle power) has been found to be more relevant for functional ability than absolute values of muscle power. 14 , 17 , 18 Therefore, the main goals of the present investigation conducted in a large European cohort were (i) to assess the relationship between relative STS power and age; (ii) to calculate minimal clinically important difference (MCID) values for relative STS power and its underlying measures; and (iii) to provide normative data and functionally relevant cut‐off points for relative STS power and its underlying measures.
Methods
Participants
A total of 9320 older subjects (6161 older women and 3159 older men) were included in the present investigation. Participants belonged to different European cohorts and research studies grouped in four nationalities (Table 1): (i) Belgium (n = 1083; 60–93 years old): from a large‐scale cohort study by the first and third generation of the Flemish Policy Research Centre on Sport 19 , 20 , 21 and other interventional studies 22 , 24 , 25 ; (ii) Denmark (n = 719; 60–94 years old): from the Copenhagen Sarcopenia Study 2 and the Falls cohort 26 ; (iii) Portugal (n = 4856; 65–103 years): from a Portuguese cohort 27 ; and (iv) Spain (n = 2662; 65–91 years old): from the EXERNET multi‐centre study. 28 Briefly, these cohorts included representative samples of community‐dwelling older people aged 60 years and over living in Belgium (Flemish area), Denmark (Copenhagen metropolitan area), Portugal (five sampling areas covering the entire mainland of Portugal), and Spain (six sampling areas covering the entire territory of Spain). In addition, 586 subjects (318 women) aged 20–60 years from the Copenhagen sarcopenia study were included 29 in order to describe the relationship between age and relative STS muscle power. Having any co‐morbidity was not a reason for exclusion; however, those older people that were unable to walk independently or diagnosed with dementia, or acute neuromuscular or joint injury were excluded due to the characteristics of the tests. All the subjects gave their written informed consent; all the procedures were performed in accordance with the Helsinki Declaration; and the corresponding local ethical committees of the participating cohorts approved the studies.
Table 1.
Main characteristics of the young and middle‐aged, and older female and male study participants
| Young and middle‐aged adults | Older adults | ||||
|---|---|---|---|---|---|
| Denmark | Belgium | Denmark | Portugal | Spain | |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Women (n) | 318 | 559 | 405 | 3164 | 2033 |
| Age (years) | 42.9 ± 10.6 a | 68.1 ± 6.2 b | 74.0 ± 8.2 b | 75.4 ± 7.4 b | 72.1 ± 5.3 b |
| Body mass (kg) | 67.9 ± 10.4 a | 69.1 ± 11.03 | 67.8 ± 12.4 | 67.1 ± 11.71,4 | 68.6 ± 10.63 |
| Height (m) | 1.68 ± 0.06 a | 1.60 ± 0.06 b | 1.63 ± 0.06 b | 1.54 ± 0.07 b | 1.53 ± 0.06 b |
| BMI (kg·m−2) | 24.1 ± 6.6 a | 27.1 ± 4.3 b | 25.6 ± 4.6 b | 28.3 ± 4.5 b | 29.4 ± 4.3 b |
| 30 s STS test (reps) | 24.9 ± 6.6 a | 13.0 ± 3.22,4 | 15.0 ± 5.51,3,4 | 13.2 ± 5.12,4 | 14.1 ± 3.41,2,3 |
| Men (n) | 268 | 524 | 314 | 1692 | 629 |
| Age (years) | 43.4 ± 10.4 a | 68.2 ± 6.02,3,4 | 72.4 ± 7.41,3 | 75.5 ± 7.51,2,4 | 72.1 ± 5.41,3 |
| Body mass (kg) | 83.9 ± 11.9 a | 81.1 ± 10.8 b | 83.8 ± 14.5 b | 73.1 ± 12.3 b | 77.5 ± 11.1 b |
| Height (m) | 1.82 ± 0.07 a | 1.72 ± 0.06 b | 1.77 ± 0.07 b | 1.63 ± 0.08 b | 1.65 ± 0.07 b |
| BMI (kg·m−2) | 25.4 ± 3.5 a | 27.3 ± 3.44 | 26.6 ± 4.13,4 | 27.6 ± 3.92,4 | 28.3 ± 3.51,2,3 |
| 30 s STS test (reps) | 25.6 ± 5.8 a | 14.2 ± 3.12,4 | 16.6 ± 6.11,3,4 | 14.2 ± 5.22,4 | 15.0 ± 3.51,2,3 |
BMI, body mass index; reps, number of repetitions; SD, standard deviation; STS, sit‐to‐stand.
Superscript numbers denote significant differences compared with older people from Belgium1, Denmark2, Portugal3, and Spain4.
Significant differences compared with older people.
Significant differences among all older people groups.
Anthropometrics and body composition
Participants removed shoes, socks, and heavy clothes during the anthropometric and body composition analyses. A stadiometer and scale device was used to record the height and body mass of the participants. Body mass index (BMI) was calculated as the ratio between body mass and height squared (kg·m−2). Additionally, body composition was assessed in the Spanish 30 and Danish participants. 26 , 29 Dual energy X‐ray absorptiometry (DXA) (iDXA, Lunar, GE Healthcare, USA) was utilized to assess regional lean mass in the Danish participants, whereas bioelectrical impedance analysis (Tanita BC 418‐MA, Tanita Corp., Japan) was used to estimate regional skeletal muscle mass in the Spanish participants by a cross‐validated equation 31 recommended to estimate muscle mass values provided by DXA scans in older European populations. 32 Legs skeletal muscle index (SMI) was then calculated as legs skeletal muscle mass divided by height squared (kg·m−2).
Physical performance
Physical performance was assessed by various functional tests in the different cohorts/research studies: habitual GS, maximal GS, the timed up‐and‐go (TUG) test, and the 6 min walking test (6MWT). Gait velocity during each test was calculated as the ratio between the walked distance and the recorded time (m·s−1). Older subjects with either a habitual GS below 0.8 m·s−1, 7 maximal GS below 1.13 m·s−1, 33 TUG velocity below 0.43 m·s−1, 34 or 6MWT velocity below 0.83 m·s−1, 35 were categorized as mobility‐limited older subjects.
Sit‐to‐stand muscle power test
Muscle power was assessed by the 30 s STS muscle power test 15 in all the included studies. Briefly, the subjects performed as many STS repetitions as possible in 30 s on a standardized armless chair from the sitting position—buttocks touching the chair—to the full standing position, with their arms crossed over the chest. Verbal encouragement was given throughout the test. The participants were allowed to try one to two times before the definitive measure was annotated. STS muscle power produced when propelling the body centre of mass vertically during the concentric (upward) phase of the STS task was assessed by a previously validated equation 14 , 15 :
where body mass is indicated in kg, g is gravity (i.e. 9.81 m·s−2), body height and chair height are indicated in m, time is indicated in s (i.e. 30 s), n of reps indicates the number of repetitions performed during the STS test, and the other values (i.e. 0.9 and 0.5) are biomechanics‐derived coefficients. Then relative STS muscle power (W·kg−1) was calculated as STS mean muscle power normalized to total body mass; allometric STS muscle power (W·m−2) was calculated as the product of relative STS muscle power and BMI (or STS muscle power normalized to height squared); and specific STS muscle power (W·kg−1) was calculated as the ratio between allometric STS muscle power and legs SMI (or STS muscle power normalized to legs muscle mass).
Statistical analysis
Data are presented as mean ± standard deviation (SD) unless otherwise stated. Normality was assessed and confirmed by looking at the skewness and kurtosis values derived from the included variables (all ranged between −1.0 and 1.0). Linear mixed effects models were used to assess baseline differences including age group (<60 and ≥60 years old), study cohort (Belgium, Denmark, Portugal, and Spain), and sex (female and male) as fixed factors, and participants as a random factor. The models were calculated using maximum likelihood estimation and the best‐fitting covariance structure according to −2 log likelihood values. Bonferroni's corrections were applied to pairwise comparisons. The characteristics of the well‐functioning and mobility‐limited groups of older subjects were compared by linear mixed effects models including functional mobility status (well‐functioning and mobility‐limited), sex (female and male), and study cohort (Belgium, Denmark, Portugal, and Spain) as fixed factors, and participants as a random factor. The relationship between age and relative STS muscle power was assessed using segmented (piecewise) linear regression analyses in all the subjects (young, middle‐aged, and older adults). 36 Briefly, we used an iterative approach by which several age knots were evaluated (30, 35, 40, 45, 50, 55, 60, 65, 70, 75, and 80 years) at different age intervals (20–45, 20–50, 25–55, 30–60, 35–65, 40–70, 45–75, 50–80, 55–85, 60–90, and 65–95 years, respectively). Subsequently, a single regression model was created considering the age knots at which a statistically significant change in slope was observed. MCID values and 95% confidence intervals (CI) in relative, allometric, and specific STS muscle power were calculated based on previous evidence showing that a change of at least 2 repetitions is required in the 30 s STS test to be considered an MCID. 37 Thus, we calculated what a 2‐rep change would represent in our older participants in terms of STS power values. Normative data for relative STS muscle power and its related variables (allometric STS muscle power, BMI, specific STS muscle power, and SMI of the lower limbs) were calculated in the group of older people without mobility limitations using standard statistical methods. Several categories were created according to the calculated percentiles 38 : extremely low (percentile <3), very low (percentile 3 to 10), low (percentile 10 to 25), normal (percentile 25 to 75), high (percentile 75 to 90), very high (percentile 90 to 97), and extremely high (percentile >97). Furthermore, optimal cut‐off values for the recognition of older subjects with mobility limitations were assessed by receiver operator characteristic (ROC) curves for the following variables: relative STS muscle power, allometric STS muscle power, specific STS muscle power, BMI, and legs SMI. Consequently, area under the curve (AUC) values were reported and cut‐off values were selected based on the best trade‐off between sensitivity and specificity according to the highest product of both. Finally, age‐adjusted logistic regression analyses were used to calculate odds ratios (OR) and 95% CI of low relative STS muscle power associated with mobility limitations. Statistical analyses were performed using SPSS v20 (SPSS Inc., Chicago, Illinois, USA), and the level of significance was set at α = 0.05 using two‐tailed testing.
Results
Comparison of well‐functioning and mobility‐limited older adults
A total of 1129 older women (18.3%) and 510 older men (16.1%) had mobility limitations (i.e. habitual GS < 0.8 m·s−1, maximal GS < 1.13 m·s−1, TUG velocity < 0.43 m·s−1, or 6MWT velocity < 0.83 m·s−1). Mobility‐limited subjects were older, presented higher BMI values (women only), and performed fewer repetitions during the 30 s STS test than older subjects without mobility limitations (all P < 0.001) (Table 2). Mobility‐limited older women had lower relative STS muscle power (1.79 ± 0.93 vs. 2.95 ± 0.84 W·kg−1), lower allometric STS muscle power (51.5 ± 27.7 vs. 82.7 ± 24.4 W·m−2), and lower specific STS muscle power (10.6 ± 4.1 vs. 16.7 ± 4.0 W·kg−1) than older women without mobility limitations (all P < 0.001), but higher levels of legs SMI (5.08 ± 0.73 vs. 4.94 ± 0.47 kg·m−2; P = 0.015) (Figure 1A). In comparison with older men without mobility limitations, mobility‐limited older men presented lower relative STS power (2.12 ± 0.94 vs. 3.66 ± 1.03 W·kg−1), lower allometric STS power (59.0 ± 28.5 vs. 100.4 ± 29.7 W·m−2), lower specific STS power (11.4 ± 4.8 vs. 18.9 ± 5.0 W·kg−1) (all P < 0.001), and lower legs SMI (5.42 ± 0.72 vs. 5.58 ± 0.58 kg·m−2; P = 0.023) (Figure 1B).
Table 2.
Comparison of mean characteristics between older subjects with and without mobility limitations
| Well‐functioning older subjects | Mobility‐limited older subjects | P value a | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Women (n [%]) | 5032 [81.7] | 1129 [18.3] | |
| Age (years) | 72.0 ± 6.0 | 80.4 ± 7.3 | <0.001 |
| Body mass (kg) | 68.1 ± 11.1 | 66.6 ± 12.6 | 0.465 |
| Height (m) | 1.55 ± 0.07 | 1.52 ± 0.07 | <0.001 |
| BMI (kg·m−2) | 28.3 ± 4.5 | 28.8 ± 5.0 | <0.001 |
| 30 s STS test (reps) | 14.6 ± 3.9 | 9.2 ± 4.4 | <0.001 |
| Men (n [%]) | 2649 [83.9] | 510 [16.1] | |
| Age (years) | 71.8 ± 6.4 | 80.7 ± 7.6 | <0.001 |
| Body mass (kg) | 77.0 ± 12.4 | 73.0 ± 13.8 | <0.001 |
| Height (m) | 1.67 ± 0.09 | 1.62 ± 0.09 | <0.001 |
| BMI (kg·m−2) | 27.5 ± 3.6 | 27.8 ± 4.6 | 0.360 |
| 30 s STS test (reps) | 15.6 ± 4.2 | 9.6 ± 4.2 | <0.001 |
BMI, body mass index; reps, number of repetitions; SD, standard deviation; STS, sit‐to‐stand.
Bold values indicate significant differences between older subjects with and without mobility limitations.
Adjusted for study cohort.
Figure 1.
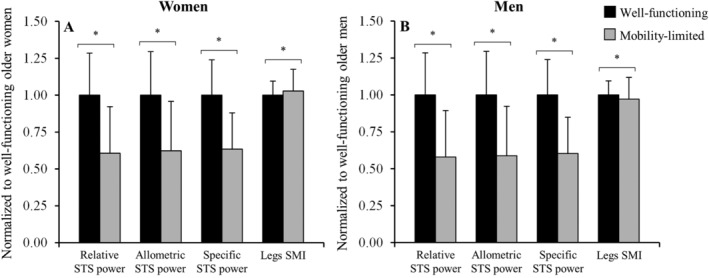
Comparison between older subjects with (grey bars) and without (black bars) mobility limitations regarding sit‐to‐stand (STS) power measures and legs skeletal muscle index (SMI), separately in women (A) and men (B), and adjusted for study cohort. Data are normalized to sex‐specific values found in the well‐functioning groups. *Significant differences noted between older subjects with and without mobility limitations (P < 0.05).
Association of relative sit‐to‐stand muscle power with age
The association between relative STS muscle power and age in women and men is displayed in Figure 2A and 2B. Women exhibited no change between 20 and 30 years (0.00 ± 0.02 W·kg−1·year−1; P = 0.842), a non‐significant decrease between 30 and 50 years (−0.05 ± 0.03 W·kg−1·year−1; P = 0.089), and significant decreases above the ages of 50 (−0.10 ± 0.02 W·kg−1·year−1) and 80 years (−0.07 ± 0.01 W·kg−1·year−1) (both P < 0.001). Similarly, men experienced a non‐significant increase between 20 and 30 years (0.02 ± 0.03 W·kg−1·year−1; P = 0.584), a non‐significant decrease between 30 and 50 years (−0.05 ± 0.04 W·kg−1·year−1; P = 0.226), and significant decreases above the ages of 50 (−0.13 ± 0.02 W·kg−1·year−1) and 80 years (−0.08 ± 0.01 W·kg−1·year−1) (both P < 0.001).
Figure 2.
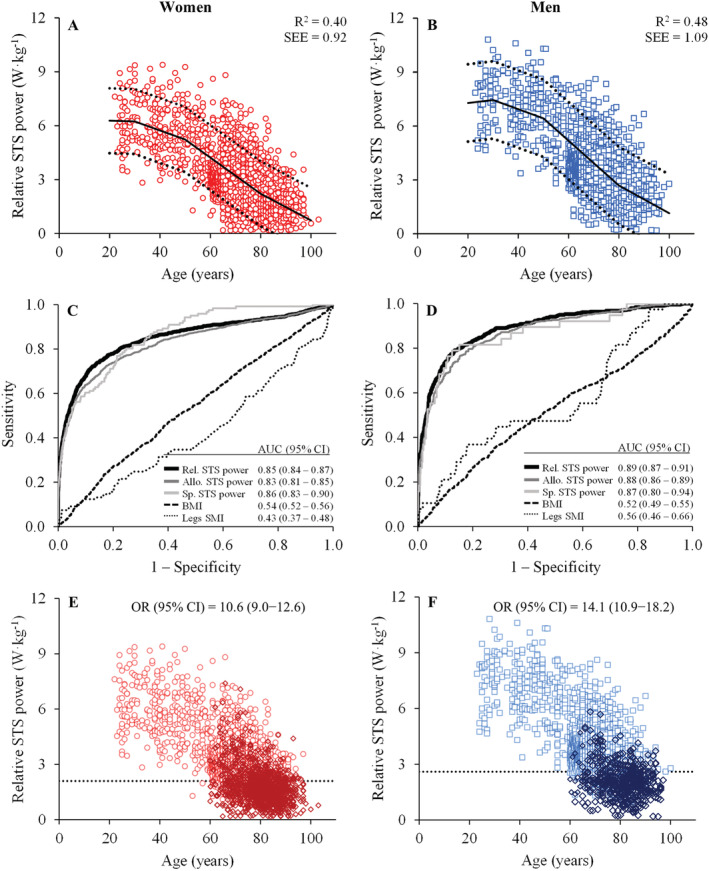
Trajectories of relative sit‐to‐stand (STS) muscle power throughout the lifespan in women (A; red open circles; n = 6479) and men (B; blue open squares; n = 3427) [regression lines (continuous), 95% prediction intervals (dashed), coefficient of determination (R 2), and standard error of the estimate (SEE) obtained by segmented regression analysis]; and receiver operator characteristic curve plot for women (C) and for men (D). Area under the curve (AUC) with 95% confidence intervals (CI) indicate the ability of the different measures to discriminate between well‐functioning and mobility‐limited older subjects. Finally, the participants above and below the cut‐off points for low relative muscle power (dashed lines) are shown in (E) for women (red open circles; cut‐off point = 2.1 W·kg−1) and in (F) for men (blue open squares; cut‐off point = 2.6 W·kg−1). Subjects exhibiting mobility limitations (open diamonds) are differentiated from those without mobility limitations. Allo., allometric; BMI, body mass index; CI, confidence interval; OR, odds ratio; Rel., relative; SMI, skeletal muscle index; Sp., specific; STS, sit‐to‐stand.
Minimal clinically important differences in sit‐to‐stand power variables
An MCID of 2 repetitions in the STS test corresponded to 0.33 W·kg−1 (95% CI: 0.17 to 0.50 W·kg−1) in women and 0.42 W·kg−1 (95% CI: 0.19 to 0.64 W·kg−1) in men in terms of relative STS muscle power, 9.4 W·m−2 (95% CI: 4.7 to 14.1 W·m−2) in women and 11.5 W·m−2 (95% CI: 4.9 to 18.1 W·m−2) in men in terms of allometric STS muscle power, and 1.78 W·kg−1 (95% CI: 0.78 to 2.78 W·kg−1) in women and 2.08 W·kg−1 (95% CI: 0.87 to 3.28 W·kg−1) in men in terms of specific STS muscle power.
Normative data and functionally relevant cut‐off points for low sit‐to‐stand muscle power and its underlying variables
Normative data regarding relative, allometric, and specific STS power, BMI, and legs SMI are presented in Table 3 for both older women and older men.
Table 3.
Normative data for older women and men regarding relative, allometric, and specific STS power, BMI, and legs SMI
| Extremely low | Very low | Low | Normal | High | Very high | Extremely high | |
|---|---|---|---|---|---|---|---|
| (P 3) | (P 10) | (P 25) | (P 50) | (P 75) | (P 90) | (P 97) | |
| Older women | |||||||
| Relative STS power (W·kg −1 ) | |||||||
| Belgium | 1.53 | 1.89 | 2.26 | 2.71 | 3.16 | 3.64 | 4.01 |
| Denmark | 1.69 | 2.22 | 2.64 | 3.23 | 4.00 | 4.98 | 6.27 |
| Portugal | 1.59 | 2.03 | 2.44 | 2.97 | 3.54 | 4.20 | 5.07 |
| Spain | 1.68 | 2.04 | 2.37 | 2.76 | 3.18 | 3.63 | 4.18 |
| All | 1.62 | 2.02 | 2.40 | 2.85 | 3.39 | 4.00 | 4.82 |
| Allometric STS power (W·m −2 ) | |||||||
| Belgium | 43.0 | 54.0 | 67.6 | 82.0 | 99.9 | 120.0 | 147.0 |
| Denmark | 42.5 | 55.7 | 67.3 | 82.3 | 103.8 | 127.4 | 148.1 |
| Portugal | 40.7 | 48.5 | 59.8 | 72.8 | 85.7 | 98.5 | 112.5 |
| Spain | 47.7 | 56.8 | 67.4 | 80.3 | 93.2 | 106.3 | 121.2 |
| All | 44.2 | 54.9 | 66.6 | 80.2 | 95.3 | 112.8 | 136.9 |
| BMI (kg·m −2 ) | |||||||
| Belgium | 20.3 | 22.4 | 24.2 | 26.5 | 29.5 | 32.9 | 35.8 |
| Denmark | 20.2 | 21.2 | 22.5 | 24.8 | 27.7 | 31.9 | 34.8 |
| Portugal | 21.2 | 23.0 | 24.9 | 27.3 | 29.9 | 32.3 | 34.7 |
| Spain | 22.3 | 24.3 | 26.2 | 28.9 | 31.6 | 34.4 | 37.6 |
| All | 21.3 | 23.1 | 25.3 | 28.0 | 30.8 | 33.8 | 37.0 |
| Specific STS power (W·kg −1 ) | |||||||
| Denmark | 9.2 | 11.7 | 14.1 | 16.6 | 20.4 | 25.1 | 29.3 |
| Spain | 10.0 | 12.1 | 14.0 | 16.3 | 18.8 | 21.3 | 24.5 |
| All | 9.9 | 12.0 | 14.0 | 16.3 | 19.0 | 21.6 | 25.4 |
| Legs SMI (kg·m −2 ) | |||||||
| Denmark | 3.95 | 4.24 | 4.48 | 4.90 | 5.37 | 5.83 | 6.27 |
| Spain | 4.27 | 4.44 | 4.63 | 4.88 | 5.18 | 5.45 | 5.74 |
| All | 4.23 | 4.41 | 4.61 | 4.89 | 5.19 | 5.51 | 5.84 |
| Older men | |||||||
| Relative STS power (W·kg −1 ) | |||||||
| Belgium | 2.10 | 2.52 | 2.99 | 3.51 | 4.00 | 4.38 | 4.97 |
| Denmark | 2.20 | 2.91 | 3.29 | 4.24 | 5.40 | 6.58 | 7.91 |
| Portugal | 1.92 | 2.40 | 2.92 | 3.50 | 4.15 | 4.88 | 5.80 |
| Spain | 2.19 | 2.62 | 2.97 | 3.49 | 4.02 | 4.56 | 5.36 |
| All | 2.03 | 2.53 | 2.99 | 3.55 | 4.17 | 4.92 | 6.09 |
| Allometric STS power (W·m −2 ) | |||||||
| Belgium | 50.3 | 64.6 | 77.8 | 95.8 | 115.7 | 137.7 | 161.9 |
| Denmark | 51.6 | 71.2 | 87.1 | 110.6 | 145.3 | 175.1 | 203.2 |
| Portugal | 55.4 | 67.4 | 79.5 | 94.8 | 109.5 | 121.8 | 141.7 |
| Spain | 58.6 | 71.2 | 83.0 | 97.4 | 114.0 | 134.2 | 152.1 |
| All | 53.4 | 66.6 | 80.2 | 97.1 | 115.9 | 139.0 | 165.4 |
| BMI (kg·m −2 ) | |||||||
| Belgium | 21.2 | 23.3 | 25.2 | 27.1 | 29.3 | 31.9 | 35.3 |
| Denmark | 20.3 | 22.3 | 24.0 | 26.1 | 28.7 | 31.8 | 34.8 |
| Portugal | 21.3 | 23.2 | 25.3 | 27.8 | 30.6 | 33.3 | 36.8 |
| Spain | 22.1 | 24.3 | 26.1 | 28.0 | 30.2 | 33.0 | 35.2 |
| All | 21.2 | 23.2 | 25.1 | 27.3 | 29.7 | 32.2 | 34.8 |
| Specific STS power (W·kg −1 ) | |||||||
| Denmark | 9.9 | 13.2 | 15.4 | 19.1 | 24.4 | 28.7 | 34.0 |
| Spain | 11.1 | 13.3 | 15.5 | 17.9 | 21.1 | 23.6 | 27.7 |
| All | 10.8 | 13.3 | 15.5 | 18.2 | 21.8 | 25.4 | 30.5 |
| Legs SMI (kg·m −2 ) | |||||||
| Denmark | 4.47 | 4.93 | 5.37 | 5.83 | 6.25 | 6.74 | 7.21 |
| Spain | 4.75 | 4.94 | 5.16 | 5.42 | 5.68 | 5.99 | 6.32 |
| All | 4.70 | 4.94 | 5.20 | 5.49 | 5.90 | 6.29 | 6.80 |
BMI, body mass index; P, percentile; SMI, skeletal muscle index; STS, sit‐to‐stand.
Finally, optimal sex‐specific cut‐off values to discriminate between older subjects with and without mobility limitations, as well as sensitivity and specificity values, are reported in Table 4 for all the older participants included in the current study. Of note, the cut‐off point for low relative STS muscle power was 2.1 W·kg−1 in older women (AUC [95% CI] = 0.85 [0.84–0.87]; sensitivity = 73.7% and specificity = 86.0%) and 2.6 W·kg−1 in older men (AUC [95% CI] = 0.89 [0.87–0.91]; sensitivity = 79.0% and specificity = 86.6%) (Figure 2C and 2D). The OR [95% CI] for having mobility limitations was 10.6 [9.0–12.6] among older women with low relative STS muscle power, and 14.1 [10.9–18.2] among older men with low relative STS muscle power (both P < 0.001) (Figure 2E and 2F). Cut‐off values for the different nationalities are reported in Supporting Information, Table S1 .
Table 4.
Optimal cut‐off points to discriminate between older subjects with and without mobility limitations, and sensitivity and specificity values
| Women | Men | |||||
|---|---|---|---|---|---|---|
| Cut‐off point | Sensitivity (%) | Specificity (%) | Cut‐off point | Sensitivity (%) | Specificity (%) | |
| Relative STS power (W·kg−1) | 2.1 | 73.7 | 86.0 | 2.6 | 79.0 | 86.6 |
| Allometric STS power (W·m−2) | 61.5 | 72.4 | 82.5 | 75.4 | 79.6 | 81.5 |
| Specific STS power (W·kg−1) | 13.6 | 76.9 | 78.6 | 14.2 | 81.6 | 84.9 |
| BMI (kg·m−2) | 28.8 | 48.1 | 58.7 | 28.2 | 47.9 | 58.8 |
| Legs SMI (kg·m−2) | 4.8 | 33.9 | 59.6 | 5.3 | 44.7 | 71.5 |
BMI, body mass index; SMI, skeletal muscle index; STS, sit‐to‐stand.
Discussion
The present investigation conducted on 9906 subjects (6479 women and 3427 men) found that relative muscle power declined steeply after the fifth decade of life in both women and men. Notably, older women and men with mobility limitations had decreased relative, allometric, and specific STS muscle power compared with older women and men without mobility limitations. In contrast, only mobility‐limited older men, but not older women, reported decreased levels of legs SMI when compared with their well‐functioning counterparts. In addition, this study provided MCID, normative, and cut‐off values to allow an adequate monitoring of relative muscle power during aging and/or inactivity in clinical and other health‐related settings.
There is compelling evidence showing the importance of maintaining and/or increasing muscle power at older age. Kuo et al. 39 demonstrated that having decreased muscle power was associated with late‐life disability after controlling for several potential cofounders, such as age, sex, BMI, cognitive performance, physical activity, inflammation, and co‐morbidities. Metter et al. 12 showed that having decreased muscle power was a risk factor for all‐cause mortality, independently of muscle strength, muscle mass, and physical activity. Steves et al. 11 found higher muscle power levels to protect from 10 year cognitive decline and magnetic resonance imaging‐assessed brain atrophy in middle‐aged and older female twins after controlling for genetics and early life environment shared by twins, and for other variables such as physical activity, co‐morbidities, and telomere length. In line with this evidence, a meta‐analysis demonstrated that power training provides greater benefits to older adults' functional ability than strength training. 40 Therefore, with the advent of older 41 and more disabled 42 populations, the monitoring and treatment of low muscle power during aging should be considered of outmost importance.
Nevertheless, the assessment of muscle power is rarely conducted in the clinical setting. Current guidelines give preference to the assessment of sarcopenia, despite acknowledging muscle power as a more relevant measure for older adults. 5 The reason for that includes the lack of standardized and feasible protocols to assess muscle power 13 and normative data that allow the identification of low muscle power among older adults. 5 However, the STS muscle power test 14 , 15 is a feasible test that can be performed in a few minutes (after providing proper explanation and familiarization to the subject) and only requires a chair and a stopwatch. This test has been demonstrated to be valid to assess muscle power in older adults when compared with the assessment of muscle power in the leg press exercise using a linear position transducer, 14 to muscle power values obtained from the Nottingham power rig, 15 and to STS power obtained with a force platform. 43 In addition, both test–retest 44 and inter‐rater 45 reliability of STS testing have been found to be excellent in older subjects. STS muscle power has also been shown to be superior to time‐based or repetition‐based STS performance, handgrip strength, and muscle power obtained with the Nottingham power rig in terms of their association with other relevant outcomes such as GS or cognitive function. 14 , 15
Jointly, it appears that the STS muscle power test may be an excellent candidate to be used in the clinical setting. In further support to this notion, low relative STS muscle power has recently been found to have a greater overall effect on physical function, frailty, disability in activities of daily living, and quality of life than sarcopenia. 16 , 46 Low relative STS muscle power has also been recently associated with an increased 9 year all‐cause mortality risk among older people. 47 Moreover, previously missed normative and cut‐off values for muscle power have been provided in the current study. Accordingly, an operational algorithm to guide health professionals to identify low relative muscle power among older adults has been proposed (Figure 3), 16 that could be implemented alone or in combination with the sarcopenia algorithm. 32 Of note, the operational algorithm for low relative muscle power presents some advantages over the one proposed for the diagnosis of sarcopenia 32 : Firstly, DXA scanning is not necessary to diagnose low relative muscle power (Part A in Figure 3), which reduces the costs associated with DXA examination for sarcopenia diagnosis; secondly, it facilitates the identification of the specific outcomes that need to be improved to revert low relative muscle power (e.g. high levels of BMI blunting the presence of adequate levels of muscle function 48 is not regarded in the sarcopenia algorithm); and thirdly, although DXA examination may be required in some subjects to elucidate the components that need to be improved to revert low allometric muscle power (Part B in Figure 3), the proportion of older adults requiring DXA scanning is considerably lower than the proportion of older individuals requiring DXA examination for sarcopenia diagnosis (10% vs. 25%, respectively). The latter is accomplished by using sex‐specific cut‐off points of BMI that help identify older people at a higher risk of low legs SMI (sensitivity = 90% in older women and 90% in older men; specificity = 84% in older women and 74% in older men).
Figure 3.
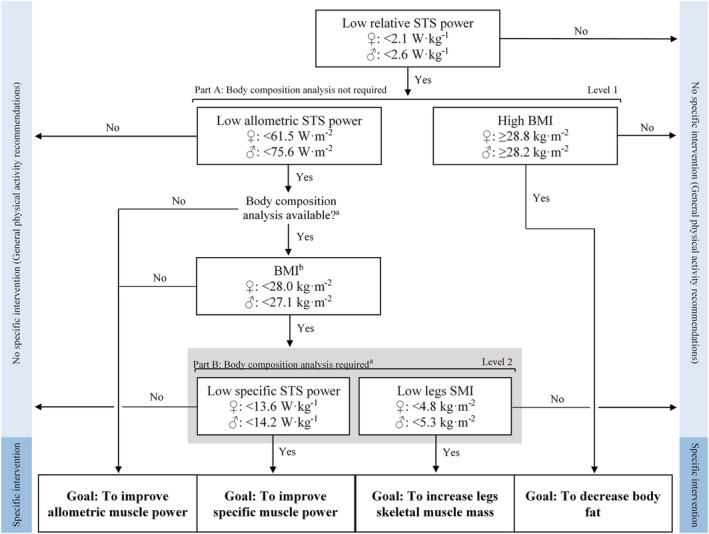
Updated operational algorithm to identify low relative STS power and its related causes. Older adults presenting low relative muscle power should participate in a specific intervention designed to improve the physiological components accounting for low relative muscle power. Part A of the algorithm can be accomplished without body composition examination. Older subjects with low allometric muscle power and low BMIb should be evaluated on their body composition when available in order to determine the specific causes of low allometric muscle power. aBody composition assessment can be conducted by DXA or bioelectrical impedance analysis. bReceiver operator characteristic curves in older women yielded: AUC [95% CI] = 0.92 [0.91–0.93], sensitivity = 89.5%, and specificity = 83.9%; and for older men: AUC [95% CI] = 0.87 [0.85–0.89], sensitivity = 89.6%, and specificity = 74.1%. BMI, body mass index; SMI, skeletal muscle index; STS, sit‐to‐stand.
A surprising finding was that higher legs SMI values were reported in mobility‐limited older women compared with their well‐functioning counterparts. This may be caused by older people having higher levels of legs SMI also being those with higher levels of obesity, which would lead to a higher risk of mobility limitations. This hypothesis was tested by excluding those subjects with obesity (i.e. BMI ≥ 30 kg·m−2) from a secondary analysis that showed that mobility‐limited older men had lower legs SMI than older men without mobility limitations (5.1 ± 0.6 vs. 5.4 ± 0.5 kg·m−2, respectively; P = 0.002), while a trend was noted when comparing mobility‐limited and well‐functioning older women (4.6 ± 0.5 vs. 4.7 ± 0.3 kg·m−2, respectively; P = 0.081). Therefore, unlike older men, higher levels of legs muscle mass in older women do not seem to be a protective factor when they are accompanied by increased adiposity levels. These results coincide with previous evidence showing that muscle mass is not an independent predictor of mobility limitations. 49 , 50 Still, skeletal muscle mass is an important metabolic regulator 51 , 52 , 53 , 54 and in combination with specific muscle power contributes to allometric muscle power output, so its relevance should not be undervalued. However, high levels of specific muscle power may compensate low levels of muscle mass to reach an adequate level of allometric muscle power. Age‐related reductions in cross‐sectional area of type II muscle fibres 55 , 56 and in neuromuscular excitation rate 57 among others can lead to an accentuated loss in specific muscle power with age, while legs SMI level may be well preserved in women until the age of 75 years and in men until the age of 65 years. 36
In terms of variations in STS power with age, although the absolute rate of decline in relative STS power seemed to peak between 50 and 80 years in both women and men (cf. Figure 2), percentage losses increased progressively from 30 years to oldest age: 30–50 years old (women = 0.8–0.9%; men = 0.7–0.8%), 50–80 years old (women = 1.9–4.4%; men = 2.0–4.5%), and >80 years old (women = 3.4–9.2%; men = 2.9–6.4%). These findings underline that interventions aimed at preventing a substantial loss of relative muscle power with aging should be conducted in the age window of 30 to 50 years. Another age window critical to preserve functional ability at old age is 50 to 80 years. Approximately 10% of older subjects aged 60–70 years and 20% of those aged 70–80 years had low relative muscle power. Older people below these cut‐off points exhibited a more than 10‐fold increase in their odds of having mobility limitations compared with older subjects with adequate levels of relative muscle power. Consequently, individuals aged 50–80 years should be strongly encouraged to take part in interventions aimed at improving relative muscle power to postpone the onset of mobility limitations and disability later in life. Although the primary focus should be on prevention, people older than 80 years can still benefit greatly from interventions aiming to improve relative muscle power. 58 In this sense, any improvement of at least 0.33 W·kg−1 in women and 0.42 W·kg−1 in men in terms of relative muscle power can be considered an MCID. However, given the curvilinear relationship between muscle power and physical function, 59 , 60 future studies should be conducted to assess MCID values in older people with different baseline levels of muscle power.
Regarding subjects with starting values of power above the cut‐off points, relative, allometric, and specific muscle power can be monitored according to normative data presented here. The classification of well‐muscle functioning subjects in different categories may help people gain awareness on the importance of muscle power and encourage subjects to maintain and/or improve their power measures, thus reducing their likelihood of presenting mobility limitations and disability later in life. 39 , 61
Finally, we should highlight the excellent AUC values yielded by the reported STS power measures in relation with their ability to discriminate between mobility‐limited and well‐functioning subjects in a large cohort composed of 9320 older subjects. On the contrary, AUC values for BMI and legs SMI were modest. However, the cut‐off points for BMI and legs SMI are still necessary to explain, for example, why some older subjects may present low relative muscle power and adequate levels of allometric muscle power at the same time.
Several limitations of the present study should be acknowledged and considered when interpreting the results. The cross‐sectional nature of our data may limit our conclusions due to a potential refusal or survival effect. These limitations however can also affect longitudinal studies. There are reports showing comparable patterns of decline in muscle power between cross‐sectional and longitudinal designs. 62 In this same line, we cannot establish a cause–effect relationship between low relative muscle power and mobility limitations, but the prognostic value of low muscle power in relation to incident disability, 61 cognitive decline, 11 and mortality 12 has already been demonstrated. In this sense, future longitudinal studies may benefit from the use of the proposed operational algorithm of low relative muscle power to obtain standardized results across studies and further evidence on the impact of low relative muscle power in various outcomes. In addition, the relationship between muscle power and age was assessed including participants younger than 60 years old only from the Danish cohort, given that the other cohorts did not include participants younger than 60 years. Mobility limitations were assessed through different physical tests in the original cohort studies. Finally, physical activity levels were not investigated in the current study. Additional studies considering this aspect may provide further insights on the importance of an active lifestyle in the maintenance of muscle power during aging.
In conclusion, the present cross‐sectional study found annual percentage losses in relative muscle power to increase progressively from the age of 30 years (~1%) to the oldest age (~6–9%), in a population consisting of 9906 people aged 20–103 years from several European cohorts/research studies. Notably, the provided sex‐specific cut‐off points for low relative muscle power were proved to discriminate satisfactorily between older subjects with and without mobility limitations. In addition, the cut‐off points reported for the different components of relative muscle power can help identify the specific domains that need to be improved to revert low relative muscle power (i.e. specific muscle power, muscle mass, and/or body mass). Finally, normative data for relative muscle power and its determining variables can be used to categorize and monitor older subjects with adequate starting levels of relative muscle power during aging.
Funding
This work was supported by the Ministerio de Educación, Cultura y Deporte of the Government of Spain (Grant FPU014/05106 to J.A.), by the Biomedical Research Networking Center on Frailty and Healthy Aging (CIBERFES) and FEDER funds from the European Union (Grant CB16/10/00477), by the Ministerio de Educación y Ciencia (Red EXERNET DEP2005‐00046), by the Portuguese Foundation for Science and Technology (Grant SFRH/BPD/115977/2016 to P.B.J.), and by the Flemish Government and the Research Foundation Flanders (Grant G052105).
Conflict of interest
The authors declare no competing interests.
Ethical guidelines statement
All authors comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. This study was approved by the corresponding local ethical committees of the participating cohorts and was performed in accordance with the ethical standards laid down in the 1965 Declaration of Helsinki and its later amendments. All participants gave their informed consent prior to their inclusion in the study.
Supporting information
Table S1. Optimal cut‐off points to discriminate between older subjects with and without mobility limitations, and sensitivity and specificity values, per country
Alcazar J., Alegre L. M., Van Roie E., Magalhães J. P., Nielsen B. R., González‐Gross M., Júdice P. B., Casajús J. A., Delecluse C., Sardinha L. B., Suetta C., and Ara I. (2021) Relative sit‐to‐stand power: aging trajectories, functionally relevant cut‐off points, and normative data in a large European cohort, Journal of Cachexia, Sarcopenia and Muscle, 12, 921–932, 10.1002/jcsm.12737
References
- 1. WHO . World Report on Ageing and Health. Geneva, Switzerland: World Health Organization; 2015. [Google Scholar]
- 2. Suetta C, Haddock B, Alcazar J, Noerst T, Hansen O, Ludvig H, et al. The Copenhagen Sarcopenia Study: lean mass, muscle strength, muscle power and physical function in a Danish cohort aged 20–93 years. J Cachexia Sarcopenia Muscle 2019;10:1316–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dodds RM, Kuh D, Sayer AA, Cooper R. Can measures of physical performance in mid‐life improve the clinical prediction of disability in early old age? Findings from a British birth cohort study. Exp Gerontol 2018;110:118–124. [DOI] [PubMed] [Google Scholar]
- 4. WHO . The International Classification of Functioning, Disability and Health. Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- 5. Beaudart C, Rolland Y, Cruz‐Jentoft AJ, Bauer JM, Sieber C, Cooper C, et al. Assessment of muscle function and physical performance in daily clinical practice: a position paper endorsed by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Calcif Tissue Int 2019;105:1–14. [DOI] [PubMed] [Google Scholar]
- 6. Reid KF, Pasha E, Doros G, Clark DJ, Patten C, Phillips EM, et al. Longitudinal decline of lower extremity muscle power in healthy and mobility‐limited older adults: influence of muscle mass, strength, composition, neuromuscular activation and single fiber contractile properties. Eur J Appl Phyisiol 2014;114:29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, et al. Age‐associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol 2003;95:1851–1860. [DOI] [PubMed] [Google Scholar]
- 8. Pearson SJ, Young A, Macaluso A, Devito G, Nimmo MA, Cobbold M, et al. Muscle function in elite master weightlifters. Med Sci Sports Exerc 2002;34:1199–1206. [DOI] [PubMed] [Google Scholar]
- 9. Foldvari M, Clark M, Laviolette LC, Bernstein MA, Kaliton D, Castaneda C, et al. Association of muscle power with functional status in community‐dwelling elderly women. J Gerontol A Biol Sci Med Sci 2000;55:M192–M199. [DOI] [PubMed] [Google Scholar]
- 10. Martinikorena I, Martínez‐Ramírez A, Gómez M, Lecumberri P, Casas‐Herrero A, Cadore EL, et al. Gait variability related to muscle quality and muscle power output in frail nonagenarian older adults. J Am Med Dir Assoc 2016;17:162–167. [DOI] [PubMed] [Google Scholar]
- 11. Steves CJ, Mehta MM, Jackson SH, Spector TD. Kicking back cognitive ageing: leg power predicts cognitive ageing after ten years in older female twins. Gerontology 2016;62:138–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Metter EJ, Talbot LA, Schrager M, Conwit RA. Arm‐cranking muscle power and arm isometric muscle strength are independent predictors of all‐cause mortality in men. J Appl Phys (Bethesda, Md: 1985) 2004;96:814–821. [DOI] [PubMed] [Google Scholar]
- 13. Alcazar J, Guadalupe‐Grau A, García‐García FJ, Ara I, Alegre LM. Skeletal muscle power measurement in older people: a systematic review of testing protocols and adverse events. J Gerontol A Biol Sci Med Sci 2017;73:914–924. [DOI] [PubMed] [Google Scholar]
- 14. Alcazar J, Losa‐Reyna J, Rodriguez‐Lopez C, Alfaro‐Acha A, Rodriguez‐Manas L, Ara I, et al. The sit‐to‐stand muscle power test: an easy, inexpensive and portable procedure to assess muscle power in older people. Exp Gerontol 2018;112:38–43. [DOI] [PubMed] [Google Scholar]
- 15. Alcazar J, Kamper RS, Aagaard P, Haddock B, Prescott E, Schnorh P, et al. Relation between leg extension power and the 30‐s sit‐to‐stand muscle power in older adults: validation and translation to functional performance. Sci Reports 2020;10:16337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Losa‐Reyna J, Alcazar J, Rodríguez‐Gómez I, Alfaro‐Acha A, Alegre LM, Rodriguez Manas L, et al. Low relative mechanical power in older adults: an operational definition and algorithm for its application in the clinical setting. Exp Gerontol 2020;142:111141. [DOI] [PubMed] [Google Scholar]
- 17. Alcazar J, Rodriguez‐Lopez C, Ara I, Alfaro‐Acha A, Rodriguez‐Gomez I, Navarro‐Cruz R, et al. Force‐velocity profiling in older adults: an adequate tool for the management of functional trajectories with aging. Exp Gerontol 2018;108:1–6. [DOI] [PubMed] [Google Scholar]
- 18. Skelton DA, Greig C, Davies JM, Young A. Strength, power and related functional ability of healthy people aged 65–89 years. Age Ageing 1994;23:371–377. [DOI] [PubMed] [Google Scholar]
- 19. Wijndaele K, Duvigneaud N, Matton L, Duquet W, Thomis M, Beunen G, et al. Muscular strength, aerobic fitness, and metabolic syndrome risk in Flemish adults. Med Sci Sports Exerc 2007;39:233–240. [DOI] [PubMed] [Google Scholar]
- 20. Charlier R, Mertens E, Lefevre J, Thomis M. Muscle mass and muscle function over the adult life span: a cross‐sectional study in Flemish adults. Arch Gerontol Geriatr 2015;61:161–167. [DOI] [PubMed] [Google Scholar]
- 21. Charlier R, Knaeps S, Mertens E, Van Roie E, Delecluse C, Lefevre J, et al. Age‐related decline in muscle mass and muscle function in Flemish Caucasians: a 10‐year follow‐up. Age (Dordrecht, Netherlands) 2016;38:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Van Roie E, Delecluse C, Coudyzer W, Boonen S, Bautmans I. Strength training at high versus low external resistance in older adults: effects on muscle volume, muscle strength, and force‐velocity characteristics. Exp Gerontol 2013;48:1351–1361. [DOI] [PubMed] [Google Scholar]
- 23. Van Roie E, Delecluse C, Opdenacker J, De Bock K, Kennis E, Boen F. Effectiveness of a lifestyle physical activity versus a structured exercise intervention in older adults. J Aging Phys Act 2010;18:335–352. [DOI] [PubMed] [Google Scholar]
- 24. Van Hoecke AS, Delecluse C, Bogaerts A, Boen F. The long‐term effectiveness of need‐supportive physical activity counseling compared with a standard referral in sedentary older adults. J Aging Phys Act 2014;22:186–198. [DOI] [PubMed] [Google Scholar]
- 25. Bogaerts A, Delecluse C, Claessens AL, Coudyzer W, Boonen S, Verschueren SM. Impact of whole‐body vibration training versus fitness training on muscle strength and muscle mass in older men: a 1‐year randomized controlled trial. J Gerontol A Biol Sci Med Sci 2007;62:630–635. [DOI] [PubMed] [Google Scholar]
- 26. Christensen MG, Piper KS, Dreier R, Suetta C, Andersen HE. Prevalence of sarcopenia in a Danish geriatric out‐patient population. Dan Med J 2018;65:A5485. [PubMed] [Google Scholar]
- 27. Marques EA, Baptista F, Santos R, Vale S, Santos DA, Silva AM, et al. Normative functional fitness standards and trends of portuguese older adults: cross‐cultural comparisons. J Aging Phys Act 2014;22:126–137. [DOI] [PubMed] [Google Scholar]
- 28. Pedrero‐Chamizo R, Gómez‐Cabello A, Delgado S, Rodríguez‐Llarena S, Rodríguez‐Marroyo JA, Cabanillas E, et al. Physical fitness levels among independent non‐institutionalized Spanish elderly: the elderly EXERNET multi‐center study. Arch Gerontol Geriatr 2012;55:406–416. [DOI] [PubMed] [Google Scholar]
- 29. Suetta C. Copenhagen sarcopenia study—time to implement assessment of muscle mass and muscle function as a clinical target. Med Sci Sports Exerc 2017;49:434. [Google Scholar]
- 30. Gomez‐Cabello A, Pedrero‐Chamizo R, Olivares PR, Luzardo L, Juez‐Bengoechea A, Mata E, et al. Prevalence of overweight and obesity in non‐institutionalized people aged 65 or over from Spain: the elderly EXERNET multi‐centre study. Obes Rev: an official Journal of the International Association for the Study of Obesity 2011;12:583–592. [DOI] [PubMed] [Google Scholar]
- 31. Sergi G, De Rui M, Veronese N, Bolzetta F, Berton L, Carraro S, et al. Assessing appendicular skeletal muscle mass with bioelectrical impedance analysis in free‐living Caucasian older adults. Clin Nutr (Edinburgh, Scotland) 2015;34:667–673. [DOI] [PubMed] [Google Scholar]
- 32. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2018;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Middleton A, Fulk GD, Herter TM, Beets MW, Donley J, Fritz SL. Self‐selected and maximal walking speeds provide greater insight into fall status than walking speed reserve among community‐dwelling older adults. Am J Phys Med Rehabil 2016;95:475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shumway‐Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community‐dwelling older adults using the Timed Up & Go Test. Phys Ther 2000;80:896–903. [PubMed] [Google Scholar]
- 35. Solway S, Brooks D, Lacasse Y, Thomas S. A qualitative systematic overview of the measurement properties of functional walk tests used in the cardiorespiratory domain. Chest 2001;119:256–270. [DOI] [PubMed] [Google Scholar]
- 36. Alcazar J, Aagaard P, Haddock B, Kamper R, Krarup S, Prescott E, et al. Age‐ and sex‐specific changes in lower‐limb muscle power throughout the lifespan. J Gerontol A Biol Sci Med Sci 2020;75:1369–1378. [DOI] [PubMed] [Google Scholar]
- 37. Wright AA, Cook CE, Baxter GD, Dockerty JD, Abbott JH. A comparison of 3 methodological approaches to defining major clinically important improvement of 4 performance measures in patients with hip osteoarthritis. J Orthop Sports Phys Ther 2011;41:319–327. [DOI] [PubMed] [Google Scholar]
- 38. Ramirez‐Velez R, Correa‐Bautista JE, Garcia‐Hermoso A, Cano CA, Izquierdo M. Reference values for handgrip strength and their association with intrinsic capacity domains among older adults. J Cachexia Sarcopenia Muscle 2019;10:278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kuo HK, Leveille SG, Yen CJ, Chai HM, Chang CH, Yeh YC, et al. Exploring how peak leg power and usual gait speed are linked to late‐life disability: data from the National Health and Nutrition Examination Survey (NHANES), 1999–2002. Am J Phys Med Rehabil 2006;85:650–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tschopp M, Sattelmayer MK, Hilfiker R. Is power training or conventional resistance training better for function in elderly persons? A meta‐analysis Age Ageing 2011;40:549–556. [DOI] [PubMed] [Google Scholar]
- 41. Commission E . The 2018 ageing report. In: Union E editor. 2017.
- 42. Kyu HH, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi H, et al. Global, regional, and national disability‐adjusted life‐years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England) 2018;392:1859–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Baltasar‐Fernandez I, Alcazar J, Rodriguez‐Lopez C, Losa‐Reyna J, Alonso‐Seco M, Ara I, et al. Sit‐to‐stand muscle power test: comparison between estimated and force plate‐derived mechanical power and their association with physical function in older adults. Exp Gerontol 2021;145:111213. [DOI] [PubMed] [Google Scholar]
- 44. Tiedemann A, Lord SR, Sherrington C. The development and validation of a brief performance‐based fall risk assessment tool for use in primary care. J Gerontol A Biol Sci Med Sci 2010;65:896–903. [DOI] [PubMed] [Google Scholar]
- 45. Wallmann HW, Evans NS, Day C, Neelly KR. Interrater reliability of the five‐times‐sit‐to‐stand test. Home Health Care Manag Pract 2013;25:13–17. [Google Scholar]
- 46. Bahat G, Kilic C, Eris S, Karan MA. Power versus sarcopenia: associations with functionality and physical performance measures. J Nutr Health Aging 2021;25:13–17. [DOI] [PubMed] [Google Scholar]
- 47. Alcazar J, Navarrete‐Villanueva D, Mañas A, Gómez‐Cabello A, Pedrero‐Chamizo R, Alegre LM, et al. ‘Fat but powerful’ paradox: association of muscle power and adiposity markers with all‐cause mortality in older adults from the EXERNET multicentre study. Br J Sports Med. 2021; 10.1136/bjsports-2020-103720 [DOI] [PubMed] [Google Scholar]
- 48. Yang M, Ding X, Luo L, Hao Q, Dong B. Disability associated with obesity, dynapenia and dynapenic‐obesity in Chinese older adults. J Am Med Dir Assoc 2014;15:e11–6. [DOI] [PubMed] [Google Scholar]
- 49. Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well‐functioning older persons. J Gerontol A Biol Sci Med Sci 2005;60:324–333. [DOI] [PubMed] [Google Scholar]
- 50. Bouchard DR, Heroux M, Janssen I. Association between muscle mass, leg strength, and fat mass with physical function in older adults: influence of age and sex. J Aging Health 2011;23:313–328. [DOI] [PubMed] [Google Scholar]
- 51. McMurray RG, Soares J, Caspersen CJ, McCurdy T. Examining variations of resting metabolic rate of adults: a public health perspective. Med Sci Sports Exerc 2014;46:1352–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Muller MJ, Bosy‐Westphal A, Kutzner D, Heller M. Metabolically active components of fat‐free mass and resting energy expenditure in humans: recent lessons from imaging technologies. Obes Rev 2002;3:113–122. [DOI] [PubMed] [Google Scholar]
- 53. Akasaki Y, Ouchi N, Izumiya Y, Bernardo BL, Lebrasseur NK, Walsh K. Glycolytic fast‐twitch muscle fiber restoration counters adverse age‐related changes in body composition and metabolism. Aging Cell 2014;13:80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. LeBrasseur NK, Walsh K, Arany Z. Metabolic benefits of resistance training and fast glycolytic skeletal muscle. Am J Physiol Endocrinol Metab 2011;300:E3–E10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. McPhee JS, Cameron J, Maden‐Wilkinson T, Piasecki M, Yap MH, Jones DA, et al. The contributions of fiber atrophy, fiber loss, in situ specific force, and voluntary activation to weakness in sarcopenia. J Gerontol A Biol Sci Med Sci 2018;73:1287–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Larsson L, Grimby G, Karlsson J. Muscle strength and speed of movement in relation to age and muscle morphology. J Appl Physiol Respir Environ Exerc Physiol 1979;46:451–456. [DOI] [PubMed] [Google Scholar]
- 57. Reid KF, Pasha E, Doros G, Clark DJ, Patten C, Phillips EM, et al. Longitudinal decline of lower extremity muscle power in healthy and mobility‐limited older adults: influence of muscle mass, strength, composition, neuromuscular activation and single fiber contractile properties. Eur J Appl Physiol 2014;114:29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Losa‐Reyna J, Baltasar‐Fernandez I, Alcazar J, Navarro‐Cruz R, Garcia‐Garcia FJ, Alegre LM, et al. Effect of a short multicomponent exercise intervention focused on muscle power in frail and pre frail elderly: a pilot trial. Exp Gerontol 2019;115:114–121. [DOI] [PubMed] [Google Scholar]
- 59. Alcazar J, Rodriguez‐Lopez C, Ara I, Alfaro‐Acha A, Mañas‐Bote A, Guadalupe‐Grau A, et al. The force‐velocity relationship in older people: reliability and validity of a systematic procedure. Int J Sports Med 2017;38:1097–1104. [DOI] [PubMed] [Google Scholar]
- 60. Bean JF, Kiely DK, Herman S, Leveille SG, Mizer K, Frontera WR, et al. The relationship between leg power and physical performance in mobility‐limited older people. J Am Geriatr Soc 2002;50:461–467. [DOI] [PubMed] [Google Scholar]
- 61. Hicks GE, Shardell M, Alley DE, Miller RR, Bandinelli S, Guralnik J, et al. Absolute strength and loss of strength as predictors of mobility decline in older adults: the InCHIANTI study. J Gerontol Series A Biol Sci Med Sci 2012;67:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Metter EJ, Conwit R, Tobin J, Fozard JL. Age‐associated loss of power and strength in the upper extremities in women and men. J Gerontol A Biol Sci Med Sci 1997;52:B267–B276. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Optimal cut‐off points to discriminate between older subjects with and without mobility limitations, and sensitivity and specificity values, per country


