Abstract
Background
Myosteatosis has been associated with shorter overall survival in cancer patients. The increase in ectopic fat might not be limited to skeletal muscle only and might also extend to other sites such as the liver, resulting in non‐alcoholic fatty liver disease (NAFLD). In this study, we assessed the relationship between myosteatosis and NAFLD and their association with overall survival in patients with colorectal liver metastases undergoing partial hepatectomy.
Methods
Patients were selected from a prospective cohort of 289 consecutive patients with colorectal liver metastases. All patients with a preoperative computed tomography (CT)‐scan and liver biopsy obtained during surgery were included. If available a second pre‐operative CT scan was used to calculate changes in body composition over time. Muscle radiation attenuation was defined as the average Hounsfield units on CT of all muscle tissue at the L3 level. Liver biopsies were graded by a liver pathologist using the steatosis, activity, and fibrosis scoring system for NAFLD.
Results
Two‐hundred and eighteen patients had an available liver biopsy of which 131 patients had two available pre‐operative CT scans with an average time interval of 3.2 months. One‐hundred and thirty‐five (62%) biopsies were classified as NAFLD. In multivariable Cox‐regression analysis, NAFLD [hazard ratio (HR): 1.8, 95%‐confidence interval (CI) 1.0–3.0, P = 0.037], increase in myosteatosis (HR 1.8, 95%‐CI 1.1–2.9, P = 0.018), and skeletal muscle loss (HR 1.7, 95%‐CI 1.0–2.9, P = 0.035) were independently associated with shorter overall survival while high visceral adipose tissue fat content was associated with longer overall survival (HR: 0.7, 95%‐CI 0.5–0.9, P = 0.014).
Conclusions
Ectopic fat content of liver as well as skeletal muscle tissue is independently associated with shorter overall survival in patients with colorectal liver metastases, while increased visceral adipose tissue fat content is associated with longer overall survival.
Keywords: Body composition, Non‐alcoholic fatty liver disease, Skeletal muscle loss, Sarcopenia, Myosteatosis, Colorectal liver metastases
Background
Colorectal liver metastases (CRLM) develop in up to 70% of all colorectal cancer cases and are responsible for around 320 000 annual deaths in high‐income countries making it the second most lethal cancer type. 1 , 2 In around 30% of metastatic patients, the metastases are confined to the liver. 3 Apart from liver transplantation, partial hepatectomy is currently the only option for curation with a median 5 year survival rate of 36–47%. 4 , 5 , 6 Around 30% of patients with CRLM are eligible for surgery. As operative mortality is low (<5%), survival is probably mostly related to oncologic outcome and/or host characteristics such as body composition and comorbidity. In recent years, associations between body composition and outcome are being studied increasingly. We recently demonstrated that low visceral adipose tissue mass and low skeletal muscle mass (sarcopenia) as assessed by analysis of pre‐operative computed tomography (CT) scans are associated with shorter overall survival in patients with CRLM. 7 Other studies also found relationships between low skeletal muscle mass and decreased overall survival 8 as well as increased postoperative complications. 9 Next to skeletal muscle and adipose tissue mass, a CT scan can provide estimations of tissue fat content through the tissue specific radiation attenuation, which is expressed as the average radiodensity in Hounsfield units (HUs). 10 A low skeletal radiation attenuation (myosteatosis) is associated with increased tissue triglyceride content. 11 In visceral and subcutaneous adipose tissue, a low radiation attenuation probably is an indicator of high adipose tissue lipid content as it is associated with increased adipocyte size, high adipose tissue mass, and increased serum lipoprotein levels in obese individuals. 12 , 13 While adipose tissue fat content has been poorly studied in cancer, myosteatosis has been associated with decreased overall survival in several cancer types. 14 , 15 , 16 However, the aetiology of myosteatosis is not fully understood. Previous studies found associations of myosteatosis with increased adipose tissue mass 7 and systemic inflammation 7 , 17 in patients with colorectal cancer. In healthy adults, myosteatosis has been shown to be related to systemic hyperinsulinemia and insulin resistance. 18 It could be hypothesized that insulin resistance and systemic inflammation driven by adiposity and/or tumour factors lead to impaired fat storage in adipocytes and increased lipolysis. Subsequent increased plasma free fatty acids would promote increased lipid accumulation in skeletal muscle tissue. However, the systemic component of the proposed pathophysiology in this hypothesis suggests that steatosis might not be limited to skeletal muscle tissue alone. Liver tissue for instance could also be affected. In non‐oncologic patients with non‐alcoholic fatty liver disease (NAFLD), liver steatosis has been shown to be associated with both hepatic and skeletal muscle insulin resistance. 19 In addition, NAFLD has been associated with low skeletal muscle mass in obese patients. 20 The direct association between myosteatosis and liver steatosis has not been studied, however. Also, virtually all studies on body composition and/or myosteatosis in cancer patients use only one CT scan, providing a mere snap shot of the patient's body composition without insight into skeletal muscle loss/gain or increase/decrease in myosteatosis over time. In this study, we assessed the relationship between body composition, myosteatosis, adipose tissue fat content, and liver steatosis and their associations with disease‐free and overall survival in CRLM patients who had undergone partial hepatectomy, using serial CT scans over time and liver biopsies.
Patients and methods
Patients
Patients were selected from a prospective cohort of 289 patients undergoing partial hepatectomy between 2008 and 2013, at the Maastricht University Medical Centre, the Netherlands. Patients were selected for inclusion if they met the following criteria: pathology‐proven colorectal liver metastasis, two preoperative CT scans available with a 1–6 months interval in between, and a surgical biopsy of unaffected liver tissue. Patients with poor quality CT scans or large radiation artefacts were excluded from the study.
Data collection
The primary outcome of this study was overall survival, expressed in months from the day of surgery. Secondary outcomes included disease‐free survival, expressed in months from day of surgery until recurrence, and major complications. A validated liver surgery‐specific composite endpoint was used as outcome parameter for major complications. 21 The composite endpoint included the following major (Dindo–Clavien ≥ 3) liver surgery specific complications occurring within 90 days after surgery: ascites, post‐operative liver failure, bile leakage, intra‐abdominal abscess, intra‐abdominal haemorrhage, and operative mortality. Radical resection was defined as a cancer‐free surgical margin of more than 1 mL. The Fong‐score was calculated to estimate the risk of tumour recurrence (one point each: node‐positive primary tumour, initial disease‐free interval < 12 months, >1 liver metastasis, largest metastasis > 5 cm, and CEA > 200 ng/mL). 22 Recorded patient characteristics included age, sex, body mass index, American Society of Anaesthesiologists classification, comorbidities (cardiac, pulmonal, renal, and diabetes mellitus), and the administration of neoadjuvant chemotherapy.
Liver biopsies
Surgical liver biopsies were performed during partial hepatectomy from an unaffected part of the liver (without metastases) at minimal 3 cm depth. Biopsies were fixed in formalin for 24 h and embedded in paraffin. Thereafter, 4 μm sections were stained with haematoxylin & eosin. One blinded pathologist classified the liver tissue according to the steatosis, activity, and fibrosis (SAF) scoring system as NAFLD with NASH, NAFLD without NASH or no NAFLD. 23 According to the SAF scoring system, NAFLD was defined as a steatosis score of at least Grade 1 (5% to 33% steatotic hepatocytes) and NAFLD with NASH was defined as at least Grade 1 of all three SAF features (steatosis, hepatocellular ballooning, and lobular inflammation).
Computed tomography scan analysis
Pre‐operative CT scans were selected if they were performed within 4 months before surgery. If available, an additional earlier CT scan performed within 1–6 months before the pre‐operative CT scan was selected to assess preoperative body composition changes over time. Patients received intravenous contrast during scanning. Average slice thickness was 4.2 ± 1.1 mm; tube voltage was 120 kV. One blinded researcher trained in radiologic anatomy and body composition analysis performed all CT scan analyses using sliceOmatic 5.0 (TomoVision, Magog, Canada) software for Microsoft Windows® as described previously. 15 In short, a single CT‐slice at the level of the third lumbar vertebra (L3) was selected for each scan. Using predefined HU ranges, the total areas of skeletal muscle (−29 to 150 HU), visceral adipose tissue (VAT, −150 to −50 HU), and subcutaneous adipose tissue (SAT, −190 to −30 HU) were assessed. Total adipose tissue (TAT) area was calculated by adding up VAT and SAT area. Tissue area (cm2) was adjusted for height to calculate the L3‐index (cm2/m2), which corresponds well with total body muscle and adipose tissue mass. 24 In addition, the tissue specific radiation attenuation was calculated as the average HU value of the total tissue area for muscle, VAT, and SAT separately. A high tissue fat content is associated with a low radiation attenuation. The measurement errors (% coefficient of variation) for tissue area and radiation attenuation measurements were 0.65% and 0.60%, respectively. To correct for sex‐related and age‐related body composition differences, Z‐scores were calculated for the L3‐index and radiation attenuation of each tissue. A Z‐score is the number of standard deviations that each patient differs from his or her sex‐specific and age‐specific mean value within the study cohort and was calculated using the following formula:
Z is the patient's Z‐score, is the patient's CT value, μ is the sex‐specific and age‐specific mean, and σ the sex‐specific and age‐specific standard deviation derived from the present cohort. Z‐scoring facilitates body composition comparison among patients with different sex and age. Changes in body composition over time were calculated using the following formula:
Δbody composition is the pre‐operative change in body composition for a specific parameter in %/100 days in which a negative Δ signifies tissue loss and a positive Δ signifies gain of tissue area. A similar formula was used for radiation attenuation. A negative Δ for radiation attenuation signifies an increase in tissue fat content while a positive Δ signifies a decrease in tissue fat content. CT preop is the pre‐operative CT value (closest to surgery), CT2 is the additional CT value (before pre‐operative scan), and n days is the number of days between the pre‐operative and additional CT scans.
Statistical analysis
A value of ≤ − 2% change in any body composition parameter was considered a loss of tissue or radiation attenuation, which was well above the minimal detectable change (0.65% and 0.60%, respectively). 25 Data were analysed using IBM SPSS statistics 23 for Microsoft Windows®. Follow‐up was determined using the Kaplan–Meier estimate of potential follow‐up. 26 Patient characteristics were assessed by either a χ 2 test, independent t‐test, or one‐way analysis of variance where appropriate. For correlations, Pearson's correlation coefficient (r p ) was used. Correlations were visualized in a correlation heatmap using R 3.4.1 for Microsoft Windows®. Univariable and multivariable Cox‐regression analyses were used to assess the associations between NAFLD/body composition parameters and overall survival/disease‐free survival. Univariable and multivariable logistic regression was used to assess the association with major complications (composite endpoint). For regression analysis, three models were used: Model 1 was the unadjusted univariable analysis. Model 2 was the multivariable analysis including all known confounding variables which included sex, age, American Society of Anaesthesiologists, comorbidity, neoadjuvant chemotherapy, radical resection, and FONG score. Model 3 was the final multivariable analysis in which all liver and body composition variables were added to Model 2 and selected by backward elimination with a P value of <0.1 as condition to keep the variable in the model. Eligible variables were as follows: liver steatosis; pre‐operative Z‐score of muscle, VAT, SAT, and TAT L3‐index; pre‐op Z‐score of muscle, VAT, and SAT radiation attenuation; Δ muscle, VAT, SAT, and TAT area; and Δ muscle, VAT, and SAT radiation attenuation. Associations among different fat storage compartments in patients were visualized in a proportional Venn diagram using eulerAPE (University of Kent, Canterbury, United Kingdom). 27 A P value of <0.05 was considered statistically significant.
Results
Patient characteristics
Of the 289 patients in the prospected cohort, 218 patients were selected for analysis. Sixty‐one patients did not have a CT scan available, eight patients had poor quality scans, and two patients did not have a liver biopsy available. There were no significant differences in patient characteristics between excluded and included patients. Of the 218 patients, 131 patients had a second CT scan available prior to the pre‐operative CT scan within the pre‐defined time‐frame. Patients with a second CT‐scan available had significantly more often neoadjuvant chemotherapy and a higher rate of metachronous metastasis compared with patients with only one pre‐operative CT scan available (82% vs. 49%, P < 0.001 and 79% vs. 49%, P < 0.001, respectively). Median time between pre‐operative CT scan and surgery was 47 days [interquartile range (IQR): 28–68 days], and median time between the pre‐operative CT scan and second CT scan was 94 days (IQR: 75–119 days). Median follow‐up was 56 months (IQR: 36–72 months). Patient characteristics are shown in Table 1.
Table 1.
Patient characteristics
| Characteristic | Total | No NAFLD | NAFLD | P value |
|---|---|---|---|---|
| (n = 218) | (n = 83) | (n = 135) | ||
| Male (n, %) | 142 (65.1%) | 55 (66%) | 87 (64%) | 0.784 |
| Age (years) | 63.8 ± 10.5 | 62.2 ± 11.4 | 64.8 ± 9.8 | 0.084 |
| BMI (kg/m2) | 26.3 ± 3.8 | 25.0 ± 3.5 | 27.1 ± 3.7 | <0.001 |
| ASA classification (n, %) | ||||
| 1 | 35 (16.1%) | 13 (16%) | 22 (16%) | 0.491 |
| 2 | 132 (60.6%) | 47 (56%) | 85 (63%) | |
| ≥3 | 51 (23.4%) | 23 (28%) | 28 (21%) | |
| Comorbidity (n, %) | 134 (62%) | 46 (55%) | 88 (65%) | 0.150 |
| Cardiovascular | 97 (45%) | 33 (40%) | 64 (47%) | 0.270 |
| Pulmonary | 21 (10%) | 9 (11%) | 12 (9%) | 0.635 |
| Renal | 7 (3%) | 4 (5%) | 3 (2%) | 0.431 |
| Diabetes mellitus | 19 (9%) | 3 (3.6%) | 16 (12%) | 0.036 |
| Other | 21 (10%) | 8 (10%) | 13 (10%) | 0.998 |
| Neoadjuvant chemotherapy (n, %) | 150 (69%) | 58 (69%) | 92 (68%) | 0.789 |
| Radical resection (n, %) | 145 (67%) | 59 (71%) | 86 (64%) | 0.262 |
| Fong‐score > 2 (n, %) | 72 (33%) | 26 (31%) | 46 (34%) | 0.675 |
| Synchronous metastasis (n, %) | 71 (33%) | 51 (61%) | 96 (71%) | 0.139 |
| Node positive primary (n, %) | 140 (64%) | 53 (64%) | 87 (64%) | 0.930 |
| Largest metastasis ≥ 5 cm (n, %) | 29 (13%) | 13 (16%) | 16 (12%) | 0.421 |
| >1 metastasis (n, %) | 128 (59%) | 47 (57%) | 81 (60%) | 0.623 |
| CEA ≥ 200 ng/mL (n, %) | 6 (3%) | 2 (2%) | 4 (3%) | 1.000 |
| Liver steatosis grade (n, %) | ||||
| 0 | 83 (38%) | 83 (100%) | — | <0.001 |
| 1 | 106 (48%) | — | 106 (78%) | |
| 2 | 21 (10%) | — | 21 (16%) | |
| 3 | 8 (4%) | — | 8 (6%) | |
| Tissue L3‐index (Z‐score) | ||||
| Muscle | 0 ± 1.0 | −0.20 ± 0.91 | 0.12 ± 1.00 | 0.022 |
| VAT | 0 ± 1.0 | −0.46 ± 0.91 | 0.28 ± 0.93 | <0.001 |
| SAT | 0 ± 1.0 | −0.30 ± 0.89 | 0.18 ± 1.00 | <0.001 |
| TAT | 0 ± 1.0 | −0.42 ± 0.92 | 0.26 ± 0.94 | <0.001 |
| Tissue radiation attenuation (Z‐score) | ||||
| Muscle | 0 ± 1.0 | 0.03 ± 1.05 | −0.02 ± 0.96 | 0.752 |
| VAT | 0 ± 1.0 | 0.43 ± 1.07 | −0.27 ± 0.83 | <0.001 |
| SAT | 0 ± 1.0 | 0.32 ± 1.22 | −0.20 ± 0.75 | 0.001 |
| ΔTissue L3‐index (%/100 days) a | ||||
| Muscle | −2.4 ± 9.7 | −1.5 ± 9.4 | −2.9 ± 9.9 | 0.425 |
| VAT | 10.4 ± 43.3 | 20.8 ± 60.2 | 3.7 ± 26.1 | 0.060 |
| SAT | 3.6 ± 20.2 | 7.4 ± 25.3 | 1.1 ± 15.8 | 0.114 |
| TAT | 5.1 ± 22.6 | 10.4 ± 30.6 | 1.7 ± 14.9 | 0.065 |
| ΔTissue radiation attenuation (%/100 days) a | ||||
| Muscle | 5.7 ± 23.3 | 6.3 ± 25.5 | 5.3 ± 21.9 | 0.801 |
| VAT | 1.2 ± 7.5 | 1.4 ± 9.3 | 1.1 ± 6.1 | 0.859 |
| SAT | 1.4 ± 7.8 | 1.5 ± 9.4 | 1.4 ± 6.7 | 0.948 |
Patient characteristics and differences in characteristics between non‐NAFLD and NAFLD patients. Continuous variables are depicted as mean ± standard deviation.
ASA, American Society of Anaesthesiologists; BMI, body mass index; CEA, carcinoembryonic antigen; NAFLD, non‐alcoholic fatty liver disease; SAT, subcutaneous adipose tissue; TAT, total adipose tissue; VAT, visceral adipose tissue.
Differences in body composition over time were assessed in 131 patients.
Body composition analysis
Sex‐specific and age‐specific preoperative body composition variables are shown in Table 2. In general, males were more muscular (P < 0.001) and had more visceral adipose tissue (P < 0.001), while females had more subcutaneous adipose tissue (P = 0.001). Adipose tissue mass and especially muscle fat content increased with age in both males and females (P = 0.001). Of the 131 patients with consecutive CT scans, 66 (59%), 77 (59%), 80 (61%), and 82 (63%) patients experienced ≥2% loss of skeletal muscle tissue, VAT, SAT, and TAT L3‐index, respectively. Concerning radiation attenuation, 56 (43%), 40 (31%), and 36 (28%) had a ≥2% decrease in respectively muscle tissue, VAT, and SAT. Correlations among the various body composition parameters are visualized in Figure 1. Adipose tissue L3‐indexes were highly correlated with adipose tissue radiation attenuation, but only mildly with muscle radiation attenuation. There were no strong correlations between single pre‐operative CT‐parameters and changes over time.
Table 2.
Sex‐specific and age‐specific body composition parameters assessed by computed tomography scan
| Sex | Age (years) | n (%) | L3‐index (cm2/m2) | Radiation attenuation (HU) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Muscle | VAT | SAT | TAT | Muscle | VAT | SAT | |||
| Male | <60 | 33 (15%) | 51.6 ± 10.0 | 55.9 ± 29.8 | 62.4 ± 30.6 | 118.3 ± 54.1 | 38.0 ± 7.0 | −93.0 ± 5.4 | −98.0 ± 8.9 |
| 60–70 | 67 (31%) | 49.9 ± 7.7 | 56.6 ± 28.8 | 50.9 ± 22.5 | 107.5 ± 44.6 | 34.0 ± 8.2 | −91.9 ± 6.8 | −94.3 ± 7.4 | |
| >70 | 42 (19%) | 49.0 ± 7.3 | 74.7 ± 29.2 | 60.0 ± 21.4 | 134.7 ± 42.1 | 31.3 ± 7.0 | −93.2 ± 5.7 | −93.6 ± 7.8 | |
| Female | <60 | 30 14(%) | 40.0 ± 5.1 | 28.2 ± 15.8 | 73.3 ± 25.6 | 101.6 ± 39.2 | 37.4 ± 9.2 | −88.5 ± 9.1 | −101.8 ± 6.0 |
| 60–70 | 30 (14%) | 40.2 ± 6.2 | 47.0 ± 30.9 | 94.8 ± 49.9 | 141.9 ± 73.9 | 32.9 ± 8.6 | −89.6 ± 9.0 | −99.5 ± 10.7 | |
| >70 | 16 (7%) | 38.2 ± 6.0 | 45.7 ± 19.4 | 86.3 ± 29.2 | 132.0 ± 43.0 | 26.7 ± 7.6 | −90.4 ± 6.9 | −98.7 ± 7.5 | |
Body composition parameters were assessed by analysing a single pre‐operative computed tomography slice at the level of the third lumbar vertebra (L3). Total tissue mass was estimated by assessing the total tissue area at L3 and dividing it by height squared. The radiation attenuation is the average Hounsfield unit value of the total tissue area at L3. A lower radiation attenuation is associated with increased tissue fat content. Values are depicted as mean ± standard deviation.
HU, Hounsfield units; SAT, subcutaneous adipose tissue; TAT, total adipose tissue; VAT, visceral adipose tissue.
Figure 1.
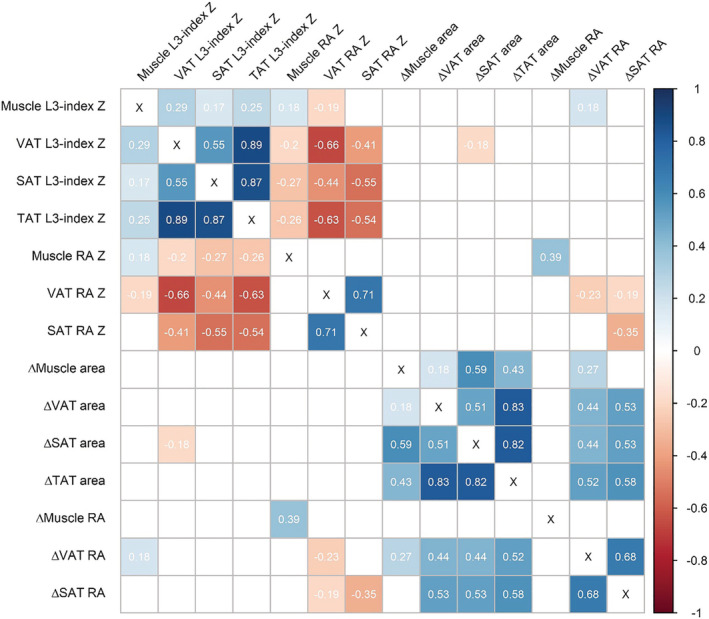
Correlation matrix of all CT body composition variables. Values represent Pearson correlation coefficients (r). Only significant correlations (<0.05) are shown, white squares indicate insignificant correlations. L3‐indexes are a representation of whole‐body tissue mass and have been Z‐scored for sex and age. Δ parameters indicate changes between two pre‐operative CT scans in %/100 days. CT, computed tomography; RA, radiation attenuation; SAT, subcutaneous adipose tissue; TAT, total adipose tissue; VAT, visceral adipose tissue.
Liver biopsies
According to the SAF score, 83 patients had no NAFLD, 133 patients had NAFLD without NASH, and two patients had NAFLD with NASH. Patient characteristics and differences between NAFLD and non‐NAFLD patients are shown in Table 1. The majority of NAFLD patients had mild steatosis (78%). NAFLD patients had diabetes mellitus and higher skeletal muscle significantly more often, and higher VAT, SAT, and TAT masses. VAT and SAT radiation attenuation were significantly lower in NAFLD patients (P < 0.001 and P = 0.001, respectively), whereas skeletal muscle tissue radiation attenuation was not different (P = 0.752). Figure 2 visualizes the co‐existence of increased fat content/low radiation attenuation in liver, skeletal muscle, and VAT. Twenty‐five per cent of patients had all three features (liver steatosis, myosteatosis, and high fat content in VAT).
Figure 2.
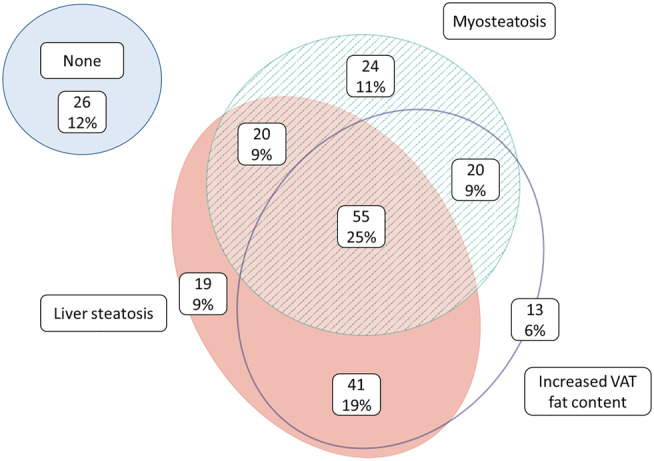
Venn diagram depicting the co‐existence of lipid accumulation in liver, skeletal muscle, and visceral adipose tissue. Numbers represent n patients. Liver steatosis was defined as at least steatosis Grade 1 on histology. Myosteatosis was defined as a skeletal muscle radiation attenuation Z‐score of <0. Increased VAT fat content was defined as a VAT radiation attenuation Z‐score of <0. VAT, visceral adipose tissue.
Survival analysis and major complications
In multivariable Cox‐regression analysis, NAFLD [hazard ratio (HR) 1.8, 95%‐confidence interval (CI) 1.0–3.0, P = 0.037], decreas in muscle radiation attenuation over time (HR 1.8, 95%‐CI 1.1–2.9, P = 0.018), and skeletal muscle index loss over time (HR 1.7, 95%‐CI 1.0–2.9, P = 0.035) were associated with shorter overall survival (Figure 3). In addition, VAT radiation attenuation Z‐score was associated with shorter overall survival (HR 1.4, 95%‐CI 1.1–1.9, P = 0.014) indicating that a high‐fat content of VAT was protective. NAFLD (HR 1.8, 95%‐CI 1.0–3.0, P = 0.021), decrease in muscle radiation attenuation over time (HR 1.8, 95%‐CI 1.1–3.0), and SAT radiation attenuation Z‐score (HR 1.5, 95%‐CI 1.2–2.0, P = 0.001) were associated with shorter disease‐free survival (Figure 4). In addition, radical resection was associated with longer overall survival and disease‐free survival (both HR 0.4, 95%‐CI 0.2–0.6, P < 0.001), while comorbidity was associated with shorter disease‐free survival (HR 2.2, 95%‐CI 1.2–4.3, P = 0.016). There were no significant associations between major complications and any of the studied variables in multivariable logistic regression analysis. VAT and SAT radiation attenuation had similar associations with both overall survival and disease‐free survival. As they were collinear, only one (VAT RA or SAT RA) variable was included per multivariable analysis (the variable with the strongest association).
Figure 3.
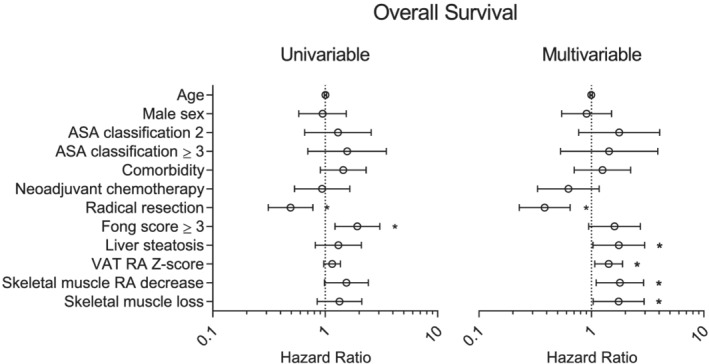
Cox regression analysis for overall survival. Liver steatosis and body composition variables were only kept in the multivariable model if P < 0.05. Liver steatosis was defined as a steatosis grade of ≥1. Z‐scores are included as continuous variables. Radiation attenuation (RA) is the average Hounsfield unit value of a certain type of tissue; a low RA is associated with a high tissue triglyceride content. Decrease/loss was defined as a Δ of <−2%/100 days. *P < 0.05. ASA, American Society of Anaesthesiologists; RA, radiation attenuation; VAT, visceral adipose tissue.
Figure 4.
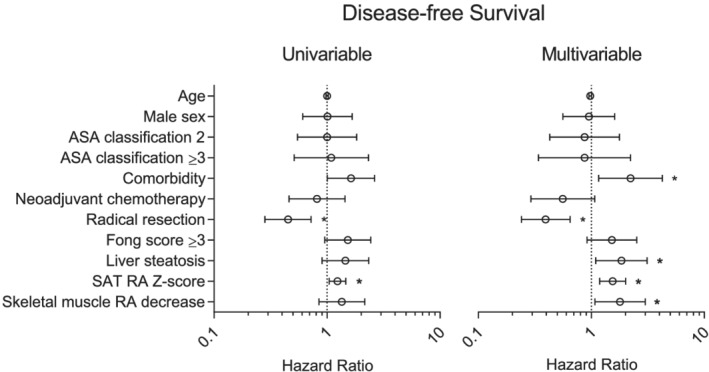
Cox‐regression analysis for disease‐free survival. Liver steatosis and body composition variables were eliminated from the multivariable model by stepwise backward elimination if P > 0.05. Liver steatosis was defined as a steatosis grade of ≥1. Z‐scores are included as continuous variables. Radiation attenuation (RA) is the average Hounsfield unit value of a certain type of tissue; a low RA is associated with a high tissue triglyceride content. Decrease was defined as a Δ of < −2%/100 days. *P < 0.05. ASA, American Society of Anaesthesiologists; RA, radiation attenuation; SAT, subcutaneous adipose tissue.
Discussion
In this study, we found that ectopic fat deposition in liver and pre‐operative decrease in skeletal muscle radiation attenuation over time is independently associated with shorter overall and disease‐free survival after liver resection for CRLM, while a high adipose tissue fat content on the pre‐operative CT scan was related to increased survival. Whereas we expected that steatosis of liver and skeletal muscle tissue would occur simultaneously, they can occur independently from each other. Liver steatosis was associated with increased adipose tissue fat content, whereas myosteatosis was not, suggesting a different aetiology.
Liver steatosis has been studied mostly in non‐oncologic patients with obesity‐associated NAFLD. Liver steatosis is highly related to obesity, especially visceral adiposity. 28 A tracer study in obese NAFLD patients demonstrated that around 60% of liver triglycerides are formed from adipose tissue‐derived fatty acids; de novo lipogenesis contributed up to 25% (five times more than in healthy individuals), possibly due to insulin resistance. 29 Myosteatosis has been studied less extensively than liver steatosis. There is a low correlation between myosteatosis and obesity. 30 Goodplaster et al. found that myosteatosis was associated with insulin resistance independent of visceral fat in non‐oncologic subjects. 31 In colorectal cancer patients, myosteatosis has been associated with systemic inflammation. 17 While we did not assess systemic inflammation in the present study, we recently demonstrated that myosteatosis was associated with elevated serum C‐reactive protein levels as well as increased visceral fat in patients with CRLM, suggesting multiple possible aetiologies of myosteatosis. 7 As systemic inflammation is a hallmark of cancer cachexia, 32 its relationship with myosteatosis warrants further investigation.
One of the key findings of the present study was that the location of fat storage (adipose tissue vs. ectopic) showed opposing associations with survival. The protective effect of high adipose tissue fat content is a relatively new finding as most other studies on body composition did not report on adipose tissue radiation attenuation. Silvério et al. found up‐regulated expression hormone‐sensitive lipase and adipose triglyceride lipase in white adipose tissue of patients with cancer cachexia, 33 which could potentially lead to triglyceride depletion (and increased radiation attenuation). Adipose tissue mass had a similar association with survival, as is to be expected with a correlation of −0.66 between VAT radiation attenuation and VAT L3‐index. We previously reported lower overall survival in CRLM patients with a low VAT L3‐index in a different cohort. 7 In addition, Ebadi et al. found that in a cohort of 1746 cancer patients, a low SAT L3‐index was independently associated with poor survival. 34 Moreover, in the presence of sarcopenia, patients with high SAT L3‐index had the longest survival. Furthermore, Choe et al. reported that patients with colorectal cancer who had an increase in VAT after surgery had better overall survival. 35 In contrast, van Vledder et al. found a decreased disease‐free survival in men with CRLM and central obesity, but they did not perform a multivariable analysis for this variable. 8 Lee et al. reported decreased overall survival in colorectal cancer patients with visceral obesity. 36 However, this cohort is difficult to compare with our patient cohort as they studied colorectal cancer patients without liver metastasis in an adjuvant chemotherapy setting. To our knowledge, there are no studies that assessed changes in skeletal muscle fat content over time preoperatively, although many studies reported that baseline myosteatosis was associated with decreased survival in patients with lung, pancreatic, hepatocellular, or colorectal cancer. 14 , 15 , 16 In line with the results in the present study, we did not find an association between preoperative myosteatosis based on a single‐CT scan analysis and survival in CRLM patients in a previous study. 7 A possible explanation could be that CRLM patients are generally not severely cachectic but rather in a pre‐cachectic state without profound myosteatosis. The patients with an increase in skeletal muscle fat content before surgery could be progressing towards profound myosteatosis, possibly explaining the observed association with survival in the present study.
Previously, the relationship between body composition and liver steatosis had only been studied in non‐oncologic patients with NAFLD, almost exclusively in Korea. Lee et al. found in a cross‐sectional nationwide study including 15 132 subjects that sarcopenia was associated with NAFLD 20 and liver fibrosis 37 independent of obesity and insulin resistance. However, they used a NAFLD prediction model for identification of NAFLD instead of liver biopsies. In a biopsy proven NAFLD cohort, Koo et al. found that sarcopenia was independently associated with NASH and liver fibrosis. 38 However, they used bioelectrical impedance analysis for body composition assessment, which can overestimate as well as underestimate skeletal muscle mass compared with CT scan or dual‐energy X‐ray absorptiometry based analysis. 24 Other studies in NAFLD patients reported similar associations between sarcopenia and NAFLD/NASH. 39 , 40 , 41 In the present study, we did not find a relationship between skeletal muscle mass and liver steatosis (NAFLD). This may be explained by the following: (i) we assessed liver steatosis and skeletal muscle mass by different methods, applying the gold standard techniques; (ii) we studied a cohort of CRLM patients who were not necessarily obese or at risk of having liver steatosis, resulting in lower grades of liver steatosis (79% mild) and only two patients with NASH; (iii) as patients with high adipose tissue mass also tend to have high skeletal muscle mass, 7 patients with liver steatosis are likely to have both more adipose tissue and more skeletal muscle tissue. This also explains why patients with diabetes mellitus in our cohort had a relatively high skeletal mass, which is in contrast with a study by Park et al. who found lower skeletal muscle mass in (non‐oncologic) patients with type 2 diabetes mellitus. 42 Because patients with diabetes are more likely to be obese, they also are more likely to have more skeletal muscle mass, as is the case in our cohort. Thus, to assess body composition in the diabetes mellitus subgroup, skeletal muscle mass should be corrected for adipose tissue mass which was not possible considering the small number of patients with diabetes in our cohort (n = 19). Nevertheless, this shows the importance of elaborate assessment of patients (i.e. deep phenotyping). While we found that increased adipose tissue fat content has a favourable association with overall survival (in‐line with the obesity‐paradox 43 ), we also found that liver steatosis was associated with worse survival, while liver steatosis in turn was associated with increased adipose tissue mass and fat content. Adipose tissue mass can consequently be low or high relative to the patient's sex, age, race, and inflammatory status. This illustrates the complex different pathophysiological mechanisms at work in a single patient and advocates that body composition should never be solely assessed without taking other confounding characteristics into account.
The importance of collecting prospective data at multiple time points is highlighted in the present study. Single time‐point body composition parameters such as sarcopenia and myosteatosis can often be misinterpreted as muscle loss. Our data indicate that there is little to no correlation between pre‐operative body composition determined using a single‐CT scan analysis and tissue loss or gain over time. Few studies have evaluated body composition changes over time, all in a chemotherapy setting instead of a pre‐operative setting like in the present study. Rutten et al. assessed CT scans before and after neoadjuvant chemotherapy for ovarian cancer and found that both skeletal muscle loss and VAT loss were associated with shorter overall survival. 25 Blauwhoff‐Buskermolen et al. only assessed skeletal muscle mass in colorectal cancer patients before and after receiving chemotherapy and also found an association with overall survival. 44 Our findings are in line with these studies because we also found an association between skeletal muscle loss and overall survival. We could not detect an association with VAT loss, as was described by Rutten et al., but the latter did not assess changes in VAT radiation attenuation in their model, possibly explaining this difference.
The present study has some limitations that should be considered. Firstly, it should be noted that by only selecting patients with two available pre‐operative CT scans, we induced some selection bias as these patients were more likely to receive neoadjuvant chemotherapy, for which we corrected in the multivariable analysis. Secondly, we used different modalities to assess steatosis in skeletal muscle (CT scan) and liver (histologic assessment). The reason we used histologic assessment for liver biopsies is that this still is the gold standard. 45 However, recent advancements in non‐contrast‐enhanced CT scan analysis of liver steatosis have shown it to be an acceptable non‐invasive alternative for histologic assessment. 46 , 47 Unfortunately, non‐contrast‐enhanced CT scans were not available for most patients. Nonetheless, this is a promising technique to be used in future studies as it is non‐invasive, does not suffer from sampling error, and could be used to assess changes over time. Finally, we used adipose tissue radiation attenuation as a marker of adipose tissue fat content. The assumption that low adipose tissue radiation attenuation reflects high‐fat content is reasonable because a high correlation between CT adipose tissue radiation attenuation and adipocyte size (assessed on tissue biopsies) has been reported in obese individuals. 13 However, few studies 7 , 48 on adipose tissue radiation attenuation have been conducted in cancer patients and studies comparing CT‐radiation attenuation with tissue biopsy triglyceride content should be conducted to further support our findings.
In conclusion, we found that ectopic fat content in liver as well as skeletal muscle tissue is independently associated with shorter overall survival in patients with CRLM, while increased visceral adipose tissue fat content is associated with longer overall survival. Assessing body composition changes over time adds valuable information to regular single time‐point body composition assessment and should be encouraged in future studies in both perioperative and palliative settings.
Funding
David van Dijk is supported as a PhD candidate by the Netherlands Organization for Scientific Research (NWO Grant 022.003.011).
Conflict of interest
D.D., J.Z., K.K., V.B., C.D., S.R., and S.O.D. declare that they have no conflict of interest.
Ethical statement
The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 49
van Dijk D. P. J., Zhao J., Kemter K., Baracos V. E., Dejong C. H. C., Rensen S. S., and Olde Damink S. W. M. (2021) Ectopic fat in liver and skeletal muscle is associated with shorter overall survival in patients with colorectal liver metastases, Journal of Cachexia, Sarcopenia and Muscle, 12, 983–992, 10.1002/jcsm.12723
References
- 1. Jemal A, Bray F, Center M, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- 2. van de Velde CJH. Treatment of liver metastases of colorectal cancer. Ann Oncol 2006;17:727. [DOI] [PubMed] [Google Scholar]
- 3. Weiss L, Grundmann E, Torhorst J, Hartveit F, Moberg I, Eder M, et al. Haematogenous metastatic patterns in colonic carcinoma: an analysis of 1541 necropsies. J Pathol 1986;150:195–203. [DOI] [PubMed] [Google Scholar]
- 4. Rees M, Tekkis PP, Welsh FK, O'Rourke T, John TG. Evaluation of long‐term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg 2008;247:125–135. [DOI] [PubMed] [Google Scholar]
- 5. de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick RD, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi‐institutional analysis of 1669 patients. Ann Surg 2009;250:440–448. [DOI] [PubMed] [Google Scholar]
- 6. Morris EJ, Forman D, Thomas JD, Quirke P, Taylor EF, Fairley L, et al. Surgical management and outcomes of colorectal cancer liver metastases. Br J Surg 2010;97:1110–1118. [DOI] [PubMed] [Google Scholar]
- 7. Van Dijk DP, Krill M, Farshidfar F, Li T, Rensen SS, Olde Damink SW, et al. Host phenotype is associated with reduced survival independent of tumor biology in patients with colorectal liver metastases. J Cachexia Sarcopenia Muscle 2019;10:123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Vledder MG, Levolger S, Ayez N, Verhoef C, Tran TC, Ijzermans JN. Body composition and outcome in patients undergoing resection of colorectal liver metastases. Br J Surg 2012;99:550–557. [DOI] [PubMed] [Google Scholar]
- 9. Peng PD, van Vledder MG, Tsai S, De Jong MC, Makary M, Ng J, et al. Sarcopenia negatively impacts short‐term outcomes in patients undergoing hepatic resection for colorectal liver metastasis. HPB (Oxford) 2011;13:439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aubrey J, Esfandiari N, Baracos VE, Buteau FA, Frenette J, Putman CT, et al. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol (Oxf) 2014;210:489–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol (1985) 2000;89:104–110. [DOI] [PubMed] [Google Scholar]
- 12. Dadson P, Rebelos E, Honka H, Juárez‐Orozco LE, Kalliokoski KK, Iozzo P, et al. Change in abdominal, but not femoral subcutaneous fat CT‐radiodensity is associated with improved metabolic profile after bariatric surgery. Nutr Metab Cardiovasc Dis 2020;30:2363–2371. [DOI] [PubMed] [Google Scholar]
- 13. Côté JA, Nazare JA, Nadeau M, Leboeuf M, Blackburn L, Després JP, et al. Computed tomography‐measured adipose tissue attenuation and area both predict adipocyte size and cardiometabolic risk in women. Adipocyte 2016;5:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martin L, Birdsell L, MacDonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013;31:1539–1547. [DOI] [PubMed] [Google Scholar]
- 15. van Dijk DP, Bakens MJ, Coolsen MMM, Rensen SS, van Dam RM, Bours MJ, et al. Low skeletal muscle radiation attenuation and visceral adiposity are associated with overall survival and surgical site infections in patients with pancreatic cancer. J Cachexia Sarcopenia Muscle 2017;8:317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fujiwara N, Nakagawa H, Kudo Y, Tateishi R, Taguri M, Watadani T, et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol 2015;63:131–140. [DOI] [PubMed] [Google Scholar]
- 17. Malietzis G, Johns N, Al‐Hassi HO, Knight SC, Kennedy RH, Fearon KC, et al. Low muscularity and myosteatosis is related to the host systemic inflammatory response in patients undergoing surgery for colorectal cancer. Ann Surg 2016;263:320–325. [DOI] [PubMed] [Google Scholar]
- 18. Miljkovic I, Cauley JA, Wang PY, Holton KF, Lee CG, Sheu Y, et al. Abdominal myosteatosis is independently associated with hyperinsulinemia and insulin resistance among older men without diabetes. Obesity (Silver Spring) 2013;21:2118–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Deivanayagam S, Mohammed BS, Vitola BE, Naguib GH, Keshen TH, Kirk EP, et al. Nonalcoholic fatty liver disease is associated with hepatic and skeletal muscle insulin resistance in overweight adolescents. Am J Clin Nutr 2008;88:257‐62‐62–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee Y‐H, Jung K, Kim S, Yoon HJ, Yun YJ, Lee BW, et al. Sarcopaenia is associated with NAFLD independently of obesity and insulin resistance: nationwide surveys (KNHANES 2008–2011). J Hepatol 2015;63:486–493. [DOI] [PubMed] [Google Scholar]
- 21. van den Broek MA, van Dam RM, van Breukelen GJ, Bemelmans MH, Oussoultzoglou E, Pessaux P, et al. Development of a composite endpoint for randomized controlled trials in liver surgery. Br J Surg 2011;98:1138–1145. [DOI] [PubMed] [Google Scholar]
- 22. Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bedossa P, Consortium F. Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatology (Baltimore, Md) 2014;60:565–575. [DOI] [PubMed] [Google Scholar]
- 24. Mourtzakis M, Prado CMM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 2008;33:997–1006. [DOI] [PubMed] [Google Scholar]
- 25. Rutten IJG, Van Dijk DPJ, Kruitwagen RFPM, Beets‐Tan RGH, Olde Damink SWM, Van Gorp T. Changes in skeletal muscle mass during neoadjuvant chemotherapy are related to survival in ovarian cancer. J Cachexia Sarcopenia Muscle 2016;7:458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schemper M, Smith TL. A note on quantifying follow‐up in studies of failure time. Control Clin Trials 1996;17:343–346. [DOI] [PubMed] [Google Scholar]
- 27. Micallef L, Rodgers P. eulerAPE: drawing area‐proportional 3‐Venn diagrams using ellipses. PLoS ONE 2014;9:e101717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marchesini G, Moscatiello S, Di Domizio S, Forlani G. Obesity‐associated liver disease. J Clin Endocrinol Metab 2008;93:80. [DOI] [PubMed] [Google Scholar]
- 29. Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 2005;115:1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr 2000;71:885–892. [DOI] [PubMed] [Google Scholar]
- 31. Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes 1997;46:1579–1585. [DOI] [PubMed] [Google Scholar]
- 32. Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab 2012;16:153–166. [DOI] [PubMed] [Google Scholar]
- 33. Silvério R, Lira FS, Oyama LM, do Nascimento CM, Otoch JP, Alcântara PS, et al. Lipases and lipid droplet‐associated protein expression in subcutaneous white adipose tissue of cachectic patients with cancer. Lipids Health Dis 2017;16:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ebadi M, Martin L, Ghosh S, Field CJ, Lehner R, Baracos VE, et al. Subcutaneous adiposity is an independent predictor of mortality in cancer patients. Br J Cancer 2017;117:148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Choe EK, Park KJ, Ryoo SB, Moon SH, Oh HK, Han EC. Prognostic impact of changes in adipose tissue areas after colectomy in colorectal cancer patients. J Korean Med Sci 2016;31:1571–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee CS, Murphy DJ, McMahon C, Nolan B, Cullen G, Mulcahy H, et al. Visceral adiposity is a risk factor for poor prognosis in colorectal cancer patients receiving adjuvant chemotherapy. J Gastrointest Cancer 2015;46:243–250. [DOI] [PubMed] [Google Scholar]
- 37. Lee Y, Kim S, Song K, Park JY, Kim DY, Ahn SH, et al. Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: nationwide surveys (KNHANES 2008‐2011). Hepatology 2016;63:776–786. [DOI] [PubMed] [Google Scholar]
- 38. Koo BK, Kim D, Joo SK, Kim JH, Chang MS, Kim BG, et al. Sarcopenia is an independent risk factor for non‐alcoholic steatohepatitis and significant fibrosis. J Hepatol 2017;66:123–131. [DOI] [PubMed] [Google Scholar]
- 39. Hong HC, Hwang SY, Choi HY, Yoo HJ, Seo JA, Kim SG, et al. Relationship between sarcopenia and nonalcoholic fatty liver disease: the Korean Sarcopenic Obesity Study. Hepatology (Baltimore, Md) 2014;59:1772–1778. [DOI] [PubMed] [Google Scholar]
- 40. Petta S, Ciminnisi S, di Marco V, Cabibi D, Cammà C, Licata A, et al. Sarcopenia is associated with severe liver fibrosis in patients with non‐alcoholic fatty liver disease. Aliment Pharmacol Ther 2017;45:510–518. [DOI] [PubMed] [Google Scholar]
- 41. Kim H, Kim C, Park C‐H, Choi JY, Han K, Merchant AT, et al. Low skeletal muscle mass is associated with non‐alcoholic fatty liver disease in Korean adults: the Fifth Korea National Health and Nutrition Examination Survey. Hepatobiliary Pancreat Dis Int 2016;15:39–47. [DOI] [PubMed] [Google Scholar]
- 42. Park SW, Goodpaster BH, Lee JS, Kuller LH, Boudreau R, De Rekeneire N, et al. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care 2009;32:1993–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Strulov Shachar S, Williams GR. The obesity paradox in cancer‐moving beyond BMI. Cancer Epidemiol Biomarkers Prev 2017;26:13–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Blauwhoff‐Buskermolen S, Versteeg KS, de van der Schueren MA, den Braver NR, Berkhof J, Langius JAE, et al. Loss of muscle mass during chemotherapy is predictive for poor survival of patients with metastatic colorectal cancer. J Clin Oncol Off J Am Soc Clin Oncol 2016;34:1339–1344. [DOI] [PubMed] [Google Scholar]
- 45. Kogachi S, Noureddin M. Noninvasive evaluation for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Clin Ther 2021;43:455–472. [DOI] [PubMed] [Google Scholar]
- 46. Pickhardt PJ, Graffy PM, Reeder SB, Hernando D, Li K. Quantification of liver fat content with unenhanced MDCT: phantom and clinical correlation with MRI proton density fat fraction. AJR Am J Roentgenol 2018;211:W151–w7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Graffy PM, Sandfort V, Summers RM, Pickhardt PJ. Automated liver fat quantification at nonenhanced abdominal CT for population‐based steatosis assessment. Radiology 2019;293:334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. West MA, van Dijk DPJ, Gleadowe F, Reeves T, Primrose JN, Abu Hilal M, et al. Myosteatosis is associated with poor physical fitness in patients undergoing hepatopancreatobiliary surgery. J Cachexia Sarcopenia Muscle 2019;10:860–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]


