Abstract
Background
Elderly classical Hodgkin lymphoma (cHL) (ecHL) is a rare disease with dismal prognosis and no standard treatment. Fitness‐based approaches may help design appropriate treatments. Sarcopenia has been associated with an increased risk of treatment‐related toxicities and worse survival in various solid tumours, but its impact in ecHL is unknown. The aim of this retrospective multicentre study was to investigate the prognostic role of sarcopenia in ecHL.
Methods
We included newly diagnosed >64 years old cHL patients who performed a baseline comprehensive geriatric assessment and high‐dose computed tomography (CT) or 18fluorine‐fluorodeoxyglucose positron emission tomography/CT before any treatment. Sarcopenia was measured as skeletal muscle index (SMI, cm2/m2) by the analysis of high‐dose CT or low‐dose positron emission tomography/CT images at the L3 level. The specific cut‐offs for the SMI were determined by receiver operator curve analysis and compared with those proposed in literature and studied in diffuse large B‐cell lymphoma. Survival functions [progression‐free survival [PFS] and overall survival (OS)] were calculated for the whole population and for different subgroups defined as per different sarcopenia cut‐off levels.
Results
We included 154 patients (median age 71 years old, 76 female). The median L3‐SMI was 42 cm2/m2. The specific cut‐off derived in our male population was 45 cm2/m2; using this cut‐off, 27 male patients (35%) were defined as sarcopenic. After a median follow‐up of 5.9 years, the overall 5‐year PFS and OS rates were 53% and 65%, respectively, and were significantly shorter in sarcopenic male patients compared with non‐sarcopenic (PFS 31% vs. 61%, P = 0.008; OS 51% vs. 74%, P = 0.042). Applying diffuse large B‐cell lymphoma‐derived sarcopenic thresholds, there were no significant differences between sarcopenic and non‐sarcopenic patients for both PFS and OS, with a sole exception of a significant reduced PFS in sarcopenic male patients using Namakura cut‐off. The comprehensive geriatric assessment‐determined frail functional status was an independent adverse prognostic factor for both female and male patients.
Conclusions
Baseline evaluation of sarcopenia through radiological examinations performed for ecHL staging may help define a proportion of male patients with unfavourable outcome with current treatment strategies. Also the functional status evaluation could allow to identify a frail subgroup of patients with worse outcome.
Keywords: Hodgkin lymphoma, Sarcopenia, Geriatric assessment, Elderly
Introduction
The incidence of classical Hodgkin lymphoma (cHL) in Western countries is around 2–2.5 cases per 100 000 inhabitants per year, with a mild prevalence in male individuals. 1 Among recent data from the Surveillance, Epidemiology, and End Results (SEER) programme, evidence of an age‐related bi‐modal incidence pattern in cHL persists, and the incidence of cHL in patients ≥ 65 years is 4.0/100 000 per year. 2 cHL in elderly patients is still a rare condition, but in the next years, the proportion of people ≥65 years will become much larger as a consequence of the expected increase in median life in Western countries.
The prognosis of elderly cHL patients is much worse than that of younger cHL patients. Many studies provide evidence that the unfavourable outcome of elderly cHL patients is partly related to clinical and biological differences of the disease itself, such as more frequent advanced stage, B symptoms, infra‐diaphragmatic presentation, elevated erythrocyte sedimentation rate levels, higher prevalence of mixed cellularity histopathology and Epstein‐Barr virus infection. 3 , 4 , 5 Moreover, elderly cHL patients present specific age‐related conditions and comorbidities that could increase the risk of complications after anti‐tumour therapy and compromise the prognosis.
A standard treatment for elderly cHL patients does not exist, even in first line setting. The ABVD regimen (adriamycin, bleomycin, vinblastine, dacarbazine) is considered gold standard for young cHL patients, but has significant toxicities in the elderly that limit its use above the age of 60–65 years. The use of a less intensive approach translates into a reduction of the expected toxicity, but unfortunately, it is counterbalanced by a non‐optimal efficacy. 6 , 7 , 8 , 9 For that reason, the attention of many authors has focused on trying to ameliorate the ABVD scheme, by substituting or removing single drugs and/or by combining an ABVD backbone with the anti‐CD30 monoclonal antibody brentuximab vedotin. 10 , 11
A tailored treatment, based on patient's fitness status, could help successfully balance efficacy and toxicity of chemotherapy. For this purpose the Fondazione Italiana Linfomi (FIL) has adopted a simplified comprehensive geriatric assessment (sCGA) based on a model that divides patients into three categories (FIT, UNFIT, and FRAIL) classified according to age, activities of daily living (ADL), instrumental ADL (IADL), and the Cumulative Illness Rating Scale for Geriatrics (CIRS‐G). In the FIL experience, the age cut‐off for the definition of elderly patients was 65 years. The FIL Elderly Project has recently validated this model in a prospective multicentre observational study in the setting of diffuse large B‐cell lymphoma (DLBCL) patients. 12 , 13 , 14
Alongside the use of these geriatric scales, in recent years the evaluation of the so‐called ‘sarcopenia’ (Greek ‘sarx’ or flesh + ‘penia’ or loss) 15 has been introduced as a parameter related to patient's frailty. Sarcopenia is now considered a syndrome characterized by a progressive and generalized loss of skeletal muscle mass and strength with an increased risk of adverse outcomes such as physical disability, poor quality of life and death. 16 In 2010, the European Working Group on Sarcopenia in Older People (EWGSOP) developed a practical clinical definition and consensus diagnostic criteria for age‐related sarcopenia that were updated in 2018. 17 , 18 The EWGSOP recommends using the presence of both low muscle mass and low muscle function (strength or performance) for the diagnosis of sarcopenia. Specifically, sarcopenia is probable when low muscle strength is detected; the diagnosis is confirmed by the presence of low muscle quantity or quality. 18
A wide variety of tests and tools are now available for the characterization of sarcopenia in clinical practice and in research settings. Computed tomography (CT) and magnetic resonance imaging, routinely used in staging of malignancies, are still considered the gold standard for non‐invasive assessment of muscle quantity/mass. 19 The use of CT has several advantages, such as accurate quantification of muscle mass, differentiation between fat and muscle, and wide availability as a routine test in cancer. Muscle mass can be reported as muscle cross‐sectional area of specific muscle groups or body locations [skeletal muscle area (SMA)], adjusted for body size in different ways, that is, using height squared (SMA/height2), weight (SMA/weight) or body mass index (SMA/BMI). In particular, CT images of a specific lumbar vertebral landmark (L3) significantly correlated with whole‐body muscle. 20 , 21 Muscle quality is also impaired in sarcopenia; this term has been used to describe microscopic and macroscopic aspects of muscle architecture and composition. 22 Magnetic resonance imaging, CT, and bioelectrical impedance analysis have been used to assess muscle quality in research settings, but data are limited and controversial. 23 There are so far no data about the role of positron emission tomography (PET), known to be the best imaging for stage definition in cHL, in the evaluation of sarcopenia.
Sarcopenia, in most cases evaluated by CT, is known to be associated with an increased risk of treatment‐related toxicity and worse survival outcome in various solid tumours. 24 , 25 , 26 Very few studies have been published so far about the role of sarcopenia in elderly patients with lymphoma. In a French study of 82 elderly patients with DLBCL treated with R‐CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone) or R‐miniCHOP, sarcopenic cases had a higher revised International Prognostic Index (R‐IPI) and a higher risk of death. 27 In a Japanese study of 207 DLBCL patients treated with R‐CHOP or R‐CHOP‐like regimens, male (but not female) sarcopenic subjects had worse progression‐free survival (PFS). 28 A Korean study of 187 DLBCL patients treated with R‐CHOP showed that treatment‐related mortality (21.7% vs. 5%, P = 0.002) and early discontinuation of therapy (32.6% vs. 14.9%, P = 0.008) were more common in the sarcopenic group compared with the non‐sarcopenic group; the 5 year PFS (35.3% vs. 65.8%, P < 0.001) and the 5 year overall survival (OS) (37.3% vs. 68.1%, P < 0.001) were significantly lower in the sarcopenic group. 29 In multivariate analysis, sarcopenia was one of the independent poor prognostic factors for PFS and OS. By contrast, in a subgroup analysis of a large retrospective Canadian experience, elderly DLBCL sarcopenic patients seem to perform better than non‐sarcopenic ones. 30
As far as we know, there are no published studies regarding the role of sarcopenia in elderly patients affected with cHL. The aim of this study was to describe the prevalence of sarcopenia in elderly cHL patients, and to evaluate the impact of sarcopenic status on patients' survival.
Methods
Patients
We retrospectively analysed all elderly patients, defined as age >64 years, diagnosed with cHL and consecutively evaluated between January 2006 and December 2018 at the four participating centres. All >64‐year‐old patients with untreated cHL and availability of a pre‐treatment CT scan or PET/CT scan were included. This study was approved by the ethical committees of the participating centres. Patients were staged and treated accordingly to local practice; patients who did not receive any anti‐cancer treatment or who received radiotherapy only were included in this analysis.
18Fluorine‐fluorodeoxyglucose positron emission tomography/computed tomography imaging
All available data on baseline 18fluorine‐fluorodeoxyglucose (18F‐FDG) PET/CT (before any treatment), interim PET/CT (after two to four cycles of chemotherapy), and end‐of‐treatment PET/CT have been recorded.
The 18F‐FDG PET/CT acquisition was performed according to standard operating procedures, 31 using a Discovery STE (D‐STE) or Discovery 690 (D‐690) scanner (General Electric, Milwaukee, WI, USA), or on a Biograph TruePoint PET/CT tomograph (Siemens Medical Solutions, Erlangen, Germany). For all scanners, a standard non‐contrast free‐breathing helical low‐dose CT was obtained for attenuation correction and anatomical correlation. The D‐STE acquisition parameters were 120 kV, fixed tube current ~73 mAs (40–160 mAs), eight slices × 3.75 mm and 3.27 mm interval, pitch 1:5, tube rotation 0.8 s. The D‐690 and Biograph TruePoint acquisition parameters were 120 kV, fixed tube current ~60 mAs (40–100 mAs), 64 slices × 3.75 mm and 3.27 mm interval, pitch 0.984:1, tube rotation 0.5 s. The PET/CT images were reconstructed using a 512 × 512 matrix and iterative reconstruction, 3.75 mm slice thickness and 3.25 mm interval, standard filter with a window setting with 400 Hounsfiled units (HU) of window width, and 40 HU of window level.
The PET images were analysed both visually by expert nuclear medicine physicians, and interim PET/CT was interpreted applying the Deauville 5‐point scale and treatment response was defined according to the Lugano classification 32 , 33 ; 18F‐FDG PET/CT were judged as complete metabolic response for Deauville scores 1–3 and not complete metabolic response for Deauville scores 4 and 5.
Computed tomography imaging
High‐dose CT imaging was performed on different local scanners of different CT manufacturers: General Electric, Siemens, Toshiba, and Philips scanner. The acquisition parameters were as follows: tube voltage settings selected were 100, 120, and 130 kV, and tube current in a range of values from 65 to 389 mAs. The CT images were reconstructed using a 512 × 512 matrix and standard filter. Contrast enhancement venous phase images were used for the measurements in all cases.
Imaging analysis
High‐dose CT or low‐dose CT of PET/CT images were analysed by two researchers for the measurements of adipose and muscular tissues using Slice‐O‐Matic software V4.2 (Montreal, Quebec, Canada Tomovision), which enables specific tissue demarcation using the previously reported HU thresholds.
Skeletal muscle area was assessed from a single axial slice at the third lumbar (L3) level considering psoas, paraspinal, abdominal transverse rectum, internal, and external obliques muscles, because the skeletal muscle in this region has been shown to represent to the whole‐body tissue quantities. 27 To measure the SMA (cm2), CT HU thresholds ranged from −29 to 150 were considered. Subsequently, SMA was normalized for stature, as has been reported elsewhere 20 and was referred to as the L3 skeletal muscle index (L3 SMI, in cm2/m2).
For those cases for whom digital records of high‐dose CT were unavailable, low‐dose CT of PET/CT scans were used as reference to perform these measurements. This choice was taken due to the optimal reproducibility and agreement between low‐dose and high‐dose CT in adipose and muscular measurements in our experience (data not published).
As previously studied in DLBCL population, 27 , 28 to identify sarcopenia, we considered gender‐specific cut‐offs of SMI of 39 and 34.4 cm2/m2 for female and 55 cm2/m2 and 47.1 cm2/m2 for male patients. Moreover, we calculated a specific threshold of SMI for our population applying receiver operator curve (ROC) analysis and we used it also for out analyses.
Clinical data collection and definitions
Clinical data were collected by reviewing medical records. Collected data included demographics (year of birth, gender), patient‐related data at diagnosis (age at diagnosis, height, weight, BMI, Eastern Cooperative Oncology Group Performance Status), disease‐related data at diagnosis (histology, Ann Arbor stage, presence of B symptoms, bulk disease, extranodal sites, lactic acid dehydrogenase (LDH) level normal vs. elevated, Hasenclever score), treatment‐related data [chemotherapy, combined modality treatment, radiotherapy (RT) only, no treatment; among chemotherapy ABVD‐like treatments vs. others]. Data were recorded also regarding functional status evaluation at baseline: patients were categorized as FIT, UNFIT, or FRAIL according to comprehensive geriatric assessment (CGA) evaluation. 12 For those patients for whom a CGA was not performed, data on ADL, IADL, or CIRS‐G were recorded if available. Focusing on sarcopenia, 18 muscle mass data (as described earlier), and metabolic data on PET/CT scans (data not presented here) were collected. Finally, follow‐up data included last visit date, disease, and survival status at last visit.
Statistical analysis
Continuous covariates were dichotomized with the cut‐off reported in literature. The categorical variables were summarized as absolute and percentage frequencies, and comparison by groups were performed using the Fisher's exact test and, if appropriate, χ 2 test.
The endpoints of the study were the PFS, the OS, and the disease‐free survival (DFS). The PFS was measured from the date of diagnosis to the date of progression or relapse or death for any cause; patients alive at last clinical contact were considered censored. The OS was measured from the date of diagnosis to the date of death for any cause or last clinical contact. The DFS was measured from the date of first complete remission to the date of progression or relapse or death from any cause; patients alive at last clinical contact were considered censored. The survival functions were estimated and plotted using the Kaplan–Meier methodology, with the 95% confidence interval (95% CI). Comparison by groups were performed by log‐rank test, and the effect of covariates were estimates as hazard ratio (HR) by means of Cox proportional hazard (PH) regression, with 95% CI, either in univariable or multivariable analysis. The proportionality of hazard assumption was checked after the Cox regression by means of scaled Schoenfeld residuals. From the analysis of the ROC at 5 years of follow‐up, the optimal cut‐point maximizing the Youden function was estimated. Further, the log (HR) as function of SMI in continuous form was evaluated, by means of Cox PH restricted cubic spline regression. We assessed the importance of predictors based on their bootstrap inclusion fractions, when 1000 replications were run in the Cox PH model. We used a backward elimination selection approach with a cut‐off of 0.05. 34 All statistical tests were two‐sided.
Results
Patients characteristics
A total of 167 patients were included according to the eligibility criteria and L3‐SMI was evaluable in 154 (study population) out of 167. The study population comprised 76 female and 78 male patients, with a median age at diagnosis of 71 years, with 15% of patients aged 80 years or older. Almost half of the patients (47%) presented with B symptoms, advanced stage disease was present in 68%, extranodal involvement was present in 39%, while bulky disease was rare (8%).
Comprehensive geriatric assessment evaluation was performed in 122 out of 154 patients: 66% were FIT, 16% UNFIT, and 18% FRAIL.
Median BMI was 24.3 kg/m2: 57% were normal, 36% overweight, and 7% obese.
The median L3‐SMI was 42 cm2/m2.
Most patients (77%) were treated with ABVD‐like chemotherapy, while 20% received other (less intensive) chemotherapy and 3% received RT only. Apart from the five patients who received RT only, 23% of patients were treated with combined‐modality therapy.
Table 1 summarizes the clinical characteristics of the study population.
Table 1.
Characteristics of the study population
| Variable | Status | N | n (%) | Missing |
|---|---|---|---|---|
| Age (median 71 years) | ≥80 | 154 | 23 (15) | — |
| Gender | Male | 154 | 78 (51) | — |
| Symptoms | B | 154 | 73 (47) | — |
| Bulky | Yes | 154 | 13 (8) | — |
| Extranodal sites | Yes | 154 | 60 (39) | — |
| Stage | III–IV | 154 | 105 (68) | — |
| LDH | >Upper normal level | 148 | 66 (45) | 6 |
| Hasenclever IPS | ≥3 | 50 | 33 (66) | 104 |
| CGA | 122 | 32 | ||
| FIT | 81 (67) | — | ||
| UNFIT | 20 (16) | — | ||
| FRAIL | 21 (17) | — | ||
| BMI (median 24.2 kg/m2) | 152 | 2 | ||
| Normal | 85 (56) | — | ||
| Overweight | 53 (35) | — | ||
| Obese | 14 (9) | — | ||
| Histology | 154 | — | ||
| Nodular sclerosis | 59 (39) | — | ||
| Mixed cellularity | 24 (15) | — | ||
| Lymphocyte rich | 7 (5) | — | ||
| Lymphocyte deplete | 2 (1) | — | ||
| Classic, not specified | 62 (40) | — | ||
| Treatment | 153 | 1 | ||
| ABVD | 117 (76) | — | ||
| Other CHT | 31 (21) | — | ||
| Radiotherapy only | 5 (3) | — | ||
| Radiotherapy | Yes | 153 | 41 (27) | 1 |
ABVD, adriamycin, bleomycin, vinblastine, dacarbazine; BMI, body mass index; CGA, comprehensive geriatric assessment; CHT, chemotherapy; LDH, lactic acid dehydrogenase.
All patients underwent baseline PET/CT before any treatment, 100 performed an interim PET/CT, and 126 underwent PET/CT at completion of first line therapy. All but 22 patients underwent high‐dose CT scan at baseline; for those 22 patients low‐dose CT of PET/CT scans were used as reference to perform muscle mass measurements.
Survival analysis of the whole population
Overall, with a median follow‐up duration of 5.9 years (95CI, 4.7–6.6 years), the 5 year PFS and OS rates are 53% (95% CI, 45–61%) and 65% (95% CI, 56–72%), respectively.
In univariate analysis, the presence of B symptoms (P = 0.016), advanced stage at diagnosis (P = 0.016), a FRAIL (CGA) functional status (P = 0.019) and Deauville score (DS) 4–5 at PET2 evaluation (P < 0.001) significantly impact PFS, while age ≥80 years, gender, bulky, extranodal sites, LDH levels, Hasenclever IPS score, and BMI do not.
In univariate analysis, age ≥80 years (P = 0.005), presence of B symptoms (P = 0.035), advanced stage at diagnosis (P = 0.007), a FRAIL (CGA) functional status (P < 0.001) and DS 4–5 at PET2 evaluation (P = 0.009) significantly impact OS, while gender, bulky, extranodal sites, LDH levels, IPS score, and BMI do not.
Sarcopenia in classical Hodgkin lymphoma patients
Applying conventional gender‐specific cut‐offs of sarcopenia measures as L3‐SMI (<39 cm2/m2 for female and <55 cm2/m2 for male patients), 27 113 patients (73%) resulted sarcopenic. There was a significant prevalence of male gender in sarcopenic patients with 66 male and 47 female patients (84% vs. 62%, P = 0.002), while sarcopenic patients were not older than the non‐sarcopenic patients [mean age 72 vs. 73 years, P not significant (NS)]. Being male (P = 0.002) and having a normal BMI (P < 0.001) were associated with a low L3‐SMI. In contrast, age at diagnosis, stage, and CGA subgroup, did not correlate with L3‐SMI (Table 2).
Table 2.
Sarcopenia in classical Hodgkin lymphoma patients applying conventional and Nakamura gender‐specific cut‐offs of L3‐SMI
|
Sarcopenic cut‐offs of L3‐SMI (<39 cm2/m2 for F and <55 cm2/m2 for M) | |||
|---|---|---|---|
| Variable (categorical) | Sarcopenia yes, n (%) | P value | |
| Age | <80 | 93 (70) | 0.131 |
| ≥80 | 20 (87) | ||
| Gender | Male | 66 (84) | 0.002 |
| Female | 47 (62) | ||
| Stage | I–II | 33 (67) | 0.249 |
| III–IV | 80 (76) | ||
| CGA | FIT | 52 (72) | 0.381 |
| UNFIT | 12 (60) | ||
| FRAIL | 17 (81) | ||
| BMI | Normal | 69 (81) | <0.001 |
| Oversize | 38 (72) | ||
| Obese | 4 (29) | ||
| Treatment | ABVD | 85 (73) | 0.654 |
| CHT | 24 (77) | ||
| Only RT | 3 (60) | ||
| PET2 Deauville Score | − | 52 (73) | 0.786 |
| + | 19 (79) | ||
| Response (EoT) | CR | 21 (78) | 0.805 |
| <CR | 70 (74) | ||
| Sarcopenic Nakamura cut‐offs of L3‐SMI (<34.4 cm2/m2 for F and <47 cm2/m2 for M) | |||
|---|---|---|---|
| Variable (categorical) | Sarcopenia yes, n (%) | P value | |
| Age | <80 | 56 (42) | 1.000 |
| ≥80 | 10 (43) | ||
| Gender | Male | 42 (54) | 0.006 |
| Female | 24 (32) | ||
| Stage | I–II | 15 (31) | 0.038 |
| III–IV | 51 (49) | ||
| CGA | FIT | 34 (42) | 0.963 |
| UNFIT | 9 (45) | ||
| FRAIL | 9 (43) | ||
| BMI | Normal | 47 (55) | 0.001 |
| Oversize | 18 (34) | ||
| Obese | 0 (0) | ||
| Treatment | ABVD | 49 (42) | 0.053 |
| CHT | 17 (55) | ||
| Only RT | 0 (0) | ||
| PET2 Deauville score | − | 32 (45) | 0.813 |
| + | 12 (50) | ||
| Response (EoT) | CR | 13 (48) | 0.828 |
| <CR | 42 (45) | ||
ABVD, adriamycin, bleomycin, vinblastine, dacarbazine; BMI, body mass index; CGA, comprehensive geriatric assessment; CHT, chemotherapy; CR, complete remission; EoT, end of treatment; F, female; M, male; PET2, PET scan after two ABVD/ABVD‐like cycles; RT, radiotherapy.
The 5 years PFS rates are 52% (95% CI, 42–62%) and 57% (95% CI, 39–71%), respectively, in the sarcopenic vs. non‐sarcopenic group. The 5 years OS rates are 65% (95% CI, 54–74%) and 65% (95% CI, 45–79%), respectively, in the sarcopenic vs. non‐sarcopenic group (Figure 1A). There was no difference between sarcopenic and non‐sarcopenic patients for 5 years PFS (P = 0.998) and 5 years OS (P = 0.954).
Figure 1.
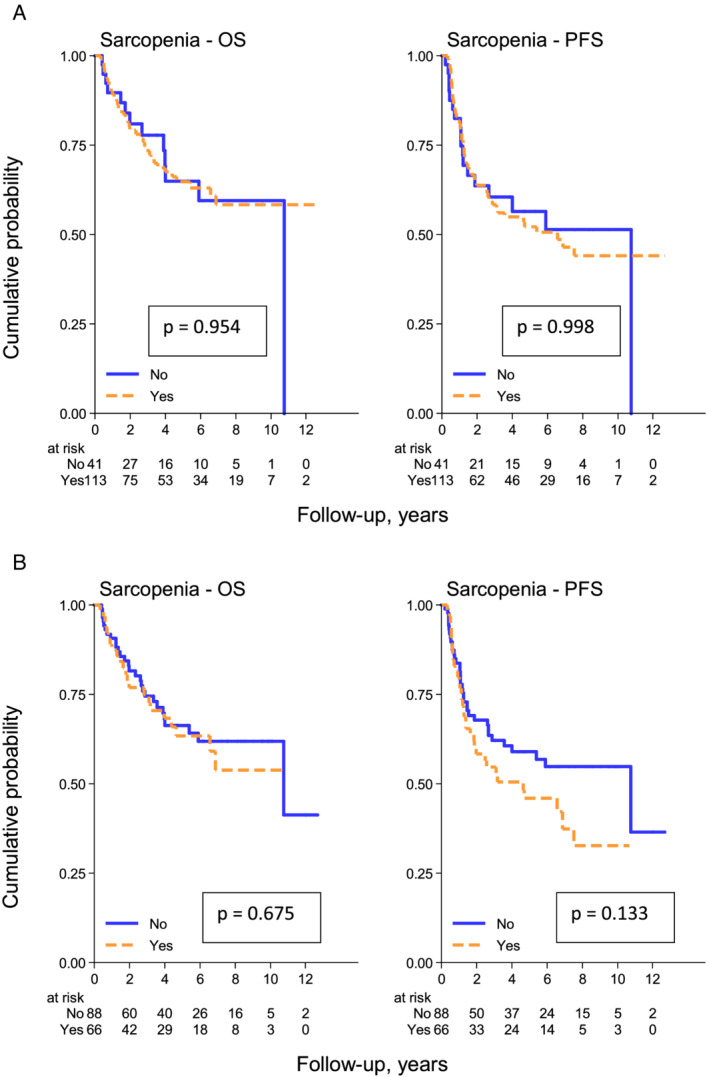
(A) (above) Overall survival (OS) and progression‐free survival (PFS) according to sarcopenic status defined by ‘conventional’ cut‐offs [<39 cm2/m2 (F) and <55 cm2/m2 (M)]. (B) (below) OS and PFS according to sarcopenic status defined by Nakamura cut‐offs [<34.4 cm2/m2 (F) and <47 cm2/m2 (M)].
Sarcopenia in classical Hodgkin lymphoma patients according to Nakamura cut‐offs
Applying other gender‐specific cut‐offs of L3‐SMI (<34.4 cm2/m2 for female and <47 cm2/m2 for male patients) as suggested by Nakamura, 28 66 patients (43%) were defined as sarcopenic. Also in this analysis, there was a prevalence of male among sarcopenic patients (54% vs. 32%, P = 0.006), while no significant differences in age were detected (mean age of 72 and 73 years, respectively).
Being male (P = 0.006), presenting with advanced stage (P = 0.038) and having a normal BMI (P < 0.001) correlated with a low L3‐SMI, while age at diagnosis and CGA subgroups did not (Table 2).
The 5 year PFS rates are 46% (95% CI, 33–58%) and 59% (95% CI, 47–69%), respectively, in the sarcopenic vs. non‐sarcopenic group. The 5 years OS rates are 63% (95% CI, 49–75%) and 66% (95% CI, 54–76%), respectively, in the sarcopenic vs. non‐sarcopenic group. There was no difference between sarcopenic and non‐sarcopenic patients for 5 years PFS (P = 0.133) and 5 years OS (P = 0.675) (Figure 1B).
Subgroup analyses by gender
We performed an analysis by gender, using conventional cut‐offs [<39 cm2/m2 (F) and <55 cm2/m2 (M)].
In female patients, the 5 year PFS rates are 61% (95% CI, 45–74%) and 46% (95% CI, 26–64%), respectively, in the sarcopenic vs. non‐sarcopenic group (P = 0.114), while the 5 year OS rates are 67% (95% CI, 51–79%) and 55% (95% CI, 31–73%), respectively, in the sarcopenic vs. non‐sarcopenic group (P = 0.292).
In male patients, the 5 year PFS rates are 45% (95% CI, 32–58%) and 80% (95% CI, 40–95%), respectively, in the sarcopenic vs. non‐sarcopenic group (P = 0.110), while the 5 years OS rates are 63% (95% CI, 48–75%) and 89% (95% CI, 43–98%), respectively, in the sarcopenic vs. non‐sarcopenic group (P = 0.232). (Figure 2).
Figure 2.
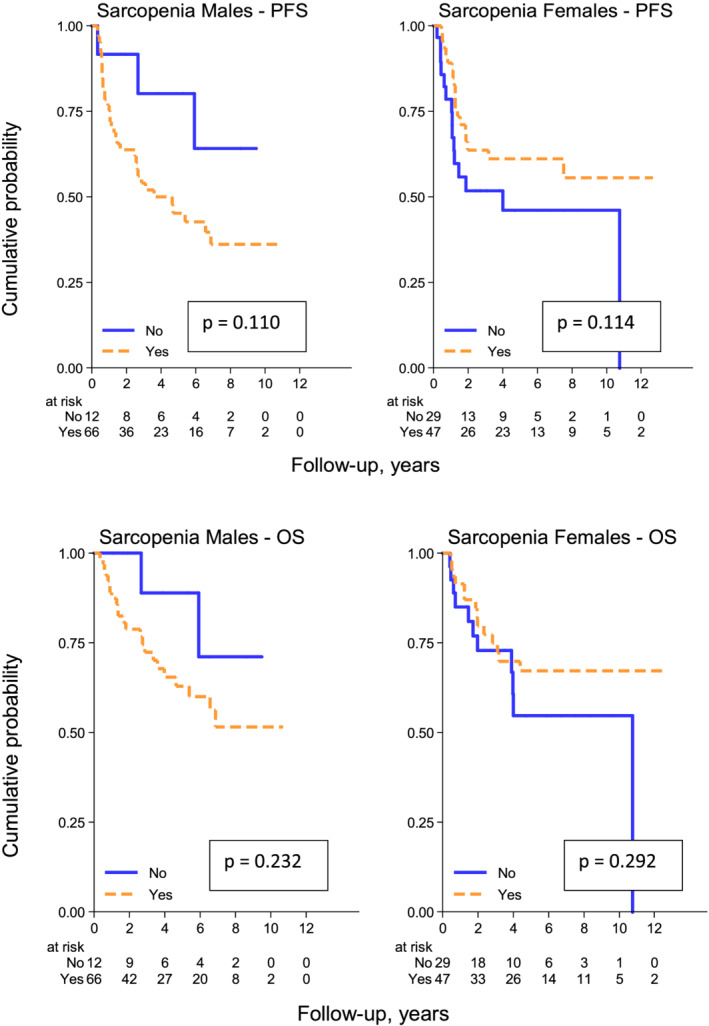
Progression‐free survival (above) and overall survival (below) according to sarcopenic status defined by ‘usual’ cut‐offs [<39 cm2/m2 (F) and <55 cm2/m2 (M)] in male and female patients.
Subsequently, we performed an analysis by gender using Nakamura cut‐offs [<34.4 cm2/m2 (F) and <47.1 cm2/m2 (M)].
In female patients, the 5 year PFS rates are 57% (95% CI, 35–74%) and 55% (95% CI, 40–68%), respectively, in the sarcopenic vs. non‐sarcopenic group (P = 0.832), while the 5 year OS rates are 64% (95% CI, 41–80%) and 63% (95% CI, 46–76%), respectively, in the sarcopenic vs. non‐sarcopenic group (P = 0.759).
In male patients, the 5 year PFS rates are 39% (95% CI, 22–55%) and 64% (95% CI, 45–77%), respectively, in the sarcopenic vs. non‐sarcopenic group (P = 0.040), while the 5 years OS rates are 64% (95% CI, 45–79%) and 71% (95% CI, 51–84%), respectively, in the sarcopenic vs. non‐sarcopenic group (P = 0.386) (Figure 3).
Figure 3.
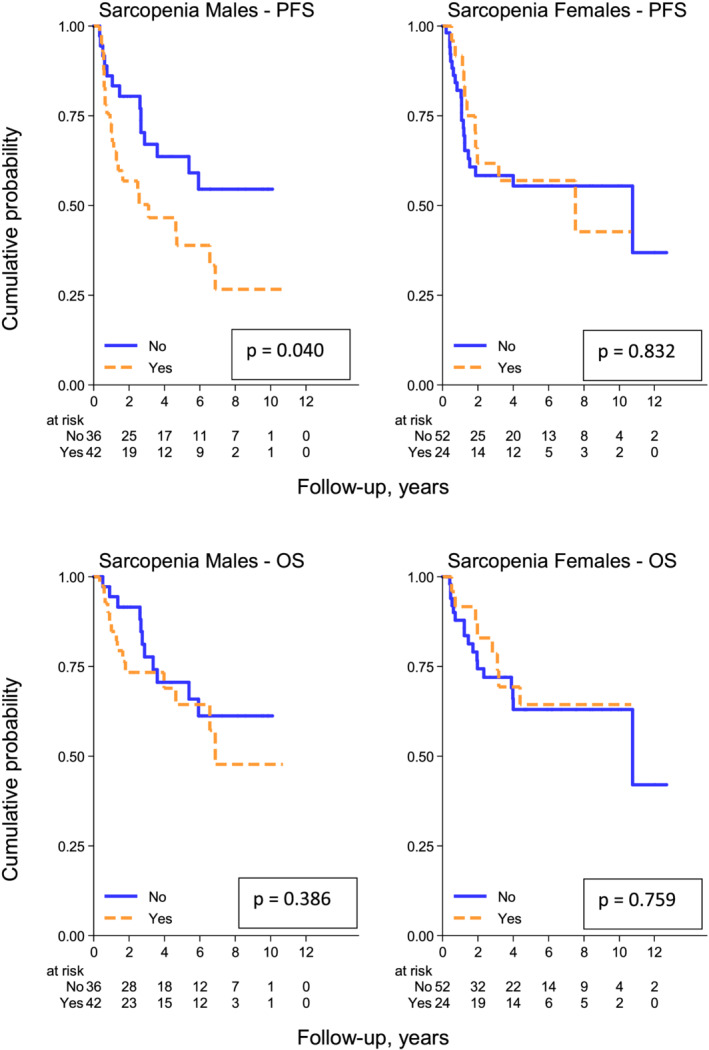
Progression‐free survival (above) and overall survival (below) according to sarcopenic status defined by Nakamura cut‐offs [<34.4 cm2/m2 (F) and <47 cm2/m2 (M)] in male and female patients.
Subgroup analyses by gender with specific population cut‐offs
With the aim to define sex‐specific cut‐offs in our cHL population, subsequent ROCs analyses (Figure 4) were performed, finding a cut‐off point of 45 cm2/m2 for male patients. Using this cut‐off, 27 out of 76 male patients were defined as sarcopenic (35%).
Figure 4.
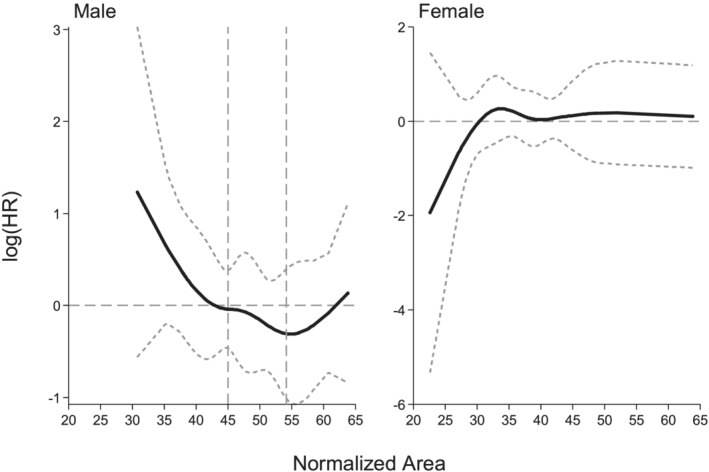
With receiver operator curve–area under the curve at 5‐yr of follow‐up the cut‐point for male patients was 45 (area under the curve = 0.65, 95% confidence interval 0.53–0.78 and cut‐point 45, 95% confidence interval 38–52; after 250 bootstrap replications). In female patients, the hazard ratio was not associated to sarcopenia.
The 5 year PFS rates in male patients are 31% (95% CI, 12–51%) and 61% (95% CI, 44–73%), respectively, in the sarcopenic vs. non‐sarcopenic group (P = 0.008).
The 5 year OS rates in male patients are 51% (95% CI, 29–85%) and 74% (95% CI, 58–85%), respectively, in the sarcopenic vs. non‐sarcopenic group (P = 0.042) (Figure 5).
Figure 5.
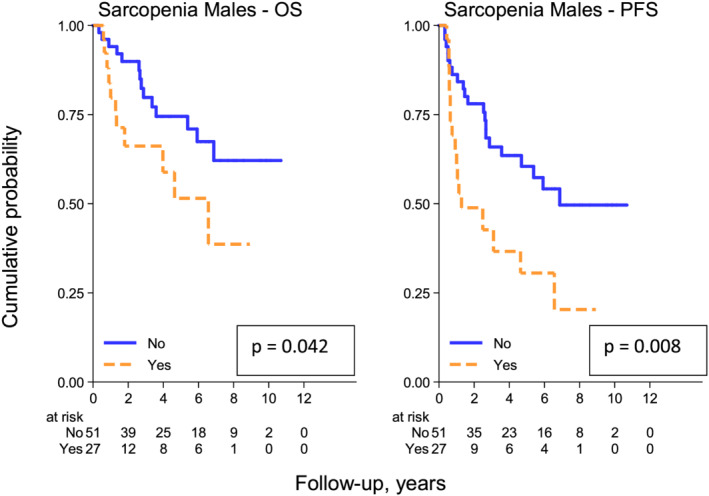
Overall survival (OS) and progression‐free survival (PFS) according to sarcopenic status defined by our specific population cut‐off [<45 cm2/m2] in male patients.
In multivariable analysis crossed by gender and adjusted by Stages III and IV, age, and CGA FRAIL status, sarcopenic male patients showed a worse outcome in PFS (HR 3.09, 95% CI 1.43–6.64, P = 0.004) and, although with less evidence, in OS (HR 2.69–7.46, P = 0.057), while sarcopenic female patients did not. Age as continuous variable had a significant impact in terms of both PFS and OS (Table 3).
Table 3.
Univariate and multivariate analyses for overall survival and progression‐free survival
| Variable | Status | Overall survival | Progression free survival | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | ||||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | ||
| Age, continuous | By 1 year | 1.09 (1.04–1.14) | <0.001 | 1.11 (1.03–1.20) | 0.005 | 1.06 (1.02–1.10) | 0.003 | 1.07 (1.01–1.14) | 0.016 |
| Gender | Male | 1.00 | 1.00 | ||||||
| Female | 0.93 (0.55–1.56) | 0.780 | 1.56 (0.64–3.80) | 0.332 | 0.87 (0.56–1.35) | 0.535 | 1.49 (0.71–3.13) | 0.288 | |
| Symptoms | A | 1.00 | 1.00 | ||||||
| B | 1.77 (1.04–3.01) | 0.035 | 1.74 (1.11–2.72) | 0.016 | |||||
| Bulky | No | 1.00 | 1.00 | ||||||
| Yes | 1.37 (0.55–3.43) | 0.502 | 1.95 (0.97–3.91) | 0.060 | |||||
| Extranodal sites | No | 1.00 | 1.00 | ||||||
| Yes | 1.38 (0.81–2.35) | 0.243 | 1.32 (0.83–2.08) | 0.231 | |||||
| Stage | I–II | 1.00 | 1.00 | ||||||
| III–IV | 2.34 (1.26–4.34) | 0.007 | 1.75 (0.83–3.70) | 0.144 | 1.85 (1.12–3.06) | 0.016 | 1.46 (0.81‐2.64) | 0.208 | |
| LDH | ≤UNL | 1.00 | 1.00 | ||||||
| >UNL | 1.04 (0.59–1.80) | 0.901 | 1.19 (0.75–1.89) | 0.468 | |||||
| Hasenclever IPS | 1 to 2 | 1.00 | 1.00 | ||||||
| 3 to 7 | 0.99 (0.33–2.96) | 0.983 | 0.82 (0.32–2.11) | 0.688 | |||||
| CGA | FIT | 1.00 | 1.00 | ||||||
| UNFIT | 1.22 (0.50–3.03) | 0.660 | 0.53 (0.18–1.61) | 0.264 | 1.00 (0.48–2.08) | 0.995 | 0.60 (0.26–1.43) | 0.250 | |
| FRAIL | 3.95 (2.00–7.83) | <0.001 | 2.82 (1.12–7.07) | 0.028 | 2.06 (1.12–3.77) | 0.019 | 1.78 (0.85–3.71) | 0.126 | |
| BMI | Normal | 1.00 | 1.00 | ||||||
| >Normal | 0.77 (0.43–1.36) | 0.366 | 0.76 (0.47–1.24) | 0.274 | |||||
| PET2, Deauville score | 1–3 (−) | 1.00 | 1.00 | ||||||
| 4–5 (+) | 3.02 (1.31–6.96) | 0.009 | 3.22 (1.67–6.18) | <0.001 | |||||
| Sarcopenia Lanic | No | 1.00 | 1.00 | ||||||
| Yes | 0.98 (0.52–1.85) | 0.954 | 1.01 (0.59–1.71) | 0.998 | |||||
| Sarcopenia Nakamura | No | 1.00 | 0.675 | 1.00 | |||||
| Yes | 1.13 (0.64–1.98) | 1.43 (0.90–2.29) | 0.133 | ||||||
| Sarcopenia—male | 2.29 (1.03–5.10) | 0.042 | 2.69 (0.97–7.46) | 0.057 | 2.43 (1.27–4.66) | 0.008 | 3.09 (1.43–6.64) | 0.004 | |
| Sarcopenia—female | 0.65 (0.30–1.44) | 0.292 | 0.50 (0.21–1.20) | 0.120 | 0.57 (0.29–1.14) | 0.114 | 0.53 (0.25–1.13) | 0.100 | |
BMI, body mass index; CGA, comprehensive geriatric assessment; CI, confidence interval; HR, hazard ratio; LDH, lactic acid dehydrogenase; PET2, PET scan after two ABVD/ABVD‐like cycles; UNL, upper normal level.
Disease progression in sarcopenic patients
The cause of death for patients with and without sarcopenic status was comparable (72% and 68% for progression disease, respectively). Considering the number of cycles of chemotherapy done, a relevant difference in treatment between patients with and without sarcopenia did not emerge (full cycles 89% vs. 85%, P = 0.483).
Considering DFS, the overall rates at 5 years were 87% (95% CI, 64–96%) for non‐sarcopenic and 57% (35–74%) for sarcopenic patients, respectively, with HR 3.38 (95% CI, 1.18–9.62; P = 0.023). The difference was higher in male patients (n = 34, 88% vs. 39% at 5 years, P = 0.002) than in female patients (n = 26, 86% vs. 69% at 5 years, P = 0.272).
Discussion
Malignancy‐related sarcopenia typically presents as muscle atrophy with a decrease in muscle cells size and number 21 and it is mainly caused by the presence of a systemic inflammatory response that induces protein degradation. Systemic inflammation in lymphoma patients may be induced by cytokines released by the lymphoma itself or as a response of the host to lymphoma cells. 35 In this way, at least in the DLBCL setting, sarcopenia may be related to an increased biological aggressiveness of the lymphoma that translates into systemic inflammation and loss of skeletal muscle. 36 However, at the best of our knowledge, no data have been published on the frequency and role of sarcopenia in the setting of cHL.
In our study, we choose to focus on elderly cHL patients, who are normally markedly underrepresented in clinical studies on cHL but that constitute an absolute medical need, both for the rising incidence of cHL in this range of age that is expected in the next decades due to the general ageing of Western countries populations and for the difficult of balancing the goal of efficacy and the risk of toxicities in this setting.
For this reason, the primary objective of the study was to determine the frequency of sarcopenia in an elderly cHL population, and accordingly to the L3‐SMI cut‐off levels proposed by Lanic, 27 a broad proportion of patients (73%) in our study resulted sarcopenic. Due to the lack of consensus of optimal cut‐off levels to define sarcopenia in lymphoma patients, we subsequently estimated the frequency of sarcopenia in our population according to the cut‐off published by Nakamura 28 and 43% of the patients resulted to be sarcopenic. However, because no data are available in the specific setting of elderly cHL, we tried to identify specific cut‐offs that could be applied to this population: we were not able to define new cut‐off levels for female patients, but according to our ROC analyses, elderly cHL male patients could be defined as sarcopenic using an L3‐SMI cut‐off level of 45 cm2/m2.
Secondary endpoint of our study was to correlate the sarcopenic status of the patients with their outcomes in terms of PFS and OS, and we approached this endpoint applying the same step‐by‐step ‘cut‐off choosing’ procedure described above. In our study population, we could not observe any differences in terms of both OS and PFS nor with the sarcopenic cut‐off levels by Lanic or with those defined by Nakamura. However, like in the Nakamura DLBCL setting, we investigated if any differences were detectable in male vs. female patients, and we could observe that ‘Nakamura‐defined’ cHL sarcopenic male patients showed a worse outcome in terms of PFS, while no differences in terms of OS were observed. Using the male cHL specific cut‐off of 45 cm2/m2, we could observe a significant difference in PFS (P = 0.008) and OS (P = 0.042). In multivariate analysis adjusted by Stages III and IV, age ≥80, and CGA FRAIL status, this cut‐off value retained its statistical significance in terms of PFS, and it showed a similar trend in terms of OS although not reaching statistical significance due to the lower number of events observed in our dataset. We do not have definite answers to the emerging questions of what the causes of this wide ‘cut‐off’ levels heterogeneity could be, but it is a matter of fact that we should consider that these values result from the analysis of widely different populations (i.e. in terms of patients number, histology, stage of disease, comorbidities, etc.). This might explain why we could observe a different cut‐off level in cHL male patients, that in any case could be considered as a new ‘reference‐level’ to detect sarcopenia in the cHL setting. Unfortunately, due to the retrospective nature of our study, we cannot draw conclusions about what the causes of the different effect of sarcopenia between male and female patients could be. However, the role of different body fat composition with its consequence in terms of drug distribution should be taken into account, 28 and a combined approach including both sarcopenia and adipopenia evaluation at baseline could probably better define populations of patients with different outcomes. 37 We should also consider that, both for the retrospective nature and for the onco‐haematological setting of the study, we focused on muscle mass evaluation to define sarcopenic patients, but it is possible and even probable that a full evaluation according to EWGSOP2 criteria could better define and stratify sarcopenic patients balancing this gender‐related effect on body composition.
Another secondary endpoint of the study was to define if any correlations exist between sarcopenia and patient functional status. At the best of our knowledge, it is the first protocol that presents data on a consistent number of elderly cHL patients functionally evaluated adopting a CGA including ADL‐IADL‐CIRSG baseline evaluations and a chronological cut‐off level (80 years), even if multiple experiences have brought attention on the role of one or more of these parameters in cHL patients. 6 , 9 , 38 As described earlier, this simplified CGA approach has become a useful and progressively implemented tool in the setting of elderly patients with lymphoma, and it is an easy‐to‐do and time‐saving approach that could be proposed in outpatient setting and takes less than 10 min to be fully completed. In this study, a FRAIL functional status at baseline has emerged as a patient‐related adverse prognostic factor for both PFS and OS in univariate analysis and it retained its prognostic role at least in terms of PFS even in multivariate analysis. However, we were not able to observe significant interactions between the patient's functional status and sarcopenic level through different cut‐offs (and as muscle mass continuous variable, data not shown). Due to the retrospective nature of this study, it is important to verify if this simplified CGA approach retains its usefulness and prognostic importance in cHL in a prospective collection, and among other elderly cHL related risk factors. This is the aim of a large prospective multicentre FIL trial currently running in Italy.
Even if no strong answers to a possible interaction between sarcopenia and patient's functional status emerge from this study, we believe that our experience may be helpful in a field in which we are facing with a hard‐to‐cure disease and where there have been several attempts to investigate single biological factors or to propose new chemotherapy cycles. Our contribute mainly relies on a new perspective on elderly cHL patients approach that is ‘patient‐centred’ in terms of functional evaluation through CGA and in terms of evaluation of sarcopenia as possible prognostic variable using patient‐level radiological tools commonly primarily used in lymphoma staging. Once more, this approach worths to be verified in a prospective setting and enriched by a more complete evaluation of the performance/strength reduction that contribute to a full definition of sarcopenia 18 ; the prospective observational trial on elderly cHL ongoing in Italy has been recently amended to include full evaluations of these variables.
Finally, even if beyond the scope of the study, we could confirm in a wide population the impact of some ‘lymphoma‐related’ cHL prognostic factors even in the setting of the elderly population, such as advanced stage at diagnosis, the presence of B symptoms and the importance of obtaining an early complete response (DS 1 to 3) for patients undergoing ABVD/ABVD‐like treatments as recently demonstrated. 39
Our study contains some limitations, like its retrospective nature, the relatively low sample of population, the heterogeneous management approaches to our patients and the absence of collection of treatment toxicities, which may influence prognostic evaluation.
Conclusions
Baseline evaluation of sarcopenia through radiological exams performed for cHL staging may help define a proportion of male patients with unfavourable outcome with current treatment strategies. Functional status evaluation may help identify a FRAIL subgroup of patients (both male and female) with particularly worse outcome. Prospective studies are warranted to better define in real‐life settings whether these easy and patient‐level approaches retain their significance and utility and could be used to improve a treatment tailoring in the setting of elderly cHL.
Conflict of interest
All authors have no conflicts of interest to declare.
Funding
No funding was provided for this study.
Acknowledgements
The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia, and Muscle: update 2017. 40
Zilioli V. R., Albano D., Arcari A., Merli F., Coppola A., Besutti G., Marcheselli L., Gramegna D., Muzi C., Manicone M., Camalori M., Ciammella P., Colloca G., and Tucci A. (2021) Clinical and prognostic role of sarcopenia in elderly patients with classical Hodgkin lymphoma: a multicentre experience, Journal of Cachexia, Sarcopenia and Muscle, 12, 1042–1055, 10.1002/jcsm.12736
References
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011. Mar‐Apr;61:69–90. [DOI] [PubMed] [Google Scholar]
- 2. Shenoy P, Maggioncalda A, Malik N, Flowers CR. Incidence patterns and outcomes for Hodgkin lymphoma patients in the United States. Adv Hematol 2011;2011:725219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Erdkamp FL, Breed WP, Bosch LJ, Wijnen JT, Blijham GB. Hodgkin disease in the elderly. A registry‐based analysis. Cancer 1992. Aug 15;70:830–834. [DOI] [PubMed] [Google Scholar]
- 4. Engert A, Ballova V, Haverkamp H, Pfistner B, Josting A, Dühmke E, et al. Hodgkin's lymphoma in elderly patients: a comprehensive retrospective analysis from the German Hodgkin's Study Group. J Clin Oncol 2005. Aug 1;23:5052–5060. [DOI] [PubMed] [Google Scholar]
- 5. Diepstra A, van Imhoff GW, Schaapveld M, Diepstra A, van Imhoff GW, Schaapveld M, et al. Latent Epstein‐Barr virus infection of tumor cells in classical Hodgkin's lymphoma predicts adverse outcome in older adult patients. J Clin Oncol 2009. Aug 10;27:3815–3821. [DOI] [PubMed] [Google Scholar]
- 6. Levis A, Anselmo AP, Ambrosetti A, Adamo F, Bertini M, Cavalieri E, et al. VEPEMB in elderly Hodgkin's lymphoma patients. Results from an Intergruppo Italiano Linfomi (IIL) study. Ann Oncol 2004. Jan;15:123–128. [DOI] [PubMed] [Google Scholar]
- 7. Levis A, Depaoli L, Bertini M, Botto B, Ciravegna G, Freilone R, et al. Results of a low aggressivity chemotherapy regimen (CVP/CEB) in elderly Hodgkin's disease patients. Haematologica 1996. Sep‐Oct;81:450–456. [PubMed] [Google Scholar]
- 8. Salvi F, Luminari S, Tucci A, Massidda S, Liberati AM, Stelitano C, et al. Bleomycin, vinblastine and dacarbazine with nonpegylated liposomal doxorubicin (MBVD) in elderly (≥70 years) or cardiopathic patients with Hodgkin lymphoma: a phase‐II study from Fondazione Italiana Linfomi (FIL). Leuk Lymphoma 2019. Dec;60:2890–2898. [DOI] [PubMed] [Google Scholar]
- 9. Zallio F, Tamiazzo S, Monagheddu C, Merli F, Ilariucci F, Stelitano C, et al. Reduced intensity VEPEMB regimen compared with standard ABVD in elderly Hodgkin lymphoma patients: Results from a randomized trial on behalf of the Fondazione Italiana Linfomi (FIL). Br J Haematol 2016. Mar;172:879–888. [DOI] [PubMed] [Google Scholar]
- 10. Stamatoullas A, Brice P, Bouabdallah R, Mareschal S, Camus V, Rahal I, et al. Outcome of patients older than 60 years with classical Hodgkin lymphoma treated with front line ABVD chemotherapy: frequent pulmonary events suggest limiting the use of bleomycin in the elderly. Br J Haematol 2015. Jul;170:179–184. [DOI] [PubMed] [Google Scholar]
- 11. Evens AM, Advani RH, Helenowski IB, Fanale M, Smith SM, Jovacnovic BD, et al. Multicenter phase II study of sequential brentuximab vedotin and doxorubicin, vinblastine, and dacarbazine chemotherapy for older patients with untreated classical Hodgkin lymphoma. J Clin Oncol 2018. Oct 20;36:3015–3022. [DOI] [PubMed] [Google Scholar]
- 12. Tucci A, Martelli M, Rigacci L, Riccomagno P, Cabras MG, Salvi F, et al. Comprehensive geriatric assessment is an essential tool to support treatment decisions in elderly patients with diffuse large B‐cell lymphoma: a prospective multicenter evaluation in 173 patients by the Lymphoma Italian Foundation (FIL). Leuk Lymphoma 2015. Apr;56:921–926. [DOI] [PubMed] [Google Scholar]
- 13. Merli F, Luminari S, Rossi G, Mammi C, Marcheselli L, Ferrari A, et al. Outcome of elderly frail patients with diffuse large B‐cell lymphoma (DLBCL) prospectively identified by comprehensive geriatric assessment (CGA). Results from a study of the Intergruppo Italiano Linfomi (IIL). Leuk Lymphoma 2014. Jan;55:38–43. [DOI] [PubMed] [Google Scholar]
- 14. Merli F, Luminari S, Tucci A, Arcari A, Rigacci L, Hawkes E, et al. Simplified geriatric assessment in older patients with diffuse large B‐cell lymphoma: the prospective elderly project of the Fondazione Italiana Linfomi. J Clin Oncol 2021. Feb 12; JCO2002465;39:1214–1222. [DOI] [PubMed] [Google Scholar]
- 15. Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr 1997. May;127:990S–991S. [DOI] [PubMed] [Google Scholar]
- 16. Lang T, Streeper T, Cawthon P, Baldwin K, Taaffe DR, Harris TB. Sarcopenia: etiology, clinical consequences, intervention, and assessment. Osteoporos Int 2010. Apr;21:543–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019. Jan 1;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beaudart C, McCloskey E, Bruyere O, Cesari M, Rolland Y, Rizzoli R, et al. Sarcopenia in daily practice: assessment and management. BMC Geriatr 2016. Oct 5;16:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A pratctical and precise approach to quantification of body composition in cancer using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 2008;33:997–1006. [DOI] [PubMed] [Google Scholar]
- 21. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011. May;12:489–495. [DOI] [PubMed] [Google Scholar]
- 22. Mc Gregor RA, Cameron‐Smith D, Poppins SD. It is not just muscle mass: a review of muscle quality, composition and metabolism during ageing as determinants of muscle function and mobility in later life. Longev Healthspan 2014. Dec 1;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heymsfield SB, Gonzalez MC, Lu J, Jia G, Zheng J. Skeletal muscle mass and quality: evolution of modern measurement concepts in the context of sarcopenia. Proc Nutr Soc 2015. Nov;74:355–366. [DOI] [PubMed] [Google Scholar]
- 24. Prado CM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res 2009. Apr 15;15:2920–2926. [DOI] [PubMed] [Google Scholar]
- 25. Martin L, Birdsell L, Macdonald N, Reyman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013. Apr 20;31:1539–1547. [DOI] [PubMed] [Google Scholar]
- 26. Go S‐I, Park MJ, Song H‐N, Kang MH, Park HJ, Jeon KN, et al. Sarcopenia and inflammation are independent predictors of survival in male patients newly diagnosed with small cell lung cancer. Support Care Cancer 2016. May;24:2075–2084. [DOI] [PubMed] [Google Scholar]
- 27. Lanic H, Kraut‐Tauzia J, Modzelewski R, Clatot F, Mareschal S, Picquenot JM, et al. Sarcopenia is an independent prognostic factor in elderly patients with diffuse large B‐cell lymphoma treated with immunochemotherapy. Leuk Lymphoma 2014. Apr;55:817–823. [DOI] [PubMed] [Google Scholar]
- 28. Nakamura N, Hara T, Shibata Y, Matsumoto T, Nakamura H, Ninomiya S, et al. Sarcopenia is an independent prognostic factor in male patients with diffuse large B‐cell lymphoma. Ann Hematol 2015. Dec;94:2043–2053. [DOI] [PubMed] [Google Scholar]
- 29. Go S‐I, Park MJ, Song H‐N, Kim HG, Kang MH, Lee HR, et al. Prognostic impact of sarcopenia in patients with diffuse large B‐cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Cachexia Sarcopenia Muscle 2016. Dec;7:567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chu MP, Lieffers J, Ghosh S, Belch A, Chua NS, Fontaine A, et al. Skeletal muscle density is an independent predictor of diffuse large B‐cell lymphoma outcomes treated with rituximab‐based chemoimmunotherapy. J Cachexia Sarcopenia Muscle 2017;8:298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boellaard R, Delgado‐Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging 2015;42:328–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non‐Hodgkin lymphoma: the Lugano classification. J Clin Oncol 2014. Sep 20;32:3059–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Müeller SP, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol 2014. Sep 20;32:3048–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sauerbrei W, Schumacher M. A bootstrap resampling procedure for model building: application to the Cox regression model. Stat Med 1992. Dec;11:2093–2109. [DOI] [PubMed] [Google Scholar]
- 35. Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 2012;21:309–322. [DOI] [PubMed] [Google Scholar]
- 36. Dodson S, Baracos VE, Jatoi A, Evans WJ, Cella D, Dalton JT, et al. Muscle wasting in cancer cachexia: clinical implications, diagnosis, and emerging treatment strategies. Annu Rev Med 2011;62:265–279. [DOI] [PubMed] [Google Scholar]
- 37. Camus V, Lanic H, Kraut J, Modzelewski R, Clatot F, Picquenot JM, et al. Prognostic impact of fat tissue loss and cachexia assessed by computed tomography scan in elderly patients with diffuse large B‐cell lymphoma treated with immunochemotherapy. Eur J Haematol 2014;93:9–18. [DOI] [PubMed] [Google Scholar]
- 38. Evens AM, Helenowski I, Ramsdale E, Nabhan C, Karmali R, Hanson B, et al. A retrospective multicenter analysis of elderly Hodgkin lymphoma: outcomes and prognostic factors in the modern era. Blood 2012. Jan 19;119:692–695. [DOI] [PubMed] [Google Scholar]
- 39. Albano D, Mazzoletti A, Zilioli VR, Muzi C, Crucitti L, Tucci A, et al. Clinical and prognostic role of interim 18F‐FDG PET/CT in elderly Hodgkin lymphoma: a dual‐center experience. Leuk Lymphoma 2020. Jul;24:1–8. [DOI] [PubMed] [Google Scholar]
- 40. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]


