Abstract
Background Mild cognitive impairment (MCI) is an intermediate stage between normal aging and dementia. Amnestic MCI (aMCI) and non-amnestic MCI are the two subtypes of MCI with the former having a higher risk for progressing to Alzheimer's disease (AD). Compared with healthy elderly adults, individuals with MCI have specific functional alterations in the salience network (SN). However, no consistent results are documenting these changes. This meta-analysis aimed to investigate the specific functional alterations in the SN in MCI and aMCI.
Methods: We systematically searched PubMed, Embase, and Web of Science for scientific neuroimaging literature based on three research methods, namely, functional connectivity (FC), regional homogeneity (ReHo), and the amplitude of low-frequency fluctuation or fractional amplitude of low-frequency fluctuation (ALFF/fALFF). Then, we conducted the coordinate-based meta-analysis by using the activation likelihood estimation algorithm.
Results: In total, 30 functional neuroimaging studies were included. After extracting the data and analyzing it, we obtained specific changes in some brain regions in the SN including decreased ALFF/fALFF in the left superior temporal gyrus, the insula, the precentral gyrus, and the precuneus in MCI and aMCI; increased FC in the thalamus, the caudate, the superior temporal gyrus, the insula, and the cingulate gyrus in MCI; and decreased ReHo in the anterior cingulate gyrus in aMCI. In addition, as to FC, interactions of the SN with other networks including the default mode network and the executive control network were also observed mainly in the middle frontal gyrus and superior frontal gyrus in MCI and inferior frontal gyrus in aMCI.
Conclusions: Specific functional alternations in the SN and interactions of the SN with other networks in MCI could be useful as potential imaging biomarkers for MCI or aMCI. Meanwhile, it provided a new insight in predicting the progression of health to MCI or aMCI and novel targets for proper intervention to delay the progression.
Systematic Review Registration: [PROSPERO], identifier [No. CRD42020216259].
Keywords: regional homogeneity, mild cognitive impairment, salience network, amnestic mild cognitive impairment, amplitude of low-frequency fluctuation, functional connectivity, activation likelihood estimation
Introduction
Mild cognitive impairment (MCI) is universally considered as the predementia phase of Alzheimer's disease (AD) (Gao et al., 2020). About one in five community-dwelling elders aged 65 years and above suffer from MCI (Livingston et al., 2017). MCI has been divided into two subtypes, amnestic MCI (aMCI) and non-amnestic MCI (naMCI), according to the difference of impaired cognitive domain. The former manifests as memory deficit combined with or without slight impairment of other cognitive domains and converts to AD at a high rate of 10–15% per annum (Cai et al., 2020a). Some researchers have estimated that the number of patients with AD would be reduced by half if the onset of AD is delayed by five years (Butterfield and Boyd-Kimball, 2018). Furthermore, accurate diagnosis of the early or preclinical stage of AD can provide a basis for early intervention. Therefore, determining the specific change of MCI or aMCI is important. Studies have used the resting-state functional magnetic resonance imaging (rs-fMRI) for investigating neuronal integrity and functional alteration in the brain of patients with MCI (Khazaee et al., 2016; Zhu et al., 2016). Salience network (SN), which is involved in affections, attention, emotions, and switching between cognitive resources, is significantly affected in patients with MCI (Chen H. et al., 2019). Many studies have reported functional alterations in the SN in MCI when compared with healthy controls (HCs). However, there were no consistent results (Chen J. et al., 2019). Thus, our study aimed to conclude the characteristic abnormalities of the SN in MCI by analyzing previous investigations.
The rs-fMRI has been widely used to detect functional changes, such as disruptions in network integrity in the brain, and to predict future cognitive decline in normal elderly participants or MCI (Kaiser et al., 2015; Buckley et al., 2017). Functional connectivity (FC), the amplitude of low-frequency fluctuation (ALFF) or fractional amplitude of low-frequency fluctuation (fALFF), and regional homogeneity (ReHo) are the three main methods used for measuring functional changes (Harrington et al., 2017; Cheng et al., 2019). FC, based on independent component analysis (ICA) or seed regions of interest (seed-ROI) analysis, reflects the functional relationship between two or more regions of the brain (Mamah et al., 2013; Chand et al., 2017). ALFF and fALFF are used for quantitatively assessing the total voxel-wise amplitude of the local features of the brain oscillatory activities. The latter is more specific because its measure includes the entire frequency range, and it attenuates the influence of physiological noise (Han et al., 2011; Qing et al., 2017). ReHo, which is based on the similarity of neighboring voxels over a time series, can be used to determine the functional change of the local brain area. It is highly reliable for studying the consistency of local brain activity (Zang et al., 2004; Li M. G. et al., 2020). Thus, the three methods mentioned above have their virtues in investigating brain networks to understand the functional changes in MCI.
The SN, as one of the three core neurocognitive networks [the default mode network (DMN), SN, and the executive control network (ECN)], plays a vital role in cognitive function. It is a task-positive network, serving to identify relevant stimuli for guiding behavior in response to several internal and external stimuli, and is relevant for attention and the interoceptive process (Menon, 2011; Supekar et al., 2019). Many studies have reported functional changes of the SN in patients with cognitive impairment (Agosta et al., 2012; Onoda et al., 2012). In addition, multiple studies have examined the relationships between SN and other networks. Li et al. found that the SN-centered “triple-network model” was impaired in patients with cognitive decline by comparing 33 AD, 24 late MCI, and 25 control subjects (Li et al., 2019). However, the lack of data makes it difficult to find reliable specific imaging markers in the SN to predict the transformation from healthy elders to MCI or MCI to AD. Thus, concluding consistent results of alterations in the SN is essential.
Activation likelihood estimation (ALE) technique is a powerful meta-analysis method based on random-effects interference and controlling for sample size, which uses peak coordinates reported in selected studies as Gaussian probability distributions (Kollndorfer et al., 2013). It avoids the shortcoming of uncertainty of the actual abnormal position by using the coordinates of abnormal sites instead of area label (Hétu et al., 2013). A study by Chen et al. analyzed the structural brain changes in MCI using ALE and suggested that regional alternations might serve as early predictive or diagnostic biomarkers for MCI (Chen et al., 2020). Nevertheless, this study was the first one to assess functional specific changes in the SN in patients with MCI and aMCI.
Our study aimed to comprehensively analyze and conclude the specific functional alterations of brain areas in the SN and discuss the specific abnormal markers within the SN as well as its interaction with other networks in MCI. We proposed the hypothesis that (1) the three indexes (i.e., ALFF/fALFF, ReHo, and FC) of the SN would show specific neuroimaging changes in some brain regions, (2) specific changes would be different in MCI and aMCI, and (3) interactions between SN and other networks could be observed in MCI and aMCI. A detailed understanding of the specific functional alterations of the SN in MCI will provide a more profound understanding of the pathological mechanism of such patients and may help better establish rehabilitation procedures along those lines.
Materials and Methods
Search Strategy
A systematic research of studies was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement and then recorded using the suggested checklist. We systematically searched PubMed, Web of Science, and Embase for rs-fMRI scientific literature published in English between January 1, 2000 and April 1, 2021. Search keywords were as follows: (1) (salience network) AND (“mild cognitive impairment” [MeSH]) AND (“functional magnetic resonance imaging” [MeSH]) AND (Resting state) AND [(functional connectivity) OR (fc)], (2) (“mild cognitive impairment” [MeSH]) AND (“functional magnetic resonance imaging” [MeSH]) AND (Resting state) AND [(regional homogeneity) OR (ReHo)] OR (local consistency), (3) (“mild cognitive impairment” [MeSH]) AND (“functional magnetic resonance imaging” [MeSH]) AND (Resting state) AND [(amplitude of low frequency fluctuations) OR (ALFF)], and (4) (“mild cognitive impairment” [MeSH]) AND (“functional magnetic resonance imaging” [MeSH]) AND (Resting state) AND [(fractional amplitude of low frequency fluctuations) OR (fALFF)].
A total of 353 publications were initially retrieved. After careful screening, a total of 30 publications (7 FC, 13 ALFF/fALFF, 8 ReHo, and 2 ALFF/fALFF & ReHo) were included in the final analysis (Figure 1). Among the included studies, a total of 18 studies met the criteria of aMCI (4 FC, 8 ALFF/fALFF, 5 ReHo, and 1 ALFF/fALFF & ReHo). However, mainly due to the absence of detailed information on neuropsychological assessment, no study met the criteria of naMCI.
Figure 1.
Flowchart shows study selection process.
Inclusion and Exclusion Criteria
Studies related to rs-fMRI investigations on MCI were included if they (1) reported significant alterations of FC, ALFF/fALFF, or ReHo in the SN using thresholds uncorrected with spatial extent thresholds or corrected for multiple comparisons; (2) made comparisons between MCI and HCs; (3) reported information about the stereotactic space in Talairach or Montreal Neurologic Institute (MNI) coordinates; and (4) were published in English in peer-reviewed journals. The patients with MCI met the following criteria: (a) evidence of the complaint of cognitive change, (b) impairment in one or more cognitive domains, (c) preservation of the independence of function in daily life, and (d) not demented (Albert et al., 2011). The MCI group was further divided into two types, namely, the aMCI group and the naMCI group, based on whether the cognitive impairment was dominated by memory (Cooper et al., 2015). Only the study with the largest sample size, to avoid repeat count, was included.
Studies investigating patients with MCI with a history of other diseases, such as Parkinson's disease, depression, focal epilepsy, and stroke, were excluded unless they included the group of MCIs with no other concurrent diseases. Meta-analysis, reviews, animal experiments, conference meeting abstracts, case reports, studies without group-level statistics, and articles lacking necessary baseline information were not included in the analysis.
Moreover, the quality of the individual studies was evaluated using the Newcastle–Ottawa Scale (NOS) by two reviewers. Three major domains (i.e., selection, comparability, and the ascertainment of outcome) were included in this scale rates (Lo et al., 2014). At any point, any disagreement between reviewers was resolved by means of meeting and discussion among all authors to establish a consensus.
Data Extraction
Two researchers independently conducted the data extraction and then checked the accuracy of extracted data. When disagreements appeared, a third reviewer participated in the discussion until a consensus was reached.
In terms of studies focused on FC, studies investigating the FC within the SN or intra-FC between SN and other networks such as the DMN and ECN were all included, and the coordinate of brain regions that revealed significant changes was extracted. Due to the limited number of included studies, we did not further group according to the difference of methods.
In terms of studies focused on ALFF/fALFF and ReHo, which were voxel-wise whole-brain indexes, first, we reviewed all the studies investigating functional alterations in MCI based on ICA or seed ROI to summarize brain areas belong to the SN. According to the results, we extracted the coordinates of brain regions in the SN which revealed significant changes.
Data Analysis Procedure
The results of the three different methods were divided into increased group and decreased group: (a) MCI: increased ALFF/ fALFF (168 subjects, seven foci, six experiments); decreased ALFF/fALFF (321 subjects, 21 foci, 12 experiments); increased ReHo (166 subjects, eight foci, seven experiments); decreased ReHo (138 subjects, six foci, five experiments); increased FC (111 subjects, 15 foci, four experiments); decreased FC (63 subjects, 26 foci, four experiments). (b) aMCI: increased ALFF/fALFF (126 subjects, four foci, four experiments); decreased ALFF/fALFF (236 subjects, 16 foci, eight experiments); increased ReHo (78 subjects, four foci, three experiments); decreased ReHo (126 subjects, five foci, four experiments); increased FC (30 subjects, one foci, one experiments); decreased FC (47 subjects, five foci, three experiments).
Data were calculated using a Java-based version of Ginger ALE 2.3.6 (http://www.brainmap.org/ale) (Browndyke et al., 2013). Ginger ALE determined whether there was anatomical or functional convergence of differences among multiple coordinate-based human brain imaging studies (Mar, 2011). After transforming Talairach coordinates into MNI space, we sorted all the foci and basic information into six text files. These files were subsequently imported into the software to read the foci. The cluster-forming threshold at p < 0.01 was set to obtain the ALE map with a cluster-level family-wise error (FWE) correction at p < 0.05 and 1,000 permutations (Eickhoff et al., 2012).
However, among the studies included in the analysis, there was one study that reported increased FC of the SN of the left anterior insula in aMCI, compared with HCs. Only one coordinate was available, and thus, the ALE analysis was not conducted in this group (Li X. et al., 2020).
Following the ALE analysis, a jackknife sensitivity analysis was performed by iteratively repeating the same analysis, excluding one dataset each time to test the replicability of results across studies (Lyles and Lin, 2010). However, due to the limitation of the number of included studies involving different indicators, the sensitivity analysis was performed only on the group of decreased ALFF/fALFF in MCI (a total of 12 included studies).
Results
Search Results
Details about the demographics and characteristics of the included studies are summarized in Table 1. The quality assessment of the included studies ranged from low scores of 5 and moderate scores of 6–7 to high scores of 8. The result of the quality assessment is available in the Supplementary Material.
Table 1.
Demographic characteristics of the included rs-fMRI studies.
| Number | Study | Sample size(n) | Gender (M/F) | Age (years ± SD) | MMSE (SD) | Reference space | Group contrasts | Foci (n) | Threshold | Method of analysis |
|---|---|---|---|---|---|---|---|---|---|---|
| ALFF/fALFF | ||||||||||
| 1 | Cai et al. (2017) | MCI 39 | 19/20 | 72.4 ± 5.01 | 25.51 ± 2.88 | MNI | MCI>HC | 0 | p < 0.05 cor | |
| HC 38 | 19/19 | 73.92 ± 3.90 | 29.28 ± 0.88 | MCI < HC | 3 | |||||
| 2 | Cha et al. (2015) | MCI 34 | 18/16 | 68.4 ± 7.9 | 27.1 ± 2. | 1 MNI | MCI>HC | 0 | p < 0.05 cor | |
| HC 62 | 17/45 | 68.5 ± 8.0 | 28.6 ± 1.9 | MCI < HC | 4 | |||||
| 3 | Jia et al. (2015) | MCI 7 | 2/5 | 74.1 ± 7.8 | 27.0 ± 2.3 | MNI | MCI>HC | 0 | p < 0.05 cor | |
| HC 15 | 8/7 | 70.2 ± 7.1 | 29.2 ± 1.3 | MCI < HC | 1 | |||||
| 4 | Liang et al. (2014) | MCI 53 | 31/22 | 73.2 ± 7.3 | 27.1 ± 2.3 | MNI | MCI>HC | 0 | p < 0.05 cor | |
| HC 35 | 18/17 | 74.3 ± 5.9 | 28.9 ± 1.6 | MCI < HC | 1 | |||||
| 5 | Liu X. et al. (2014) | MCI 25 | 16/9 | 71.88 ± 4.09 | 27 | MNI | MCI>HC | 0 | p < 0.05 cor | |
| HC 21 | 14/7 | 72.95 ± 3.53 | 29 | MCI < HC | 1 | |||||
| 6 | Li et al. (2017a) | MCI 27 | 13/14 | 67.44 ± 8.49 | 23.52 ± 3.31 | MNI | MCI < HC | 0 | p < 0.05 cor | |
| HC 32 | 16/16 | 64.88 ± 7.54 | 27.67 ± 1.67 | MCI < HC | 2 | |||||
| 7 | Ni et al. (2016) | MCI 26 | 12/14 | 71 ± 9 | 25 ± 1.48 | MNI | MCI>HC | 2 | p < 0.01 cor | |
| HC 28 | 17/11 | 70 ± 9 | 29 ± 1.09 | MCI < HC | 0 | |||||
| 8 | Wang et al. (2011) | MCI 16 | 7/9 | 69.38 ± 7.00 | 26.50 ± 1.03 | MNI | MCI>HC | 1 | p < 0.05 cor | |
| HC 22 | 7/15 | 66.55 ± 7.67 | 28.59 ± 0.59 | MCI < HC | 1 | |||||
| 9 | Yang et al. (2018) | MCI 55 | 27/28 | 67.51 ± 9.62 | 24.66 ± 4.20 | MNI | MCI>HC | 1 | p < 0.001 uncor | |
| HC 57 | 22/35 | 63.77 ± 8.09 | 28.14 ± 2.13 | MCI < HC | 2 | |||||
| 10 | Yin et al. (2014) | MCI 11 | 2/9 | 66.6 ± 8.7 | 24.6 ± 3.2 | MNI | MCI>HC | 0 | p < 0.05 cor | |
| HC 22 | 12/10 | 62.1 ± 8.1 | 29.2 ± 1.1 | MCI < HC | 2 | |||||
| 11 | Zhao et al. (2014) | MCI 20 | 12/8 | 65.11 ± 9.92 | 25.21 ± 2.24 | MNI | MCI>HC | 1 | p < 0.01 cor | |
| HC 18 | 10/8 | 66.81 ± 7.43 | 29.31 ± 1.22 | MCI < HC | 2 | |||||
| 12 | Zhuang et al. (2019) | MCI 35 | 23/12 | 71.42 ± 4.39 | 27.61 ± 2.14 | MNI | MCI>HC | 0 | p < 0.05 cor | |
| HC 26 | 16/10 | 70.01 ± 5.58 | 28.51 ± 1.32 | MCI < HC | 1 | |||||
| 13 | Zhuang et al. (2012) | MCI 47 | 28/19 | 71.96 ± 4.78 | 26.98 ± 1.53 | MNI | MCI>HC | 0 | p < 0.05 cor | |
| HC 33 | 18/15 | 72.85 ± 3.39 | 28.18 ± 1.33 | MCI < HC | 1 | |||||
| 14 | Zhou et al. (2020) | MCI 24 | 10/14 | 69.8 ± 6.2 | 23.9 ± 3.6 | MNI | MCI>HC | 1 | p < 0.001 uncor | |
| HC 32 | 14/18 | 67.9 ± 6.4 | 28.0 ± 1.9 | MCI < HC | 0 | |||||
| 15 | Zhuang et al. (2020) | MCI 43 | 18/25 | 64.50 ± 5.64 | – | MNI | MCI>HC | 1 | p < 0.05 cor | |
| HC 29 | 7/22 | 66.79 ± 3.68 | – | MCI < HC | 0 | |||||
| ReHo | ||||||||||
| 1 | Bai et al. (2008) | MCI 20 | 10/10 | 71.3 ± 3.8 | 27.2 ± 1.6 | Talairac | MCI>HC | 0 | p < 0.005 cor | |
| HC 20 | 9/11 | 69.4 ± 3.8 | 28.3 ± 1.4 | MCI < HC | 1 | |||||
| 2 | Cai et al. (2018) | MCI 20 | 11/9 | 69.76 ± 6.48 | 25.61 ± 2.67 | MNI | MCI>HC | 1 | p < 0.01 cor | |
| HC 53 | 29/24 | 76.08 ± 6.45 | 28.2 ± 2.13 | MCI < HC | 0 | |||||
| 3 | Cha et al. (2015) | MCI 34 | 18/16 | 68.4 ± 7.9 | 27.1 ± 2.1 | MNI | MCI>HC | 0 | p < 0.05 cor | |
| HC 62 | 17/45 | 68.5 ± 8.0 | 28.6 ± 1.9 | MCI < HC | 1 | |||||
| 4 | Liu Z. et al. (2014) | MCI 12 | 1/11 | 59.3 ± 3.3 | 26.4 ± 0.9 | MNI | MCI>HC | 1 | p < 0.01 cor | |
| HC 12 | 4/8 | 60.6 ± 5.8 | 29.8 ± 0.4 | MCI < HC | 0 | |||||
| 5 | Luo et al. (2018) | MCI 32 | 17/15 | 72.43 ± 4.25 | 28.34 ± 1.68 | MNI | MCI>HC | 1 | p < 0.01 cor | |
| HC 49 | 18/31 | 73.33 ± 4.60 | 29.02 ± 1.20 | MCI < HC | 0 | |||||
| 6 | Min et al. (2019) | MCI 10 | 5/5 | 69.80 ± 2.66 | 25.90 ± 0.74 | MNI | MCI>HC | 2 | p < 0.05 cor | |
| HC 10 | 5/5 | 69.90 ± 2.60 | 29.30 ± 0.83 | MCI < HC | 0 | |||||
| 7 | Ni et al. (2016) | MCI 26 | 12/14 | 71 ± 9 | 25 ± 1.48 | MNI | MCI>HC | 1 | p < 0.05 cor | |
| HC 28 | 17/11 | 70 ± 9 | 29 ± 1.09 | MCI < HC | 0 | |||||
| 8 | Wang et al. (2015) | MCI 30 | 18/12 | 69.1 ± 5.8 | 26.2 ± 2.2 | MNI | MCI>HC | 1 | p < 0.01 cor | |
| HC 32 | 15/17 | 70.1 ± 5.5 | 28.1 ± 1.5 | MCI < HC | 0 | |||||
| 9 | Yuan X. et al. (2016) | MCI 36 | 17/19 | 66.8 ± 9.5 | 24.9 ± 3.4 | MNI | MCI>HC | 1 | p < 0.01 cor | |
| HC 46 | 19/2 | 7 64.3 ± 7.8 | 28.5 ± 2.0 | MCI < HC | 1 | |||||
| 10 | Zhang et al. (2010) | MCI 48 | – | 72.04 ± 4.42 | 27 | MNI | MCI>HC | 0 | p < 0.05 cor | |
| HC 36 | 71.64 ± 3.72 | 29 | MCI < HC | 2 | ||||||
| FC | ||||||||||
| 1 | Conwell et al. (2018) | MCI 15 | 6/9 | 71.1 ± 6.0 | 25.0 ± 3.4 | MNI | MCI>HC | 1 | p < 0.001 uncor | ICA |
| HC 15 | 6/9 | 67.3 ± 8.4 | 29.5 ± 0.6 | MCI < HC | 0 | |||||
| 2 | He et al. (2014) | MCI 18 | 10/8 | 70.2 ± 7.9 | 21.9 ± 5.0 | MNI | MCI>HC | 0 | p < 0.05 cor | ICA, Seed ROI |
| HC 21 | 1/14 | 65.0 ± 8.2 | 28.5 ± 1.4 | MCI < HC | 5 | |||||
| 3 | Liang et al. (2012) | MCI 16 | 6/10 | 68.50 ± 7.77 | 25.94 ± 1.65 | MNI | MCI>HC | 10 | p < 0.05 cor | Seed ROI |
| HC 16 | 6/10 | 67.19 ± 8.38 | 28.56 ± 0.63 | MCI < HC | 15 | |||||
| 4 | Li X. et al. (2020) | MCI 30 | 13/17 | 68.53 ± 2.97 | 25.10 ± 0.66 | MNI | MCI>HC | 1 | p < 0.001 uncor | ICA |
| HC 30 | 14/16 | 68.67 ± 3.19 | 28.20 ± 0.92 | MCI < HC | 0 | |||||
| 5 | Sarli et al. (2021) | MCI 50 | 25/25 | 67.13 ± 9.74 | 21.1 ± 4.89 | MNI | MCI>HC MCI < HC | 3 | p < 0.001 uncor | ICA, Seed ROI |
| HC 50 | 30.20 | 67.14 ± 9.71 | 28.12 ± 1.57 | 0 | ||||||
| 6 | Yi et al. (2015) | MCI 10 | 2/8 | 70.70 ± 1.71 | 23.70 ± 0.87 | MNI152 | MCI>HC | 0 | p < 0.005 uncor | ICA |
| HC 12 | 3/9 | 71.75 ± 1.21 | 27.40 ± 0.45 | MCI < HC | 1 | |||||
| 7 | Zhu et al. (2016) | MCI 19 | 7/12 | 65.7 ± 10.7 | 26.7 ± 1.6 | MNI | MCI>HC | 0 | p < 0.05 cor | Seed ROI |
| HC 28 | 11/17 | 63.8 ± 6.7 | 29.0 ± 0.8 | MCI < HC | 3 | |||||
rs-fMRI, resting-state functional magnetic resonance imaging; MNI, Montreal Neurologic Institute; MCI, mild cognitive impairment; HCs, healthy controls; SD, standard deviation; ALFF/fALFF, the amplitude of low frequency fluctuation/fractional amplitude of low-frequency fluctuation; ReHo, regional homogeneity; FC, functional connectivity; MMSE. Mini-mental state examination; ICA, independent component analysis; Seed ROI, seed regions of interest.
The brain areas of the SN were summarized as follows: (1) Use ICA: bilateral anterior insula (Yi et al., 2015), bilateral frontoinsular cortex, bilateral anterior cingulate cortex (ACC) (Han et al., 2011), bilateral dorsal ACC (dACC), bilateral insula lobule (Chand et al., 2017), right supplementary motor area (SMA), left superior temporal gyrus (STG), bilateral supramarginal gyrus (SG), left precuneus (Conwell et al., 2018; Cai et al., 2020b), preSMA (Yi et al., 2015), dorsal anterior cingulate cortex/medial prefrontal cortex (dACC/MPFC), dorsolateral prefrontal cortex (DLPFC) (He et al., 2014), bilateral caudate (Agosta et al., 2012), the thalamus (Wang et al., 2020), bilateral ventrolateral prefrontal cortex, bilateral STG/insula, midcingulate cortex/preSMA, medial orbitofrontal cortex, and bilateral orbital/frontoinsular (Agosta et al., 2012) and (2) Use seed ROI: bilateral frontoinsular cortex (Joo et al., 2017), bilateral anterior insula, bilateral dACC, dACC/MPFC, bilateral anterior insula (Zhu et al., 2016), bilateral insula (Sarli et al., 2021), and bilateral SG (Liang et al., 2012).
Meta-analysis Results
Regions With Significant Changes Between MCI and HCs
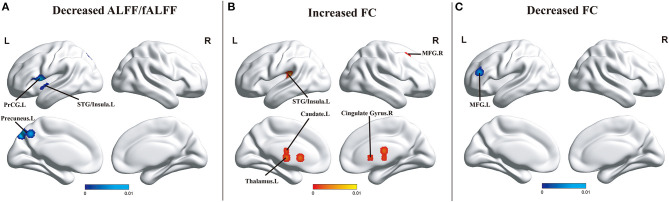
Compared with HCs, patients with MCI showed decreased ALFF/fALFF in the left STG (BA 22), left insula (BA 13), left precentral gyrus (PreCG/BA 44), and the precuneus (BA 7). No brain region presenting increased ALFF/fALFF was found. In addition, patients with MCI showed increased FC of the SN in the thalamus, the caudate, the cingulate gyrus (BA 32), the right middle frontal gyrus (MFG/BA 41), and the left STG/insula (BA 41) and decreased FC in the left MFG (BA 46) and superior frontal gyrus (SFG/BA 9). However, no brain area presenting increased ReHo or decreased ReHo was observed (Figure 2).
Figure 2.
(A) Brain regions showing decreased ALFF/fALFF in patients with MCI compared with HCs. (B) Brain regions showing increased FC in patients with MCI when compared with HCs. (C) Brain regions showing decreased FC in patients with MCI compared with HCs. The color bar represents the p-value. Areas with decreased change relative to controls are displayed in blue, and areas with increased change are displayed in red. Results are thresholded at p < 0.01 cluster-corrected and p < 0.05 family-wise error corrected. Abbreviations: MCI, mild cognitive impairment; HCs, healthy controls; ALFF/fALFF, the amplitude of low-frequency fluctuation/fractional amplitude of low-frequency fluctuation; FC, functional connectivity; MFG, middle frontal gyrus; PreCG, precentral gyrus; STG, superior temporal gyrus; R, right; L, left.
Regions With Significant Changes Between aMCI and HCs
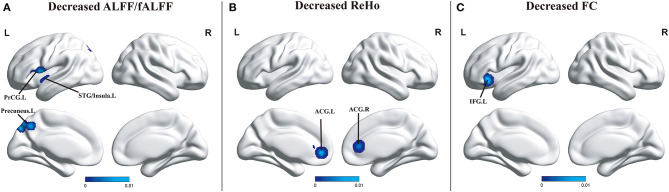
Compared with HCs, patients with aMCI revealed decreased ALFF/fALFF in the left STG (BA 22), left insula (BA 13), left PreCG (BA 44), and the precuneus (BA 7), and no brain region presenting increased ALFF/fALFF was observed, which was in line with what is seen in MCI. Differently, patients with aMCI showed decreased FC in the left inferior frontal gyrus (IFG) and decreased ReHo in the anterior cingulate gyrus (ACG). However, no brain area presenting increased FC or increased ReHo was observed (Figure 3).
Figure 3.
(A) Brain regions showing decreased ALFF/fALFF in patients with aMCI compared with HCs. (B) Brain regions showing decreased ReHo in patients with aMCI compared with HCs. (C) Brain regions showing decreased FC in patients with aMCI when compared with HCs. The color bar represents the p-value. Areas with decreased change relative to controls are displayed in blue, and areas with increased change are displayed in red. Results are thresholded at p < 0.01 cluster-corrected and p < 0.05 family-wise error corrected. Abbreviations: aMCI, amnestic mild cognitive impairment; HCs, healthy controls; ALFF/fALFF, the amplitude of low-frequency fluctuation/fractional amplitude of low-frequency fluctuation; ReHo, regional homogeneity; FC, functional connectivity; ACG, anterior cingulate gyrus; PreCG, precentral gyrus; STG, superior temporal gyrus; IFG, inferior frontal gyrus; R, right; L, left.
More details about clusters from the ALE analysis are summarized in Table 2.
Table 2.
All clusters from the ALE analysis.
| Cluster | Volume (mm3) | MNI | Maximum ALE value | Z value | Side | Anatomical regions | BA | ||
|---|---|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||||
| ALFF/fALFF | |||||||||
| MCI>HC | |||||||||
| NA | |||||||||
| MCI < HC | |||||||||
| 1 | 5,720 | −56 | 8 | 0 | 0.009969907 | 4.2067027 | Left | Superior temporal gyrus | 22 |
| 1 | 5,720 | −54 | 6 | 8 | 0.009410367 | 4.0713005 | Left | Precentral gyrus | 44 |
| 1 | 5,720 | −52 | 18 | 2 | 0.008123473 | 3.4053626 | Left | Precentral gyrus | 44 |
| 1 | 5,720 | −46 | 6 | −6 | 0.00798626 | 3.3795779 | Left | Insula | 13 |
| 1 | 5,720 | −46 | −2 | −2 | 0.007434398 | 3.2798133 | Left | Insula | 13 |
| 2 | 3,224 | −10 | −76 | 48 | 0.01004661 | 4.2205467 | Left | Precuneus | 7 |
| 2 | 3,224 | −16 | −76 | 42 | 0.00981178 | 4.1814437 | Left | Precuneus | 7 |
| 2 | 3,224 | −6 | −60 | 46 | 0.006709485 | 3.1047783 | Left | Precuneus | 7 |
| aMCI>HC | |||||||||
| NA | |||||||||
| aMCI < HC | |||||||||
| 1 | 6,928 | −56 | 8 | 0 | 0.009969907 | 4.322146 | Left | Superior temporal gyrus | 22 |
| 1 | 6,928 | −54 | 6 | 8 | 0.009410367 | 4.1647773 | Left | Precentral gyrus | 44 |
| 1 | 6,928 | −52 | 18 | 2 | 0.008123473 | 3.4509804 | Left | Precentral gyrus | 44 |
| 1 | 6,928 | −46 | 6 | −6 | 0.00798626 | 3.422147 | Left | Insula | 13 |
| 1 | 6,928 | −46 | −2 | −2 | 0.007434398 | 3.3515155 | Left | Insula | 13 |
| 2 | 4,128 | −10 | −76 | 48 | 0.01004661 | 4.335871 | Left | Precuneus | 7 |
| 2 | 4,128 | −16 | −76 | 42 | 0.00981178 | 4.297305 | Left | Precuneus | 7 |
| 2 | 4,128 | −6 | −60 | 46 | 0.006709485 | 3.1681263 | Left | Precuneus | 7 |
| ReHo | |||||||||
| MCI>HC | |||||||||
| NA | |||||||||
| MCI < HC | |||||||||
| NA | |||||||||
| aMCI>HC | |||||||||
| NA | |||||||||
| aMCI < HC | |||||||||
| 1 | 8,672 | −8 | 44 | −2 | 0.009139125 | 4.1076794 | Left | Anterior cingulate | 32 |
| 1 | 8,672 | 8 | 34 | 8 | 0.00801415 | 3.7818716 | Right | Anterior cingulate | 24 |
| FC | |||||||||
| MCI>HC | |||||||||
| 1 | 8,808 | 0 | −12 | 7.5 | 0 | 2.2455866 | Left | Thalamus | – |
| 1 | 8,808 | 0 | −7.5 | 16.5 | 0 | 2.3138068 | Left | Thalamus | – |
| 2 | 3,024 | −10.5 | 12 | 7.5 | 0.002202077 | 2.4327567 | Left | Caudate | – |
| 3 | 2,936 | −39 | −33 | 18 | 0.007414908 | 3.4869988 | Left | Superior temporal gyrus | 41 |
| 3 | 2,936 | −45 | −27 | 15 | 0.007187633 | 3.3858383 | Left | Insula | 41 |
| 4 | 2,848 | 29 | 12 | 44 | 0.008558434 | 3.769974 | Right | Middle frontal gyrus | 6 |
| 4 | 2,848 | 24 | 14 | 45 | 0.009539967 | 4.119189 | Right | Cingulate gyrus | 32 |
| MCI < HC | |||||||||
| 1 | 2,480 | −45 | 43.5 | 16.5 | 0.002824822 | 2.3455477 | Left | Middle frontal gyrus | 46 |
| 1 | 2,480 | −45 | 42 | 27 | 0.007414908 | 3.4772985 | Left | Superior frontal gyrus | 9 |
| aMCI < HC | |||||||||
| 1 | 3,848 | −34 | 24 | −8 | 0.011685779 | 4.9532757 | Left | Inferior frontal gyrus | 47 |
| aMCI>HC | |||||||||
| NA | |||||||||
BA, Brodmann area; ALE, anatomical/activation likelihood estimation; MCI, mild cognitive impairment; HC, healthy control; ALFF/fALFF, the amplitude of low-frequency fluctuation/fractional amplitude of low-frequency fluctuation; ReHo, regional homogeneity; FC, functional connectivity.
Jackknife Sensitivity Analysis
A jackknife sensitivity analysis revealed that the decreased ALFF/fALFF in the left PreCG was the most robust data replicable in all 12 datasets. The decreased ALFF/fALFF in the left STG and insula remained highly replicable in at least 11 combinations of the datasets. The decreased ALFF/fALFF in the left precuneus remained significant in at least nine combinations of the datasets. The results are presented in the Supplementary Material.
Discussion
To the best of our knowledge, this meta-analysis was the first to assess the functional integrity of the SN in MCI and aMCI. Compared with the healthy group, the specific abnormal brain regions in MCI were mainly located in the frontal lobe, including MFG, SFG, and PreCG; the limbic lobe, including cingulate gyrus; and the occipital lobe, including precuneus. Besides, there were also differences in the temporal lobe, including STG, and sub-lobar, including the thalamus and the caudate. Patients with aMCI demonstrated specific changes in the bilateral ACG and the left IFG.
Specific Imaging Abnormal Changes in SN
Both patients with MCI and patients with aMCI showed decreased ALFF/fALFF in the left STG, left insula, left PreCG, and the precuneus. This indicated that the intrinsic neural activity of these regions had significant dysfunction in MCI and aMCI when compared with HCs (Zhao et al., 2014; Zhuang et al., 2019).
The STG is related to the cognition process and social interaction and plays an important role in the function of language (Li et al., 2017b). Dysfunction of the STG may result in the impairment of language and social skills in MCI. Localized changes of the STG were present after mindfulness practice for three months in subjects with MCI (Fam et al., 2020). The insula, especially the anterior insula, is a critical node of the SN which connects many areas including the prefrontal cortex and the parietal lobe and engages in the transformation between cognitive domains (Cerliani et al., 2012). A study by Na et al. reported that the MCI subgroup revealed focal activation in the left anterior insula and ACC after 24 sessions of computerized cognitive training (Na et al., 2018). The PreCG, which overlaps with the SMA, is involved in producing and controlling the movement (Cai et al., 2017). Abnormalities in the PreCG may affect learning and memorizing and show sluggish behavior; a positive correlation between the damage of this region and dysfunction of verbal short-term memory was reported in MCI (Bi et al., 2018; Sakurai et al., 2018). Increased blood oxygen level-dependent signal activation in the PreCG in MCI after daily supplementation for 16 weeks was reported by a clinical study that investigated the curative effect of blueberry supplementation (Boespflug et al., 2018). The precuneus is associated with mental imagery, source memory, and working memory (Utevsky et al., 2014). Decreased activity of neurons in the precuneus was shown to be connected with a steeper decline of global cognition function (Verfaillie et al., 2018). Patients in the early stages of AD have been shown to present hypoperfusion or metabolic reduction of the precuneus, and a positive correlation between the weaker function of the precuneus and lower MoCA score or lower working memory performance has been reported (Miners et al., 2016; Yokosawa et al., 2020). Therefore, when converting from health to MCI, the dysfunction of these brain regions would occur, which could be considered as the neuroimaging marker of MCI. Moreover, these brain regions can be regarded as the target for intervention treatment and follow-up care.
Our meta-analysis also observed increased FC in the thalamus, the caudate, and the left STG/insula in MCI when compared with HCs. The thalamus contributes to attentional control by high-fidelity information relay to cortical regions and shifting and sustaining functional interactions across cortical areas and is conventionally recognized to be affected by AD pathology (Halassa and Kastner, 2017). Pathological studies of thalamic involvement in AD demonstrated that nearly all thalamic nuclei got extracellular amyloid deposits, as well as the presence of thalamic volume loss (Ryan et al., 2013). The caudate contributes to behavior via activating correct action schemas and selecting opportune sub-goals and is associated with various higher neurological functions. A recent study that analyzed resting-state networks in prodromal AD also detected increased FC in the caudate in patients with positive biomarkers suggestive of incipient AD (Conwell et al., 2018). Previous studies had confirmed that the reduced volume of the caudate was significant with MCI-to-AD conversion (Madsen et al., 2010). Thus, we demonstrated that increased FC of the thalamus and the caudate in the SN, which may be considered as a compensated mechanism within the SN in MCI, can also be a specific marker of converting from health to MCI. The absence of this mechanism predicts the progress from MCI to dementia.
Besides, significantly increased FC and decreased ALFF/fALFF were simultaneously observed in the left STG/insula in MCI, compared with HCs. Studies have demonstrated that the ALFF/fALFF focuses on the intrinsic neural oscillation alterations, while the FC focuses on the connective relationship of the brain (Chen et al., 2016; Zhou et al., 2020). Therefore, the increased connectivity of the SN in these two areas may make up for deficits in their neuronal activity. Moreover, we hypothesized that further dysfunction of partial neurons of some brain regions would occur in subjects when converting to MCI. However, the other neurons of these brain regions could make some compensation by increasing their own activity or by connecting with others to maintain their cognitive state. Increased alteration in the STG/insula, as well as the increased FC in the left thalamus and the caudate, observed in this study could make up for the dysfunction to some extent, and the STG/insula might be the most susceptible one. As the disease progresses, the normal functional patterns are affected and partial compensatory is necessary.
With regard to ReHo, few studies have reported significant changes in the SN in MCI. An rs-fMRI study composing 30 MCI and 32 HCs revealed an increased alteration in the left precuneus in MCI (Wang et al., 2015), and other studies reported an increase in the left lingual gyrus or the right MFG, compared with HCs (Yuan X. et al., 2016; Luo et al., 2018). Some studies have demonstrated a reduction in changes in the right SG, the left MFG, or the left ACC (Zhang et al., 2012; Yuan B. et al., 2016). However, in this study, we did not observe consistent results from the ALE analysis. This could be explained by the fact that limited rs-fMRI studies focusing on the SN to investigate ReHo changes in MCI were available. Moreover, different stages of MCI may present different changes in the SN.
In terms of patients with aMCI, compared with HCs, besides consistent results of alterations in MCI, diminished ReHo was explicitly observed in the bilateral ACG, which indicated that local synchronization of the bilateral ACG was significantly declined in aMCI (Bai et al., 2008; Zhang et al., 2010). The ACG, in healthy and clinical individuals, plays a vital role in the conflict-monitoring process in attentional tasks (Borsa et al., 2018). Luks et al. reported that in the clinical populations, the atrophy of ACC and DLPFC in the left hemisphere was directly associated with poorer attentional control accuracy in patients with neurodegenerative diseases, compared with HCs (Luks et al., 2010). It is also imperative to note that studies have confirmed the impairment of endogenously orienting attention in patients with aMCI and AD. Furthermore, several studies combining MRI and SPECT have reported that aMCI converters can augment hypometabolism in ACG, when compared with subjects who did not convert to AD (Van Dam et al., 2013; McLaughlin et al., 2014). Therefore, the specific alteration of the ACG may help to distinguish aMCI from healthy elderly and even predict whether patients with aMCI will convert to AD.
Salience Network Interaction With Other Networks
Reviewing the results of ALE analysis of the FC of the SN, interactions of the SN with DMN and ECN had been observed in MCI or aMCI. The key areas exhibiting interactions with DMN included the SFC and the ACC (Liao et al., 2010; Cunningham et al., 2017), and the ones showing interactions with ECN were mainly the SFG and the IFG (Xu et al., 2020). Recently, studies based on three large-scale functional brain networks or a triple-network model, the DMN, SN, and ECN, have been carried out (Joo et al., 2016; Li et al., 2019; Yu et al., 2019). The main functions of the three networks are not similar but they have anatomical coincident areas and co-activation. Regulating other networks might be a possible function of the SN, and dysregulated interactions among the three networks closely correlate with the cognitive dysfunction (Jilka et al., 2014; Liao et al., 2018).
The DMN, which is the main focus of neural-related rs-fMRI, is a task-negative network associated with information processing related to memory, emotion, and learning and contributes to support mind wandering during self-referential mental processing. The network is active when the individual is in the resting state (Esposito et al., 2018). The SN is coupling with the DMN, and the increased coupling within the two networks can be observed across cognitive tasks (Elton and Gao, 2015). Converging evidence indicates a linear relationship between the amyloid deposition and the dysfunction of DMN connectivity (Schultz et al., 2020). Previous studies concerning a deficit in DMN revealed a much more significant alteration in the AD group, thereby suggesting that the DMN had a progression of the connectivity deficit (Zhu et al., 2016). Consistent with previous reports, our results demonstrated that the SFG, which is one of the typical regions of the DMN, showed lower FC with the SN in MCI groups when compared with HCs (Liang et al., 2012). The ACC is the anterior part of the cingulate cortex (Torta et al., 2013). This brain region is the core one of the SN and is involved in integrating and coordinating information to guide behavior (Colangeli et al., 2016; Zeuner et al., 2016). However, the posterior part of the cingulate cortex (posterior cingulate cortex, PCC) is the core node of the DMN (Vogt, 2005; Cera et al., 2019). Studies have shown disrupted FC in the PCC in MCI when compared with HCs (Dillen et al., 2017). According to the posterior-to-anterior shift in aging theory, the cognitive performance is maintained by the recruitment of anterior regions when the function of the posterior region is impaired (Grady, 2012; Skouras et al., 2019). Compared with HCs, the increased FC of the ACC in the SN in MCI might be interpreted as a compensation mechanism to counter decay affected by impairments of posterior regions, thereby suggesting that the interaction among networks may be associated with a structural location.
The ECN governs executive functions, including problem-solving and working memory, and plays a key role in the regulation of cognition and the integration of memory and sensory information (Xu et al., 2020). Patients with MCI showed a significantly decreased FC, as well as increased FC, of the SN in the left MFG compared with HCs (He et al., 2014). The MFG is important for the execution of emotion regulation strategies (Japee et al., 2015). The executive functional impairment is considered to aggravate memory decline (Yuan B. et al., 2016). When the executive function of individuals was mildly affected, some compensatory changes were particularly necessary. This implied that impairment mechanism and compensation mechanism coexist among the SN-ECN connectivity in patients with MCI. Additionally, the IFG exhibited an aMCI-specific change for the SN-ECN connectivity in the FC in this study. The IFG, along with the hippocampus, drives encoding and retrieval of memory (Hampstead et al., 2016). This area also contributes to response inhibition, which is an important element in executive control (Jahanshahi et al., 2015). A study by He et al. reported significant differences in the internetwork FCs of the SN with the IFG in aMCI (He et al., 2014). Besides the SN-ECN connectivity, analyzing the FC of the DMN in aMCI revealed decreased FC between the DMN and the left IFG (Li et al., 2017c). Thus, the abnormality of the IFG could be considered as one of the major factors contributing to the pathological mechanism of aMCI. Studies have also reported the gradient change in the FC of the ECN from aMCI to AD (Zhu et al., 2016). This further supports the abnormality of FC as a biomarker for monitoring disease progression.
The FC-altered interactions between SN and other networks, such as DMN and ECN, were mainly present in the prefrontal lobe (SFG, MFG, and IFG) (Petrelli et al., 2020). Studies have shown that when compared with HCs, the perfusion in the frontal lobe was significantly lower in MCI (Zhang et al., 2019). A recent study reported that following 12 weeks of exercise training, there was an increase in the FC in the prefrontal lobe and insular lobe in MCI (Chirles et al., 2017). A meta-analysis for target identification in the non-invasive brain stimulation of AD and behavioral variant frontotemporal dementia demonstrated the core targets, including the MFG and SFG (Pievani et al., 2017). A systematic review of repetitive transcranial magnetic stimulation (rTMS) effects revealed that high-frequency rTMS, which targeted the left DLPFC, significantly improved the memory power (Chou et al., 2020). Thus, the prefrontal lobe, particularly the IFG in aMCI, can be a key target for intervention.
Limitations
This meta-analysis had some limitations. First, the heterogeneity is the main limitation of this study and is due to the following four aspects: (1) data sources with different demographics of the subjects and acquisition parameters; (2) data quality, such as differences in motion correction and noise, affected the results; (3) data processing schemes including divergent preprocessing protocols, seed selection methods, and threshold settings; and (4) aberrant structural abnormalities, which were related to functional connectivity, were ignored in some studies. Second, besides the MCI group, we analyzed only the aMCI group. A meta-analysis based on naMCI was not conducted due to the inadequacy of the number of relative papers. Analysis of the subtype of MCI such as early MCI or later MCI is also neglected. Finally, we excluded the literature that was not published in English; hence, the data, which we have collected, might be incomplete.
Clinical Implications
Although amounts of single studies have been done to determine the characteristics of the SN of patients with MCI and its subtype, making quantitative neuroimaging analysis is needed to summarize the neurobiology of the SN in MCI and aMCI. Uniform and precious results determine more exact targets for moderate interventions such as drug, transcranial direct current stimulation, rTMS, deep brain stimulation, and proper functional training. Core brain regions, such as STG/insula, the left MFG, and IFG, can be considered as potential targets in patients with MCI or aMCI. At the same time, these brain areas are also regarded as focuses for follow-up to understand the effect of interventions and the pathogenesis of diseases. To sum up, our results strongly demonstrated the specific characteristics of affected brain areas and provided new insights for targeted treatment and follow-up care.
Conclusions
By conducting the ALE meta-analysis in patients with MCI and aMCI to identify the functional alterations about the SN, we have demonstrated the coexistence of impairment and compensation in the SN of patients with MCI, which is observed mainly in the SFG and the insula. Moreover, interactions of the SN with other networks, including changed the SN-DME connectivity and changed the SN-ECN connectivity, are also observed in MCI or aMCI. These findings can be a potential imaging biomarker for MCI or aMCI. Meanwhile, it has also provided a new insight in predicting the progression from a healthy state to MCI and novel targets for proper intervention to delay the further decline of cognitive function. Further research about MCI with comparisons between its subtypes needs to be carried out to explore more specific biomarkers for the predementia phase of AD.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
YS designed the study under the guidance of XL and JC. YS and WX performed the study extraction, meta-analysis, and drafting of the manuscript. SC, GH, HG, CX, and WQ helped in literature search and data analyses. All authors contributed toward revising the manuscript and gave the final approval of the version to be published.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
Funding. This study was supported by the National Natural Science Foundation of China (No. 81701675); the Key Research and Development Plan (Social Development) Project of Jiangsu Province (No. BE2018608); the Cooperative Research Project of Southeast University-Nanjing Medical University (No. 2018DN0031); the Key Project supported by Medical Science and Technology Development Foundation, Nanjing Department of Health (No. JQX18005); and Innovation and Entrepreneurship Training Program for College Students in Jiangsu Province (No.201810312061X and 201910312035Z).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2021.695210/full#supplementary-material
References
- Agosta F., Pievani M., Geroldi C., Copetti M., Frisoni G. B., Filippi M. (2012). Resting state fMRI in Alzheimer's disease: beyond the default mode network. Neurobiol. Aging 33, 1564–1578. 10.1016/j.neurobiolaging.2011.06.007 [DOI] [PubMed] [Google Scholar]
- Albert M. S., DeKosky S. T., Dickson D., Dubois B., Feldman H. H., Fox N. C., et al. (2011). The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 7, 270–279. 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai F., Zhang Z., Yu H., Shi Y., Yuan Y., Zhu W., et al. (2008). Default-mode network activity distinguishes amnestic type mild cognitive impairment from healthy aging: a combined structural and resting-state functional MRI study. Neurosci. Lett. 438, 111–115. 10.1016/j.neulet.2008.04.021 [DOI] [PubMed] [Google Scholar]
- Bi X. A., Xu Q., Luo X., Sun Q., Wang Z. (2018). Weighted random support vector machine clusters analysis of resting-state fMRI in mild cognitive impairment. Front. Psychiatry 9:340. 10.3389/fpsyt.2018.00340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boespflug E. L., Eliassen J. C., Dudley J. A., Shidler M. D., Kalt W., Summer S. S., et al. (2018). Enhanced neural activation with blueberry supplementation in mild cognitive impairment. Nutr. Neurosci. 21, 297–305. 10.1080/1028415X.2017.1287833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsa V. M., Della Rosa P. A., Catricalà E., Canini M., Iadanza A., Falini A., et al. (2018). Interference and conflict monitoring in individuals with amnestic mild cognitive impairment: a structural study of the anterior cingulate cortex. J. Neuropsychol. 12, 23–40. 10.1111/jnp.12105 [DOI] [PubMed] [Google Scholar]
- Browndyke J., Giovanello K., Petrella J., Hayden K., Chiba-Falek O., Tucker K., et al. (2013). Phenotypic regional functional imaging patterns during memory encoding in mild cognitive impairment and Alzheimer's disease. Alzheimers Dement. 9, 284–294. 10.1016/j.jalz.2011.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley R. F., Schultz A. P., Hedden T., Papp K. V., Hanseeuw B. J., Marshall G., et al. (2017). Functional network integrity presages cognitive decline in preclinical Alzheimer disease. Neurology 89, 29–37. 10.1212/WNL.0000000000004059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield D. A., Boyd-Kimball D. (2018). Oxidative stress, amyloid-β peptide, and altered key molecular pathways in the pathogenesis and progression of Alzheimer's disease. J. Alzheimer's Dis. 62, 1345–1367. 10.3233/JAD-170543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai C., Huang C., Yang C., Lu H., Hong X., Ren F., et al. (2020a). Altered patterns of functional connectivity and causal connectivity in salience subnetwork of subjective cognitive decline and amnestic mild cognitive impairment. Front. Neurosci. 14:288. 10.3389/fnins.2020.00288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai C., Huang C., Yang C., Zhang X., Peng Y., Zhao W., et al. (2020b). Altered patterns of phase position connectivity in default mode subnetwork of subjective cognitive decline and amnestic mild cognitive impairment. Front. Neurosci. 14:185. 10.3389/fnins.2020.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai S., Chong T., Peng Y., Shen W., Li J., von Deneen K. M., et al. (2017). Altered functional brain networks in amnestic mild cognitive impairment: a resting-state fMRI study. Brain Imaging Behav. 11, 619–631. 10.1007/s11682-016-9539-0 [DOI] [PubMed] [Google Scholar]
- Cai S., Wang Y., Kang Y., Wang H., Kim H., von Deneen K. M., et al. (2018). Differentiated Regional homogeneity in progressive mild cognitive impairment: a study with post-hoc label. Am. J. Alzheimer's Dis. Other Dement. 33, 373–384. 10.1177/1533317518778513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cera N., Esposito R., Cieri F., Tartaro A. (2019). Altered cingulate cortex functional connectivity in normal aging and mild cognitive impairment. Front. Neurosci. 13:857. 10.3389/fnins.2019.00857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerliani L., Thomas R. M., Jbabdi S., Siero J. C., Nanetti L., Crippa A., et al. (2012). Probabilistic tractography recovers a rostrocaudal trajectory of connectivity variability in the human insular cortex. Hum. Brain Mapp. 33, 2005–2034. 10.1002/hbm.21338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha J., Hwang J. M., Jo H. J., Seo S. W., Na D. L., Lee J. M. (2015). Assessment of functional characteristics of amnestic mild cognitive impairment and Alzheimer's disease using various methods of resting-state FMRI analysis. BioMed. Res. Int. 2015:907464. 10.1155/2015/907464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chand G. B., Wu J., Hajjar I., Qiu D. (2017). Interactions of the salience network and its subsystems with the default-mode and the central-executive networks in normal aging and mild cognitive impairment. Brain Connect. 7, 401–412. 10.1089/brain.2017.0509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Li Y., Liu Q., Shi Q., Wang J., Shen H., et al. (2019). Abnormal interactions of the salience network, central executive network, and default-mode network in patients with different cognitive impairment loads caused by leukoaraiosis. Front. Neural Circuits 13:42. 10.3389/fncir.2019.00042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Chen G., Shu H., Chen G., Ward B. D., Wang Z., et al. (2019). Predicting progression from mild cognitive impairment to Alzheimer's disease on an individual subject basis by applying the CARE index across different independent cohorts. Aging 11, 2185–2201. 10.18632/aging.101883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Shu H., Wang Z., Zhan Y., Liu D., Liao W., et al. (2016). Convergent and divergent intranetwork and internetwork connectivity patterns in patients with remitted late-life depression and amnestic mild cognitive impairment. Cortex 83, 194–211. 10.1016/j.cortex.2016.08.001 [DOI] [PubMed] [Google Scholar]
- Chen S., Xu W., Xue C., Hu G., Ma W., Qi W., et al. (2020). Voxelwise meta-analysis of gray matter abnormalities in mild cognitive impairment and subjective cognitive decline using activation likelihood estimation. J. Alzheimer's Dis. 77, 1495–1512. 10.3233/JAD-200659 [DOI] [PubMed] [Google Scholar]
- Cheng H., Sun G., Li M., Yin M., Chen H. (2019). Neuron loss and dysfunctionality in hippocampus explain aircraft noise induced working memory impairment: a resting-state fMRI study on military pilots. Biosci. Trends 13, 430–440. 10.5582/bst.2019.01190 [DOI] [PubMed] [Google Scholar]
- Chirles T. J., Reiter K., Weiss L. R., Alfini A. J., Nielson K. A., Smith J. C. (2017). Exercise training and functional connectivity changes in mild cognitive impairment and healthy elders. J. Alzheimer's Dis. 57, 845–856. 10.3233/JAD-161151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou Y. H., Ton That V., Sundman M. (2020). A systematic review and meta-analysis of rTMS effects on cognitive enhancement in mild cognitive impairment and Alzheimer's disease. Neurobiol. Aging 86, 1–10. 10.1016/j.neurobiolaging.2019.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colangeli S., Boccia M., Verde P., Guariglia P., Bianchini F., Piccardi L. (2016). Cognitive reserve in healthy aging and Alzheimer's disease: a meta-analysis of fMRI studies. Am. J. Alzheimer's Dis. Other Dement. 31, 443–449. 10.1177/1533317516653826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conwell K., von Reutern B., Richter N., Kukolja J., Fink G. R., Onur O. A. (2018). Test-retest variability of resting-state networks in healthy aging and prodromal Alzheimer's disease. NeuroImage Clin. 19, 948–962. 10.1016/j.nicl.2018.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper C., Sommerlad A., Lyketsos C., Livingston G. (2015). Modifiable predictors of dementia in mild cognitive impairment: a systematic review and meta-analysis. Am. J. Psychiatry 172, 323–334. 10.1176/appi.ajp.2014.14070878 [DOI] [PubMed] [Google Scholar]
- Cunningham S. I., Tomasi D., Volkow N. D. (2017). Structural and functional connectivity of the precuneus and thalamus to the default mode network. Hum. Brain Mapp. 38, 938–956. 10.1002/hbm.23429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillen K. N. H., Jacobs H. I. L., Kukolja J., Richter N., von Reutern B., Onur Ö., et al. (2017). Functional disintegration of the default mode network in prodromal alzheimer's disease. J. Alzheimer's Dis. 59, 169–187. 10.3233/JAD-161120 [DOI] [PubMed] [Google Scholar]
- Eickhoff S., Bzdok D., Laird A., Kurth F., Fox P. (2012). Activation likelihood estimation meta-analysis revisited. NeuroImage 59, 2349–2361. 10.1016/j.neuroimage.2011.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elton A., Gao W. (2015). Task-positive functional connectivity of the default mode network transcends task domain. J. Cogn. Neurosci. 27, 2369–2381. 10.1162/jocn_a_00859 [DOI] [PubMed] [Google Scholar]
- Esposito R., Cieri F., Chiacchiaretta P., Cera N., Lauriola M., Di Giannantonio M., et al. (2018). Modifications in resting state functional anticorrelation between default mode network and dorsal attention network: comparison among young adults, healthy elders and mild cognitive impairment patients. Brain Imaging Behav. 12, 127–141. 10.1007/s11682-017-9686-y [DOI] [PubMed] [Google Scholar]
- Fam J., Sun Y., Qi P., Lau R. C., Feng L., Kua E. H., et al. (2020). Mindfulness practice alters brain connectivity in community-living elders with mild cognitive impairment. Psychiatry Clin. Neurosci. 74, 257–262. 10.1111/pcn.12972 [DOI] [PubMed] [Google Scholar]
- Gao H., Liu M., Zhao Z., Yang C., Zhu L., Cai Y., et al. (2020). Diagnosis of mild cognitive impairment and alzheimer's disease by the plasma and serum amyloid-beta 42 assay through highly sensitive peptoid nanosheet sensor. ACS Appl. Mater. Interfaces 12, 9693–9700. 10.1021/acsami.0c00370 [DOI] [PubMed] [Google Scholar]
- Grady C. (2012). The cognitive neuroscience of ageing. Nat. Rev. Neurosci. 13, 491–505. 10.1038/nrn3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa M. M., Kastner S. (2017). Thalamic functions in distributed cognitive control. Nat. Neurosci. 20, 1669–1679. 10.1038/s41593-017-0020-1 [DOI] [PubMed] [Google Scholar]
- Hampstead B. M., Khoshnoodi M., Yan W., Deshpande G., Sathian K. (2016). Patterns of effective connectivity during memory encoding and retrieval differ between patients with mild cognitive impairment and healthy older adults. Neuroimage 124, 997–1008. 10.1016/j.neuroimage.2015.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y., Wang J., Zhao Z., Min B., Lu J., Li K., et al. (2011). Frequency-dependent changes in the amplitude of low-frequency fluctuations in amnestic mild cognitive impairment: a resting-state fMRI study. Neuroimage 55, 287–295. 10.1016/j.neuroimage.2010.11.059 [DOI] [PubMed] [Google Scholar]
- Harrington D. L., Shen Q., Castillo G. N., Filoteo J. V., Litvan I., Takahashi C., et al. (2017). Aberrant intrinsic activity and connectivity in cognitively normal parkinson's disease. Front. Aging Neurosci. 9:197. 10.3389/fnagi.2017.00197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Qin W., Liu Y., Zhang X., Duan Y., Song J., et al. (2014). Abnormal salience network in normal aging and in amnestic mild cognitive impairment and Alzheimer's disease. Hum. Brain Mapp. 35, 3446–3464. 10.1002/hbm.22414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hétu S., Grégoire M., Saimpont A., Coll M., Eugène F., Michon P., et al. (2013). The neural network of motor imagery: an ALE meta-analysis. Neurosci. Biobehav. Rev. 37, 930–949. 10.1016/j.neubiorev.2013.03.017 [DOI] [PubMed] [Google Scholar]
- Jahanshahi M., Obeso I., Rothwell J. C., Obeso J. A. (2015). A fronto-striato-subthalamic-pallidal network for goal-directed and habitual inhibition. Nat. Rev. Neurosci. 16, 719–732. 10.1038/nrn4038 [DOI] [PubMed] [Google Scholar]
- Japee S., Holiday K., Satyshur M. D., Mukai I., Ungerleider L. G. (2015). A role of right middle frontal gyrus in reorienting of attention: a case study. Front. Syst. Neurosci. 9:23. 10.3389/fnsys.2015.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia B., Liu Z., Min B., Wang Z., Zhou A., Li Y., et al. (2015). The effects of acupuncture at real or sham acupoints on the intrinsic brain activity in mild cognitive impairment patients. Evid. Based Complement. Alternat. Med. 2015:529675. 10.1155/2015/529675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilka S. R., Scott G., Ham T., Pickering A., Bonnelle V., Braga R. M., et al. (2014). Damage to the salience network and interactions with the default mode network. J. Neurosci. 34, 10798–10807. 10.1523/JNEUROSCI.0518-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo S. H., Lee C. U., Lim H. K. (2017). Apathy and intrinsic functional connectivity networks in amnestic mild cognitive impairment. Neuropsychiatr. Dis. Treat. 13, 61–67. 10.2147/NDT.S123338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo S. H., Lim H. K., Lee C. U. (2016). Three large-scale functional brain networks from resting-state functional MRI in subjects with different levels of cognitive impairment. psychiatry investigation 13, 1–7. 10.4306/pi.2016.13.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser R. H., Andrews-Hanna J. R., Wager T. D., Pizzagalli D. A. (2015). Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry 72, 603–611. 10.1001/jamapsychiatry.2015.0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazaee A., Ebrahimzadeh A., Babajani-Feremi A. (2016). Application of advanced machine learning methods on resting-state fMRI network for identification of mild cognitive impairment and Alzheimer's disease. Brain Imaging Behav. 10, 799–817. 10.1007/s11682-015-9448-7 [DOI] [PubMed] [Google Scholar]
- Kollndorfer K., Krajnik J., Woitek R., Freiherr J., Prayer D., Schöpf V. (2013). Altered likelihood of brain activation in attention and working memory networks in patients with multiple sclerosis: an ALE meta-analysis. Neurosci. Biobehav. Rev. 37, 2699–2708. 10.1016/j.neubiorev.2013.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Li Y., Zheng L., Zhu X., Shao B., Fan G., et al. (2019). Abnormal brain network connectivity in a triple-network model of Alzheimer's disease. J. Alzheimer's Dis. 69, 237–252. 10.3233/JAD-181097 [DOI] [PubMed] [Google Scholar]
- Li M., Meng Y., Wang M., Yang S., Wu H., Zhao B., et al. (2017a). Cerebral gray matter volume reduction in subcortical vascular mild cognitive impairment patients and subcortical vascular dementia patients, and its relation with cognitive deficits. Brain Behav. 7:e00745. 10.1002/brb3.745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Zheng G., Zheng Y., Xiong Z., Xia R., Zhou W., et al. (2017b). Alterations in resting-state functional connectivity of the default mode network in amnestic mild cognitive impairment: an fMRI study. BMC Med. Imaging 17:48. 10.1186/s12880-017-0221-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M. G., Liu T. F., Zhang T. H., Chen Z. Y., Nie B. B., Lou X., et al. (2020). Alterations of regional homogeneity in Parkinson's disease with mild cognitive impairment: a preliminary resting-state fMRI study. Neuroradiology 62, 327–334. 10.1007/s00234-019-02333-7 [DOI] [PubMed] [Google Scholar]
- Li X., Wang F., Liu X., Cao D., Cai L., Jiang X., et al. (2020). Changes in brain function networks in patients with amnestic mild cognitive impairment: a resting-state fMRI Study. Front. Neurol. 11:554032. 10.3389/fneur.2020.554032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Jing B., Liu H., Li Y., Gao X., Li Y., et al. (2017c). Frequency-dependent changes in the amplitude of low-frequency fluctuations in mild cognitive impairment with mild depression. J. Alzheimer's Dis. 58, 1175–1187. 10.3233/JAD-161282 [DOI] [PubMed] [Google Scholar]
- Liang P., Wang Z., Yang Y., Li K. (2012). Three subsystems of the inferior parietal cortex are differently affected in mild cognitive impairment. J. Alzheimer's Dis. 30, 475–487. 10.3233/JAD-2012-111721 [DOI] [PubMed] [Google Scholar]
- Liang P., Xiang J., Liang H., Qi Z., Li K., Alzheimer's Disease NeuroImaging I. (2014). Altered amplitude of low-frequency fluctuations in early and late mild cognitive impairment and Alzheimer's disease. Curr. Alzheimer Res. 11, 389–398. 10.2174/1567205011666140331225335 [DOI] [PubMed] [Google Scholar]
- Liao W., Chen H., Feng Y., Mantini D., Gentili C., Pan Z., et al. (2010). Selective aberrant functional connectivity of resting state networks in social anxiety disorder. Neuroimage 52, 1549–1558. 10.1016/j.neuroimage.2010.05.010 [DOI] [PubMed] [Google Scholar]
- Liao Z. L., Tan Y. F., Qiu Y. J., Zhu J. P., Chen Y., Lin S. S., et al. (2018). Interhemispheric functional connectivity for Alzheimer's disease and amnestic mild cognitive impairment based on the triple network model. J. Zhejiang Univ. Sci. B 19, 924–934. 10.1631/jzus.B1800381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Bai F., Yue C., Shi Y., Yu H., Luo B., et al. (2014). The association between TOMM40 gene polymorphism and spontaneous brain activity in amnestic mild cognitive impairment. J. Neurol. 261, 1499–1507. 10.1007/s00415-014-7368-x [DOI] [PubMed] [Google Scholar]
- Liu Z., Wei W., Bai L., Dai R., You B., Chen S., et al. (2014). Exploring the patterns of acupuncture on mild cognitive impairment patients using regional homogeneity. PLoS ONE 9:e99335. 10.1371/journal.pone.0099335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston G., Sommerlad A., Orgeta V., Costafreda S. G., Huntley J., Ames D., et al. (2017). Dementia prevention, intervention, and care. Lancet 390, 2673–2734. 10.1016/S0140-6736(17)31363-6 [DOI] [PubMed] [Google Scholar]
- Lo C. K., Mertz D., Loeb M. (2014). Newcastle-Ottawa scale: comparing reviewers' to authors' assessments. BMC Med. Res. Methodol. 14:45. 10.1186/1471-2288-14-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luks T. L., Oliveira M., Possin K. L., Bird A., Miller B. L., Weiner M. W., et al. (2010). Atrophy in two attention networks is associated with performance on a Flanker task in neurodegenerative disease. Neuropsychologia 48, 165–170. 10.1016/j.neuropsychologia.2009.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X., Jiaerken Y., Huang P., Xu X. J., Qiu T., Jia Y., et al. (2018). Alteration of regional homogeneity and white matter hyperintensities in amnestic mild cognitive impairment subtypes are related to cognition and CSF biomarkers. Brain Imaging Behav. 12, 188–200. 10.1007/s11682-017-9680-4 [DOI] [PubMed] [Google Scholar]
- Lyles R. H., Lin J. (2010). Sensitivity analysis for misclassification in logistic regression via likelihood methods and predictive value weighting. Stat. Med. 29, 2297–2309. 10.1002/sim.3971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen S. K., Ho A. J., Hua X., Saharan P. S., Toga A. W., Jack C. R., Jr., et al. (2010). 3D maps localize caudate nucleus atrophy in 400 Alzheimer's disease, mild cognitive impairment, and healthy elderly subjects. Neurobiol. Aging 31, 1312–1325. 10.1016/j.neurobiolaging.2010.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamah D., Barch D. M., Repovš G. (2013). Resting state functional connectivity of five neural networks in bipolar disorder and schizophrenia. J. Affect. Disord. 150, 601–609. 10.1016/j.jad.2013.01.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar R. (2011). The neural bases of social cognition and story comprehension. Annu. Rev. Psychol. 62, 103–134. 10.1146/annurev-psych-120709-145406 [DOI] [PubMed] [Google Scholar]
- McLaughlin P. M., Anderson N. D., Rich J. B., Chertkow H., Murtha S. J. (2014). Visual selective attention in amnestic mild cognitive impairment. J. Gerontol. B. Psychol. Sci. Soc. Sci. 69, 881–891. 10.1093/geronb/gbt077 [DOI] [PubMed] [Google Scholar]
- Menon V. (2011). Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 15, 483–506. 10.1016/j.tics.2011.08.003 [DOI] [PubMed] [Google Scholar]
- Min J., Zhou X. X., Zhou F., Tan Y., Wang W. D. (2019). A study on changes of the resting-state brain function network in patients with amnestic mild cognitive impairment. Braz. J. Med. Biol. Res. 52:8244. 10.1590/1414-431x20198244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miners J. S., Palmer J. C., Love S. (2016). Pathophysiology of hypoperfusion of the precuneus in early Alzheimer's disease. Brain Pathol. (Zurich, Switzeland) 26, 533–541. 10.1111/bpa.12331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na H. R., Lim J. S., Kim W. J., Jang J. W., Baek M. J., Kim J., et al. (2018). Multimodal Assessment of neural substrates in computerized cognitive training: a preliminary study. J. Clin. Neurol. (Seoul, Korea) 14, 454–463. 10.3988/jcn.2018.14.4.454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni L., Liu R., Yin Z., Zhao H., Nedelska Z., Hort J., et al. (2016). Aberrant spontaneous brain activity in patients with mild cognitive impairment and concomitant lacunar infarction: a resting-state functional MRI study. J. Alzheimer's Dis. 50, 1243–1254. 10.3233/JAD-150622 [DOI] [PubMed] [Google Scholar]
- Onoda K., Ishihara M., Yamaguchi S. (2012). Decreased functional connectivity by aging is associated with cognitive decline. J. Cogn. Neurosci. 24, 2186–2198. 10.1162/jocn_a_00269 [DOI] [PubMed] [Google Scholar]
- Petrelli F., Dallérac G., Pucci L., Calì C., Zehnder T., Sultan S., et al. (2020). Dysfunction of homeostatic control of dopamine by astrocytes in the developing prefrontal cortex leads to cognitive impairments. Mol. Psychiatry 25, 732–749. 10.1038/s41380-018-0226-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pievani M., Pini L., Ferrari C., Pizzini F. B., Boscolo Galazzo I., Cobelli C., et al. (2017). Coordinate-based meta-analysis of the default mode and salience network for target identification in non-invasive brain stimulation of Alzheimer's disease and behavioral variant frontotemporal dementia networks. J. Alzheimer's Dis. 57, 825–843. 10.3233/JAD-161105 [DOI] [PubMed] [Google Scholar]
- Qing Z., Li W., Nedelska Z., Wu W., Wang F., Liu R., et al. (2017). Spatial navigation impairment is associated with alterations in subcortical intrinsic activity in mild cognitive impairment: a resting-state fMRI study. Behav. Neurol. 2017:6364314. 10.1155/2017/6364314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan N. S., Keihaninejad S., Shakespeare T. J., Lehmann M., Crutch S. J., Malone I. B., et al. (2013). Magnetic resonance imaging evidence for presymptomatic change in thalamus and caudate in familial Alzheimer's disease. Brain 136, 1399–1414. 10.1093/brain/awt065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai Y., Furukawa E., Kurihara M., Sugimoto I. (2018). Frontal phonological agraphia and acalculia with impaired verbal short-term memory due to left inferior precentral gyrus lesion. Case Rep. Neurol. 10, 72–82. 10.1159/000487849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarli G., De Marco M., Hallikainen M., Soininen H., Bruno G., Venneri A. (2021). Regional strength of large-scale functional brain networks is associated with regional volumes in older adults and in Alzheimer's disease. Brain Connect. 11, 201–212. 10.1089/brain.2020.0899 [DOI] [PubMed] [Google Scholar]
- Schultz A. P., Buckley R. F., Hampton O. L., Scott M. R., Properzi M. J., Peña-Gómez C., et al. (2020). Longitudinal degradation of the default/salience network axis in symptomatic individuals with elevated amyloid burden. NeuroImage Clin. 26:102052. 10.1016/j.nicl.2019.102052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skouras S., Falcon C., Tucholka A., Rami L., Sanchez-Valle R., Lladó A., et al. (2019). Mechanisms of functional compensation, delineated by eigenvector centrality mapping, across the pathophysiological continuum of Alzheimer's disease. NeuroImage Clin. 22:101777. 10.1016/j.nicl.2019.101777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K., Cai W., Krishnadas R., Palaniyappan L., Menon V. (2019). Dysregulated brain dynamics in a triple-network saliency model of schizophrenia and its relation to psychosis. Biol. Psychiatry 85, 60–69. 10.1016/j.biopsych.2018.07.020 [DOI] [PubMed] [Google Scholar]
- Torta D. M., Costa T., Duca S., Fox P. T., Cauda F. (2013). Parcellation of the cingulate cortex at rest and during tasks: a meta-analytic clustering and experimental study. Front. Hum. Neurosci. 7:275. 10.3389/fnhum.2013.00275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utevsky A. V., Smith D. V., Huettel S. A. (2014). Precuneus is a functional core of the default-mode network. J. Neurosci. 34, 932–940. 10.1523/JNEUROSCI.4227-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dam N. T., Sano M., Mitsis E. M., Grossman H. T., Gu X., Park Y., et al. (2013). Functional neural correlates of attentional deficits in amnestic mild cognitive impairment. PLoS ONE 8:e54035. 10.1371/journal.pone.0054035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verfaillie S. C. J., Slot R. E. R., Dicks E., Prins N. D., Overbeek J. M., Teunissen C. E., et al. (2018). A more randomly organized grey matter network is associated with deteriorating language and global cognition in individuals with subjective cognitive decline. Hum. Brain Mapp. 39, 3143–3151. 10.1002/hbm.24065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt B. A. (2005). Pain and emotion interactions in subregions of the cingulate gyrus. Nat. Rev. Neurosci. 6, 533–544. 10.1038/nrn1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Wang R., Li F., Lin Q., Zhao X., Hu Z. (2020). Large-scale granger causal brain network based on resting-state fMRI data. Neuroscience 425, 169–180. 10.1016/j.neuroscience.2019.11.006 [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhao X., Xu S., Yu L., Wang L., Song M., et al. (2015). Using regional homogeneity to reveal altered spontaneous activity in patients with mild cognitive impairment. BioMed. Res. Int. 2015:807093. 10.1155/2015/807093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Yan C., Zhao C., Qi Z., Zhou W., Lu J., et al. (2011). Spatial patterns of intrinsic brain activity in mild cognitive impairment and alzheimer's disease: a resting-state functional MRI study. Hum. Brain Mapp. 32, 1720–1740. 10.1002/hbm.21140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W., Chen S., Xue C., Hu G., Ma W., Qi W., et al. (2020). Functional MRI-specific alterations in executive control network in mild cognitive impairment: an ALE meta-analysis. Front. Aging Neurosci.12:578863. 10.3389/fnagi.2020.578863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Yan Y., Wang Y., Hu X., Lu J., Chan P., et al. (2018). Gradual disturbances of the Amplitude of Low-Frequency Fluctuations (ALFF) and fractional ALFF in Alzheimer spectrum. Front. Neurosci. 12:975. 10.3389/fnins.2018.00975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi D., Choe Y. M., Byun M. S., Sohn B. K., Seo E. H., Han J., et al. (2015). Differences in functional Brain Connect. alterations associated with cerebral amyloid deposition in amnestic mild cognitive impairment. Front. Aging Neurosci. 7:15. 10.3389/fnagi.2015.00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin C., Yi L., Jia L., Wang J., Liu P., Guo Y., et al. (2014). Early morphological brain abnormalities in patients with amnestic mild cognitive impairment. Transl. Neurosci. 5, 253–259. 10.2478/s13380-014-0234-6 [DOI] [Google Scholar]
- Yokosawa K., Kimura K., Takase R., Murakami Y., Boasen J. (2020). Functional decline of the precuneus associated with mild cognitive impairment: Magnetoencephalographic observations. PLoS ONE 15:e0239577. 10.1371/journal.pone.0239577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu E., Liao Z., Tan Y., Qiu Y., Zhu J., Han Z., et al. (2019). High-sensitivity neuroimaging biomarkers for the identification of amnestic mild cognitive impairment based on resting-state fMRI and a triple network model. Brain Imaging Behav. 13, 1–14. 10.1007/s11682-017-9727-6 [DOI] [PubMed] [Google Scholar]
- Yuan B., Chen J., Gong L., Shu H., Liao W., Wang Z., et al. (2016). Mediation of episodic memory performance by the executive function network in patients with amnestic mild cognitive impairment: a resting-state functional MRI study. Oncotarget 7, 64711–64725. 10.18632/oncotarget.11775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X., Han Y., Wei Y., Xia M., Sheng C., Jia J., et al. (2016). Regional homogeneity changes in amnestic mild cognitive impairment patients. Neurosci. Lett. 629, 1–8. 10.1016/j.neulet.2016.06.047 [DOI] [PubMed] [Google Scholar]
- Zang Y., Jiang T., Lu Y., He Y., Tian L. (2004). Regional homogeneity approach to fMRI data analysis. Neuroimage 22, 394–400. 10.1016/j.neuroimage.2003.12.030 [DOI] [PubMed] [Google Scholar]
- Zeuner K. E., Knutzen A., Granert O., Sablowsky S., Götz J., Wolff S., et al. (2016). Altered brain activation in a reversal learning task unmasks adaptive changes in cognitive control in writer's cramp. NeuroImage Clin. 10, 63–70. 10.1016/j.nicl.2015.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Hua R., Qing Z., Ni L., Zhang X., Zhao H., et al. (2019). Abnormal brain functional connectivity coupled with hypoperfusion measured by resting-state fMRI: an additional contributing factor for cognitive impairment in patients with Alzheimer's disease. Psychiatry Res. Neuroimaging 289, 18–25. 10.1016/j.pscychresns.2019.04.007 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Deng L., Bai F., Shi Y., Yu H., Yuan Y., et al. (2010). Alteration of resting brain function by genetic variation in angiotensin converting enzyme in amnestic-type mild cognitive impairment of Chinese Han. Behav. Brain Res. 208, 619–625. 10.1016/j.bbr.2010.01.008 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Liu Y., Jiang T., Zhou B., An N., Dai H., et al. (2012). Altered spontaneous activity in Alzheimer's disease and mild cognitive impairment revealed by regional homogeneity. Neuroimage 59, 1429–1440. 10.1016/j.neuroimage.2011.08.049 [DOI] [PubMed] [Google Scholar]
- Zhao Z., Lu J., Jia X., Chao W., Han Y., Jia J., et al. (2014). Selective changes of resting-state brain oscillations in aMCI: an fMRI study using ALFF. Biomed. Res. Int. 2014:920902. 10.1155/2014/920902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q. H., Wang K., Zhang X. M., Wang L., Liu J. H. (2020). Differential regional brain spontaneous activity in subgroups of mild cognitive impairment. Front. Hum. Neurosci. 14:2. 10.3389/fnhum.2020.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Zhou P., Alcauter S., Chen Y., Cao H., Tian M., et al. (2016). Changes of intranetwork and internetwork functional connectivity in Alzheimer's disease and mild cognitive impairment. J. Neural Eng. 13:046008. 10.1088/1741-2560/13/4/046008 [DOI] [PubMed] [Google Scholar]
- Zhuang L., Liu X., Shi Y., Liu X., Luo B. (2019). Genetic variants of PICALM rs541458 modulate brain spontaneous activity in older adults with amnestic mild cognitive impairment. Front. Neurol. 10:494. 10.3389/fneur.2019.00494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang L., Liu X., Xu X., Yue C., Shu H., Bai F., et al. (2012). Association of the interleukin 1 beta gene and brain spontaneous activity in amnestic mild cognitive impairment. J. Neuroinflammation 9:263. 10.1186/1742-2094-9-263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang L., Ni H., Wang J., Liu X., Lin Y., Su Y., et al. (2020). Aggregation of vascular risk factors modulates the amplitude of low-frequency fluctuation in mild cognitive impairment patients. Front. Aging Neurosci. 12:604246. 10.3389/fnagi.2020.604246 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.