Highlights
-
•
Clear cell adenocarcinoma of the lower urinary tract is rare and poses diagnostic challenge.
-
•
GATA3, which is frequently expressed in urothelial carcinoma, can be expressed in clear cell adenocarcinoma.
-
•
ARID1A, PBRM1, ERBB4, and SMARCA4 mutations were identified in the current CASE.
-
•
Molecular studies may aid in the diagnosis, and optimal treatment decision-making process.
Keywords: Clear cell adenocarcinoma, Molecular profiling, Urothelial carcinoma, Bladder
Funding
No internal or external funding was used.
Authors' contributions
M.A. and H.A. conceived and designed the report. H.A. and D.F. contributed to the acquisition, analysis and interpretation of data. M.A. substantially contributed to the revision of the manuscript. M.A. made substantial contribution to the preparation of the manuscript. A.L, B.M, and H·F reviewed the final drafts and, along with M.A, H·F, and D. F, approved it.
Primary clear cell carcinoma of the lower urinary tract is rare, with controversy regarding its histogenesis. Its heterogenous morphology cause diagnostic challenge, particularly with its most important differential, urothelial carcinoma. We present an advanced stage clear cell carcinoma arising from urinary bladder and urethra, with focal but strong GATA3 expression. Next generation sequencing using 324-gene panel revealed previously undocumented variants in ARID1A, PBRM1, ERBB4, and SMARCA4 genes as well as unequivocal RAD21 gene amplification. These findings suggest a distinct genomic signature which might aid not only in the tailored management, but also in diagnosis in challenging cases.
1. Introduction
Primary clear cell adenocarcinoma (CCA) of the lower urinary tract (LUT) is rare (1). Female urethra is the most common, although CCA can also occur in the urinary bladder and despite the female predominance, both genders are affected (1). Median age is in mid-50s, with initial complaints of hematuria.1 CCA shows solid, tubulocystic, and papillary features with cuboidal or columnar cells with high-grade cytologic atypia, brisk mitosis with atypical mitotic Fig. 1. CCA may resemble endometriosis, nephrogenic adenoma, and urothelial carcinoma.1
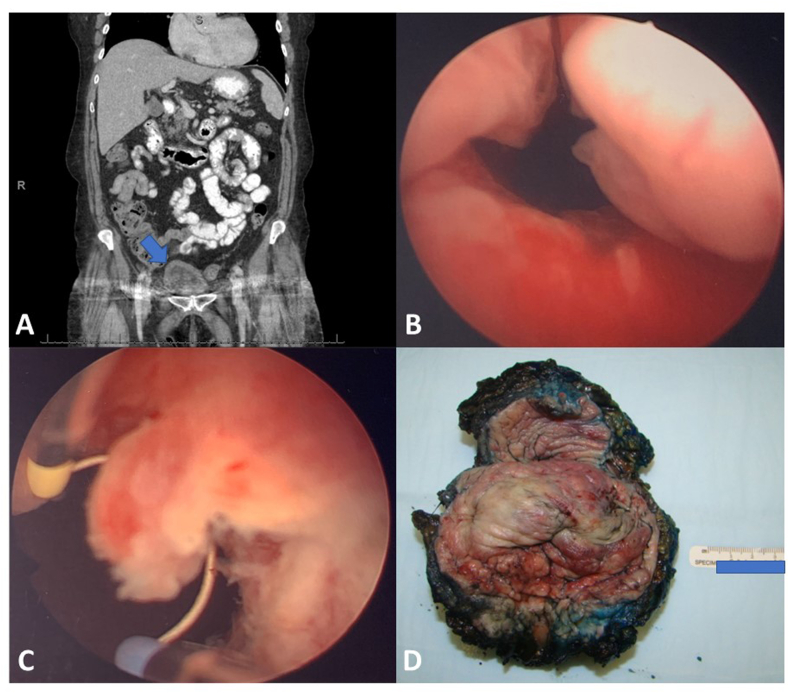
Fig. 1.
CT abdomen with contrast showed no evidence of metastatic disease prior to cystectomy. Bladder difficult to evaluate due to artifact from bilateral hip hardware, but diffuse wall thickening (Arrow) (A). Transurethral resection of the papillary/polypoid mass in the bladder neck/urethra (B, C). Cystectomy (prepped, inked, opened from anterior wall) specimen reveals (D) extensive tumor presence in bladder neck, trigone, and bilateral urinary bladder walls. Urethra was also involved (not shown).
Endometriosis/endosalpingiosis or Mullerian remnants in the LUT, nephrogenic adenoma (NA), or a differentiation of urothelial carcinoma (UCa) are proposed etiologies for the CCA origin, however, each standing hypothesis has features that support or argue against itself.1 Immunohistochemical (IHC) profile includes consistent expression of PAX8 and although focal, napsin A and HNF1B.1 As a transcription factor impacting embryogenesis and cell differentiation, GATA3 is expressed in many tumors but is particularly utilized in UCa, however, its expression status is unclear in CCA. Moreover, limited data exist on the molecular landscape of the CCA. Here we present a challenging CASE of CCA arising from urinary bladder/urethra with comprehensive molecular interrogation.
2. CASE presentation
A female in her 70s with history of total hysterectomy for fibroids was initially presented with urinary retention, abdominal distention, and pain for 3 days. Abdominopelvic computed tomography with contrast demonstrated mild bilateral hydronephrosis, no pathology was identified in patient's adnexa (Fig. 1A). Cystoscopy demonstrated narrowing of the urethra and 4–5 cm sessile mass extending to the bladder neck and part of trigone (Fig. 1B and C). Subsequent cystectomy revealed that the tumor involved the bladder apex (Fig. 1D).
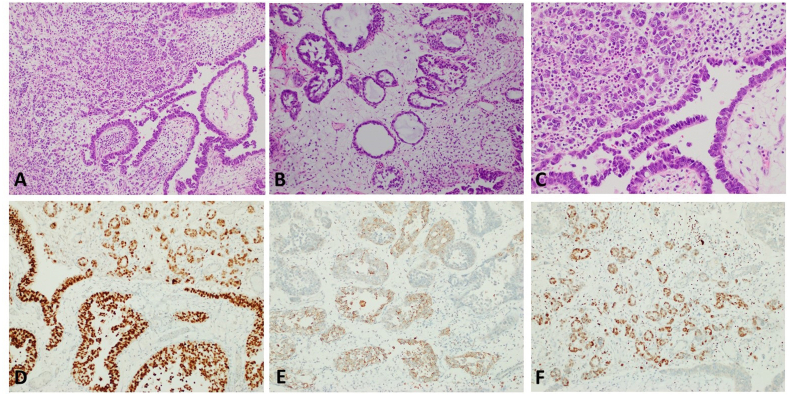
Transurethral resection showed muscle invasive tumor with mixed growth patterns including broad based papillae lined by a single layer of atypical cells (Fig. 2A) and areas of diffuse (Fig. 2A) tubular (Fig. 2A) and tubulocystic (Fig. 2B) growth. The tumor cells were pleomorphic with clear and eosinophilic cytoplasm with focal hobnailing (Fig. 2C). Frequent mitosis was present. IHC assays with appropriate controls showed diffuse positivity for PAX8 (Fig. 2D) and patchy positivity for Napsin-A (Fig. 2E) and GATA3 (Fig. 2F) while negative staining for P63. In cystectomy, extension into the urethra and bladder trigone with extension to the posterior vaginal wall. A periurethral cyst was apparent and was filled with papillary tumor with similar morphology. Multiple bilateral pelvic lymph nodes were positive for metastatic disease. Final tumor pathological staging per 8th edition of the American Joint Committee on Cancer TNM scheme was pT4N2Mx. No endometriosis or endosalpingiosis were identified in the bladder or urethra.
Fig. 2.
Microscopic evaluation of the tumor showed high-grade tumor cells lining broad based papillae (A, 100×) and tubules (B, 100×) with occasionally forming diffuse sheets (C, 100×). Immunohistochemical assays demonstrated diffuse PAX8 expression (D, 100×) with focal napsin A (E, 100×). Interestingly, GATA3 was also focally positive (F, 100×).
During follow up at post-op 5th week, a positron emission tomography scan demonstrated metastases involving T11 and L2 vertebral bodies, the right sacrum, and the right iliac bone which was confirmed with magnetic resonance imaging of the spine. Currently, the patient is receiving radiation therapy and is being evaluated for additional chemotherapy.
The tumor was sent to next generation sequencing (Foundation One, F1CDx), 324-gene panel. Microsatellite status was stable, and tumor mutational burden was 9 muts/Mb. Significant genetic alterations were as follows: ARID1A S711* (variant allelic frequency (VAF) 51.1 %, 2132C > G, NM_006015, pathogenic); PBRM1 splice site 3616 + 1G > A (VAF 9.2 %, NM_018,313, unknown significance); two distinct ERBB4 variants S303F (VAF 1.3 %, 908C > A, NM_005235, unknown significance) and S303F (VAF 2.0 %, 908C > T, NM_005235); and SMARCA4 R1189Q (VAF 3.9 %, 3566G > A, NM_003072, unknown significance). RAD21 gene amplification as well as equivocal amplifications of MYC and LYN amplifications were also noted.
3. Discussion and conclusion
CCA arising from the LUT have generated a controversy which has caused not only the ambiguity on its origins but also its nomenclature as well its classification. The earliest claim about histogenesis is the malignant transformation from endometriosis in the LUT, with documentation of CCA with concurrent endometriosis on few occasions (1). Although, obviously, presence of male CCA in the LUT is the single most convincing argument against it (1). Similarly, nephrogenic adenoma, as a well-known morphologic mimicker (1), has long been a challenging differential, and a potential candidate as a precursor lesion for CCA. Nephrogenic adenoma and CCA both express PAX8 (which was also thought as an evidence of common origin), racemase, or napsin-A (1). Urothelial carcinoma has frequent variants, with as much as 40 % squamous component present, followed by glandular differentiation in up to one fifth of cases.1 In a landmark study, Oliva et al. identified conventional urothelial carcinoma in 4 of 19 CCA arising from the urinary bladder and urethra, adding another possible explanation about the histogenesis.2 Our CASE demonstrated partial but strong GATA3 expression without urothelial carcinoma component, a biomarker that is sensitive in urothelial carcinoma with poor specificity, although its expression is not well studied in CCA.
Numerous large-scale genomic studies demonstrated distinct subgroups by mutational signature in urothelial carcinoma, particularly FGFR3/PIK3CA mutated and TP53/MDM2 mutated subgroups, respectively.3 On the other hand, Lin et al.4 identified recurrent PIK3CA (p. E545K) mutations and a TP53 variant (p.R273C) in CCA of LUT, but also identified KRAS (p.G12D) mutations with an additional APC variant (P·S2310×), and MYC amplification event in three urinary bladder CCA. Our CASE lacked FGFR3/PIK3CA or TP53/MDM2 mutations, and mutational status in our case is not compatible with urothelial carcinoma. ARID1A mutations are more frequently seen in clear cell carcinoma of the ovary (about up to 60 % of cases)5 when compared to urothelial carcinoma (about 25 % of cases),3 suggesting that CCA of the LUT may not only morphologically but also genetically resemble CCA of the ovary. RAD21 amplifications are enriched in high grade serous ovarian carcinoma,5 although its prevalence in CCA is not known. ERBB4, SMARCA4, and PBRM1 mutations are not frequent in urothelial carcinoma.3
In summary, Gata3 expression in CCA is not well studied. Further studies are needed to identify the significance of GATA3 expression in CCA of the bladder as well as molecular profiling that can help in understanding the origin of this rare entity and identifying possible common gene alterations that could be therapeutic targets in the future.
Acknowledgement
N/A.
References
- 1.Humphrey P.A., Moch H., Cubilla A.L., Ulbright T.M., Reuter V.E. The 2016 WHO classification of tumours of the urinary system and male genital organs-Part B: prostate and bladder tumours. Eur Urol. 2016;70(1):106–119. doi: 10.1016/j.eururo.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 2.Oliva E., Amin M.B., Jimenez R., Young R.H. Clear cell carcinoma of the urinary bladder: a report and comparison of four tumors of mullerian origin and nine of probable urothelial origin with discussion of histogenesis and diagnostic problems. Am J Surg Pathol. 2002;26(2):190–197. doi: 10.1097/00000478-200202000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Ross J.S., Wang K., Khaira D. Comprehensive genomic profiling of 295 cases of clinically advanced urothelial carcinoma of the urinary bladder reveals a high frequency of clinically relevant genomic alterations. Cancer. 2016;122(5):702–711. doi: 10.1002/cncr.29826. [DOI] [PubMed] [Google Scholar]
- 4.Lin C.Y., Saleem A., Stehr H., Zehnder J.L., Pinsky B.A., Kunder C.A. Molecular profiling of clear cell adenocarcinoma of the urinary tract. Virchows Arch. 2019;475(6):727–734. doi: 10.1007/s00428-019-02634-5. [DOI] [PubMed] [Google Scholar]
- 5.Jones S., Wang T.L., Shih Ie M. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science. 2010;330(6001):228–231. doi: 10.1126/science.1196333. [DOI] [PMC free article] [PubMed] [Google Scholar]