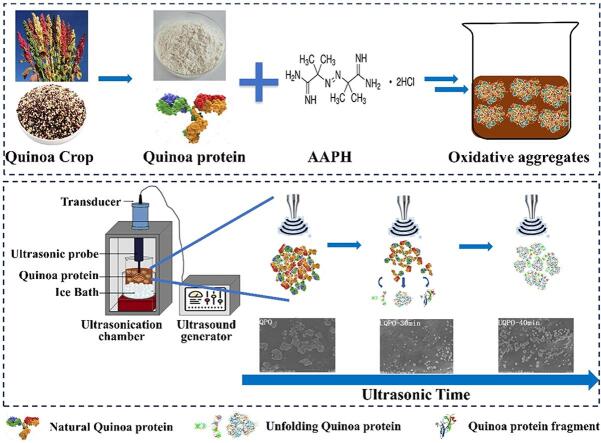
Graphic abstract
Keywords: Quinoa protein, Ultrasonic, Aggregates, Structure, Functional properties
Highlights
-
•
AAPH was used as oxidation inducer to construct the oxidative protein model.
-
•
Negative effects of oxidized protein were mainly caused by aggregation.
-
•
Ultrasound changed the intermolecular force to improve functional properties.
-
•
Excessive ultrasonic resulted in hydrophobic collapse and reaggregation.
Abstract
Protein oxidation leads to covalent modification of structure and deterioration of functional properties of quinoa protein. The objective of this study was to investigate the effects of ultrasonic treatment on the functional and physicochemical properties of quinoa protein oxidation aggregates. In this concern, 2,2′-azobis (2-amidinopropane) dihydrochloride (AAPH) was selected as oxidative modification of quinoa protein. The microstructure of quinoa protein displayed by scanning electron microscope (SEM) indicated that oxidation induced extensive aggregation, leading to carbonylation and degradation of sulfhydryl groups. Aggregation induced by oxidation had a negative effect on the solubility, turbidity, emulsifying stability. However, according to the analysis of physicochemical properties, ultrasonic significantly improved the water solubility of quinoa protein. The quinoa protein treated by ultrasonic for 30 min exhibited the best dispersion stability in water, which corresponded to the highest ζ-potential, smallest particle size and most uniform distribution. Based on the FT-IR, SDS-PAGE and surface hydrophobicity analysis, the increase of α-helix, β-turn and surface hydrophobicity caused by cavitation effect appeared to be the main mechanism of quinoa protein solubilization. In addition, the hydrophobic region of the protein was re-buried by excessive ultrasonic treatment, and the protein molecules were reaggregated by disulfide bonds. Microstructural observations further confirmed that ultrasonic treatment effectively inhibited protein aggregation and improved the functional properties of quinoa protein.
1. Introduction
Quinoa (Chenopodium quinoa Willd) is natural to the Andes mountains of South America and has been planted for more than 5000 years. With high content of protein and all essential amino acids, quinoa has no gluten protein. Quinoa contains abundant polyunsaturated fat and high dietary fiber, which can be used as low glycemic index food[1]. People with lactose intolerance, diabetes and hyperlipidemia can benefit from it[2]. In addition, quinoa is rich in vitamins, polyphenols, flavonoids, saponins, phytosterols and other active ingredients, which has the functions of enhancing immunity, anti-oxidation, anti-inflammatory, hypoglycemic and hypolipidemic effects[3]. Because of the abundant nutritional characteristics, quinoa is also known as “plant gold” and “future food”, which is one of the important cereals to deal with food security in the 21st century. However, the oil content of quinoa is about 6.0–9.5%, much higher than that of wheat, corn, barley and other common cereals except for soybean[4]. Therefore, quinoa is easy to be oxidized during storage. Due to the oxidative denaturation of quinoa protein, the groups inside the molecule recombine to form oligomers after exposure, which further form macromolecular aggregates under the action of hydrophobicity and electrostatic attraction[5]. The decrease of solubility and interfacial activity leads to the poor functional properties of quinoa proteins; hence quinoa protein is difficult to apply in food industry[6].
At present, the regulation of oxidized protein aggregates by physical means has been widely studied. Ultrasonic, pulsed electric field and high-pressure homogenization were used to change the spatial structure of protein aggregates to improve the functional properties of proteins[7]. However, high-pressure homogenization technology cannot be industrialized because of its large energy consumption and small work capacity[8]. Owing to the high requirements for environment, such as pH and temperature, pulsed electric field processing technology was difficult to operate in food processing[9]. As a non-thermal physical processing technology, ultrasonic was more suitable for application in food industry because of its green, efficient and simple operation mode[10]. The shear force generated by ultrasonic cavitation can destroy the forces that maintain protein structure, such as hydrogen bonds, Van der Waals forces and hydrophobicity. Furthermore, the range of protein particle size distribution was altered due to the increased hydrophobicity of protein surface[11]. Eventually, the aggregation degree of protein molecules was changed, which contributed to the favorable functional characteristics of protein. The oxidation of protein usually occurred before food processing. To achieve the purpose of improving protein functional properties, ultrasonic can be used to depolymerize aggregates through directly imposing on oxidized protein. The peroxy radical produced by the decomposition of AAPH was the most important free radical intermediate in lipid peroxidation, which has an important impact on the structure and properties of proteins[12]. Due to the favorable reproducibility and stability of the oxidized aggregation model, AAPH was often used as an oxidative inducer to construct oxidative protein models[13].
In this study, quinoa protein was used as the research object, and AAPH was used to construct oxidation system of quinoa protein. By simulating the actual production of oxidized protein aggregates in the process of factory storage, the oxidized protein aggregates were treated with different ultrasonic time at suitable power. Based on exploring the effect of ultrasonic treatment on the functional properties of protein, the relationship between the structure and functional properties of oxidized quinoa protein was established. Furthermore, the mechanism of structural oxidative modification of quinoa protein will be explained at molecular level. The results of this work will provide a theoretical basis for the application of ultrasonic in the development and storage of quinoa protein products.
2. Materials and methods
2.1. Materials
Quinoa (Longli No.1, harvested in 2020) was acquired from Jiaqi Agricultural Technology Co., Ltd. (Shanxi, China). Sodium azide was purchased from Haozhong Chemical Co., Ltd. (Shandong, China). Dinitrophenylhydrazine, 2, 2′–azobis [2-methylpropionamidine] dihydrochloride (AAPH), 8-anilinonaphthalenesulfonate-1-carboxylate (ANS), was purchased from Sigma-Aldrich (Shanghai, China), other chemical reagents were purchased from Yuanye Bio-Technology Co., Ltd. (Shanghai, China). All the chemical reagents used in this research were analytical grade.
2.2. Preparation of quinoa protein
Quinoa protein were prepared using a modified method described before [14]. Quinoa seeds were ground with a HK-820 grain mill (Xulang, China) using a speed of 1500 r/min, the flour was sieved through a 200 μm sieve. The flour was defatted by N-hexane (1:3 w/w) in the Soxhlet extractor, then the N-hexane was removed by evaporation. The defatted flour was dissolved in deionized water (1: 10 w/w), and the pH was adjusted to 9 by 0.5 mol/L NaOH. The solution was stirred for 4 h at 25 °C and stored at 4 °C for 16 h to maximize protein solubilization. Then the solution was centrifuged at 4 °C for 30 min at 6000 g. The pH of the supernatants was adjusted to pH 4.5 used 2 mol/L HCl, and the supernatants were centrifuged twice for 30 min at 13,000 g and 4 °C. The precipitates were re-dissolved with deionized water (1:12 w/w) and the pH was neutralized. The suspensions were freeze-dried into powder for later use. The yield and purity of quinoa protein were 62.34 ± 2.63%, 82.06 ± 1.26%.
2.3. Ultrasonic treatment of oxidized quinoa
The quinoa protein solution was prepared by dissolving quinoa protein in phosphate buffer solution with pH 7.4 (containing NaN3 0.5 mg/mL) to 10 mg/mL. AAPH was added into quinoa protein solution to concentration of 1 mmol /L. After 24 h of oxidation treatment at 37 ℃ without light, quinoa protein was dialyzed in a 14000 kDa dialysis bag at 4 ℃ for 72 h. The deionized water was changed every 6 h during the whole dialysis process. Dialyzed liquid (100 mL) was placed in the ultrasonic processor (Model JY92-II DN, Xinyi ultrasonic equipment Co., Ltd, China) under the condition of ice bath, ultrasonic temperature was controlled by circulation system. Then, the suspensions were treated under the condition of ultrasonic power of 300 W for 10, 20, 30, 40 min (Fig. S1). Ultrasonic treatments was set to a pulse mode (3 s on followed by 5 s off) with a Φ12 mm ultrasonic probe. To make sure that the energy volume density was identical, the ultrasound probe was dipped (immersed at approximately 3.0 cm) in the protein suspensions. After ultrasonic treatment, the solution was transferred to a 4 ℃ centrifuge for secondary centrifugation (9000 r/min for 20 min). Subsequently, 6 kinds of quinoa protein aggregates were obtained after freeze-drying, codes as QP, UQPO-0 min, UQPO-10 min, UQPO-20 min, UQPO-30 min, UQPO-40 min, in which they respectively represented the natural quinoa and ultrasound treated oxidized quinoa protein aggregates at 0, 10, 20, 30 and 40 min.
2.4. Determination of particle size
Particle size: The particle size of protein aggregates was measured by dynamic light scattering (Malvern Instruments Ltd., Malvern, Worcestershire, UK). Samples were adjusted to 0.5 mg/mL before pouring into the sample cell, and the particle size of quinoa protein aggregates were determined through a cumulative analysis.
2.5. Determination of turbidity and solubility
Turbidity: the samples were dissolved in deionized water to prepare the required concentration, and stirred magnetically at room temperature for 30 min[15]. The absorbance was measured by spectrophotometer (Lambda 1050 UV/VIS/NIR, PerkinElmer, Waltham, USA) at 600 nm with deionized water as blank control.
Solubility: quinoa protein was dispersed in the distilled water (1% w/w) and then centrifuged at 10,000 g for 30 min at 10 ℃, solubility of quinoa protein was expressed as a percentage of the total protein[16].
2.6. Emulsifying activity index (EAI) and emulsification stability index (ESI)
Emulsification properties of quinoa protein were measured as the method described by Dabbour et al. [17]. Quinoa protein solution (1% w/v) and corn oil were blended at the ratio of 3 : 1 in volume. After homogenizing at 24,000 r/min by a T18 homogenizer (IKA, Germany), the emulsion was diluted with 0.1% (w/v) SDS solution, and the absorbance was quantified at wavelength of 500 nm. EAI and ESI were calculated by the following equations:
| (1) |
| (2) |
Where DF representing dilution factor of 100; C representing the concentration of quinoa protein (g/ml); φ representing optical path; θrepresenting the fraction of oil phase (0.25); and A0 and A30 representing the absorbance at 0 min and 30 min.
2.7. Measurement of water binding capacity (WBC) and oil binding capacity (OBC)
WBC and OBC were determined according to the method of Timilsena et al with modification[18]. WBC and OBC were calculated with following equations:
| (3) |
Where Wa is the weight of centrifuge tube with quinoa protein and the absorbed water, Wb is the weight of centrifuge tube and quinoa protein, and Wc is the weight of quinoa protein. The OBC of quinoa protein was calculated by the same method, except the water in the above method was changed into oil.
2.8. Surface hydrophobicity and ζ-potential
The measurement of surface hydrophobicity refers to Kato’s method[19] . Quinoa protein treated by ultrasonic was diluted with phosphate buffer to obtain concentrations of 0.05–0.4 mg/mL. Then, ANS (40 μL of 8 mmol/L) was added to 4 mL of different concentrations of protein solution, and the mixtures were incubated at 25 ℃ for 30 min. The fluorescence intensity was measured using Hitachi F-7000 fluorescence spectrometer (Hitachi, Tokyo, Japan). Excitation and emission wavelength were set at 390 nm and 468 nm, the scanning slit was set at 10 nm and the scanning speed was set at 10 nm/s. The surface hydrophobicity of quinoa protein treated by ultrasonic was calculated by the initial slope value of the relative fluorescence intensity vs. concentration of protein.
ζ-potential: the determination of ζ was carried out by ZetaSizer Nano ZSP (Horiba Ltd., Kyoto, Japan) at 25 ℃. Protein sample was dispersed into the 50 mmol/L of phosphate buffer (pH 7.0) to make sure the concentration was 0.5 g/L, ζ-potential was calculated by ZetaSizer software.
2.9. Determination of free amine and carbonyl group
Free amine group: In the presence of β-mercaptoethanol, ortho-phthalaldehyde (OPA) can form a yellow compound with free α-amine group, and has a characteristic absorption peak at 340 nm. The oxidized quinoa protein samples were thoroughly mixed with OPA and then treated with 35 ℃ water bath for 2 min. The content of free amine group can be calculated according to the standard curve of L-Leucine [20].
Carbonyl group: The prepared oxidized quinoa protein was dispersed in deionized water, magnetic stirring for 2 h at room temperature, and then centrifuged at 4 ℃ for 30 min at 10,000 g. The concentration of protein was determined by biuret indicator method. The mixture of protein solution and 2,4-dinitrophenylhydrazine was used for colorimetry at 347 nm[21]. The molar number of carbonyl derivatives per mg protein was calculated by molar extinction coefficient of 22000 M−1cm−1.
| (4) |
Where G represents carbonyl concentration of quinoa protein, nmol/mg; n is dilution factor; A367 represents the absorbance at 367 nm; E is concentration of quinoa protein, mg / mL.
2.10. Determination of free sulfhydryl group and disulfide bonds
The free sulfhydryl group and disulfide bond contents of quinoa protein treated by ultrasound were determined according to the method of Ellman's reagent [22]. To determine the free sulfhydryl content, quinoa protein (2 g) was dissolved in 2 mL of Tris-Gly buffer (pH 8.0) with 0.02 mL Ellman's reagent. The solution was shaken and quickly mixed, and then incubated at 25 ℃ for 15 min. The absorbance was measured at 412 nm, with a molar extinction coefficient of 1360 M−1cm−1. Same method was used to determine the total sulfhydryl content but 8 mol/L of urea was added to the buffer solution. The content of sulfhydryl group and disulfide bond was calculated according to the following equation:
| (5) |
| (6) |
Where A412 represents the absorbance of the sample with Ellman reagent; D is the dilution factor; Ct and Cf is the final concentration of total sulfhydryls and free sulfhydryls, respectively.
2.11. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE)
The oxidized quinoa protein profiles treated by ultrasonic were exhibited by reducing or non-reducing SDS-PAGE[23]. The concentration of separation gel was 12%, and the concentration of concentrated gel was 5%. Each sample and markers (10 μL) were loaded on the gel, and the electrophoretic analysis was performed on a Bio-Rad Mini-PROTEAN II System Cell apparatus (Bio-Rad Laboratories Inc., Hercules, CA, USA) in constant voltage mode. For reducing conditions, 5% (v/v) of 2-βME was added, and samples were boiling for 5 min. Gels were stained by Coomassie Brilliant Blue R-250 and decolorized with 10% (v/v) acetic acid and 5% (v/v) ethanol.
2.12. Fourier transformed infrared spectroscopy (FT-IR)
The FT-IR spectra of raw and oxidized quinoa protein were evaluated by a Nicolet-6700 FT-IR Spectrometer (Thermo Electron Scientific Instruments Corporation, USA)[24]. The freeze-dried sample (1 mg) was thoroughly mixed and grounded with potassium bromide (100 mg) in the agate mortar. All the samples were evaluated at ambient temperature, and the range of wavenumber was 4000–400 cm−1. A total of 60 scans were taken at a resolution of 4 cm−1 with the accuracy of 0.01 cm−1.
2.13. Measurement of intrinsic fluorescence
The intrinsic fluorescence spectra of oxidized quinoa treated by ultrasound was measured with a Hitachi F-7000 fluorescence spectrometer (Hitachi, Tokyo, Japan). The excitation wavelength was 290 nm, and the emission wavelength were recorded from 310 to 460 nm at a scan speed of 2000 nm/min. The emission and excitation slits were both set at 5.0 nm[16].
2.14. Micromorphological analysis
2.14.1. Scanning electron microscope (SEM)
The surface morphology of the oxidized quinoa protein treated by ultrasonic was observed by a SM-6490LV SEM (JEOL Company, Japan). The samples were evenly spread on the sample table with conductive adhesive and sprayed with gold, then the microstructure of quinoa protein was observed[25].
2.14.2. Atomic force microscopy (AFM)
Quinoa protein solutions were diluted by deionized water to 20 μg/mL. Diluted quinoa protein suspensions (10 μL) were dropped on newly peeled mica. In order to prevent dust pollution in the air, the mica flakes with samples were placed in the clean bench and dried naturally at room temperature[26]. The morphology of quinoa protein treated by ultrasound for different time was performed using a Multimode Nanoscope IIIa atomic force microscopy (Veeco Digital Instruments, Santa Barbara, CA, USA). All the AFM images were acquired using 5 N/m elastic coefficient, and each sample was analyzed using the Nanoscope Analysis 1.4 software.
2.15. Statistical analysis
Each experiment was repeated at least three times in this research, and reported as means ± standard deviations (SD). The ANOVA significance of data were analyzed by SPSS Statistics 22 using analysis of Tukey’s HSD, and the difference was significant (P < 0.05). All graphs were presented by Origin 9.1 and GraphPad Prism V9.0.2 Software.
3. Results and discussion
3.1. Particle size and dissolution characteristics
The protein aggregation produced by oxidation greatly changed the physicochemical properties of quinoa protein. As the ultrasonic time extended, the values of protein particle, polymer dispersity index (PDI) and turbidity decreased first and then increased (Table 1). Oxidation induced partial unfolding of protein structures, and then more hydrophobic groups were exposed. The increase of protein structure volume and hydrophobic interaction reduced the surface electrostatic charge density and raised electrostatic interaction between polypeptide chains, which accelerated the formation of oxidized aggregates[27]. As such, the difference of particle size was accumulated, and the suspended matter in protein liquid conglutinated, which led to the rise of particle size, PDI and turbidity. Same results were proved by Sun et al, who found that average particle size and solubility were affected by the oxidation of proteins[28]. Huang et al also reported that the aggregation of soybean protein induced by lipoxygenase catalyzed linoleic acid oxidation showed increased turbidity, which indicated that aggregates with high molecular weight and large particle size existed in the oxidized proteins[21]. Ultrasonic can unfold the protein aggregates through cavitation, and make them dissociate and disintegrate to the greatest extent. As the ultrasonic time extended, the effect was more obvious, and the disintegration reached the maximum when the ultrasonic time was 30 min. The conformation of protein was changed by intense ultrasound. Based on this, embedding of some hydrophobic groups bring about the formation of large-scale aggregates[29]. The particle size and turbidity increased during the process of protein aggregation. On the contrary, the solubility of quinoa protein after oxidation showed the opposite trend. Due to the unfolding of protein structure induced by AAPH oxidation, covalent and noncovalent interactions of oxidized protein were weakened, and more hydrophobic groups were exposed. When the hydrophobic interaction exceeded a critical value, protein aggregation was facilitated and insoluble aggregates were produced[30]. The shear stress and cavitation provided by ultrasonic treatment impelled the macromolecular aggregates to depolymerize. Protein molecules gradually peeled off and separated from aggregates, and thereby improved the stability of protein dispersion system. If the ultrasonic time was too long, the protein molecules will re-polymerize under the hydrophobic force, and the stability of the solution will decline. Thus, the inhibition effect of ultrasound on the formation of aggregates was abated. Owing to the accelerating transformation of small molecular to medium-sized aggregates, the solubility of quinoa protein was reduced. Elsewhere, it has been reported ultrasound can change oxidative aggregation by alter the physicochemical properties of the pork myofibrillar proteins[31].
Table 1.
Effect of ultrasound on dissolution characteristics of oxidized aggregates.
| Ultrasonic condition | Particle size (nm) | PDI | Solubility (%) | Turbidity |
|---|---|---|---|---|
| QP | 442.32 ± 23.46a | 0.82 ± 0.04a | 18.36 ± 0.46f | 1.53 ± 0.02a |
| UQPO-0 min | 886.42 ± 28.34f | 1.58 ± 0.06f | 6.48 ± 0.23a | 2.52 ± 0.03f |
| UQPO-10 min | 683.28 ± 23.64e | 1.32 ± 0.04e | 9.32 ± 0.36b | 2.22 ± 0.04e |
| UQPO-20 min | 523.22 ± 14.74c | 1.18 ± 0.03d | 12.36 ± 0.48d | 2.01 ± 0.04d |
| UQPO-30 min | 494.56 ± 15.76b | 0.90 ± 0.02b | 13.78 ± 0.85e | 1.82 ± 0.03b |
| UQPO-40 min | 586.44 ± 26.84d | 1.02 ± 0.05c | 10.88 ± 0.35c | 1.93 ± 0.04c |
Values are means ± standard deviation of determinations, a–f different lowercase letters within a column indicate significant differences (p < 0.05).
3.2. Emulsion capacity and stability
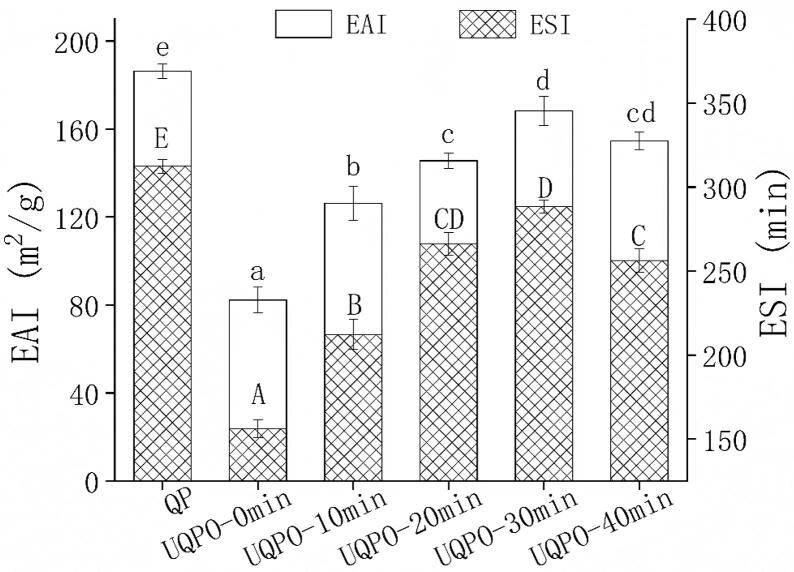
Protein molecules have both hydrophobic and hydrophilic groups, which can directly act on oil phase and water phase respectively, thus acting as emulsifiers. The EAI and ESI of oxidized quinoa protein decreased significantly compared with natural quinoa protein. The unfolding of protein structure induced by oxidation promoted the aggregation of protein molecules, which re-buried the hydrophobic groups, and resulting in the decrease of surface hydrophobicity and solubility[32]. In addition, the ROO• formed by degradation of AAPH directly attacked the main peptide chain and side chain groups of protein through hydrogen abstraction reaction. The flexibility of protein molecules was reduced, which made it unable to expand rapidly at the oil–water interface, thus reducing the emulsifying properties of quinoa protein. With the extension of ultrasonic time, the EAI and ESI increased first and then decreased, and reached the maximum when the ultrasonic time was 30 min (Fig. 1). According to Yan et al., the shear force produced by ultrasound reduced the particle size of protein and increased the specific surface area of protein, which enhanced the stability of protein solution[33]. Furthermore, the non-covalent bonds which maintained the stability of protein spatial structure was destroyed by cavitation force caused by ultrasonic[34]. Therefore, the hydrophobic group of protein was exposed, and more protein molecules spread to the water–oil interface, which improved the emulsifying properties of protein. However, the exposure of hydrophobic groups of protein reached the critical value at 30 min. The destruction of hydrophobic interaction in protein molecules and the acceleration of protein molecular motion leaded to protein aggregation gradually. When small molecules were adsorbed to the interface, they were easily replaced by macromolecular proteins[35]. The interfacial tension of emulsion increased and flocculation occurred, which caused the reduction of EAI and ESI. The results were consistent with the findings reported by Liu et al., who investigated myofibrillar proteins treated by ultrasound for long time might cause a flocculation of insoluble protein and a reaggregation of protein particles through Intermolecular force[23].
Fig. 1.
Emulsifying properties of quinoa protein treated by ultrasonic with different time. Error bars represent mean ± standard deviations (n = 5); Uppercase letters indicate significant difference of ESI (P < 0.05) with different ultrasonic treatments, lowercase letters indicate the difference of EAI with different ultrasonic treatments.
3.3. Water binding capacity and oil binding compacity
The effect of ultrasonic with varying time intervals on water and oil binding capacities of quinoa protein was shown in Fig. S2. There was a significant reduction in the combining ability of quinoa protein subjected to the oxidation treatment compared to natural quinoa protein. The highest value of WBC of oxidized quinoa protein appeared at 30 min after ultrasonic treatment, which was still a little lower than the WBC of natural quinoa protein. Similar results were observed in the soybean and chia seed protein treated by ultrasonic[18], [36]. Variable time sonification was used for Malik et al. also found that high intensity ultrasound treatment of sunflower protein can increased WBC and OBC[37]. The possible reason for the increase in the WBC of oxidized quinoa protein was related to the reduction in particle size caused by ultrasound. Interestingly, the value of WBC was decreased for longer ultrasound duration, which may be due to the interactions of protein molecules and produce aggregation. Same tendency was observed in the OBC of native and oxidized quinoa protein treated by ultrasound. The highest OBC of oxidized quinoa protein was reached at 30 min treated with ultrasound. The effect of ultrasonic exposed the hydrophobic groups of quinoa proteins to the external environment, which allowed entrapping oil molecules thus increases the values of OBC. Mir et al, reported a similar results for protein isolates from Chenopodium seeds [38].
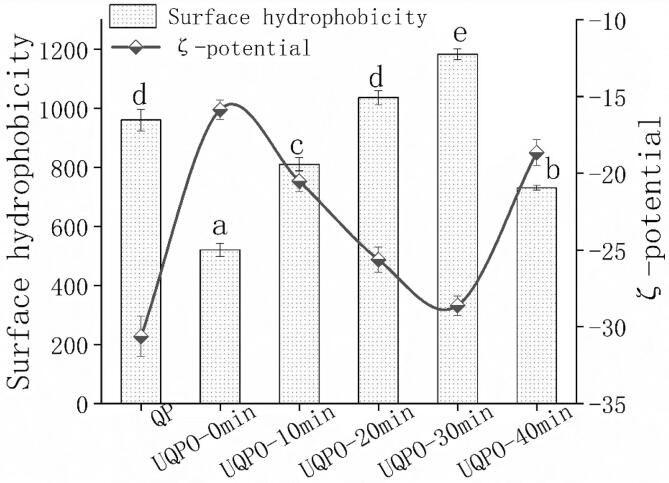
3.4. Surface hydrophobicity and ζ-potential
ζ-potential was usually used to characterize the stability of solution system, and the change of surface hydrophobicity reflected the structural change of quinoa protein. Oxidative modification can lead to protein fragmentation, crosslinking, unfolding and conformational changes, and thus the hydrophobic group of protein was exposed[39]. to increase the hydrophobic interaction. With the increase of hydrophobic interaction in the system, the protein aggregation was promoted to a certain extent, resulting in the decrease of surface hydrophobicity and electrostatic charge density. The encapsulation effect during the process of aggregation consumed the charges in the solution and reduced the ζ-potential of quinoa protein. With the increase of ultrasonic time, the protein unfolds, the insoluble aggregates were transformed into soluble aggregates, the protein molecules expand, the hydrophobic groups were exposed, and the surface hydrophobicity increased. However, the oxidized protein unfolded gradually with the extension of ultrasonic time, and the insoluble aggregates were transformed into soluble. While the protein molecules were extended and the hydrophobic groups were exposed, the surface hydrophobicity was increased accordingly. This was mainly because the cavitation phenomenon caused by ultrasonic treatment, which can also promote the depolymerization of protein(Fig. 2). The hydrophobic residues and charged groups inside the molecule were exposed to the surface, and the depolymerization effect improved with ultrasonic time. The increase of the number of free functional groups and charged particles amplified the electrostatic force in the solution, which indicated that ultrasound inhibited quinoa protein aggregation induced by oxidation[40]. However, when the ultrasonic time exceeded 30 min, the conformation of the protein changed greatly. More hydrophilic amino acid residues were water-oriented, which resulted in protein re-aggregation and reduced surface hydrophobicity and ζ-potential. Zou et al. and Cui et al. called this phenomenon as “over-processing” effect[41], [42]. In addition to the hydrophobic interaction, changes of structures and groups also play an important role during ultrasound treatment. Hence, we next focused on inspecting the changes in the main groups and conformation of aqueous suspensions of oxidized quinoa protein treated with ultrasound.
Fig. 2.
Surface hydrophobicity and ζ-potential of quinoa protein treated by ultrasonic with different time. Error bars represent mean ± standard deviations (n = 5); Lowercase letters indicate significant difference (P < 0.05) of surface hydrophobicity with different ultrasonic treatments.
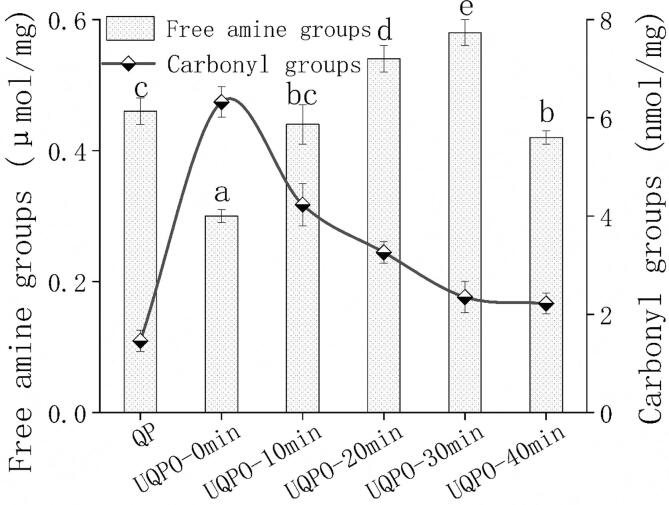
3.5. Free amine and carbonyl groups
The changes of amino and carbonyl content can characterize the unfolding and oxidation degree of protein. AAPH is a kind of water-soluble azo radical initiator, which can form peroxy radical by thermal degradation under aerobic condition. Free radical oxidation of proteins was often accompanied by the increase of carbonylation, which was mainly because peroxy radicals can break the main peptide chain and carbonyl the protein through α - amination pathway[43]. Therefore, compared with the natural quinoa protein, the carbonyl content of quinoa protein after APPH treatment increased significantly. The present result was in accordance with the research of Cheng et al., who found that ultrasonic treatment increased the carbonyl content of egg white protein[5]. Ultrasonic treatment increased the surface charge of protein and enhanced the electrostatic repulsion between particles. By improving the stability of protein dispersion, protein aggregation was inhibited and the amount of carbonyl group was reduced (Fig. 3). The content of free amine groups in oxidized quinoa protein aggregates increased first and then declined with ultrasonic time. This changing trend was basically opposite to that of carbonyl group, which may be because the newly formed carbonyl group can further react with amino groups of protein to form Schiff base, thus reducing the content of free amine groups[44]. Similar decrease of amine groups were also discovered in fish myofibrillar treated by ultrasound[45].
Fig. 3.
Free amine and carbonyl groups of quinoa protein treated by ultrasonic with different time. Error bars represent mean ± standard deviations (n = 5); Lowercase letters indicate significant difference (P < 0.05) of free amine groups with different ultrasonic treatments.
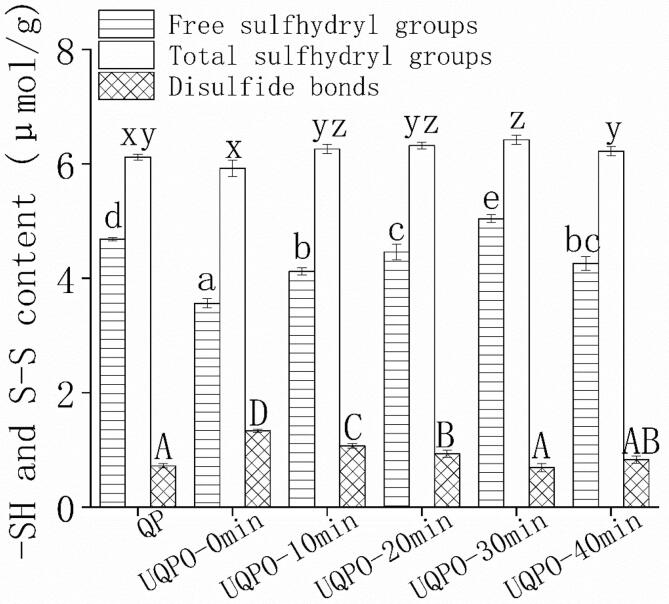
3.6. Sulfhydryl group and disulfide bonds
Cysteine was one of the most sensitive amino acids to oxidative modification, but carbonyl content of protein cannot reflect the oxidative state of cysteine. Therefore, sulfhydryl group and disulfide bond should be used to characterize the oxidation state of quinoa protein’s cysteine. Sulfhydryl group was an important functional group in protein, and the content reflected the denaturation degree of protein, which played a major role in the exertion of protein’s functional properties. Compared with the native quinoa protein, the content of sulfhydryl group in oxidized quinoa protein aggregates was up-trend, and the content descended with continue ultrasonic treatment, and the sulfhydryl group reached the maximum at 30 min, while the change trend of disulfide bond was opposite (Fig. 4). The unfolding effect of ultrasonic promoted more exposure of sulfhydryl group and more breaking of disulfide bonds[46]. With the increase of oxidation degree, intermolecular aggregation of protein occurred, which led to the decrease of free sulfhydryl groups, and the content of disulfide bonds raised correspondingly. Many studies reported increased disulfide bonds, oxidation induced the conversion of ε - amino group on lysine residue to carbonyl group of protein, and the content of amino group descended[47], [48]. The destruction of protein secondary and tertiary structure by ultrasound promoted disulfide bond breaking[13]. Due to the sulfhydryl groups exposed to the surface of protein, the content of sulfhydryl group increased. However, with the further extension of ultrasonic time, quinoa protein was denatured and extended, and hydrophobic region and free amino groups were exposed. Rising hydrophobic interaction promoted protein aggregation, the content of free amine and sulfhydryl group decreased at 40 min. The results were in agreement with the reports that disulfide bonds were mediated by ultrasound among faba bean protein[49]. Ultrasonic can regulate intermolecular polymerization and depolymerization, and then affected the functional properties of proteins.
Fig. 4.
Sulfhydryl group and disulfide bonds of quinoa protein treated by ultrasonic with different time. Error bars represent mean ± standard deviations (n = 5); Lowercase letters a-e indicate significant difference (P < 0.05) of free sulfhydryl groups, lowercase letters x-z indicate the difference of total sulfhydryl groups, and uppercase letters A-D indicate difference of disulfide bonds with different ultrasonic treatments.
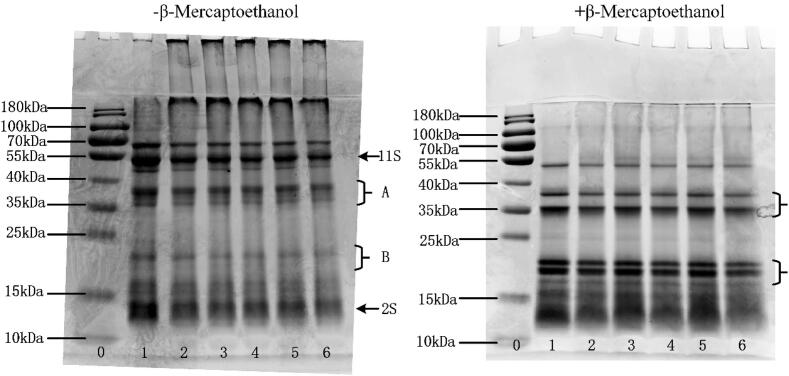
3.7. SDS-PAGE electrophoresis
The main components of quinoa protein are 11S globulin and 2S albumin, both of which are stabilized by disulfide bonds[15]. In the non-reducing gel chromatography, the most intense bands present in the molecular weight ranging around 50 kDa corresponded to 11 S globulin known as chenopodin in the natural quinoa protein. In addition, 11S globulin was composed of 32–39 kDa subunit A and 21–23 kDa subunit B, while molecular weight 2 S albumin was 14 kDa (Fig. 5). After oxidation treatment, the characteristic of 2 S and 11 S bands were significantly weakened, and lots of new bands were emerged on the top of the stacking gel. These new bands cannot penetrate the channel, which was mainly due to the formation of aggregates with large molecular weight among protein molecules[50]. Ultrasonic disintegrated the aggregated proteins and restored some protein bands. With the extension of ultrasonic treatment time, the decreasing trend of the characteristic bands of 2 S and 11 S was improved, both intensity and density of the bands increased gradually. During the oxidation process, the globulin and its subunits with high molecular weight were vulnerable to AAPH, resulting in the weakening of the intensity of subunit characteristic bands. However, the cavitation effect of ultrasound weakened the interaction between protein molecules, the spatial structure of protein molecules was destroyed[30]. Therefore, the subunit was depolymerized and the content increased. When the ultrasonic time exceeded 30 min, the depolymerized subunits were polymerized again, which affected the structural properties of protein. In reductive gel electrophoresis, disulfide bonds of quinoa protein were destroyed by β-mercaptoethanol, molecular weights of 22–23 kDa and 32–39 kDa were obviously increased. This was in accordance with the results of a previously study ascribed the increase to the shear stress turbulence effects of ultrasound[16]. It was suggested that ultrasound can improve the aggregation of quinoa protein induced by oxidation.
Fig. 5.
SDS–PAGE pattern of quinoa protein treated with different ultrasonic time, A, B represents subunit of 11S respectively. Lane 0: molecular markers (10–180 KDa), Lane 1: natural quinoa protein, Lane 2 – 6: oxidized quinoa protein treated by ultrasound for 0, 10, 20, 30, 40 min respectively.
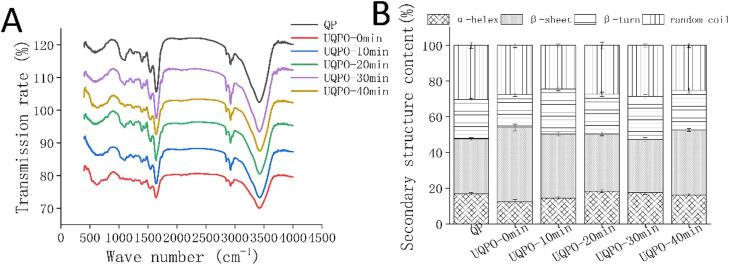
3.8. Effect of ultrasonic treatment time on FTIR analysis of quinoa protein
There are three groups of characteristic absorption bands in infrared spectrum, including amide I, II, III band. Amide I band is in the wave number range of 1600–1700 cm-1, which is mainly caused by C = O stretching and H-O–H bending vibration. The changes of protein secondary structure: α-helix, β-sheet, β-turn and random coil can be reflected in this vulnerable zone. After oxidation, the β-sheet of quinoa protein increased, while the α - helix and random coil decreased. Oxidized quinoa protein treated with ultrasonic for 10–20 min resulted in decrease in α–helix and β–turn, while β-sheet increased. Further prolongation of ultrasonic treatment for 30 min reduced theβ- sheet structure, the trend of α - helix and β - turn structure was opposite (Fig. 6).
Fig. 6.
Fourier transform infrared spectroscopy (FTIR) of quinoa protein and analysis of quinoa protein secondary structure. A: transmission rate of quinoa protein with different ultrasonic treatment, B: Secondary structure content of quinoa protein with different ultrasonic treatment.
The reverse parallel folding structure (β1) was formed in the aggregated protein molecules, and the increase of β1 structure reflected the aggregation of protein from the side. After oxidative induction, quinoa protein unfolded and the α – helix was stretched. When the exposed hydrophobic groups increased to a certain value, the balance of electrostatic repulsion and hydrophobic interaction was broken, which promoted the aggregation of protein, so the content of β-sheet structure increased, and the content of α- helix and random coil decreased[16]. This may be due to the destruction of interaction between local amino acid sequences and some protein molecules. The shear force and cavity effect induced by ultrasonic treatment can induce the unfolding of partial protein structure, which transformed insoluble aggregates into soluble aggregates. The results of oxidized quinoa protein treated for 10–20 min indicated that the change of secondary structure was related to the increase of soluble aggregate content caused by the transformation process. Accompanied by the formation of stronger intramolecular hydrogen bonds, the oscillating effect of ultrasonic caused the transformation of β - sheet into highly ordered supramolecular structures, which improved the functional properties of proteins[51]. However, the protein conformation was changed and protein aggregated again after long-term ultrasonic treatment, resulting in the decrease of α - helix content and irregular coil structure. Similarly, Yan et al. confirmed that with increases in the ultrasound time, the protein was partially denatured and re-polymerized through hydrophobic binding and other effects, resulting in the change of secondary structure[52].
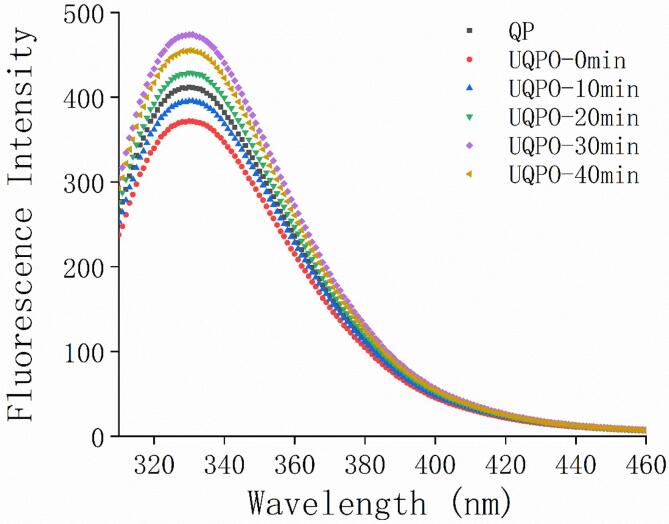
3.9. Effect of ultrasonic treatment on the intrinsic fluorescence of quinoa protein
To further elucidate the conformational changes in the tertiary structure of oxidized quinoa protein during ultrasonic treatment. Intrinsic fluorescence was used to reflect the oxidation degree of tryptophan residues and the change of microenvironment in quinoa protein. The Intrinsic fluorescence spectrum of native and ultrasound treated quinoa protein was shown in Fig. 7. The maximum fluorescence intensity of native quinoa protein was at 330 nm (λmax), which was consistent with the typical Intrinsic fluorescence spectra of tryptophan residues in the relatively hydrophobic environment reported by Mir et al. [15]. After oxidation treatment, the fluorescence intensity of quinoa protein decreased significantly and a tiny blue-shifting was appeared (330–326 nm). The blue-shifting of λmax caused by ROO• oxidation indicated that tryptophan residues previously located outside the quinoa protein molecules in polar environments were transferred to the internal non-polar environment, which was caused by the aggregation of quinoa protein. A similar trend for fluorescence spectra was found by Li et al. for rice bran protein[53]. Protein oxidation induced by lipid peroxidation products was often accompanied by fluorescence quenching[54]. Among the 20 kinds of amino acids that composing quinoa protein, tryptophan has the lowest single-electron oxidation potential energy. It was easy for ROO• to convert tryptophan residues into metastable tryptophan free radicals by hydrogen abstraction reaction. Then the radicals combined with molecular oxygen to form tryptophan peroxy radicals. Finally, those peroxy radicals were convert into kynurenine, which reduced the intrinsic fluorescence intensity[54]. After ultrasonic treatment for 10–30 min, the fluorescence intensity of quinoa protein was significantly enhanced, which indicated that the microenvironment of tryptophan residues in quinoa protein was changed, and the tertiary structure of quinoa protein was expanded[55]. More tryptophan residues were exposed to the environment with stronger polarity of molecular surface. Under the action of ultrasound, the changes of molecular conformation of quinoa protein promoted tryptophan residues closer to tyrosine residues. Meanwhile, there was an energy transfer process occurring during the molecular conformational changes. The oxidized quinoa protein aggregates were depolymerized, and the tryptophan residues were further away from the disulfide bond and other fluorescence quenching groups, which increased the fluorescence intensity. However, when the ultrasonic time reached 40 min, a notable descent was observed, indicating that quinoa protein was refolded and the chromogenic groups were reburied[56]. As a result, the λmax of fluorescence spectrum decreased owing to the fluorescence quenching phenomenon. These results suggested that ultrasound treatment can alleviate the oxidative aggregation of quinoa protein by changing the conformation of quinoa protein.
Fig. 7.
Fluorescence spectroscopy analysis of native and ultrasound treated oxidized quinoa protein.
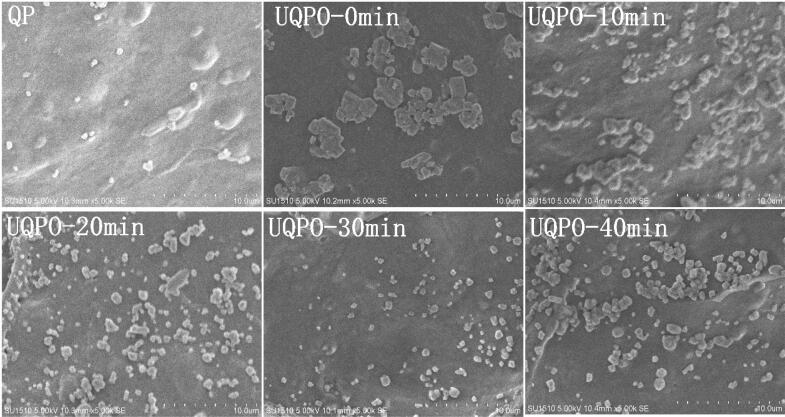
3.10. Microstructure analysis
The functional properties of quinoa protein are closely related to its microstructure. Because natural quinoa protein has good solubility, its microstructure presented uniform and smooth surface (Fig. 8). After oxidation treatment, quinoa protein appeared large aggregates with uneven distribution and larger surface roughness. However, with the intervention of ultrasound, the macromolecular of quinoa aggregates gradually disintegrated and transformed into smaller particles[57]. With the extension of ultrasonic time, the effect was more obvious, which also confirmed the relevant results of the above functional characteristics. On the one hand, quinoa protein aggregates were affected by ultrasound in liquid, and the mechanical effect of ultrasound forced the protein aggregates to disperse and depolymerize to small protein molecules. On the other hand, the cavitation of ultrasound made the structure of protein aggregates looser. With the vibration of the medium, quinoa protein molecules shed from the aggregates, and the molecular weight and particle size of the aggregates decreased[58]. When the ultrasonic power was constant, with the increase of ultrasonic time, the protein molecules will interact with each other under the action of intermolecular forces, and then aggregates were formed again. Likewise, studies conducted on the soy protein has also observed the reaggregation of soluble aggregates, which was consistent with the particle size in Table1 [59]. These results indicated that ultrasonic treatment can affect the aggregation effect of protein, and then affect the microstructure of protein.
Fig. 8.
The morphological characteristics of quinoa protein treated by ultrasonic irradiation (magnification: × 5000).
AFM images can help to explain the changes of solubility, particle size and morphology of quinoa protein after ultrasonic treatment (Fig. S3, S4). Oxidized quinoa protein showed great difference in morphology compared with the natural quinoa protein. The oxidized quinoa proteins have larger aggregation particles and higher height in the three-dimensional microstructure. After ultrasonic treatment, the height of quinoa protein decreased gradually. Huang et al. found that ultrasound promoted the dissociation of protein aggregates[60]. The protein aggregation collapsed and formed smaller particles when the ultrasound treating time was 10–30 min. The result was due to the high disaggregation of protein subunits caused by cavitation and mechanical effect of ultrasound. However, when the ultrasonic treatment time exceeded 30 min, the height of quinoa protein in AFM image was increased slightly, and some of the dissociated subunits reassembled into protein aggregates. Similar results were observed in whey and soybean protein, which indicated that long-term ultrasound will lead to the further folding of small protein particles into large aggregates[61], [62].
4. Conclusion
Quinoa protein was vulnerable to peroxyl radicals, and resulting in protein aggregation. The particle size of quinoa protein increased dramatically, but the emulsification and solubility decreased significantly. Moreover, oxidation of quinoa protein was accompanied by remarkable loss of α-helix structure and backbone fragmentation. Oxidation reduced the functional properties of quinoa protein by changing spatial conformation and aggregation morphology. However, the cavitation effect of ultrasound promoted the depolymerization of oxidized quinoa protein aggregates and inhibited the aggregation of protein fragments. Ultrasound enhanced surface hydrophobicity and ζ-potential, which can be contribute to the transformation of protein structure from order to disordered. These results indicated that ultrasound can regulate the conformation and aggregation of oxidized quinoa protein by changing the intermolecular force, thus improving functional properties of quinoa protein.
CRediT authorship contribution statement
Hongwei Cao: Methodology, Data curation, Writing - review & editing. Rulian Sun: Writing - original draft, Investigation. Junru Shi: Software, Methodology. Mengyao Li: Software, Methodology. Xiao Guan: Funding acquisition, Supervision. : . Jing Liu: Formal analysis. Kai Huang: Visualization. Yu Zhang: Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by Shanghai Sailing Program (34-20-308-004); the Capacity-Building Project of Local Universities of SSTC (20060502100); the Dawn Program of Shanghai Education Commission (19SG45) and Scientific Action and Technological Creation Program of Shanghai (20DZ2202700).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2021.105685.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Li G., Zhu F. Quinoa starch: Structure, properties, and applications. Carbohydr. Polym. 2018;181:851–861. doi: 10.1016/j.carbpol.2017.11.067. [DOI] [PubMed] [Google Scholar]
- 2.Vilcacundo R., Hernández-Ledesma B. Nutritional and biological value of quinoa. Chenopodium quinoa WilldCurr. Opin. Food Sci. 2017;14:1–6. [Google Scholar]
- 3.Ceyhun Sezgin A., Sanlier N. A new generation plant for the conventional cuisine: Quinoa (Chenopodium quinoa Willd.) Trends Food Sci. Technol. 2019;86:51–58. [Google Scholar]
- 4.Chen Y.-S., Aluwi N.A., Saunders S.R., Ganjyal G.M., Medina-Meza I.G. Metabolic fingerprinting unveils quinoa oil as a source of bioactive phytochemicals. Food Chem. 2019;286:592–599. doi: 10.1016/j.foodchem.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 5.Cheng Y., Chi Y., Geng X., Chi Y. Effect of 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH) induced oxidation on the physicochemical properties, in vitro digestibility, and nutritional value of egg white protein. LWT. 2021;143 [Google Scholar]
- 6.Hinderink E.B.A., Schröder A., Sagis L., Schroën K., Berton-Carabin C.C. Physical and oxidative stability of food emulsions prepared with pea protein fractions. LWT. 2021;146:111424. doi: 10.1016/j.lwt.2021.111424. [DOI] [Google Scholar]
- 7.Shi R., Li T., Li M., Munkh-Amgalan G., Qayum A., Bilawal A., Jiang Z. Consequences of dynamic high-pressure homogenization pretreatment on the physicochemical and functional characteristics of citric acid-treated whey protein isolate. LWT. 2021;136 [Google Scholar]
- 8.Han T., Wang M., Wang Y., Tang L. Effects of high-pressure homogenization and ultrasonic treatment on the structure and characteristics of casein. LWT. 2020;130 [Google Scholar]
- 9.Wu L., Zhao W., Yang R., Chen X. Effects of pulsed electric fields processing on stability of egg white proteins. J. Food Eng. 2014;139:13–18. [Google Scholar]
- 10.Pezeshk S., Rezaei M., Hosseini H., Abdollahi M. Impact of pH-shift processing combined with ultrasonication on structural and functional properties of proteins isolated from rainbow trout by-products. Food Hydrocolloids. 2021;118 [Google Scholar]
- 11.Zhang Y.i., Di R., Zhang H., Zhang W., Wu Z., Liu W., Yang C. Effective recovery of casein from its aqueous solution by ultrasonic treatment assisted foam fractionation: Inhibiting molecular aggregation. J. Food Eng. 2020;284:110042. doi: 10.1016/j.jfoodeng.2020.110042. [DOI] [Google Scholar]
- 12.Lv L., Lin H., Li Z., Nayak B., Ahmed I., Tian S., Chen G., Lin H., Zhao J. Structural changes of 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH) treated shrimp tropomyosin decrease allergenicity. Food Chem. 2019;274:547–557. doi: 10.1016/j.foodchem.2018.09.030. [DOI] [PubMed] [Google Scholar]
- 13.Duan X., Li M., Shao J., Chen H., Xu X., Jin Z., Liu X. Effect of oxidative modification on structural and foaming properties of egg white protein. Food Hydrocolloids. 2018;75:223–228. [Google Scholar]
- 14.G.A. Ruiz, W. Xiao, M.V. Boekel, M. Minor, M. Stieger, Effect of extraction pH on heat-induced aggregation, gelation and microstructure of protein isolate from quinoa (Chenopodium quinoa Willd), 209 (2016) 203-210. [DOI] [PubMed]
- 15.Mir N.A., Riar C.S., Singh S. Structural modification of quinoa seed protein isolates (QPIs) by variable time sonification for improving its physicochemical and functional characteristics. Ultrason. Sonochem. 2019;58 doi: 10.1016/j.ultsonch.2019.104700. [DOI] [PubMed] [Google Scholar]
- 16.Vera A., Valenzuela M.A., Yazdani-Pedram M., Tapia C., Abugoch L. Conformational and physicochemical properties of quinoa proteins affected by different conditions of high-intensity ultrasound treatments. Ultrason. Sonochem. 2019;51:186–196. doi: 10.1016/j.ultsonch.2018.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Dabbour M., He R., Mintah B., Xiang J., Ma H. Changes in functionalities, conformational characteristics and antioxidative capacities of sunflower protein by controlled enzymolysis and ultrasonication action. Ultrason. Sonochem. 2019;58 doi: 10.1016/j.ultsonch.2019.104625. [DOI] [PubMed] [Google Scholar]
- 18.Timilsena Y.P., Adhikari R., Barrow C.J., Adhikari B. Physicochemical and functional properties of protein isolate produced from Australian chia seeds. Food Chem. 2016;212:648–656. doi: 10.1016/j.foodchem.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 19.Kato A., Nakai S. Hydrophobicity Determination by a Fluorescence Probe Method and its Correlation with Surface Properties of Proteins. BBA. 1980;624:13–20. doi: 10.1016/0005-2795(80)90220-2. [DOI] [PubMed] [Google Scholar]
- 20.Mulcahy E.M., Fargier-Lagrange M., Mulvihill D.M., O'Mahony J.A. Characterisation of heat-induced protein aggregation in whey protein isolate and the influence of aggregation on the availability of amino groups as measured by the ortho-phthaldialdehyde (OPA) and trinitrobenzenesulfonic acid (TNBS) methods. Food Chem. 2017;229:66–74. doi: 10.1016/j.foodchem.2017.01.155. [DOI] [PubMed] [Google Scholar]
- 21.Huang Y., Hua Y., Qiu A. Soybean protein aggregation induced by lipoxygenase catalyzed linoleic acid oxidation. Food Res. Int. 2006;39:240–249. [Google Scholar]
- 22.Chen X., Xu X., Han M., Zhou G., Chen C., Li P. Conformational changes induced by high-pressure homogenization inhibit myosin filament formation in low ionic strength solutions. Food Res. Int. 2016;85:1–9. doi: 10.1016/j.foodres.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 23.Liu H., Zhang H., Liu Q., Chen Q., Kong B. Solubilization and stable dispersion of myofibrillar proteins in water through the destruction and inhibition of the assembly of filaments using high-intensity ultrasound. Ultrason. Sonochem. 2020;67 doi: 10.1016/j.ultsonch.2020.105160. [DOI] [PubMed] [Google Scholar]
- 24.Khatkar A.B., Kaur A., Khatkar S.K., Mehta N. Characterization of heat-stable whey protein: Impact of ultrasound on rheological, thermal, structural and morphological properties. Ultrason. Sonochem. 2018;49:333–342. doi: 10.1016/j.ultsonch.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 25.Rombouts I., Wouters A.G.B., Lambrecht M.A., Uten L., Van Den Bosch W., Vercruysse S.A.R., Delcour J.A. Food Protein Network Formation And Gelation Induced By Conductive Or Microwave Heating: A Focus On Hen Egg White. Innovative Food Sci. Emerg. Technol. 2020;66:102484. doi: 10.1016/j.ifset.2020.102484. [DOI] [Google Scholar]
- 26.Müller S.A., Müller D.J., Engel A. Assessing the structure and function of single biomolecules with scanning transmission electron and atomic force microscopes. Micron. 2011;42:186–195. doi: 10.1016/j.micron.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Dorta E., Ávila F., Fuentes-Lemus E., Fuentealba D., López-Alarcón C. Oxidation of myofibrillar proteins induced by peroxyl radicals: Role of oxidizable amino acids. Food Res. Int. 2019;126:108580. doi: 10.1016/j.foodres.2019.108580. [DOI] [PubMed] [Google Scholar]
- 28.Sun W., Cui C., Zhao M., Zhao Q., Bao Y. Effects of composition and oxidation of proteins on their solubility, aggregation and proteolytic susceptibility during processing of Cantonese sausage. Food Chem. 2011;124:336–341. [Google Scholar]
- 29.Wu W., Zhang C., Kong X., Hua Y. Oxidative modification of soy protein by peroxyl radicals. Food Chem. 2009;116:295–301. [Google Scholar]
- 30.Alavi F., Emam-Djomeh Z., Momen S., Mohammadian M., Salami M., Moosavi-Movahedi A.A. Effect of free radical-induced aggregation on physicochemical and interface-related functionality of egg white protein. Food Hydrocolloids. 2019;87:734–746. [Google Scholar]
- 31.Zhang R., Xing L., Kang D., Zhou L., Wang L., Zhang W. Effects of ultrasound-assisted vacuum tumbling on the oxidation and physicochemical properties of pork myofibrillar proteins. Ultrason. Sonochem. 2021;74 doi: 10.1016/j.ultsonch.2021.105582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li T., Wang L., Chen Z., Sun D., Li Y. Electron beam irradiation induced aggregation behaviour, structural and functional properties changes of rice proteins and hydrolysates. Food Hydrocolloids. 2019;97 [Google Scholar]
- 33.Yan S., Xu J., Zhang S., Li Y. Effects of flexibility and surface hydrophobicity on emulsifying properties: Ultrasound-treated soybean protein isolate. LWT. 2021;142 [Google Scholar]
- 34.Jiang Y., Zhou X., Zheng Y., Wang D., Deng Y., Zhao Y. Impact of ultrasonication/shear emulsifying/microwave-assisted enzymatic extraction on rheological, structural, and functional properties of Akebia trifoliata (Thunb.) Koidz. seed protein isolates. Food Hydrocolloids. 2021;112 [Google Scholar]
- 35.Cui Q., Zhang A., Li R., Wang X., Sun L., Jiang L. Ultrasonic treatment affects emulsifying properties and molecular flexibility of soybean protein isolate-glucose conjugates. Food Bioscience. 2020;38 [Google Scholar]
- 36.Li K., Li Y., Liu C.-L., Fu L., Zhao Y.-Y., Zhang Y.-Y., Wang Y.-T., Bai Y.-H. Improving interfacial properties, structure and oxidative stability by ultrasound application to sodium caseinate prepared pre-emulsified soybean oil. LWT. 2020;131 [Google Scholar]
- 37.Malik M.A., Sharma H.K., Saini C.S. High intensity ultrasound treatment of protein isolate extracted from dephenolized sunflower meal: Effect on physicochemical and functional properties. Ultrason. Sonochem. 2017;39:511–519. doi: 10.1016/j.ultsonch.2017.05.026. [DOI] [PubMed] [Google Scholar]
- 38.Mir N.A., Riar C.S., Singh S. Effect of pH and holding time on the characteristics of protein isolates from Chenopodium seeds and study of their amino acid profile and scoring. Food Chem. 2018;272:165–173. doi: 10.1016/j.foodchem.2018.08.048. [DOI] [PubMed] [Google Scholar]
- 39.Zhao C., Yin H., Yan J., Niu X., Qi B., Liu J. Structure and acid-induced gelation properties of soy protein isolate–maltodextrin glycation conjugates with ultrasonic pretreatment. Food Hydrocolloids. 2021;112 [Google Scholar]
- 40.Zhang Q.-T., Tu Z.-C., Xiao H., Wang H., Huang X.-Q., Liu G.-X., Liu C.-M., Shi Y., Fan L.-L., Lin D.-R. Influence of ultrasonic treatment on the structure and emulsifying properties of peanut protein isolate. Food Bioprod. Process. 2014;92:30–37. [Google Scholar]
- 41.Zou Y., Xu P., Wu H., Zhang M., Sun Z., Sun C., Wang D., Cao J., Xu W. Effects of different ultrasound power on physicochemical property and functional performance of chicken actomyosin. Int. J. Biol. Macromol. 2018;113:640–647. doi: 10.1016/j.ijbiomac.2018.02.039. [DOI] [PubMed] [Google Scholar]
- 42.Cui Q., Wang G., Gao D., Wang L., Zhang A., Wang X., Xu N., Jiang L. Improving the gel properties of transgenic microbial transglutaminase cross-linked soybean-whey mixed protein by ultrasonic pretreatment. Process Biochem. 2020;91:104–112. [Google Scholar]
- 43.Feng J., Berton-Carabin C.C., Ataç Mogol B., Schroën K., Fogliano V. Glycation of soy proteins leads to a range of fractions with various supramolecular assemblies and surface activities. Food Chem. 2021;343 doi: 10.1016/j.foodchem.2020.128556. [DOI] [PubMed] [Google Scholar]
- 44.Cao Y., Ma W., Huang J., Xiong Y.L. Effects of sodium pyrophosphate coupled with catechin on the oxidative stability and gelling properties of myofibrillar protein. Food Hydrocolloids. 2020;104 [Google Scholar]
- 45.Pan J., Lian H., Jia H., Li S., Hao R., Wang Y., Zhang X., Dong X. Ultrasound treatment modified the functional mode of gallic acid on properties of fish myofibrillar protein. Food Chem. 2020;320 doi: 10.1016/j.foodchem.2020.126637. [DOI] [PubMed] [Google Scholar]
- 46.Li S., Li Z., Li X., Wang P., Yu X., Fu Q., Gao S. Effect of AAPH oxidation on digestion characteristics of seed watermelon (Citrullus lanatus var) kernels protein isolates. Food Science and Human Wellness. 2020;9(4):402–410. [Google Scholar]
- 47.Ma J., Wang X., Li Q., Zhang L.i., Wang Z., Han L., Yu Q. Oxidation of myofibrillar protein and crosslinking behavior during processing of traditional air-dried yak (Bos grunniens) meat in relation to digestibility. LWT. 2021;142:110984. doi: 10.1016/j.lwt.2021.110984. [DOI] [Google Scholar]
- 48.Cheng J., Xiang R., Tang D., Zhu M., Liu X. Regulation of protein oxidation in Cantonese sausages by rutin, quercetin and caffeic acid. Meat Sci. 2021;175:108422. doi: 10.1016/j.meatsci.2020.108422. [DOI] [PubMed] [Google Scholar]
- 49.Alavi F., Chen L., Emam-Djomeh Z. Effect of ultrasound-assisted alkaline treatment on functional property modifications of faba bean protein. Food Chem. 2021;354:129494. doi: 10.1016/j.foodchem.2021.129494. [DOI] [PubMed] [Google Scholar]
- 50.Chen Y., Huang F., Xie B., Sun Z., McClements D.J., Deng Q. Fabrication and characterization of whey protein isolates- lotus seedpod proanthocyanin conjugate: Its potential application in oxidizable emulsions. Food Chem. 2021;346:128680. doi: 10.1016/j.foodchem.2020.128680. [DOI] [PubMed] [Google Scholar]
- 51.N.M. Duran, M. Galante, D. Spelzini, V. Boeris, The effect of carrageenan on the acid-induced aggregation and gelation conditions of quinoa proteins, Food Research International, 113 267-272. [DOI] [PubMed]
- 52.Yan S., Xu J., Zhang S., Li Y. Effects of flexibility and surface hydrophobicity on emulsifying properties: Ultrasound-treated soybean protein isolate. LWT- Food Science and Technology. 2021;142:110881. doi: 10.1016/j.lwt.2021.110881. [DOI] [Google Scholar]
- 53.Li F., Wu X., Wu W. Effects of oxidative modification by malondialdehyde on the in vitro digestion properties of rice bran protein. J. Cereal Sci. 2021;97 [Google Scholar]
- 54.Xu M., Lian Z., Chen X., Yao X., Lu C., Niu X., Xu M., Zhu Q. Effects of resveratrol on lipid and protein co-oxidation in fish oil-enriched whey protein isolate emulsions. Food Chem. 2021;365 doi: 10.1016/j.foodchem.2021.130525. [DOI] [PubMed] [Google Scholar]
- 55.Kumari S., Gupta O.P., Mishra C.B., Thimmegowda V., Krishnan V., Singh B., Sachdev A., Dahuja A. Gamma irradiation, an effective strategy to control the oxidative damage of soy proteins during storage and processing. Radiat. Phys. Chem. 2020;177 [Google Scholar]
- 56.Zhu Z., Bassey A.P., Khan I.A., Huang M., Zhang X. Inhibitory mechanism of catechins against advanced glycation end products of glycated myofibrillar protein through anti-aggregation and anti-oxidation. LWT. 2021;147 [Google Scholar]
- 57.Cueto M., Porras-Saavedra J., Farroni A., Alamilla-Beltrán L., Schöenlechner R., Schleining G., Buera P. Physical and mechanical properties of maize extrudates as affected by the addition of chia and quinoa seeds and antioxidants. J. Food Eng. 2015;167:139–146. [Google Scholar]
- 58.Flores-Jiménez N.T., Ulloa J.A., Silvas J.E.U., Ramírez J.C.R., Ulloa P.R., Rosales P.U.B., Carrillo Y.S., Leyva R.G. Effect of high-intensity ultrasound on the compositional, physicochemical, biochemical, functional and structural properties of canola (Brassica napus L.) protein isolate. Food Res. Int. 2019;121:947–956. doi: 10.1016/j.foodres.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 59.Lha B., Wz A., Dy C., Lm A., Hma B. Solubility and aggregation of soy protein isolate induced by different ionic liquids with the assistance of ultrasound - ScienceDirect. Int. J. Biol. Macromol. 2020;164:2277–2283. doi: 10.1016/j.ijbiomac.2020.08.031. [DOI] [PubMed] [Google Scholar]
- 60.Huang L., Zhang W., Yan D., Ma L., Ma H. Solubility and aggregation of soy protein isolate induced by different ionic liquids with the assistance of ultrasound. Int. J. Biol. Macromol. 2020;164:2277–2283. doi: 10.1016/j.ijbiomac.2020.08.031. [DOI] [PubMed] [Google Scholar]
- 61.Shen X., Fang T., Gao F., Guo M. Effects of ultrasound treatment on physicochemical and emulsifying properties of whey proteins pre- and post-thermal aggregation. Food Hydrocolloids. 2017;63:668–676. [Google Scholar]
- 62.Huang L., Ding X., Li Y., Ma H. The aggregation, structures and emulsifying properties of soybean protein isolate induced by ultrasound and acid. Food Chem. 2019;279:114–119. doi: 10.1016/j.foodchem.2018.11.147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.