Abstract
Increasing evidence has shown that the metabolism and clearance of molecular targeted agents, such as sorafenib, plays an important role in mediating the resistance of HCC cells to these agents. Metabolism of sorafenib is performed by oxidative metabolism, which is initially mediated by CYP3A4. Thus, targeting CYP3A4 is a promising approach to enhance the sensitivity of HCC cells to chemotherapeutic agents. In the present work, we examined the association between CYP3A4 and the prognosis of HCC patients receiving sorafenib. Using the online tool miRDB, we predicted that has-microRNA-4277 (miR-4277), an online miRNA targets the 3’UTR of the transcript of cyp3a4. Furthermore, overexpression of miR-4277 in HCC cells repressed the expression of CYP3A4 and reduced the elimination of sorafenib in HCC cells. Moreover, miR-4277 enhanced the sensitivity of HCC cells to sorafenib in vitro and in vivo. Therefore, our results not only expand our understanding of CYP3A4 regulation in HCC, but also provide evidence for the use of miR-4277 as a potential therapeutic in advanced HCC.
Keywords: advanced hepatocellular carcinoma, cytochrome P450 3A4, miR-4277, chemoresistance, sorafenib, metabolism or clearance
Introduction
Though many advances have been made in the treatment of advanced HCC, it remains a major challenge for China’s public health (1–3). In China, more than 80 million people suffer from viral liver disease or related acute and chronic liver diseases (1). These patients have a high risk of developing HCC (1, 4). Furthermore, most HCC patients are diagnosed with advanced disease, so they are not candidates for radical treatment strategies like surgery or liver transplantation (5–7). At present, drug treatment strategies for advanced HCC are limited, they are mainly based on various molecular targeted therapeutics (8–10), or immune checkpoint inhibitors targeting PD-1/PD-L1 (programmed cell death-1/programmed cell death ligand-1) (11–13). Among them, molecular-targeted agents like sorafenib have been used in clinical treatment for many years, though several problems remain: the efficacy of sorafenib varies based on the patient, and resistance is common; sorafenib treatment often induces serious side effects (14, 15); newer agents such as lenvatinib, regorafenib, and carbozantinib (8–10), have the same chemical parent ring structure (1-(4- (pyridin-4-yloxy)phenyl) urea) as sorafenib. Therefore, it is important to understand the mechanisms of resistance to sorafenib and other agents, and to study and explore sensitization strategies for HCC cells to molecular targeted agents. This will not only provide more choice for the patient, but it will also help improve the efficacy of combination therapies using targeted agents and/or immune checkpoint inhibitors.
The metabolism and clearance mechanisms of sorafenib and other exogenous agents in HCC cells are crucial factors in the development of HCC chemoresistance (16, 17). Via a systemic analysis, Feng et al. (18) and Shao et al. (19) found that sorafenib can act as a ligand/agonist to activate PXR/NR1I2 (pregnane X receptor/nuclear receptor subfamily 1 group I member 2) and induce expression of downstream genes involved in chemoresistance, including cyp3a4 and abcb1 (ATP-binding cassette, sub-family B, member 1). Ultimately, this accelerates elimination of the therapeutic and results in drug resistance through negative feedback regulation (18, 19). Although related studies have expanded our understanding of PXR in HCC, much remains unclear; PXR is not the only metabolism-related nuclear receptor in HCC cells, and the CAR/NR1I3 (constitutive androstane receptor/nuclear receptor subfamily 1 group I member 3) may have similar functions to PXR (20, 21). By inhibiting the activity of PXR alone, CAR may have a compensatory effect on the function of PXR. Moreover, targeting oxidative metabolism, which is mediated by CYP3A4 in the initial step of sorafenib elimination in HCC cells, represents a promising approach to enhance the sensitivity of these cells to targeted agents (22, 23). This makes CYP3A4 more advantageous to target compared with PXR or CAR. Both PXR and CAR mediate the expression of CYP3A4 to eliminate sorafenib. By inhibiting CYP3A4, compensatory effects between PXR and CAR can be avoided.
MicroRNA is a type of small non-coding RNA transcribed by RNA polymerase II (24–27). In mammalian cells, miRNA can directly affect the 3’UTR of the target mRNA to degrade it in a sequence-specific manner and silence gene expression (28–30). Because of this feature, miRNAs are widely used as anti-cancer therapeutics. By predicting miRNAs that target certain sets of oncogenes, one can identify novel anti-cancer miRNAs that can be added to lentiviral particles to reduce expression of the target oncogene and sensitize cells to targeted agents. Our study used the online tool miRDB to identify miR-4277, a potential repressor of CYP3A4 expression. We infected HCC cells with lentiviral particles containing pre-miR-4277 and confirmed the effect of miR-4277 on CYP3A4 and the elimination of sorafenib.
Materials and Methods
Cell Lines and Reagents
The HCC cell lines, MHCC97-H, HepG2, BEL7402 or SMMC7721, were grown in our lab and described previously (18, 19). The clinical specimens of advanced HCC were also descripted in our previous work (18, 19). The use of human subjects was approved by the ethics committee of the Fifth Medical Center, General Hospital of Chinese PLA (People’s Liberation Army). All assays were carried out in accordance with the Helsinki Declaration. Sorafenib, lenvatinib, cabozantinib, regorafenib, anlotinib, and apatinib were chemically synthesized by Dr. Shuang Cao at the Wuhan Institute of Technology, Wuhan City, Hubei Province of China. The potential cyp3a4’s inhibitor, ketoconazole, amprenavir or diltiazem, was also gifts from Dr. Shuang Cao at the Wuhan Institute of Technology, Wuhan City, Hubei Province of China. All agents were initially prepared as powders purified to >99% by using the HPLC (high performance liquid chromatography) ( Supplemental Table 1 ). The miR-4277 was a microRNA potentially targeting to cyp3a4’s 3’UTR via an online tool, miRDB, and the full-length sequences of has-pre-miR-4277, wild-type cyp3a4 and cyp3a4, and miR-4277 mutated at its targeting sites (two targeting sites of miR-4277 located in the 3’UTR of cyp3a4: 1st site of 496th – 503th nt [a 8mer site]; 2nd site of 984th – 991st nt [a 8mer site]) for the 3’UTR were chemically synthesized and prepared as lentiviral particles: (1) Luc-1 (the luciferase reporter with the wild type of the 1st miR-4277 targeting site), (2) Luc-2 (the luciferase reporter with the wild type of the 2nd miR-4277 targeting site), (3) Luc-3 (the luciferase reporter with the wild type of the 1st and 2nd miR-4277 targeting site), (4) Luc-4 (the luciferase reporter with the wild type of the 2nd and the mutated 1st miR-4277 targeting site), (5) CYP3A4 mutations (CYP3A4Mut1 [the vector of cyp3a4 with the mutation of the 1st miR-4277 targeting site], CYP3A4Mut2 [the vector of cyp3a4 with the mutation of the 2nd miR-4277 targeting site], or CYPMut [the vector of cyp3a4 with the mutation of the 1st and 2nd miR-4277 targeting site]).
Quantitative PCR
The endogenous mRNA levels of cyp3a4 in HCC clinical specimens were identified using quantitative polymerase chain reaction (qPCR) in accordance with methods described by Wang et al. (20) and Ma et al. (20, 25). The primers used were: (1) cyp3a4, forward sequence 5’-CCGAGTGGATTTCCTTCAGCTG-3’, reverse sequence 5’-TGCTCGTG GTTTCATAGCCAGC-3’; (2) β-actin, forward sequence 5’-CACCATTGGCAATGA GCGGTTC-3’, reverse sequence 5-AGGTCTTTGCGGAT GTCCACGT-3’; (3) PXR/NR1I2 (31), forward sequence 5’-CCCACCTCAGA AGACAAAGC-3’, reverse sequence, 5’-GAACCCCAGACCCTACACAA-3’ (4); CAR/NR1I3 (31): forward sequence, 5’-TACTGTGCTTCGTGCTCCTG-3’, Reverse sequence, 5’-CCTGG TCTTCGGGTTCAAG-3’. The results (the relative expression level [folds of β-Actin]) of PXR or CAR was shown as heat-map.
Western Blot
MHCC97-H cells were cultured and TACT transfected with plasmids, such as miR-4277 or cyp3a4; the cells were then harvested and proteins were extracted as previously described by Wei et al. (32) and Jia et al. (32, 33). Antibodies against CYP3A4 (Cat. No.: ab124921) and β-Actin (Cat. No.: ab8226) were purchased from the Abcam Corporation, Cambridge, UK). The images of western blot was quantitatively analyzed by Image J Software (National Institutes of Health [NIH], Bethesda, Maryland, USA).
Assessment of Sorafenib Elimination in HCC Cells
HCC cells were used to examine the rate of elimination of sorafenib (18, 19, 34, 35). For cell-based experiments, HCC cells were treated with 1 μmol/L of sorafenib for 12 h. After treatment, the cells were harvested at a series of time-points. For in vivo experimentation, HCC cells were cultured and subcutaneously injected into mice to generate tumors. When tumor volume reached 2000 mm3, a solution of sorafenib was directly injected into the tumors. After injection, the tumors were excised at a series of time-points. Next, sorafenib was extracted from MHCC97-H cells or tumors using the acetonitrile (ACN). The sustaining amount of sorafenib at each time-point was measured using liquid chromatography–mass spectrometry/mass spectrometry (LC-MS/MS) and the in vitro/in vivo half-life of sorafenib was determined (18, 19, 34, 35).
Assessment of Cell Survival
After transfection or treated with potential inhibitor of cyp3a4, HCC cells were treated with a series concentrations of sorafenib, lenvatinib, cabozantinib, regorafenib, anlotinib, or apatinib (10 μmol/L, 3 μmol/L, 1 μmol/L, 0.3 μmol/L, 0.1 μmol/L, 0.03 μmol/L, or 0.01 μmol/L). After 48 h, MTT was performed to measure ell survival, and the IC50 value of each agent was calculated (36–38).
Nude Mouse Tumor Model
All animal experiments were reviewed approved by the Institutional Animal Care and Use Committee, the Fifth Medical Center, Chinese PLA. All animal experiments were performed in accordance with the UK Animals (Scientific Procedures) Act, 1986 and the associated guidelines. Female nude mice were purchased from the Si-Bei-Fu Corporation, Beijing China. Following transfection, MHCC97-H cells were prepared as a single-cell suspension and subcutaneously injected into nude mice (39, 40). The mice orally received sorafenib once every two days. Tumors from the mice were harvested, and the volumes and weights were measured (39, 40).
Statistical Analysis
SPSS 9.0 statistical software (IBM Corporation, Armonk, NY, USA) was used for all statistical analyses. Statistics were calculated using two-way ANOVA with the Bonferroni correction. IC50 values and the half-lie values (t1/2 values) were calculated using Origin software (Origin 6.1; OriginLab Corporation, Northampton, MA, USA). The *P < 0.05 being statistically significant between groups. The heat-map of the qPCR results were obtained according to the methods by Zhou et al. (41) and Yin et al. (42).
Results
High Levels of cyp3a4 mRNA Expression Are Associated With Poor Prognosis in HCC Patients Receiving Sorafenib
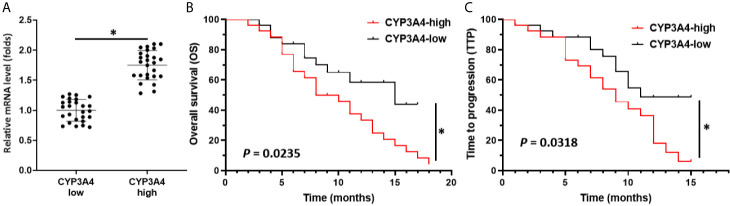
Though it has been suggested that cyp3a4 participates in the resistance of HCC cells to sorafenib, the clinical significance of cyp3a4 requires further analysis. As shown in Figure 1 , the endogenous mRNA levels of cyp3a4 were examined in 52 clinical specimens from patients with advanced HCC ( Figure 1 and Table 1 ). According to the median expression level of cyp3a4 in HCC tissue samples, patients were divided into two groups: a cyp3a4 high expression group [cyp3a4-high] and a cyp3a4 low expression group [cyp3a4-low]) ( Figure 1A ). We then performed survival analysis ( Figures 1B, C ). The prognosis of patients in the cyp3a4 high expression group [cyp3a4-high] treated with sorafenib, as measured by TTP (time to progress) and OS (overall survival), was significantly worse than that of the cyp3a4 low expression group [cyp3a4-low]) ( Figures 1B, C and Table 1 ). Therefore, high levels of cyp3a4 appear to be associated with poor prognosis of HCC patients receiving sorafenib.
Figure 1.
High levels of cyp3a4 is associated with poor prognosis in advanced HCC. (A) The endogenous mRNA levels of cyp3a4 were assessed in 52 clinical specimens from patients with advanced HCC. According to the median expression level of cyp3a4 in HCC tissue samples, patients were divided into two groups: a cyp3a4 high expression group [cyp3a4-high] and a cyp3a4 low expression group [cyp3a4-low]) (B, C). Survival analysis included analysis of TTP and OS. *P < 0.05.
Table 1.
CYP3A4 expression and clinical outcome of sorafenib treatment.
| CYP3A4 mRNA expression | P | ||
|---|---|---|---|
| High (n = 26) | Low (n = 26) | ||
| TTP | 9.0 | 11.0 | 0.032 |
| 6.1-11.8 (M) | 9.5-12.9 (M) | ||
| OS | 8.0 | 15.0 | 0.023 |
| 4.8-11.2 (M) | 6.9-23.1 (M) | ||
TTP, time to progress; OS, overall survival; PR, partial remission; CR, complete remission; SD, stable of disease; M, months.
miR-4277 Represses the Expression of cyp3a4 by Targeting its 3’UTR
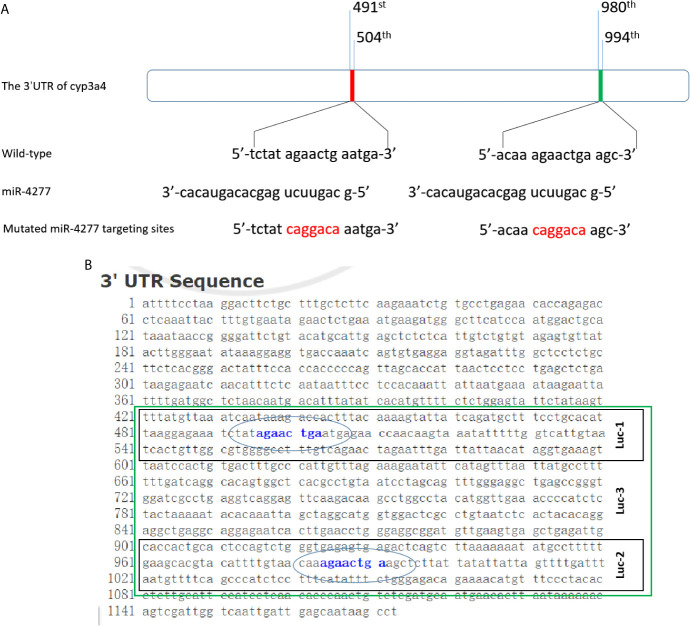
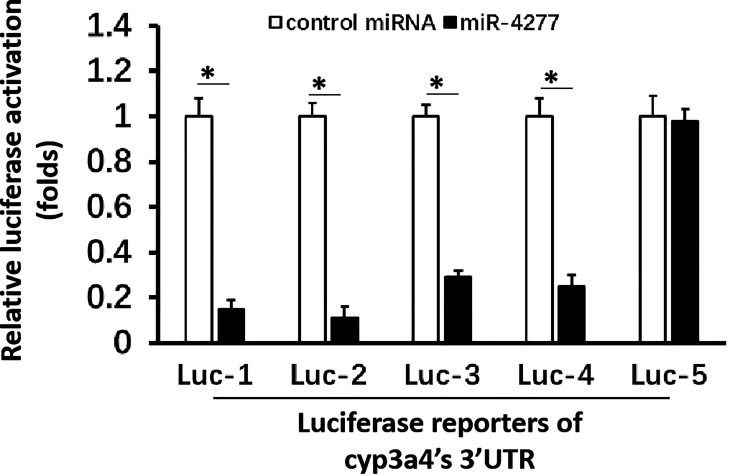
To explore CYP3A4 as a potential anti-cancer target, miR-4277 was identified via the online tool miRDB as a microRNA that could potentially target cyp3a4. The potential binding sites of miR-4277 in the 3′-UTR of cyp3a4 as well as in wild type cyp3a4 and cyp3a4 with mutated miR-4277 binding sites are shown in Figure 2A . To confirm the effects of miR-4277 on cyp3a4 expression, we used a luciferase reporter construct for cyp3a4 and transfected cells with miR-4277. As shown in Figures 2B and 3 , overexpression of miR-4277 repressed the activation of Luc-1 (the luciferase reporter with the wild type of the 1st miR-4277 targeting site), Luc-2 (the luciferase reporter with the wild type of the 2nd miR-4277 targeting site), Luc-3 (the luciferase reporter with the wild type of the 1st and 2nd miR-4277 targeting site), and Luc-4 (the luciferase reporter with the wild type of the 2nd and the mutated 1st miR-4277 targeting site). Moreover, miR-4277 did not affect the activation of Luc-5 (the luciferase reporter with the wild type of the 2nd and the mutated 1st miR-4277 targeting site) ( Figure 3 ).
Figure 2.
miR-4277 is predicted to target the 3’UTR of cyp3a4. (A) The sequences of miR-4277 and cyp3a4 are shown as schematic diagrams. (B) The luciferase reporters containing the 3’UTR region of cyp3a4 containing the miR-4277 binding site are shown as a schematic diagram.
Figure 3.
miR-4277 represses the activation of cyp3a4’s 3’UTR luciferase reporters in MHCC97-H cells. The effect of miR-4277 on the activation of Luc-1 (the luciferase reporter with the wild type of the 1st miR-4277 targeting site) or Luc-2 (the luciferase reporter with the wild type of the 2nd miR-4277 targeting site), Luc-3 (the luciferase reporter with the wild type of the 1st and 2nd miR-4277 targeting site), Luc-4 (the luciferase reporter with the wild type of the 2nd and the mutated 1st miR-4277 targeting site), or Luc-5 (the luciferase reporter with the wild type of the 2nd and the mutated 1st miR-4277 targeting site) was examined. The results are shown as histograms. *P < 0.05.
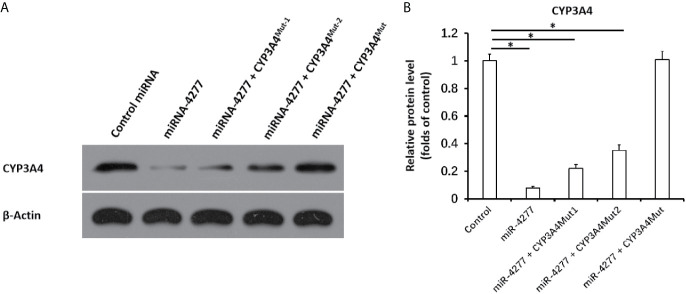
Next, the effect of miR-4277 on the CYP3A4 protein expression was examined via western blot. As shown in Figure 4 , miR-4277 not only inhibited the protein expression of the endogenous CYP3A4 in MHCC97-H cells, but also the expression of CYP3A4MUT1 or CYP3A4Mut2. Transfection of miR-4277 did not affect the expression of CYP3A4MUT ( Figure 4 ). Therefore, these results suggest that miR-4277 targets the 3’UTR of cyp3a4 in a sequence specific manner.
Figure 4.
miR-4277 represses the expression of CYP3A4 in MHCC97-H cells. The effect of miR-4277 on wild type CYP3A4 or CYP3A4 mutations (CYP3A4Mut1 [the vector of cyp3a4 with the mutation of the 1st miR-4277 targeting site], CYP3A4Mut2 [the vector of cyp3a4 with the mutation of the 2nd miR-4277 targeting site], or CYPMut [the vector of cyp3a4 with the mutation of the 1st and 2nd miR-4277 targeting site]) in MHCC97-H cells was measured by western blot. The results are shown as blots (A) or the quantitative results (B). *P < 0.05.
miR-4277 Reduces the Elimination of Sorafenib in HCC Cells
Our results above suggest that miR-4277 represses the expression of CYP3A4 in MHCC97-H cells; therefore, we assessed whether miR-4277 could affect the elimination of sorafenib via LC-MS/MS. As shown in Table 2 , overexpression of miR-4277 reduces elimination of sorafenib in cultured MHCC97-H cells; the half-life (t1/2) of sorafenib in MHCC97-H cells was also decreased in the presence of miR-4277 ( Table 2 ). Transfection of CYP3A4Mut but not CYP3A4Mut1 or CYPMut2 blocked the effect of miR-4277 on the reduction in t1/2 of sorafenib ( Table 2 ). Therefore, miR-4277 blocked elimination of sorafenib in HCC cells by targeting the 3’UTR of cyp3a4.
Table 2.
The effect of miR-4277 on Sorafenib in cultured MHCC97-H cells.
| Groups | t1/2 of Sorafenib (hours) | IC50 of Sorafenib (μmol/L) |
|---|---|---|
| control miRNA | 21.60 ± 1.34 | 0.70 ± 0.09 |
| miR-4277 | 38.46 ± 9.97 | 0.15 ± 0.01 |
| miR-4277 + CYP3A4Mut-1 | 22.11 ± 6.59 | 0.22 ± 0.14 |
| miR-4277 + CYP3A4Mut-2 | 24.68 ± 8.63 | 0.35 ± 0.05 |
| miR-4277 + CYP3A4Mut | 19.88 ± 2.71 | 0.92 ± 0.20 |
miR-4277 Enhances the Sensitivity of HCC Cells to Targeted Agents
To further examine the effect of miR-4277 on cyp3a4 in HCC cells, the effects of other targeted agents were assessed by MTT. As shown in Table 2 , overexpression of miR-4277 enhanced the sensitivity of MHCC97-H cells to sorafenib, with a decrease in its IC50 value. Transfection of CYP3A4Mut but not CYP3A4Mut1 or CYPMut2 blocked the effect of miR-4277 on the reduction in t1/2 value for sorafenib. Similar results were obtained for five other targeted agents, including regorafenib, lenvatinib, anlotinib, cabozantinib, and apatinib ( Table 3 ). Therefore, miR-4277 appears to enhance the sensitivity of HCC cells to multiple targeted agents by targeting the 3’UTR of cyp3a4.
Table 3.
miR-4277 enhanced the sensitivity of MHCC97-H cells to molecular targeted agents, regorafenib, lenvatinib, anlotinib, cabozantinib, or apatinib.
| Groups | Regorafenib | Lenvatinib | Anlotinib | Cabozantinib | Apatinib |
|---|---|---|---|---|---|
| IC50 values of the agents on MHCC97-H cells (μmol/L) | |||||
| control miRNA | 0.67 ± 0.49 | 0.55 ± 0.20 | 0.75 ± 0.03 | 0.51 ± 0.19 | 0.96 ± 0.33 |
| miR-4277 | 0.22 ± 0.04 | 0.12 ± 0.06 | 0.10 ± 0.01 | 0.10 ± 0.04 | 0.26 ± 0.07 |
| miR-4277 + CYP3A4Mut-1 | 0.33 ± 0.32 | 0.41 ± 0.01 | 0.30 ± 0.12 | 0.25 ± 0.06 | 0.52 ± 0.27 |
| miR-4277 + CYP3A4Mut-2 | 0.35 ± 0.09 | 0.28 ± 0.11 | 0.45 ± 0.35 | 0.30 ± 0.21 | 0.46 ± 0.30 |
| miR-4277 + CYP3A4Mut | 0.71 ± 0.28 | 0.60 ± 0.20 | 0.78 ± 0.55 | 0.56 ± 0.15 | 0.98 ± 0.37 |
miR-4277 Inhibits the Growth and Induces Sensitization of HCC Tumors to Sorafenib in Nude Mice
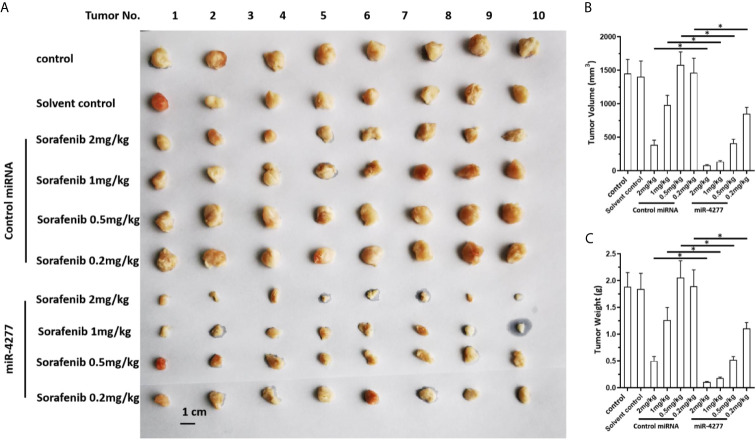
The effect of miR-4277 to induce sensitivity to sorafenib was further confirmed in a nude mice model. As shown in Figure 5 , sorafenib inhibited the subcutaneous growth of HCC cells in a dose-dependent manner, and transfected with miR-4277 further enhanced this effect ( Figure 5 ).
Figure 5.
miR-4277 enhances the antitumor effects of sorafenib in inhibiting the growth of HCC cells in a nude mouse model. MHCC97-H cells were transfected with vectors (control miR or miR-4277) and subcutaneously injected into nude mice. The mice then received the indicated dose of sorafenib via oral administration. The results are shown as tumor images (A), tumor volumes (B), or tumor weights (C). *P < 0.05.
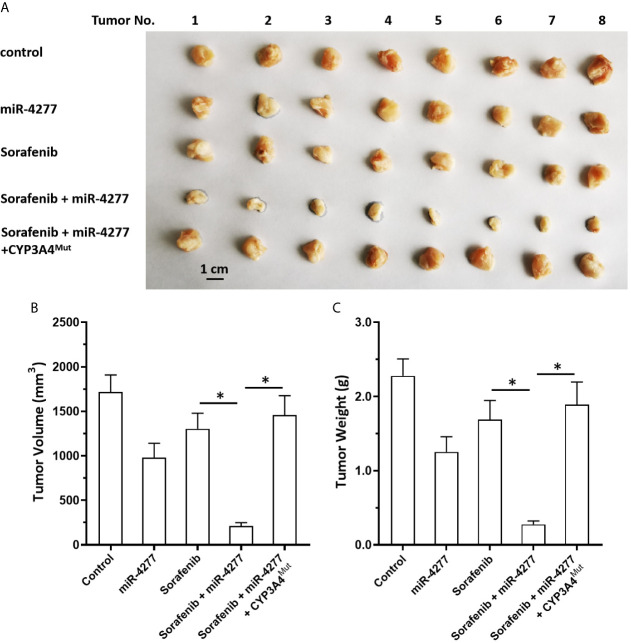
Moreover, the specificity of miR-4277 for cyp3a4 was examined in our subcutaneous tumor model. As shown in Figure 6 , MHCC97-H cells were transfected with control, miR-4277, or miR-4277 + CYP3A4Mut (the expression vectors of cyp3a4 with the mutated binding sites of miR-4277). Transfection of miR-4277 inhibited the subcutaneous growth of MHCC97-H cells, and a 0.5 mg/kg dose of sorafenib had no effect ( Figure 6 ). Transfection of miR-4277 enhanced the sensitivity of MHCC97-H cells to sorafenib, whereas CYP3A4Mut blocked the effect of miR-4277 ( Figure 6 ).
Figure 6.
miR-4277 enhances the antitumor effect of sorafenib in a nude mouse model. MHCC97-H cells were transfected with vectors (control miR, miR-4277, or miR-4277 + CYP3A4Mut) and subcutaneously injected into nude mice. The mice then received the indicated dose of sorafenib via oral administration. The results are shown as tumor images (A), tumor volumes (B), or tumor weights (C). *P < 0.05.
The elimination of sorafenib in subcutaneous tumors were also examined to further confirm the effect of miR-4277. As shown in Table 4 , overexpression of miR-4277 reduced the rates of sorafenib elimination in tumor tissues formed by MHCC97-H cells, and the t1/2 of sorafenib in these tissues was also decreased. Transfection of CYP3A4Mut but not CYP3A4Mut1 or CYPMut2 blocked this effect. These results further confirm the effect of miR-4277 on sorafenib elimination.
Table 4.
Effect of miR-4277 on the clearance of sorafenib in the subcutaneous tumors formed by MHCC97-H cells.
| Groups | t1/2 of Sorafenib (hours) |
|---|---|
| control miRNA | 45.68 ± 5.31 |
| miR-4277 | 72.79 ± 7.52 |
| miR-4277 + CYP3A4Mut-1 | 52.38 ± 16.19 |
| miR-4277 + CYP3A4Mut-2 | 48.74 ± 28.50 |
| miR-4277 + CYP3A4Mut | 36.67 ± 12.58 |
The Endogenous mRNA Level of PXR and CAR in Clinical Specimens
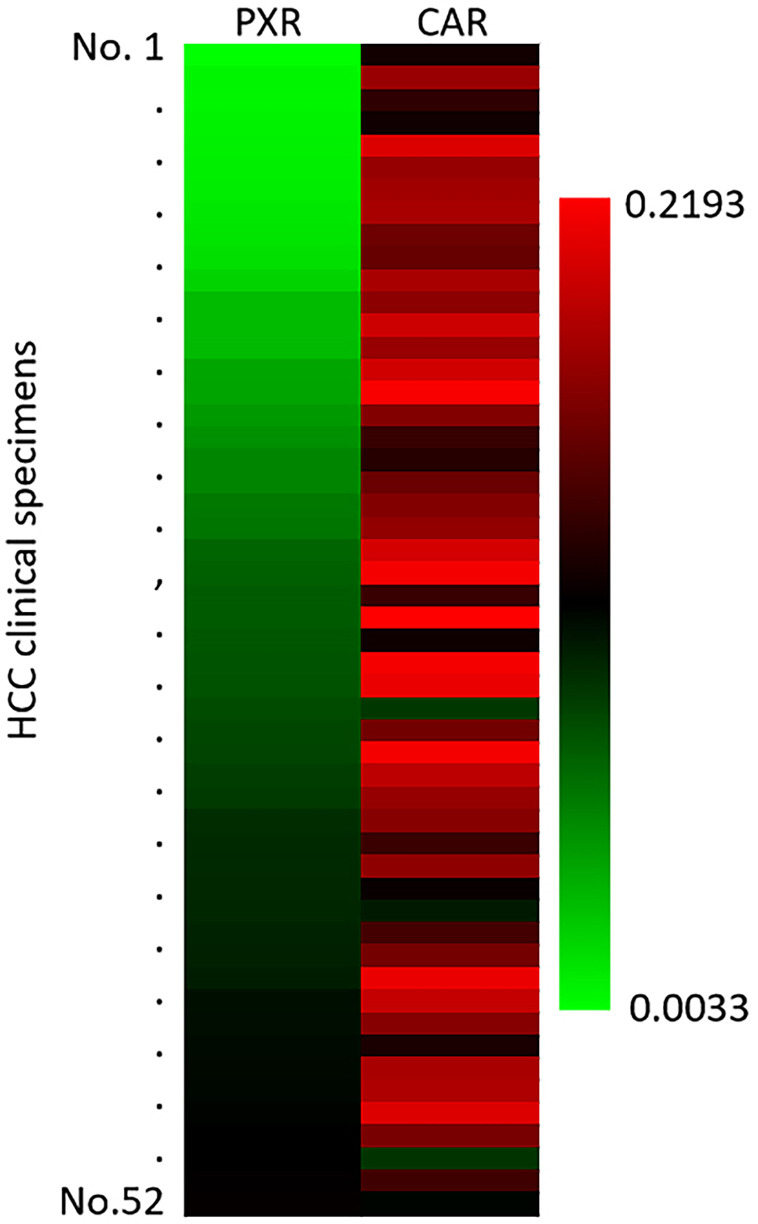
The above results mainly focus on the roles of miR-4277/cyp3a4 in HCC cells. To further confirm the clinical significance of cyp3a4, the expression level of PXR and CAR in HCC clinical specimens was examined by qPCR. As shown in Figure 7 , the expression of PXR or CAR were detected in the HCC clinical specimens. The expression level of CAR was also higher than that of PXR ( Figure 7 ). Therefore, the endogenous mRNA level of PXR and CAR are positive in HCC clinical specimens and the compensatory effect between PXR and CAR cannot be ignored.
Figure 7.
The endogenous expression of PXR or CAR in HCC clinical specimens. The endogenous expression of PXR or CAR in HCC clinical specimens was examined by qPCR. The results were shown as heat-map from the relative expression level (folds of β-Actin).
miR-4277 Also Enhances the Sensitivity of Sorafenib in Some Other HCC Cell Lines
Next, the effect of miR-4277 on sorafenib was examined in some other HCC cell lines. As shown in Table 5 , transfection with miR-4277 decelerated the metabolism or clearance of sorafenib in HepG2, BEL-7402 or SMMC-7721 cells, and the t1/2 values of sorafenib increased, respectively ( Table 5 ). Moreover, transfection of miR-4277 also enhances the sensitivity of these three HCC cell lines to sorafenib ( Table 5 ), and the IC50 values of sorafenib decreased, respectively ( Table 5 ). Transfection of CYP3A4Mut almost blocked the effect of miR-4277 on sorafenib’s metabolism/clearance rates or the antitumor activation on HCC cells. Therefore, miR-4277 also enhances the sensitivity of sorafenib in some other HCC cell lines by targeting cyp3a4’s 3’UTR.
Table 5.
The effect of miR-4277 on sorafenib in HCC cells.
| Sorafenib on HCC cells | Groups | HepG2 | BEL-7402 | SMMC-7721 |
|---|---|---|---|---|
| t1/2 values (h) | control | 30.30 ± 3.31 | 28.20 ± 8.67 | 25.70 ± 2.43 |
| miR-4277 | 56.43 ± 1.90 | 42.40 ± 3.33 | 46.25 ± 2.88 | |
| miR-4277 + CYP3A4Mut | 25.74 ± 4.47 | 26.36 ± 3.79 | 27.39 ± 6.93 | |
| IC50 values (μmol/L) | control | 2.78 ± 0.59 | 1.79 ± 0.18 | 1.95 ± 0.54 |
| miR-4277 | 0.41 ± 0.07 | 0.28 ± 0.05 | 0.73 ± 0.33 | |
| miR-4277 + CYP3A4Mut | 3.56 ± 0.82 | 2.88 ± 0.35 | 1.92 ± 0.55 |
The Potential Inhibitor of CYP3A4 Enhances the Sensitivity of MHCC97-H Cells to Sorafenib
Furthermore, the effect of potential inhibitors of cyp3a4 were used. As shown in Table 6 , treatment of 1μmol/L concentration of ketoconazole, amprenavir or diltiazem, decelerated the metabolism or clearance of sorafenib in MHCC87-H cells, and the t1/2 values of sorafenib increased, respectively ( Table 6 ). Treatment of these agents also enhances the sensitivity of MHCC97-H cells to sorafenib ( Table 6 ), and the IC50 values of sorafenib decreased, respectively ( Table 6 ). The effect of ketoconazole is much greater than amprenavir or diltiazem. Therefore, the inhibited of CYP3A4 both via miR-4277 or inhibitors could enhance the sensitivity of HCC cells to sorafenib.
Table 6.
The effect of potential inhibitors of CYP3A4 on sorafenib in MHCC97-H cells.
| Cell lines | Sorafenib on MHCC97-H | |
|---|---|---|
| t1/2 values (h) | IC50 values (μmol/L) | |
| Solvent control | 23.63 ± 0.70 | 0.68 ± 0.11 |
| ketoconazole | 44.17 ± 2.86 | 0.08 ± 0.01 |
| amprenavir | 33.85 ± 3.92 | 0.42 ± 0.05 |
| diltiazem | 37.40 ± 0.43 | 0.34 ± 0.10 |
Discussion
Currently, targeted therapy remains a first-line choice for patients with advanced HCC; however, the overall clinical benefit of these therapies are unsatisfactory (43–45). It is important to block chemoresistance pathways in HCC to improve sensitivity to targeted agents. Zhu et al. (14) systematically summarized the possible mechanisms of resistance of HCC cells to sorafenib and discerned the compensatory effects of various signal pathways, including the epithelial-mesenchymal transition and signals from the tumor stem cell environment, may increase resistance to sorafenib (14). Although studies like these are beneficial to expand our understanding of sorafenib resistance in advanced HCC, several issues persist. The liver is the body’s regulatory center for exogenous metabolism and clearance (46, 47). HCC arises from hepatocytes, and the metabolism and clearance of exogenous agents may be specific to the ability of HCC cells to tolerate sorafenib (18, 19). Feng et al. (18) showed that sorafenib can function as a ligand/agonist to induce PXR transcription factor activity and accelerate the metabolism and clearance rate of sorafenib. It does this by inducing the expression of drug resistance genes downstream of PXR, such as cyp3a4 or abcb1 (ATP-binding cassette, sub-family B, member 1), and through a similar negative feedback mechanism that induces resistance of HCC cells to sorafenib itself. This means that HCC patients with high background expression levels of PXR may not be sensitive to sorafenib (18). With long-term treatment, sorafenib can also induce the activity of PXR in HCC cells and the expression of drug-resistant genes, which ultimately leads to multidrug resistance (18). CYP3A4 is an important regulator of sorafenib metabolism and clearance in HCC cells as it can mediate the oxidative metabolism of sorafenib (48). Our study identified that a microRNA that can act on the 3’UTR of cyp3a4: miR-4277. We found that it could down-regulate the expression levels of CYP3A4 in HCC cells, reduce elimination of sorafenib, and ultimately enhance the sensitivity of HCC cells to sorafenib. Therefore, miR-4277 and cyp3a4 represent ideal targets that can be modulated to overcome the resistance of HCC cells to targeted therapy. Our results also showed that the potential inhibitors of CYP3A4 had the similar effect of miR-4277 on sorafenib in HCC cells. The effect of ketoconazole is significantly stronger than amprenavir or diltiazem (49–55). Since ketoconazole is also considered to be an inhibitor of PXR, this needs to be discussed in depth.
The transcription of cyp3a4 is mainly mediated by PXR, but it can also regulated by CAR (56–58). In cancerous cells, particularly in HCC, the activity of PXR may also be compensated by CAR. Using miRNA or PXR antagonists alone to down-regulate the activity of PXR may be an inefficient method of fully blocking the resistance of malignant tumor cells to anti-tumor agents. CAR can also induce the resistance of tumor cells to anti-tumor agents. Wang et al. (20) previously used miR-4271 to down-regulate the expression levels of CAR, which significantly enhanced the sensitivity of tumor cells to anlotinib (20). Our results also examined the expression of PXR and CAR in HCC’s clinical specimens. Therefore, we selected CYP3A4 in our study to avoid the compensation effects of PXR and CAR in HCC cells.
miRNA is an important type of non-coding RNA that induces the post-transcriptional silencing of target genes’ expression in a sequence-specific manner by targeting the 3’UTR (26). This feature makes miRNA an ideal strategy for anti-tumor gene therapy; tumors can be treated by cloning the full pre-miRNA sequence into a vector and delivering it in lentiviral particles (26). Transfection of lentiviral particles into tumor cells or via intratumor tissue injection can down-regulate the expression levels of certain oncogenes (26). Li et al. (59), Yang et al. (60), and Li et al. (61) showed that miR-140-3p, miR-30c, and miR-148a can inhibit the expression of PXR and CYP3A4, respectively, by acting on the 3’UTR of PXR and reduce the elimination of anti-cancer agents in tumor cells. Our study used an online tool to identify miR-4277 as a regulator of cyp3a4 expression. This miRNA had the highest score among all miRNAs tested from the miRDB database. We then constructed mutants to confirm the effect of miR-4277 on cyp3a4. Transfecting HCC cells with miR-4277 decreased the expression levels of CYP3A4 and enhanced the sensitivity of HCC cells to targeted agents. Besides miR-4277, there are also some other miRNAs could target to cyp3a4. Ekström et al. (62), Tang et al. (63), Gill et al. (64), Huang et al. (65), Li et al. (66) and Zastrozhin et al. (67) suggested that the miR-27 family, miR-142, miR-200a, miR-150 or miR-328 could targets to cyp3a4 (62–67). Therefore, our results extended the knowledge of miRNAs on cyp3a4.
It is worth mentioning that in our study, elimination of sorafenib was measured via LC-MS/MS. We observed that miR-4277 could prolong the half-life of sorafenib in HCC cells and tissues and reduce its elimination. Several other targeted agents were tested for their ability to kill HCC cells; however, we failed to detect the presence of these agents in HCC cells, mainly due to the lack of efficient protocols for assessing the levels of the compounds. In the future, LC-MS/MS methodology for these and other targeted agents will likely be established well enough to determine the effect of miR-4277 on their elimination rate in HCC cells.
Furthermore, Drug interactions mediated by CYP3A4 are not only closely related to clinical treatment and drug contraindications, but also an important mechanism of anti-tumor drug resistance (68, 69). CYP3A4 is not only closely related to the metabolism and drug resistance of cytotoxic chemotherapy agents such as paclitaxel and camptothecin, but also closely related to the metabolism and drug resistance of molecularly targeted agents such as Imatinib, Gefitinib and Pazopanib (70–75). Therefore, the results of this study can be extended to other types of malignant tumor molecular targeted therapy in the future.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the ethics committee of the Fifth Medical Center, General Hospital of Chinese PLA. The patients/participants provided their written informed consent to participate in this study. The animal study was reviewed and approved by the Institutional Animal Care and Use Committee, the Fifth Medical Center, Chinese PLA.
Author Contributions
SX, JH, and BL conceived the main ideas and wrote the paper. XL, BZ, and ZW supervised the study. XH, HS, QJ, YC, and SY developed major methodologies, databases, reagents, and primary experiments. XH, HS, and QJ analyzed different aspects of the results. All authors contributed to the article and approved the submitted version.
Funding
This work is supported by National Science and Technology Major Project of China, Chinese Government, Chinese Government (new techniques and new schemes for clinical treatment of severe hepatitis B [liver failure]; NO 2017ZX10203201004).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Authors all deeply thanks Prof. and Dr. Shuang Cao in Wuhan Institute of Technology.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.735447/full#supplementary-material
References
- 1. Polaris Observatory Collaborators Global Prevalence, Treatment, and Prevention of Hepatitis B Virus Infection in 2016: A Modelling Study. Lancet Gastroenterol Hepatol (2018) 3(6):383–403. 10.1016/S2468-1253(18)30056-6 [DOI] [PubMed] [Google Scholar]
- 2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71(3):209–49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2019. CA Cancer J Clin (2019) 69(1):7–34. 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 4. Wang F-S, Fan J-G, Zhang Z, Gao B, Wang H-Y. The Global Burden of Liver Disease: The Major Impact of China. Hepatology (2014) 60(6):2099–108. 10.1002/hep.27406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jia L, Gao Y, He Y, Hooper JD, Yang P. HBV Induced Hepatocellular Carcinoma and Related Potential Immunotherapy. Pharmacol Res (2020) 159:104992. 10.1016/j.phrs.2020.104992 [DOI] [PubMed] [Google Scholar]
- 6. Forner A, Reig M, Bruix J. Hepatocellular Carcinoma. Lancet (2018) 391(10127):1301–14. 10.1016/S0140-6736(18)30010-2 [DOI] [PubMed] [Google Scholar]
- 7. Lin AJ, Baranski T, Chaterjee D, Chapman W, Foltz G, Kim H. Androgen-Receptor-Positive Hepatocellular Carcinoma in a Transgender Teenager Taking Exogenous Testosterone. Lancet (2020) 396(10245):198. 10.1016/S0140-6736(20)31538-5 [DOI] [PubMed] [Google Scholar]
- 8. Roskoski R, Jr. Properties of FDA-approved Small Molecule Protein Kinase Inhibitors: A 2021 Update. Pharmacol Res (2021) 165:105463. 10.1016/j.phrs.2021.105463 [DOI] [PubMed] [Google Scholar]
- 9. Roskoski R., Jr. Properties of FDA-approved Small Molecule Protein Kinase Inhibitors: A 2020 Update. Pharmacol Res (2020) 152:104609. 10.1016/j.phrs.2019.104609 [DOI] [PubMed] [Google Scholar]
- 10. Roskoski R., Jr. Properties of FDA-approved Small Molecule Protein Kinase Inhibitors. Pharmacol Res (2019) 144:19–50. 10.1016/j.phrs.2019.03.006 [DOI] [PubMed] [Google Scholar]
- 11. Ying H, Zhang X, Duan Y, Lao M, Xu J, Yang H, et al. Non-Cytomembrane PD-L1: An Atypical Target for Cancer. Pharmacol Res (2021) 23:105741. 10.1016/j.phrs.2021.105741 [DOI] [PubMed] [Google Scholar]
- 12. Shergold AL, Millar R, Nibbs RJB. Understanding and Overcoming the Resistance of Cancer to PD-1/PD-L1 Blockade. Pharmacol Res (2019) 145:104258. 10.1016/j.phrs.2019.104258 [DOI] [PubMed] [Google Scholar]
- 13. Torrens L, Montironi C, Puigvehí M, Mesropian A, Leslie J, Haber PK, et al. Immunomodulatory Effects of Lenvatinib Plus anti-PD1 in Mice and Rationale for Patient Enrichment in Hepatocellular Carcinoma. Hepatology (2021). 10.1002/hep.32023 [DOI] [PubMed] [Google Scholar]
- 14. Zhu YJ, Zheng B, Wang HY, Chen L. New Knowledge of the Mechanisms of Sorafenib Resistance in Liver Cancer. Acta Pharmacol Sin (2017) 38(5):614–22. 10.1038/aps.2017.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. He Y, Luo Y, Huang L, Zhang D, Wang X, Ji J, et al. New Frontiers Against Sorafenib Resistance in Renal Cell Carcinoma: From Molecular Mechanisms to Predictive Biomarkers. Pharmacol Res (2021) 170:105732. 10.1016/j.phrs.2021.105732 [DOI] [PubMed] [Google Scholar]
- 16. Shao QP, Wei C, Yang J, Zhang WZ. miR-3609 Decelerates the Clearance of Sorafenib in Hepatocellular Carcinoma Cells by Targeting EPAS-1 and Reducing the Activation of the Pregnane X Receptor Pathway. Onco Targets Ther (2020) 13:7213–27. 10.2147/OTT.S246471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nagai K, Fukuno S, Yamamoto K, Omotani S, Hatsuda Y, Myotoku M, et al. Downregulation of Organic Cation Transporter 1 and Breast Cancer Resistance Protein With the Induction of Pregnane X Receptor in Rat Kidney Impaired by Doxorubicin. Pharmazie (2019) 74(12):744–6. 10.1691/ph.2019.9703 [DOI] [PubMed] [Google Scholar]
- 18. Feng F, Jiang Q, Cao S, Cao Y, Li R, Shen L, et al. Pregnane X Receptor Mediates Sorafenib Resistance in Advanced Hepatocellular Carcinoma. Biochim Biophys Acta Gen Subj (2018) 1862(4):1017–30. 10.1016/j.bbagen.2018.01.011 [DOI] [PubMed] [Google Scholar]
- 19. Shao Z, Li Y, Dai W, Jia H, Zhang Y, Jiang Q, et al. ETS-1 Induces Sorafenib-Resistance in Hepatocellular Carcinoma Cells Via Regulating Transcription Factor Activity of PXR. Pharmacol Res (2018) 135:188–200. 10.1016/j.phrs.2018.08.003 [DOI] [PubMed] [Google Scholar]
- 20. Wang C, Ding S, Sun B, Shen L, Xiao L, Han Z, et al. Hsa-miR-4271 Downregulates the Expression of Constitutive Androstane Receptor and Enhances In Vivo the Sensitivity of Non-Small Cell Lung Cancer to Gefitinib. Pharmacol Res (2020) 161:105110. 10.1016/j.phrs.2020.105110 [DOI] [PubMed] [Google Scholar]
- 21. Chai SC, Lin W, Li Y, Chen T. Drug Discovery Technologies to Identify and Characterize Modulators of the Pregnane X Receptor and the Constitutive Androstane Receptor. Drug Discov Today (2019) 24(3):906–15. 10.1016/j.drudis.2019.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harding JJ, Kelley RK, Tan B, Capanu M, Do GK, Shia J, et al. Phase Ib Study of Enzalutamide With or Without Sorafenib in Patients With Advanced Hepatocellular Carcinoma. Oncologist (2020) 25(12):e1825–36. 10.1634/theoncologist.2020-0521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chang YT, Hernandez D, Alonso S, Gao M, Su M, Ghiaur G, et al. Role of CYP3A4 in Bone Marrow Microenvironment-Mediated Protection of FLT3/ITD AML From Tyrosine Kinase Inhibitors. Blood Adv (2019) 3(6):908–16. 10.1182/bloodadvances.2018022921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen S, Gao C, Yu T, Qu Y, Xiao GG, Huang Z. Bioinformatics Analysis of a Prognostic miRNA Signature and Potential Key Genes in Pancreatic Cancer. Front Oncol (2021) 11:641289. 10.3389/fonc.2021.641289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ma Y, Chai N, Jiang Q, Chang Z, Chai Y, Li X, et al. DNA Methyltransferase Mediates the Hypermethylation of the microRNA 34a Promoter and Enhances the Resistance of Patient-Derived Pancreatic Cancer Cells to Molecular Targeting Agents. Pharmacol Res (2020) 160:105071. 10.1016/j.phrs.2020.105071 [DOI] [PubMed] [Google Scholar]
- 26. Yang B, Wang C, Xie H, Wang Y, Huang J, Rong Y, et al. MicroRNA-3163 Targets ADAM-17 and Enhances the Sensitivity of Hepatocellular Carcinoma Cells to Molecular Targeted Agents. Cell Death Dis (2019) 10(10):784. 10.1038/s41419-019-2023-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ma D-B, Qin M-M, Shi L, Ding X-M. MicroRNA-6077 Enhances the Sensitivity of Patients-Derived Lung Adenocarcinoma Cells to Anlotinib by Repressing the Activation of Glucose Transporter 1 Pathway. Cell Signal (2019) 64:109391. 10.1016/j.cellsig.2019.109391 [DOI] [PubMed] [Google Scholar]
- 28. Liu Y, Zhang Y, Xiao B, Tang N, Hu J, Liang S, et al. MiR-103a Promotes Tumour Growth and Influences Glucose Metabolism in Hepatocellular Carcinoma. Cell Death Dis (2021) 12(6):618. 10.1038/s41419-021-03905-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang P, Yan Q, Liao B, Zhao L, Xiong S, Wang J, et al. The HIF1α/Hif2α-miR210-3p Network Regulates Glioblastoma Cell Proliferation, Dedifferentiation and Chemoresistance Through EGF Under Hypoxic Conditions. Cell Death Dis (2020) 11(11):992. 10.1038/s41419-020-03150-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li DM, Chen QD, Wei GN, Wei J, Yin JX, He JH, et al. Hypoxia-Induced miR-137 Inhibition Increased Glioblastoma Multiforme Growth and Chemoresistance Through LRP6. Front Oncol (2021) 10:611699. 10.3389/fonc.2020.611699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Duan YB, Zhu JB, Yang JX, Liu GQ, Bai X, Qu N, et al. Regulation of High-Altitude Hypoxia on the Transcription of CYP450 and UGT1A1 Mediated by PXR and CAR. Front Pharmacol (2020) 11:574176. 10.3389/fphar.2020.574176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wei L, Lun Y, Zhou X, He S, Gao L, Liu Y, et al. Novel Urokinase-Plasminogen Activator Inhibitor SPINK13 Inhibits Growth and Metastasis of Hepatocellular Carcinoma In Vivo . Pharmacol Res (2019) 143:73–85. 10.1016/j.phrs.2019.03.009 [DOI] [PubMed] [Google Scholar]
- 33. Jia H, Liu M, Wang X, Jiang Q, Wang S, Santhanam RK, et al. Cimigenoside Functions as a Novel γ-Secretase Inhibitor and Inhibits the Proliferation or Metastasis of Human Breast Cancer Cells by γ-Secretase/Notch Axis. Pharmacol Res (2021) 169:105686. 10.1016/j.phrs.2021.105686 [DOI] [PubMed] [Google Scholar]
- 34. Gao X, Chen H, Huang X, Li H, Liu Z, Bo X. ARQ-197 Enhances the Antitumor Effect of Sorafenib in Hepatocellular Carcinoma Cells Via Decelerating its Intracellular Clearance. Onco Targets Ther (2019) 12:1629–40. 10.2147/OTT.S196713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Du Y, Shi X, Ma W, Wen P, Yu P, Wang X, et al. Phthalates Promote the Invasion of Hepatocellular Carcinoma Cells by Enhancing the Interaction Between Pregnane X Receptor and E26 Transformation Specific Sequence 1. Pharmacol Res (2021) 169:105648. 10.1016/j.phrs.2021.105648 [DOI] [PubMed] [Google Scholar]
- 36. Zou XZ, Zhou XH, Feng YQ, Hao JF, Liang B, Jia MW. Novel Inhibitor of OCT1 Enhances the Sensitivity of Human Esophageal Squamous Cell Carcinoma Cells to Antitumor Agents. Eur J Pharmacol (2021) 907:174222. 10.1016/j.ejphar.2021.174222 [DOI] [PubMed] [Google Scholar]
- 37. Wang Y, Liu S, Chen Q, Ren Y, Li Z, Cao S. Novel Small Molecular Inhibitor of Pit-Oct-Unc Transcription Factor 1 Suppresses Hepatocellular Carcinoma Cell Proliferation. Life Sci (2021) 277:119521. 10.1016/j.lfs.2021.119521 [DOI] [PubMed] [Google Scholar]
- 38. Jie Y, Liu G, Mingyan E, Li Y, Xu G, Guo J, et al. Novel Small Molecule Inhibitors of the Transcription Factor ETS-1 and Their Antitumor Activity Against Hepatocellular Carcinoma. Eur J Pharmacol (2021) 906:174214. 10.1016/j.ejphar.2021.174214 [DOI] [PubMed] [Google Scholar]
- 39. Sun H, Feng F, Xie H, Li X, Jiang Q, Chai Y, et al. Quantitative Examination of the Inhibitory Activation of Molecular Targeting Agents in Hepatocellular Carcinoma Patient-Derived Cell Invasion Via a Novel In Vivo Tumor Model. Anim Model Exp Med (2019) 2(4):259–68. 10.1002/ame2.12085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Feng F, Li X, Li R, Li B. The Multiple-Kinase Inhibitor Lenvatinib Inhibits the Proliferation of Acute Myeloid Leukemia Cells. Anim Model Exp Med (2019) 2(3):178–84. 10.1002/ame2.12076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhou W, Gao Y, Tong Y, Wu Q, Zhou Y, Li Y. Anlotinib Enhances the Antitumor Activity of Radiofrequency Ablation on Lung Squamous Cell Carcinoma. Pharmacol Res (2021) 164:105392. 10.1016/j.phrs.2020.105392 [DOI] [PubMed] [Google Scholar]
- 42. Yin F, Feng F, Wang L, Wang X, Li Z, Cao Y. SREBP-1 Inhibitor Betulin Enhances the Antitumor Effect of Sorafenib on Hepatocellular Carcinoma Via Restricting Cellular Glycolytic Activity. Cell Death Dis (2019) 10(9):672. 10.1038/s41419-019-1884-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Roberts JL, Poklepovic A, Booth L, Dent P. The Multi-Kinase Inhibitor Lenvatinib Interacts With the HDAC Inhibitor Entinostat to Kill Liver Cancer Cells. Cell Signal (2020) 70:109573. 10.1016/j.cellsig.2020.109573 [DOI] [PubMed] [Google Scholar]
- 44. Zhu M, Li L, Lu T, Yoo H, Zhu J, Gopal P, et al. Uncovering Biological Factors That Regulate Hepatocellular Carcinoma Growth Using Patient-Derived Xenograft Assays. Hepatology (2020) 72(3):1085–101. 10.1002/hep.31096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Feng GS, Hanley KL, Liang Y, Lin X. Improving the Efficacy of Liver Cancer Immunotherapy: The Power of Combined Preclinical and Clinical Studies. Hepatology (2021) 73(Suppl 1)(Suppl 1):104–14. 10.1002/hep.31479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li L, Welch MA, Li Z, Mackowiak B, Heyward S, Swaan PW, et al. Mechanistic Insights of Phenobarbital-Mediated Activation of Human But Not Mouse Pregnane X Receptor. Mol Pharmacol (2019) 96(3):345–54. 10.1124/mol.119.116616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mackowiak B, Hodge J, Stern S, Wang H. The Roles of Xenobiotic Receptors: Beyond Chemical Disposition. Drug Metab Dispos (2018) 46(9):1361–71. 10.1124/dmd.118.081042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Karbownik A, Stachowiak A, Urjasz H, Sobańska K, Szczecińska A, Grabowski T, et al. The Oxidation and Hypoglycaemic Effect of Sorafenib in Streptozotocin-Induced Diabetic Rats. Pharmacol Rep (2020) 72(1):254–9. 10.1007/s43440-019-00021-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lee-Montiel FT, Laemmle A, Charwat V, Dumont L, Lee CS, Huebsch N, et al. Integrated Isogenic Human Induced Pluripotent Stem Cell-Based Liver and Heart Microphysiological Systems Predict Unsafe Drug-Drug Interaction. Front Pharmacol (2021) 12:667010. 10.3389/fphar.2021.667010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kar A, Mukherjee PK, Saha S, Banerjee S, Goswami D, Matsabisa MG, et al. Metabolite Profiling and Evaluation of CYP450 Interaction Potential of ‘Trimada’- an Ayurvedic Formulation. J Ethnopharmacol (2021) 266:113457. 10.1016/j.jep.2020.113457 [DOI] [PubMed] [Google Scholar]
- 51. Mendes MS, Hatley O, Gill KL, Yeo KR, Ke AB. A Physiologically Based Pharmacokinetic - Pharmacodynamic Modelling Approach to Predict Incidence of Neutropenia as a Result of Drug-Drug Interactions of Paclitaxel in Cancer Patients. Eur J Pharm Sci (2020) 150:105355. 10.1016/j.ejps.2020 [DOI] [PubMed] [Google Scholar]
- 52. Dufek MB, Bridges AS, Thakker DR. Intestinal First-Pass Metabolism by Cytochrome p450 and Not P-Glycoprotein Is the Major Barrier to Amprenavir Absorption. Drug Metab Dispos (2013) 41(9):1695–702. 10.1124/dmd.113.052191 [DOI] [PubMed] [Google Scholar]
- 53. Posada MM, Morse BL, Turner PK, Kulanthaivel P, Hall SD, Dickinson GL. Predicting Clinical Effects of CYP3A4 Modulators on Abemaciclib and Active Metabolites Exposure Using Physiologically Based Pharmacokinetic Modeling. J Clin Pharmacol (2020) 60(7):915–30. 10.1002/jcph.1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Li S, Yu Y, Jin Z, Dai Y, Lin H, Jiao Z, et al. Prediction of Pharmacokinetic Drug-Drug Interactions Causing Atorvastatin-Induced Rhabdomyolysis Using Physiologically Based Pharmacokinetic Modelling. BioMed Pharmacother (2019) 119:109416. 10.1016/j.biopha.2019.109416 [DOI] [PubMed] [Google Scholar]
- 55. Boof ML, Alatrach A, Ufer M, Dingemanse J. Interaction Potential of the Dual Orexin Receptor Antagonist ACT-541468 With CYP3A4 and Food: Results From Two Interaction Studies. Eur J Clin Pharmacol (2019) 75(2):195–205. 10.1007/s00228-018-2559-5 [DOI] [PubMed] [Google Scholar]
- 56. Li H, Wang YG, Ma ZC, Yun-Hang G, Ling S, Teng-Fei C, et al. A High-Throughput Cell-Based Gaussia Luciferase Reporter Assay for Measurement of CYP1A1, CYP2B6, and CYP3A4 Induction. Xenobiotica (2021) 51(7):752–63. 10.1080/00498254.2021.1918800 [DOI] [PubMed] [Google Scholar]
- 57. Ly JQ, Wong S, Liu L, Li R, Messick K, Chang JH. Investigating the Utility of Humanized PXR-CAR-CYP3A4/7 Mouse Model to Assess CYP3A-Mediated Induction. Drug Metab Dispos (2021) 49(7):540–7. 10.1124/dmd.121.000439 [DOI] [PubMed] [Google Scholar]
- 58. Preiss LC, Liu R, Hewitt P, Thompson D, Georgi K, Badolo L, et al. Deconvolution of CYP Induction Mechanisms in HepaRG Nuclear Hormone Receptor Knockout Cells. Drug Metab Dispos (2021) DMD–AR-2020-000333. 10.1124/dmd.120.000333 [DOI] [PubMed] [Google Scholar]
- 59. Li J, Zhao J, Wang H, Li X, Liu A, Qin Q, et al. MicroRNA-140-3p Enhances the Sensitivity of Hepatocellular Carcinoma Cells to Sorafenib by Targeting Pregnenolone X Receptor. Onco Targets Ther (2018) 11:5885–94. 10.2147/OTT.S179509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yang H, Ren L, Wang Y, Bi X, Li X, Wen M, et al. FBI-1 Enhanced the Resistance of Triple-Negative Breast Cancer Cells to Chemotherapeutic Agents Via the miR-30c/PXR Axis. Cell Death Dis (2020) 11(10):851. 10.1038/s41419-020-03053-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Li B, Feng F, Jia H, Jiang Q, Cao S, Wei L, et al. Rhamnetin Decelerates the Elimination and Enhances the Antitumor Effect of the Molecular-Targeting Agent Sorafenib in Hepatocellular Carcinoma Cells Via the miR-148a/PXR Axis. Food Funct (2021) 12(6):2404–17. 10.1039/d0fo02270e [DOI] [PubMed] [Google Scholar]
- 62. Ekström L, Skilving I, Ovesjö M-L, Aklillu E, Nylén H, Rane A, et al. miRNA-27b Levels Are Associated With CYP3A Activity In Vitro and In Vivo . Pharmacol Res Perspect (2015) 3(6):e00192. 10.1002/prp2.192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tang Q-J, Lin H-M, He G-D, Liu J-E, Wu H, Li X-X, et al. Plasma miR-142 Accounting for the Missing Heritability of CYP3A4/5 Functionality Is Associated With Pharmacokinetics of Clopidogrel. Pharmacogenomics (2016) 17(14):1503–17. 10.2217/pgs-2016-0027 [DOI] [PubMed] [Google Scholar]
- 64. Gill P, Bhattacharyya S, McCullough S, Letzig L, Mishra PJ, Luo C, et al. MicroRNA Regulation of CYP 1a2, CYP3A4 and CYP2E1 Expression in Acetaminophen Toxicity. Sci Rep (2017) 7(1):12331. 10.1038/s41598-017-11811-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Huang Z, Wang M, Liu L, Peng J, Guo C, Chen X, et al. Transcriptional Repression of CYP3A4 by Increased miR-200a-3p and miR-150-5p Promotes Steatosis In Vitro . Front Genet (2019) 10:484. 10.3389/fgene.2019.00484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Li X, Tian Y, Tu M-J, Ho PY, Batra N, Yu A-M. Bioengineered miR-27b-3p and miR-328-3p Modulate Drug Metabolism and Disposition Via the Regulation of Target ADME Gene Expression. Acta Pharm Sin B (2019) 9(3):639–47. 10.1016/j.apsb.2018.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zastrozhin MS, Yu Skryabin V, Smirnov VV, Petukhov AE, Pankratenko EP, Zastrozhina AK, et al. Effects of Plasma Concentration of Micro-RNA Mir-27b and CYP3A4*22 on Equilibrium Concentration of Alprazolam in Patients With Anxiety Disorders Comorbid With Alcohol Use Disorder. Gene (2020) 739:144513. 10.1016/j.gene.2020.144513 [DOI] [PubMed] [Google Scholar]
- 68. Schütz R, Müller M, Geisslinger F, Vollmar A, Bartel K, Bracher F. Synthesis, Biological Evaluation and Toxicity of Novel Tetrandrine Analogues. Eur J Med Chem (2020) 207:112810. 10.1016/j.ejmech.2020.112810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Atasilp C, Chansriwong P, Sirachainan E, Reungwetwattana T, Sirilerttrakul S, Chamnanphon M, et al. Effect of Drug Metabolizing Enzymes and Transporters in Thai Colorectal Cancer Patients Treated With Irinotecan-Based Chemotherapy. Sci Rep (2020) 10(1):13486. 10.1038/s41598-020-70351-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kawahara B, Faull KF, Janzen C, Mascharak PK. Carbon Monoxide Inhibits Cytochrome P450 Enzymes CYP3A4/2C8 in Human Breast Cancer Cells, Increasing Sensitivity to Paclitaxel. J Med Chem (2021) 64(12):8437–46. 10.1021/acs.jmedchem.1c00404 [DOI] [PubMed] [Google Scholar]
- 71. Xing H, Luo X, Li Y, Fan C, Liu N, Cui C, et al. Effect of Verapamil on the Pharmacokinetics of Hydroxycamptothecin and its Potential Mechanism. Pharm Biol (2020) 58(1):152–6. 10.1080/13880209.2020.1717550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Adiwidjaja J, Boddy AV, McLachlan AJ. Implementation of a Physiologically Based Pharmacokinetic Modeling Approach to Guide Optimal Dosing Regimens for Imatinib and Potential Drug Interactions in Paediatrics. Front Pharmacol (2020) 10:1672. 10.3389/fphar.2019.01672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wang C, Ying L, Jin M, Zhang F, Shi D, Dai Y, et al. An Investigation Into Possible Interactions Among Four Vascular Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitors With Gefitinib. Cancer Chemother Pharmacol (2021) 87(1):43–52. 10.1007/s00280-020-04191-0 [DOI] [PubMed] [Google Scholar]
- 74. Azam C, Claraz P, Chevreau C, Vinson C, Cottura E, Mourey L, et al. Association Between Clinically Relevant Toxicities of Pazopanib and Sunitinib and the Use of Weak CYP3A4 and P-gp Inhibitors. Eur J Clin Pharmacol (2020) 76(4):579–87. 10.1007/s00228-020-02828-w [DOI] [PubMed] [Google Scholar]
- 75. Schmitz LM, Hageneier F, Rosenthal K, Busche T, Brandt D, Kalinowski J, et al. Recombinant Expression and Characterization of Novel P450s From Actinosynnema Mirum. Bioorg Med Chem (2021) 42:116241. 10.1016/j.bmc.2021.116241 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding authors.