Abstract
Muscle glycogen depletion has been proposed as one of the main causes of fatigue during exercise. However, few studies have addressed the contribution of liver glycogen to exercise performance. Using a low-intensity running protocol, here, we analyzed exercise capacity in mice overexpressing protein targeting to glycogen (PTG) specifically in the liver (PTGOE mice), which show a high concentration of glycogen in this organ. PTGOE mice showed improved exercise capacity, as determined by the distance covered and time ran in an extenuating endurance exercise, compared with control mice. Moreover, fasting decreased exercise capacity in control mice but not in PTGOE mice. After exercise, liver glycogen stores were totally depleted in control mice, but PTGOE mice maintained significant glycogen levels even in fasting conditions. In addition, PTGOE mice displayed an increased hepatic energy state after exercise compared with control mice. Exercise caused a reduction in the blood glucose concentration in control mice that was less pronounced in PTGOE mice. No changes were found in the levels of blood lactate, plasma free fatty acids, or β-hydroxybutyrate. Plasma glucagon was elevated after exercise in control mice, but not in PTGOE mice. Exercise-induced changes in skeletal muscle were similar in both genotypes. These results identify hepatic glycogen as a key regulator of endurance capacity in mice, an effect that may be exerted through the maintenance of blood glucose levels.
Keywords: exercise, glycogen, glucose, protein targeting to glycogen (PTG), liver metabolism, ATP
Abbreviations: FFAs, free fatty acids; G6P, glucose-6-phosphate; G6pase, glucose-6-phosphatase; GS, glycogen synthase; Nr4a3, NR4A member 3; Pdk4, pyruvate dehydrogenase kinase 4; PEPCK, phosphoenolpyruvate carboxykinase; Pgc1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; PTG, protein targeting to glycogen; PTGOE, overexpressing PTG specifically in the liver; TAGs, triacylglycerols
Glycogen is the storage form of glucose in mammals. Glycogen synthesis is catalyzed by glycogen synthase (GS). There are two mammalian isoforms of GS. One, encoded by the Gys2 gene, is expressed only in the liver (1), whereas a second gene, Gys1, is expressed in the skeletal muscle, cardiac muscle, adipose tissue, kidneys, and brain (2). GS is regulated by covalent phosphorylation (3) and allosteric effectors (4). Dephosphorylation and thus activation of GS is catalyzed by protein phosphatase 1. A family of scaffolding proteins target the protein phosphatase 1 catalytic subunit to glycogen particles, where the enzymes of glycogen metabolism are concentrated. There are seven scaffolding proteins, among them the ubiquitously distributed protein targeting to glycogen (PTG). Overexpression of PTG has been used to activate GS and thus promote glycogen synthesis (5, 6, 7, 8, 9, 10).
Carbohydrate and fat are the main substrates used during prolonged endurance-type exercise (11, 12, 13). The relative contribution of each is determined primarily by the intensity and duration of exercise (11, 12). During moderate- to high-intensity exercise, carbohydrate is the main source of substrate. Given that carbohydrate stores in the form of glycogen (primarily in the liver and muscle) are relatively small, endurance-type exercise performance/capacity is often limited by endogenous carbohydrate availability (13).
The importance of muscle glycogen availability during prolonged exercise has received much attention over the last 50 years (13, 14, 15), and a strong association has been reported between muscle glycogen depletion, impaired muscle performance, and fatigue (16, 17, 18, 19, 20, 21). In contrast, little attention has been paid to the contribution of liver glycogen during exercise. Liver glycogen stores are mobilized during exercise in response to the increased glucose demands of contracting skeletal muscle (22, 23, 24). Accordingly, continuous exercise lasting >60 min in humans substantially depletes liver glycogen (25). Interestingly, in humans, liver glycogen sparing in an endurance-trained state may account partly for training-induced performance/capacity adaptations during prolonged (>90 min) exercise (13). Liver GS KO mice, which have a 95% reduction in the liver glycogen content, have a decreased capacity for exhaustive high-intensity running compared with control mice. This difference disappears after an overnight fast, which reduces the liver glycogen of control mice to levels comparable with those of Gys2 KO animals (26). These results indicate that the lack of liver glycogen impairs endurance capacity. We sought to analyze endurance capacity in animals overexpressing PTG specifically in the liver (PTGOE mice) and that maintain relatively high levels of hepatic glycogen even after fasting. To extrapolate our results to endurance sports such as marathon running or cycling, a low-intensity running protocol was used. We show that PTGOE mice indeed have a greater endurance capacity than control animals. Importantly, fasting did not significantly reduce the capacity of PTGOE mice. All together, these results highlight the role of liver glycogen in resistance to fatigue.
Results
Generation of mice with liver-specific PTG overexpression
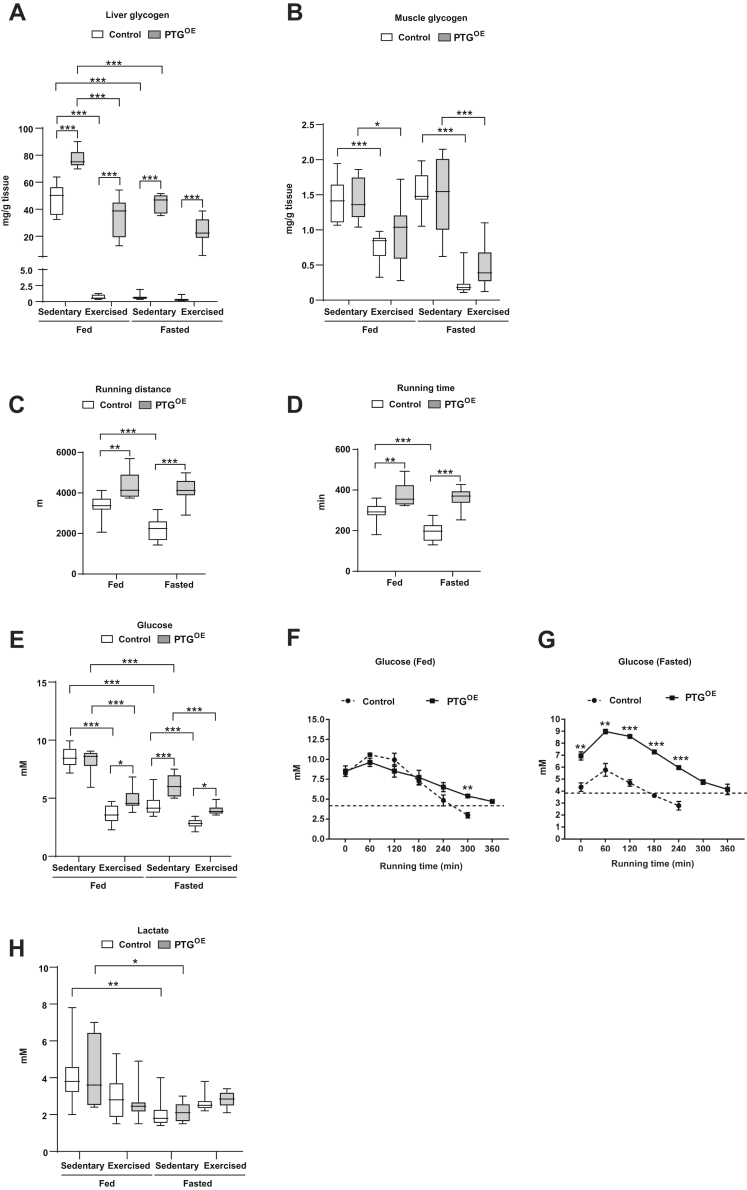
To study the role of increased liver glycogen storage in endurance capacity, we generated PTGOE mice (see Experimental procedures). The mRNA level of Ptg in the livers of these mice was 8-fold greater than that of control animals (Fig. S1A). PTGOE mice showed an increase in hepatic glycogen compared with control mice both in fed and fasting conditions (Fig. 1A), as previously described (27). There was no difference in the glycogen content of muscles (Fig. 1B). Before the exercise experiments, the body weight was equivalent between control and PTGOE mice. After overnight fasting, the weight of the animals of both genotypes was similarly diminished compared with fed mice (Fig. S2).
Figure 1.
PTGOEmice showed enhanced endurance exercise capacity.A, liver glycogen, (B) muscle glycogen, (C) running distance, (D) running time, (E–G) blood glucose, and (H) blood lactate in sedentary and exercised control and PTGOE mice under fed and fasting conditions (n = 8–12 in experiments A–E and H; n = 3 in experiments F and G). All values are the mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. PTG, protein targeting to glycogen; PTGOE, overexpressing PTG specifically in the liver.
PTGOE mice showed enhanced endurance exercise capacity
To assess capacity for endurance exercise, control and PTGOE mice were forced to run to exhaustion under fed and fasting conditions. PTGOE mice ran significantly more (≈30% of the distance and of the time ran) than control mice both in fed and fasting conditions (Fig. 1, C and D). Interestingly, fasting induced a decrease (≈35% of the distance and of the time ran) for endurance exercise capacity in control mice but not in PTGOE animals (Fig. 1, C and D). Upon completion of the exercise, liver glycogen stores were totally depleted in fed control mice but not in fed PTGOE ones, which were able to maintain liver glycogen at 30 mg/g of tissue (Fig. 1A). Strikingly, glycogen stores were not depleted in fasted PTGOE mouse livers even after exhaustive exercise (Fig. 1A). Muscle glycogen was depleted similarly in both genotypes under fed and fasting conditions (Fig. 1B).
It has been suggested that a decrease in systemic glucose limits endurance exercise (28). In our experiments, basal blood glucose was similar between genotypes under fed conditions. However, fasting and exercise caused a reduction in blood glucose concentration in control mice, and this reduction was less pronounced in PTGOE mice (Fig. 1E). We also monitored blood glucose every hour during the run-to-exhaustion test. Glucose levels were maintained above 3.9 mM (70 mg/dl) for longer in PTGOE mice than control animals in both fed (Fig. 1F) and fasting conditions (Fig. 1G). In fact, in fasting conditions, glucose levels were higher in PTGOE mice throughout the test. Lactate is a gluconeogenic precursor released from skeletal muscle during exercise and taken up by the liver to be converted to pyruvate and used in gluconeogenesis. Blood lactate was similar between the two genotypes in all conditions (Fig. 1H).
Liver and muscle metabolites
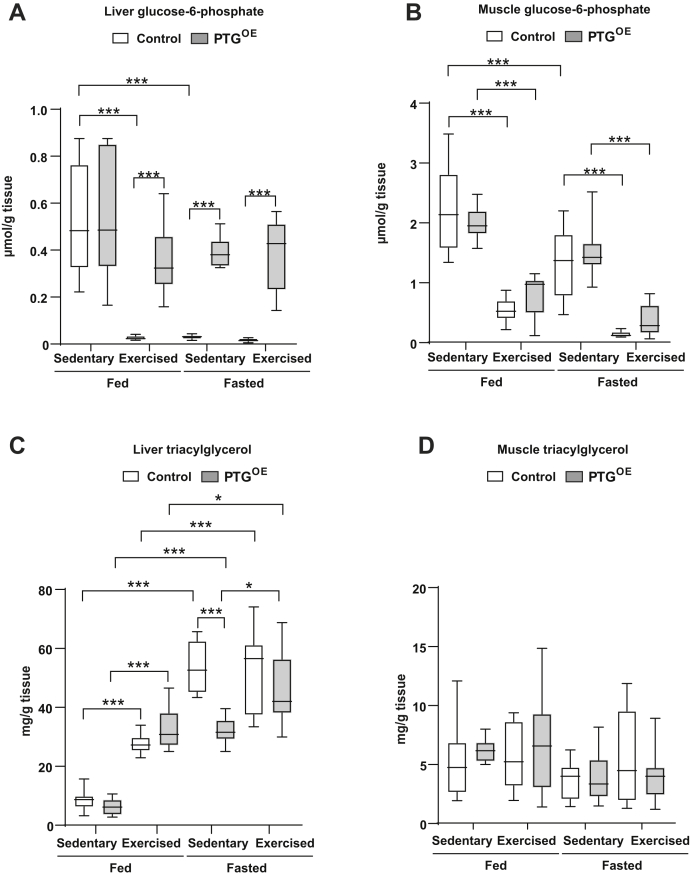
It has been described that the levels of G6P in the liver decrease immediately after exercise (29). Liver G6P was almost undetectable after exercise and/or fasting in control mice. In contrast, PTGOE mice maintained relatively high levels of G6P, about 65% of that observed in control fed animals, after exercise and also in fasting conditions (Fig. 2A). In the muscle, G6P was similarly depleted after exercise in both genotypes under fed and fasting conditions (Fig. 2B).
Figure 2.
Liver and muscle metabolites.A, liver glucose-6-phosphate, (B) muscle glucose-6-phosphate, (C) liver triacylglycerol, and (D) muscle triacylglycerol in sedentary and exercised control and PTGOE mice under fed and fasting conditions (n = 8–12 in all experiments). All values are the mean ± SEM. ∗p < 0.05 and ∗∗∗p < 0.001. PTG, protein targeting to glycogen; PTGOE, overexpressing PTG specifically in the liver.
Hepatic TAGs acutely accumulate during and after exercise in rodents (30). We found that exercise and fasting increased hepatic TAG concentration in fed control and PTGOE mice (Fig. 2C). Fasted PTGOE mice showed lower liver TAG levels than control mice before exercise, but after exercise, the concentration of TAG was similar in the two genotypes (Fig. 2C). Exercise did not significantly alter TAG concentration in muscle (Fig. 2D).
Plasma hormones and metabolites
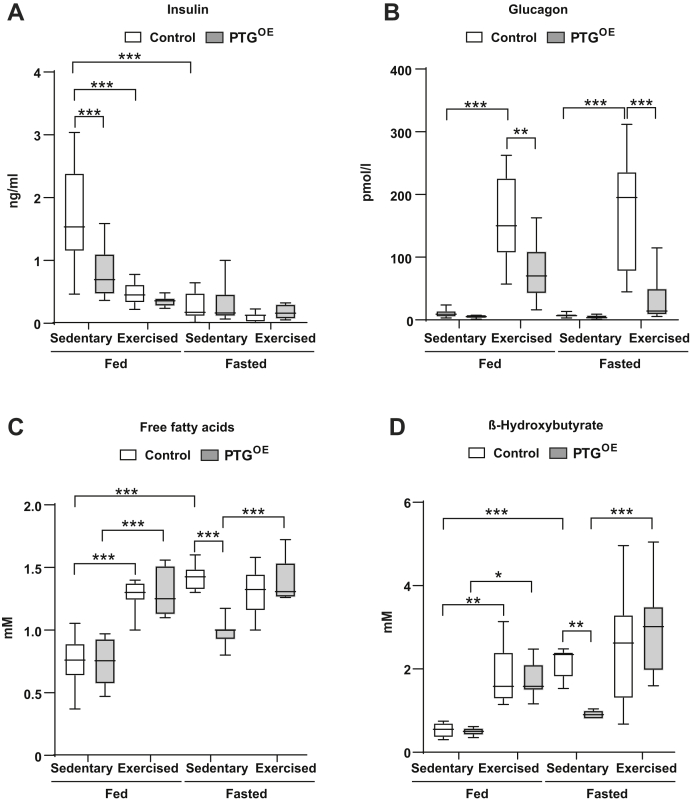
Exercise is characterized by complex endocrine responses (31). If exercise is sustained, a decrease in insulin secretion and increases in glucagon are observed (32). In fed control mice, plasma insulin was significantly reduced after exercise (Fig. 3A). Insulin concentration was already lower in resting PTGOE mice, as previously described (27) and it was not further reduced after exercise. Glucagon increased dramatically after exercise in both genotypes, but this increment was lower in PTGOE mice under fed and fasting conditions (Fig. 3B).
Figure 3.
Plasma hormones and metabolites.A, plasma insulin, (B) plasma glucagon, (C) plasma free fatty acids, and (D) plasma β-hydroxybutyrate in sedentary and exercised control and PTGOE mice under fed and fasting conditions (n = 8–12 in all experiments). All values are the mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. PTG, protein targeting to glycogen; PTGOE, overexpressing PTG specifically in the liver.
During exercise, FFAs and β-hydroxybutyrate become a major fuel source for other organs (33, 34). Under fed conditions, exercise increased plasma FFAs and β-hydroxybutyrate levels similarly in both genotypes (Fig. 3, C and D). In fasted conditions, plasma FFAs (Fig. 3C) and β-hydroxybutyrate (Fig. 3D) were lower in sedentary PTGOE than control mice as we previously described (27). However, exercise increased the levels of FFAs and β-hydroxybutyrate to similar levels in both genotypes (Fig. 3, C and D).
Liver and muscle nucleotides
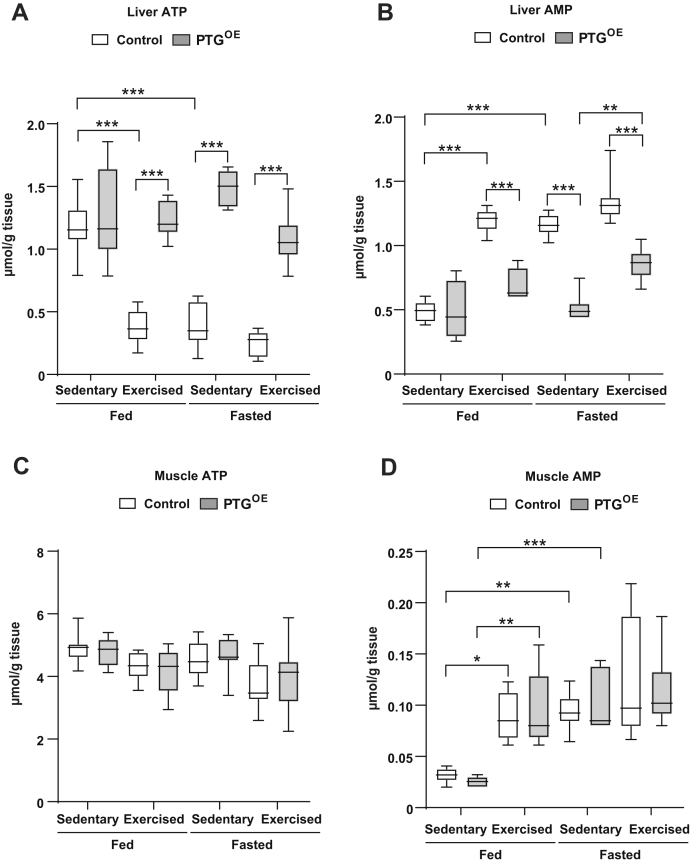
Exercise causes a drop in hepatic ATP (31). Our results showed that liver ATP concentration decreased and AMP concentration increased in control mice after exercise (Fig. 4, A and B). Fasted control mice also showed lower ATP and higher AMP levels than fed control counterparts (Fig. 4, A and B), as previously shown (35). Remarkably, the ATP and AMP levels of exercised fed and fasted PTGOE mice were maintained at similar levels to those of fed sedentary mice. The energy state of muscle is less sensitive to changes in metabolic demand than that of the liver (36). Therefore, muscle ATP levels were similar in all the groups (Fig. 4C). However, AMP concentration was significantly increased after exercise and fasting similarly in both genotypes (Fig. 4D).
Figure 4.
Liver and muscle nucleotides.A, liver ATP, (B) liver AMP, (C) muscle ATP, and (D) muscle AMP in sedentary and exercised control and PTGOE mice under fed and fasting conditions (n = 8–12 in all experiments). All values are the mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. PTG, protein targeting to glycogen; PTGOE, overexpressing PTG specifically in the liver.
Liver metabolism enzymes
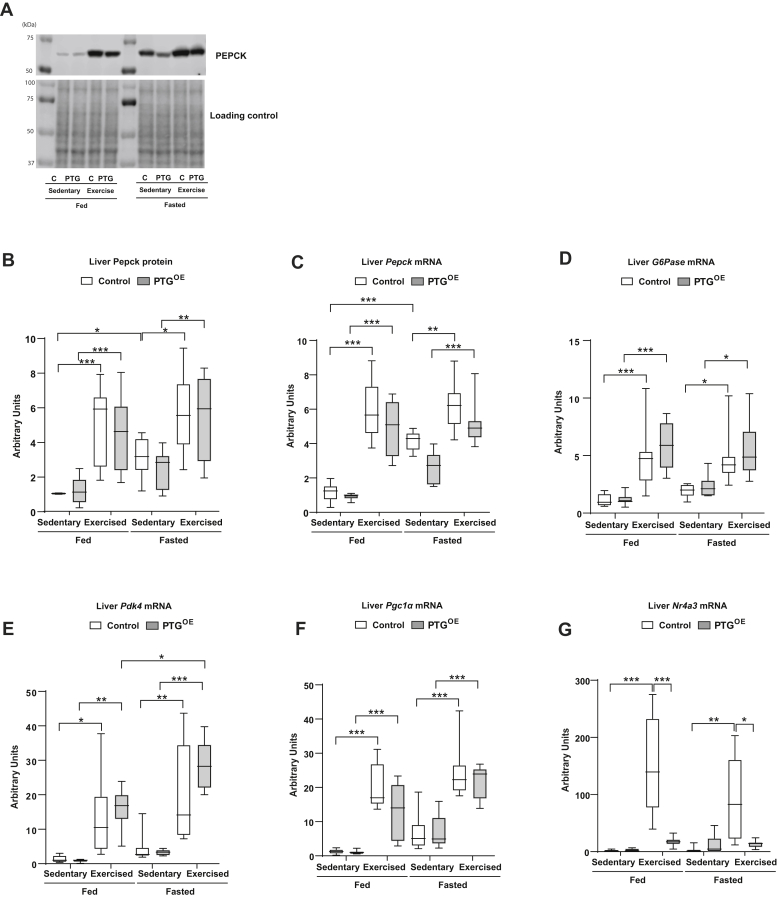
The liver has to adapt to an enormous metabolic challenge during exercise. We studied the expression of enzymes known to be regulated by exercise in this organ (37). The protein level of Pepck was higher at exhaustion in control and PTGOE mice under fed and fasting conditions (Fig. 5, A and B). The mRNA expression of Pepck and glucose-6-phosphatase (G6pase) was similarly upregulated in control and PTGOE mice after exercise (Fig. 5, C and D). Pyruvate dehydrogenase kinase 4 (Pdk4) phosphorylates and inactivates the pyruvate dehydrogenase complex, thus inhibiting glucose oxidation and promoting gluconeogenesis (38). Pdk4 mRNA expression was also similarly upregulated in exercised control and PTGOE mice (Fig. 5E). Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (Pgc1α) has been identified as an important regulator of hepatic gluconeogenesis (39). The mRNA expression of Pgc1α was increased in exercised mice compared with sedentary mice, but there were no changes between the genotypes (Fig. 5F). NR4A orphan nuclear receptors are induced by multiple extracellular signals and hormones in a cell type–specific manner (40, 41). In the liver, NR4A receptors are transcriptional modulators of hepatic glucose metabolism. Interestingly, we found that hepatic gene expression of NR4A member 3 (Nr4a3) was increased in control exercise mice but not in PTGOE mice (Fig. 5G). We also measured the hepatic expression of genes implicated in glycogen metabolism. The expression of Gys2 was upregulated in control but not in PTGOE mice after exercise and fasting (Fig. S1B). Glycogen phosphorylase was downregulated after fasting in control but not in PTGOE mice (Fig. S1C). Glycogen branching enzyme (Gbe-1) and phosphoglucomutase (Pgm2) were upregulated after fasting in control mice but not in PTGOE mice (Fig. S1, D and E). Sedentary fed PTGOE animals showed a lower glucokinase (Gk) mRNA expression than control mice (Fig. S1F), probably as a consequence of hypoinsulinemia. Moreover, Gk gene expression was downregulated after exercise in control but not in PTGOE mice under fed conditions (Fig. S1F).
Figure 5.
Liver metabolism enzymes.A, representative Western blot image of Pepck and loading control, (B) Pepck protein expression, (C) Pepck mRNA expression, (D) G6pase mRNA expression, (E) Pdk4 mRNA expression, (F) Pgc1α mRNA expression, and (G) Nr4a3 mRNA expression in sedentary and exercised control and PTGOE mice under fed and fasting conditions (n = 8–12 in all experiments). All values are the mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. G6pase, glucose-6-phosphatase; Nr4a3, NR4A member 3; Pdk4, pyruvate dehydrogenase kinase 4; PEPCK, phosphoenolpyruvate carboxykinase; Pgc1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; PTG, protein targeting to glycogen; PTGOE, overexpressing PTG specifically in the liver.
Muscle metabolism enzymes
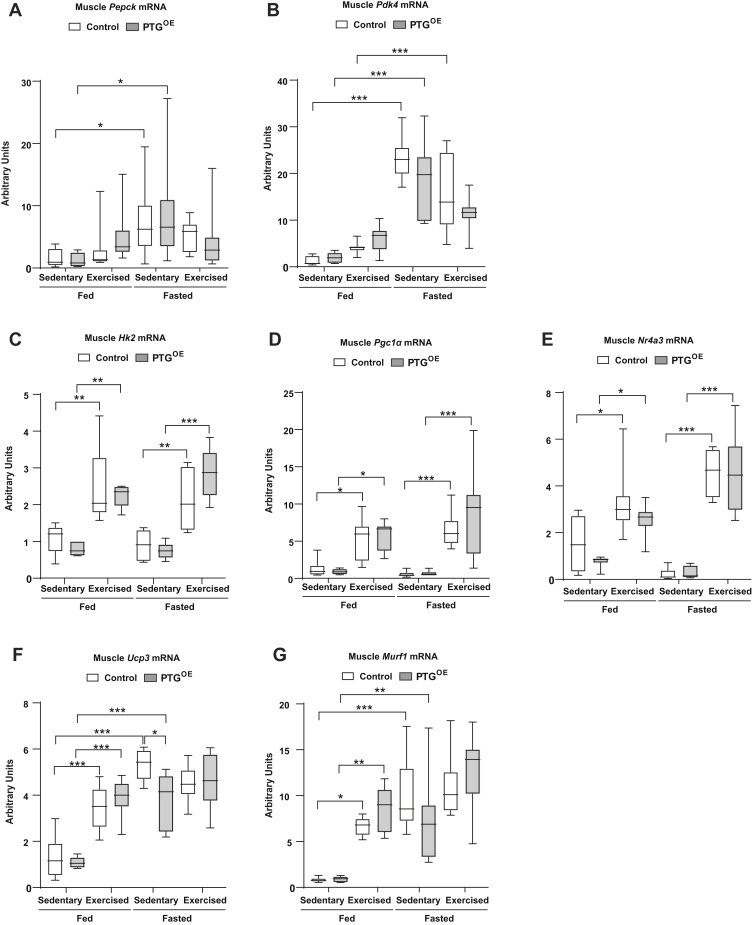
Exercise is associated with profound changes in skeletal muscle. We sought to determine whether the increased exercise capacity observed in PTGOE mice is associated with changes in some of the main genes related to muscle metabolism. In muscle, fasting, but not exercise, induced the expression of Pepck (Fig. 6A) and Pdk4 (Fig. 6B) in both genotypes. Exercise induced an increase in the expression of hexokinase-2 (Fig. 6C), Pgc1α (Fig. 6D), and Nr4a3 (Fig. 6E) in muscle under fed and fasting conditions, but there were no differences between the genotypes. Exercise upregulated the expression of uncoupling protein 3 (Fig. 6F) and muscle RING-finger protein-1 (Fig. 6G) under fed but not under fasting conditions because fasting already induced an increase in these genes.
Figure 6.
Muscle gene expression.A, Pepck mRNA expression, (B) Pdk4 mRNA expression, (C) Hk2 mRNA expression, (D) Pgc1α mRNA expression, (E) Nr4a3 mRNA expression, (F) Ucp3 mRNA expression, and (G) Murf1 mRNA expression in sedentary and exercised control and PTGOE mice under fed and fasting conditions (n = 8–12 in all experiments). All values are the mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. Hk2, hexokinase-2; Murf1, RING-finger protein-1; Nr4a3, NR4A member 3; Pdk4, pyruvate dehydrogenase kinase 4; PEPCK, phosphoenolpyruvate carboxykinase; Pgc1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; PTG, protein targeting to glycogen; PTGOE, overexpressing PTG specifically in the liver; Ucp3, uncoupling protein 3.
Discussion
Many mechanisms may be involved in the occurrence of fatigue, including changes in the internal environment (blood, extracellular fluid), changes within muscle fibers, and effects on the central nervous system (central fatigue) (42). It has been proposed that depletion of liver and muscle glycogen leads to exhaustion (13, 16). The importance of muscle glycogen for performance was subsequently confirmed in numerous studies in humans (43, 44, 45). However, studies addressing the contribution of liver glycogen to exercise capacity are limited in humans presumably because of the methodological limitations when attempting to assess liver glycogen content in these subjects (13). In an effort to elucidate the role of hepatic glycogen in endurance capacity, we generated PTGOE mice with a high concentration of hepatic glycogen. Using a low-intensity running protocol, we found that these mice were able to run for greater distances and longer, thereby indicating that liver glycogen is strongly associated with endurance capacity. Fasting completely depleted liver glycogen in control mice, and fasted animals ran significantly less than fed mice, as previously described (26). In contrast, PTGOE mice maintained sizeable concentrations of hepatic glycogen even after fasting and showed a similar endurance capacity to fed animals. These results confirm a key role of liver glycogen in exercise performance in mice.
In this study, we show that the key factor by which increased liver glycogen stores contributes to endurance capacity is the preservation of blood glucose. Hepatic glucose output is the primary source of the increased glucose available to exercising muscle (46). During prolonged exercise, glucose utilization by working muscle may outstrip glucose production, resulting in the gradual development of hypoglycemia (46). Several studies show that the strongest correlation to endurance is the maintenance of blood glucose above 3.9 mM (70 mg/dl) (28, 47). We found an increase in blood glucose after 1 h of exercise and a gradual decline during the running test in both genotypes, but blood glucose declined more slowly in PTGOE mice. Fed control mice showed hypoglycemia (blood glucose levels <3.9 mM) at the time of exhaustion, that is, after 5 h of running in fed conditions or after 3 h of running in fasting conditions. In contrast, PTGOE mice maintained a blood glucose concentration above 3.9 mM throughout the test in both conditions, probably because of higher glycogenolysis, in keeping with higher glycogen stores, which is reflected in the maintenance of substantial levels of G6P even at exhaustion. Moreover, the fall in blood glucose during prolonged exercise is accompanied by a reduction in insulin (48) and a rise in glucagon concentrations (49), particularly if a degree of hypoglycemia ensues. PTGOE mice showed a lower glucagon concentration than control mice after running under fed and fasting conditions. This observation is in line with the absence of hypoglycemia in these mice. Moreover, plasma insulin concentration under fed condition was lower in sedentary PTGOE mice than control mice (27, 35). This observation could be explained by the improved glucose tolerance observed in these mice (27). Furthermore, the levels of insulin were not reduced after exercise in PTGOE mice.
Gluconeogenesis is important for maintaining blood glucose levels during exercise (50), and this process is regulated at the transcriptional level by key enzymes, namely Pepck and G6pase, which are increased during exercise (51). At exhaustion, the hepatic expression of Pepck, G6pase, Pdk4, and Pgc1α was equally upregulated in fed control and PTGOE mice, thereby indicating that gluconeogenesis was similarly increased in the livers of both groups of mice. Thus, the ability of PTGOE mice to preserve glucose levels during exercise was not the result of an increased expression of these genes.
The NR4A family of nuclear hormone receptors has been shown to regulate varied processes across a host of tissues (52). Nr4a3, also known as NOR1, has been identified as one of the most exercise responsive genes in the skeletal muscle of humans (53), but the role of hepatic Nr4a3 in exercise has not been explored. Interestingly, exercise induced the expression of hepatic Nr4a3 in control but not in PTGOE mice, which could be explained by the circulating levels of glucagon. Indeed, the hepatic expression of Nr4a3 is potently induced by glucagon in vivo in a cAMP-dependent manner (54), and PTGOE mice showed lower plasma glucagon at exhaustion than control mice.
During exercise, adipose tissue lipolysis is stimulated by hormonal changes, resulting in increased availability and tissue utilization of FFAs. In the circulation, the concentration of β-hydroxybutyrate increased after exercise, indicating an acceleration of ketogenesis. Plasma FFAs and β-hydroxybutyrate were similar in both genotypes after exercise, indicating that differences in adipose tissue lipolysis did not affect endurance capacity. Fasting and exercise in mice induced hepatic steatosis as a result of excessive uptake of circulating FFAs derived from adipose tissue lipolysis. The amount of hepatic TAG content was lower in fasted PTGOE mice than control mice, which could be attributable to a lower flux of FFAs reaching the liver, as previously described (27). However, hepatic steatosis was present to a similar extent in exercised control and PTGOE mice, and this finding correlates with the amount of circulating FFAs detected.
Exercise results in a deficit in the energy status of the liver as defined by increased hepatic AMP concentrations and decreased ATP concentrations (36). Strikingly, exercised PTGOE mice maintained ATP and AMP at the same level as sedentary counterparts, both in fed and fasting conditions. Thus, increasing liver glycogen maintained hepatic energy status during exercise. In this regard, we previously demonstrated that liver glycogen maintains hepatic energy state during fasting (35) and diabetes (27, 55, 56). In mice, metabolic stress and a physiological rise in glucagon caused a decrease in the hepatic energy state (57). PTGOE mice did not show higher levels of glucagon, which would explain the maintenance of hepatic energy status.
A strong association has been suggested between muscle glycogen depletion, impaired muscle performance, and fatigue development during exercise (16, 17). Thus, we analyzed whether the increased exercise capacity observed in PTGOE mice is associated with changes in skeletal muscle. We found that muscle glycogen, G6P, TAG, ATP, and AMP content were similarly changed in both genotypes after exercise. This observation thus indicates that these metabolites did not have an effect on endurance capacity in this model. Plasma lactate, an indicator for muscle glycolysis, was similar in both genotypes, thus indicating that lactate was not related to the increased performance in PTGOE mice. We also analyzed genes whose gain of function in skeletal muscle increases endurance performance in mice (58). The expression of Pepck, hexokinase-2, Pgc1α, Nr4a3, uncoupling protein 3, and RING-finger protein-1 in skeletal muscle was similar in both genotypes, thus indicating that they were not involved in the increased performance of the PTGOE mice.
In conclusion, these results identify hepatic glycogen as a key regulator of endurance capacity in mice, an effect that may be exerted through the maintenance of blood glucose. Thus, in endurance sports such as marathon running and long-distance cycling, increasing liver glycogen stores could maintain blood glucose and delay the onset of hypoglycemia or “hitting the wall.”
Experimental procedures
Animals
All procedures were approved by the Barcelona Science Park Animal Experimentation Committee and carried out in accordance with the European Community Council Directive and the National Institutes of Health guidelines for the care and use of laboratory animals. PTGOE mice were generated as previously described (27). Briefly, the PTG cDNA under the control of the ubiquitous CAG promoter (cytomegalovirus immediate early enhancer/chicken b-actin promoter fusion) was introduced into an innocuous locus by homologous recombination. A loxP-flanked transcription stop cassette was included between the CAG promoter and the PTG cDNA to allow the expression to depend on the action of a Cre recombinase. The resulting mouse line was bred with an albumin promoter Cre recombinase–expressing animal (The Jackson Laboratory), which drove the expression of PTG specifically to the liver. The PTGOE mice were backcrossed to C57BL/6. Studies were performed in 5-month-old male mice. Ad libitum–fed animals or, when indicated, 16-h overnight-fasted animals were used. Fed and fasted mice were enrolled in the exercise protocol at 8.00 AM. For fasted mice, food was removed at 4.00 PM on the day before the experiment, and animals were fasted for 16 h until the next morning at 8.00 AM when the experimental runs started. Immediately after reaching exhaustion, the mice were sacrificed by cervical dislocation, and the tissues (liver and muscle) were collected in liquid nitrogen and stored at −80 °C for further analysis. Littermates of the same age were used as controls.
Exercise protocol
A motorized, speed-controlled treadmill was used to exercise the mice (LE8710; Harvard Apparatus). Before running until exhaustion, mice were preadapted to the treadmill for four consecutive days (3 min/day). For the experimental run, mice ran at 8.00 AM on a treadmill set at a 20° incline with an initial belt speed of 12 cm/s. The speed was increased by 1 cm/s at 2, 5, 10, 20, 30, 40, 50, and 60 min after initiation of the exercise, followed by the exhaustion run at 20 cm/s until mice failed.
Blood metabolite and hormone levels
Whole-blood samples were collected from the tail into EDTA-microvettes (Sarstedt Inc) and were then centrifuged at 1000g for 10 min at 4 °C. Supernatants were aliquoted and stored at −20 °C. Plasma insulin and glucagon were determined by ELISA (Crystal Chem). Plasma free fatty acids (FFAs) (Abcam) and β-hydroxybutyrate (Sigma-Aldrich) were measured using a commercial kit.
Blood glucose and lactate levels were measured using a glucometer (Bayer Contour Next, Bayer Healthcare) and a lactometer (Lactate Scout 4 analyzer, EKF Diagnostics) with tail-tip bleeding.
Biochemical analysis
Glycogen content was determined in samples of frozen tissue by measuring amyloglucosidase-released glucose from glycogen as previously described (59). The intracellular concentration of ATP and AMP was measured by HPLC as previously described (55). Hepatic and muscle triacylglycerols (TAGs) were quantified in 3 mol/l KOH and 65% ethanol extracts as previously described (60). The intracellular concentration of glucose-6-phosphate (G6P) was measured in perchloric acid extracts with a fluorometric assay, as described (61).
Western blot analysis
Liver samples were homogenized in 50 mM Tris/HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 5 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 50 mM NaF, 1% NP-40, 1 mM PMSF, and a protease inhibitor cocktail tablet (Roche). Immunoblot analysis of homogenates was performed using the following antibodies: phosphoenolpyruvate carboxykinase (PEPCK) (a kind gift from Dr E. Beale) at a dilution of 1:100.000. Proteins were detected by the ECL method (Immobilon Western Chemiluminescent HRP Substrate, Millipore, Sigma-Aldrich). The loading control of the WB membrane was performed using the REVERT total protein stain.
RNA extraction and quantitative RT-PCR
Liver and muscle RNA extraction, RT-PCR, and quantitative real-time PCR analysis were performed as previously described (62). The following TaqMan probes (Applied Biosystems) were used for quantitative real-time PCR: Ptg (Mm01204084_m1), Gys2 (Mm00523953_m1), Pygl (Mm01289790_m1), Gbe-1 (Mm00472359_m1), Pgm2 (Mm00728285_s1), Gk (Mm00439129_m1), Pepck (Mm00440636_m1), G6pase (Mm00839363_m1), Pdk4 (Mm01166879_m1), Pgc1α (Mm00447180_m1), Nr4a3 (Mm00450071_g1), Ucp3 (Mm00494077_m1), Murf1 (Mm01185221_m1), Hk2 (Mm00443385_m1), and 18S (Mm03928990_g1). 18S was used as a housekeeping gene.
Statistical analysis
Data are expressed as the mean ± SEM. p Values were calculated using two-way ANOVA with post hoc Tukey’s test as appropriate or two-tailed t test.
Data availability
The data are available on request from the corresponding author (Iliana López-Soldado, IRB Barcelona, iliana.lopez@irbbarcelona.org).
Supporting information
This article contains supporting information.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
We wish to thank Anna Adrover, Emma Veza, Vanessa Hernandez, and Laura I. Alcaide for technical assistance. We also thank Tanya Yates for correcting the English version of the manuscript.
IRB Barcelona is the recipient of a Severo Ochoa Award of Excellence from MINECO (Government of Spain). The project also received funding from “La Marató de TV3” Foundation (Barcelona, Spain) (201613-10).
Author contributions
I. L.-S. and J. J. G. conceptualization; I. L.-S. data curation; I. L.-S. formal analysis; I. L.-S. investigation; I. L.-S. and J. D. methodology; I. L.-S., J. J. G., and J. D. writing–original draft; I. L.-S. project administration; I. L.-S., J. J. G., and J. D. writing–review and editing; J. J. G. supervision; J. J. G. and J. D. funding acquisition.
Funding and additional information
This study was supported by a grant BFU2017-84345-P from MINECO to J. G. and J. D. and the CIBER de Diabetes y Enfermedades Metabólicas Asociadas (ISCIII, Ministerio de Ciencia e Innovación).
Edited by Qi-Qun Tang
Supporting information
References
- 1.Kaslow H.R., Lesikar D.D., Antwi D., Tan A.W. L-type glycogen synthase. Tissue distribution and electrophoretic mobility. J. Biol. Chem. 1985;260:9953–9956. [PubMed] [Google Scholar]
- 2.Kaslow H.R., Lesikar D.D. Isozymes of glycogen synthase. FEBS Lett. 1984;172:294–298. doi: 10.1016/0014-5793(84)81144-8. [DOI] [PubMed] [Google Scholar]
- 3.Ros S., Garcia-Rocha M., Dominguez J., Ferrer J.C., Guinovart J.J. Control of liver glycogen synthase activity and intracellular distribution by phosphorylation. J. Biol. Chem. 2009;284:6370–6378. doi: 10.1074/jbc.M808576200. [DOI] [PubMed] [Google Scholar]
- 4.von Wilamowitz-Moellendorff A., Hunter R.W., Garcia-Rocha M., Kang L., Lopez-Soldado I., Lantier L., Patel K., Peggie M.W., Martinez-Pons C., Voss M., Calbó J., Cohen P.T., Wasserman D.H., Guinovart J.J., Sakamoto K. Glucose-6-phosphate-mediated activation of liver glycogen synthase plays a key role in hepatic glycogen synthesis. Diabetes. 2013;62:4070–4082. doi: 10.2337/db13-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gasa R., Jensen P.B., Berman H.K., Brady M.J., DePaoli-Roach A.A., Newgard C.B. Distinctive regulatory and metabolic properties of glycogen-targeting subunits of protein phosphatase-1 (PTG, GL, GM/RGl) expressed in hepatocytes. J. Biol. Chem. 2000;275:26396–26403. doi: 10.1074/jbc.M002427200. [DOI] [PubMed] [Google Scholar]
- 6.Berman H.K., O'Doherty R.M., Anderson P., Newgard C.B. Overexpression of protein targeting to glycogen (PTG) in rat hepatocytes causes profound activation of glycogen synthesis independent of normal hormone- and substrate-mediated regulatory mechanisms. J. Biol. Chem. 1998;273:26421–26425. doi: 10.1074/jbc.273.41.26421. [DOI] [PubMed] [Google Scholar]
- 7.Jensen J., Rustad P.I., Kolnes A.J., Lai Y.C. The role of skeletal muscle glycogen breakdown for regulation of insulin sensitivity by exercise. Front. Physiol. 2011;2:112. doi: 10.3389/fphys.2011.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Auge E., Pelegri C., Manich G., Cabezon I., Guinovart J.J., Duran J., Vilaplana J. Astrocytes and neurons produce distinct types of polyglucosan bodies in Lafora disease. Glia. 2018;66:2094–2107. doi: 10.1002/glia.23463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duran J., Gruart A., Garcia-Rocha M., Delgado-Garcia J.M., Guinovart J.J. Glycogen accumulation underlies neurodegeneration and autophagy impairment in Lafora disease. Hum. Mol. Genet. 2014;23:3147–3156. doi: 10.1093/hmg/ddu024. [DOI] [PubMed] [Google Scholar]
- 10.Mir-Coll J., Duran J., Slebe F., Garcia-Rocha M., Gomis R., Gasa R., Guinovart J.J. Genetic models rule out a major role of beta cell glycogen in the control of glucose homeostasis. Diabetologia. 2016;59:1012–1020. doi: 10.1007/s00125-016-3871-1. [DOI] [PubMed] [Google Scholar]
- 11.van Loon L.J., Greenhaff P.L., Constantin-Teodosiu D., Saris W.H., Wagenmakers A.J. The effects of increasing exercise intensity on muscle fuel utilisation in humans. J. Physiol. 2001;536:295–304. doi: 10.1111/j.1469-7793.2001.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romijn J.A., Coyle E.F., Sidossis L.S., Gastaldelli A., Horowitz J.F., Endert E., Wolfe R.R. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am. J. Physiol. 1993;265:E380–E391. doi: 10.1152/ajpendo.1993.265.3.E380. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez J.T., Fuchs C.J., Betts J.A., van Loon L.J. Liver glycogen metabolism during and after prolonged endurance-type exercise. Am. J. Physiol. Endocrinol. Metab. 2016;311:E543–E553. doi: 10.1152/ajpendo.00232.2016. [DOI] [PubMed] [Google Scholar]
- 14.Cermak N.M., van Loon L.J. The use of carbohydrates during exercise as an ergogenic aid. Sports Med. 2013;43:1139–1155. doi: 10.1007/s40279-013-0079-0. [DOI] [PubMed] [Google Scholar]
- 15.Egan B., Zierath J.R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013;17:162–184. doi: 10.1016/j.cmet.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Shulman R.G., Rothman D.L. The “glycogen shunt” in exercising muscle: A role for glycogen in muscle energetics and fatigue. Proc. Natl. Acad. Sci. U. S. A. 2001;98:457–461. doi: 10.1073/pnas.98.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hermansen L., Hultman E., Saltin B. Muscle glycogen during prolonged severe exercise. Acta Physiol. Scand. 1967;71:129–139. doi: 10.1111/j.1748-1716.1967.tb03719.x. [DOI] [PubMed] [Google Scholar]
- 18.Pederson B.A., Cope C.R., Irimia J.M., Schroeder J.M., Thurberg B.L., Depaoli-Roach A.A., Roach P.J. Mice with elevated muscle glycogen stores do not have improved exercise performance. Biochem. Biophys. Res. Commun. 2005;331:491–496. doi: 10.1016/j.bbrc.2005.03.206. [DOI] [PubMed] [Google Scholar]
- 19.Pederson B.A., Cope C.R., Schroeder J.M., Smith M.W., Irimia J.M., Thurberg B.L., DePaoli-Roach A.A., Roach P.J. Exercise capacity of mice genetically lacking muscle glycogen synthase: In mice, muscle glycogen is not essential for exercise. J. Biol. Chem. 2005;280:17260–17265. doi: 10.1074/jbc.M410448200. [DOI] [PubMed] [Google Scholar]
- 20.Pederson B.A., Schroeder J.M., Parker G.E., Smith M.W., DePaoli-Roach A.A., Roach P.J. Glucose metabolism in mice lacking muscle glycogen synthase. Diabetes. 2005;54:3466–3473. doi: 10.2337/diabetes.54.12.3466. [DOI] [PubMed] [Google Scholar]
- 21.Xirouchaki C.E., Mangiafico S.P., Bate K., Ruan Z., Huang A.M., Tedjosiswoyo B.W., Lamont B., Pong W., Favaloro J., Blair A.R., Zajac J.D., Proietto J., Andrikopoulos S. Impaired glucose metabolism and exercise capacity with muscle-specific glycogen synthase 1 (gys1) deletion in adult mice. Mol. Metab. 2016;5:221–232. doi: 10.1016/j.molmet.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petersen K.F., Price T.B., Bergeron R. Regulation of net hepatic glycogenolysis and gluconeogenesis during exercise: Impact of type 1 diabetes. J. Clin. Endocrinol. Metab. 2004;89:4656–4664. doi: 10.1210/jc.2004-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wasserman D.H., Spalding J.A., Lacy D.B., Colburn C.A., Goldstein R.E., Cherrington A.D. Glucagon is a primary controller of hepatic glycogenolysis and gluconeogenesis during muscular work. Am. J. Physiol. 1989;257:E108–E117. doi: 10.1152/ajpendo.1989.257.1.E108. [DOI] [PubMed] [Google Scholar]
- 24.Wolfe R.R., Nadel E.R., Shaw J.H., Stephenson L.A., Wolfe M.H. Role of changes in insulin and glucagon in glucose homeostasis in exercise. J. Clin. Invest. 1986;77:900–907. doi: 10.1172/JCI112388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stevenson E.J., Thelwall P.E., Thomas K., Smith F., Brand-Miller J., Trenell M.I. Dietary glycemic index influences lipid oxidation but not muscle or liver glycogen oxidation during exercise. Am. J. Physiol. Endocrinol. Metab. 2009;296:E1140–E1147. doi: 10.1152/ajpendo.90788.2008. [DOI] [PubMed] [Google Scholar]
- 26.Irimia J.M., Meyer C.M., Peper C.L., Zhai L., Bock C.B., Previs S.F., McGuinness O.P., De Paoli-Roach A., Roach P.J. Impaired glucose tolerance and predisposition to the fasted state in liver glycogen synthase knock-out mice. J. Biol. Chem. 2010;285:12851–12861. doi: 10.1074/jbc.M110.106534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopez-Soldado I., Zafra D., Duran J., Adrover A., Calbo J., Guinovart J.J. Liver glycogen reduces food intake and attenuates obesity in a high-fat diet-fed mouse model. Diabetes. 2015;64:796–807. doi: 10.2337/db14-0728. [DOI] [PubMed] [Google Scholar]
- 28.Fan W., Waizenegger W., Lin C.S., Sorrentino V., He M.X., Wall C.E., Li H., Liddle C., Yu R.T., Atkins A.R., Auwerx J., Downes M., Evans R.M. PPARdelta promotes running endurance by preserving glucose. Cell Metab. 2017;25:1186–1193.e1184. doi: 10.1016/j.cmet.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knudsen J.G., Bienso R.S., Hassing H.A., Jakobsen A.H., Pilegaard H. Exercise-induced regulation of key factors in substrate choice and gluconeogenesis in mouse liver. Mol. Cell. Biochem. 2015;403:209–217. doi: 10.1007/s11010-015-2351-0. [DOI] [PubMed] [Google Scholar]
- 30.Gorski J., Nowacka M., Namiot Z., Puch U. Effect of prolonged exercise on the level of triglycerides in the rat liver. Eur. J. Appl. Physiol. Occup. Physiol. 1988;57:554–557. doi: 10.1007/BF00418461. [DOI] [PubMed] [Google Scholar]
- 31.Trefts E., Williams A.S., Wasserman D.H. Exercise and the regulation of hepatic metabolism. Prog. Mol. Biol. Transl. Sci. 2015;135:203–225. doi: 10.1016/bs.pmbts.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wasserman D.H. Four grams of glucose. Am. J. Physiol. Endocrinol. Metab. 2009;296:E11–21. doi: 10.1152/ajpendo.90563.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evans M., Cogan K.E., Egan B. Metabolism of ketone bodies during exercise and training: Physiological basis for exogenous supplementation. J. Physiol. 2017;595:2857–2871. doi: 10.1113/JP273185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frayn K.N. Fat as a fuel: Emerging understanding of the adipose tissue-skeletal muscle axis. Acta Physiol. (Oxf.) 2010;199:509–518. doi: 10.1111/j.1748-1716.2010.02128.x. [DOI] [PubMed] [Google Scholar]
- 35.Lopez-Soldado I., Bertini A., Adrover A., Duran J., Guinovart J.J. Maintenance of liver glycogen during long-term fasting preserves energy state in mice. FEBS Lett. 2020;594:1698–1710. doi: 10.1002/1873-3468.13770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Camacho R.C., Donahue E.P., James F.D., Berglund E.D., Wasserman D.H. Energy state of the liver during short-term and exhaustive exercise in C57BL/6J mice. Am. J. Physiol. Endocrinol. Metab. 2006;290:E405–E408. doi: 10.1152/ajpendo.00385.2005. [DOI] [PubMed] [Google Scholar]
- 37.Hoene M., Lehmann R., Hennige A.M., Pohl A.K., Haring H.U., Schleicher E.D., Weigert C. Acute regulation of metabolic genes and insulin receptor substrates in the liver of mice by one single bout of treadmill exercise. J. Physiol. 2009;587:241–252. doi: 10.1113/jphysiol.2008.160275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holness M.J., Bulmer K., Smith N.D., Sugden M.C. Investigation of potential mechanisms regulating protein expression of hepatic pyruvate dehydrogenase kinase isoforms 2 and 4 by fatty acids and thyroid hormone. Biochem. J. 2003;369:687–695. doi: 10.1042/BJ20021509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoon J.C., Puigserver P., Chen G., Donovan J., Wu Z., Rhee J., Adelmant G., Stafford J., Kahn C.R., Granner D.K., Newgard C.B., Spiegelman B.M. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413:131–138. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- 40.Winoto A., Littman D.R. Nuclear hormone receptors in T lymphocytes. Cell. 2002;109 Suppl:S57–66. doi: 10.1016/s0092-8674(02)00710-9. [DOI] [PubMed] [Google Scholar]
- 41.Pei L., Castrillo A., Chen M., Hoffmann A., Tontonoz P. Induction of NR4A orphan nuclear receptor expression in macrophages in response to inflammatory stimuli. J. Biol. Chem. 2005;280:29256–29262. doi: 10.1074/jbc.M502606200. [DOI] [PubMed] [Google Scholar]
- 42.Ament W., Verkerke G.J. Exercise and fatigue. Sports Med. 2009;39:389–422. doi: 10.2165/00007256-200939050-00005. [DOI] [PubMed] [Google Scholar]
- 43.Pernow B., Saltin B. Availability of substrates and capacity for prolonged heavy exercise in man. J. Appl. Physiol. 1971;31:416–422. doi: 10.1152/jappl.1971.31.3.416. [DOI] [PubMed] [Google Scholar]
- 44.Gollnick P.D., Piehl K., Saubert C.W.t., Armstrong R.B., Saltin B. Diet, exercise, and glycogen changes in human muscle fibers. J. Appl. Physiol. 1972;33:421–425. doi: 10.1152/jappl.1972.33.4.421. [DOI] [PubMed] [Google Scholar]
- 45.Bangsbo J., Graham T.E., Kiens B., Saltin B. Elevated muscle glycogen and anaerobic energy production during exhaustive exercise in man. J. Physiol. 1992;451:205–227. doi: 10.1113/jphysiol.1992.sp019161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wahren J., Ekberg K. Splanchnic regulation of glucose production. Annu. Rev. Nutr. 2007;27:329–345. doi: 10.1146/annurev.nutr.27.061406.093806. [DOI] [PubMed] [Google Scholar]
- 47.Li L.O., Grevengoed T.J., Paul D.S., Ilkayeva O., Koves T.R., Pascual F., Newgard C.B., Muoio D.M., Coleman R.A. Compartmentalized acyl-CoA metabolism in skeletal muscle regulates systemic glucose homeostasis. Diabetes. 2015;64:23–35. doi: 10.2337/db13-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wahren J., Felig P., Ahlborg G., Jorfeldt L. Glucose metabolism during leg exercise in man. J. Clin. Invest. 1971;50:2715–2725. doi: 10.1172/JCI106772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Felig P., Wahren J., Hendler R., Ahlborg G. Plasma glucagon levels in exercising man. N. Engl. J. Med. 1972;287:184–185. doi: 10.1056/NEJM197207272870412. [DOI] [PubMed] [Google Scholar]
- 50.John-Alder H.B., McAllister R.M., Terjung R.L. Reduced running endurance in gluconeogenesis-inhibited rats. Am. J. Physiol. 1986;251:R137–142. doi: 10.1152/ajpregu.1986.251.1.R137. [DOI] [PubMed] [Google Scholar]
- 51.Banzet S., Koulmann N., Simler N., Sanchez H., Chapot R., Serrurier B., Peinnequin A., Bigard X. Control of gluconeogenic genes during intense/prolonged exercise: Hormone-independent effect of muscle-derived IL-6 on hepatic tissue and PEPCK mRNA. J. Appl. Physiol. (1985) 2009;107:1830–1839. doi: 10.1152/japplphysiol.00739.2009. [DOI] [PubMed] [Google Scholar]
- 52.Herring J.A., Elison W.S., Tessem J.S. Function of Nr4a orphan nuclear receptors in proliferation, apoptosis and fuel utilization across tissues. Cells. 2019;8:1373. doi: 10.3390/cells8111373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pillon N.J., Gabriel B.M., Dollet L., Smith J.A.B., Sardon Puig L., Botella J., Bishop D.J., Krook A., Zierath J.R. Transcriptomic profiling of skeletal muscle adaptations to exercise and inactivity. Nat. Commun. 2020;11:470. doi: 10.1038/s41467-019-13869-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pei L., Waki H., Vaitheesvaran B., Wilpitz D.C., Kurland I.J., Tontonoz P. NR4A orphan nuclear receptors are transcriptional regulators of hepatic glucose metabolism. Nat. Med. 2006;12:1048–1055. doi: 10.1038/nm1471. [DOI] [PubMed] [Google Scholar]
- 55.Lopez-Soldado I., Fuentes-Romero R., Duran J., Guinovart J.J. Effects of hepatic glycogen on food intake and glucose homeostasis are mediated by the vagus nerve in mice. Diabetologia. 2017;60:1076–1083. doi: 10.1007/s00125-017-4240-4. [DOI] [PubMed] [Google Scholar]
- 56.Lopez-Soldado I., Guinovart J.J., Duran J. Increasing hepatic glycogen moderates the diabetic phenotype in insulin-deficient akita mice. J. Biol. Chem. 2021;296:100498. doi: 10.1016/j.jbc.2021.100498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berglund E.D., Lee-Young R.S., Lustig D.G., Lynes S.E., Donahue E.P., Camacho R.C., Meredith M.E., Magnuson M.A., Charron M.J., Wasserman D.H. Hepatic energy state is regulated by glucagon receptor signaling in mice. J. Clin. Invest. 2009;119:2412–2422. doi: 10.1172/JCI38650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yaghoob Nezhad F., Verbrugge S.A.J., Schonfelder M., Becker L., Hrabe de Angelis M., Wackerhage H. Genes whose gain or loss-of-function increases endurance performance in mice: A systematic literature review. Front. Physiol. 2019;10:262. doi: 10.3389/fphys.2019.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garcia-Rocha M., Roca A., De La Iglesia N., Baba O., Fernandez-Novell J.M., Ferrer J.C., Guinovart J.J. Intracellular distribution of glycogen synthase and glycogen in primary cultured rat hepatocytes. Biochem. J. 2001;357:17–24. doi: 10.1042/0264-6021:3570017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Salmon D.M., Flatt J.P. Effect of dietary fat content on the incidence of obesity among ad libitum fed mice. Int. J. Obes. 1985;9:443–449. [PubMed] [Google Scholar]
- 61.Zhu A., Romero R., Petty H.R. An enzymatic fluorimetric assay for glucose-6-phosphate: Application in an in vitro Warburg-like effect. Anal. Biochem. 2009;388:97–101. doi: 10.1016/j.ab.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ros S., Zafra D., Valles-Ortega J., Garcia-Rocha M., Forrow S., Dominguez J., Calbo J., Guinovart J.J. Hepatic overexpression of a constitutively active form of liver glycogen synthase improves glucose homeostasis. J. Biol. Chem. 2010;285:37170–37177. doi: 10.1074/jbc.M110.157396. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are available on request from the corresponding author (Iliana López-Soldado, IRB Barcelona, iliana.lopez@irbbarcelona.org).