Highlights
-
•
No partial or complete responses to pembrolizumab and lenvatinib therapy were observed in advanced or recurrent UCS.
-
•
Median PFS and OS following pembrolizumab and lenvatinib therapy is similar to traditional cytotoxic regimens.
-
•
Pembrolizumab and lenvatinib therapy was only used in patients who had failed two or more lines of therapy.
Keywords: Uterine carcinosarcoma, Pembrolizumab, Lenvatinib
Abstract
Objective(s)
To investigate the efficacy of pembrolizumab plus lenvatinib as a second-line or later-line therapy in women with advanced or recurrent uterine carcinosarcoma (UCS).
Methods
A single-institution pharmacy database was queried for women with advanced or recurrent UCS who were prescribed concurrent pembrolizumab and lenvatinib. Patient demographic, oncologic, and immunotherapy outcomes data were recorded. Univariate analysis summarized progression-free survival (PFS) and overall survival (OS).
Results
Seven patients with advanced or recurrent UCS were treated with combination pembrolizumab and lenvatinib, with a median age of 63.0 years. The majority had stage III or IV disease (n = 6, 85.7%) and had failed two or more lines of therapy (n = 7, 100.0%), and a minority were MMR deficient (n = 1, 14.3%) or PD-L1+ (n = 1, 14.3%). No partial or complete responses were observed. The median PFS was 2.6 months (95% CI, 0.9–11.2 months), and the median OS was 2.8 months (95% CI, 2.4-NE).
Conclusions
In this small, retrospective series, we demonstrate that pembrolizumab and lenvatinib combination therapy may not be highly active in UCS and may be associated with similar PFS and OS as traditional cytotoxic regimens. Further study is warranted to assess the efficacy of this regimen in more targeted cohorts of women with advanced or recurrent UCS.
1. Introduction
Uterine carcinosarcomas (UCS) account for less than 5% of endometrial cancers (EC), yet this aggressive variant has a poor prognosis accounting for 15% of uterine cancer deaths (Siegel et al., 2014, Brooks et al., 2004, Cimbaluk et al., 2007). Studies have demonstrated that women diagnosed with uterine carcinosarcomas have significantly worse survival compared to those with high-grade endometrioid, serous, or clear cell carcinomas (Zhang et al., 2015). Despite recent molecular studies suggesting that the sarcomatous element is secondary to metaplasia of the carcinomatous component, prognosis remains much bleaker than other histologic sub-types (Gotoh et al., 2019, Cherniack et al., 2017, Zhao et al., 2016). In a large retrospective analysis of over 900 women with UCS from the United States and Japan, patients with a high-grade carcinoma and heterologous sarcoma had a 5-year progression-free survival (PFS) of 34%, those with a high-grade carcinoma and homologous sarcoma had a 5-year PFS of 46%, and those with a low-grade carcinoma and homologous sarcoma had a 5-year PFS of 60% (Matsuo et al., 2016).
The primary management for UCS involves a multi-modality approach, typically including cytotoxic chemotherapy and surgery in the setting of operable tumors, with complete cytoreduction associated with improved overall survival (Harano et al., 2016). GOG 261 demonstrated that the combination of carboplatin with paclitaxel was associated with better PFS (16 vs 12 months; HR 0.73, p < .01) and OS (37 vs 29 months; HR 0.87, p < .01) compared with ifosfamide with paclitaxel in women with advanced or recurrent UCS (Powell et al., 2019). Unfortunately, in the setting of second-line and later-line therapies, median response rate to cytotoxic therapy is less than 10% with median PFS of approximately two months (Matsuzaki et al., 2021). Collectively, these studies demonstrate that new therapeutic approaches for women with recurrent UCS are urgently needed.
The KEYNOTE-146 study by Makker et al. was a single-arm, open-label, multicenter trial that enrolled 108 patients with metastatic EC (Makker et al., 2020). In this trial, combination therapy comprising pembrolizumab and lenvatinib demonstrated an impressive response rate of 38% with a PFS of 7.4 months and overall survival of 16.7 months, with many patients experiencing durable responses to treatment (Makker et al., 2020). Although women with UCS were not enrolled in this trial, many believe UCS shares tumor biology with serous carcinomas, which made up 32.4% of the KEYNOTE-146 study population (Makker et al., 2020, Menczer et al., 2005, Vitale et al., 2017, Livasy et al., 2006, Emoto et al., 2004). Understanding the biologic activity of this regimen in women with UCS is essential to improving oncologic outcomes in women. The objective of this study was to report preliminary data for efficacy of pembrolizumab and lenvatinib combination therapy with advanced or recurrent UCS in a retrospective single institution cohort study.
2. Methods
2.1. Study design
The study is an Institutional Review Board approved, single-institution retrospective case series including all women with advanced or recurrent uterine carcinosarcomas treated with combination therapy with pembrolizumab and lenvatinib at the Cleveland Clinic. The institution’s pharmacy database was queried for patients with concurrent prescriptions for pembrolizumab and lenvatinib, and those with a diagnosis of uterine carcinosarcoma confirmed on final surgical pathology were included. Consent for use of patient health information was obtained prior to starting the study.
2.2. Data collection
Patient demographics were extracted from the electronic medical record, including age, race, body mass index (kg/m2), Eastern Cooperative Oncology Group (ECOG) performance status score prior to start of immunotherapy, and medical comorbidities. Oncologic variables included stage, primary cancer treatment, route of initial surgery, prior lines of treatment, PD-L1 status (positive, negative, or unknown), and MMR status (proficient, deficit, or unknown). PD-L1 status was defined as positive if there was membranous staining in at least 1% of viable tumor cells. Immunotherapy variables included start and end date, number of cycles, reason for discontinuation, radiological response using the Immunotherapy Response Evaluation Criteria in Solid Tumors (iRECIST) criteria, whether the patient required dose reduction or steroids during immunotherapy, progression or recurrence date, last follow-up, current status, and date of death (Seymour et al., 2017). All data was collected and stored securely in a RedCAP database (Harris et al., 2019, Harris et al., 2009).
2.3. Statistical analysis
Continuous variables were reported using medians and interquartile range. Categorical factors and ordinal variables were described as frequencies and percentages. For right censored PFS and OS, time to progression or death was defined as the difference in months from immunotherapy start date to progression date or death date. Kaplan-Meier plot was created for PFS and OS. Statistical analysis was performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC).
3. Results
3.1. Patient demographics and oncologic characteristics
Seven eligible patients with advanced or recurrent uterine carcinosarcoma were treated with combination pembrolizumab and lenvatinib at the Cleveland Clinic. Patient demographics are displayed in Table 1. The median age at the start of immunotherapy was 63.0 years (IQR 58.0, 64.0 years). The majority of patient had stage III or IV disease (85.7%), an ECOG score of 0 or 1 (71.4%), and comorbid hypertension (57.1%). All patients had undergone cytoreductive surgery followed by adjuvant chemotherapy (42.9%) or adjuvant chemoradiation (57.1%). Pembrolizumab and lenvatinib was the third line of treatment for most included patients (71.4%), with zero instances of being used as a second-line therapy. Few tumors were known to be MMR deficient (14.3%) or PD-L1 positive (14.3%).
Table 1.
Patient and oncologic characteristics.
| Variable | N = 7 |
|---|---|
| Age | 63.0 [58.0, 64.0] |
| Race | |
| White | 3 (42.9) |
| Black | 4 (57.1) |
| BMI | 27.0 [19.0, 29.0] |
| ECOG Score | |
| 0 | 4 (57.1) |
| 1 | 1 (14.3) |
| 2 | 2 (28.6) |
| Medical Comorbidities | |
| HTN | 4 (57.1) |
| HLD | 3 (42.9) |
| DM | 2 (28.6) |
| VTE | 1 (14.3) |
| PVD | 1 (14.3) |
| Pulmonary Disease | 1 (14.3) |
| Renal Disease | 0 (0.00) |
| Stage | |
| I | 1 (14.3) |
| III | 4 (57.1) |
| IV | 2 (28.6) |
| Primary Cancer Treatment | |
| Surgery + Chemotherapy | 3 (42.9) |
| Surgery + Chemotherapy + Radiotherapy | 4 (57.1) |
| Number of Prior Lines of Therapy | |
| 2 | 5 (71.4) |
| 3 | 1 (14.3) |
| 4 | 1 (14.3) |
| Prior Bevacizumab | |
| Yes | 2 (28.6) |
| No | 5 (71.4) |
| MMR Status | |
| MMR proficient | 6 (85.7) |
| MMR deficient | 1 (14.3) |
| PD-L1 Status | |
| Positive | 1 (14.3) |
| Negative | 6 (85.7) |
BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; HTN, hypertension; HLD, hyperlipidemia; DM, diabetes mellitus; VTE, venous thromboembolic disease; PVD, peripheral vascular disease; CKD, chronic kidney disease.
Statistics presented as Median [P25, P75], N (column %).
3.2. Oncologic outcomes
Starting lenvatinib dose ranged from 10 mg to 20 mg, with a starting mean dose of 16 mg/day (standard deviation, 4.0). Only two of seven patients (28.6%) required lenvatinib dose reductions for treatment-related AEs, with a final mean dose of 14.3 mg/day (standard deviation, 5.3). Treatment-related AEs requiring dose reduction in this series included poor appetite leading to hospitalization for failure to thrive in one patient, and plantar-palmar erythrodysesthesia syndrome and mucositis in the other. No patients discontinued treatment due to treatment-related toxicity.
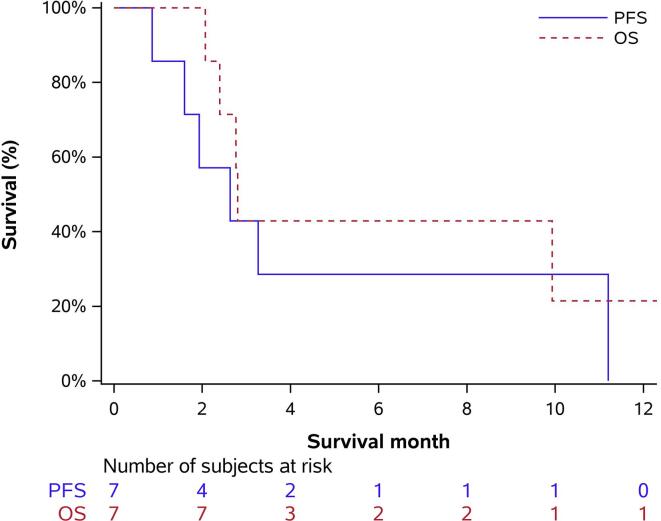
The median follow-up duration for the cohort was 2.8 months (IQR 2.4, 9.9 months). Imaging was typically performed every 3 cycles while receiving pembrolizumab and lenvatinib combination therapy, unless otherwise clinically indicated. No complete or partial responses were observed. Disease progression was noted in 5 patients (71.4%), while 1 patient achieved stable disease followed by progression (14.3%) and the other achieved stable disease without progression (14.3%). PFS was 2.6 months (95% CI, 0.9–11.2 months) with 6-month progression free survival of 28.6% (95% CI, 0.0, 62.0). Furthermore, OS was 2.8 months (95% CI, 2.4-NE) with 6-month overall survival of 42.9% (6.2, 79.5) (Table 2, Fig. 1).
Table 2.
Oncologic outcomes.
| Variable | N = 7 |
|---|---|
| Cycles of Immunotherapy | |
| 2 | 2 (28.6) |
| 3 | 1 (14.3) |
| 4 | 2 (28.6) |
| 6 | 1 (14.3) |
| 16 | 1 (14.3) |
| Reason for Stopping Immunotherapy* | |
| Progression | 6 (100.0) |
| Response (iRECIST) | |
| Progression | 5 (71.4) |
| Stable disease followed by progression | 1 (14.3) |
| Stable disease | 1 (14.3) |
| Lenvatinib Starting Dose | |
| 20 mg | 3 (42.9) |
| 14 mg | 3 (42.9) |
| 10 mg | 1 (14.3) |
| Lenvatinib Dose Reductions | |
| Yes | 2 (28.6) |
| No | 5 (71.4) |
| Steroids During Immunotherapy | |
| Yes | 2 (28.6) |
| No | 5 (71.4) |
| Current Status | |
| Alive with disease | 2 (28.6) |
| Dead of disease | 5 (71.4) |
| Currently on Immunotherapy | |
| Yes | 1 (14.3) |
| No | 6 (85.7) |
| Follow-up Duration (months) | 2.8 [2.4, 9.9] |
iRECIST, immunotherapy response evaluation criteria in solid tumors.
Statistics presented as Median [P25, P75], N (column %).
*Data not available for all subjects. Patients stopped immunotherapy N = 6.
Fig. 1.
Kaplan-Meier plot for progression-free survival and overall survival of patients with advanced or recurrent uterine carcinosarcoma receiving pembrolizumab and lenvatinib combination therapy.
4. Discussion
The prognosis of women diagnosed with UCS is quite dismal, with a median overall survival of 23 months, which is significantly worse than women diagnosed with other EC histologies including high-grade endometrioid, serous, or clear cell carcinomas (Zhang et al., 2015, Matsuzaki et al., 2021). The standard management according to National Comprehensive Cancer Network (NCCN) guidelines in women with UCS is surgical management followed by a combination of adjuvant platinum-based chemotherapy with or without radiotherapy (NCCN Clinical Practice Guidelines in Oncology, n.d, Matsuo et al., 2017). Unfortunately, despite upfront multi-modality therapy, even women with early-stage UCS have high risk for recurrence and death from their disease (Matsuzaki et al., 2021). There is a significant, unmet need to advance therapeutic options for women with recurrent uterine carcinosarcoma (see Table 3).
Table 3.
Patient details.
| ID | Stage | MMR | PDL1 | Prior lines | # Cycles | iRECIST | PFS (m) | OS (m) |
|---|---|---|---|---|---|---|---|---|
| 1 | IV | MMRp | PDL1- | 2 | 4 | Progression | 3.3 | Dead of disease at 9.9 months |
| 2 | III | MMRp | PDL1- | 2 | 2 | Progression | 0.9 | Dead of disease at 2.8 months |
| 3 | IV | MMRd | PDL1+ | 2 | 2 | Progression | 1.6 | Dead of disease at 2.4 months |
| 4 | III | MMRp | PDL1- | 2 | 4 | Progression | 2.6 | Dead of disease at 2.8 months |
| 5 | III | MMRp | PDL1- | 4 | 3 | Progression | 1.9 | Dead of disease at 2.1 months |
| 6 | III | MMRp | PDL1- | 3 | 6 | Stable | – | Alive with disease at 4.4 months |
| 7 | I | MMRp | PDL1- | 2 | 16 | Stable then progression | 11.2 | Alive with disease at 12.6 months |
MMRp, mismatch repair proficient; MMRd, mismatch repair deficient; PFS, progression-free survival; OS, overall survival.
The KEYNOTE-146 study was a promising turn of events in the treatment of recurrent EC, with pembrolizumab and lenvatinib combination therapy generating an overall response rate of 38.0% (Makker et al., 2020). Notably, women with UCS were not included in this trial. Given the similarities of UCS with other EC high-risk histologies, pembrolizumab and lenvatinib was considered as a novel approach for improving outcomes in the previously treated UCS population, but data was limited to inform its efficacy. To this end, in this small, retrospective series of women with advanced or recurrent UCS, we demonstrate that pembrolizumab and lenvatinib combination therapy has limited efficacy with no complete or partial responses, and a 28.6% rate of stable disease.
In our cohort of women with advanced or recurrent UCS who underwent pembrolizumab and lenvatinib combination therapy, the median PFS and OS were 2.6 months and 2.8 months, respectively. In a pooled analysis of studies by Matsuzaki et al. of women undergoing salvage chemotherapy for uterine carcinosarcoma, second-line and later-line therapies demonstrated a median response rate of only 5.5% and median PFS of approximately 2 months (Matsuzaki et al., 2021). Our findings suggest a similar PFS compared to prior historical studies, and no objective radiographic response among a series of seven patients.
Interestingly, PD-L1 status was negative in the majority of patients (85.7%). Given recent studies demonstrating PD-L1 expression in 58% of UCS, this cohort of patients does not follow the expected distribution of PD-L1 status, and therefore these findings may not be generalizable to all patients (Engerud et al., 2020). The majority of patients were also MMR-proficient (85.7%). Further study is needed to understand how UCS response to pembrolizumab and lenvatinib combination therapy is impacted by the tumor’s immunohistochemical signature.
An important consideration with pembrolizumab and lenvatinib combination therapy is patient tolerability and toxicity. The KEYNOTE-146 study reported a 62.9% rate of dose reductions of lenvatinib for treatment-related adverse effects (AEs) with a mean dose intensity of lenvatinib of 14.4 mg/day (standard deviation, 4.3) (Makker et al., 2020). In contrast, in our series, only two of seven patients (28.6%) required dose reductions for treatment-related AEs, with a final mean dose of lenvatinib of 14.3 mg/day (standard deviation, 5.3). No patients discontinued treatment due to treatment-related toxicity, although this must be interpreted in the context of four of seven patients (57.1%) dying within three months of treatment initiation. Although five of seven patients discontinued therapy within four cycles (71.4%), recent studies show that all-grade immune-related adverse events present within 8.4 weeks of initiation of PD-1 and PD-L1 inhibitors, and within the first 2 months of initiation of lenvatinib therapy (Haddad et al., 2017, Tang et al., 2021). Toxicity appears not to bias the response rate of this case series more than that seen in larger trials. Despite this, it is essential that patients are adequately counselled regarding the side effect profile of this regimen, in light of the poor efficacy, prior to starting treatment.
There are several significant limitations to consider in the interpretation of our results. Primarily, our findings are limited by the small sample size, including only seven patients with previously treated UCS, without a control cohort who received an alternative line of therapy. Similarly, given the retrospective nature, all prior treatment lines were at the discretion of the primary gynecologic oncologist, as well as the starting dose of lenvatinib and the approach taken towards dose reduction and steroid administration. Notably, in the KEYNOTE-146 study, 51.1% patients received only one prior line of treatment, 38.3% patients received two prior lines of treatment, and 10.6% patients received three or more prior lines of treatment (Makker et al., 2020). Our cohort of women included 0.0% with only one prior line of treatment, and 28.6% with three or more prior lines of treatment, representative of a more heavily-pretreated population. Despite a higher number of prior lines, only two of the seven patients (28.6%) had previously received bevacizumab. Such a trend in number of prior lines of treatment shifts the focus towards more aggressive or refractory tumor biologies, and therefore introduces a bias towards a worse response rate and PFS. Further study is needed to determine whether this regimen would be more active in patients earlier in their disease recurrence.
Despite these limitations, our study is of the first to report the limited activity of pembrolizumab and lenvatinib combination therapy in women with advanced and recurrent UCS and contributes relevant, timely information to the literature. Our single institution, retrospective data suggest that pembrolizumab and lenvatinib combination therapy is not highly active in UCS and is associated with similar PFS and OS as similar traditional cytotoxic regimens. Further study is warranted to assess the efficacy of this regimen in more targeted cohorts of women with advanced or recurrent UCS.
Author contribution
JH, LC, and PR conceived of the idea and designed the study. MY performed the computations. AJP obtained and evaluated archived tumor for PD-L1 and MMR status. JH wrote the manuscript with support from LC, RD, PR.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
None.
References
- Brooks S.E., Zhan M., Cote T., Baquet C.R. Surveillance, epidemiology, and end results analysis of 2677 cases of uterine sarcoma 1989–1999. Gynecol. Oncol. 2004;93:204–208. doi: 10.1016/j.ygyno.2003.12.029. [DOI] [PubMed] [Google Scholar]
- Cherniack A.D., Shen H., Walter V., Stewart C., Murray B.A., Bowlby R., Hu X., Ling S., Soslow R.A., Broaddus R.R., Zuna R.E., Robertson G., Laird P.W., Kucherlapati R., Mills G.B., Akbani R., Ally A., Auman J.T., Balasundaram M., Balu S., Baylin S.B., Beroukhim R., Bodenheimer T., Bogomolniy F., Boice L., Bootwalla M.S., Bowen J., Broaddus R., Brooks D., Carlsen R., Cho J., Chuah E., Chudamani S., Cibulskis K., Cline M., Dao F., David M., Demchok J.A., Dhalla N., Dowdy S., Felau I., Ferguson M.L., Frazer S., Frick J., Gabriel S., Gastier-Foster J.M., Gehlenborg N., Gerken M., Getz G., Gupta M., Haussler D., Hayes D.N., Heiman D.I., Hess J., Hoadley K.A., Hoffmann R., Holt R.A., Hoyle A.P., Huang M., Hutter C.M., Jefferys S.R., Jones S.J.M., Jones C.D., Kanchi R.S., Kandoth C., Kasaian K., Kerr S., Kim J., Lai P.H., Lander E., Lawrence M.S., Lee D., Leraas K.M., Leshchiner I., Levine D.A., Lichtenberg T.M., Lin P., Liu J., Liu W., Liu Y., Lolla L., Lu Y., Ma Y., Maglinte D.T., Marra M.A., Mayo M., Meng S., Meyerson M., Mieczkowski P.A., Moore R.A., Mose L.E., Mungall A.J., Mungall K., Naresh R., Noble M.S., Olvera N., Parker J.S., Perou C.M., Perou A.H., Pihl T., Radenbaugh A.J., Ramirez N.C., Rathmell W.K., Roach J., Robertson A.G., Sadeghi S., Saksena G., Salvesen H.B., Schein J.E., Schumacher S.E., Sheth M., Shi Y., Shih J., Simons J.V., Sipahimalani P., Skelly T., Sofia H.J., Soloway M.G., Sougnez C., Sun C., Tam A., Tan D., Tarnuzzer R., Thiessen N., Thorne L.B., Tse K., Tseng J., Van Den Berg D.J., Veluvolu U., Verhaak R.G.W., Voet D., von Bismarck A., Wan Y., Wang Z., Wang C., Weinstein J.N., Weisenberger D.J., Wilkerson M.D., Winterhoff B., Wise L., Wong T., Wu Y., Yang L., Zenklusen J.C., (Julia) Zhang J., Zhang H., Zhang W., chun Zhu J., Zmuda E., Zhang J. Integrated molecular characterization of uterine carcinosarcoma. Cancer Cell. 2017;31:411–423. doi: 10.1016/j.ccell.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimbaluk D., Rotmensch J., Scudiere J., Gown A., Bitterman P. Uterine carcinosarcoma: immunohistochemical studies on tissue microarrays with focus on potential therapeutic targets. Gynecol. Oncol. 2007;105:138–144. doi: 10.1016/j.ygyno.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Emoto M., Charnock-Jones D.S., Licence D.R., Ishiguro M., Kawai M., Yanaihara A., Saito T., Hachisuga T., Iwasaki H., Kawarabayashi T., Smith S.K. Localization of the VEGF and angiopoietin genes in uterine carcinosarcoma. Gynecol. Oncol. 2004;95:474–482. doi: 10.1016/j.ygyno.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Engerud H., Berg H.F., Myrvold M., Halle M.K., Bjorge L., Haldorsen I.S., Hoivik E.A., Trovik J., Krakstad C. High degree of heterogeneity of PD-L1 and PD-1 from primary to metastatic endometrial cancer. Gynecol. Oncol. 2020;157:260–267. doi: 10.1016/j.ygyno.2020.01.020. [DOI] [PubMed] [Google Scholar]
- Gotoh O., Sugiyama Y., Takazawa Y., Kato K., Tanaka N., Omatsu K., Takeshima N., Nomura H., Hasegawa K., Fujiwara K., Taki M., Matsumura N., Noda T., Mori S. Clinically relevant molecular subtypes and genomic alteration-independent differentiation in gynecologic carcinosarcoma. Nat. Commun. 2019;10 doi: 10.1038/s41467-019-12985-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad R.I., Schlumberger M., Wirth L.J., Sherman E.J., Shah M.H., Robinson B., Dutcus C.E., Teng A., Gianoukakis A.G., Sherman S.I. Incidence and timing of common adverse events in Lenvatinib-treated patients from the SELECT trial and their association with survival outcomes. Endocrine. 2017;56:121–128. doi: 10.1007/s12020-017-1233-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harano K., Hirakawa A., Yunokawa M., Nakamura T., Satoh T., Nishikawa T., Aoki D., Ito K., Ito K., Nakanishi T., Susumu N., Takehara K., Watanabe Y., Watari H., Saito T. Optimal cytoreductive surgery in patients with advanced uterine carcinosarcoma: a multi-institutional retrospective study from the Japanese gynecologic oncology group. Gynecol. Oncol. 2016;141:447–453. doi: 10.1016/j.ygyno.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P.A., Taylor R., Minor B.L., Elliott V., Fernandez M., O’Neal L., McLeod L., Delacqua G., Delacqua F., Kirby J., Duda S.N. The REDCap consortium: building an international community of software platform partners. J. Biomed. Inform. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livasy C.A., Reading F.C., Moore D.T., Boggess J.F., Lininger R.A. EGFR expression and HER2/neu overexpression/amplification in endometrial carcinosarcoma. Gynecol. Oncol. 2006;100:101–106. doi: 10.1016/j.ygyno.2005.07.124. [DOI] [PubMed] [Google Scholar]
- Makker V., Taylor M.H., Aghajanian C., Oaknin A., Mier J., Cohn A.L., Romeo M., Bratos R., Brose M.S., DiSimone C., Messing M., Stepan D.E., Dutcus C.E., Wu J., Schmidt E.V., Orlowski R., Sachdev P., Shumaker R., Herraez A.C. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J. Clin. Oncol. 2020;38:2981–2992. doi: 10.1200/JCO.19.02627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo K., Takazawa Y., Ross M.S., Elishaev E., Podzielinski I., Yunokawa M., Sheridan T.B., Bush S.H., Klobocista M.M., Blake E.A., Takano T., Matsuzaki S., Baba T., Satoh S., Shida M., Nishikawa T., Ikeda Y., Adachi S., Yokoyama T., Takekuma M., Fujiwara K., Hazama Y., Kadogami D., Moffitt M.N., Takeuchi S., Nishimura M., Iwasaki K., Ushioda N., Johnson M.S., Yoshida M., Hakam A., Li S.W., Richmond A.M., Machida H., Mhawech-Fauceglia P., Ueda Y., Yoshino K., Yamaguchi K., Oishi T., Kajiwara H., Hasegawa K., Yasuda M., Kawana K., Suda K., Miyake T.M., Moriya T., Yuba Y., Morgan T., Fukagawa T., Wakatsuki A., Sugiyama T., Pejovic T., Nagano T., Shimoya K., Andoh M., Shiki Y., Enomoto T., Sasaki T., Fujiwara K., Mikami M., Shimada M., Konishi I., Kimura T., Post M.D., Shahzad M.M., Im D.D., Yoshida H., Omatsu K., Ueland F.R., Kelley J.L., Karabakhtsian R.G., Roman L.D. Significance of histologic pattern of carcinoma and sarcoma components on survival outcomes of uterine carcinosarcoma. Ann. Oncol. 2016;27:1257–1266. doi: 10.1093/annonc/mdw161. [DOI] [PubMed] [Google Scholar]
- Matsuo K., Machida H., Ragab O.M., Takiuchi T., Pham H.Q., Roman L.D. Extent of pelvic lymphadenectomy and use of adjuvant vaginal brachytherapy for early-stage endometrial cancer. Gynecol. Oncol. 2017;144:515–523. doi: 10.1016/j.ygyno.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki S., Klar M., Matsuzaki S., Roman L.D., Sood A.K., Matsuo K. Uterine carcinosarcoma: contemporary clinical summary, molecular updates, and future research opportunity. Gynecol. Oncol. 2021;160:586–601. doi: 10.1016/j.ygyno.2020.10.043. [DOI] [PubMed] [Google Scholar]
- Menczer J., Kravtsov V., Levy T., Berger E., Glezerman M., Avinoach I. Expression of c-kit in uterine carcinosarcoma. Gynecol. Oncol. 2005;96:210–215. doi: 10.1016/j.ygyno.2004.09.045. [DOI] [PubMed] [Google Scholar]
- NCCN Clinical Practice Guidelines in Oncology, n.d. https://www.nccn.org/professionals/physician_gls/default.aspx (accessed March 24, 2021).
- Powell M.A., Filiaci V.L., Hensley M.L., Huang H.Q., Moore K.N., Tewari K.S., Copeland L.J., Secord A.A., Mutch D.G., Santin A., Richards W., Warshal D.P., Spirtos N.M., Disilverstro P., Ioffe O., Miller D.S. A randomized phase 3 trial of paclitaxel (P) plus carboplatin (C) versus paclitaxel plus ifosfamide (I) in chemotherapy-naive patients with stage I-IV, persistent or recurrent carcinosarcoma of the uterus or ovary: an NRG Oncology trial. J. Clin. Oncol. 2019;37:5500. doi: 10.1200/jco.2019.37.15_suppl.5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour L., Bogaerts J., Perrone A., Ford R., Schwartz L.H., Mandrekar S., Lin N.U., Litière S., Dancey J., Chen A., Hodi F.S., Therasse P., Hoekstra O.S., Shankar L.K., Wolchok J.D., Ballinger M., Caramella C., de Vries E.G.E. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18:e143–e152. doi: 10.1016/S1470-2045(17)30074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R., Ma J., Zou Z., Jemal A. Cancer statistics, 2014, CA. Cancer J. Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- Tang S.Q., Tang L.L., Mao Y.P., Li W.F., Chen L., Zhang Y., Guo Y., Liu Q., Sun Y., Xu C., Ma J. The pattern of time to onset and resolution of immune-related adverse events caused by immune checkpoint inhibitors in cancer: a pooled analysis of 23 clinical trials and 8,436 patients. Cancer Res. Treat. 2021;53:339–354. doi: 10.4143/CRT.2020.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale S.G., Laganà A.S., Capriglione S., Angioli R., Lucia V., Rosa L., Lopez S., Valenti G., Sapia F., Sarpietro G., Butticè S., Tuscano C., Fanale D., Tropea A., Rossetti D. Target therapies for uterine carcinosarcomas: current evidence and future perspectives. Mdpi. Com. 2017 doi: 10.3390/ijms18051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Hu W., Jia N., Li Q., Hua K., Tao X., Wang L., Feng W. Uterine carcinosarcoma and high-risk endometrial carcinomas. Int. J. Gynecol. Cancer. 2015;25:629–636. doi: 10.1097/IGC.0000000000000350. [DOI] [PubMed] [Google Scholar]
- Zhao S., Bellone S., Lopez S., Thakral D., Schwab C., English D.P., Black J., Cocco E., Choi J., Zammataro L., Predolini F., Bonazzoli E., Bi M., Buza N., Hui P., Wong S., Abu-Khalaf M., Ravaggi A., Bignotti E., Bandiera E., Romani C., Todeschini P., Tassi R., Zanotti L., Odicino F., Pecorelli S., Donzelli C., Ardighieri L., Facchetti F., Falchetti M., Silasi D.A., Ratner E., Azodi M., Schwartz P.E., Mane S., Angioli R., Terranova C., Quick C.M., Edraki B., Bilgüvar K., Lee M., Choi M., Stiegler A.L., Boggon T.J., Schlessinger J., Lifton R.P., Santin A.D. Mutational landscape of uterine and ovarian carcinosarcomas implicates histone genes in epithelial-mesenchymal transition. Proc. Natl. Acad. Sci. USA. 2016;113:12238–12243. doi: 10.1073/pnas.1614120113. [DOI] [PMC free article] [PubMed] [Google Scholar]