Abstract
Several intracellular pathways that contribute to the adaptation or maladaptation to environmental challenges mediate the vulnerability and resilience to chronic stress. The activity of the hypothalamic-pituitary-adrenal (HPA) axis is fundamental for the proper maintenance of brain processes, and it is related to the functionality of the isoform alfa and beta of the glucocorticoid receptor (Gr), the primary regulator of HPA axis. Among the downstream effectors of the axis, the scaffolding protein RACK1 covers an important role in regulating synaptic activity and mediates the transcription of the neurotrophin Bdnf. Hence, by employing the chronic mild stress (CMS) paradigm, we studied the role of the Grβ-RACK1-Bdnf signaling in the different susceptibility to chronic stress exposure. We found that resilience to two weeks of CMS is paralleled by the activation of this pathway in the ventral hippocampus, the hippocampal subregion involved in the modulation of stress response. Moreover, the results we obtained in vitro by exposing SH-SY5Y cells to cortisol support the data we found in vivo. The results obtained add novel critical information about the link among Gr, RACK1 and Bdnf and the resilience to chronic stress, suggesting novel targets for the treatment of stress-related disorders, including depression.
Keywords: Stress, Glucocorticoid receptor, Rack1, Bdnf, Resilience
Graphical abstract
Highlights
-
•
Stress susceptibility is associated with an alteration of the HPA axis functionality.
-
•
GRβ-RACK1-BDNF signaling is differently modulated in vulnerable and resilient animals.
-
•
RACK1 may represent a promising target to promote resilience.
1. Introduction
From a biological point of view, stress has been defined as the result of an organism's failed attempt to respond appropriately to a physical challenge (Selye, 1998) whereas the traditional psychology definition indicates that stress occurs when a person perceives the demands of environmental stimuli to be greater than his/her ability to meet, mitigate or alter those demands (Lazarus et al., 1985). Accordingly, prolonged exposure to stressful events can alter the mechanisms that regulate normal homeostasis thus altering brain functions and leading to the development of stress-related disorders, including depression (McEwen et al., 2015).
A hallmark of stress response is the activation of the hypothalamic-pituitary-adrenal (HPA) axis, which produces adaptation to external stimuli through the so called “allostasis”. The activity of such axis in the brain is regulated by mineralocorticoid (Mr) and glucocorticoid receptors (Gr) with Gr having tenfold lower affinities for glucocorticoids (GCs) compared to Mrs; as consequence Grs are engaged when GCs levels increase, for example after stress. The human GR gene (NR3C1) is formed by nine exons (Hollenberg et al., 1985) and it has been shown that alternative splicing of the exon 9 leads to the formation of the Grα and Grβ isoforms (Oakley et al., 1996). The two isoforms differ in their carboxyl terminus, with Grβ lacking the ligand-binding domain. The general assumption was that this isoform is unable to modulate the transcription of the glucocorticoid responsive genes (Oakley et al., 1996) and that it mainly acts as a dominant-negative regulator of Grα, by affecting the transcriptional activity mediated by the Grα isoform (Bamberger et al., 1995; Kino et al., 2009b; Oakley et al., 1996). However, subsequent studies have demonstrated that the action of Grβ is not limited to the negative regulation of Grα, but it has also a proper intrinsic activity as direct controller of a plethora of genes involved in several intracellular pathways related, for example, to metabolism, inflammation, apoptosis and cell migration (Kino et al, 2009a, 2009b; Ramos-Ramírez and Tliba, 2021).
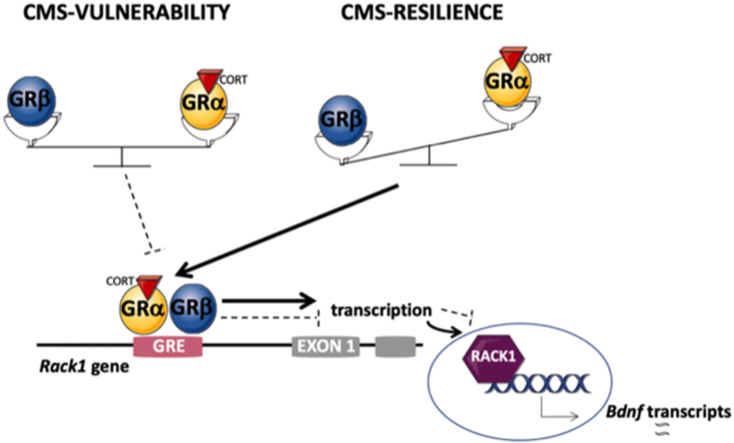
Among the genes containing in their promoter region the glucocorticoid responsive element (GRE), we focus on the receptor for activated C kinase 1 (RACK1) that responds to circulating GCs. Indeed, its transcription is inhibited by Grα in presence of elevated levels of cortisol, whereas the Grβ isoform, being the dominant-negative regulator of Grα, favors the induction of Rack1 gene expression (Buoso et al., 2017a; Racchi et al., 2017).
RACK1 has been first described as a scaffolding protein that shuttles protein kinase C (PKC) to its substrates; this action (Ron et al., 1999) is critical for nervous system development (Kershner and Welshhans, 2017; Wehner et al., 2011) as well as for proper brain functions (McGough, 2004; Sklan et al., 2006). Moreover, it has been linked with the expression of Brain derived neurotrophic factor (Bdnf) (Neasta et al., 2012), one of the major mediators of neuroplasticity (Calabrese et al., 2009). Accordingly, Rack1 silencing decreases BDNF expression both in SH-SY5Y cells and in hippocampal neurons thus highlighting that RACK1 is required for BDNF expression (He et al., 2010; Neasta et al., 2012).
Given the role of RACK1 as downstream effector of the HPA axis as well as a mediator of synaptic plasticity (Del Vecchio et al., 2009; Yaka et al., 2002; Yang et al., 2019) here we employed the chronic mild stress paradigm (CMS) to investigate whether the impact of stress on neuroplasticity occurs through the regulation of the RACK1-BDNF pathway.
The CMS paradigm is a well-established animal model to study depression in rodents (Willner, 2017). In fact, it has been associated with the development of depressive-like symptoms, including anhedonia and cognitive deficits (Brivio et al., 2021; Calabrese et al, 2017a, 2020) but also with alteration of GR activity (Brivio et al., 2021; Calabrese et al., 2020) and synaptic plasticity (Calabrese et al., 2017a; Luoni et al., 2015). Of note, the sucrose consumption test has been extensively employed in CMS protocols (Papp, 2012; Willner, 2017) to dissect two different subgroups of rats developing, or not, the anhedonic-like behaviour, thus determining two populations that are vulnerable or resilient, respectively, to chronic stress. Accordingly, by testing the animals in this task we had the possibility to investigate the Grβ-RACK1 pathway in the two subpopulations of stressed rats. The in vivo analysis was paralleled by in vitro studies employing SH-SY5Y cell exposed to cortisol to further dissect the mechanisms altered following a stressful condition. The in vivo analyses were conducted in the ventral subregion of hippocampus, known to be responsible for emotional behavior (Fanselow and Dong, 2010), stress vulnerability and resilience (Anacker et al., 2016) and involved in the regulation of the HPA axis (Anacker et al., 2011). Our results support the contribution of Grβ-RACK1-BDNF pathway in promoting resilience to chronic stress.
2. Material and methods
2.1. Chemicals, culture media and supplements
Cortisol (PubChem CID:5754) and mifepristone (PubChem CID:55245) were obtained from Sigma Aldrich (St. Louis, MO, USA). They were dissolved in DMSO at concentration of 1 mM and 10 mM and frozen (−20 °C) in stock aliquots. Stock aliquots were diluted at a final concentration in culture media at the time of use (final concentration of DMSO in culture medium <0.1 %). Cell culture media and all supplements were obtained from Sigma Aldrich. Anti-RACK1 (sc17754) and anti-SRp30c (sc-134036) were obtained from Santa Cruz Biotechnology. Primers and probes of Grα, Grβ, Rack1, total Bdnf and 18S were purchased by Eurofins genomics whereas Bdnf long 3′UTR and Bdnf isoform IV were bought by Life technologies Italia. Anti-BDNF (SAB 1405514) and anti β-tubulin (T0198) were purchased from Sigma Aldrich. Anti lamin A/C (612162) was obtained from BD Biosciences (Franklin Lakes, NJ, USA). Host-specific peroxidase-conjugated IgG secondary antibodies were purchased from Thermo Scientific Inc. (Waltham,MA, USA). Electrophoresis reagents were purchased from Bio-Rad (Richmond, CA, USA).
2.2. Cell cultures and treatments
Human neuroblastoma SH-SY5Y cells from the European Collection of Cell Cultures (ECACC No. 94030304) were cultured in a medium with equal amounts of Eagle's minimum essential medium and Nutrient Mixture Ham's F-12, supplemented with 10 % heat-inactivated fetal bovine serum (FBS), 2 mM glutamine, 0.1 mg/ml streptomycin, 100 IU/ml penicillin and 1X MEM non-essential amino acid solution (M7145, Merck) at 37 °C in 5 % CO2-containing, and 95 % air atmosphere. SH-SY5Y cells were treated for different times with increasing concentrations of cortisol (0.1, 0.5, 1 and 5 μM) according to previous data (Buoso et al., 2011; Del Vecchio et al., 2009). To demonstrate the role of glucocorticoid receptor, cells were treated for 1 h with 10 μM mifepristone before the addition of cortisol for 24 h. Other specific details of times and concentrations are also given in figure legends.
2.3. Animals
Adult male (post natal day 70) Wistar rats (Charles River, Germany) were adapted, housed in groups of 10, to the animal facilities one month before the start of the experiment. During the whole experiment animals were singly housed in standard conditions (12-h light/dark, constant temperature (22 ± 2 °C) and humidity (50 ± 5 %)). All procedures used in this study have conformed to the rules and principles of the 86/609/EEC Directive and have been approved by the Local Bioethical Committee at the Institute of Pharmacology, Polish Academy of Sciences, Krakow, Poland. All efforts were made to minimize animal suffering, to reduce the number of animals used and the animal studies comply with the ARRIVE guidelines.
2.4. Stress procedure and behavioral test
Before starting of the experiment, rats were trained to consume 1 % sucrose solution as previously described (Calabrese et al., 2020) and sucrose consumption test (SCT) (one bottle test) was performed at weekly intervals throughout the duration of the study. Sucrose intake was calculated by measuring pre-weighted bottles filled with the 1 % sucrose solution. The cut off to discriminate among vulnerable and resilient animals was set at 50 %: vulnerable rats consumed approximately 50 % less sucrose solution in comparison to the baseline (training values), whereas resilient animals showed similar levels of sucrose intake to their baseline, despite the CMS procedure.
Animals were randomly divided into two groups: one group of animals was unstressed (No stress group) and the other one was exposed to the CMS procedure for a period of 2 consecutive weeks. The protocol consists of periods of: food or water deprivation, 45-degree cage tilt, intermittent illumination, soiled cage, paired housing, low intensity stroboscopic illumination, periods of no stress (see (Calabrese et al., 2017) for details). Based on the result of the sucrose consumption test carried out following the first 2 weeks of stress, two groups of animals were identified: those showing a significative reduction of sucrose intake in comparison to the control group, ie. developing an anhedonic-like behavior, (CMS vulnerable) and those that did not develop anhedonia (CMS resilient).
24 h after the final stressor animals were decapitated (between 9:10 and 12:25 a.m.), the trunk blood was collected in tubes with ethylenediaminetetraacetic acid and the ventral hippocampus (vHip) was dissected from the whole brain according to the plates 34–43 of the atlas of Paxinos and Watson (2004) and stored at −80 °C.
2.5. Measurement of corticosterone plasma levels
After collection, blood specimens were centrifuged for 20 min at 3000 g at 4 °C for the separation of plasma. Corticosterone plasma levels were assessed with the IBL international enzyme linked immunosorbent assay according to manufacturers’ instructions.
2.6. RNA preparation and gene expression analysis by quantitative real-time PCR
Total RNA was isolated using the PureZol RNA isolation reagent (Bio-Rad Laboratories, Italy) and quantified by spectrophotometric analysis as previously described (Brivio et al., 2020). Samples were treated with DNase (ThermoFisher scientific, Italy) to avoid DNA contamination. Real-time polymerase chain reaction (RT-PCR) was performed to assess Rack1, total Bdnf, Bdnf long 3′UTR and Bdnf isoform IV mRNA levels. RNA was analyzed by TaqMan qRT-PCR instrument (CFX384 real time system, Bio-Rad Laboratories, Italy) using the iScriptTM one-step RT-PCR kit for probes (Bio-Rad Laboratories, Italy) (see (Brivio et al., 2020) for details). Samples were run in 384 well formats in triplicate as multiplexed reactions with the normalizing internal control 18s. Primers and probes sequences of the mouse genes analyzed were listed in Table 1.
Table 1.
a) Sequences of forward and reverse primers and probes purchased from Eurofins MWG-Operon. b) Probes purchased from Life Technologies.
| a) Gene | Forward primer | Reverse primer | Probe |
|---|---|---|---|
| Grα | GCGACAGAAGCAGTTGAGTCATC | CCATGCCTCCACGTAACTGTTAG | TGAAGTGATAGCACAGCAGACAGTGA |
| Grβ | GCGCTTGAGGCTAAGATAGCT | CCCATGTTTCTGCCTCTTTCTTTG | AGTCTGCCTTCAGAATGCCTGTCA |
| Rack1 | CTCTTTGGCTTGGTCTGCTG | ATACACGCACCAAGTTGTCG | ATGGCCAGACTCTGTTTGCT |
| Total Bdnf | AAGTCTGCATTACATTCCTCGA | GTTTTCTGAAAGAGGGACAGTTTAT | TGTGGTTTGTTGCCGTTGCCAAG |
| 18s | GTAACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGCG | TGCAATTATTCCCCATGAACGAGG |
| b) Gene | Accession number | Assay ID | |
| Bdnf long 3′UTR | EF125675 | Rn02531967_s1 | |
| Bdnf IV | EF125679 | Rn01484927_m1 |
qPCR for human RACK1 and 18S were performed as indicated in (Buoso et al, 2017a, 2020).
Transcript quantification was performed with 2(−ΔΔCT) method.
2.7. Subcellular fractionation
Western blot analysis was used to investigate RACK1 in the subcellular fractions. In brief, 3.5 × 106 SH-SY5Y cells were seeded in 100 mm dishes and treated for 24 h with 1 μM or 5 μM cortisol and cellular fractionation conducted as described in detail in refs (Buoso et al, 2012, 2013).
Ventral hippocampi were manually homogenized using a glass-glass potter in a pH 7.4 cold buffer containing 0.32 M sucrose, 0.1 mM EGTA, 1 mM HEPES solution in the presence of a complete set of proteases (Roche) and phosphatases (Sigma-Aldrich) inhibitors. The total homogenates were centrifuged at 1000 g for 10 min at 4 °C to obtain a pellet enriched in nuclear components, which were suspended in a buffer (20 mM HEPES, 0.1 mM dithiothreitol (DTT), 0.1 mM EGTA) containing proteases and phosphatases inhibitors. Supernatants obtained were further centrifuged at 10000 g for 15 min at 4 °C to obtain the pellet corresponding to the membrane fractions that was re-suspended in the same buffer prepared for the nuclear fractions. The purity of the fractions obtained was previously detailed (Brivio et al., 2019). Total protein content was measured according to the Bradford Protein Assay procedure (Bio-Rad Laboratories), using bovine serum albumin (BSA) as a calibration standard.
2.8. Protein extraction and Western blot analysis
The expression of RACK1, mBDNF and β-tubulin in cell homogenates and in the subcellular fractions obtained by vHip fractionation were assessed by Western blot analysis. Briefly, cells were treated and then collected, washed twice with PBS 1X, centrifuged, and lysed in 100 μL of homogenization buffer (50 mM Tris−HCl pH 7.5, 150 mM NaCl, 5 mM EDTA, 0.5 % Triton X-100 and protease inhibitor mix). The protein content was measured using the Bradford method using BSA as a calibration standard. Western blotting samples were prepared mixing the cell lysate with sample buffer (125 mM Tris−HCl pH 6, 8.4 % SDS, 20 % glycerol, 6 % β-mercaptoethanol, 0.1 % bromophenol) and denaturing at 95 °C for 5 min. Equivalent amounts of extracted protein (10 μg) were electrophoresed into an appropriate % SDS-PAGE under reducing conditions. The proteins were then transferred onto a nitrocellulose membrane (Amersham, Little Chalfont, UK) that was blocked in 5 % w/v BSA, 1X TBS, 0.1 % Tween-20 for 1 h with gentle shaking. The proteins were visualized using primary antibodies diluted in 5 % w/v BSA, 1X TBS, 0.1 % Tween-20 for RACK1 (1:1000), SRp30c (1:500), mBDNF (1:500) and β-tubulin (1:1000) as indicated in (Buoso et al, 2013, 2017a). In all experiments, immuno-reactivity was measured using host specific secondary IgG peroxidase conjugated antibodies (1:7000 diluted) and developed using enhanced chemiluminescence reagent (Pierce, Thermo Fisher Scientific, Rockford, IL, USA). For signal detection Amersham Imager 680 (GE, Healthcare) was used. After Western blot acquisition, bands optical analysis was performed with the ImageJ program (W. Rasband, Research Service Branch, National Institute of Mental Health, National Institutes of Health, Bethesda, MD and Laboratory for Optical and Computational Instrumentation, University of Wisconsin). Bands relative densities were expressed as arbitrary units and normalized over control sample run under the same conditions.
2.9. Statistical analysis
Each experimental group consists of 4–8 rats. In vitro data consist of at least three independent experiments. Data are presented as means ± SEM. Statistical analyses were conducted with GraphPad Prism version 7 (GraphPad Software, San Diego, CA, USA) and outliers were determined with GraphPad outlier calculator (alpha = 0.05). Statistical differences were determined by analysis of variance (one-way ANOVA) followed, when significant, by the Tukey multiple comparison post hoc test. In all reported statistical analyses, effects were designated as significant if the p value was <0.05.
3. Results
3.1. The anhedonic-like behavior induced by chronic stress was positively correlated with circulating corticosterone levels
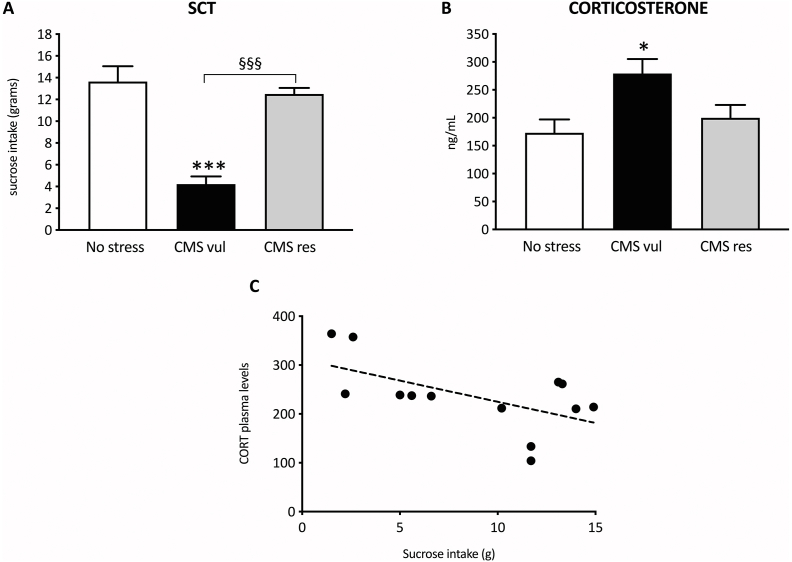
SCT has been employed to identify vulnerable and resilient rats to chronic stress exposure. As shown in Fig. 1A, we found a significative effect of stress in the one-way ANOVA analysis (F2-21 = 28, p < 0.001). A subpopulation of stressed animals was identified, from now on named vulnerable (CMS vul), showing an anhedonic-like phenotype, as indicated by the reduction of sucrose intake in comparison to unstressed animals (−69 %, p < 0.001 vs No stress). Conversely, another group of rats, from now on named resilient (CMS res), showed a similar sucrose intake to unstressed animals, with an increased sucrose intake with respect to the vulnerable animals (+195 %, p < 0.001 vs CMS vul).
Fig. 1.
Analysis of the sucrose consumption test (A) and corticosterone plasma levels (B) in vulnerable and resilient animals exposed to two weeks of CMS. Panel C shows a correlation analysis between sucrose intake and corticosterone plasma levels in CMS vul and CMS res animals (Pearson's product-moment correlation). *p < 0.05, ***p < 0.001 vs No stress; §§§p < 0.001 vs CMS vul (one-way ANOVA with Tukey's multiple comparison test).
Similarly, one-way ANOVA revealed a significant effect of stress on plasma corticosterone levels (F2-18 = 28, p = 0.0203), with the CMS-vul group specifically showing increased circulating levels of corticosterone in comparison to the No stress group (+61 %, p < 0.05 vs No stress) (Fig. 1B).
Moreover, we examined a possible covariation within sucrose intake and corticosterone plasma levels in the two subpopulations of stressed animals. This analysis revealed that sucrose intake negatively correlates with corticosterone levels (r = 0.5869, r2 = 0.3445, p = 0.0350), as shown in Fig. 1C, suggesting a relationship among the development of the anhedonic-like behavior and the alteration of the HPA axis functionality.
3.2. Chronic stress promoted the splicing of Grβ isoform mediated by SRSF9 in resilient animals
Alternative splicing of the gene encoding for the Gr, mediated by the serine/arginine-rich proteins (SRp) (Jain et al., 2012), leads to the formation of the Grα and Grβ isoforms that differ for their carboxyl terminus and, consequently, for their biological effects (Oakley et al., 1996; Yudt et al., 2003).
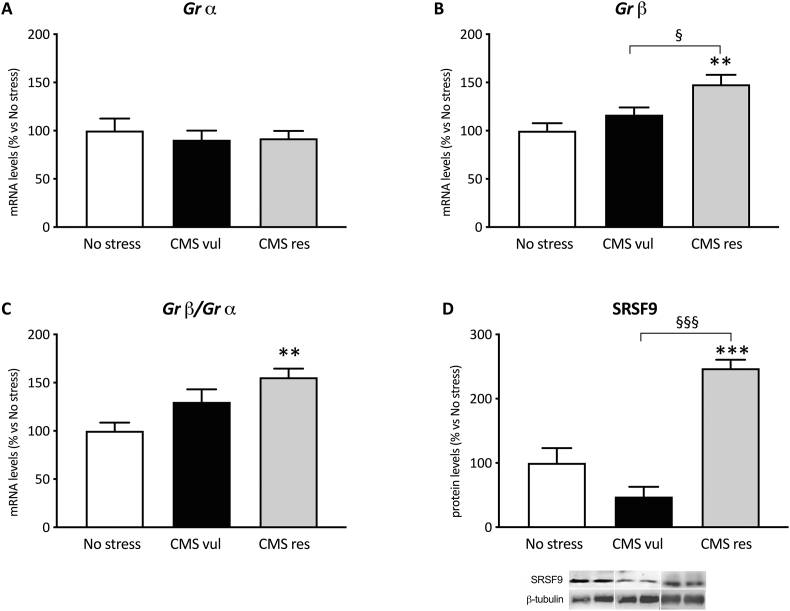
As shown in Fig. 2A, chronic stress did not modulate the gene expression of Grα (one-way ANOVA: F2-21 = 0.250, p = 0.7808), while we observed a significant effect of the one-way ANOVA analysis on Grβ mRNA levels (F2-21 = 8.15 p = 0.0024) (Fig. 2B), which were selectively enhanced in resilient animals in comparison not only to no stress animals (+48 %, p < 0.01 vs No stress) but also to vulnerable animals (+27 %, p < 0.05 vs CMS vul). Accordingly, as indicated in Fig. 2C, the ratio between Grβ/Grα was significantly modulated, as indicated by the one-way ANOVA results (F2-21 = 7.32, p = 0.0039), with such ratio being increased specifically in CMS-res group (+56 %, p < 0.01 vs No stress).
Fig. 2.
Analysis of Grα (A), Grβ (B) mRNA levels, Grβ/Grα ratio (C) and SRSF9 protein levels (D) in vulnerable and resilient animals exposed to two weeks of CMS. Data are expressed as percentage of non-stressed animals (set at 100 %). **p < 0.01, ***p < 0.001 vs No stress; § <0.05, §§§p < 0.001 vs CMS vul (one-way ANOVA with Tukey's multiple comparison test).
Moreover, we observed a significant effect of the one-way ANOVA (F2-9 = 33.8, p < 0.001) on the protein expression of SRSF9 (also known as SRp30c), the SR splicing factor that specifically mediates the formation of Grβ isoform. Indeed, in line with the increased mRNA levels of Grβ (Fig. 2B), we found that resilient animals specifically showed an overexpression of SRSF9 protein levels with respect to unstressed animals (+147 %, p < 0.001 vs No stress) and to CMS vulnerable group (+421 %, p < 0.001 vs CMS vul) (Fig. 2D).
The enhancement of Grβ levels, coupled with the increased protein level of SRSF9, suggests a requirement of this specific isoform to promote resilience thus counteracting the negative effects of chronic stress exposure.
3.3. Resilient animals showed enhancement of RACK1 at both transcriptional and translational level
The human gene RACK1 contains the GRE sequence, which mediates the cortisol physiological inhibition of RACK1 (Del Vecchio et al., 2009).
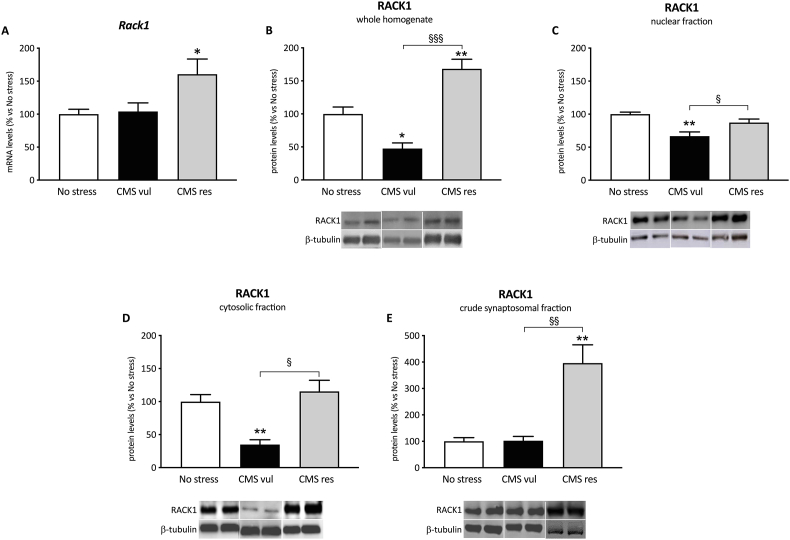
As shown in Fig. 3A, we observed a significant effect of stress on Rack1 gene expression (F2-21 = 4.53 p = 0.0232), with its mRNA levels being upregulated specifically in CMS res (+60 %, p < 0.05 vs No stress) in comparison to unstressed animals.
Fig. 3.
Analysis of Rack1 mRNA levels (A) and RACK1 protein levels in the whole homogenate (B), in the nucleus (C), in the cytosol (D) and in the crude synaptosomal fraction (E), in vulnerable and resilient animals exposed to two weeks of CMS. Data are expressed as percentage of non-stressed animals (set at 100 %). *p < 0.05, **p < 0.01 vs No stress; §p < 0.05, §§p < 0.05, §§§p < 0.001 vs CMS-vul (one-way ANOVA with Tukey's multiple comparison test).
At translational levels, one-way ANOVA revealed a significant effect of stress on RACK1 protein levels in the whole homogenate (F2-9 = 28.2, p < 0.001), in the cytosol (F2-9 = 12.1, p = 0.0028), in the crude synaptosomal fraction (F2-9 = 16.6, p = 0.010) as well as in the nuclear fraction (F2-9 = 11.1, p = 0.0037).
Accordingly, in the whole homogenate, we observed a decrease of RACK1 in vulnerable animals in comparison to No stress group (−52 %, p < 0.05 vs No stress) and an increased expression in resilient animals with respect not only to no stress (+68 %, p < 0.01 vs No stress) but also to CMS vul group (+254 %, p < 0.001 vs CMS vul) (Fig. 3B). As shown in the Fig. 3C, we found a similar modulation in the nuclear fraction, with RACK1 being downregulated in vulnerable animals (−33 %, p < 0.01 vs No stress) and upregulated in resilient group vs vulnerable group (+31 %, p < 0.05 vs CMS vul).
Similarly, in the cytosolic fraction RACK1 expression was decreased in vulnerable animals (−65 %, p < 0.01 vs No stress) and increased in resilient vs vulnerable animals (+220 %, p < 0.01 vs CMS vul) (Fig. 3D). Moreover, in the crude synaptosomal fraction (Fig. 3E), RACK1 protein levels were specifically enhanced in resilient animals with respect to no stress (+295 %, p < 0.01 vs No stress) and CMS vul animals (+287 %, p < 0.01 vs CMS vul).
All in all, these data highlighted the strict connection among the behavioral phenotype, glucocorticoids and RACK1 expression.
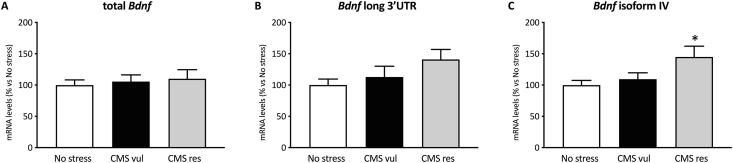
3.4. Bdnf isoform IV was up-regulated in CMS-resilient rats
Bdnf, the main neurotrophin essential for synaptic plasticity, has been linked with the activity of Rack1 in the central nervous system through the activation of cAMP signaling (Neasta et al, 2012, 2016).
As shown in Fig. 4A and B, we did not observe a significant effect of stress on total Bdnf and Bdnf long 3′UTR mRNA levels (one-way ANOVA: F2-21 = 0.195, p = 0.8240; F2-19 = 2.13, p = 0.1469). By contrast, we observed a significant effect of stress in the one-way ANOVA analysis on Bdnf isoform IV expression (F2-20 = 3.69, p = 0.0432), with its mRNA levels being upregulated in resilient animals in comparison to the No stress group (+45 %, p < 0.05 vs No stress) (Fig. 4C).
Fig. 4.
Analysis of total Bdnf (A), Bdnf long 3′UTR (B) and Bdnf isoform IV (C) mRNA levels in vulnerable and resilient animals exposed to two weeks of CMS. Data are expressed as percentage of non-stressed animals (set al 100 %). *p < 0.05 vs No stress (one-way ANOVA with Tukey's multiple comparison test).
These data suggest that resilience to CMS is possibly associated with an increased expression of Bdnf isoform IV, which might be mediated by the association of RACK1 with the promoter IV region of Bdnf (He et al., 2010).
3.5. Cortisol-induced RACK1 negative regulation was correlated with mature BDNF down-regulation in vitro
In the attempt to link the activation of the Gr-RACK1-Bdnf pathway more tightly to stress-induced cortisol release, we shifted our approach to in vitro experiments to be able to dissect a putative underlying mechanism. Accordingly, we exposed SH-SY5Y cells to increasing concentration of cortisol and analyzed the results at different times.
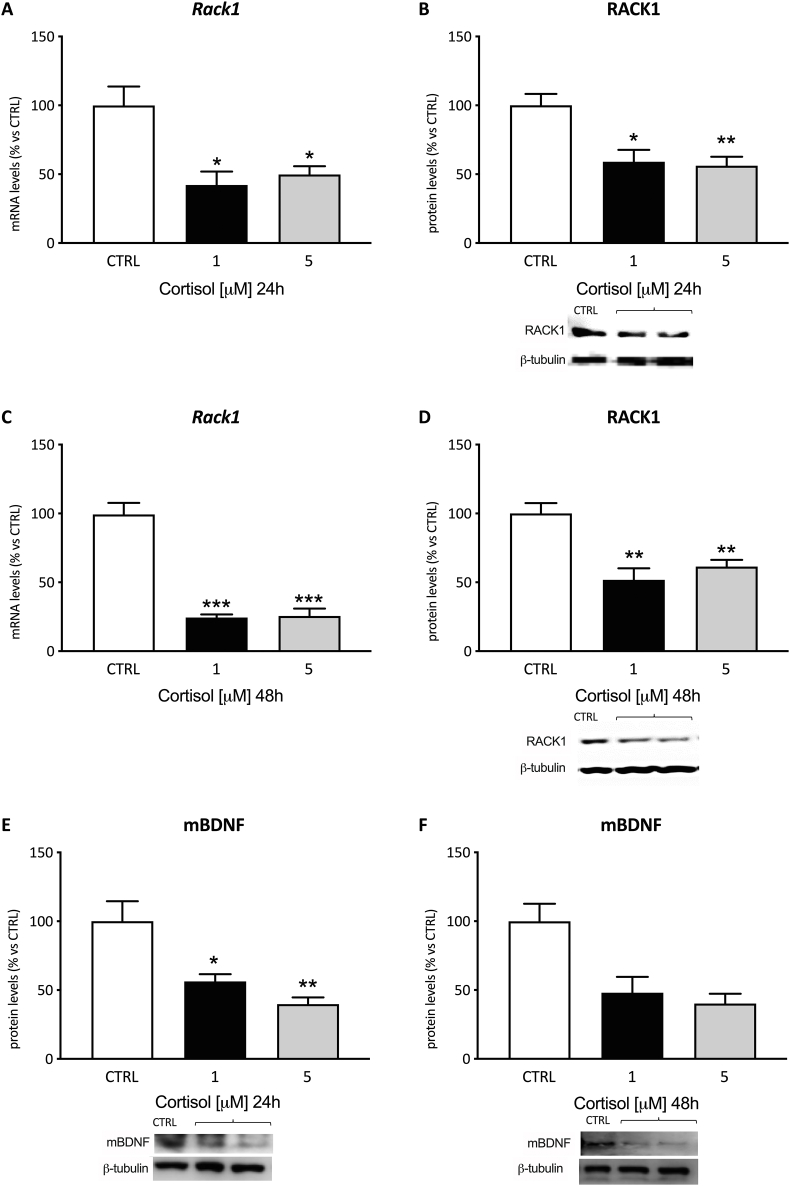
Specifically, we tested the SH-SY5Y cells with 0.1, 0.5, 1 and 5 μM of cortisol for 6, 12, 24 and 48 h, to mimic acute and chronic stressors (Choi et al., 2021; Silva et al., 2019). As reported in Supplementary Figure 1, regardless of the cortisol concentrations herein used, Rack1 gene and protein expression were not altered at the earliest time points (6 and 12 h) while we observed a decrease of Rack1 mRNA and protein levels at 24 and 48 h with 1 and 5 μM of cortisol. These results, in line with our previous promoter luciferase findings (Del Vecchio et al., 2009), show that stress-mimicking cortisol treatments (1 and 5 μM) induced a significant down-regulation of Rack1 expression.
As showed in Fig. 5 (and in Supplementary Figure 1), we found a significative effect of cortisol in the one-way ANOVA analysis on Rack1 mRNA level at 24 h (F2-6 = 9.3, p = 0.0145). Indeed, Rack1 mRNA levels were significantly downregulated by both 1 μM (−57.8 %, p < 0.05 vs CTRL) and 5 μM (−50.2 %, p < 0.05 vs CTRL) cortisol concentrations (Fig. 5A). In line, one-way ANOVA revealed a significant effect of cortisol also on RACK1 protein levels at the same time point (F2-15 = 9.05, p = 0,0026) with cortisol specifically inducing a significant decrease of RACK1 protein levels with both 1 μM (−41 %, p < 0.05 vs CTRL) and 5 μM (−43.8 %, p < 0.01 vs CTRL) treatments (Fig. 5B). Moreover, we observed a similar modulation at 48 h on both Rack1 mRNA (Fig. 5C) and protein levels (Fig. 5D) (one-way ANOVA: F2-16 = 10.6, p = 0.0011; F2-6 = 52.8, p = 0.0002, respectively) that were reduced after cortisol administration (mRNA: 1 μM: −74.8 %, p < 0.001 vs CTRL; 5 μM: −73.8 %, p < 0.001 vs CTRL; protein: 1 μM: −51.7 %, p < 0.01 vs CTRL; 5 μM: −61.5 %, p < 0.01 vs CTRL).
Fig. 5.
Effects of stress-mimicking cortisol concentrations on Rack1 and mBDNF expression. Analysis of Rack1 mRNA and protein levels (A-D) in SH-SY5Y cells exposed to either 1 or 5 µM cortisol or vehicle control (DMSO < 0.1%) for 24 h (A-B) and 48 h (C-D). Analysis of mBDNF protein levels (E-F) in SH-SY5Y cells treated with either 1 or 5 µM cortisol or vehicle control (DMSO < 0.1%) for 24 h (E) and 48 h (F). Data are expressed as percentage of CTRL (set at 100%). *p<0.05, **p < 0.01 vs CTRL (one-way ANOVA with Tukey’s multiple comparison test).
To confirm the cortisol-mediated modulation of RACK1, we treated the cells with the GR inhibitor mifepristone (RU486), and we observed that it abolished the cortisol-induced RACK1 protein down-regulation (Supplementary Figure 2) confirming that RACK1 expression involves Gr-mediated gene transcription.
Next, we investigated whether cortisol would reduce mature BDNF (mBDNF) protein levels via inhibition of RACK1 nuclear translocation. We observed a significant effect of the one-way ANOVA on the protein expression of mBDNF both at 24 h (F2-10 = 9.06, p = 0.0057) and at 48 h (F2-13 = 9.102, p = 0.0034). Our data showed that cortisol reduced RACK1 nuclear translocation (Supplementary Figure 3) that eventually resulted in a significant down-regulation of mBDNF protein levels with 1 μM (−43.72 %, p < 0.05 vs CTRL) and 5 μM (−60.13 %, p < 0.01 vs CTRL) cortisol concentrations at 24 h (Fig. 5E). Similarly, mBDNF expression was decreased at 48 h with both 1 μM (−51.96 %, p < 0.01 vs CTRL) and 5 μM (−59.77 %, p < 0.01 vs CTRL) cortisol concentrations (Fig. 5F) in line with literature data demonstrating that RACK1 silencing significantly decreases BDNF expression in both SH-SY5Y cells and hippocampal neurons, thus highlighting that RACK1 is required for BDNF expression (He et al., 2010; Neasta et al., 2012).
Taken together, in vitro data identify the Gr-RACK1-Bdnf pathway as a target of stress-induced cortisol release.
4. Discussion
In this study we demonstrated that chronic stress differently affected the Grβ-RACK1-Bdnf pathway, which appears to be selectively activated in animals resilient to the negative external cues.
In line with our previous evidence (Calabrese et al., 2017), we found that ~70 % of stressed animals developed the anhedonic-like behavior whereas the remaining ~30 % was resilient to chronic stress exposure showing the normal hedonic phenotype as the non-stressed rats. Interestingly, we observed that the vulnerability and resilience to the CMS paradigm were correlated with a specific activity of the HPA axis. Indeed, as expected, chronic stress led to elevated levels of circulating corticosterone suggesting a stress-mediated alteration of the functionality of the HPA axis (McEwen, 2007; Miller et al., 2007). By contrast, we found that resilience was correlated with normal corticosterone plasma levels, in line with the notion that the mechanisms that promote resilience may be protective against HPA axis overactivation (DeVries et al., 2003; Ong et al., 2006).
To better understand the underlying mechanisms, we observed that animals resilient to chronic stress showed overexpression of the beta isoform of Gr and of the SRSF9 protein, the serine/arginine-rich protein that mediates the post-transcriptional modification leading specifically of Grβ.
In previous experiments, we demonstrated that the expression of both GRβ and SRSF9 was enhanced by the treatment with the functional antagonist of glucocorticoids dehydroepiandrosterone (DHEA), supporting the link among glucocorticoids and Grβ-SRSF9 mediated splicing (Buoso et al., 2017a; Pinto et al., 2015). Moreover, given the regulation of Rack1 by glucocorticoids through the binding with the GRE sites on its promoter, we investigated whether cortisol could modulate the activity of mapped Rack1 promoter. By using two different constructs, the Δ1 and the Δ6 (obtained through Δ1 deletion) lacking GRE binding sequence, we demonstrated a GRE-dependent inhibition of Rack1 promoter transcriptional activity in SH-SY5Y cells. Indeed, only the luciferase activity of Δ1 construct was significantly reduced suggesting a role of glucocorticoids in RACK1 regulation (Del Vecchio et al., 2009). Accordingly, we observed that prolonged DHEA treatment counteracts the negative effect of cortisol on RACK1 expression by modulating GRβ (Buoso et al., 2011, Buoso et al., 2017b; Muller et al., 2004), an effect that was supported by the silencing of Grβ with a specific small-interfering RNA (Pinto et al., 2015).
In our CMS protocol, we found that Rack1 gene and protein expression was increased in resilient animals, whereas vulnerable animals showed reduced levels of Rack1. Accordingly, given the role of RACK1 as scaffolding anchoring protein that, through PKC activation, regulates several membrane-related pathways (Adams et al., 2011; Ron et al., 1994; Yang et al., 2019), the enhancement of RACK1 in the crude synaptosomal fraction of resilient animals may be required to modulate synaptic activity thus promoting the coping of this group of rats with the stress procedure.
Moreover, to further link the connection among glucocorticoids and Rack1, we mimicked a prolonged stressful condition by treating the SH-SY5Y cells with 1 and 5 μM of cortisol for 24 and 48 h and observed an overall reduction of both the gene and protein expression of Rack1, an effect that was abolished by the GR inhibitor mifepristone, thus confirming that Rack1 expression involves Gr-mediated gene transcription.
RACK1 has been demonstrated to be involved in the spatial and temporal orchestration of signaling cascades mediated by Bdnf (Neasta et al., 2012) and it has been also reported to display a role as a mediator of chromatin remodeling in an exon-specific expression of Bdnf gene. Indeed, it has been shown that nuclear RACK1 is connected to the chromatin complex through its interaction with histone H3 and H4 thus leading to the dissociation of the transcription repressor MeCP2 from the promoter and the subsequent induction of the promoter-controlled transcription of BDNF exon IV (He et al., 2010). Here, we found that animals resilient to chronic stress showed increased Bdnf isoform IV expression, a modulation that may be driven by the binding of RACK1 with the promoter of the promoter IV region of Bdnf (He et al., 2010), whereas the in vitro stress-mimicking cortisol concentrations not only significantly reduced RACK1 expression but also its nuclear translocation, ultimately leading to a strong decrease of mBDNF, further supporting the existence of a correlation between RACK1 and mBDNF expression.
Accordingly, accumulating data indicated the negative effects of chronic stress on Bdnf expression (Miao et al., 2020), as well as the role of Bdnf in promoting resilience (Krishnan et al., 2007; Taliaz et al., 2011).
Our results are in line with the evidence recently reviewed by Ramos-Ramirez and Tilba showing that Grβ does not act exclusively as antagonist of Grα, but also it can directly induce or repress the expression of a large number of genes regardless of Grα antagonism (Ramos-Ramírez and Tliba, 2021).
Under our experimental conditions, the enhancement of Grβ seems to be a strategy set in motion by the resilient subpopulation of stressed rats to face with the negative effects of chronic stress.
Moreover, further studies are needed to demonstrate that the modulation of Grβ-SRSF9, RACK1 and Bdnf IV is causally, and not correlatively, related to stress resilience as reported in the present work, although we must emphasize that our in vitro data provide evidence to this effect.
A limitation of our study is that corticosterone levels are measured only at one time point, which does not allow us to provide indications about changes in the circadian release of the hormone or on the potential impairment in the negative feedback functionality. Furthermore, we are aware that the basal levels of corticosterone, measured in the control group, are quite high, an effect that could be due to several reasons, such as for instance, the strain of the rats used (Kühn et al., 1983) and the type of ELISA kit employed for the analyses (Kinn Rød et al., 2017).
All in all, our in vivo data coupled with the in vitro approach suggest that the activation of the Grβ-RACK1-Bdnf cascade compensates and provides resilience to the detrimental effects caused by chronic stress. Interestingly, our data add novel critical information for the discovery of novel targets for the treatment of stress-related disorders, including depression.
Funding and disclosure
This work is supported by a grant from MIUR Progetto Eccellenza (2018–2022) to the Department of Pharmacological and Biomolecular Sciences, University of Milan, Milan, Italy and by Ministero dell’Istruzione, dell’Università e della Ricerca (PRIN2017, Project number 2017MLC3NF).
The behavioral part of this work has been supported by the statutory activity of the Maj Institute of Pharmacology Polish Academy of Sciences (Krakow, Poland) to M.P. None of the funding bodies had any role in designing the study.
CRediT authorship contribution statement
Paola Brivio: Conceptualization, Investigation, Methodology, Data curation, Formal analysis, Writing – original draft. Erica Buoso: Conceptualization, Methodology, Data curation, Formal analysis, Writing – original draft. Mirco Masi: Methodology, Formal analysis. Maria Teresa Gallo: Methodology. Piotr Gruca: Methodology. Magdalena Lason: Methodology. Ewa Litwa: Methodology. Mariusz Papp: Methodology, Writing – review & editing. Fabio Fumagalli: Writing – review & editing. Marco Racchi: Conceptualization, Writing – review & editing. Emanuela Corsini: Conceptualization, Funding acquisition, Writing – review & editing. Francesca Calabrese: Conceptualization, Investigation, Formal analysis, Funding acquisition, Writing – original draft.
Declaration of competing interest
The authors have no conflict of interest to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2021.100372.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Adams D.R., Ron D., Kiely P.A. RACK1, A multifaceted scaffolding protein: structure and function. Cell Commun. Signal. 2011;9:22. doi: 10.1186/1478-811X-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker C., Scholz J., O'Donnell K.J., Allemang-Grand R., Diorio J., Bagot R.C., Nestler E.J., Hen R., Lerch J.P., Meaney M.J. Neuroanatomic differences associated with stress susceptibility and resilience. Biol. Psychiatr. 2016;79:840–849. doi: 10.1016/j.biopsych.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker C., Zunszain P.A., Carvalho L.A., Pariante C.M. The glucocorticoid receptor: pivot of depression and of antidepressant treatment? Psychoneuroendocrinology. 2011;36:415–425. doi: 10.1016/j.psyneuen.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamberger C.M., Bamberger A.M., de Castro M., Chrousos G.P. Glucocorticoid receptor beta, a potential endogenous inhibitor of glucocorticoid action in humans. J. Clin. Invest. 1995;95:2435–2441. doi: 10.1172/JCI117943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brivio P., Corsini G., Riva M.A., Calabrese F. Chronic vortioxetine treatment improves the responsiveness to an acute stress acting through the ventral hippocampus in a glucocorticoid-dependent way. Pharmacol. Res. 2019;142:14–21. doi: 10.1016/j.phrs.2019.02.006. [DOI] [PubMed] [Google Scholar]
- Brivio P., Sbrini G., Riva M.A., Calabrese F. Acute stress induces cognitive improvement in the novel object recognition task by transiently modulating Bdnf in the prefrontal cortex of male rats. Cell. Mol. Neurobiol. 2020;40:1037–1047. doi: 10.1007/s10571-020-00793-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brivio P., Sbrini G., Tarantini L., Parravicini C., Gruca P., Lason M., Litwa E., Favero C., Riva M.A., Eberini I., Papp M., Bollati V., Calabrese F. Stress modifies the expression of glucocorticoid-responsive genes by acting at epigenetic levels in the rat prefrontal cortex: modulatory activity of lurasidone. Int. J. Mol. Sci. 2021;22:6197. doi: 10.3390/ijms22126197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buoso E., Biundo F., Lanni C., Aiello S., Grossi S., Schettini G., Govoni S., Racchi M. Modulation of Rack-1/PKCβII signalling by soluble AβPPα in SH-SY5Y cells. Curr. Alzheimer Res. 2013;10:697–705. doi: 10.2174/15672050113109990145. [DOI] [PubMed] [Google Scholar]
- Buoso E., Biundo F., Lanni C., Schettini G., Govoni S., Racchi M. AβPP intracellular C-terminal domain function is related to its degradation processes. J. Alzheim. Dis. 2012;30:393–405. doi: 10.3233/JAD-2012-111961. [DOI] [PubMed] [Google Scholar]
- Buoso E., Galasso M., Ronfani M., Serafini M.M., Lanni C., Corsini E., Racchi M. Role of spliceosome proteins in the regulation of glucocorticoid receptor isoforms by cortisol and dehydroepiandrosterone. Pharmacol. Res. 2017;120:180–187. doi: 10.1016/j.phrs.2017.03.019. [DOI] [PubMed] [Google Scholar]
- Buoso E., Galasso M., Serafini M.M., Ronfani M., Lanni C., Corsini E., Racchi M. Transcriptional regulation of RACK1 and modulation of its expression: role of steroid hormones and significance in health and aging. Cell Signal. 2017;35:264–271. doi: 10.1016/j.cellsig.2017.02.010. [DOI] [PubMed] [Google Scholar]
- Buoso E., Lanni C., Molteni E., Rousset F., Corsini E., Racchi M. Opposing effects of cortisol and dehydroepiandrosterone on the expression of the receptor for Activated C Kinase 1: implications in immunosenescence. Exp. Gerontol. 2011;46:877–883. doi: 10.1016/j.exger.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Buoso E., Masi M., Galbiati V., Maddalon A., Iulini M., Kenda M., Sollner Dolenc M., Marinovich M., Racchi M., Corsini E. Effect of estrogen-active compounds on the expression of RACK1 and immunological implications. Arch. Toxicol. 2020;94:2081–2095. doi: 10.1007/s00204-020-02756-9. [DOI] [PubMed] [Google Scholar]
- Calabrese F., Brivio P., Gruca P., Lason-Tyburkiewicz M., Papp M., Riva M.A. Chronic mild stress-induced alterations of local protein synthesis: a role for cognitive impairment. ACS Chem. Neurosci. 2017;8:817–825. doi: 10.1021/acschemneuro.6b00392. [DOI] [PubMed] [Google Scholar]
- Calabrese F., Brivio P., Gruca P., Lason-Tyburkiewicz M., Papp M., Riva M.A. Chronic mild stress-induced alterations of local protein synthesis: a role for cognitive impairment. ACS Chem. Neurosci. 2017;8:817–825. doi: 10.1021/acschemneuro.6b00392. [DOI] [PubMed] [Google Scholar]
- Calabrese F., Brivio P., Sbrini G., Gruca P., Lason M., Litwa E., Niemczyk M., Papp M., Riva M.A. Effect of lurasidone treatment on chronic mild stress-induced behavioural deficits in male rats: the potential role for glucocorticoid receptor signalling. J. Psychopharmacol. 2020;34:420–428. doi: 10.1177/0269881119895547. [DOI] [PubMed] [Google Scholar]
- Calabrese F., Molteni R., Racagni G., Riva M.A. Neuronal plasticity: a link between stress and mood disorders. Psychoneuroendocrinology. 2009;34:S208–S216. doi: 10.1016/j.psyneuen.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Choi G.E., Lee H.J., Chae C.W., Cho J.H., Jung Y.H., Kim J.S., Kim S.Y., Lim J.R., Han H.J. BNIP3L/NIX-mediated mitophagy protects against glucocorticoid-induced synapse defects. Nat. Commun. 2021;12:487. doi: 10.1038/s41467-020-20679-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Vecchio I., Zuccotti A., Pisano F., Canneva F., Lenzken S.C., Rousset F., Corsini E., Govoni S., Racchi M. Functional mapping of the promoter region of the GNB2L1 human gene coding for RACK1 scaffold protein. Gene. 2009;430:17–29. doi: 10.1016/j.gene.2008.10.005. [DOI] [PubMed] [Google Scholar]
- DeVries A.C., Glasper E.R., Detillion C.E. Social modulation of stress responses. Physiol. Behav. 2003;79:399–407. doi: 10.1016/S0031-9384(03)00152-5. [DOI] [PubMed] [Google Scholar]
- Fanselow M.S., Dong H.W. Are the dorsal and ventral Hippocampus functionally distinct structures? Neuron. 2010 doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He D.-Y., Neasta J., Ron D. Epigenetic regulation of BDNF expression via the scaffolding protein RACK1. J. Biol. Chem. 2010;285:19043–19050. doi: 10.1074/jbc.M110.100693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg S.M., Weinberger C., Ong E.S., Cerelli G., Oro A., Lebo R., Thompson E.B., Rosenfeld M.G., Evans R.M. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985;318:635–641. doi: 10.1038/318635a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A., Wordinger R.J., Yorio T., Clark A.F. Spliceosome protein (SRp) regulation of glucocorticoid receptor isoforms and glucocorticoid response in human trabecular meshwork cells. Investig. Opthalmology Vis. Sci. 2012;53:857. doi: 10.1167/iovs.11-8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershner L., Welshhans K. RACK1 is necessary for the formation of point contacts and regulates axon growth. Dev. Neurobiol. 2017;77:1038–1056. doi: 10.1002/dneu.22491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinn Rød A.M., Harkestad N., Jellestad F.K., Murison R. Comparison of commercial ELISA assays for quantification of corticosterone in serum. Sci. Rep. 2017;7:6748. doi: 10.1038/s41598-017-06006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kino T., Manoli I., Kelkar S., Wang Y., Su Y.A., Chrousos G.P. Glucocorticoid receptor (GR) β has intrinsic, GRα-independent transcriptional activity. Biochem. Biophys. Res. Commun. 2009;381:671–675. doi: 10.1016/j.bbrc.2009.02.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kino T., Su Y.A., Chrousos G.P. Human glucocorticoid receptor isoform beta: recent understanding of its potential implications in physiology and pathophysiology. Cell. Mol. Life Sci. 2009;66:3435–3448. doi: 10.1007/s00018-009-0098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V., Han M.-H., Graham D.L., Berton O., Renthal W., Russo S.J., LaPlant Q., Graham A., Lutter M., Lagace D.C., Ghose S., Reister R., Tannous P., Green T.A., Neve R.L., Chakravarty S., Kumar A., Eisch A.J., Self D.W., Lee F.S., Tamminga C.A., Cooper D.C., Gershenfeld H.K., Nestler E.J. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Kühn E., Bellon K., Huybrechts L., Heyns W. Endocrine differences between the wistar and sprague-dawley laboratory rat: influence of cold adaptation. Horm. Metab. Res. 1983;15:491–498. doi: 10.1055/s-2007-1018767. [DOI] [PubMed] [Google Scholar]
- Lazarus R.S., DeLongis A., Folkman S., Gruen R. Stress and adaptational outcomes. The problem of confounded measures. Am. Psychol. 1985;40:770–785. doi: 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- Luoni A., Macchi F., Papp M., Molteni R., Riva M.A. Lurasidone exerts antidepressant properties in the chronic mild stress model through the regulation of synaptic and neuroplastic mechanisms in the rat prefrontal cortex. Int. J. Neuropsychopharmacol. 2015;18 doi: 10.1093/ijnp/pyu061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 2007 doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McEwen B.S., Bowles N.P., Gray J.D., Hill M.N., Hunter R.G., Karatsoreos I.N., Nasca C. Mechanisms of stress in the brain. Nat. Neurosci. 2015;18:1353–1363. doi: 10.1038/nn.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGough N.N.H. RACK1 and brain-derived neurotrophic factor: a homeostatic pathway that regulates alcohol addiction. J. Neurosci. 2004;24:10542–10552. doi: 10.1523/JNEUROSCI.3714-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Z., Wang Y., Sun Z. The relationships between stress, mental disorders, and epigenetic regulation of BDNF. Int. J. Mol. Sci. 2020;21:1375. doi: 10.3390/ijms21041375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G.E., Chen E., Zhou E.S. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol. Bull. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Muller C., Cluzeaud F., Pinon G.M., Rafestin-Oblin M.-E., Morfin R. Dehydroepiandrosterone and its 7-hydroxylated metabolites do not interfere with the transactivation and cellular trafficking of the glucocorticoid receptor. J. Steroid Biochem. Mol. Biol. 2004;92:469–476. doi: 10.1016/j.jsbmb.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Neasta J., Fiorenza A., He D.-Y., Phamluong K., Kiely P.A., Ron D. Activation of the cAMP pathway induces RACK1-dependent binding of β-actin to BDNF promoter. PloS One. 2016;11 doi: 10.1371/journal.pone.0160948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neasta J., Kiely P.A., He D.-Y., Adams D.R., O'Connor R., Ron D. Direct interaction between scaffolding proteins RACK1 and 14-3-3ζ regulates brain-derived neurotrophic factor (BDNF) transcription. J. Biol. Chem. 2012;287:322–336. doi: 10.1074/jbc.M111.272195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley R.H., Sar M., Cidlowski J.A. The human glucocorticoid receptor β isoform: expression, biochemical properties, and putative function. J. Biol. Chem. 1996;271:9550–9559. doi: 10.1074/jbc.271.16.9550. [DOI] [PubMed] [Google Scholar]
- Ong A.D., Bergeman C.S., Bisconti T.L., Wallace K.A. Psychological resilience, positive emotions, and successful adaptation to stress in later life. J. Pers. Soc. Psychol. 2006;91:730–749. doi: 10.1037/0022-3514.91.4.730. [DOI] [PubMed] [Google Scholar]
- Papp M. Models of affective illness: chronic mild stress in the rat. Curr. Protoc. Pharmacol. 2012;57 doi: 10.1002/0471141755.ph0509s57. [DOI] [PubMed] [Google Scholar]
- Paxinos G., Watson C. 2004. The Rat Brain in Stereotaxic Coordinates - the New Coronal Set. English. [DOI] [PubMed] [Google Scholar]
- Pinto A., Malacrida B., Oieni J., Serafini M.M., Davin A., Galbiati V., Corsini E., Racchi M. DHEA modulates the effect of cortisol on RACK1 expression via interference with the splicing of the glucocorticoid receptor. Br. J. Pharmacol. 2015;172:2918–2927. doi: 10.1111/bph.13097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racchi M., Buoso E., Ronfani M., Serafini M.M., Galasso M., Lanni C., Corsini E. Role of hormones in the regulation of RACK1 expression as a signaling checkpoint in immunosenescence. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18071453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Ramírez P., Tliba O. Glucocorticoid receptor β (GRβ): beyond its dominant-negative function. Int. J. Mol. Sci. 2021;22:3649. doi: 10.3390/ijms22073649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D., Chen C.H., Caldwell J., Jamieson L., Orr E., Mochly-Rosen D. Cloning of an intracellular receptor for protein kinase C: a homolog of the beta subunit of G proteins. Proc. Natl. Acad. Sci. Unit. States Am. 1994;91:839–843. doi: 10.1073/pnas.91.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D., Jiang Z., Yao L., Vagts A., Diamond I., Gordon A. Coordinated movement of RACK1 with activated betaIIPKC. J. Biol. Chem. 1999;274:27039–27046. doi: 10.1074/jbc.274.38.27039. [DOI] [PubMed] [Google Scholar]
- Selye H. A syndrome produced by diverse nocuous agents. J. Neuropsychiatry Clin. Neurosci. 1998;10:230–231. doi: 10.1176/jnp.10.2.230a. 1936. [DOI] [PubMed] [Google Scholar]
- Silva J.M., Rodrigues S., Sampaio-Marques B., Gomes P., Neves-Carvalho A., Dioli C., Soares-Cunha C., Mazuik B.F., Takashima A., Ludovico P., Wolozin B., Sousa N., Sotiropoulos I. Dysregulation of autophagy and stress granule-related proteins in stress-driven Tau pathology. Cell Death Differ. 2019;26:1411–1427. doi: 10.1038/s41418-018-0217-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklan E.H., Podoly E., Soreq H. RACK1 has the nerve to act: structure meets function in the nervous system. Prog. Neurobiol. 2006;78:117–134. doi: 10.1016/j.pneurobio.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Taliaz D., Loya A., Gersner R., Haramati S., Chen A., Zangen A. Resilience to chronic stress is mediated by hippocampal brain-derived neurotrophic factor. J. Neurosci. 2011;31:4475–4483. doi: 10.1523/JNEUROSCI.5725-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehner P., Shnitsar I., Urlaub H., Borchers A. RACK1 is a novel interaction partner of PTK7 that is required for neural tube closure. Development. 2011;138:1321–1327. doi: 10.1242/dev.056291. [DOI] [PubMed] [Google Scholar]
- Willner P. The chronic mild stress (CMS) model of depression: history, evaluation and usage. Neurobiol. Stress. 2017 doi: 10.1016/j.ynstr.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaka R., Thornton C., Vagts A.J., Phamluong K., Bonci A., Ron D. NMDA receptor function is regulated by the inhibitory scaffolding protein, RACK1. Proc. Natl. Acad. Sci. 2002;99:5710–5715. doi: 10.1073/pnas.062046299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Yang C., Zhu Q., Wei M., Li Y., Cheng J., Liu F., Wu Y., Zhang J., Zhang C., Wu H. Rack1 controls parallel fiber–purkinje cell synaptogenesis and synaptic transmission. Front. Cell. Neurosci. 2019;13 doi: 10.3389/fncel.2019.00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudt M.R., Jewell C.M., Bienstock R.J., Cidlowski J.A. Molecular origins for the dominant negative function of human glucocorticoid receptor beta. Mol. Cell Biol. 2003;23:4319–4330. doi: 10.1128/MCB.23.12.4319-4330.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.