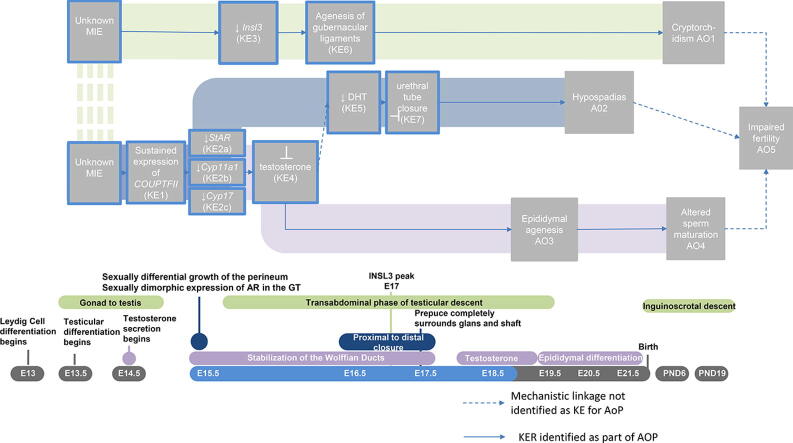
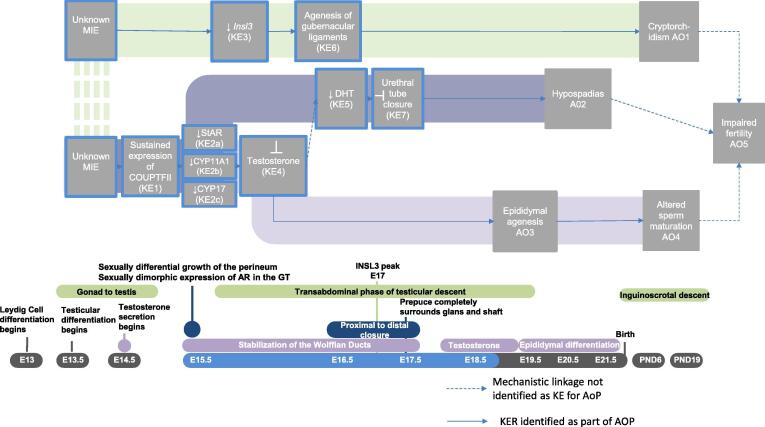
Graphical abstract
Abbreviations: AGD, Anogenital distance; AO, Adverse Outcome; AOP, Adverse Outcome Pathway; DBP, Dibutyl phthalate; DEHP, Di(2-ethylhexyl)phthalate; DPP, Dipentyl phthalate; DHT, 5α-dihydrotestosterone; E, Embryonic day (ED1=GD1 gestational day 1); GD, Gestational day (GD1=ED1 embryonic day 1); KE, Key event; KER, Key event relationship; LMWP, low molecular weight phthalate straight chain length of the esterified alcohols between 3 and 6 carbon atoms; MPW, male programming window
Keywords: Male programming window, Phthalate, Adverse outcome pathway, Adverse outcome pathway network
Highlights
-
•
A putative AOP network for the androgen sensitive development window is described.
-
•
Developmental biology data and chemical stressor data were integrated as the basis.
-
•
The network does not converge at testosterone.
-
•
Depicting gestational timing should be promoted for developmental AOPs.
-
•
Grouping of KEs into one KE should be avoided for broader use of AOPs and networks.
Abstract
Structured approaches like the adverse outcome pathway (AOP) framework offer great potential for depicting complex toxicological processes in a manner that can facilitate informed integration of mechanistic information in regulatory decisions. While this concept provides a structure for organizing evidence and facilitates consistency in evidence integration; the process, inputs, and manner in which AOPs and AOP networks are developed is still evolving. Following the OECD guiding principles of AOP development, we propose three AOPs for male reproductive tract abnormalities and derive a putative AOP network. The AOPs were developed using a fundamental understanding of the developmental biology of the organs of interest, paying close attention to the gestational timing of key events (KEs) to very specifically inform the domain of life stage applicability for the key event relationships (KERs). Chemical stressor data primarily from studies on low molecular weight phthalates (LMWPs) served to ‘bound’ the pathways of focus in this dynamic period of development and were integrated with the developmental biology data through an iterative process to define KEs and conclude on the extent of evidence in support of the KERs. The AOPs developed describe the linkage between 1) a decrease in Insl3 gene expression and cryptorchidism, 2) the sustained expression of Coup-tfII and hypospadias and 3) the sustained expression of Coup-tfII and altered Wolffian duct development/ epididymal agenesis. A putative AOP network linking AOP2 and AOP3 through decreased steroidogenic biosynthetic protein expression and converging of all AOPS at the population level impaired fertility adverse outcome is proposed. The network depiction specifies and displays the KEs aligned with their occurrence in gestational time. The pathways and network described herein are intended to catalyze collaborative initiatives for expansion into a larger network to enable effective data collection and inform alternative approaches for identifying stressors impacting this sensitive period of male reproductive tract development.
1. Introduction
Structured frameworks like the adverse outcome pathway (AOP) framework and associated AOP networks offer great potential for depicting complex toxicological processes in a manner which can facilitate informed integration of mechanistic information in chemical safety assessments (OECD, 2018, OECD, 2020, Villeneuve et al., 2014a). Conceptually, the AOP framework organizes data into a series of biological events spanning multiple levels of biological organization, providing a hypothesized path between two anchors: a molecular initiating event (MIE) and an adverse outcome (AO) (Ankley et al., 2010, Villeneuve et al., 2014b, OECD, 2018). The pathway is then characterized using key events (KEs) and key event relationships (KERs) (Villeneuve et al., 2014a, Villeneuve et al., 2014b, Knapen et al., 2015). AOPs are often constructed initially as unidirectional linear pathways from MIE through AO. They are recognized to be oversimplifications of the complexity of the biological systems which they represent (Knapen et al., 2018) but aim to depict how an explicit perturbation by any stressor may lead to an adverse effect (i.e. they are chemically agnostic). Because AOPs, as well as the components of each AOP, cannot be considered in isolation, the interactions among pathways, stressors, taxa, and life stages are critical to AOP development and also highlight the importance of AOP networks. Networks are simply an assembly of two or more AOPs that share one or more KEs and allow for better accommodation of more realistic biological scenarios (OECD, 2018, Knapen et al., 2018). They can depict all of the possible AOs resulting from a given MIE, or all of the different MIEs through which a given AO can be caused. Networks acknowledge that even a single stressor may induce toxicity via multiple mechanisms and/or interact with multiple targets, the outcome of which may be fully dependent on the occurrence of the perturbation and sequence of events relative to windows of biological exposure. In this way, networks can provide a pragmatic representation of the biological complexity and highlight all of the possible perturbation points through which stressors may evoke AOs in a sensitive life stage.
Though AOPs capture the toxicodynamic portion of a toxicity pathway and are therefore chemically agnostic, focusing on data from one stressor “class” can be a useful means for defining or narrowing the scope of an AOP/AOP network development effort. The term “rat phthalate syndrome” (Foster, 2006) was coined to encompass a group of effects observed in male rats from exposures to low molecular weight phthalates (LMWP) (C3-C6 backbone) during a critical developmental window of male sexual differentiation. These effects include reproductive abnormalities characterized by malformations of the epididymis, vas deferens, seminal vesicles, prostate, external genitalia (hypospadias), cryptorchidism and testicular injury together with permanent changes (feminization) in the retention of nipples/areolae (sexually dimorphic structures in rodents) and demasculinization of the growth of the perineum resulting in a reduced anogenital distance (AGD) (Foster, 2006). Despite decades of research on “rat phthalate syndrome”, the understanding of relationships between conditions and identification of early initiators remains obscure. For LMWPs, it remains unclear if the multitude of effects are different manifestations of a shared mechanism, have interrelated but diverging mechanistic underpinnings, or represent outcomes of distinct biological pathways that share a common window of developmental susceptibility and/or common chemical trigger. Clarifying what is known and not know about the connectivity between biological events and life stage applicability of events during development will facilitate transferability of the knowledge gleaned from these widely studied substances into new applications such as informing chemical test strategies and alternative methods development.
To facilitate a more generalized and long term utility of the biological response pathways embodied in “rat phthalate syndrome”, the objective herein was to define AOPs and a putative network associated with adverse male reproductive tract outcomes informed by developmental biology and the life stage specificity of KEs. We took a targeted approach that started with attaining a fundamental biological understanding of the pathways that could possibly lead to hypospadias, cryptorchidism and epididymal agenesis. As these outcomes could occur via a multitude of pathways, the next step involved evaluation of the empirical evidence from primarily (but not exclusively) LMWP data to further inform the causal linkages between KEs. The three AOPs were subsequently combined in a simple putative AOP network that aligns with the temporality of the underlying biological processes specific to the androgen sensitive window of biological development. Refining life stage applicability was a key focus of this effort and recommendations for improving information capture for AOPs relevant to reproductive development were made. The AOP conceptual paradigm recognizes the need for collaboration, interdisciplinary interaction and presents itself as a “living” construct where AOPs and AOP networks are modified, refined, and expanded as understanding and knowledge advances (Villeneuve et al., 2014a). The network described herein is meant to serve as a starting point for network expansion efforts to include additional MIEs and AOs relevant to this developmental window. Simplifying the complex developmental biology into a pragmatic AOP network is an important first step towards identification of the critical pathways and critical few network nodules important for advancing alternative methods development for this susceptible life stage.
2. Approach
2.1. AOP development
The focus of this effort was to develop AOPs in a manner compliant with the principles and guidance set out by the OECD (OECD, 2018). Systematic review guidance for AOP development was not available at the onset of the project, and is, at the time of publication, still being drafted. Systematic methods were considered and initially employed; however, given the broad scope of the assessment, it was determined that fully implementing such methods was not feasible. Rather, a targeted approach which built on the existing knowledge base was used to further elucidate the putative AOPs herein.
To define KEs and KERs in male rat reproductive tract abnormality pathways, two comprehensive literature searches were conducted in PubMed. Both search strategies captured rodent studies in primary and secondary (i.e. review) articles with no date exclusion applied. Citation mining was performed when relevant review articles were identified. The first search captured mechanistic data related to development of the reproductive tract in the rodent male programming window (MPW) and used key terms (e.g. development, hypospadias, cryptorchidism, epididymal malformations) for the target organs (epididymis, Wolffian duct, penis, testis). Articles of relevance were those providing mechanistic insight to the normal and abnormal development of the target organs of interest during gestation. The second search captured studies evaluating the effects of LMWP exposure during the MPW and used search terms such as phthalate and male reproductive tract or hypospadias, or cryptorchidism, or androgen, or testosterone, or epididymal agenesis, or testicular dysgenesis; or phthalate syndrome. Articles were selected when the exposure spanned the MPW, reported effects on the target organs, and/or provided mechanistic insight into phthalate mediated developmental toxicity. In some cases, studies reporting effects on the target organs from exposures outside of the MPW were also captured to inform on the life stage specificity of the KERs. Subsequent targeted investigative searches were performed (in both the biology and toxicology fields) to inform critical decision points (as described more below).
To establish AOPs, the molecular and cellular events that may lead to cryptorchidism, hypospadias and epididymal malformations were identified from the development biology literature and categorized by level of biological organization and gestational timing. The timing of in utero events are referred to as both embryonic day (E) and gestational day (GD) in the literature and these designations were used interchangeably in this manuscript, as to retain the designations utilized in the relied upon references. Based on the fundamental understanding of biology, more than one MIE could be responsible for mediating the AOs. Therefore we narrowed our focus to defining the KERs applicable to “rat phthalate syndrome” by initially considering empirical evidence from the LMWP literature. Using an iterative process of integrating data, KEs from the developmental biology literature were ‘ruled in’ when evidence from the stressor literature supported it; or KEs were ‘ruled out’ when counter evidence was present. When evidence for a KE in relation to the entire AOP was minimal or confounded, targeted investigative literature searches were performed and supporting information was assembled from either the developmental biology literature and/or non-LMWP chemical stressors shown to induce the AOs of interest.
2.2. Evidence integration and weight of evidence evaluation
Confidence in each AOP was established using the weight of evidence (WoE) considerations per the recommendations in the OECD AOP Developer’s Handbook (OECD, 2018). The first type of evidence assembled as part of the AOP evaluation was biological plausibility. Biological plausibility refers to the extent the relationship between an upstream and downstream KE is supported by an understanding of normal biological function (versus a response to a specific stressor). Weight of evidence in support of biological plausibility for the KERs was assessed consistent with the OECD handbook (OECD, 2018).
High: KER is well understood based on extensive previous documentation; established mechanistic basis and broad acceptance.
Moderate: KER is plausible based on analogy to accepted biological relationships but biological relationship/scientific understanding not completely established.
Low: empirical support for an association exists between KEs but structural or functional relationship between KEs is not understood.
The second type of evidence assembled as part of the AOP evaluation was the extent of empirical evidence for the KERs. The level of empirical concordance for the KERs was assessed by determining whether upstream KEs occurred at lower doses and earlier time points than downstream KEs and/or the frequency and incidence of an upstream KE was greater than the downstream KE at the same dose. A quantitative characterization of the KERs was out of scope of this effort. Rather focus was given to the qualitative evidence supporting dose, temporal and incidence concordance of KERs (taking care to identify where evidence was limited), and particularly to the concordance across multiple KEs in support of the AOP. Weight of empirical evidence for the KERs and AOP was assessed consistent with the OECD handbook (OECD, 2018) as defined below:
High: there is dependent change in events following exposure to a wide range of stressors (extensive evidence for dose, temporal and incidence concordance) and few data gaps or conflicting data. In this case, dose, temporal and incidence concordance had to be demonstrated in a well-designed study or when combining data across multiple studies for at least three LMWPs. The number of studies reporting a dose response effect of both observations for at least three LMWPs was also considered.
Moderate: dependent change in events is demonstrated following exposure to a small number of specific stressors and some evidence inconsistent with the expected pattern that can be explained by factors such as experimental design. In this case, dose temporal and incidence concordance demonstrated in a well-designed study for at least one LMWP. The number of studies reporting a dose response effect of both observations for two or more LMWPs was also considered.
Low: dependent change in events following exposure to a specific stressor is identified in limited or no studies (i.e. endpoints never measured in the same study or not at all), and/or lacking evidence of temporal or dose–response concordance, or identification of significant inconsistences in empirical dataset that do not align with the expected pattern for the hypothesized AOP.
Indirect (or “other”) observational evidence was also relied upon to inform the extent of empirical evidence for KERs. This type of evidence considered how the pattern of observations within the collective and broad data set (e.g. across animal strains, stressors) supported or refuted a given KER. Indirect evidence was considered in addition to the empirical concordance determinants above for an overall conclusion on weight of empirical evidence.
The biological plausibility and empirical evidence ratings were assessed together to conclude on the overall support for the AOP. Biological plausibility ranks highest among the different types of evidence used to support the AOPs (OECD, 2018, OECD, 2020) as it requires an understanding of the structural and functional relationships in normal biology and therefore weighted higher in the overall assessment. Empirical evidence primarily reflected dose response/incidence and temporal relationship data from studies with LMWP and therefore served to increase confidence in the AOP. The available data did not allow for assessment of the confidence in supporting data for essentiality of KEs within the AOP. Experimental approaches for establishing essentiality of KEs, such as genetic knockout animal models or genetic overexpression models, underpin the basic developmental biology understanding and, as such, were part of the biological plausibility evaluation.
2.3. AOP network development
To establish an AOP network that respects the gestational timing of KE/KERs, each AOP was evaluated for confluence of KEs, considering both the nature of the KE as well as its temporal occurrence in development. AOPs were linked through putative shared KEs as the basis to define an AOP network.
The numbering for the MIEs, KEs, and AOs described herein refer to the AOP network shown in Fig. 4. Supplementary Material provide detailed descriptions of empirical evidence and biological plausibility for KEs/KERs to facilitate independent assessment of the interpretations drawn in this analysis.
Fig. 4.
Putative network to male reproductive AOs initiated in the MPW. The figure should be read from left to right and is organized in developmental time from top to bottom. Everything sharing the same vertical plane is thought to occur in that developmental window. The horizontal planes depict: 1) linear pathway network, 2) biological process and 3) gestational timing of events (E = embryonic day). KEs outlined in blue are those impacted during the MPW. The solid arrows indicate KER identified as part of the AOP and the dashed arrows indicate a mechanistic link where the event was not identified as a “key” part of the AOP. Hypothesized AOPs are underscored by a colored pathway- green for AOP1, blue for AOP2 and purple for AOP3. The dashed green between the MIE’s indicate they may be unique, as depicted, or shared. For the network it was determined that enough evidence existed to assume the unknown MIE for AOP’s 2 and 3 is shared as the two AOPs share the same early KE’s and it is unlikely that two distinct MIE’s would converge on the same early KE. AOP1 could not be linked to the other AOPs with any known early KE’s therefore, until otherwise demonstrated, the MIE is depicted as a unique event. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3. Results
3.1. AOPs
Male reproductive development is highly dynamic and complex. As such, many perturbations of the system could possibly lead, in a causal manner, to the AOs of interest. To narrow the scope, attention was given to molecular and cellular perturbations during the male programming window (MPW) of development (GD15.5–18.5) (Sharpe, 2020). LMWP literature largely shows the importance of GD 15.5–18.5 as the window in which the initiating event(s) that leads to LMWP mediated AOs occurs (Howdeshell et al., 2017, Kortenkamp, 2020); however, the timing of the other KEs in the outcome pathways has been less rigorously defined. The time between KEs in male reproductive development could be milliseconds, days or in some instances months before the AO is realized. The alignment of the KEs and KERs in the three AOPs described below aims to respect gestational timing by carefully considering the window of chemical exposure and life stage specificity of when observational measurements were taken in the studies relied upon to build the AOP. In this way, for example, testosterone levels measured in adulthood would not be considered to provide much value in informing on hormonal changes during gestation.
3.1.1. AOP1: INSL3 decrease to cryptorchidism
Cryptorchidism is an absence of one or both testes from the scrotum and reflects an alteration in the normal process of testicular descent. Testicular descent is characterized as a 2-stage process, with each phase regulated by different hormones and anatomical mechanisms. The first phase (transabdominal phase) beings around GD15.5 in rodents (Hutson et al., 1997, Hutson et al., 2013, Hutson et al., 2016, Klonisch et al., 2004) and is characterized by regression of the cranial suspensory ligament (CSL) and swelling of the gubernaculum to position the testes at the bottom of the abdomen. In this phase, CSL regression is initiated by testosterone (Amann and Veeramachanemi, 2007; Van der Schoot and Emmen, 1996), and the swelling reaction in the gubernaculum is stimulated primarily by insulin-like hormone 3 (INSL3) with some studies implicating secondary augmentation by androgens (Adham et al., 2002, Bogatcheva et al., 2003, Gorlov et al., 2002; Nef et al. 1999; Overbeek et al., 2001, Tomiyama et al., 2003; Zimmerman et al., 1999). The second phase of testicular descent (inguinoscrotal process) is largely controlled by androgens and occurs at approximately postnatal week 3 and involves movement of the testes from the bottom of the abdomen to the base of the scrotum (Klonisch et al 2004, Zimmerman et al., 1999).
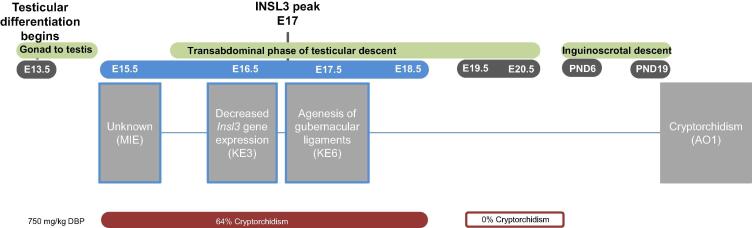
In rats, gestational exposure during the MPW to dibutyl phthalate (DBP) has been associated with increased incidence of cryptorchidism (Welsh et al., 2008, van den Driesche et al., 2017, van den Driesche et al., 2020). van den Driesche and colleagues (2017) exemplified the window of susceptibility for initiating this AO showing exposure to DBP during GD 15.5–18.5 only resulted in cryptorchidism, whereas exposure to DBP during GD 19.5–21.5 did not (Fig. 1). This narrow window of susceptibility implies the hormonal and anatomical mechanisms of relevance in deriving a pathway initiated by LMWPs and leading to cryptorchidism are restricted to the transabdominal phase of testicular descent (Hutson et al., 1997, Klonisch et al., 2004, Amann and Veeramachaneni, 2007, Nation et al., 2011). Evaluation of the events occurring during this phase of development as well as observational data on LMWPs suggest a pathway with two KEs initiated by an unknown MIE leading to one AO: 1) decreased Insl3 gene expression; 2) agenesis of the gubernaculum ligaments; 3) cryptorchidism (AO) (Fig. 1).
Fig. 1.
INSL3 reduction to cryptorchidism pathway (AOP1). Leydig cells (in the testes) are the cellular target for KE3, the gubernaculum is the target organ for KE6, and the testes the target organ for the AO, cryptorchidism (AO1). KEs outlined in blue are proposed to occur during the MPW. Events are aligned to the developmental timeline such that events sharing the same vertical plane occur in the same developmental window. The horizontal planes depict: 1) biological process occurring, 2) embryonic day (E), 3) AOP, and 4) subset of data that supports the pathway being specific to the indicated developmental window. This data demonstrates exposure to DBP during the MPW (filled red bar), but not later in gestation (open red bar) induces cryptorchidism (van den Driesche et al. 2017). Note: KEs are numbered according to the network (Fig. 4). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The first known event in this pathway was decreased Insl3 gene expression in Leydig cells of the testis (KE3) (Fig. 1). Developmental biology studies support an essential role of this testicular factor in normal gubernacular outgrowth and testicular descent (Nef and Parada, 1999, Zimmermann et al., 1999). INSL3 interacts with relaxin-family peptide receptor 2 (RXFP2) to stimulate gubernacular growth through an unclear mechanism (Bogatcheva et al., 2003). Developmental expression of Insl3 transcripts coincides with the onset of testicular descent with the transcript first detected as early as GD13.5 in mice (Feng et al., 2009). INSL3 protein is first detectable in rats at GD15.5, expression peaks at GD17.5–19 before returning to low levels at GD 21.5 (McKinnell et al., 2005). Very little is known about the regulation of Insl3 in development hindering the ability to hypothesize the upstream MIE for AOP1. One study implicated COUP-TFII as being an important regulator of Insl3 in Leydig cells (Mendoza-Villarroel et al., 2014). In this study, a significant decrease in Insl3 mRNA levels and an absence of testosterone were apparent in mice with prepubertal depletion of COUP-TFII. LMWPs, however, are associated with increased expression of COUP-TFII (van den Driesche et al., 2012) casting doubt on the role of COUP-TFII as the MIE for this pathway. Two other transcription factors, SF-1 and DAX-1, have also been shown to affect mouse Insl3 expression in vitro. SF-1 enhanced Insl3 promoter activity and increased transcription whereas, DAX-1 inhibited promoter activity and decreased transcription of Insl3 (Adham et al., 2004). In the rat testis, Insl3 has a relatively short promoter region characterized by three putative binding sites for SF-1 transcription factors (Ivell and Bathgate, 2002, Sadeghian et al., 2005). It is interesting to speculate that displacement of SF-1 from the Insl3 promoter via COUP-TFII might create a common thread with AOP2; however data and mechanistic understanding are currently too limited to support this as the MIE for AOP1.
The level of confidence in the KERs in AOP1 based on biological plausibility is summarized in Table 1. The confidence in the KERs downstream of the MIE is high, based on well documented mechanistic understanding in the developmental biology literature that strongly support a role for INSL3 and altered gubernacular development during the transabdominal phase of testicular descent (Table 1). There is also extensive literature describing and evaluating the ability of LMWPs to induce cryptorchidism and the general consensus in this literature concludes a role for INSL3 in mediating the effects (Foster, 2006, Wilson et al., 2008, Howdeshell et al., 2015, Gray et al., 2016). The developmental biology literature supports well the proposed KERs and a high degree of confidence in AOP1.
Table 1.
Assessment of biological plausibility and empirical evidence in support of the KERs in AOP1.
| KER (Adjacency of KEs) WoE Conclusion |
WoE Rationale |
|---|---|
| Biological Plausibility WoE | |
| KE3 leads to AO1 (Indirect) High |
Established developmental biological knowledge based on molecular biology studies (e.g. Insl3 knockout studies) support INSL3 is necessary in promoting normal testicular descent (Overbeek et al., 2001, Gorlov et al., 2002, Bogatcheva et al., 2003, Tomiyama et al., 2003, Rugarli and Langer, 2012, Imaizumi et al., 2015). |
| KE3 leads to KE 6 (Direct) High |
Established developmental biological knowledge based on molecular biology studies (e.g. Insl3 knockout and mutation studies) support INSL3 disruption impairs gubernacular development (Nef and Parada, 1999, Zimmermann et al., 1999, Overbeek et al., 2001, Adham et al., 2002, Gorlov et al., 2002, Bogatcheva et al., 2003, Tomiyama et al., 2003). |
| KE6 leads to AO1 (Direct) High |
Established developmental biological knowledge based on targeted molecular biology studies and observational associations support abnormal gubernacular development impedes testes descent (Ivell and Hartung, 2003, Adham and Agoulnik, 2004, Klonisch et al., 2004, Amann and Veeramachaneni, 2007, Hutson et al., 2013, Hutson et al., 2016, Hadziselimovic, 2017). Established biological knowledge that the higher testicular temperature environment resulting from the physical location of cryptorchid testes (away from the scrotum) causes germ cell depletion and infertility (Setchell, 1998). |
| Empirical Evidence WoE | |
| KE3 lead to AO1 (Indirect) High |
Dose, temporal and incidence concordance well supported in a single study on DPP (Table 2), and for a small number of LMWPs when combining data across multiple studies (Supplemental Table 2: BBP data limited, DBP and DEHP consistent with KER). Pattern of observations within a broader biological dataset supports the KER (Supplemental Table 3). A dependent change in both events is observed in the same study for a number of LMWPs (Supplemental Table 1). |
| KE3 leads to KE6 (Direct) Moderate |
Dose, temporal and incidence concordance well supported in a single study on DPP (Table 2), support for concordance is challenged across a small number of LMWPs when combining data across multiple studies (Supplemental Table 2: BBP data limited, DBP consistent and DEHP inconsistent but data limited). Pattern of observations within the broader dataset supports the KER (Supplemental Table 3). A dependent change in both events is observed in a same study for a number of LMWPs (Supplemental Table 1). Inconsistent data possibly implicating failed CSL regression (and not gubernacular agenesis) in the LMWP literature (Supplemental Table 4) is comparatively minimal to the evidence supporting involvement of the gubernaculum. |
| KE6 leads to AO1 (Direct) Moderate |
Dose, temporal and incidence concordance well supported in a single study on DPP (Table 2). Support for concordance is challenged across a small number of LMWPs when combining data across multiple studies (Supplemental Table 2: BBP and DEHP consistent with the KER; DBP inconsistent but data limited). A dependent change in both events is observed in the same study for a number of LMWPs (Supplemental Table 1). Inconsistent data possibly implicating failed CSL regression (see above). |
KE3 = decreased Insl3 expression; KE6 = agenesis of the gubernaculum; AO1 = cryptorchidism; KER = key event relationships; WoE = weight of evidence.
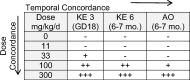
The extent of empirical support for the KERs in AOP1 is summarized in Table 1 and range from moderate to high. Though a dose responsive effect following exposure to a small number of LMWPs is supported for KE3, KE6 and AO1 (KE increased in incidence/severity with increasing dose of phthalate, See Supplemental Table 1), empirical evidence to support concordance of KERs downstream from the MIE ranged from low to high. One study with DPP, shows strong dose, temporal and incidence concordance between Insl3 expression, gubernaculum effects and testicular descent (Table 2) (Grey et al., 2016), supporting high confidence in the AOP. As all KEs were very rarely measured in a single study, concordance of KEs for a wider range of LMWPs was assessed by compiling observations from multiple studies (Supplemental Table 2). The low frequency of observations for KE6 in this data set was a notable limitation in the confidence assessment of KER concordance. Variation in experimental design, measurement methods, and measurement time points also challenged the calibration of these data. Overall, concordance between KEs was moderately supported when combining data across multiple studies for DBP and DEHP, and not well supported for BBP (likely due to limited data). Indirect (or ‘other’) empirical evidence from the broad biological data set depicts a pattern of observations consistent with the KERs and includes 1) Wistar rats exposed to LMWPs had a higher incidence of gubernacular lesions and lower Insl3 mRNA levels compared to Sprague Dawley rats with a higher level of Insl3 and no gubernacular lesions; 2) LMWP-exposed rat phenotype was identical to that observed in Insl3 KO mice which displayed freely moving testes that were not attached to the abdominal wall by the CSL or gubernaculum; 3) undescended testes and gubernacular malformations co-occurred in adult male Sprague-Dawley rats exposed in utero to a mixture of phthalates (Supplemental Table 3). In totality, these evidence support moderate confidence in the AOP based on consideration of empirical concordance and indirect observational evidence.
Table 2.
Dose, temporal and incidence concordance of KEs in AOP1. Observations as reported by Gray et al. (2016) on dipentyl phthalate (DPP) support concordance of KEs. A dash (−) indicates no effect; a plus (+) indicates effect observed; number of pluses indicates increased severity/incidence. Severity/incidence of effect weighting are provided as part of Supplemental Table 2. An AOP supported by empirical data shows early events occurring at lower doses and with higher severity than later events. GD = gestational day; mo. = months.
 |
Considering the major decision points and uncertainties in this pathway: in the transabdominal phase of testicular descent, the position of the testis is a function of both the swelling of the gubernaculum and the regression of the CSL (Klonisch et al., 2004, Nation et al., 2011). Early biology studies implicate a role for androgens in CSL regression and propose inappropriate outgrowth of the CSL could lead to cryptorchidism (van der Schoot and Emmen, 1996, Emmen et al., 1998). Because LMWPs impact androgens during GD 15.5–18.5, it is also important to consider an alternative AOP where reduced androgen and impaired CSL regression could lead to cryptorchidism. Analysis of the available literature show comparatively little evidence to support a role for the CSL (Supplemental Table 4). The LMWP literature does not substantiate well, a link between stunting of the CSL and transabdominal testicular descent failure although there are only a few studies that capture CSL observations (Gray et al., 2000, Gray et al., 2009); moreover, the developmental biology literature does not support well impaired CSL regression as a primary cause of cryptorchidism during the transabdominal phase (van der Schoot and Emmen, 1996, Emmen et al., 1998, Adham and Agoulnik, 2004, Kassim et al., 2010, Mamoulakis et al., 2015). It is also important to consider an alternative AOP where reduced androgen could work in concert with INSL3 or alone to cause gubernacular agenesis. Particularly as the severity of LMWP mediated cryptorchidism has been correlated with greater androgen impact in some studies (Howdeshell et al., 2015, van den Driesche et al., 2017). However the pattern of observations in the collective LMWP data set are inconsistent with a causal link between testosterone (Supplemental Table 4). Most notably, experiments in different rat strains (Supplemental Table 3) show a strain-specific sensitivity to LMWP mediated cryptorchidism implicating INSL3 rather than testosterone in testicular descent (Wilson et al., 2007). LMWP studies in Sprague-Dawley rats show a greater reduction in testosterone levels is associated with lower rates of cryptorchidism as compared to studies in Wistar rats demonstrating a greater impact on INSL3 and a higher incidence of cryptorchidism and gubernacular lesions (Mylchreest et al., 1998, Mylchreest et al., 1999, Mylchreest and Foster, 2000, Mylchreest et al., 2000, McKinnell et al., 2005, Wilson et al., 2007). The developmental biology literature also supports a change in testosterone is not necessary to alter gubernacular development. Developmental biology evidence does show testosterone plays an important role in the postnatal inguinoscrotal phase of testis descent (Nation et al., 2009, Hutson et al., 2013, Hutson et al., 2016, Hadziselimovic, 2017) as is described in AOP288 (https://aopwiki.org/); however, the life stage specificity of AOP288 to postnatal development excludes the relevance of these KEs to cryptorchidism initiated during the MPW. Based on limited evidence, a role for the CSL was ruled out.
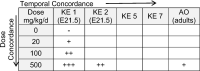
Table 4.
Dose-response and temporal concordance of KE in AOP2. Observations as reported in van den Driesche et al. (2012) on DBP support concordance of early KEs with the AO. A dash (-) indicates no effect; a plus (+) indicates effect observed; number of pluses indicates increased severity/incidence; a blank cell indicates event not measured. Severity/incidence of effect weighting are provided as part of Supplemental Table 5. An AOP supported by empirical data shows early events occurring at lower doses and with higher severity than later events.
 |
Considering the pathway as a whole, the extent of evidence is concluded as high in support of AOP1 upon consideration of high biological plausibility, moderate empirical evidence, and uncertainties. Confidence is high in support of the life stage relevance of this AOP during fetal life and more specifically the gestational timing of the MIE, KE1 and KE6 to the MPW of fetal development (van den Driesche et al., 2017). Despite the unknown MIE, confidence in this AOP for use in regulatory application is considered high based on the confidence in the downstream KERs, however clarity on the MIE may improve advancement of alternative approaches capable of screening or identifying developmental stressors.
3.1.2. AOP2: sustained expression of coup-TFII to hypospadias
Development of the anogenital tract is a complex process which is temporally and spatially dependent (Supplemental Fig. 1). Signaling needs to occur in a complex continuum to have appropriate differentiation of the anorectal tract and genitourinary tract, as well as appropriate growth of external genitals. In the early stages coordinated signaling across time is hormonally independent, and needs to occur in various disparate structures simultaneously for normal development to occur. Later, signaling becomes hormonally responsive in order to sexually differentiate the genital tract into the male or female form. Disruption of this process can lead to various malformations of differing severity depending upon what signals are disrupted and at what time point. The precursor to the external genital is the genital tubercle (GT). Growth of the GT consists of two phases. The first phase consists of the initial outgrowth and patterning of the GT, which occurs in both males and females, and results in the bipotential GT (Miyagawa et al., 2009, Petiot et al., 2005). This phase occurs from roughly GD10.5 when the genital swellings first appear to GD15/15.5 which is the start of sexual differentiation of the GT (Ipulan et al., 2014; Murishma et al., 2015). The end of Phase I coincides with the start of the male programing window (MPW) (Petiot et al., 2005; Welsch et al., 2008; van den Driesche et al., 2012, Miyagawa et al., 2009). During the first phase growth and patterning occurs the same in males and females, with no, as yet, discernable differences. The second phase is hormonally mediated and entails either continued growth and differentiation of the penis, or the arrest of outgrowth and differentiation into the clitoris (Petiot et al., 2005, Suzuki et al., 2014).
Of interest to this assessment were processes specific to development of the external male genitalia and the disruptions leading to hypospadias. Penile malformations elicited by prenatal exposure to LMWPs in rats are typified by the development of hypospadias arising from a proximal shift in the urethral meatus (Supplemental Table 1; (Foster, 2006). Using a rat model, gestational exposure to DBP during the MPW was sufficient to cause hypospadias, with gestational exposures outside this gestational window unable to elicit this abnormality (van den Driesche et al., 2017). A similar effect pattern is observed after treatment with androgen receptor (AR) agonist flutamide (Foster and Harris, 2005) and 5-α reductase inhibitor finasteride (Clark et al., 1993) where treatment at GD17/GD18 and GD16-17, respectively, induced hypospadias but treatment earlier or later did not. Even though each stressor operates via a distinct molecular initiating event, exposure of each only induces hypospadias during a short developmental time window in rats, which supports the developmental occurrences during this window as being critical androgen mediated events in normal penile development.
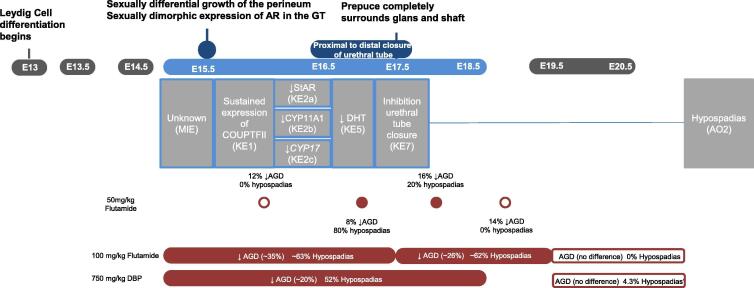
This narrow window means the anatomical mechanisms of relevance include growth of the GT as well as the formation of the internal urethra and proper positioning of the urethral meatus at the tip of the penis (Georgas et al., 2015). Evaluation of molecular events occurring during urethral development together with observational data on LMWPs suggest a pathway with four KEs initiated by an unknown MIE leading to one AO: 1) sustained COUP-TFII expression in Leydig cells; 2) decreased steroidogenic biosynthetic protein expression (StAR, CYP11A1, CYP17); 3) decreased 5α-dihydrotestosterone (DHT); 4) inhibition of urethral tube closure (decrease in Mafb expression) and 5) hypospadias (Fig. 2).
Fig. 2.
Sustained expression of COUP-TFII to hypospadias pathway (AOP2). Leydig cells are the cellular target for KE1 & KE2, the genital tubercle is the target organ for KE5 & KE7, and the penis is the target organ for the AO, hypospadias (AO2). KEs outlined in blue are proposed to occur during the MPW. Events are aligned to the developmental timeline such that events sharing the same vertical plane occur in the same developmental window. The horizontal planes depict: 1) biological process occurring, 2) embryonic day (E), 3) AOP, and 4) subset of data that supports the pathway being specific to the indicated developmental window. These data demonstrate a single 50 mg/kg dose (Foster and Harris 2005) or repeated 100 mg/kg dosing (Welsh et al., 2008) of the AR antagonist flutamide needs to occur within the MPW to induce hypospadias. Repeat dosing with 750 mg/kg/d DBP (van den Driesche et al., 2017) leads to a high incidence of hypospadias when delivered during the MPW. Exposure during the late gestational window (open bar) result in a low incidence (in the range of background) of hypospadias (4.3%). Note: KEs are numbered according to the network (Fig. 4). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The first proposed event in this pathway is sustained expression of COUP-TFII in Leydig cells of the testis (KE1). Greater than 85% of Leydig cells are COUP-TFII positive at E15.5, the beginning of the male programing window and roughly the time when testosterone production begins to increase. Following E15.5 there is a decrease in Coup-TFII positive Leydig cells with only ~10% of the cells remaining positive by E21.5 which is consistent with the increasing levels of androgen during this developmental window (van den Driesche et al 2012). The highest levels of COUP-TFII positive cells precede steroid synthesis and increases in steroid levels are dose dependently associated with a decline in COUP-TFII expression, supporting the potential for a causal relationship between the decline in COUP-TFII and steroidogenesis. COUP-TFII has been shown to act as a transcriptional repressor of gene expression, possibly through competition for occupancy of binding sites. The ability of COUP-TFII to suppress SF1 activation of KE2 (Shibata et al., 2003) genes is central to the proposed AOP. Plummer et al. (2007) demonstrated impacts on the expression of steroidogenesis genes by DBP exposure only during the time period when COUP-TFII is decreasing during normal development (GD 17.5 and GD 19.5) with no effect on GD15.5 before COUP-TFII expression normally declines in Leydig cells. COUP-TF II is an orphan nuclear receptor which interacts with the corepressors N-CoR, SMRT and RIP13, to silence transcription by active repression and transrepression (Qiu et al., 1994, Bailey et al., 1997). LMWPs have been shown to activate some members of the nuclear receptor superfamily, (i.e. PPARs) (Bility et al., 2004). The MIE may be specific ligand activation of COUP-TFII, or potential disruption of required cofactors. Further research is required to identify precise MIE for COUP-TFII sustained expression.
One of the important features of this pathway is that androgen effects are mediated by DHT rather than testosterone and this is supported by an abundance of literature demonstrating a role for DHT in urethral tube development (Table 3). The site of androgen synthesis changes during development with androgen precursors first synthesized in fetal Leydig cells on GD 14.5 in rodents when cholesterol was transported to the mitochondria by steroidogenic acute regulatory protein (StAR). In fetal Leydig cells, lack of a key conversion enzyme (HSD17β3) means conversion of cholesterol to testosterone occurs outside the Leydig cells (Wen et al., 2016). Conversion of testosterone to the more potent DHT occurs at the site of DHT action (i.e. DHT target tissues). This information supports that testosterone is not present in the biological compartment (i.e. Leydig cells) specified for KE1 and KE2 during this life stage. As testosterone is not the active androgen responsible for the differentiation of the genital tubercle (target organ) for this AOP, it was not included as a KE in the AOP for hypospadias. The proposed AOP aims to depict the critical few events necessary to progress from the unknown MIE to AO. Assessment of testosterone could be used as an indirect measurement of KE2 or KE5, as it is one of a few intermediary steps between these KEs. Anogenital distance (AGD), however, is considered a more reliable indicator of DHT levels during the male programming window. Using anogenital distance (AGD) as a biomarker gives an assessment of DHT level throughout the whole MPW window (McIntyre et al., 2001). It is important to emphasize that while AGD is an important indicator of the DHT environment during development (Dean et al., 2012, Dean and Sharpe, 2013, Suzuki et al., 2015) a change in AGD is not a KE in this AOP because a reduction in AGD is not essential for hypospadias to occur. It is the change in DHT (which the AGD measurement reflects) which is the causal event. This has been demonstrated in knock out studies in mice where AGD effects were disassociated from hypospadias (Suzuki et al., 2014). As such AGD is considered a useful biomarker but not a KE in the progression of this AOP.
Table 3.
Assessment of biological plausibility and empirical evidence in support of the KERs in AOP2.
| KER (Adjacency of KEs) WoE Conclusion |
WoE Rationale |
|---|---|
| Biological Plausibility WoE | |
| KE 1 leads to KE 2 (Direct) Moderate |
Established mechanistic basis that SF-1 regulates StAR, Cyp11a1, and Cyp17 genes. Coup-TFII is a known transcriptional repressor and steroidogenesis genes have SF-1 and COUP-TFII overlapping binding sites in the promoter. Limited biological and mechanistic evidence that COUP-TFII displaces SF-1 from binding to target genes leading to down regulation of genes critical for steroidogenesis (van den Driesche et al., 2012). Some evidence based on dose response studies with COUP-TF1 which shares a conserved binding domain with COUP-TFII (Shibata et al., 2003). |
| KE2 leads to KE5 (Direct) High |
Established biological knowledge of the steroidogenic process and that enzymatic disruption impacts male hormones (DHT) levels (Hasegawa et al., 2000; Hu et. al., 2002; Barsoum and Yao 2006; Miller and Walter 2007; Turcu and Auchus 2015). |
| KE5 leads to AO1(Indirect) High |
Established biological knowledge across a range of chemicals (flutamide, finasteride) and clinical outcomes that disruption of DHT (reduced production or interference with receptor binding) causes hypospadias (Kim et al., 2002, Turcu and Auchus, 2015). Established mechanistic basis that the importance of testosterone in external genital development is exclusively as a precursor for DHT; testosterone does not play a role. (Hib and Ponzio, 1995, Tian and Russell, 1997). |
| KE5 leads to KE7 (Direct) Moderate |
Strong but limited biological evidence that MAFB (serving as an indicator of urethral tube closure) is androgen dependent (sexually dimorphic expression, co-localization with AR receptor expression, and AR dependent expression) (Suzuki et al., 2014). |
| KE7 leads to AO2(Direct) High |
Established biological knowledge based on Mafb-/- mice with hypospadias phenotype (Suzuki et al., 2014). Limited mechanistic basis: transcription factor MAFB expressed in the mesenchymal cell population of the genital tubercle adjacent to the urethral plate (cell population critical for urethral tube formation and closure) (Nishida et al., 2008, Miyagawa et al., 2009, Suzuki et al., 2014). |
| KE2 leads to AO2 (Indirect) Moderate |
Male Cyp11a1 KO mice have external female genitalia which demonstrates a direct link with the development of male external genitalia and a male like urethra (Hu et al., 2002). |
| Empirical Evidence WoE | |
| KE1 leads to KE2* (Direct) Moderate |
Dose and incidence concordance well supported in a single study on DBP (Table 4). Dose, incidence concordance challenged when combining data across multiple studies for DBP (Supplemental Table 6). Pattern of observations within the broader dataset supports a relationship between KEs (Supplemental Table 7). |
| KE2* leads to KE5** (Direct) Moderate |
Dose and incidence concordance well supported in a single study on DPP (Supplemental Table 5). Dose and incidence concordance supported when combining data across multiple studies for DBP (Supplemental Table 6). A dependent change in both events is observed in the same study for a number of LMWPs (Supplemental Table 1) |
| KE5** leads to AO2 (Indirect) Moderate |
Dose and incidence concordance well supported in a single study on DPP (Supplemental Table 5). Data lacking to support concordance when combining data across multiple studies for DBP (Supplemental Table 6). Association between severity of DHT impact (as measured by severity of AGD reduction) and hypospadias incidence has been demonstrated (Supplemental Table 7). A dependent change in both events is observed in the same study for a number of LMWPs (Supplemental Table 1). |
| KE5** lead to KE7 (Direct) Low |
Data on markers of urethral tube disruption (KE7) were not found. Pattern of observations within the broader dataset supports the KER (Supplemental Table 7) |
| KE7 leads to AO2 (Direct) Low |
Data on markers of urethral tube disruption (KE7) were not found in. Pattern of observations within the broader dataset supports the KER (Supplemental Table 7). |
*The WoE assessment focused on data measuring steroidogenesis gene transcription/protein expression. **The WoE assessment relied on AGD as a surrogate measure for DHT during the MPW. Testosterone was not used as a surrogate for KE2 or KE5 in this WoE assessment. KE1 = sustained Coup-TFII expression; KE2 = decreased steroidogenic biosynthetic protein expression (StAR, CYP11A1, CYP17); KE5 = decreased DHT; KE7 = inhibition of urethral tube closure; AO2 = hypospadias; KER = key event relationships; WoE = weight of evidence.
Appropriate DHT signaling is necessary for proper penile development but not sufficient as additional factors need to occur to ensure the proper closure of the urethral tube, which, if disrupted, lead to hypospadias, suggesting alternative androgen independent AOPs for hypospadias exist as well. Evidence of the lack of sufficiency is provided by data from Mafb knockout (KO) mice as these animals demonstrate a failure in urethral tube formation and closure despite having similar levels of DHT in the GTs as their wild type counter parts (Suzuki et al., 2014). Mafb KO also show no discernable difference from wild type animals in AGD measures, which further supports this measure as a reliable marker of DHT levels and not a causal factor in the AOP (Suzuki et al., 2014). One confounding factor reported by Foster and Harris (2005) showed a single exposure to flutamide on GD 16 or GD 19 impacted AGD, but did not lead to hypospadias. These gestational days coincides with the period before proximal fusion of the urethra begins, and after urethra fusion is complete which is consistent with the proposed AO but suggests AGD may not be a perfect indicator as it is influenced outside of the window of effect. We suggest the addition of KE7, inhibition of urethral tube closure, and propose Mafb gene expression as an indicator of disruption, to further capture the AO progression. Unlike some of the other genes which are critical for normal GT growth, deletion of Mafb does not affect development of the GT prior to the critical androgen dependent programming window (Suzuki et al., 2014). Additional work suggest that Fgf10 and FgfR2, also appear to be critical for proper urethral tube closure, though these genes appear to be related to both growth and fusion events (Petiot et al., 2005, Leung et al., 2016). Temporal expression patterns of Mafb are consistent with developmental timing of urethral tube closure as gene expression is evident by GD16.5, the start of proximal fusion (Suzuki et al., 2014) and the KO animals provide direct evidence that MAFB has a key role in urethral tube closure. There is also strong biological plausibility for a KER between DHT and Mafb expression. Data from Androgen receptor (Ar) KO mice, provide evidence of a causal relationship between DHT and Mafb expression, as Ar KO male GTs have down regulated Mafb signaling as compared to wild type animals (Suzuki et al., 2014). Addition of a KE specific for urethral tube closure may also facilitate network expansion as it differentiates the proposed AOP based on androgen dependent mesenchymal differentiation to form the urethra and what would be a separate AOP for androgen-dependent organ size control, which would branch after DHT. (Suzuki et al., 2014). As no experimental evidence provided dose and temporal concordance data for Mafb expression, further work would be needed to explore this potential KE for AOP2 and the utility of Mafb as a marker.
The level of confidence in the KERs of AOP2 based on consideration of biological plausibility is summarized in Table 3 and range from low to high. In general, the developmental biology supports well the latter half of the AOP, with reduced insight provided into the early/upstream KEs. The biological and mechanistic understanding of the steroidogenesis pathway is well documented and the importance of DHT in urethral tube development broadly demonstrated and accepted. However, the ability of the transcriptional repressor COUP-TFII to suppress steroidogenesis genes has not been widely established. Additionally, the mechanistic underpinnings of DHTs effects on the urethral tube and necessity of another key molecular event in the urethral tube downstream of DHT is not understood. Considering the pathway as a whole, the biological plausibility is moderate based on the well-established link between androgen and urethral tube development; but limited understanding of upstream regulation of steroidogenesis genes.
The extent of empirical support for the KERS in AOP2 is summarized in Table 3. Though a dose responsive effect following exposure to LMWPs is supported for most KE, empirical evidence to support concordance of KERs ranged from low to moderate. A study by van den Driesche et al (2012) demonstrates well dose and incidence concordance of the early KEs (Table 4), however measurement of these events on the same gestational day (e21.5) prevents assessment of temporal concordance and did not measure KE5 and KE7. A study by Gray et al. (2016), demonstrates dose, incidence and temporal concordance between KE2, KE5 and AO2 (Supplemental Table 5), but does not measure KE1 or KE7. Compiling observations from multiple studies on DBP with various experimental designs provides moderate support for concordance between KERs (Supplemental Table 6), with KE7 continuing to be a key observational gap in the LMWP literature. KERs are supported by indirect observational evidence showing 1) overlapping DNA promotor binding sites for SF-1, a transcription factor important for steroidogenic gene transcription, and COUP-TFII on StAR, Cyp11A1 and Cyp17, and 2) co-localization of AR expression and Mafb in cell population of GT critical for urethral tube closure (Supplemental Table 7). Considering the pathway as a whole, the extent of empirical evidence in support of the sequence of events laid out in AOP2 is concluded as moderate upon consideration of empirical concordance and indirect observational evidence.
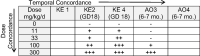
Table 6.
Dose, temporal and incidence concordance of KEs in AOP. Observations as reported in Gray et al. (2016) on DPP, supports concordance of a subset of KEs. A dash (−) indicates no effect; a plus (+) indicates effect observed; number of pluses indicates increased severity/incidence; a blank cell indicates event not measured. Note for KE2, the severity of effect at a given dose varied among the steroidogenesis genes. The severity indices here reflects an average impact between StAR, CYP17 and CYP11. Severity/incidence of effect weighting are provided as part of Supplemental Table 9. While sperm parameters were reported, parameters of impact on sperm maturation were not reported. An AOP supported by empirical concordance shows early events occurring at lower doses and with higher severity than later events. GD = gestational day; mo. = months.
 |
The KEs in this AOP are linked to developmental events which are thought to occur on specific gestation days, and the proposed AOP is disruption of the occurrence of events, not regression of said event. However some of the data suggest this may be incorrect as the AO occurs in animals treated with a stressor after completion of the developmental event. Specifically the developmental literature evaluated suggests urethral tube closure is complete by E17.5, however in one study (Foster and Harris 2005) hypospadias are observed after a single treatment on GD18 and in a second study (van den Driesche et al., 2017) hypospadias occur after treatment begins on GD19.5 (Fig. 2). These data could suggest an alternative to KE5 or could be explained by discrepancies in the data. In van den Driesche et al., (2017) only a single animal was observed with mild glandular hypospadias arising from late window exposure (750 mg/kg DBP), which could be due to a non-chemically mediated background incidence effect, or from a fetus with a delayed gestational stage (resulting in exposure during the window of sensitivity). A mild glandular hypospadias would be consistent with disruption at the very end of urethral tube development. The incidence level of hypospadias in Foster and Harris (2005) of 20% suggests a true chemically mediated event and not background incidence, however the discrepancy could be due to how the AOP was constructed, rather than an error in the data. The developmental landmarks described in Fig. 2 were identified from mouse literature (Petiot et al., 2005, Georgas et al., 2015, Ipulan et al., 2016) and designated by E whereas the chemical challenge data were developed in rats and designated by GD in the studies (Foster and Harris, 2005, Welsh et al., 2008). The Foster and Harris (2005) data could indicate there is not a direct alignment between mouse and rat development and closure of the urethral tube in rat occurs later, sometime between GD18 and GD19.
A difference in developmental timing, and thus window of susceptibility, is supported by data in mice that show when mice are treated with flutamide at E17.5 and E18.5 hypospadias do not occur (Zheng et al., 2015), which is consistent with the developmental information in mice indicating urethral closure is complete by E17.5. The MPW, which is defined as the window of susceptibility for this AOP, has been less well defined in mice. Some references indicate sexually dimorphic differences beginning at GD15 (Seifert et al., 2008) and other indicate the MPW is set at GD 15.5, the same as rats (Cohn, 2011, Miyagawa et al., 2009). Some of the primarily literature in mice demonstrates that flutamide treatment between GD15.5–16.5 and GD16.5–17.5 causes demasculinization of the external genitalia, whereas treatment between GD13.5–14.5 and GD14.5–15.5 has no effect on male genital development (Miyagawa et al., 2009). Consistent with the male data, females in this study treated with testosterone propionate (TP) during the window of sensitivity (E15.5-E16.5) develop masculinized genitalia (Miyagawa et al., 2009). These data are consistent with a MPW in mice similar to that observed in rats. However the data generated by Zheng et al. (2015) suggests the MPW in mice may be different. Treatment of male mice with flutamide did not have any impacts when it occurred at E17.5-E18.5 whereas treatment on E12.5–E13.5, E13.5–E14.5, E14.5–E15.5, or E15.5–E16.5 resulted in varying degrees of hypospadias (Zheng et al., 2015). Though treatment from E15.5-E16.5 is consistent, the earlier treatments lie outside the window of susceptibility. The data from Zheng et al. (2015) suggest the AOP as defined here should be taxonomically limited to rats until the discrepancies in the mouse data are resolved. In addition species differences in phthalate literature (reviewed in Johnson et al., 2012) suggest the MIE may be taxonomically restrained.
Considering the pathway as a whole, the extent of evidence is concluded as moderate in support of AOP2 upon consideration of moderate biological plausibility, moderate empirical evidence, and uncertainties. Confidence is high in support of the life stage relevance of this AOP during fetal life and more specifically the gestational timing of the KE upstream of the AO to the MPW of fetal development (Foster and Harris, 2005, Welsh et al., 2008, van den Driesche et al., 2017). The reduced confidence in the early KERs should be considered in applications of this AOP. Clarity on the MIE and increased confidence in early KERs, would improve utility in regulatory applications and inform the advancement of alternative approaches capable of screening or identifying developmental stressors.
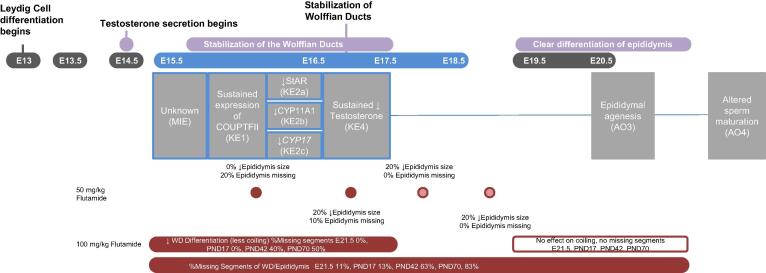
3.1.3. AOP3: sustained COUP-TFII to epididymal agenesis and altered sperm maturation
The Wolffian ducts (WD) are the progenitors of the epididymis, vas deferens and seminal vesicles. There are three developmental processes that are considered to be important during the development of the WD: (1) mesonephros formation, (2) stabilization (~GD 15.5–18.5) and differentiation (~GD19-birth) of the WD, and (3) postnatal differentiation (Murashima et al., 2015). The WD develops in both the male and female embryo as part of the first process. However, during the second process, regression of the duct occurs in females whereas androgen mediated stabilization (E 15–18) and retention of the ducts occurs in males (Murashima et al., 2015; Shaw and Renfree, 2014; Welsh et al., 2007). Following stabilization in males, tubular elongation, and morphological differentiation occur to form the epididymis, vas deferens, and seminal vesicle. Ductal elongation and coiling of the epididymis continue from ~ E18 until after birth, with regions of the epididymis becoming morphologically distinct after birth (Murashima et al., 2015).
Epididymal malformations are one of the most prevalent AOs observed following exposure to LMWPs in the MPW (Mylchreest et al., 1998, Barlow and Foster, 2003, Foster, 2006). The malformations have been most commonly assessed in adult animals that were exposed as fetuses in utero and are broadly characterized as “epididymal agenesis” describing reduced number of ducts, malformed, absent, or partial epididymides; in the fetus (~GD21.5) the outcome is described as decreased coiling (Mylchreest and Foster, 2000, Mylchreest et al., 2002, Barlow and Foster, 2003). Targeted studies with LMWPs and the AR antagonist, flutamide, support exposure from GD 15.5–17.5 as necessary and sufficient to induce epididymal agenesis (Foster and Harris, 2005, Welsh et al., 2007, Wilson et al., 2007). This timing overlaps with the stabilization phase (~GD 15.5–18.5) of Wolffian duct (WD) development (the progenitor of the epididymis), a process known to be facilitated by androgens (Hannema and Hughes, 2007, Welsh et al., 2007, Swain, 2017). Combining what is known about the mechanisms of WD stabilization and observational data on LMWPs (Supplemental Table 1), AOP3 can be described by the following events: 1) sustained expression of COUP-TFII, 2) decreased steroidogenic biosynthetic protein expression (StAR, CYP11A1, CYP17), 3) reduced testosterone levels at the site of action in the WD, 4) altered WD development/epididymal agenesis, 5) altered sperm maturation (Fig. 3).
Fig. 3.
Sustained COUP-TFII to epididymal agenesis and altered sperm maturation (AOP3). Leydig cells are the cellular target for KE1, the epididymis is the target organ leading to altered sperm maturation as the AOs. KEs outlined in blue are proposed to occur during the MPW. KEs are aligned to the developmental timeline such that events sharing the same vertical plane occur in the same developmental window. The horizontal planes depict: 1) biological process occurring, 2) embryonic day (E), 3) AOP, and 4) subset of data that supports the pathway being specific to the indicated developmental window. These data demonstrate a single dose of the AR antagonist flutamide causes different effects on the epididymis (when measured at PND95-105) depending on when in gestation the exposure occurs (red circles indicates timing of single exposure) (Foster and Harris, 2005); extending the duration of flutamide exposure to include the late gestational window (red filled bars) leads to an earlier onset of observable epididymal lesions (E21.5) and higher incidence later in life (PND17, PND42 & PND70) than flutamide exposure during the MPW only; and treatment during the late gestational window (open bar) has no effects on the epididymis (E21.5, PND17, PND42, & PND70). Note: KEs are numbered according to the network (Fig. 4). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Unlike AOP2 where DHT acts as the active local androgen, testosterone is thought to act directly on the WD to mediate stabilization (Shaw and Renfree, 2014, Murashima et al., 2015). Due to the gestational timing of early KEs in the MPW, we consider the target organ of testosterone action to be the WD (and not the epididymis). As experimental observations tend to be measured late in gestation or postnatally (i.e. after WD differentiation), the AO is characterized in the epididymis. Some studies have indicated that the epididymal changes induced during gestation increase in incidence/severity (Fig. 3) and/or may not manifest as clear malformations until adulthood (Barlow and Foster, 2003). Therefore, while the life stage applicability of the AOP is fetal life, the optimal timing to measure the AO may be in later life stages.
The level of confidence in the KERs of AOP3 based on biological plausibility is summarized in Table 5 and range from low to high. While the basic understanding of WD development is sufficient to formulate a pathway, the fundamental understanding of the mechanisms regulating WD development is limited. A role of testosterone in the stabilization phase of WD development has been established, however the upstream regulating events and role for other androgens are knowledge gaps. Considering the pathway as a whole, the biological plausibility for AOP3 is concluded to be moderate.
Table 5.
Assessment of biological plausibility and empirical evidence in support of the KERs in AOP3.
| KER | |
|---|---|
| (Adjacency of KEs) | |
| WoE Conclusion | WoE Rationale |
| Biological Plausibility WoE | |
| KE1 leads to KE 2 | |
| (Direct) | |
| Moderate | See Table 3 |
| KE2 leads to KE4 (Direct) High |
Established biological knowledge of the steroidogenic process and that disruption of steroidogenic enzymes impacts male hormone (testosterone) levels (Barsoum and Yao, 2006, Hasegawa et al., 2000, Hu et al., 2002, Miller and Walter, 2007, Turcu and Auchus, 2015). |
| KE1 leads to AO3 (Indirect) Low |
Temporal and tissue-specific COUP-TFII ablation clarified its role in developmental processes (Qin et al., 2008, Lin et al., 2011). Data assessing the essentiality of COUP-TFII in WD, while limited, implicates COUP-TFII as an active promoter of WD elimination (Zhao et al., 2017). This evidence does not support this KER, however, as it implicates COUP-TFII in a direct role in the WD itself rather than in the testes. |
| KE2 leads to AO3 (Indirect) Moderate |
While plausible that impacting the key rate limiting process in testosterone synthesis (i.e. StAR delivery of cholesterol, to the inner mitochondrial membrane) will have dramatic impact on testosterone levels and subsequent impacts on epididymal development, data to support this are not available. Male mice lacking StAR have normal epididymis and some capacity for steroidogenesis (Caron et al., 1997). Male Cyp11a1 null mice have a smaller epididymis than their wild type counter parts. Histological sections of the Cyp11a1 null epididymis revealed reduced tubule size, smaller columnar epithelium, and absence of microvilli (Hu et al., 2002). Male Cyp17a1 KO mice lack Wolffian derivatives and are infertile (Aherrahrou et al., 2020). Data from these KO animals support that a cumulative effect on expression of these genes would plausibly impact epididymal formation. |
| KE4 leads to AO3 (Direct) High |
Androgen broadly proposed in the developmental biology literature as a primary factor in WD stabilization with a direct role for testicular testosterone accepted (Higgins et al., 1989, Hannema and Hughes, 2007, Shaw and Renfree, 2014, Murashima et al., 2015, Swain, 2017). Targeted studies with androgen receptor antagonist, flutamide, during the period of WD stabilization (GD 15.5–17.5) leads to epididymal agenesis (Foster and Harris, 2005, Welsh et al., 2007). |
| AO3 leads to AO4 (Direct) High |
Well accepted role of the epididymis in supporting sperm maturation. Fertility is dependent on sperm maturation in the epididymis (Joseph et al., 2009). |
| Empirical Evidence WoE | |
| KE1 leads to KE2* | |
| (Direct) | |
| Moderate | See Table 3 |
| KE2* leads to KE4 (Direct) Moderate |
Dose and incidence concordance well supported in a single study on DPP (Table 6) and when data were combined across multiple studies for DBP (Supplemental Table 9). Change in both events is observed in the same study for a number of LMWPs (Supplemental Table 1). |
|
KE4 leads to AO3 (Indirect) High |
Dose, temporal and incidence concordance supported in a single study on DPP (Table 6). Dose and incidence concordance supported for DBP when data were combined across multiple studies (Supplemental Table 9). Pattern of observations within the broader dataset supports a relationship between KEs (Supplemental Table 11). Change in both events is observed in the same study for a number of LMWPs (Supplemental Table 1) |
|
AO3 leads to AO4 (Direct) Low |
Data on sperm maturation parameters limited. Change in both events is observed in the same study for DBP (Supplemental Table 1). |
*This assessment focused on data measuring steroidogenesis gene transcription/protein expression. Testosterone was not used as a surrogate for KE2 or KE5 in this WoE assessment. KE1 = sustained expression of COUP-TFII; KE2 = decreased steroidogenic biosynthetic protein expression (StAR, Cyp11a, Cyp17); KE4 = reduced testicular testosterone levels; AO3 = altered Wolffian duct (WD) development/epididymal agenesis; AO4 altered sperm maturation.
BP = biological plausibility; KER = key event relationships; DS KE = downstream key event; US KE = upstream key event; ND = not determined; WoE = weight of evidence.
The extent of empirical support for the KERs in AOP3 is summarized in Table 5 and range from low to high. Though a dose responsive effect following exposure to a small number of LMWPs is supported for KE1, KE2, KE4, AO3 (KE increased in incidence/severity with increasing dose of LMWP, See Supplemental Table 1), empirical evidence to support concordance of KERs was limited for most KERs. In particular, all of the KEs in AOP3 were never measured together in a single study. For example, data from Gray et al. (2016) supports well dose, temporal and incidence concordance amongst KE2, KE3 and AO3 following exposure to DPP (Table 6), however does not measure KE1 or AO4. Evaluating temporal concordance for early KEs was also hindered based on when data were collected. For example, van den Driesche et al (2012) (Supplemental Table 10) supports dose and incidence concordance amongst KE1, KE2 and KE3, however all measurements were collected on the same day, and downstream KEs (AO3 and AO4) were not assessed. As all KEs were not measured in a single study, concordance of KEs was assessed by compiling observations from multiple studies on DBP. Variation in experimental design and measurement time points challenged this analysis, which ultimately does not support well a dose and temporal concordance between KERs (Supplemental Table 9). Confidence in the KER between testosterone and epididymal agenesis is increased upon consideration of indirect observational evidence (Supplemental Table 11) showing 1) SD rats exposed to LMWPs have a higher incidence of epididymal agenesis and a greater decrease in testosterone compared to Wistar rats comparably exposed which show a lesser effect on testosterone levels and lower incidence of epididymal agenesis; 2) the epididymal lesion described following LMWP exposure during the MPW is the same characterization of the lesion following exposure in the same gestational window to the AR antagonist flutamide. The limited number of studies evaluating sperm maturation parameters (versus spermatogenesis) was a notable data gap within the collective database. In totality, the limited availability of evidence to evaluate concordance of KERs supports a conclusion of moderate empirical evidence for the sequence of events laid out in AOP3.
Considering the major decision points and uncertainties in this pathway: the initial events in AOP3 are shared with AOP2 with an unknown MIE occurring between GD 15.5 and GD 18.5, leading to sustained expression of Coup-tfII in fetal Leydig cells. Increased COUP-TFII levels may promote increased steroidogenesis from fetal Leydig cells and/or play a direct role in WD regression independent from or enhanced by reduced androgen synthesis. Coup-tfII knockout models show COUP-TFII to be a suppressor of the mesenchyme-epithelium cross-talk responsible for WD regression (Zhao et al., 2017) which suggests sustained COUP-TFII alone could destabilize the WD leading to abnormal development. As the temporal window of WD stabilization overlaps with the MPW, it has been proposed that androgens may antagonize COUP-TFII in WD mesenchymal cells to support stabilization potentially through androgen effects on growth factor signaling (Hannema and Hughes, 2007, Swain, 2017, Zhao et al., 2017). As mentioned earlier, COUP-TFII is an orphan nuclear receptor and LMWPs have been shown to activate some members of the nuclear receptor superfamily, (i.e. PPARs) (Bility et al., 2004). Therefore plausible alternatives to the AOP shown in Fig. 3 include one where sustained COUP-TFII in the WD is necessary and sufficient to cause epididymal agenesis or where sustained COUP-TFII in the WD is further enhanced by the reduction of testosterone in the testes. Difficulty separating and experimentally manipulating androgen signaling and COUP-TFII in target tissues challenges the ability to untangle these interdependent processes. Whether or not initial KEs occur in the WD, LMWP data suggest androgen plays a role in either directly mediating or modulating the events of this pathway (Supplemental Table 12). Currently the biological plausibility of testicular testosterone impacting WD stabilization rather than COUP-TFII alone is better supported when considering the developmental biology literature and chemical stressor data together.
The specificity of gestational timing for the KE/KERs in AOP3 is another point of uncertainty in this analysis. As described above, experimental evidence supports the gestational timing of the early KEs to be during the MPW (Foster and Harris, 2005, Welsh et al., 2007, Wilson et al., 2007). However, the development and differentiation of the WD depends on continuous androgen stimulation that extends beyond the MPW and into postnatal life (Shaw and Renfree, 2014). We consider this long duration of androgen dependence notable to the proposed AOP for two reasons. First, many of the KERs defined in AOP3 likely have relevance beyond the restricted gestational timing proposed here. As shown in Fig. 3, a single 50 mg/kg exposure to the AR antagonist flutamide on GD19 (outside the MPW) adversely affects epididymis size. Likewise, the LMWP literature supports that the AOs of AOP3 can arise when exposure occurs both inside and outside the MPW (Kay et al., 2014, Czubacka et al., 2021). Therefore, KERs downstream from the MIE are plausibily relevant later in development and in other life stages. Second, reduced testosterone may need to be sustained for days (or longer) to impact the epididymis. As shown in Fig. 3, exposure to the AR antagonist flutamide from GD15 until birth results in an earlier onset of observable epididymal impacts and higher incidence in adulthood than exposures restricted to the MPW only. It has been shown that in utero exposure to DBP at high doses during the MPW only produces a sustained reduction in testosterone levels that persists into adulthood (van den Driesche et al., 2017). Therefore, it is possible a sustained reduction in testosterone that extends over a period of days or longer is necessary to elicit AO3. The refinement in the KE description of AO3 may also inform the life stage restriction of this AOP. As shown in Fig. 3, the nature of the epididymal effects may vary depending on timing of exposure, likely due to the differentiation status of the WD at the time of the reduced testosterone perturbation. As mentioned earlier, studies tend to collect multiple KE measurements simultaneously, capturing a snapshot in time typically after the MPW. This challenges the confidence in the gestational timing of KEs/KERs specified here. More detail on the nature, function and cellular state of the MIE would also inform life stage specificity. At this time however, available data support that later KERs in this AOP are plausibly relevant to other life stages; and the duration of reduced testosterone sufficient for manifesting the AO is unclear with initiation of reduced testosterone levels plausibly restricted to the MPW (Supplemental Table 12).
Considering the pathway as a whole, the extent of evidence is concluded as moderate in support of AOP3 upon consideration of moderate biological plausibility, moderate empirical evidence, and uncertainties. Confidence is high in support of the life stage relevance of the entire AOP during fetal life based on the restricted gestational timing of the MIE to the MPW (Foster and Harris, 2005, Welsh et al., 2007, Wilson et al., 2007). However, the confidence is low with respect to the duration of testosterone reduction needed to manifest the AOs. Confidence in this AOP for use in regulatory application is considered high if the uncertainties described are respected. Clarity on the MIE may improve advancement of alternative approaches capable of screening or identifying developmental stressors.
3.2. Putative AOP network for male reproductive outcomes
The three AOPs described in this work were assembled into a putative AOP network that aims to more realistically represent the biology occurring during this narrow window of gestational development. Identification of shared KEs amongst the AOPs considered both the sameness of the biological or physiological state and sameness of the gestational timing of when this same KE occurred in each pathway. As a general description, the network portrays primarily a divergent motif (Knapen et al., 2018) with the androgen independent pathway, AOP1, possibly sharing an unknown MIE with the androgen dependent pathways, AOP2 and AOP3, with all 3 pathways sharing a common population level AO (i.e. impaired fertility). A lack of a convergence point at testosterone clearly depicts a variable role of testosterone in all three networked AOPs. The confidence in the network is low due to the unidentified MIEs. These AOPs are considered to be open and under development and should be iteratively refined and elaborated via incorporation of new/existing relevant data.
Attention to gestational timing of KEs and KERs was a key focus throughout this effort and care was taken to align the KEs/KERs with the gestational timing of their causal importance in the network depiction. In doing so, the life stage applicability of this network is defined in a refined manner. The evidence on LMWPs clearly supports the timing of the stressor perturbation as needing to occur during the MPW to initiate all three AOPs. This means the function or structure of the MIE(s) may likely be restricted to this narrow window of development. While the unknown identity of the MIE(s) makes this impossible to conclude, it is clear that the life stage applicability of at least one early KE/KER in this network is restricted to the MPW. Unlike AOP1/2, KERs downstream from the MIE in AOP3 are plausibly relevant to other life stages. For example the KER of reduced testosterone (KE4) leading to epididymal agenesis (AO3) is likely relevant in postnatal life and the KER of reduced testosterone (KE4) leading to altered sperm maturation relevant into adulthood. The life stage applicability of an AOP is restricted by the most restrictive KER. A simple illustration of this concept is that cryptorchidism cannot be induced in adulthood. While some KERs in an AOP leading to cryptorchidism may be functionally relevant during adulthood, an AOP describing cryptorchidism is restricted to development.
Considering the major uncertainties in this network, the unidentified MIE is a clear data gap and limits confidence in the network. A common MIE is reasonably plausible based on indirect empirical evidence that exposure during a discrete window of development (the MPW) is necessary and sufficient for a common chemical initiator (LMWPs) to induce all three AOs. However, it is equally plausible that separate initiating events could be responsible. In either case it is likely the MIE(s) occurs in fetal Leydig cells. With further delineation of these pathways and developmental events, it could be determined that AOP1 belongs in the network, or that AOPs 2 and 3 only converge on KE1 and KE2 before diverging again. While the assumption of a common MIE is plausible, an undefined MIE means the inclusion of AOP1 in this network is uncertain. Potential MIEs were explored as part of this effort and some were discussed already in earlier sections. Reduced activation of PPARα has been proposed as responsible for reduced steroidogenesis (AOP#18 https://aopwiki.org) (Nepelska et al., 2017), however the confidence in this MIE is questioned based on studies describing the lack of PPARα-responsive gene expression in the fetal testis following LMWP exposure, inability of PPARα antagonists to reduce fetal testis steroidogenic gene expression or testosterone production (Hannas et al., 2011) as well as other empirical evidence (as discussed in (Arzuaga et al., 2019, Gray et al., 2016, Clewell et al., 2020). Further evaluation of the molecular biology literature plausibly implicates the nuclear receptor NR4A1 as a possible MIE based on its demonstrated ability to regulate INSL3 (Robert et al., 2006) and testosterone biosynthesis genes (HSD3B2, rat Cyp17a1, mouse Hsd3b1, and mouse StAR promoters) (Zhang and Mellon, 1997, Hong et al., 2004, Martin and Tremblay, 2005). More recently, phospholipase A2 (cPLA2) enzyme inhibition has been proposed as a possible MIE responsible for reduced steroidogenesis (Clewell et al., 2020). In this proposal, inhibition of cPLA2 leads to inhibition of arachidonic acid (AA) release from intracellular stores resulting in reduced steroidogenesis gene transcription. The plausibility of this is supported in the literature based on role of AA in mediating StAR gene transcription. The biological plausibility of cPLA2 or AA leading to reduced INSL3 was not addressed in this proposal with overall confidence in this MIE concluded as low by Clewell et al. (2020). The uncertainty in the MIE denotes the putative nature of this proposed network. The limitation this uncertainty poses to the utility of this network will depend on the specific network application.
A lack of empirical data in this instance to support the convergence point at the AO (impaired fertility) at the population level is also a point of uncertainty worth a brief mention. Clearly the testes, epididymis, and penis all serve a role in male fertility, supporting a biologically plausible connection point. While LMWPs have been associated with effects on fertility indices e.g. sperm, fertility index (Kay et al., 2014, Yost et al., 2019), careful evaluation of these data to inform the KER (i.e. initiated in the MPW, relevance of indices to the target organs) was out of scope of this effort. The uncertainty in the population level convergence point adds to the putative nature of this proposed network.
Expansion of the putative network (Fig. 4) was explored via connectivity to information available in the AOP-Wiki (https://aopwiki.org). The AOP-Wiki was searched for KE titles of similar nature to those herein. Hits were eliminated if the information in the title, KER description and/or life stage applicability domain clearly indicated a mismatch (see Supplemental Table 13 for search terms and results). The remaining KEs and associated KERs and AOPs were evaluated for relevance to this network. Three KEs were identified as similar in title and description to those herein: KE #1613, Decrease, dihydrotestosterone levels (KE#5, herein); KE#1616 cryptorchidism (AO1, herein); and KE#406 impaired fertility (AO5). Taking KE#1613 (decrease, DHT levels) first, only 1 of the 4 associated KERs (KER # 1935: Decrease, DHT levels leads to decrease AR activation) was concluded as relevant as it would refine further the mechanistic events occurring between KE5 and KE7 in AOP2. Regarding KE#1616 (cryptorchidism), the KE is relevant as it describes the same AO captured in AOP1, however the associated KER (#1938 impaired inguinoscrotal phase leads to malformation, cryptorchidism) is irrelevant as it refers to the second phase of testis descent which is outside of the narrowly defined gestational window herein. This AOP however would be relevant to a network expansion effort aimed at depicting the sequential nature of AOPs across developmental stages leading to cryptorchidism.
4. Discussion
The objective of this review was to develop AOPs as a means to evaluate the biological plausibility and empirical evidence supporting (or refuting) the linkage and gestational timing of biological events involved in mediating male reproductive tract abnormalities during fetal development. As it is a large effort to describe all of the possible perturbations through which stressors may lead to male reproductive tract abnormalities, we focused our efforts on characterizing the pathways through which LMWPs perturb biology during the androgen sensitive window of development. As it is also a large effort to describe the pathways responsible for all of the male reproductive tract effects associated with LMWP exposure, we focused on the pathways leading to cryptorchidism, hypospadias and epididymal agenesis. The AOPs described range in confidence from moderate to high and operate through androgen independent (AOP1, Fig. 1) and androgen-dependent (AOPs 2 and 3; Fig. 2, Fig. 3, respectively) mechanisms. With regard to the impact of androgens, both testosterone and DHT play an important role. To more realistically represent the complexity of the biology and possible stressor perturbation points during this dynamic period of development, a putative network was established. As testosterone was not a common KE across pathways, the putative network does not converge in the center in a bow-tie manner at testosterone as has been previously illustrated in the literature (Howdeshell et al., 2017, Clewell et al., 2020). Instead we propose a putative network linking AOP2 and AOP3 through decreased steroidogenic biosynthetic protein expression with convergence of all AOPS at the population level impaired fertility AO. While extensive data were relied upon to underpin the proposed AOPs, the confidence in each of the AOPs varies and gaps remain, including the MIEs for all AOPs. Through development of KER descriptions and WoE evaluation in a manner consistent with the OECD AOP framework (OECD, 2018), this work provides an ample contribution towards a holistic understanding of what is known and not known about the possible perturbation points and AOs relevant to this narrow window of gestational development.
A key challenge in generating this AOP network depicting processes that are dynamic, highly interconnected and occurring in a very short window of developmental time was avoiding inference and assumption due to study design and information limitations. Detailed time course studies restricted to the 3 day window of relevance (i.e. MPW) were limited making it difficult to assign cause and effect and untangle early androgen dependent pathways from those initiated later in gestation. Available studies tended to involve high dose levels to ensure induction of reproductive abnormalities, hindering dose concordance assessments. The ability to assess cause and effect was also limited by the timing of observations. Studies often assessed endpoints simultaneously rather than sequentially over time; and observations were often made after the gestational time points of interest. For example, measurement of testosterone outside of the MPW, and in particular in postnatal or adult animals, was considered of limited utility for informing the prenatal biology occurring in the MPW, especially if the stressor exposure was not limited to the MPW. Reliance on developmental biology literature was useful for clarifying, to some extent, where extrapolation or inference of the stressor observational data was reasonable versus unfounded. Targeted studies using lower potency substances targeting multiple MIEs with careful dose spacing and observational time points may prove useful to further separate intertwined processes. Identification, standardization and utilization of biomarkers of in utero effect are also seemingly useful in efforts to improve confidence and clarity in the network. For example AGD measured post-partum is a recognized biomarker for a change in DHT levels in utero (Sharpe, 2020), and as such serves as an in-life measurement that allows for additional observations at later time points in post-natal development. Unique experimental designs are likely needed to improve confidence in the proposed KERs and proposed network convergence/divergence points.
Defining the taxonomic applicability domain for these AOPs was out of scope of this effort. However, it should be emphasized that variations in mechanisms (molecular and anatomical) and gestational timing are expected to pose varying degrees of taxonomic restriction to these AOPs. In this effort, evidence underpinning biological plausibility (i.e. the developmental biology literature) was primarily from mice while the empirical evidence (i.e. the stressor data) was primarily from rats. As there are differences in androgen responses following in utero LMWP exposure between mice and rats (Johnson et al., 2012) and regulation of testosterone in fetal development is different in rodents than humans (Sharpe 2020), taxonomic relevance is particularly important to clarify for regulatory utility of these AOPs. For example, it is known that there is a fundamental difference in the regulation of testosterone production in the human fetal testis compared to rats (Scott et al., 2009, Sharpe, 2020). The disparity in steroidogenesis regulation between these two species is consistent with findings in fetal human testes where exposure to LMWPs, both in in vitro culture and as testis xenotransplants in immune compromised mice, results in no suppression of testosterone production (Lambrot et al., 2009, Mitchell et al., 2010, Heger et al., 2012). This suggests a potential impact to the taxonomic relevance of AOP2 and AOP3 in particular, as they are androgen dependent. To advance development of alternative methods and predictive models of human relevance, understanding of the taxonomic variations is important to consider and incorporate into network expansion efforts.
Previous publications have proposed other designs for male reproductive AOP networks based on the effects of LMWPs. These include an interrelated, ‘bow-tie’ network arising from both androgen-dependent and androgen-independent pathways (Arzuaga et al., 2019, Kortenkamp, 2020) with some merging clearly at the cellular testosterone response (Howdeshell et al., 2017, Clewell et al., 2020). The purpose for which these networks were developed varies as does the breadth of biological pathways they have integrated. However a notable commonality between them was the use of broadly defined network nodes (or KEs), where several biological, cellular or target organ states were collapsed or “grouped” into a single KE. For example, some presented INSL3 and androgen in a single networking node (Howdeshell et al., 2015, Howdeshell et al., 2017, Arzuaga et al., 2019), some grouped various hormones into a generalized ‘androgen response’ (Arzuaga et al., 2019, Kortenkamp, 2020), and most commonly the AOs were merged or grouped in a manner depicting all reproductive malformations as equivalent or able to substitute for another (Howdeshell et al., 2015, Howdeshell et al., 2017, Arzuaga et al., 2019, Kortenkamp, 2020). The grouping of KEs and manner in which KEs were described in these published networks (Howdeshell et al., 2015, Howdeshell et al., 2017, Arzuaga et al., 2019, Clewell et al., 2020, Kortenkamp, 2020) challenged our ability to build upon these efforts and hindered our ability to identify and address inconsistencies.
A KE is meant to define a single measurable event within a specific biological level of organization, with a KER meant to define a causal and predictive relationship between KEs (OECD, 2018). As discussed in Villeneuve et al. (2018a), the extent of biological resolution to include in an AOP is determined by the developer, and an AOP can be reduced to a minimal few KEs if the weight of correlative evidence is sufficient. If a KE encompasses several changes this may prove problematic to the base utility of the AOP. For example, it is easily foreseen how grouping of KEs will make quantification of KERs more difficult and obscure the biological understanding needed for development of alternative testing approaches for these outcomes. In the network presented in Fig. 4, the independence of cryptorchidism from testosterone is well supported, as well as the reliance of hypospadias on DHT rather than testosterone. Refining the KEs to specify androgens could provide regulators an additional level of understanding when considering compounds with less well-studied effects, For example, a stressor could theoretically produce offspring with normal testosterone levels but also show cryptorchidism (due to impacts on INSL3) or hypospadias (due to inhibitory effect on the enzymatic conversion of testosterone to DHT). Arguably, the network depiction in Fig. 4 may obscure connectivity of the biology as testosterone is not represented as the mechanistic precursor to DHT in AOP2. As steroidogenesis is a multi-step process, the level of biological resolution in the KE descriptions and KE selections will impact network points. Just as the grouping of KEs in the previously published networks (Howdeshell et al., 2015, Howdeshell et al., 2017, Arzuaga et al., 2019, Clewell et al., 2020, Kortenkamp, 2020) challenged our ability to build upon these efforts, our choices of KE descriptors could hinder linkage to other AOPs. More clarity on the extent of biological resolution needed for KEs will likely come as more AOPs relevant to male reproductive development are established.
An important area of focus during the construction of the AOPs/AOP network herein, was on gestational temporality and aligning the KE/KERs with gestational timing. Publications depicting AOPs/AOP networks associated with perturbation of androgen mediated reproductive development have, for the most part, acknowledged the importance of GD15.5–18.5 as necessary and sufficient for LMWPs to initiate the AOPs (i.e. trigger the MIE) (Howdeshell et al., 2017, Arzuaga et al., 2019, Clewell et al., 2020, Kortenkamp, 2020). However they are not so careful to define the window of development of the other KEs, or depict the separation in developmental time among networked AOPs. For example, Howdeshell et al (2017) utilizes an AOP network to depict the multiple mechanisms and MIEs by which LMWPs in combination with other stressors can interfere with androgen singling to disrupt male reproductive development to help inform an approach for cumulative risk assessment. The network depiction appears to link together the transabdominal phase of testicular descent (influenced by INSL3 and relevant during gestation in rats), and the inguinoscrotal phase of testicular descent (influenced by androgen and relevant during postnatal development in rats) in a manner portraying these pathways co-occur in biological time. However these two phases of testicular descent occur weeks apart in development. If cumulative risk assessment is concerned with how chemicals behave when they are present together, it may be important to distinguish pathways in a network that do not co-occur in biological time as this informs what is plausible with respect to the trajectory of outcomes. In addition, the inguinoscrotal phase of testicular descent (the androgen dependent phase) occurs in utero in humans and postnatally in rodents (Picut et al., 2017). This species difference in life stage applicability of the AOPs may restrict the taxonomic validity of the network or result in different network connections based on other species differences during this period of development. During development, life stage considerations may be the most restrictive boundary of how broadly data may be extrapolated, including potential human relevance. Life stage specificity is also important for informing when data become evidence in a chemical hazard assessment (e.g., testosterone levels measured in adulthood provides little value in informing on the ability of a chemical to influence testosterone levels during gestation), for guiding experimental design (e.g. duration of exposure or optimal observation time point), and may be key to reconciling inconsistencies in a chemicals ability to elicit effects across a group of studies with a wide range of experimental designs.
Current OECD AOP guidance (OECD, 2018) identifies life stage relevance as an important consideration in bounding the biological domain of applicability of an AOP. In the AOP-wiki, structured terms can be selected to identify life stage relevance with options to clarify further in a free text field. These structured terms are fairly broad (e.g. developmental, fetal, juvenile) compared to the refinement to gestational days/weeks depicted herein. To facilitate broader use of AOPs, incorporation of more refined life stage information beyond ‘in utero’ or ‘fetal’ life stage designation (e.g. gestational timing) should be encouraged. Incorporation of life stage information to include gestational timing in the KE details will facilitate generation of AOP networks that depict the toxicodynamics during a narrow development stage. This would allow for sequential network depictions of critical molecular and morphological progressions that more appropriately capture the complexity of developmental biology. Unfortunately, there is variation in the literature for the different fetal developmental stages in a given species and standard milestones across taxa that can be readily applied do not exist (Picut et al., 2017). However, this may be important to tackle in order to facilitate more informed linkages in the AOP-wiki between shared KEs and re-use of information by AOP-wiki users.
The OECD has recognized the importance of standardized organization of information as critical to support confidence in use of AOPs and guidance for AOP development is available from many sources (Villeneuve et al., 2014a, Fay et al., 2017, OECD, 2017, Knapen et al., 2018, OECD, 2018). However, the manner in which AOPs are developed and the pathway specificity with which they are characterized is still evolving and this experience suggests further clarity of the OECD AOP development standards is warranted. First, and as discussed above, variation in KE naming conventions, limited KE descriptions, and level of mechanistic abstraction when selecting KEs continues to challenge the development of sharable KEs. Deviations from the current OECD KE naming conventions may be needed to ensure appropriate level of biological refinement to improve connectivity among AOPs (Leist et al., 2017, Villeneuve et al., 2018b). As well, guidance on how explicitly to characterize an unknown MIE would improve utility of these knowledge gaps. For example, inclusion of maximal information on biological context and speculative molecular targets would avoid propagation of fragmented information and could facilitate appropriate networking particularly if a plausible MIE is entered into the AOP-Wiki (https://aopwiki.org) at a future date. Second, standardizing approaches to data collection may be a beneficial advancement of the AOP development process. In this effort, potential AOPs were first characterized based on developmental biology knowledge, followed by multiple rounds of targeted investigative literature searches (in both the biology and toxicology fields) to inform biological plausibility and empirical evidence to support KERs. While step-wise and meticulous, clear documentation of the key questions that drove the literature searches and clarity of literature relied upon (versus excluded) would have enhanced transparency in WoE underpinning these AOPs. Expanding the AOP framework to include standards for evidence identification has been suggested as a means to improve confidence (De Vries et al., 2021, Kleinstreuer et al., 2016; Leist et al., 2017); however the standards would have to be flexible to accommodate the iterative nature of the AOP development process and evergreen status of AOPs. Finally, inclusion of fit-for-purpose criteria may alleviate the evidence evaluation burden on AOP practitioners. It is currently the case that sufficiency of an AOP for a given purpose is user determined, even for AOPs that have gone through the OECD AOP review program (OECD, 2014, 2020). Indeed the OECD endorsement of AOPs refers to confidence in the scientific review process that the AOP has undergone rather than endorsement that the AOP is sufficiently complete for use in regulatory application (OECD, 2020). This means the AOP practitioner must commit to understanding the developers scope, decision points and evidence basis, to independently conclude if the AOP is fit for their intended use. In this effort succinctly capturing the basis of WoE while achieving adequate transparency was a big challenge. Reviewing the lengthy evidence contained herein poses a heavy burden on any user of these AOPs. Establishing “readiness for use criteria” (e.g. as proposed by Coady et al. (2019)) is likely important if the full potential of AOPs is to be realized.
The breadth of data and interdisciplinary knowledge exist to advance next generation toxicity test methods and the AOP framework represents a conceptual construct to facilitate and foster collaborative efforts to this end. The AOP network herein provides a foundation to advance additional efforts. Efforts to clarify how changes at the molecular level may relate to the AOs of concern by mining high-throughput, high- content databases could inform the MIE or lead to more rapid development of additional networked AOPs. The systems approach to identifying an array of potential molecular targets involved in male reproductive development by Leung et al. (2016) provides insights to this end; as does a recent publication by Gray et al. (2021) evaluating genomic responses in fetal testes exposed to an array of chemicals. Identifying the MIE would rapidly advance identification of a targeted in vitro assay for inclusion in an integrated testing approach for male reproductive development. Future efforts could also involve advancing quantification of KERs through computational analysis of legacy dose response data to generate mathematical prediction models. A clear next step towards collaborative utility of this network is entry of these AOPs into the AOP-Wiki.
CRediT authorship contribution statement
Christine M. Palermo: Conceptualization, Formal analysis, Writing - review & editing. Jennifer E. Foreman: Conceptualization, Formal analysis, Writing - review & editing, Visualization. Daniele S. Wikoff: Writing - original draft, Writing - review & editing. Isabel Lea: Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Christine Palermo and Jennifer Foreman are employees of a producer of high molecular weight phthalates. Daniel Wikoff and Isabel Lea are employees of ToxStrategies, a private consulting firm that provides services on toxicology and risk assessment issues to private and public organizations..
Acknowledgments
Acknowledgements
The authors gratefully acknowledge the efforts and work of a significant number of contributing authors and collaborators who have participated in the development, application evolution and advancement of the adverse outcome pathway concept including the OECD Extended Advisory Group on Molecular Screening and Toxicogenomics and contributors to the OECD-Sponsored AOP knowledgebase. Special thanks to Katy Goyak at ExxonMobil Biomedical Sciences for her insights during conceptualization and manuscript review.
This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. ExxonMobil Biomedical Sciences provided financial support to ToxStrategies for preparation of this manuscript. No authors received personal fees.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crtox.2021.07.002.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Adham I.M., Agoulnik A.I. Insulin-like 3 signalling in testicular descent. Int. J. Androl. 2004;27(5):257–265. doi: 10.1111/j.1365-2605.2004.00481.x. [DOI] [PubMed] [Google Scholar]
- Adham I.M., Steding G., Thamm T., Büllesbach E.E., Schwabe C., Paprotta I., Engel W. The overexpression of the insl3 in female mice causes descent of the ovaries. Mol Endocrinol (Baltimore, Md.) 2002;16(2):244–252. doi: 10.1210/mend.16.2.0772. [DOI] [PubMed] [Google Scholar]
- Aherrahrou R., Kulle A.E., Alenina N., Werner R., Vens-Cappell S., Bader M., Schunkert H., Erdmann J., Aherrahrou Z. CYP17A1 deficient XY mice display susceptibility to atherosclerosis, altered lipidomic profile and atypical sex development. Sci Rep. 2020;10:8792. doi: 10.1038/s41598-020-65601-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann R.P., Veeramachaneni D.N.R. Cryptorchidism in common eutherian mammals. Reproduction (Cambridge, England). 2007;133:541–561. doi: 10.1530/REP-06-0272. [DOI] [PubMed] [Google Scholar]
- Ankley G.T., Bennett R.S., Erickson R.J., Hoff D.J., Hornung M.W., Johnson R.D., Mount D.R., Nichols J.W., Russom C.L., Schmieder P.K., Serrrano J.A., Tietge J.E., Villeneuve D.L. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ. Toxicol. Chem. 2010;29(3):730–741. doi: 10.1002/etc.v29:310.1002/etc.34. [DOI] [PubMed] [Google Scholar]
- Arzuaga X., Walker T., Yost E.E., Radke E.G., Hotchkiss A.K. Use of the Adverse Outcome Pathway (AOP) framework to evaluate species concordance and human relevance of Dibutyl phthalate (DBP)-induced male reproductive toxicity. Reprod. Toxicol. 2020;96:445–458. doi: 10.1016/j.reprotox.2019.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey P.J., Dowhan D.H., Franke K., Burke L.J., Downes M., Muscat G.E. Transcriptional repression by COUP-TF II is dependent on the C-terminal domain and involves the N-CoR variant, RIP13delta1. J Steroid Biochem Mol Biol. 1997;63:165–174. doi: 10.1016/s0960-0760(97)00079-4. [DOI] [PubMed] [Google Scholar]
- Barlow N.J., Foster P.M.D. Pathogenesis of male reproductive tract lesions from gestation through adulthood following in utero exposure to Di(n-butyl) phthalate. Toxicol. Pathol. 2003;31(4):397–410. doi: 10.1080/01926230390202335. [DOI] [PubMed] [Google Scholar]
- Barsoum I., Yao H.-C. The road to maleness: from testis to Wolffian duct. Trends Endocrinol Metab. 2006;17(6):223–228. doi: 10.1016/j.tem.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bility M.T., Thompson J.T., McKee R.H., David R.M., Butala J.H., Vanden Heuvel J.P., Peters J.M. Activation of mouse and human peroxisome proliferator-activated receptors (PPARs) by phthalate monoesters. Toxicol Sci. 2004;82:170–182. doi: 10.1093/toxsci/kfh253. [DOI] [PubMed] [Google Scholar]
- Bogatcheva N.V., Truong A., Feng S., Engel W., Adham I.M., Agoulnik A.I. GREAT/LGR8 is the only receptor for insulin-like 3 peptide. Mol. Endocrinol. (Baltimore Md.) 2003;17(12):2639–2646. doi: 10.1210/me.2003-0096. [DOI] [PubMed] [Google Scholar]
- Caron K.M., Soo S.-C., Wetsel W.C., Stocco D.M., Clark B.J., Parker K.L. Targeted disruption of the mouse gene encoding steroidogenic acute regulatory protein provides insights into congenital lipoid adrenal hyperplasia. PNAS. 1997;94(21):11540–11545. doi: 10.1073/pnas.94.21.11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell R.A., Leonard J.A., Nicolas C.I., Campbell J.L., Yoon M., Efremenko A.Y., McMullen P.D., Andersen M.E., Clewell H.J., Phillips K.A., Tan Y.-M. Application of a combined aggregate exposure pathway and adverse outcome pathway (AEP-AOP) approach to inform a cumulative risk assessment: A case study with phthalates. Toxicol In Vitro. 2020;66:104855. doi: 10.1016/j.tiv.2020.104855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R.L., Anderson C.A., Prahalada S., Robertson R.T., Lochry E.A., Leonard Y.M., Stevens J.L., Hoberman A.M. Critical developmental periods for effects on male rat genitalia induced by finasteride, a 5α-reductase inhibitor. Toxicol. Appl. Pharmacol. 1993;119(1):34–40. doi: 10.1006/taap.1993.1041. [DOI] [PubMed] [Google Scholar]
- Coady K., Browne P., Embry M., Hill T., Leinala E., Steeger T., Maślankiewicz L., Hutchinson T. When are adverse outcome pathways and associated assays “fit for purpose” for regulatory decision-making and management of chemicals? Integr Environ Assess Manag. 2019;15(4):633–647. doi: 10.1002/ieam.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn M.J. Development of the external genitalia: conserved and divergent mechanisms of appendage patterning. Dev. Dyn. 2011;240(5):1108–1115. doi: 10.1002/dvdy.22631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czubacka E., Czerczak S., Kupczewska-Dobecka M. The overview of current evidence on the reproductive toxicity of dibutyl phthalate. Int. J. Occup. Med. Environ. Health. 2021;34(1):15–37. doi: 10.13075/ijomeh.1896.01658. [DOI] [PubMed] [Google Scholar]
- Dean A., Sharpe R.M. Clinical review: anogenital distance or digit length ratio as measures of fetal androgen exposure: relationship to male reproductive development and its disorders. J. Clin. Endocrinol. Metab. 2013;98:2230–2238. doi: 10.1210/jc.2012-4057. [DOI] [PubMed] [Google Scholar]
- Dean A., Smith L.B., Macpherson S., Sharpe R.M. The effect of dihydrotestosterone exposure during or prior to the masculinization programming window on reproductive development in male and female rats. Int. J. Androl. 2012;35:330–339. doi: 10.1111/j.1365-2605.2011.01236.x. [DOI] [PubMed] [Google Scholar]
- De Vries R.B.M., Angrish M., Browne P., Brozek J., Rooney A.A., Wikoff D.S., Whaley P., Edwards S.W. Applying evidence-based methods to the development and use of adverse outcome pathways. ALTEX. 2021;38:336–347. doi: 10.14573/altex.2101211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmen J.M., McLuskey A., Grootegoed J.A., Brinkmann A.O. Androgen action during male sex differentiation includes suppression of cranial suspensory ligament development. Hum Reprod. 1998;13(5):1272–1280. doi: 10.1093/humrep/13.5.1272. [DOI] [PubMed] [Google Scholar]
- Fay K.A., Villeneuve D.L., LaLone C.A., Song Y., Tollefsen K.E., Ankley G.T. Practical approaches to adverse outcome pathway development and weight-of-evidence evaluation as illustrated by ecotoxicological case studies. Environ. Toxicol. Chem. 2017;36(6):1429–1449. doi: 10.1002/etc.3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, S., Ferlin, A., Truong, A., Bathgate, R., Wade, J. D., Corbett, S., et al., 2009. INSL3/RXFP2 signaling in testicular descent. Ann N Y Acad Sci. 1160, 197-204.10.1111/j.1749-6632.2009.03841.x [DOI] [PMC free article] [PubMed]
- Foster P.M.D. Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. Int J Androl. 2006;29(1):140–147. doi: 10.1111/j.1365-2605.2005.00563.x. [DOI] [PubMed] [Google Scholar]
- Foster P.M.D., Harris M.W. Changes in androgen-mediated reproductive development in male rat offspring following exposure to a single oral dose of flutamide at different gestational ages. Toxicol. Sci. 2005;85:1024–1032. doi: 10.1093/toxsci/kfi159. [DOI] [PubMed] [Google Scholar]
- Georgas, K. M., Armstrong, J., Keast, J. R., Larkins, C. E., McHugh, K. M., Southard-Smith, E. M., et al., 2015. An illustrated anatomical ontology of the developing mouse lower urogenital tract. Development (Cambridge, England). 142, 1893-1908.10.1242/dev.117903 [DOI] [PMC free article] [PubMed]
- Gorlov I.P., Kamat A., Bogatcheva N.V., Jones E., Lamb D.J., Truong A., Bishop C.E., McElreavey K., Agoulnik A.I. Mutations of the GREAT gene cause cryptorchidism. Hum. Mol. Genet. 2002;11:2309–2318. doi: 10.1093/hmg/11.19.2309. [DOI] [PubMed] [Google Scholar]
- Gray Jr, L. E., Furr, J., Tatum-Gibbs, K. R., Lambright, C., Sampson, H., Hannas, B. R., Wilson, V. S., Hotchkiss, A., Foster, P. M. D., 2016. Establishing the “Biological Relevance” of Dipentyl Phthalate Reductions in Fetal Rat Testosterone Production and Plasma and Testis Testosterone Levels. Toxicological Sciences. 149, 178-191.10.1093/toxsci/kfv224. [DOI] [PMC free article] [PubMed]
- Gray L.E, Lambright C.S., Conley J.M., Evans N, Furr J.R., Hannas B.R., Wilson V.S., Sampson H., Foster P.M.D., 2021. Genomic and Hormonal Biomarkers of Phthalate-Induced Male Rat Reproductive Developmental Toxicity Part II: A Targeted RT-qPCR Array Approach that Defines a Unique Adverse Outcome Pathway. Toxicol Sci. Epub ahead of print. doi: 10.1093/toxsci/kfab053. [DOI] [PMC free article] [PubMed]
- Gray L.E., Jr., Barlow N.J., Howdeshell K.L., Ostby J.S., Furr J.R., Gray C.L. Transgenerational effects of Di (2-ethylhexyl) phthalate in the male CRL:CD(SD) rat: added value of assessing multiple offspring per litter. Toxicol Sci. 2009;110:411–425. doi: 10.1093/toxsci/kfp109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray L.E., Jr., Ostby J., Furr J., Price M., Veeramachaneni D.N., Parks L. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol Sci. 2000;58:350–365. doi: 10.1093/toxsci/58.2.350. [DOI] [PubMed] [Google Scholar]
- Hadziselimovic F. On the descent of the epididymo-testicular unit, cryptorchidism, and prevention of infertility. Basic Clin Androl. 2017;27:21. doi: 10.1186/s12610-017-0065-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannas B.R., Lambright C.S., Furr J., Howdeshell K.L., Wilson V.S., Gray L.E., Jr. Dose-response assessment of fetal testosterone production and gene expression levels in rat testes following in utero exposure to diethylhexyl phthalate, diisobutyl phthalate, diisoheptyl phthalate, and diisononyl phthalate. Toxicol Sci. 2011;123:206–216. doi: 10.1093/toxsci/kfr146. [DOI] [PubMed] [Google Scholar]
- Hannema S.E., Hughes I.A. Regulation of Wolffian duct development. Horm. Res. 2007;67(3):142–151. doi: 10.1159/000096644. [DOI] [PubMed] [Google Scholar]
- Hasegawa T., Zhao L., Caron K.M., Majdic G., Suzuki T., Shizawa S., Sasano H., Parker K.L. Developmental roles of the steroidogenic acute regulatory protein (StAR) as revealed by StAR knockout mice. Mol. Endocrinol. (Baltimore Md.) 2000;14(9):1462–1471. doi: 10.1210/mend.14.9.0515. [DOI] [PubMed] [Google Scholar]
- Heger N.E., Hall S.J., Sandrof M.A., McDonnell E.V., Hensley J.B., McDowell E.N., Martin K.A., Gaido K.W., Johnson K.J., Boekelheide K. Human fetal testis xenografts are resistant to phthalate-induced endocrine disruption. Environ Health Perspect. 2012;120(8):1137–1143. doi: 10.1289/ehp.1104711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hib J., Ponzio R. The abnormal development of male sex organs in the rat using a pure antiandrogen and a 5 alpha-reductase inhibitor during gestation. Acta Physiol. Pharmacol. Ther. Latinoam. 1995;45:27–33. [PubMed] [Google Scholar]
- Higgins S.J., Young P., Cunha G.R. Induction of functional cytodifferentiation in the epithelium of tissue recombinants. II. Instructive induction of Wolffian duct epithelia by neonatal seminal vesicle mesenchyme. Development. 1989;106:235–250. doi: 10.1242/dev.106.2.235. [DOI] [PubMed] [Google Scholar]
- Hong C.Y., Park J.H., Ahn R.S., Im S.Y., Choi H.-S., Soh J., Mellon S.H., Lee K. Molecular mechanism of suppression of testicular steroidogenesis by proinflammatory cytokine tumor necrosis factor alpha. Mol. Cell Biol. 2004;24(7):2593–2604. doi: 10.1128/MCB.24.7.2593-2604.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howdeshell K.L., Hotchkiss A.K., Gray L.E. Cumulative effects of antiandrogenic chemical mixtures and their relevance to human health risk assessment. Int. J. Hyg. Environ. Health. 2017;220(2):179–188. doi: 10.1016/j.ijheh.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howdeshell K.L., Rider C.V., Wilson V.S., Furr J.R., Lambright C.R., Gray L.E. Dose addition models based on biologically relevant reductions in fetal testosterone accurately predict postnatal reproductive tract alterations by a phthalate mixture in rats. Toxicol. Sci. 2015;148(2):488–502. doi: 10.1093/toxsci/kfv196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M.-C., Hsu N.-C., El Hadj N.B., Pai C.-I., Chu H.-P., Wang C.-K.-L., Chung B.-C. Steroid deficiency syndromes in mice with targeted disruption of Cyp11a1. Mol. Endocrinol. 2002;16:1943–1950. doi: 10.1210/me.2002-0055. [DOI] [PubMed] [Google Scholar]
- Hutson J.M., Hasthorpe S., Heyns C.F. Anatomical and functional aspects of testicular descent and cryptorchidism. Endocr. Rev. 1997;18:259–280. doi: 10.1210/edrv.18.2.0298. [DOI] [PubMed] [Google Scholar]
- Hutson J., Li R., Vikraman J., Loebenstein M. What animal models of testicular descent and germ cell maturation tell us about the mechanism in humans. Eur. J. Pediatric Surgery. 2016;26(05):390–398. doi: 10.1055/s-0036-1592196. [DOI] [PubMed] [Google Scholar]
- Hutson J.M., Southwell B.R., Li R., Lie G., Ismail K., Harisis G., Chen N. The regulation of testicular descent and the effects of cryptorchidism. Endocr. Rev. 2013;34:725–752. doi: 10.1210/er.2012-1089. [DOI] [PubMed] [Google Scholar]
- Imaizumi N., Kwang Lee K., Zhang C., Boelsterli U.A. Mechanisms of cell death pathway activation following drug-induced inhibition of mitochondrial complex I. Redox Biol. 2015;4:279–288. doi: 10.1016/j.redox.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipulan L.A., Suzuki K., Matsushita S., Suzuki H., Okazawa M., Jacinto S., Hirai S.-I., Yamada G. Development of the external genitalia and their sexual dimorphic regulation in mice. Sexual Development. 2014;8(5):297–310. doi: 10.1159/000357932. [DOI] [PubMed] [Google Scholar]
- Ipulan L.A., Raga D., Suzuki K., Murashima A., Matsumaru D., Cunha G., Yamada G. Investigation of sexual dimorphisms through mouse models and hormone/hormone-disruptor treatments. Differentiation. 2016;91(4-5):78–89. doi: 10.1016/j.diff.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Ivell R., Bathgate R.A.D. Reproductive biology of the relaxin-like factor (RLF/INSL3) Biol. Reprod. 2002;67:699–705. doi: 10.1095/biolreprod.102.005199. [DOI] [PubMed] [Google Scholar]
- Ivell R., Hartung S. The molecular basis of cryptorchidism. Mol. Hum. Reprod. 2003;9:175–181. doi: 10.1093/molehr/gag025. [DOI] [PubMed] [Google Scholar]
- Joseph A., Yao H., Hinton B.T. Development and morphogenesis of the Wolffian/epididymal duct, more twists and turns. Dev. Biol. 2009;325(1):6–14. doi: 10.1016/j.ydbio.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K.J., Heger N.E., Boekelheide K. Of mice and men (and rats): phthalate-induced fetal testis endocrine disruption is species-dependent. Toxicol Sci. 2012;129:235–248. doi: 10.1093/toxsci/kfs206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassim N.M., Russell D.A., Payne A.P. Does the cranial suspensory ligament have a role in cryptorchidism? Cells Tissues Organs. 2010;191(4):307–315. doi: 10.1159/000260062. [DOI] [PubMed] [Google Scholar]
- Kay, V. R., Bloom, M. S., Foster, W. G., 2014. Reproductive and developmental effects of phthalate diesters in males. Crit. Rev. Toxicol. 44, 467–498.10.3109/10408444.2013.875983. [DOI] [PubMed]
- Kim K.S., Liu W., Cunha G.R., Russell D.W., Huang H., Shapiro E., Baskin L.S. Expression of the androgen receptor and 5 alpha-reductase type 2 in the developing human fetal penis and urethra. Cell Tissue Res. 2002;307:145–153. doi: 10.1007/s004410100464. [DOI] [PubMed] [Google Scholar]
- Kleinstreuer N.C., Sullivan K., Allen D., Edwards S., Mendrick D.L., Embry M., Matheson J., Rowlands J.C., Munn S., Maull E., Casey W. Adverse outcome pathways: From research to regulation scientific workshop report. Regul. Toxicol. Pharmacol. 2016;76:39–50. doi: 10.1016/j.yrtph.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klonisch T., Fowler P.A., Hombach-Klonisch S. Molecular and genetic regulation of testis descent and external genitalia development. Dev. Biol. 2004;270(1):1–18. doi: 10.1016/j.ydbio.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Knapen D., Angrish M.M., Fortin M.C., Katsiadaki I., Leonard M., Margiotta-Casaluci L., Munn S., O'Brien J.M., Pollesch N., Smith L.C., Zhang X., Villeneuve D.L. Adverse outcome pathway networks I: Development and applications. Environ. Toxicol. Chem. 2018;37(6):1723–1733. doi: 10.1002/etc.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapen D., Vergauwen L., Villeneuve D.L., Ankley G.T. The potential of AOP networks for reproductive and developmental toxicity assay development. Reprod. Toxicol. 2015;56:52–55. doi: 10.1016/j.reprotox.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Kortenkamp A. Which chemicals should be grouped together for mixture risk assessments of male reproductive disorders? Mol. Cell Endocrinol. 2020;499:110581. doi: 10.1016/j.mce:2019.110581. [DOI] [PubMed] [Google Scholar]
- Lambrot R., Muczynski V., Lécureuil C., Angenard G., Coffigny H., Pairault C., Moison D., Frydman R., Habert R., Rouiller-Fabre V. Phthalates impair germ cell development in the human fetal testis in vitro without change in testosterone production. Environ. Health Perspect. 2009;117(1):32–37. doi: 10.1289/ehp.11146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leist M., Ghallab A., Graepel R., Marchan R., Hassan R., Bennekou S.H., Limonciel A., Vinken M., Schildknecht S., Waldmann T., Danen E., van Ravenzwaay B., Kamp H., Gardner I., Godoy P., Bois F.Y., Braeuning A., Reif R., Oesch F., Drasdo D., Höhme S., Schwarz M., Hartung T., Braunbeck T., Beltman J., Vrieling H., Sanz F., Forsby A., Gadaleta D., Fisher C., Kelm J., Fluri D., Ecker G., Zdrazil B., Terron A., Jennings P., van der Burg B., Dooley S., Meijer A.H., Willighagen E., Martens M., Evelo C., Mombelli E., Taboureau O., Mantovani A., Hardy B., Koch B., Escher S., van Thriel C., Cadenas C., Kroese D., van de Water B., Hengstler J.G. Adverse outcome pathways: opportunities, limitations and open questions. Arch. Toxicol. 2017;91(11):3477–3505. doi: 10.1007/s00204-017-2045-3. [DOI] [PubMed] [Google Scholar]
- Leung M.C., Phuong J., Baker N.C., Sipes N.S., Klinefelter G.R., Martin M.T., McLaurin K.W., Setzer R.W., Darney S.P., Judson R.S., Knudsen T.B. Systems toxicology of male reproductive development: profiling 774 chemicals for molecular targets and adverse outcomes. Environ. Health Perspect. 2016;124(7):1050–1061. doi: 10.1289/ehp.1510385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F.-J., Qin J., Tang K., Tsai S.Y., Tsai M.-J. Coup d'Etat: an orphan takes control. Endocr. Rev. 2011;32:404–421. doi: 10.1210/er.2010-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamoulakis C., Antypas S., Sofras F., Takenaka A., Sofikitis N. Testicular descent. Hormones (Athens) 2015;14 doi: 10.14310/horm.2002.1634. [DOI] [PubMed] [Google Scholar]
- Martin L.J., Tremblay J.J. The human 3beta-hydroxysteroid dehydrogenase/Delta5-Delta4 isomerase type 2 promoter is a novel target for the immediate early orphan nuclear receptor Nur77 in steroidogenic cells. Endocrinology. 2005;146:861–869. doi: 10.1210/en.2004-0859. [DOI] [PubMed] [Google Scholar]
- McIntyre B.S., Barlow N.J., Foster P.M. Androgen-mediated development in male rat offspring exposed to flutamide in utero: permanence and correlation of early postnatal changes in anogenital distance and nipple retention with malformations in androgen-dependent tissues. Toxicol. Sci. 2001;62:236–249. doi: 10.1093/toxsci/62.2.236. [DOI] [PubMed] [Google Scholar]
- McKinnell, C., Sharpe, R. M., Mahood, K., Hallmark, N., Scott, H., Ivell, R., et al., 2005. Expression of insulin-like factor 3 protein in the rat testis during fetal and postnatal development and in relation to cryptorchidism induced by in utero exposure to di (n-Butyl) phthalate. Endocrinology. 146, 4536-4544 [DOI] [PubMed]
- Mendoza-Villarroel R.E., Robert N.M., Martin L.J., Brousseau C., Tremblay J.J. The nuclear receptor NR2F2 activates star expression and steroidogenesis in mouse MA-10 and MLTC-1 Leydig cells. Biol. Reprod. 2014;91:26. doi: 10.1095/biolreprod.113.115790. 10.1095/biolreprod.113.115790. [DOI] [PubMed] [Google Scholar]
- Miller W.L., Walter L. Steroidogenic acute regulatory protein (StAR), a novel mitochondrial cholesterol transporter. BBA. 2007;1771(6):663–676. doi: 10.1016/j.bbalip.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Mitchell R.T., Saunders P.T.K., Childs A.J., Cassidy-Kojima C., Anderson R.A., Wallace W.H.B., Kelnar C.J.H., Sharpe R.M. Xenografting of human fetal testis tissue: a new approach to study fetal testis development and germ cell differentiation. Hum. Reprod. 2010;25(10):2405–2414. doi: 10.1093/humrep/deq183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagawa S., Satoh Y., Haraguchi R., Suzuki K., Iguchi T., Taketo M.M. Genetic interactions of the androgen and Wnt/beta-catenin pathways for the masculinization of external genitalia. Mol. Endocrinol. 2009;23:871–880. doi: 10.1210/me.2008-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashima A., Xu B., Hinton B.T. Understanding normal and abnormal development of the Wolffian/epididymal duct by using transgenic mice. Asian J. Androl. 2015;17:749–755. doi: 10.4103/1008-682X.155540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylchreest E., Cattley R.C., Foster P.M. Male reproductive tract malformations in rats following gestational and lactational exposure to Di(n-butyl) phthalate: an antiandrogenic mechanism? Toxicol Sci. 1998;43:47–60. doi: 10.1006/toxs.1998.2436. [DOI] [PubMed] [Google Scholar]
- Mylchreest E., Foster P.M. DBP exerts its antiandrogenic activity by indirectly interfering with androgen signaling pathways. Toxicol. Appl. Pharmacol. 2000;168:174–175. doi: 10.1006/taap.2000.9031. [DOI] [PubMed] [Google Scholar]
- Mylchreest E., Sar M., Cattley R.C., Foster P.M.D. Disruption of androgen-regulated male reproductive development by di(n-butyl) phthalate during late gestation in rats is different from flutamide. Toxicol. Appl. Pharmacol. 1999;156(2):81–95. doi: 10.1006/taap.1999.8643. [DOI] [PubMed] [Google Scholar]
- Mylchreest E., Sar M., Wallace D.G., Foster P.M.D. Fetal testosterone insufficiency and abnormal proliferation of Leydig cells and gonocytes in rats exposed to di(n-butyl) phthalate. Reprod. Toxicol. 2002;16(1):19–28. doi: 10.1016/S0890-6238(01)00201-5. [DOI] [PubMed] [Google Scholar]
- Mylchreest E., Wallace D.G., Cattley R.C., Foster P.M. Dose-dependent alterations in androgen-regulated male reproductive development in rats exposed to Di(n-butyl) phthalate during late gestation. Toxicol Sci. 2000;55:143–151. doi: 10.1093/toxsci/55.1.143. [DOI] [PubMed] [Google Scholar]
- Nation T.R., Balic A., Southwell B.R., Newgreen D.F., Hutson J.M. The hormonal control of testicular descent. Pediatr. Endocrinol. Rev. 2009;7:22–31. [PubMed] [Google Scholar]
- Nation T.R., Buraundi S., Balic A., Farmer P.J., Newgreen D., Southwell B.R., Hutson J.M. The effect of flutamide on expression of androgen and estrogen receptors in the gubernaculum and surrounding structures during testicular descent. J. Pediatr. Surg. 2011;46(12):2358–2362. doi: 10.1016/j.jpedsurg.2011.09.026. [DOI] [PubMed] [Google Scholar]
- Nef S., Parada L.F. Cryptorchidism in mice mutant for Insl3. Nat. Genet. 1999;22(3):295–299. doi: 10.1038/10364. [DOI] [PubMed] [Google Scholar]
- Nepelska, M., Odum, J., Munn, S., 2017. Adverse Outcome Pathway: Peroxisome Proliferator-Activated Receptor α Activation and Reproductive Toxicity—Development and Application in Assessment of Endocrine Disruptors/Reproductive Toxicants Applied In Vitro Toxicology. 3, 234-249.http://doi.org/10.1089/aivt.2017.0004
- Nishida H., Miyagawa S., Matsumaru D., Wada Y., Satoh Y., Ogino Y., Fukuda S., Iguchi T., Yamada G. Gene expression analyses on embryonic external genitalia: identification of regulatory genes possibly involved in masculinization processes. Congenital Anomalies. 2008;48(2):63–67. doi: 10.1111/j.1741-4520.2008.00180.x. [DOI] [PubMed] [Google Scholar]
- OECD, 2017. Revised Guidance Document on Developing and Assessing Adverse Outcome Pathways. Series on Testing & Assessment. No. 184. Series on Testing & Assessment. No. 184.
- OECD, 2018. Users' Handbook supplement to the Guidance Document for developing and assessing Adverse Outcome Pathways. doi:https://doi.org/10.1787/5jlv1m9d1g32-en. last accessed May 18, 2021.
- OECD, 2020. Draft Guidance Document for the scientific review of Adverse Outcome Pathways. https://www.oecd.org/chemicalsafety/testing/Draft_GD_AOP_scientific_review_27_July.pdf. last accessed May 18, 2021.
- Overbeek, P. A., Gorlov, I. P., Sutherland, R. W., Houston, J. B., Harrison, W. R., Boettger-Tong, H. L., Bishop, C. E., Agoulnik, A. I., 2001. A transgenic insertion causing cryptorchidism in mice. Genesis (New York, N.Y.: 2000). 30, 26-35. [DOI] [PubMed]
- Petiot A., Perriton C.L., Dickson C., Cohn M.J. Development of the mammalian urethra is controlled by Fgfr2-IIIb. Development. 2005;132:2441–2450. doi: 10.1242/dev.01778. [DOI] [PubMed] [Google Scholar]
- Picut C.A., Ziejewski M.K., Stanislaus D. Comparative Aspects of pre- and postnatal development of the male reproductive system. Birth Defects Res. 2017;110(3):190–227. doi: 10.1002/bdr2.1133. [DOI] [PubMed] [Google Scholar]
- Plummer S., Sharpe R.M., Hallmark N., Mahood I.K., Elcombe C. Time-dependent and compartment-specific effects of in utero exposure to Di(n-butyl) phthalate on gene/protein expression in the fetal rat testis as revealed by transcription profiling and laser capture microdissection. Toxicol. Sci. 2007;97(2):520–532. doi: 10.1093/toxsci/kfm062. [DOI] [PubMed] [Google Scholar]
- Qin J., Tsai M.-J., Tsai S.Y., Mueller U. Essential roles of COUP-TFII in Leydig cell differentiation and male fertility. PLoS ONE. 2008;3(9):e3285. doi: 10.1371/journal.pone.0003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y., Tsai S.Y., Tsai M.-J. COUP-TF an orphan member of the steroid/thyroid hormone receptor superfamily. Trends Endocrinol Metab. 1994;5(6):234–239. doi: 10.1016/1043-2760(94)P3081-H. [DOI] [PubMed] [Google Scholar]
- Robert N.M., Martin L.J., Tremblay J.J. The orphan nuclear receptor NR4A1 regulates insulin-like 3 gene transcription in leydig cells1. Biol. Reprod. 2006;74:322–330. doi: 10.1095/biolreprod.105.044560. [DOI] [PubMed] [Google Scholar]
- Rugarli E.I., Langer T. Mitochondrial quality control: a matter of life and death for neurons. EMBO J. 2012;31:1336–1349. doi: 10.1038/emboj.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghian H., Anand-Ivell R., Balvers M., Relan V., Ivell R. Constitutive regulation of the Insl3 gene in rat Leydig cells. Mol. Cell. Endocrinol. 2005;241(1-2):10–20. doi: 10.1016/j.mce.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Scott H.M., Mason J.I., Sharpe R.M. Steroidogenesis in the fetal testis and its susceptibility to disruption by exogenous compounds. Endocr Rev. 2009;30:883–925. doi: 10.1210/er.2009-0016. [DOI] [PubMed] [Google Scholar]
- Seifert A.W., Harfe B.D., Cohn M.J. Cell lineage analysis demonstrates an endodermal origin of the distal urethra and perineum. Dev. Biol. 2008;318(1):143–152. doi: 10.1016/j.ydbio.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setchell B.P. The Parkes Lecture. Heat and the testis. J Reprod Fertil. 1998;114:179–194. doi: 10.1530/jrf.0.1140179. [DOI] [PubMed] [Google Scholar]
- Sharpe R.M. Androgens and the masculinization programming window: human-rodent differences. Biochem Soc Trans. 2020;48:1725–1735. doi: 10.1042/bst20200200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G., Renfree M.B. Wolffian duct development. Sex Dev. 2014;8:273–280. doi: 10.1159/000363432. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Suzuki K., Yamada G. Systematic analyses of murine masculinization processes based on genital sex differentiation parameters. Dev. Growth Differ. 2015;57:639–647. doi: 10.1111/dgd.12247. [DOI] [PubMed] [Google Scholar]
- Shibata H., Kurihara I., Kobayashi S., Yokoto K., Suda N., Saito I., Saruta T. Regulation of differential COUP-TF-coregulator interactions in adrenal cortical steroidogenesis. The Journal Of Steroid Biochemistry And Molecular Biology. 2003;85:449–456. doi: 10.1016/s0960-0760(03)00217-6. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Numata T., Suzuki H., Raga D.D., Ipulan L.A., Yokoyama C. Sexually dimorphic expression of Mafb regulates masculinization of the embryonic urethral formation. Proc. Natl. Acad. Sci. U.S.A. 2014;111:16407–16412. doi: 10.1073/pnas.1413273111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain, A., 2017. Ductal sex determination. Science (New York, N.Y.). 357, 648-648.10.1126/science.aao2630. [DOI] [PubMed]
- Tian H., Russell D.W. Expression and regulation of steroid 5 alpha-reductase in the genital tubercle of the fetal rat. Dev Dyn. 1997;209:117–126. doi: 10.1002/(sici)1097-0177(199705)209:1<117::Aid-aja11>3.0.Co;2-z. [DOI] [PubMed] [Google Scholar]
- Tomiyama H., Hutson J.M., Truong A., Agoulnik A.I. Transabdominal testicular descent is disrupted in mice with deletion of insulinlike factor 3 receptor. J. Pediatr. Surg. 2003;38:1793–1798. doi: 10.1016/j.jpedsurg.2003.08.047. [DOI] [PubMed] [Google Scholar]
- Turcu A.F., Auchus R.J. The next 150 years of congenital adrenal hyperplasia. J. Steroid Biochem. Mol. Biol. 2015;153:63–71. doi: 10.1016/j.jsbmb.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Driesche S., Kilcoyne K.R., Wagner I., Rebourcet D., Boyle A., Mitchell R. Experimentally induced testicular dysgenesis syndrome originates in the masculinization programming window. JCI Insight. 2017;2 doi: 10.1172/jci.insight.91204. e91204 e91204, 10.1172/jci.insight.91204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Driesche S., Shoker S., Inglis F., Palermo C., Langsch A., Otter R. Systematic comparison of the male reproductive tract in fetal and adult Wistar rats exposed to DBP and DINP in utero during the masculinisation programming window. Toxicol Lett. 2020;335:37–50. doi: 10.1016/j.toxlet.2020.10.006. [DOI] [PubMed] [Google Scholar]
- van den Driesche S., Walker M., McKinnell C., Scott H.M., Eddie S.L., Mitchell R.T. Proposed role for COUP-TFII in regulating fetal Leydig cell steroidogenesis, perturbation of which leads to masculinization disorders in rodents. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0037064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Schoot P., Emmen J.M. Development, structure and function of the cranial suspensory ligaments of the mammalian gonads in a cross-species perspective; their possible role in effecting disturbed testicular descent. Hum Reprod Update. 1996;2:399–418. doi: 10.1093/humupd/2.5.399. [DOI] [PubMed] [Google Scholar]
- Villeneuve D.L., Angrish M.M., Fortin M.C., Katsiadaki I., Leonard M., Margiotta-Casaluci L. Adverse outcome pathway networks II: Network analytics. Environ. Toxicol. Chem. 2018;37:1734–1748. doi: 10.1002/etc.4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve D.L., Landesmann B., Allavena P., Ashley N., Bal-Price A., Corsini E., Halappanavar S., Hussell Representing the process of inflammation as key events in adverse outcome pathways. Toxicol. Sci. 2018;163:346–352. doi: 10.1093/toxsci/kfy047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve D.L., Crump D., Garcia-Reyero N., Hecker M., Hutchinson T.H., LaLone C.A. Adverse outcome pathway (AOP) development I: Strategies and principles. Toxicol. Sci. 2014;142:312–320. doi: 10.1093/toxsci/kfu199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve D.L., Crump D., Garcia-Reyero N., Hecker M., Hutchinson T.H., LaLone C.A. Adverse outcome pathway development II: Best practices. Toxicol. Sci. 2014;142:321–330. doi: 10.1093/toxsci/kfu200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M., Saunders P.T., Fisken M., Scott H.M., Hutchison G.R., Smith L.B., Sharpe R.M. Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. J. Clin. Invest. 2008;118:1479–1490. doi: 10.1172/jci34241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M., Saunders P.T.K., Sharpe R.M. The critical time window for androgen-dependent development of the wolffian duct in the rat. Endocrinology. 2007;148:3185–3195. doi: 10.1210/en.2007-0028. [DOI] [PubMed] [Google Scholar]
- Wen Q., Cheng C.Y., Liu Y.X. Development, function and fate of fetal Leydig cells. Semin Cell Dev. Biol. 2016;59:89–98. doi: 10.1016/j.semcdb.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson V.S., Blystone C.R., Hotchkiss A.K., Rider C.V., Gray L.E., Jr. Diverse mechanisms of anti-androgen action: impact on male rat reproductive tract development. Int. J. Androl. 2008;31:178–187. doi: 10.1111/j.1365-2605.2007.00861.x. [DOI] [PubMed] [Google Scholar]
- Wilson V.S., Howdeshell K.L., Lambright C.S., Furr J., Earl L., Gray Differential expression of the phthalate syndrome in male Sprague-Dawley and Wistar rats after in utero DEHP exposure. Toxicol. Lett. 2007;170:177–184. doi: 10.1016/j.toxlet.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Yost E.E., Euling S.Y., Weaver J.A., Beverly B.E.J., Keshava N., Mudipalli A. Hazards of diisobutyl phthalate (DIBP) exposure: a systematic review of animal toxicology studies. Environ. Int. 2019;125:579–594. doi: 10.1016/j.envint.2018.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Mellon S.H. Multiple orphan nuclear receptors converge to regulate rat P450c17 gene transcription: novel mechanisms for orphan nuclear receptor action. Mol. Endocrinol. 1997;11:891–904. doi: 10.1210/mend.11.7.9940. [DOI] [PubMed] [Google Scholar]
- Zhao F., Franco H.L., Rodriguez K.F., Brown P.R., Tsai M.J., Tsai S.Y., Yao H.H. Elimination of the male reproductive tract in the female embryo is promoted by COUP-TFII in mice. Science. 2017;357:717–720. doi: 10.1126/science.aai9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z., Armfield B.A., Cohn M.J. Timing of androgen receptor disruption and estrogen exposure underlies a spectrum of congenital penile anomalies. Proc. Natl. Acad. Sci. U.S.A. 2015;112:E7194–E7203. doi: 10.1073/pnas.1515981112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann S., Steding G., Emmen J.M., Brinkmann A.O., Nayernia K., Holstein A.F., Engel W., Adham I.M. Targeted disruption of the Insl3 gene causes bilateral cryptorchidism. Mol. Endocrinol. (Baltimore Md.) 1999;13:681–691. doi: 10.1210/mend.13.5.0272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.