Abstract
Although lobectomy remains the gold-standard surgical treatment for non–small-cell lung cancer, the frequency of thoracoscopic segmentectomy is increasing. Multiple factors must be considered in the choice of the procedure, ranging from adequate surgical planning or simulation, tumor localization, and identification of the intersegmental plane to severing the intersegmental plane to achieve an oncologically safe surgical margin with no or minimal manual palpation and different landmarks. In this article, we present an overview of methods for each procedural step of thoracoscopic segmentectomy, from preoperative planning to division of the intersegmental plane.
Keywords: Thoracoscopy, Segmentectomy, Lung neoplasms, Simulation
Introduction
Lobectomy remains the gold-standard surgical treatment for primary lung cancer [1], but the frequency of thoracoscopic sublobar resection (SLR)—especially anatomical segmentectomy, which can achieve relatively secure and sufficient surgical margins—is increasing [2]. This phenomenon might be partially explained by the following reasons. First, with the popularization of low-dose computed tomography (CT), small lung cancers, such as ground-glass–opacity nodules (GGNs)—for which anatomical segmentectomy can achieve the same oncologic outcomes as lobectomy—have been detected more frequently. The characteristics of patients are also changing; physicians are more likely to encounter younger patients with small tumors for whom it is appropriate to consider a surgical extent that would spare lung parenchyma if a second cancer should develop [3]. Finally, there are increasingly many elderly patients with comorbidities in whom lung parenchyma should be preserved to reduce their postoperative morbidity and mortality [4]. In addition to primary lung cancer, segmentectomy can be performed for metastatic lung lesions or other non-malignant lung diseases, including inflammatory pseudotumors, congenital bronchial atresia, and pulmonary sequestration [5-9].
Relative to lobectomy, anatomical segmentectomy requires a thorough assessment of each patient’s lung anatomy and a better understanding of the branching patterns of the pulmonary vessels and bronchi [10]. In addition, numerous variations and anomalies of the pulmonary vessels and bronchus constitute issues to be overcome before performing segmentectomy. Although thoracoscopic segmentectomy has become a more common surgical procedure, surgeons should continue to be familiar with various methods, considering multiple factors such as the surgical margin, venous drainage of the tumor, and lung anatomy to conserve.
The purpose of this article was to present an overview of methods for each step of thoracoscopic segmentectomy, from preoperative planning to division of the intersegmental plane.
Preoperative planning and simulation
Segmentectomy is more complicated technically than lobectomy because of the anatomical complexity and variations of peripheral vessels and bronchi. Nagashima et al. [11,12] and Shimizu et al. [13] reported that the prevalence of variations of bronchovascular patterns differed [14], sometimes significantly, from those noted by previous reports [15,16]. In addition, unplanned procedures, such as additional wedge resection or completion lobectomy, are of concern during thoracoscopic segmentectomy [17]. Recently, 3-dimensional (3D) imaging based on high-resolution CT has become more prevalent to help thoracic surgeons perform thoracoscopic segmentectomy by revealing the patient’s anatomy in detail [18-22]. With this method, it is possible to: (1) specify the exact lesion location within a pulmonary segment, (2) define the segmental pulmonary artery or vein branches and bronchial tree divisions to identify possible anatomical variations, (3) calculate and integrate safety margins to prevent or decrease locoregional recurrence, (4) calculate the volume of the resected or nonresected segment, and (5) provide evidence to suggest alternative treatments in compromised patients for whom the 3D model was not suitable for anatomical segmentectomy due to anatomical or oncological reasons.
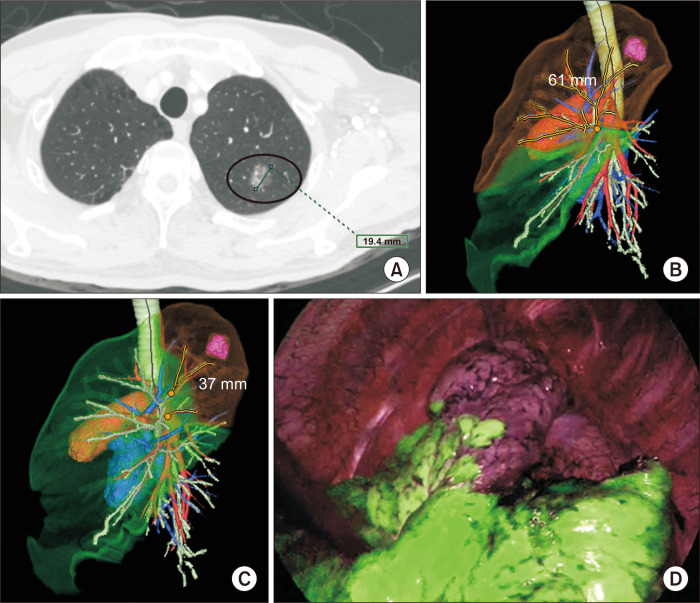
After our institution adopted a 3D workstation (SYNAPSE VINCENT: Fujifilm Medical, Tokyo, Japan) in October 2018, thin-section CT images were transferred to the 3D workstation to generate virtual 3D segmentectomy images. These simulation images were displayed on a monitor alongside the actual thoracoscopic monitor. For example, a female patient who presented with a persistent, 1.9-cm, part-solid GGN (ground-glass opacity percentage=92%) on thin-section chest CT (Fig. 1A) was referred to our department and deemed suitable for SLR, with an adequate surgical margin. Although 3D reconstruction showed the safety margin (>60 mm) to be sufficient for upper-division segmentectomy, the remaining lung volume (28% of the left upper lobe [LUL] volume) was too small, suggesting insufficient preservation of the lung parenchyma (Fig. 1B). However, another 3D simulation (Fig. 1C) revealed that a safe surgical margin (37 mm) and preservation of lung parenchyma (74% of the LUL volume) could be simultaneously achieved when performing apicoposterior segmentectomy. Based on these results, apicoposterior segmentectomy was performed (Fig. 1D), and the parenchymal resection margin was measured as 3.0 cm on the pathologic examination.
Fig. 1.
(A) Axial view chest computed tomography (17-mm part-solid ground-glass nodule in the left upper lobe [LUL]). (B) A 3-dimensional (3D) simulation of left upper division segmentectomy (virtual surgical margin: 61 mm, preserved lung volume: 392 mL [28% of the total LUL volume]). (C) A 3D simulation of left apicoposterior segmentectomy (virtual surgical margin: 37 mm, preserved lung volume: 1,042 mL [74% of the total LUL volume]). (D) Corresponding surgical near-infrared thoracoscopy image after systemic indocyanine green injection.
However, it is important to interpret and utilize 3D models carefully in this context. Most importantly, the CT images and the consequent 3D model do not reflect lung collapse or deformity. Accordingly, the surgical margin from the 3D simulation is frequently overestimated relative to the gross or pathologic surgical margin [23].
General surgical technique
Exposure of the hilar structures
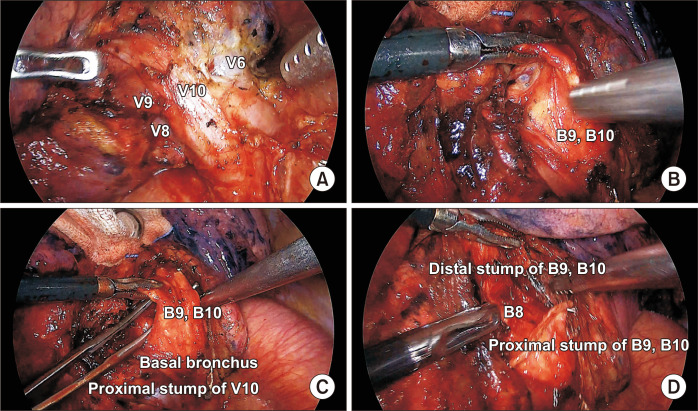
The hilar structures should be exposed as much as possible to confirm the vascular or bronchial anatomy. For example, the left inferior pulmonary vein was dissected and exposed, as shown in Fig. 2A, when performing LS9,10 segmentectomy from the posterior side; then, the segmental bronchus was exposed and grasped with atraumatic graspers (Fig. 2B). The lateral and back sides of the LS9,10 segmental bronchi were dissected and isolated from the pulmonary artery branch with scissors or a tissue dissector (Fig. 2C) and divided with an endolinear stapler (Fig. 2D).
Fig. 2.
(A) Full exposure of the left inferior pulmonary vein from the posterior side. (B) Grasping and lifting of the segmental bronchus. (C) Dissection of the lateral and posterior borders of the target bronchus. (D) Division and lifting of the distal stump of the target bronchus.
Lifting the distal stump of the vessels or bronchus
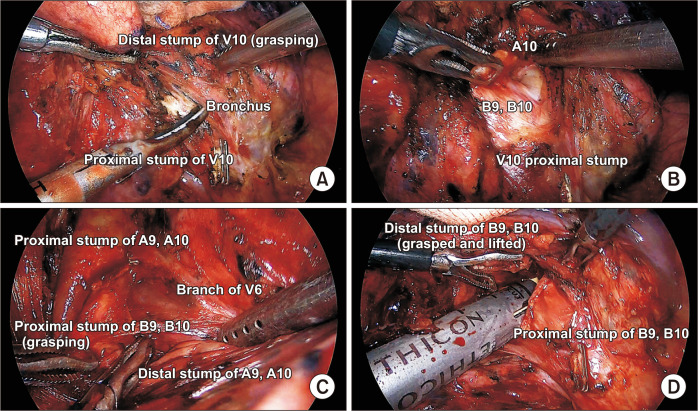
Grasping and lifting the distal stump of the vessels or bronchus help to expose posterior segmental hilar structures [24]. For example, Fig. 3A shows that lifting distal V10 enabled easy exposure of B9, 10. In addition, grasping and further dissection of the resected bronchus help to expose and isolate the pulmonary artery branch (A10), as seen in Fig. 3B. Lifting the resected hilar structure also facilitates identification of the intersegmental vein branches and planes (Fig. 3C). Lastly, further dissection around resected hilar structures can make room for staplers (Fig. 3D). The technique is discussed in detail below.
Fig. 3.
(A) Lifting the distal stump of V10 to expose the target bronchus. (B) Grasping and lifting of B9,10 to expose the posterior pulmonary artery. (C) Exposure of the intersegmental vein branch after division of A9,10 and B9,10. (D) Stapler insertion into the hilar pocket.
Identification of the intersegmental plane
It is important to recognize and mark the intersegmental plane during anatomical segmentectomy. Several techniques have been introduced and established, as discussed below and summarized in Table 1.
Table 1.
Comparison of techniques to identify the intersegmental plane during thoracoscopic segmentectomy
| Method | Inflation-deflation line | Intravenous indocyanine green | Virtual-assisted lung mapping | |
|---|---|---|---|---|
| Target segment deflated | Target segment inflated | |||
| Advantages | - No preparation is necessary - Easy and quick |
- Less interference with the surgical view - Relatively easy |
- Easy and quick - Able to create the demarcation around the hilum |
- Provides geometric information on the lung surface |
| Disadvantages | - Interference with the surgical view - Prolongation of the operation time - Intersegmental lines could be unclear due to collateral ventilation |
- Some preparation or help of the anesthesiologist is needed - The needle injection method has been discarded due to air embolism - Intersegmental lines could be unclear due to collateral ventilation |
- Correct identification of the pulmonary artery is necessary - An emphysematous lung that is less-perfused may be misleading or confusing - Infrared thoracoscopy is required |
- Requires preoperative preparation - Basic instruments and some facilities are needed |
Inflation-deflation method
Before dividing the clamped bronchus, the anesthesiologist is asked to ventilate the lung; thereafter, differential deflation and inflation of the segment to be removed are expected to develop, helping to delineate the intersegmental planes [25]. Although this is the most common method, its utility is less than satisfactory because the developing line is not as clear as desired due to collateral ventilation through Cohn’s pores. Tsubota [26] reported using a method involving inflation of the whole lung; in this context, division of the segmental bronchus and opening of the stump of the preserved bronchus allowed the gas inside the preserved segments to escape. Okada et al. [27] suggested the utility of selective jet ventilation under flexible bronchoscopy in the segment to be resected. Other methods have been reported to inflate or keep the resected segment inflated, including bronchial ligation with a slip-knot method after inflation or direct inflation by inserting a butterfly needle into the segmental bronchus [28,29].
Perfusion method
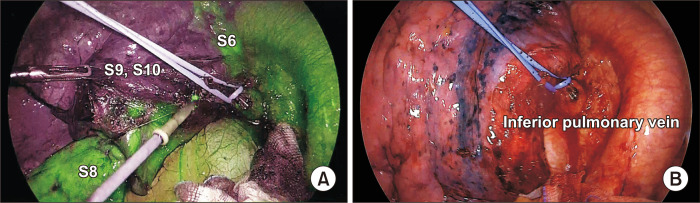
It is often difficult to delineate the intersegmental line in patients with emphysema using the inflation-deflation method because the lung parenchyma is likely to exhibit an advanced state of collateral respiratory structures, and inflated lungs typically hamper the surgical view during thoracoscopic segmentectomy, often resulting in delays in the procedure. To overcome these limitations of the inflation technique, Misaki et al. [30] introduced a novel method for visualizing adjacent segments using a near-infrared camera following systemic injection of indocyanine green (ICG). In this approach, after segmental pulmonary artery ligation or division, an intravenous injection of ICG creates a fluorescent demarcation between preserved (fluorescent) and targeted (nonfluorescent) segments (Fig. 4A) [31,32]. However, it often is difficult to stain and dissect the correct intersegmental line within the few minutes before the ICG dye is washed out. However, Ito et al. [33] proposed a new technique in which the pulmonary vein of the entire lobe is clamped temporarily, prolonging ICG visualization (Fig. 4B).
Fig. 4.
(A) Demarcation of the intersegmental plane with electrocautery after systemic indocyanine green (ICG) injection. (B) Temporary pulmonary vein clamping to prevent ICG washout.
Virtual-assisted pulmonary mapping
This technique was developed as a bronchoscopic lung- marking method to avoid the inherent complications of CT-guided percutaneous lung marking. In virtual-assisted pulmonary mapping, multiple marks are made under the lung surface and provide geometric information for localizing a lesion in 3D space [34].
Division of the intersegmental plane
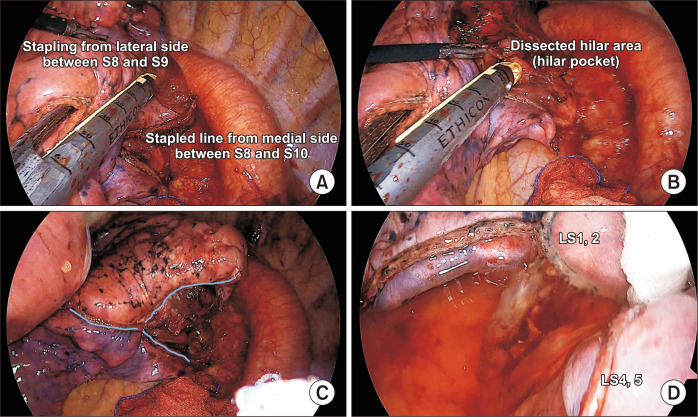
The optimal method for dissecting the intersegmental plane remains controversial. The 2 main approaches to developing the intersegmental plane involve using either stapling devices or energy instruments such as electrocautery or advanced bipolar energy. The use of energy devices has been considered advantageous for preserving pulmonary function due to the avoidance of squeezing or folding of the lungs by stapler devices [27]. However, it is possible that their use may lead to more postoperative complications, such as prolonged air leakage or bronchopleural fistula [35]. Recently, a randomized controlled trial reported a higher incidence of postoperative complications (e.g., air leakage) in the electrocautery group, while no difference was found in the loss of lung function [36]. However, there remain concerns that the effect of preserving lung function would be reduced by lung-folding involving multiple stapling actions when performing thoracoscopic complex segmentectomy. There are 2 main principles to follow to facilitate better inflation of preserved segments after thoracoscopic segmentectomy. First, the lung should be stapled from the periphery to the hilum. If multiple intersegmental planes need to be cut, peripheral stapling at different angles should be started and continued until reaching the center or hilum (Fig. 5A). Second, when the stapler reaches the hilum, it should be placed in the pocket (Fig. 5B) created by lifting the resected hilar structures as described above to involve the hilar structures of the target segment and to avoid cutting into the preserved segments. The cartilage, rather than the anvil of a stapler, should be placed toward the hilum to minimize the risk of vascular damage. Triangular (in a Mercedes-Benz mark-like fashion) (Fig. 5C) or V-shaped (or U-shaped) (Fig. 5D) stapling can be performed using these technical principles.
Fig. 5.
(A) Peripheral stapling at different angles when starting the division of the intersegmental plane. (B) Placement of the stapler tip into the hilar pocket after further dissection of the hilar area. (C) Triangular stapled line after left 9,10 segmentectomy. (D) A U-shaped (or V-shaped) stapled line after left 3 segmentectomy
Conclusion
Anatomical segmentectomy with thoracoscopy involves recognizing the correct 3D anatomy of the lung segment and accurately identifying the location of the lesion preoperatively or intraoperatively. Identifying and separating the intersegmental plane correctly and safely are important steps for completing thoracoscopic segmentectomy, with some technical pitfalls. In each step, different methods might be adopted based on the physician’s preferences or situation. However, a thorough understanding of the advantages and limitations of each technique can help surgeons to perform lung segmentectomy more accurately.
Footnotes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Howington JA, Blum MG, Chang AC, Balekian AA, Murthy SC. Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e278S–e313S. doi: 10.1378/chest.12-2359. [DOI] [PubMed] [Google Scholar]
- 2.Cao C, D'Amico T, Demmy T, et al. Less is more: a shift in the surgical approach to non-small-cell lung cancer. Lancet Respir Med. 2016;4:e11–2. doi: 10.1016/S2213-2600(16)00024-2. [DOI] [PubMed] [Google Scholar]
- 3.Gu C, Wang R, Pan X, et al. Sublobar resection versus lobectomy in patients aged ≤35 years with stage IA non-small cell lung cancer: a SEER database analysis. J Cancer Res Clin Oncol. 2017;143:2375–82. doi: 10.1007/s00432-017-2499-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Traibi A, Grigoroiu M, Boulitrop C, et al. Predictive factors for complications of anatomical pulmonary segmentectomies. Interact Cardiovasc Thorac Surg. 2013;17:838–44. doi: 10.1093/icvts/ivt292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiono S, Okumura T, Boku N, et al. Outcomes of segmentectomy and wedge resection for pulmonary metastases from colorectal cancer. Eur J Cardiothorac Surg. 2017;51:504–10. doi: 10.1093/ejcts/ezw322. [DOI] [PubMed] [Google Scholar]
- 6.Berry MF. Role of segmentectomy for pulmonary metastases. Ann Cardiothorac Surg. 2014;3:176–82. doi: 10.3978/j.issn.2225-319X.2014.02.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melloni G, Carretta A, Ciriaco P, et al. Inflammatory pseudotumor of the lung in adults. Ann Thorac Surg. 2005;79:426–32. doi: 10.1016/j.athoracsur.2004.07.077. [DOI] [PubMed] [Google Scholar]
- 8.Igai H, Kamiyoshihara M, Nagashima T, Shimizu K. Anatomical segmentectomy for pneumothorax associated with congenital bronchial atresia. Eur J Cardiothorac Surg. 2013;43:198. doi: 10.1093/ejcts/ezs377. [DOI] [PubMed] [Google Scholar]
- 9.Inoue T, Oizumi H, Nakamura M, Sadahiro M. Port-access thoracoscopic anatomical segmentectomy for pediatric intralobar pulmonary sequestration. Thorac Cardiovasc Surg Rep. 2014;3:42–4. doi: 10.1055/s-0034-1377065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimizu K, Nakazawa S, Nagashima T, Kuwano H, Mogi A. 3D-CT anatomy for VATS segmentectomy. J Vis Surg. 2017;3:88. doi: 10.21037/jovs.2017.05.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagashima T, Shimizu K, Ohtaki Y, et al. An analysis of variations in the bronchovascular pattern of the right upper lobe using three-dimensional CT angiography and bronchography. Gen Thorac Cardiovasc Surg. 2015;63:354–60. doi: 10.1007/s11748-015-0531-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagashima T, Shimizu K, Ohtaki Y, et al. Analysis of variation in bronchovascular pattern of the right middle and lower lobes of the lung using three-dimensional CT angiography and bronchography. Gen Thorac Cardiovasc Surg. 2017;65:343–9. doi: 10.1007/s11748-017-0754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimizu K, Nagashima T, Ohtaki Y, et al. Analysis of the variation pattern in right upper pulmonary veins and establishment of simplified vein models for anatomical segmentectomy. Gen Thorac Cardiovasc Surg. 2016;64:604–11. doi: 10.1007/s11748-016-0686-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang M, Mao N, Zhang K, et al. Analysis of the variation pattern in left upper division veins and establishment of simplified vein models for anatomical segmentectomy. Ann Transl Med. 2020;8:1515. doi: 10.21037/atm-20-6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyden EA, Scannell JG. An analysis of variations in the bronchovascular pattern of the right upper lobe of 50 lungs. Am J Anat. 1948;82:27–73. doi: 10.1002/aja.1000820103. [DOI] [PubMed] [Google Scholar]
- 16.Yamashita H. Variations in the pulmonary segments and the bronchovascular trees. In: Yamashita H, editor. Roentgenologic anatomy of the lung. Igaku-Shoin; Tokyo: 1978. pp. 70–107. [Google Scholar]
- 17.Gossot D, Lutz JA, Grigoroiu M, Brian E, Seguin-Givelet A. Unplanned procedures during thoracoscopic segmentectomies. Ann Thorac Surg. 2017;104:1710–7. doi: 10.1016/j.athoracsur.2017.05.081. [DOI] [PubMed] [Google Scholar]
- 18.Shimizu K, Nakano T, Kamiyoshihara M, Takeyoshi I. Segmentectomy guided by three-dimensional computed tomography angiography and bronchography. Interact Cardiovasc Thorac Surg. 2012;15:194–6. doi: 10.1093/icvts/ivs202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikeda N, Yoshimura A, Hagiwara M, Akata S, Saji H. Three dimensional computed tomography lung modeling is useful in simulation and navigation of lung cancer surgery. Ann Thorac Cardiovasc Surg. 2013;19:1–5. doi: 10.5761/atcs.ra.12.02174. [DOI] [PubMed] [Google Scholar]
- 20.Akiba T. Utility of three-dimensional computed tomography in general thoracic surgery. Gen Thorac Cardiovasc Surg. 2013;61:676–84. doi: 10.1007/s11748-013-0336-z. [DOI] [PubMed] [Google Scholar]
- 21.Oizumi H, Kanauchi N, Kato H, et al. Anatomic thoracoscopic pulmonary segmentectomy under 3-dimensional multidetector computed tomography simulation: a report of 52 consecutive cases. J Thorac Cardiovasc Surg. 2011;141:678–82. doi: 10.1016/j.jtcvs.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 22.Chen-Yoshikawa TF, Date H. Update on three-dimensional image reconstruction for preoperative simulation in thoracic surgery. J Thorac Dis. 2016;8(Suppl 3):S295–301. doi: 10.3978/j.issn.2072-1439.2016.02.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato M, Murayama T, Nakajima J. Concepts and techniques: how to determine and identify the appropriate target segment in anatomical pulmonary segmentectomy? J Thorac Dis. 2019;11:972–86. doi: 10.21037/jtd.2019.02.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato M, Murayama T, Nakajima J. Techniques of stapler-based navigational thoracoscopic segmentectomy using virtual assisted lung mapping (VAL-MAP) J Thorac Dis. 2016;8(Suppl 9):S716–30. doi: 10.21037/jtd.2016.09.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wells FC, Milstein BB. Thoracic surgical techniques. Bailliere Tindall; London: 1990. [Google Scholar]
- 26.Tsubota N. An improved method for distinguishing the intersegmental plane of the lung. Surg Today. 2000;30:963–4. doi: 10.1007/s005950070056. [DOI] [PubMed] [Google Scholar]
- 27.Okada M, Mimura T, Ikegaki J, Katoh H, Itoh H, Tsubota N. A novel video-assisted anatomic segmentectomy technique: selective segmental inflation via bronchofiberoptic jet followed by cautery cutting. J Thorac Cardiovasc Surg. 2007;133:753–8. doi: 10.1016/j.jtcvs.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 28.Oizumi H, Kato H, Endoh M, Inoue T, Watarai H, Sadahiro M. Techniques to define segmental anatomy during segmentectomy. Ann Cardiothorac Surg. 2014;3:170–5. doi: 10.3978/j.issn.2225-319X.2014.02.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamiyoshihara M, Kakegawa S, Morishita Y. Convenient and improved method to distinguish the intersegmental plane in pulmonary segmentectomy using a butterfly needle. Ann Thorac Surg. 2007;83:1913–4. doi: 10.1016/j.athoracsur.2006.06.052. [DOI] [PubMed] [Google Scholar]
- 30.Misaki N, Chang SS, Gotoh M, Yamamoto Y, Satoh K, Yokomise H. A novel method for determining adjacent lung segments with infrared thoracoscopy. J Thorac Cardiovasc Surg. 2009;138:613–8. doi: 10.1016/j.jtcvs.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Misaki N, Chang SS, Igai H, Tarumi S, Gotoh M, Yokomise H. New clinically applicable method for visualizing adjacent lung segments using an infrared thoracoscopy system. J Thorac Cardiovasc Surg. 2010;140:752–6. doi: 10.1016/j.jtcvs.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 32.Tarumi S, Misaki N, Kasai Y, Chang SS, Go T, Yokomise H. Clinical trial of video-assisted thoracoscopic segmentectomy using infrared thoracoscopy with indocyanine green. Eur J Cardiothorac Surg. 2014;46:112–5. doi: 10.1093/ejcts/ezt565. [DOI] [PubMed] [Google Scholar]
- 33.Ito A, Takao M, Shimamoto A, Shimpo H. Prolonged intravenous indocyanine green visualization by temporary pulmonary vein clamping: real-time intraoperative fluorescence image guide for thoracoscopic anatomical segmentectomy. Eur J Cardiothorac Surg. 2017;52:1225–6. doi: 10.1093/ejcts/ezx233. [DOI] [PubMed] [Google Scholar]
- 34.Sato M, Kuwata T, Yamanashi K, et al. Safety and reproducibility of virtual-assisted lung mapping: a multicentre study in Japan. Eur J Cardiothorac Surg. 2017;51:861–8. doi: 10.1093/ejcts/ezw395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohtsuka T, Goto T, Anraku M, et al. Dissection of lung parenchyma using electrocautery is a safe and acceptable method for anatomical sublobar resection. J Cardiothorac Surg. 2012;7:42. doi: 10.1186/1749-8090-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen X, Jin R, Xiang J, et al. Methods for dissecting intersegmental planes in segmentectomy: a randomized controlled trial. Ann Thorac Surg. 2020;110:258–64. doi: 10.1016/j.athoracsur.2020.02.013. [DOI] [PubMed] [Google Scholar]