Abstract
Introduction
Malignant triton tumor (MTT) is an extremely rare variant of the malignant peripheral nerve sheath tumors (MPNSTs) with rhabdomyosarcomatous differentiation, which was first described in 1932 by Mason. MTT affects, in most cases, patients under 35 years of age, and it is usually manifested as a mass that may or not be painful. However, the incidence in pediatric patients is atypical. This tumor presents an aggressive course and limited survival rate, and the prognosis is different between individuals with or without a concomitant diagnosis of neurofibromatosis type 1 (NF1). Currently, the recommended treatment is surgical resection, and adjuvant chemotherapy and radiotherapy, but its efficacy is not yet clear.
Presentation of the case
A 13-year-old female patient was referred to the pediatric oncology service due to the presence of an abdominal mass and weight loss, initially diagnosed with Wilms' tumor. After extensive investigation, surgical resection, and immunohistopathological evaluation, the diagnosis of malignant triton tumor was confirmed. The patient also underwent cycles of chemotherapy after resection, and is currently awaiting immunotherapy.
Discussion and conclusion
Malignant triton tumor is extremely rare and difficult to diagnose, especially in children or young people, age groups in which the incidence of the disease is even lower. This may be the reason it is rarely suspected, and it was a great challenge for the clinical care team. It is essential to consider and investigate this possibility of differential diagnosis, as patients diagnosed with this malignant tumor have a low survival rate and poor prognosis.
Keywords: Malignant triton tumor, Malignant peripheral nerve sheath tumors, Rhabdomyosarcomatous differentiation, Case report
Highlights
-
•
Malignant triton tumor (MTT) is an extremely rare histological subtype of MPNSTs.
-
•
MTT is an MPNST with rhabdomyosarcomatous differentiation.
-
•
MTT is an extremely rare type of neoplasia, especially in pediatric patients.
-
•
The kidney is an uncommon growth site of MTT.
-
•
MTT presents a complex anatomopathological and immunohistochemical diagnosis.
1. Introduction
Malignant peripheral nerve sheath tumors (MPNSTs), also called malignant schwannomas or neurogenic sarcomas, are soft tissue sarcomas (STSs) that arise from Schwann cells, within pre-existing neurofibromas or pluripotent cells of the neural crest with a lower degree of differentiation [1]. These tumors may contain, in 15% of cases, differentiation in other cellular components [2], such as epithelial glandular tissues, squamous cells, cartilage or bone [3], [4]. This represents approximately 5–10% of all STSs, and the incidence in the general population is 0.001% [2], and mainly affects individuals aged 20 to 50 years old [5].
Malignant triton tumor (MTT) is an extremely rare histological subtype of MPNSTs with rhabdomyosarcomatous differentiation, the most frequent among the heterologous elements of MPNSTs [6], having been first described in 1932 by Mason [1]. MTT is an aggressive tumor with a poor prognosis, affecting, in most cases, patients under 35 years of age, and the average age of incidence is 31.7 years [7]. The occurrence in pediatric patients is rare [8], and may be sporadic or occur in conjunction with neurofibromatosis type 1 (NF1) [7], [9], with 50–70% of MTTs occurring in those with this genetic condition [10], [11]. Furthermore, irradiation is considered a risk factor for the development of its sporadic form [9].
MTT is manifested as a mass that may or may not be painful. Regarding the estimated 5-year survival rate, studies have shown a variation between 5 and 20% for MTT [2], [12], [13] and 34–60% for MPNST [12], [13], and the morbidity rates of MTT are equal for both sexes [14]. However, the forms associated with NF1 are more commonly found in men and children, while sporadic forms have a higher incidence in women and older individuals [11]. When MTT affects the extremities, head, or neck, the prognosis is better than when it occurs in the retroperitoneum, buttock, or trunk, and the reason for this variation in prognosis is still unclear [5], though when associated with metastasis (31.4% of cases) [15] or with local recurrence (43–50% of cases), the tumor prognosis is even worse [12]. Furthermore, two important studies have shown that size, location and a more advanced stage of the tumor are associated with prognosis and survival [16].
In terms of histological findings, MPNSTs have spindle-shaped cells with hyperchromatic nuclei and numerous mitotic figures [2]. Concerning immunohistochemistry and immunoreactivity of MTT, protein S-100, loss of histone expression (H3K27M), myoglobin, desmin, sarcomeric actin, and myogenin of skeletal muscle can be found [6], [17].
In this article, we present a case of a 13-year-old female patient who was diagnosed with a giant MTT located in the kidney, an exceedingly rare growth site of this type of tumor [18]. The patient did not present a concomitant diagnosis of NF1. We have described the patient's clinical findings and diagnostic process, histomorphological, immunohistochemical and cytogenetic characteristics, therapeutic approach, and tumor prognosis. This case report is in line with the SCARE Criteria [20].
2. Presentation of the case
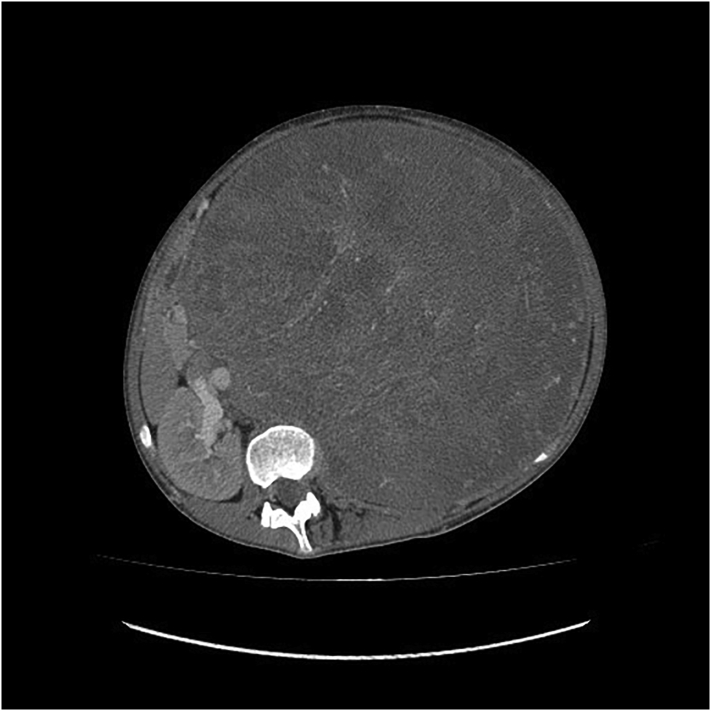
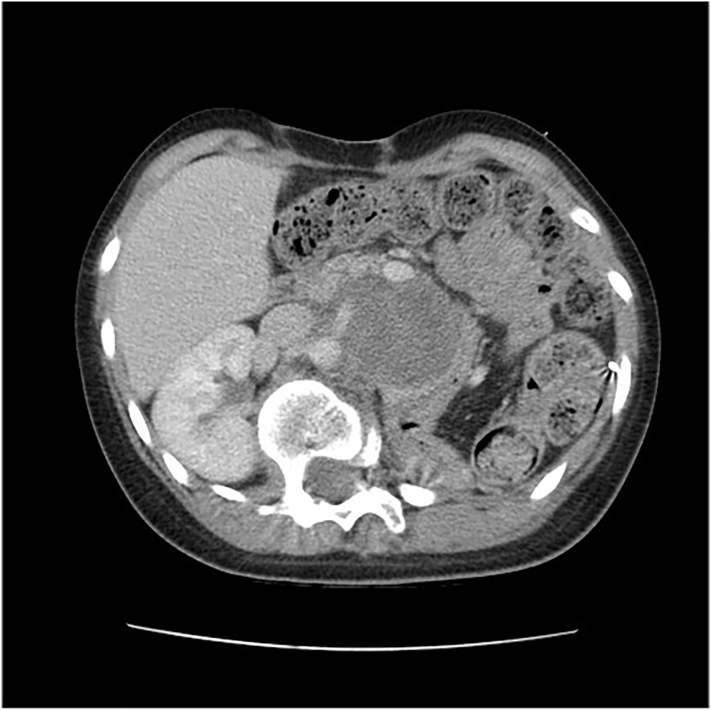
The patient is a previously healthy female, 13 years of age, with neither a family history of cancer nor relevant genetic information who was referred to the pediatric oncology service due to the presence of an abdominal mass and weight loss. The patient had been under examination for scoliosis in the orthopedic service for five months when she noticed an increase in abdominal volume in the last four weeks. The patient reported weight loss of 12 kg (from her usual weight of 57 kg to 45 kg at admission) in this period. Abdominal computed tomography (CT) performed before admission revealed a left retroperitoneal lesion measuring 24 × 20 × 20 cm, with probable renal origin (Fig. 1).
Fig. 1.
Abdominal computed tomography (CT) revealed a left retroperitoneal lesion measuring 24 × 20 × 20 cm.
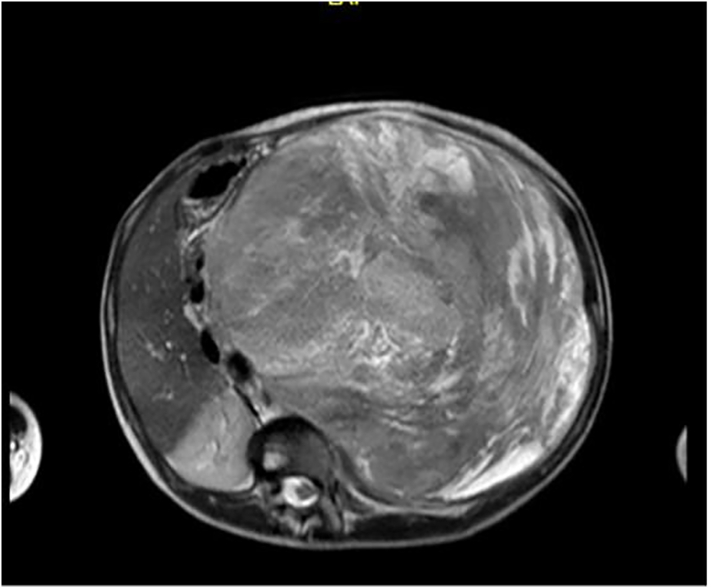
Upon admission, staging was performed with chest CT, nuclear magnetic resonance (MRI) of the abdomen, and bone scintigraphy. Staging revealed nonspecific micronodules in the basal segment of the left lung, osteoblastic activity in the distal third of the left costal arch, and confirmed a predominantly solid 24 × 20 × 20 cm lesion, which crossed the midline, maintaining close proximity to the abdominal aorta, celiac trunk, and inferior mesenteric artery (Fig. 2). The mass displaced the pancreas, liver, spleen, stomach, duodenum and other intestinal loops. Given this scenario, the care team decided to perform a percutaneous biopsy of the lesion.
Fig. 2.
MRI showing a 24 × 20 × 20 cm retroperitoneal mass in close contact with the aorta, celiac trunk and inferior mesenteric artery.
The patient was submitted to an ultrasound-guided biopsy. Histological analysis of the lesion showed spindle cells with elongated nuclei and a high mitotic index. An immunohistochemical study was performed, which was positive for WT1 (focal positive), desmin (focal positive), and Ki67 positive in 20% of the lesion. The Pathology team favored the diagnosis of Wilms' tumor (nephroblastoma) with a stromal component.
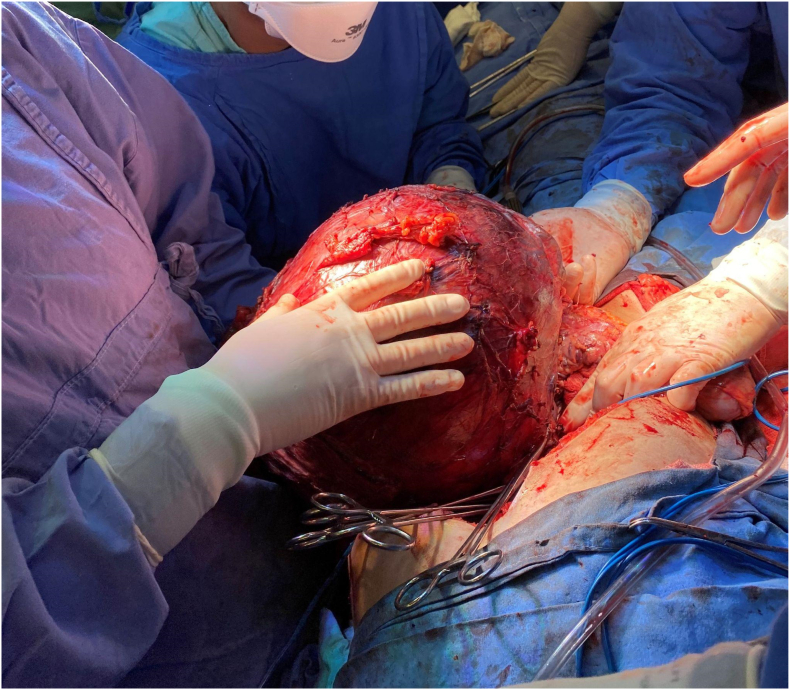
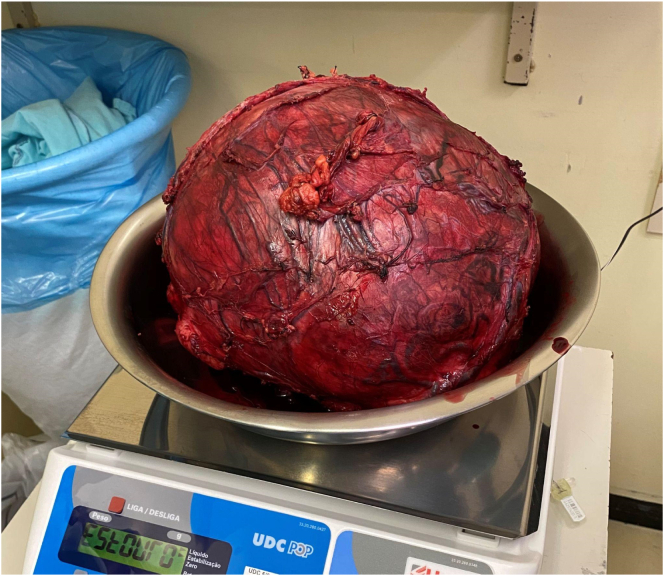
Chemotherapy was performed for four weeks. Initially, six weeks of treatment were scheduled, but the disease progressed. The patient underwent exploratory laparotomy which did not show lymph node, liver, and peritoneal implants, though there was a large mass firmly attached to the transverse mesocolon, the distal pancreas, and the spleen. Radical resection of the lesion was performed with distal pancreatectomy and splenectomy by the pediatric surgery team, coordinated by the surgeon-in-chief in July 2020. The lesion capsule was ruptured at the end of the procedure. A surgical piece weighing approximately 7 kg was removed (Fig. 3, Fig. 4).
Fig. 3.
Posterior dissection and separation of the great vessels of the abdomen.
Fig. 4.
Tumor weight was approximately 7 kg.
The patient evolved favorably in the postoperative period. During the first few days, she had low diet acceptance, but was discharged from the surgical follow-up on the 11th postoperative day, maintaining good diuresis, good pain control, eating and evacuating, without complications related to the surgical wound. The anatomical pathology of the surgical specimen again favored the diagnosis of Wilms' tumor, exclusively composed of stromal components, with free margins. The patient then underwent a new chemotherapy cycle with ifosfamide, carboplatin, and etoposide, according to the protocol of the International Society of Pediatric Oncology (SIOP).
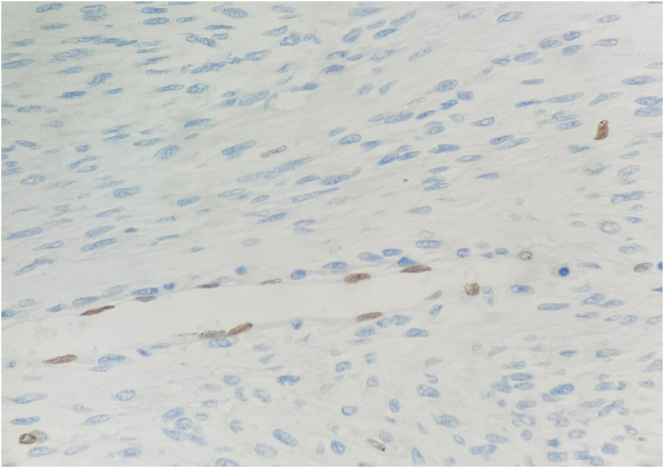
After the fourth week of chemotherapy, the patient experienced weight loss, nausea, and backache. She underwent restaging with computed tomography of the abdomen which revealed an expansive para-aortic/paravertebral retroperitoneal lesion measuring 8.3 × 6.5 × 5.8 cm (Fig. 5). Due to early local recurrence, the pathological examination was sent to the Brazilian Renal Tumor Group, being reviewed with the support laboratory of the Brazilian Society of Pediatric Oncology. The review favored the diagnosis of malignant peripheral nerve sheath tumor with areas of rhabdomyoblastic differentiation and squamous epithelium, characteristics of triton tumor. Moreover, Fig. 5 reveals the loss of histone expression (H3K27M), which is a marker of malignant peripheral nerve sheath tumor, as histone methylation occurs in the neoplastic cells.
Fig. 5.
Endothelial cells with loss of histone expression (H3K27M).
At this time, we decided to change the chemotherapy regimen to ifosfamide and doxorubicin, according to the protocol for non-rhabdomyosarcomas. However, the patient showed progression of the lesion, and the chemotherapy regimen was changed once again to vincristine, irinotecan, and temozolomide. The recurrent mass was considered inoperable due to its unfavorable location, which surrounded the abdominal vessels, especially the celiac trunk (Fig. 6). Therefore, owing to the poor response to clinical treatment and the absence of the possibility of operating the residual tumor, palliative management was defined, together with the oncology team. Currently, the patient is undergoing outpatient clinical follow-up. The patient is clinically well with no postoperative complications, and is awaiting immunotherapy.
Fig. 6.
Abdominal computed tomography (CT) revealing a recurrent 8.3 × 6.5 × 5.8 cm retroperitoneal lesion dislocating and with wide surface of contact (180–360°) with the celiac trunk. The lesion is in close contact with the superior mesenteric artery, surrounding it.
3. Discussion and conclusion
Malignant triton tumor is an extremely rare variant of the MPNSTs with rhabdomyosarcomatous differentiation, typically found on the head, neck, extremities, and trunk, and there are case reports that have shown this tumor growing in places such as the nasal cavity, paranasal sinus, lumbar spine, parapharyngeal space, rectum, mediastinum, palate, tibial nerve, esophagus, cerebellopontine angle, and acoustic nerve. It rarely affects the kidney, brain, buttock, retroperitoneum, thoracic spine, pelvis, genitourinary tract, and thigh [1], [2], [3], [5], [7], [14], [19].
MTT is extremely rare and difficult to diagnose, especially in children or young people, age groups in which the incidence of the disease is even lower, thus it is rarely suspected. Moreover, pathologists also often have difficulty finding the anatomopathological and immunohistochemical patterns of the tumor, which may be due to the rarity of the disease. Therefore, professionals rarely see patients with this condition.
In the case presented, there was also difficulty in determining the diagnosis. Thus, it was misdiagnosed twice as Wilms' tumor. Misdiagnosis was influenced by clinical and histopathological findings which were highly consistent with Wilms' tumor: a kidney tumor in a pediatric patient who was positive for WT1 (focal positive), desmin (focal positive), and Ki67 in 20% of the lesion. Moreover, due to the extremely low prevalence of MTT, especially in the renal site and in pediatric patients, diagnosis was not initially suspected.
After revision of the histopathological slides by the Brazilian Renal Tumor Group due to local recurrence, a new analysis revealed the presence of spindle-shaped cells and loss of histones expression (H3K27M), definitively establishing the diagnosis of malignant triton tumor. The delay in correctly treating the patient was a serious problem, as the poor prognosis of the disease associated with delayed treatment can cause a more unfavorable outcome.
The treatment of MTT is similar to that of rhabdomyosarcoma, which includes primary surgery and adjuvant chemotherapy and radiotherapy, although the impact of the latter two on the improvement of the outcome is not clear [4]. Currently, multimodal management seems to be the best option [19]. Since the lesion was refractory to treatment, palliative management was decided following multidisciplinary discussion. The patient is undergoing clinical follow-up since the surgical resection in July 2020. The patient is clinically well with no complications after the surgery. She is in good general condition and asymptomatic, currently awaiting immunotherapy.
Despite being an extremely rare disease, with significant diagnostic difficulty, it is essential to consider and investigate this possibility of differential diagnosis, as patients with this malignant tumor have a low survival rate and poor prognosis. Therefore, more cases and studies must be published in order to support professionals potentially exposed to this diagnosis.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Sources of funding
This case report did not receive funding or grants.
Ethical approval
This case report is exempt from ethical approval in our institution.
Consent for publication
Written informed consent was obtained from the patient ascent (the patient is 13 years old) for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
CRediT authorship contribution statement
Rafael Bittencourt Bins: Conceptualization; Project administration; Writing - original draft; Writing – review & editing.
Carlos Eduardo Pinzon: Conceptualization; Project administration; Writing - original draft; Writing – review & editing.
Leonardo Dantas da Silva Pereira: Writing – original draft.
Marina Bertuol: Writing – original draft.
Paola Maria Borlin Santis Isolan: Supervision.
Eliane Emy Takamatu: Supervision.
Research registration
N/a
Guarantors
Rafael Bittencourt Bins.
Carlos Eduardo Pinzon.
Declaration of competing interest
None.
Acknowledgements
Not applicable.
Contributor Information
Rafael Bittencourt Bins, Email: rbins@hcpa.edu.br.
Carlos Eduardo Pinzon, Email: e.carlospinzon@gmail.com.
Leonardo Dantas da Silva Pereira, Email: ldspereira@hcpa.edu.br.
Marina Bertuol, Email: marbertuol@hcpa.edu.br.
Paola Maria Brolin Santis Isolan, Email: pisolan@hcpa.edu.br.
Eliziane Emy Takamatu, Email: etakamatu@hcpa.edu.br.
References
- 1.Brtko J., Sejnová D., Ondková S., Macejová D. Malignant triton tumour exhibits a complete expression pattern of nuclear retinoid and rexinoid receptor subtypes. Gen. Physiol. Biophys. 2009 Dec;28(4):425–427. doi: 10.4149/gpb_2009_04_425. [DOI] [PubMed] [Google Scholar]
- 2.Seddighzadeh R.P., Brower S., Tzeng J., Serur A. Malignant triton tumor below the peritoneal reflection: a case report. J. Surg. Case Rep. 2020 Jun 19;2020(6) doi: 10.1093/jscr/rjaa171. PMID: 32595924; PMCID: PMC7303023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aldlyami E., Dramis A., Grimer R.J., Abudu A., Carter S.R., Tillman R.M. Malignant triton tumour of the thigh–a retrospective analysis of nine cases. Eur. J. Surg. Oncol. 2006 Sep;32(7):808–810. doi: 10.1016/j.ejso.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Velagaleti G.V., Miettinen M., Gatalica Z. Malignant peripheral nerve sheath tumor with rhabdomyoblastic differentiation (malignant triton tumor) with balanced t(7;9)(q11.2;p24) and unbalanced translocation der(16)t(1;16)(q23;q13) Cancer Genet. Cytogenet. 2004 Feb;149(1):23–27. doi: 10.1016/S0165-4608(03)00278-4. [DOI] [PubMed] [Google Scholar]
- 5.Isla A., Gutierrez M., Casillas M., Gil J.L. Malignant triton tumor in the thoracic spine. Childs Nerv. Syst. 2000 Apr;16(4):256–259. doi: 10.1007/s003810050509. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt D., Harms D., Leuschner I. Cytokeratin expression in malignant triton tumor. Pathol. Res. Pract. 1990 Aug;186(4):507–511. doi: 10.1016/S0344-0338(11)80470-X. discussion 511–3. [DOI] [PubMed] [Google Scholar]
- 7.Jaing T.H., Chuang C.C., Jung S.M., Wu C.T., Tseng C.K., Chen C.S. Malignant triton tumor of the cervical spine: report of one case and review of the literature. Pediatr. Neonatol. 2015 Feb;56(1):58–61. doi: 10.1016/j.pedneo.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Hou Z., Wang C., Li L., Dong L. Retroperitoneal malignant triton tumor in an infant: a case report and literature review. Transl. Pediatr. 2020 Aug;9(4):567–572. doi: 10.21037/tp.2020.03.12. PMID: 32953555; PMCID: PMC7475308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mijovic Z., Mihailovic D., Zivkovic N., Kostov M., Zivkovic S., Stojanovic N. A rare case of retroperitoneal malignant triton tumor invading renal vein and small intestine. Vojnosanit. Pregl. 2013 Mar;70(3):322–325. doi: 10.2298/vsp1303322m. [DOI] [PubMed] [Google Scholar]
- 10.Gallego A., Pontones J.L., Ramos D., Boronat F. Malignant triton tumor of the kidney. New location not previously reported. Urol. Int. 2017;99(1):121–123. doi: 10.1159/000434639. Epub 2015 Jul 7. PMID: 26159374. [DOI] [PubMed] [Google Scholar]
- 11.Engel E.E., Brassesco M.S., Valera E.T., Nogueira-Barbosa M.H., Yamashita M.E., Scrideli C.A., Tone L.G. Clinico-genetic aspects of a pediatric non-neurofibromatosis type 1 malignant triton tumor with loss of chromosome X. Pediatr. Blood Cancer. 2012 Dec 15;59(7):1320–1323. doi: 10.1002/pbc.24197. [DOI] [PubMed] [Google Scholar]
- 12.Alina B., Sebastian J.A., Gerardo C. Malignant triton tumors in sisters with clinical neurofibromatosis type 1. Case Rep. Oncol. Med. 2015;2015 doi: 10.1155/2015/405351. Epub 2015 May 31. PMID: 26114002; PMCID: PMC4465693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamran S.C., Howard S.A., Shinagare A.B., Krajewski K.M., Jagannathan J.P., Hornick J.L., Ramaiya N.H. Malignant peripheral nerve sheath tumors: prognostic impact of rhabdomyoblastic differentiation (malignant triton tumors), neurofibromatosis 1 status and location. Eur. J. Surg. Oncol. 2013 Jan;39(1):46–52. doi: 10.1016/j.ejso.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Zhao A., Ding D., Li X., Wang J. Malignant triton tumor in a child: case report and literature review. Cancer Manag. Res. 2019 Dec;24(11):10759–10766. doi: 10.2147/CMAR.S221110. PMID: 31920385; PMCID: PMC6935315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McConnell Y.J., Giacomantonio C.A. Malignant triton tumors–complete surgical resection and adjuvant radiotherapy associated with improved survival. J. Surg. Oncol. 2012 Jul 1;106(1):51–56. doi: 10.1002/jso.23042. [DOI] [PubMed] [Google Scholar]
- 16.Wong W.W., Hirose T., Scheithauer B.W., Schild S.E., Gunderson L.L. Malignant peripheral nerve sheath tumor: analysis of treatment outcome. Int. J. Radiat. Oncol. Biol. Phys. 1998 Sep 1;42(2):351–360. doi: 10.1016/s0360-3016(98)00223-5. [DOI] [PubMed] [Google Scholar]
- 17.Bian Y., Yongbo X., Xi Z., Zhao D., Wu H., Liu Y. A series of 10 malignant triton tumors in one institution. Medicine (Baltimore) 2019 Sep;98(36) doi: 10.1097/MD.0000000000016797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alharbi B. Malignant peripheral nerve sheath tumor of kidney-a case report. Int. J. Surg. Case Rep. 2013;4(10):914–916. doi: 10.1016/j.ijscr.2013.07.013. Epub 2013 Aug 3. PMID: 23995478; PMCID: PMC3785930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okur F.V., Oguz A., Karadeniz C., Citak C., Bayik P., Boyunaga O. Malignant triton tumor of the pelvis in a 2-year-old boy. J. Pediatr. Hematol. Oncol. 2006 Mar;28(3):173–176. doi: 10.1097/01.mph.0000201419.05182.29. [DOI] [PubMed] [Google Scholar]
- 20.Agha R.A, Franchi T., Sohrabi C., Mathew G. SCARE Group. The SCARE 2020 Guideline: Updating Consensus Surgical CAse REport (SCARE) Guidelines. Int J Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]