Highlights
-
•
Paint particles are often overlooked in the micro-debris and microplastic pools.
-
•
The sources, behaviour and impacts of paint in the marine environment are reviewed.
-
•
Paint particle emissions may be as high as 35% of the synthetic micro-debris input.
-
•
Paint is regularly detected in sea surface trawls, sediments and animal digestive tracts.
-
•
Hazardous additives in micro-paint particles render them more harmful than microplastics.
Keywords: Paint particles, Microplastics, Marine environment, Composition, Impacts, Toxicity
Abstract
Because paint particles consist of a resin (polymer) combined with one or more additives, they bear compositional similarities with microplastics. Despite these shared characteristics, however, paint particles are often undetected, deliberately overlooked or evade classification in the pool of micro-debris (all synthetic debris of < 5 mm in size), and in particular in the marine setting where an extensive body of microplastic literature exists. Accordingly, the present paper provides a critical insight into the physico-chemical properties, sources, distributions, behaviour and toxicity of paint particles in the marine environment.
Paint particles contain a greater proportion of additives than plastics and, consequently, are more brittle, angular, opaque, dense, heterogeneous and layered than microplastics of equivalent dimensions. Land-based sources of paint particles, including deteriorating or disturbed coatings on roads and building, are transported to the ocean with other microplastics via urban runoff, water treatment facilities and the atmosphere. However, inputs of paint particles are enhanced significantly and more directly by the disturbance, erosion and weathering of coatings on coastal structures, boats and ships. Estimates of paint particle emissions to the marine environment vary widely, with calculated contributions to the total synthetic micro-debris input as high as 35%. Upper estimates are consistent with available (albeit limited) quantitative information on the relative abundance of paint particles amongst synthetic material captured by sea surface trawls and ingested by marine animals. Of greatest environmental concern is the high chemical toxicity of paint particles compared with similarly-sized microplastics and other synthetic debris. This results from the contemporary and historical use of high concentrations of hazardous inorganic additives in marine antifouling and land-based paints, and the relatively ready mobilisation of harmful ions, like Cu+/Cu2+, TBT+, Pb2+ and CrO42−, from the matrix. Recommendations arising from this review include greater use of particulate capturing devices, waste collection systems and recycling facilities during paint disturbance, raising awareness of the potential impacts of discarded paint amongst users, and alerting the microplastic community to the significance of paint particles and developing means by which they are isolated from environmental samples.
1. Introduction
The recent scientific, policy and management literature contains a plethora of studies on microplastics in the environment, and in particular in the marine environment (Abbasi et al., 2018; Dauvergne, 2018; Liubartseva et al., 2018; Kane and Clare, 2019; Henderson and Green, 2020; Kor and Mehdinia, 2020). Microplastics have been operationally defined as synthetic or semisynthetic materials constructed of polymers and additives that are < 5 mm in diameter (Arthur et al., 2009), with nanoplastics defined with a 1000 nm upper size limit (Gigault et al., 2018). Microplastics may be further divided into primary particles, like pre-production pellets, clothing fibres and exfoliating beads used in cosmetic products that are < 5 mm before entering the environment, and secondary particles that are broken down from larger debris in situ to fragments, films, foams and fibres of < 5 mm in size. Microplastics may also be classified according to additional physical or chemical attributes, like polymer type, colour, roughness, transparency and shape (Gauci et al., 2019; Bikker et al., 2020).
Paint is a pigmented and usually opaque surface coating that has decorative, protective or other specific technical properties (OECD 2009, Ory et al., 2018). Paint consists of polymers and additives and dried paint particles of < 5 mm in size derived from the deterioration or removal of surface applications (paint particles) should, strictly, be classified as microplastics according to the definition above. However, there is relatively little systematic study of paint particles in the current marine scientific literature (Galafassi et al., 2019; Gaylarde et al., 2021). This may, in part, be attributed to the omission of paint in marine litter guidelines and the consequent inconsistencies regarding its classification in the microplastic literature (OSPAR Commission, 2010). Thus, some investigations include paint particles amongst the microplastic cohort (Lima et al., 2014; Cardoza et al., 2018; Haave et al., 2019) while others operationally, incidentally or deliberately exclude them or treat them differently when reporting or characterising samples (Free et al., 2014; Baini et al., 2018; Ferreira et al., 2019; Lacerda et al., 2019). In this paper, the similarities in and differences between microplastics and equivalently sized paint particles are critically evaluated based on their uses, physico-chemical characteristics, sources, behaviours and relative abundances in the environment. The focus is on the marine setting in which both types of solid clearly exist, but where the connection is often distinctly lacking or overlooked.
2. Plastics versus paints
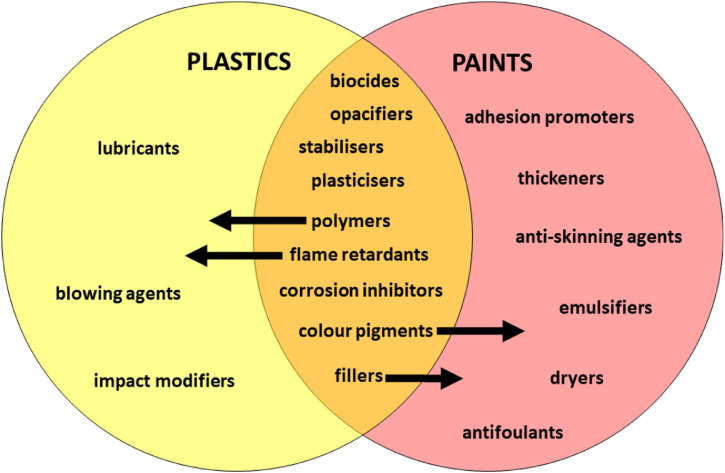
There are fundamental similarities in and differences between plastics and paints which are conceptualised in Fig. 1. Thus, plastics are constructed of one or more synthetic polymer whose properties may be customised by functional additives and fillers, while paints consist of fine, natural or synthetic polymeric particles (the binder or resin) and additives and fillers that are held together on a surface as a “plastic-like” film when cured. Frequently used synthetic polymers in consumer and industrial plastics include acrylonitrile butadiene styrene, polyethylene, polypropylene, polyethylene terephthalate (PET), polystyrene and polyvinyl chloride (PVC), while most paints are based on acrylic, alkyd, polyurethane, epoxy or chlorinated rubber binders.
Fig. 1.
The chemical constituents of plastics and paints. Polymers, pigments, fillers and certain additives are common to both materials (with the arrow showing the direction of greater abundance) while other additives are more specific to either general or speciality plastics or paints.
Some additives are common to both plastics and paints, like inorganic fillers and colourants, while others are specific to or more commonly employed in either plastics or paints because of differences in the manufacture, processing and function of the materials. For example, dryers, emulsifiers and adhesion promoters are critical to the storage and application of many kinds of paint whereas blowing agents, lubricants and impact modifiers are more important for the performance and durability of certain plastics. In general, the mass content of additives (including pigments) and fillers is significantly greater in dried paint films than in plastics and, consequently, the polymeric content is usually much greater in the latter. There is also a smaller range of additives available for plastics because the higher processing temperatures required constrain the selection of chemicals, and in particular colour pigments, based on thermal stability.
These differences generally mean that paint particles are denser, more brittle, more angular, less transparent and chemically more heterogeneous than microplastics. Moreover, the nature of paint application results in a layered structure (of identical or different formulations) that may be contaminated by residue from the underlying substrate. These characteristics may account for why paints are deliberately (through appearance under a microscope; Imhof et al. 2016; Horton et al., 2017) or operationally (via density separation or chemical treatment; Coppock et al., 2017; Rodrigues et al., 2020) excluded from the overall micro-debris pool in environmental samples. (Micro-debris is defined as all synthetic debris of < 5 mm in size, including fibres, tire-wear particles and other vehicle-derived solids, pellets, beads and paints.)
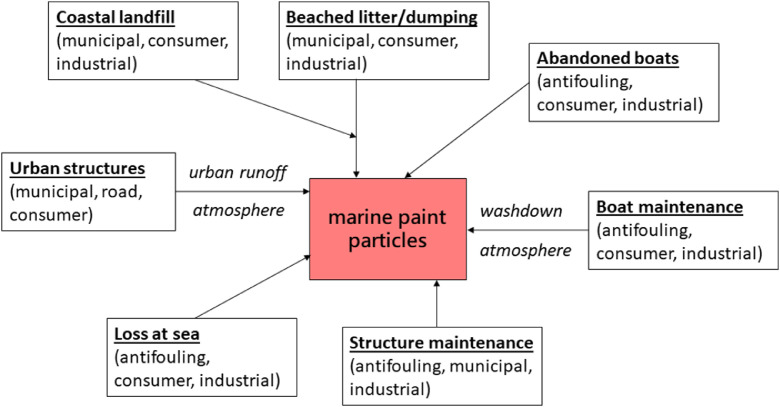
3. Sources of paint particles to the marine environment
The broad sources of paint particles to the marine environment are shown in Fig. 2, where paints are defined according to their principal users or applications; a selection of various, more specific sources is also illustrated in Fig. 3. In theory, and as with microplastics, sources can be divided into primary and secondary, with the former representing fine (< 5 mm) particles transported into the environment via runoff or the atmosphere and the latter encompassing particles that are formed in situ from, for example, the weathering of larger fragments or the deterioration or damage to coastal structures and boats. Ultimately, however, most paint particles are formed by the same mechanisms acting on the paint film and such a classification does not bear any relationship with physical or chemical properties or environmental behaviour. Mechanisms of deterioration are UV degradation of the binding polymer and intentional or unintentional mechanical disturbance of the coating (damage, wear and tear, maintenance or removal).
Fig. 2.
Sources of paint particles (from different users and applications) to the marine environment.
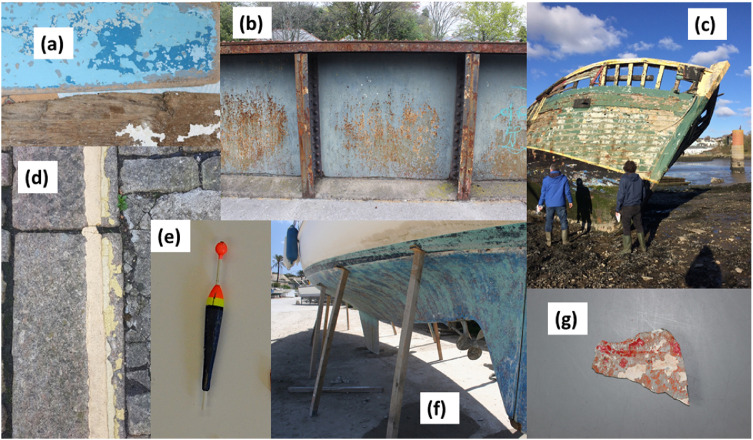
Fig. 3.
Images illustrating some of the specific sources of paint particles to the marine environment. (a) Painted wooden boards that had been dumped on a beach, (b) paint peeling off a road bridge, (c) paint deteriorating from the hull and rails of an abandoned ship, (d) crumbling yellow road paint, (e) a painted, beached fishing float, (f) the hull of a boat having recently been power-sanded, and (g) a fragment of painted plastic retrieved from a beach near a coastal landfill site. (Images courtesy of Andrew Turner, Madeleine Lewis and Christina Muller-Karanassos).
The poor management of municipal waste containing unused paint or painted structures (including building waste) and packaging may act as a direct source of paint particles to the coastal environment through littering and failing coastal landfills (Pope et al., 2011). More generally, however, the urban environment represents a rich and varied indirect source of paint particles that are generated when decorative, anticorrosive or safety paints on private and public buildings, road surfaces, and municipal structures and street furniture undergo natural deterioration or are deliberately disturbed during maintenance, repair or removal (Jartun et al., 2009; Turner and Solman, 2016; Horton et al., 2017; Lee et al., 2018). More directly, large coastal structures like bridges and decommissioned naval establishments may act as locally significant sources of paint particles to the marine environment (Finkelstein et al., 2003; Shu et al., 2015). However, perhaps of broader concern in this respect is the uncontrolled release of paint particles from boats that are abandoned, being recycled or undergoing repair (Reddy et al., 2003; Singh and Turner, 2009; Rees et al., 2014; Soon et al., 2021). Regarding the latter, maintenance of hulls in the leisure boat sector is generally unregulated and yet produces significant quantities of toxic antifouling paints particles of a range of sizes from the scraping, sanding, stripping, sand-blasting and hydro-blasting of spent coatings. Furthermore, older formulations that are disturbed often contain metals and biocides that are now restricted or prohibited (Eklund et al., 2014; Soroldoni et al., 2018).
Paint particles from these sources are transported to the aquatic environment as airborne particulates that are subject to fallout, with road runoff or treated waste water, or via washdown from boat maintenance facilities or coastal structures undergoing repair. The significance of the local transport of airborne paint particles has been established from the levels and signatures of contamination of soils and roof top dusts of residential and municipal buildings in the vicinity of roads, dry docks, boatyards and harbours (Decelis and Vella, 2007; Jartun et al., 2009; Turner, 2013; Eklund et al., 2014; Sakata et al., 2017; Meza-Figueroa et al., 2018). Given the particle size range generated by sanding (in the range of < 50 nm to a few μm in diameter; Koponen et al., 2009), however, the potential range of airborne transport of paint particles is considerable. For example, microplastics of dimensions orders of magnitude greater (albeit less dense and usually fibrous) appear to have the propensity to be transported thousands of km with regional air masses (Bergmann et al., 2019; Brahney et al., 2020) while geosolids towards the upper size limit generated by sanding may be transported globally (Mahowald et al., 2014).
Paint particles are also generated while ships are at sea. For example, groundings and collisions are known to generate large quantities of antifouling paint around the location of impaction (Negri et al., 2002; Jones, 2007; van der Schyff et al., 2020), while vessels navigating through ice or fishing activities generating friction between painted surfaces and rope may also act as more diffuse sources (Negri and Marshall, 2009; Song et al., 2014). It is also likely that paint particles are generated more passively with the general wear and tear (erosion) of hull, waterline, topside and deck coatings (Dibke et al., 2021).
4. Inputs of paint particles to the marine environment
Estimating the quantities of paint particles entering the marine environment or their contribution to the total marine microplastic pool is fraught with difficulties and uncertainties. One of the fundamental considerations in this respect is whether the mass of dried paint should be converted to the mass of polymer by subtracting the weights of additives and fillers present before direct quantitative comparisons are made with microplastics. While this approach is often favoured for flux calculations and inventories, additives and fillers are integral components of both paints and microplastics that, from physical, environmental and toxicological perspectives, should not be ignored.
Input estimates are generally based on the quantities of paints manufactured for or sold in different sectors and quantitative assumptions about longevity, removal, disposal and retention by waste facilities, and are usually compared with estimates for other types of microplastic based on equivalent assumptions (Sundt et al., 2014; Lassen et al., 2015; Verschoor et al., 2016). In a recent example, Hann et al. (2018) estimated emissions of different types of microplastics to the European aquatic environment using data, information and assumptions on paints published in earlier OECD reports (OECD, 2005; OECD 2009) and supplied by the European Council of the Paint, Printing Ink, and Artist's Colours Industry (CEPE). Neglecting inputs from poor waste management or the degradation of larger plastic waste, lower, middle and upper estimates for each microplastic category considered were derived (Table 1). For building, marine and road marking paints, estimates were based on figures for consumption, coupled with assumptions on rates of removal during maintenance, wear and tear and polymer degradation, and rates of retention, entrapment or loss by water treatment facilities, road-side sedimentation devices, road cleaning, adjacent soils and asphalt surfaces. Not factored into the estimates for paint were emissions to water when uncured, disturbance of layers of paint below the top coat (for example, primers and base coats), weathering of marine paints while in service or at the end-of-life, and the direct input of airborne dusts. Within these assumptions and constraints, estimates for paint particle emissions to surface waters range from about 12,000 to 30,000 tonnes, or between about 10 and 17% of total microplastic inputs. Presumably, inputs to the marine environment are related to these estimates but with modification of building and road paint inputs by settlement and entrapment in rivers.
Table 1.
Estimates of annual microplastic emissions (in tonnes and by category) to European surface waters (Hann et al., 2018). Upper, middle and lower estimates are based on different assumptions about removal and retention rates.
| Upper | Middle | Lower | |
|---|---|---|---|
| Automotive Tyres | 136,000 | 94,000 | 52,000 |
| Pellets | 78,000 | 41,000 | 3,000 |
| Washing of Clothing | 23,000 | 13,000 | 4,000 |
| Road Markings | 21,000 | 15,000 | 10,000 |
| Building Paint | 8,000 | 5,000 | 2,000 |
| Fishing Gear | 5,000 | 2,600 | 500 |
| Automotive Brakes | 5,000 | 2,000 | 100 |
| Artificial Turf | 3,000 | 2,000 | 300 |
| Marine Paint | 400 | 400 | 400 |
| Total | 280,600 | 176,300 | 71,800 |
| Total paint | 29,400 | 20,400 | 12,400 |
Given the many sources of marine paint particles neglected in the calculations above, it is likely that the emission figures for each paint category in Table 1 have been underestimated. In their calculations, for example, McAdams and Angelskår (2020) considered all paint applied to industrial and marine steel assets (not just marine paints, and about 42 billion litres per annum) and assumed a 20-year coating lifetime, or a 5% loss per year, and 50% efficiency of waste retrieval. A resulting 2-3 million tonnes of paint particles was predicted to enter the oceans annually, representing a highly significant fraction of the estimated 8 million tonnes of total plastic entering the marine environment each year (Jambeck et al., 2015). The calculated relative contribution of paint to the latter estimate is still subject to many uncertainties but is more consistent with measurements of the relative abundance of micro-debris constituents (that include paint) obtained by surface trawls in the ocean and as reported below.
5. Presence and abundance of paint particles in the marine environment
The presence of protective and antifouling paint particles in sediment deposits in the vicinity of boatyards, marinas, harbours and abandoned boats has been well-documented from visual or microscopic inspection of sieved samples (Thomas et al., 2003; Turner, 2010; Takahashi et al., 2012; Eklund et al., 2014; Rees et al., 2014; Costa et al., 2016; Lagerström et al., 2016; Soroldoni et al., 2018; Abreu et al., 2020). Based on sediment contamination by Cu, Singh and Turner (2009) estimated antifouling paint particle abundance of up to 1% on a mass basis in a tidal inlet of southwest England, while direct counting of antifouling paint particles in sediments from a Brazilian lagoon by Soroldoni et al. (2018) revealed mass contamination of up to 4.4%.
In contrast, very little quantitative information exists on paint particles in intertidal and benthic marine sediments more remote from significant point sources. Thus, in the coastal environment, Díez-Minguito et al. (2020) refer to paint “sheets” in sediments from the Ría de Vigo, northwest Spain, at water depths of up to 40 m, while Haave et al. (2019) report paint particles in a Norwegian fjord at depths of up to 330 m. Along the strandline of a sandy beach in eastern England, Latuta (2019) report that almost one half of microplastics retrieved by density separation were paint-based. In the open ocean, Fischer et al. (2015) mention paint chips being present in sediments from the northwest Pacific Kuril–Kamchatka Trench and its adjacent abyssal plain at depths of around 5 km. Unfortunately, however, none of these studies provide clear quantitative information on the paint particles observed, nor characteristics that could determine their origin.
A greater body of more quantitative data exist for paint particles captured by plankton trawls at or near the sea surface. In some cases, visible or chemical characterisation of particles has revealed that the protective or antifouling paints of the research vessel or trawl frame was a significant (and sometimes dominant) source created by shedding from the hull or collisions between the vessel and sampling equipment (Rummel et al., 2016; Bagaev et al., 2017; Eriksen et al., 2018; Lacerda et al., 2019; Suaria et al., 2020). However, in other cases, trawls deployed from unpainted vessels or paint sample characteristics sufficiently different to those of formulations applied to the research vessel or trawl frame have allowed external sources to be inferred.
Lima et al. (2014) found that paint particles contributed nearly 30% of all microplastics and up to about 0.1 particle m−3 in 300 μm nets trawled in the Goiana estuary, Brazil. The greatest concentrations were encountered in the bottom waters and during the rainy season, coincident with the period of most intensive fishing activities. Kang et al. (2015a) found alkyd-based paint particles contributed 20 to 50% of suspended microplastics in the mouth of Nakdong River, Korea, with abundances of up to about 1 m−3 captured by a 330 μm Manta trawl but up to 230 m−3 captured by a finer, 50 μm hand net. Dibke et al. (2012) recently estimated that up to 80% of the microplastics sampled from surface waters of the German Bight (North Sea) using an on-board deck-wash system were ship paints based on epoxy, acryl and chlorinated rubber binders; the remaining material was dominated by polyethylene, polypropylene and PET derived from packaging and mismanaged waste. Paint particles have also been reported amongst microplastic debris retrieved from plankton trawls in the North Atlantic (Morét-Ferguson et al., 2010), Black Sea (Öztekin and Bat, 2017), tropical and equatorial western Atlantic Ocean (Ivar do Sul et al., 2014; Garcia et al., 2020), Adriatic Sea (Suaria et al., 2016) and Gulf of Oman (Aliabad et al., 2019), and over the Great Barrier Reef (Hall et al., 2015; Jensen et al., 2019). In the coastal zone of Korea, Song et al. (2014) observed paint particles in the sea surface microlayer, or the boundary layer between the atmosphere and ocean, sampled to a depth of 400 μm using a metal sieve. The mean particle abundance was found to be about 200 L−1 or 150 m−2 compared with an abundance of other microplastics of 16 L−1 or 13 m−2. Significantly, there was a distinct increase in paint particle number with decreasing size (> 1000 μm to < 50 μm) which was attributed to the gradual breakdown of the floating stock of paint particles into smaller pieces.
Quantitatively and qualitatively, the observations and measurements above are subject to various limitations and uncertainties. Specifically, in sediment, where harsh chemical treatment (e.g. peroxidation or acidification) and flotation are employed to isolate microplastics, the abundance of paint particles is predicted to be under-reported because of their greater density and lower chemical stability (see below). In seawater, paint particle concentrations are likely underestimated because of the inherent size limits of the capturing devices or detection methods and the propensity for paint particles to fragment to sizes below these limits during sample processing. Nevertheless, it is clear that paint particles are a significant, if not the dominant form of microplastic at or near the water column surface and within intertidal and benthic sediments in many marine settings. Accordingly, it would appear that the sources, processes and assumptions involved in estimates of paint inputs into the aquatic environment (and as exemplified in Table 1) require re-evaluation.
6. Environmental behaviour, transport and exposure of paint particles
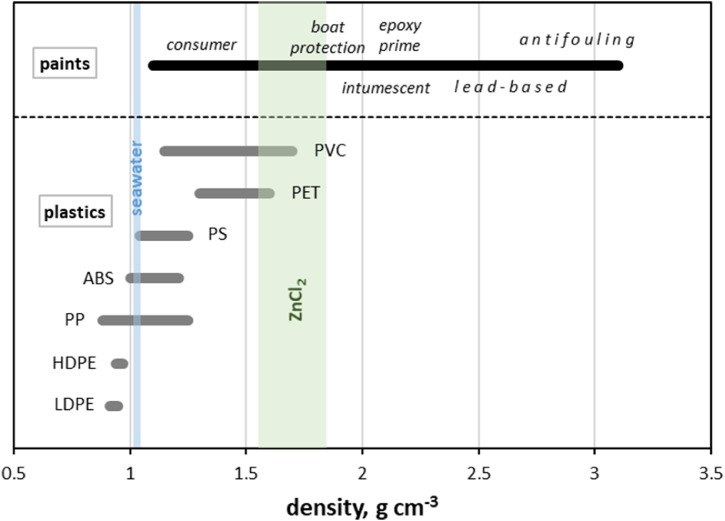
One of the key physical factors that determines the transport, behaviour and fate of plastics in natural waters or engineered systems (for example, water treatment facilities) is density (de la Fuente et al., 2021). This property also forms the basis by which microplastics are extracted (through flotation) from environmental samples like soils and sediments (Miller et al., 2017). Density ranges for common plastics and paints are compared in Fig. 4. For plastics, the lowest values represent the pure polymer while maximum values denote formulations loaded with the highest concentrations of mineral or glass fibre filler, plasticiser or flame retardant according to the Omnexus material selection platform (SpecialChem, 2021). For dried paints, the overall density range and noted formulations are based on information in the scientific and commercial literature. Also annotated on Fig. 4 are the density range of seawater and the density range of solutions of ZnCl2 that are commonly used to extract microplastics from environmental samples (Rodrigues et al., 2020).
Fig. 4.
Density ranges of common plastics (LDPE = low density polyethylene; HDPE = high density polyethylene; PP = polypropylene; ABS = acrylonitrile butadiene styrene; PET = polyethylene terephthalate; PVC = polyvinyl chloride) and dried paint formulations. Annotated are the density ranges of seawater and solutions of ZnCl2 commonly employed to isolate microplastics from sediment. Sources of information: Powell and Zinn (1983); Ruble (2002); Brockenbrough (2009); Cobb (2009); Fontana and Grass (2010); PPG Aerospace (2014); Soroldoni et al. (2018); SpecialChem (2021); Zhejiang Brother Guidepost Paint (2021).
Low density plastics like polyethylene are predicted to float in seawater while high density plastics like PET and PVC are predicted to sink. Plastics of intermediate density or with a relatively low density but broad range, like polypropylene and polystyrene, are expected to exhibit negative or positive buoyancy depending on the content and nature of additives and fillers, the precise density of ambient seawater and any biofouling present. For paints, however, all formulations are, ultimately, predicted to sink in the marine environment based on density considerations because of the greater abundance of inorganic additives and fillers in the dried formulations. This means that, in theory, paint particles of equivalent dimensions and shapes to microplastics are more readily deposited and less mobile in aquatic systems. Regarding extraction by flotation in ZnCl2 solution, the majority of microplastics should be isolated when a relatively high concentration of the salt is employed, but many types of paint particles, including those with clear marine origins, would evade capture by this approach (Haave et al., 2019). That is, conventional extraction methods are predicted to underestimate the stock of micro-sized, synthetic polymeric particulates.
Empirical studies on the settling characteristics of antifouling paints in artificial estuarine water (salinity = 15) have shown that, above a diameter of 1 mm, settling is related to density and size, but below 180 μm most particles remain at the surface without agitation and settle only after the surface tension had been broken with stirring (Soroldini et al., 2018). Accordingly, the authors suggested that the size range of paint particles generated in local harbours and boatyards could be transported several tens of km from their point of origin in a lagoonal estuary within 24 h, depending on local hydrodynamic and meteorological conditions. More generally, surface tension, augmented in natural seawater by biogenic material (Song et al., 2014) and broken only by energy equivalent to wind speeds > 6.6 m s−1 (Wurl et al., 2011), coupled with long-range airborne transport, may account for the ready detection of paint particles at or near the sea surface in locations remote from any immediate point sources and as discussed earlier.
With paint particles encountered at the sea surface and in the pelagic and benthic zones it is not surprising that they have been detected amongst other micro-debris in the digestive tracts (and sometimes gills) of various marine animals, including birds, fish, crustaceans, cetaceans, turtles and invertebrates, and as summarised in Table 2. In many cases, paint particles are noted without detailed or systematic quantification or characterisation and critical information on abundance, sources, selectivity, impacts and fate is lacking. Where paint particles have been counted and classified in fish guts, however, the contribution of this material to the MP cohort on a number basis may be as high as 35% (Cardoza et al., 2018), with blue often noted as the dominant colour (Herrera et al., 2019). Paint particles may be ingested passively while suspended in the water or attached to dietary material like algae (Russell et al., 2011), or mistaken for items of food like zooplankton because of similarities in colour, size and texture (Kang et al., 2015b). In controlled laboratory experiments involving a range of suspension- and deposit-feeding invertebrates, ground antifouling paint particle composites appear to be ingested without any avoidance mechanisms evident (Turner et al., 2008; Turner et al., 2009; Muller-Karanssos et al., 2019). Likewise, studies in the avian literature suggest that paint particles may be incidentally, or even deliberately ingested by seabirds and waterfowl while foraging for food (Molnar, 1983; Sileo and Fefer, 1987; Turner, 2010).
Table 2.
A compilation of reports of paint particle ingestion by marine animals. Asterisks denote reports of particles in the gills as well as the digestive tract; ns = not stated.
| Animal | Location | Size, mm | Paint type | Reference |
|---|---|---|---|---|
| Laysan albatross, Diomedea immutabilis | Midway Atoll, North Pacific | ns | building paint | Sileo and Fefer (1987) |
| Cape petrel, Daption capense | Equatorial Pacific | ns | ns | Laist (1997) |
| Green sea turtle, Chelonia mydas | Central Pacific | ns | ns | Russell et al. (2011) |
| Various pelagic and benthic fish | Portuguese coast | < 4.8 | alkyd | Neves et al. (2015) |
| Amberstrip scad, Decapterus muroadsi | Southern Pacific subtropical gyre | < 5 | alkyd/epoxy | Ory et al. (2018) |
| Green sea turtles, Chelonia mydas | Great Barrier Reef | 1-2.5 | acrylic | Caron et al. (2018) |
| Various pelagic and benthic fish | Southern Pacific subtropical gyre | < 5 | acrylic | Markic et al. (2018) |
| Mauve stinger, Pelagia noctiluca | Tyrrhenian Sea | 3 | zinc-rich | Macali et al. (2018) |
| Atlantic bigeye, Priacanthus arenatus | Brazilian coast | < 10 | ns | Cardoza et al. (2018) |
| Various cetaceans | Irish coast | ns | ns | Lusher et al. (2018) |
| Pelagic fish* | Musa estuary and Persian Gulf | < 0.3 | antifouling | Abbasi et al. (2018) |
| Ragworm, Hediste diversicolor | Plymouth Sound, SW England | < 2.6 | antifouling | Muller-Karanassos et al. (2019) |
| Atlantic chub mackerel, Scomber colias | Canary Island coast | < 5 | ns | Herrera et al. (2019) |
| Various estuarine snooks | Goiana estuary, Brazil | < 5 | ns | Ferreira et al. (2019) |
| Guri catfish, Genidens geniden | Laguna estuarine system, Brazil | < 5 | ns | Dantas et al. (2019) |
| Benthic jellyfish, Cassiopea xamachana | Florida estuaries | ns | ns | Iliff et al. (2020) |
| Mangrove crabs* | Hong Kong | > 0.01 | ns | Not et al. (2020) |
| Harbour porpoise, Phocoena phocoena | German coast | > 0.1 | acrylic/alkyd | Philipp et al. (2021) |
7. Paint particle toxicity and impacts on biota
From a general ecological and health perspective, exposure to paint particles is likely to exert similar impacts on marine organisms as exposure to microplastics of equivalent dimensions (Yin et al., 2018; Bringer et al., 2020; Maes et al., 2020). A greater concern regarding contemporary and historical paint particles, however, is the presence and availability of additives that are hazardous. While asbestos, cadmium compounds and certain phthalate esters and brominated flame retardants have been employed in speciality paints, of more general concern are lead-based compounds that were common in historical paints with wide-ranging applications (including road markings, buildings and shipping) and biocidal additives that are fundamental to marine antifouling formulations.
A variety of lead compounds were used as drying catalysts (lead acetate, octoate and naphthenate), pigments for colour, opacity and protection (e.g. lead carbonates, sulphates, oxides, chromates), and, on metal, corrosion inhibitors (e.g. lead tetroxide, calcium plumbate). Restrictions have eliminated the intentional use of lead in paints in many sectors but legacy coatings remain an important source of the metal to the environment (Mielke et al., 2008; Turner and Lewis, 2018). Copper(I)-based compounds have played a critical role in antifouling paints for many decades, with other metal-based biocides based on compounds of tributyl tin, lead, arsenic and mercury phased out but still encountered on the hulls of older boats and abandoned vessels (Eklund and Eklund, 2014; Rees et al., 2014). Various organic- and organo-metallic “booster” compounds with herbicidal properties have often been added at smaller concentrations to antifouling paints, with many undergoing restrictions but encountered in historical boat coatings (Parks et al., 2010).
While lead compounds and certain biocides have also been added to plastics, both their concentrations and mobilities are significantly greater in paint particles. For example, the maximum lead content of beached microplastics (mainly polyolefins) retrieved from south west England was about 5000 mg kg−1 (Massos and Turner, 2017), with PVC fragments having concentrations in the range 10,000 to 20,000 mg kg−1 (Turner and Solman, 2016). In contrast, the lead content of paint fragments derived from the hulls of abandoned boats can regularly exceed 20% by weight (Rees et al., 2014), or an order of magnitude higher than the maximum concentration reported for PVC. Regarding biocides, the typical content in protected plastics is < 1,000 mg kg−1 (Dylingowski and, 2004). This contrasts with Cu(I) concentrations in the dry films of contemporary and historical antifouling formulations of up to 50% by weight (Muller-Karanassos et al., 2019).
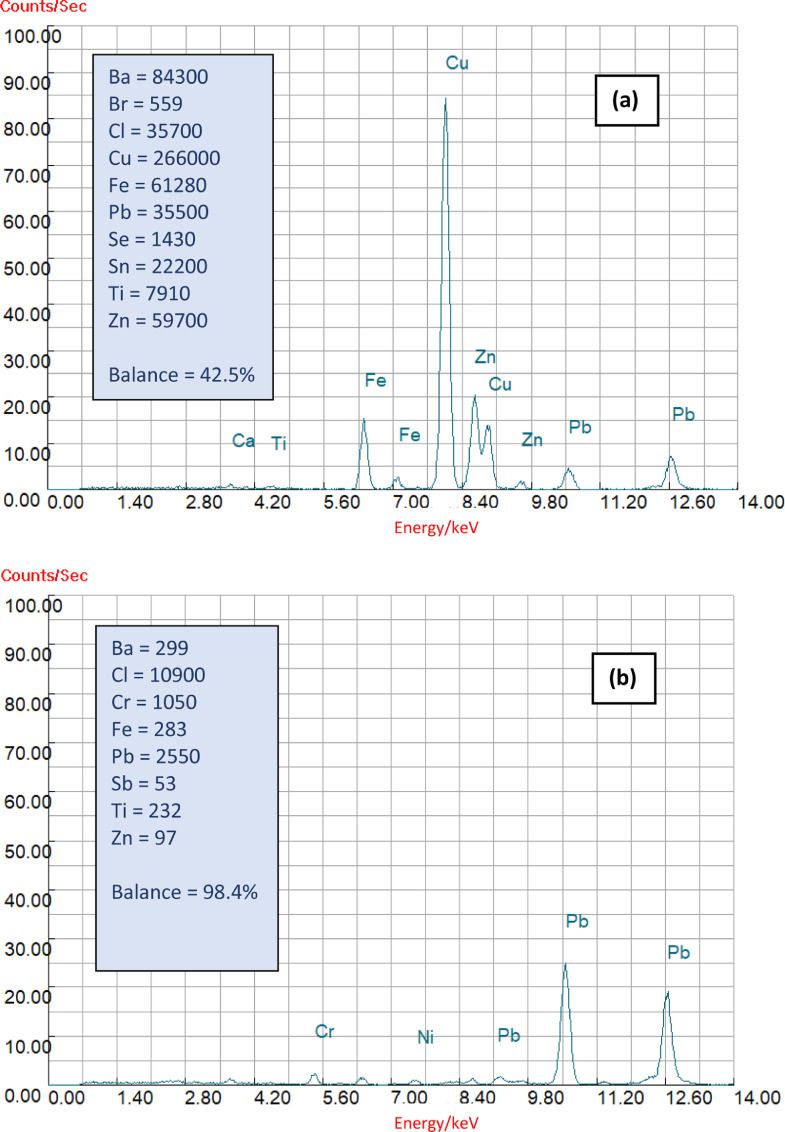
A specific chemical comparison between a fragment of polyethylene pigmented with PbCrO4 that had been retrieved from the strandline of a beach and a copper-based antifouling paint fragment taken from an abandoned boat is shown in Fig. 5. Here, X-ray fluorescent spectra acquired under the same operating conditions are illustrated over the energy range 0 to 14 keV, with selected principal secondary X-ray peaks identified. Annotated in the boxes, and where quantification was possible by fundamental parameters, are concentrations of elements detected in each sample in mg kg−1. Clearly, the number and magnitude of peaks and overall elemental concentrations are greater in the antifouling fragment but the percentage balance (also annotated), consisting of light elements not detected by the instrument (Z < 11) and indicative of the polymer concentration, is considerably lower. More generally, these observations are also consistent with the greater abundance of additives and lower polymer content of paints relative to plastics discussed earlier.
Fig. 5.
X-ray fluorescent spectra, with selected peaks identified, acquired over the range 0 to 14 keV using a Niton XL3t GOLDD+ for (a) an antifouling paint fragment and (b) a pigmented, beached plastic. Elemental concentrations, where quantified, are annotated in mg kg−1 along with the percentage balance (comprising of light elements not detected by the instrument). (Author's unpublished data.)
The greater mobility of hazardous chemicals in paint particles relative to plastics is evident from differences in the quantities of toxic metal ions (e.g. Cu+/Cu2+, TBT+, Pb2+, CrO42−) released from each particle type into physiological solutions that simulate the mammalian or avian stomach (Turner and Radford, 2010; Smith and Turner, 2020) and from the more stringent guidelines and regulations that deal with toxic metals in paint (Gooch, 1993). Factors that account for a greater mobility of metals in paint than in plastic include a higher additive to polymer/binder ratio in the dried formulation, the brittleness of weathered paint and its propensity to readily fragment into smaller particles of high surface area, and the more ready aging and degradation of the paint binder than the plastic polymer. More specifically, in hard, ablative and self-polishing antifouling paints, biocides are designed to leach out or the entire formulations dissolve at controlled rates into the surrounding medium.
The greater solubility of hazardous additives in paint than in plastic also results in a higher toxicity. Thus, in the aquatic environment, while chemicals leaching from microplastics, including Pb, have been shown to be toxic to bacteria, invertebrates and fish (Silva et al., 2016; Boyle et al., 2020; Sarkur et al., 2020), the quantities of plastic relative to water volume used to generate leachates are well in excess of levels encountered in the environment. By contrast, poisoning of birds through the ingestion of leaded paint particles has been documented in situ (Molnar, 1983; Finkelstein et al., 2003) and concentrations of antifouling paint representative of those encountered in contaminated harbour sediments have been shown to elicit toxic responses in epibenthic copepods, crustaceans and macroinvertebrates in controlled laboratory exposures. For example, Muller-Karanassos et al. (2021) report 5-day lethal and effects concentrations (LC50 and EC50, respectively) for size-fractionated particles (100 μm to 1 mm) derived from a modern Cu-based antifouling paint of 19.9 and 14.6 g per L of estuarine sediment, respectively, for the ragworm, Hediste diversicolor, and 2.3 g L−1 and 1.4 g L−1, respectively, for the common cockle, Cerrastoderma edule. Soroldoni et al. (2017) report a significant decrease in fecundity for epibenthic copepods exposed to 0.01% of modern, Cu-based antifouling paint particles (< 63 μm) in estuarine sediment and an LC50 arising from the elutriate of a preparation equivalent to 0.14% of paint. Soroldoni et al. (2020) subsequently demonstrated 10-day LC50 values for benthic microcrustaceans exposed to estuarine sediment spiked with fractionated antifouling paint particles of 0.16 to 0.45% by mass of sediment.
The biocidal properties of antifouling formulations also impact on colonisation by marine bacteria. Thus, while microplastics are rapidly colonised by microbial communities that do not differ greatly from communities on other inert surfaces like glass, rock or wood (Wright et al., 2020), antifouling surfaces provide a habitat to select particular, but sometimes diverse bacterial populations that are resistant to the active biocides (Chen et al., 2013; Flach et al., 2017). Tagg et al. (2019) analysed and compared biofilm communities on microplastics and paint particles retrieved from sediment grabs in the coastal Baltic. Alkyd- and epoxy-based paints that likely included antifouling fragments (although this was not explicitly stated) were found to support communities that were distinct from and more consistent than biofilms on polypropylene and polyvinyl chloride microplastics and on natural particles. Significantly, an abundance of taxa from the Desulfobacteraceae family on some paint particles suggests that their presence in sediment may have impacts on the sulphur metabolism cycle.
8. Concluding remarks
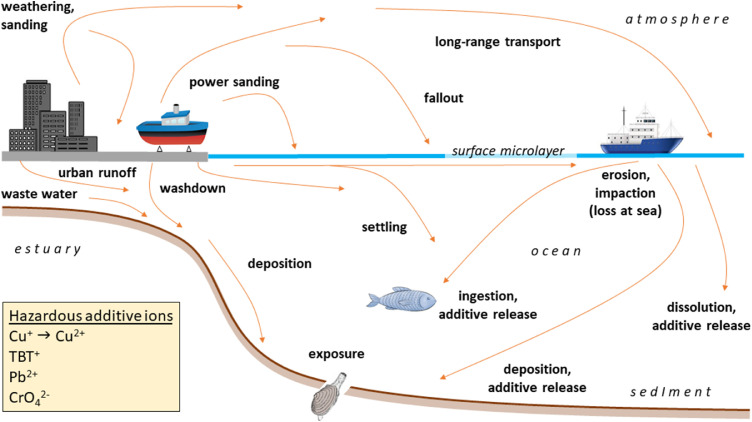
The sources, pathways, behaviour and receptors of marine paint particles described above are summarised in Fig. 6. Also shown are the ions of greatest ecotoxicological concern that are, by design or otherwise, mobilised from paint additives into seawater. Given that both the abundance and mobility of harmful additives are greater in most paint particles compared with microplastics of equivalent dimensions it is concerning that the former are often overlooked or deliberately excluded from the micro-debris pool in environmental samples (Baini et al., 2018; Ferreira et al., 2019). This omission means that quantitative comparisons of plastic to paint abundance in seawater, sediments and digestive tracts are often lacking. It is also likely that the significance of the paint pool is understated from surface or near-surface water sampling because the most mobile paint particles evade capture by the mesh size of common trawling nets (100 to a few hundred μm) while larger particles have a greater propensity to sink. In sediment, paint particle abundance is also underestimated because the densities of conventional solutions used to isolate micro-debris are lower than the densities of many common paint formulations and treatments typically used to isolate microplastics may be physically or chemically destructive to paints.
Fig. 6.
Sources, transport and impacts of paint particles in the marine environment. The metal ions shown in the box are those of greatest ecotoxicological concern that are mobilised from paint.
Clearly, a greater awareness of the potential environmental and health impacts of paints particles and development of methods for their recovery and isolation are required amongst the microplastic community, and general measures to reduce the inputs of paints to the aquatic environment by manufacturers and users are called for. Regarding the latter, potential solutions include reducing the volume of materials that require painting, improving the wear resistance and enhancing end-of-life degradation of formulations, increasing the use and efficacy of dust extraction systems during maintenance and repair, improving waste collection systems in boatyards, and incentivising the safe and sustainable disposal or recycling of boats (Turner, 2010; Rees et al., 2014; Verschoor et al., 2016).
Declaration of Competing Interest
The author declares that he has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The critical and insightful comments of three anonymous reviewers are greatly appreciated.
References
- Abbasi S., Soltani N., Keshavarzi B., Moore F., Turner A., Hassanaghaei M. Microplastics in different tissues of fish and prawn from the Musa estuary, Persian Gulf. Chemosphere. 2018;205:80–87. doi: 10.1016/j.chemosphere.2018.04.076. [DOI] [PubMed] [Google Scholar]
- Abreu F.E.L., da Silva J.N.L., Castro Í.B., Fillmann G. Are antifouling residues a matter of concern in the largest South American port? J. Hazard. Mater. 2020;398 doi: 10.1016/j.jhazmat.2020.122937. [DOI] [PubMed] [Google Scholar]
- Aliabad M.K., Nassiri M., Kor K. Microplastics in the surface seawaters of Chabahar Bay, Gulf of Oman (Makran coasts) Mar. Pollut. Bull. 2019;143:125–133. doi: 10.1016/j.marpolbul.2019.04.037. [DOI] [PubMed] [Google Scholar]
- Arthur, C., Baker, J., Bamford, H., 2009. Proceedings of the international research workshop on the occurrence, effects and fate of microplastic marine debris. Sept 9–11, 2008, NOAA Technical Memorandum NOS-OR&R30.
- Bagaev A., Mizyuk A., Khatmullina L., Isachenko I., Chubarenko I. Anthropogenic fibres in the Baltic Sea water column: Field data, laboratory and numerical testing of their motion. Sci. Total Environ. 2017;599-600:560–571. doi: 10.1016/j.scitotenv.2017.04.185. [DOI] [PubMed] [Google Scholar]
- Baini M., Foss M.C., Galli M., Caliani I., Campani T., Finoia M.G., Panti C. Abundance and characterization of microplastics in the coastal waters of Tuscany (Italy): The application of the MSFD monitoring protocol in the Mediterranean Sea. Mar. Pollut. Bull. 2018;133:543–552. doi: 10.1016/j.marpolbul.2018.06.016. [DOI] [PubMed] [Google Scholar]
- Bergmann M., Mützel S., Primple S., Tekman M.B., Trachsel J., Gerdts G. White and wonderful? Microplastics prevail in snow from the Alps to the Arctic. Sci. Adv. 2019;5:eaax1157. doi: 10.1126/sciadv.aax1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikker J., Lawson J., Wilson S., Rochman C.M. Microplastics and other anthropogenic particles in the surface waters of the Chesapeake Bay. Mar. Pollut. Bull. 2020;156 doi: 10.1016/j.marpolbul.2020.111257. [DOI] [PubMed] [Google Scholar]
- Bringer A., Thomas H., Prunier G., Dubillot E., Bossut N., Churlaud C., Clerandeau C., Le Bihanic F., Cachot J. High density polyethylene (HDPE) microplastics impair development and swimming activity of Pacific oyster D-larvae, Crassostrea gigas, depending on particle size. Environ. Pollut. 2020;260 doi: 10.1016/j.envpol.2020.113978. [DOI] [PubMed] [Google Scholar]
- Boyle D., Catarino A.I., Clark N.J., Henry T.B. Polyvinyl chloride (PVC) plastic fragments release Pb additives that are bioavailable in zebrafish. Environ. Pollut. 2020;263 doi: 10.1016/j.envpol.2020.114422. [DOI] [PubMed] [Google Scholar]
- Brahney J., Hallerud M., Heim E., Hahnenberger M., Sukumaran S. Plastic rain in protected areas of the United States. Science. 2020;368:1257–1260. doi: 10.1126/science.aaz5819. [DOI] [PubMed] [Google Scholar]
- Brockenbrough R.L. Third Edition. McGraw Hill; New York: 2009. Highway Engineering Handbook. [Google Scholar]
- Cardoza A.L.P., Farias E.G.G., Rodrigues-Filho J.L., Moteiro I.B., Scandolo T.M., Dantas D.V. Feeding ecology and ingestion of plastic fragments by Priacanthus arenatus: What's the fisheries contribution to the problem? Mar. Pollut. Bull. 2018;130:19–27. doi: 10.1016/j.marpolbul.2018.03.010. [DOI] [PubMed] [Google Scholar]
- Caron A.G.M., Thomas C.R., Berry K.L.E., Motti C.A., Ariel E., Brodie J.E. Ingestion of microplastic debris by green sea turtles (Chelonia mydas) in the Great Barrier Reef: Validation of a sequential extraction protocol. Mar. Pollut. Bull. 2018;127:743–751. doi: 10.1016/j.marpolbul.2017.12.062. [DOI] [PubMed] [Google Scholar]
- Chen C.L., Maki J.S., Rittschof D., Teo S.L.M. Early marine bacterial biofilm on a copper-based antifouling paint. Int. Biodeterior. Biodegrad. 2013;83:71–76. [Google Scholar]
- Coppock R.L., Cole M., Lindeque P.K., Queirós A.M., Galloway T.S. A small-scale, portable method for extracting microplastics from marine sediments. Environ. Pollut. 2017;230:829–837. doi: 10.1016/j.envpol.2017.07.017. [DOI] [PubMed] [Google Scholar]
- Cobb D. U.S. Consumer Product Safety Commission; Gaithersburg, MD: 2009. Study on the Effectiveness, Precision, and Reliability of X-Ray Fluorescence Spectrometry and Other Alternative Methods for Measuring Lead in paint. [Google Scholar]
- Costa L.D.F., Mirlean N., Wasserman J.C., Wallner-Kersanach M. Variability of labile metals in estuarine sediments in areas under the influence of antifouling paints, southern Brazil. Environ. Earth Sci. 2016;75:580. [Google Scholar]
- Dantas D.V., Ribeiro C.I., Frischknecht C., de C.A., Machado R., Farias E.G.G. Ingestion of plastic fragments by the Guri sea catfish Genidens genidens (Cuvier, 1829) in a subtropical coastal estuarine system. Environ. Sci. Pollut. Research. 2019 doi: 10.1007/s11356-019-04244-9. [DOI] [PubMed] [Google Scholar]
- Dauvergne P. The power of environmental norms: Marine plastic pollution and the politics of microbeads. Environmental Politics. 2018;27:579–597. [Google Scholar]
- Decelis R., Vella A.J. Contamination of outdoor settled dust by butyltins in Malta. Appl. Organomet. Chem. 2007;21:239–245. [Google Scholar]
- de la Fuente R., Drótos G., Hernàndez-García, López C., van Sebille E. Sinking microplastics in the water column: simulations in the Mediterranean Sea. Ocean Sci. 2021;17:431–453. [Google Scholar]
- Dibke C., Fischer M., Scholz-Böttcher B.M. Microplastic mass concentrations and distribution in German Bight waters by pyrolysis−gas chromatography−mass spectrometry/thermochemolysis reveal potential impact of marine coatings: Do ships leave skid marks? Environ. Sci. Technol. 2021;55:2285–2295. doi: 10.1021/acs.est.0c04522. [DOI] [PubMed] [Google Scholar]
- Díez-Minguito M., Bermúdez M., Gago J., Carretero O., Viñas L. Observations and idealized modelling of microplastic transport in estuaries: The exemplary case of an upwelling system (Ría de Vigo, NW Spain) Mar. Chem. 2020;222 [Google Scholar]
- Dylingowski P.J., Hamel R.G. Microbial degradation of plastics. In: Paulus W., editor. Directory of Microbicides for the Protection of Materials. Springer; Dordrecht: 2004. [Google Scholar]
- Eklund B., Eklund D. Pleasure boatyard soils are often highly contaminated. Environ. Manage. 2014;53:930–946. doi: 10.1007/s00267-014-0249-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund B., Johansson L., Ytreberg E. Contamination of a boatyard for maintenance of pleasure boats. J. Soils Sediments. 2014;14:955–967. [Google Scholar]
- Eriksen M., Liboiron M., Kiessling T., Charron L., Alling A., Lebreton L., Richards H., Roth B., Ory N.C., Hidalgo-Ruz V., Meerhoff E., Box C., Cummins A., Thiel M. Microplastic sampling with the AVANI trawl compared to two neuston trawls in the Bay of Bengal and South Pacific. Environ. Pollut. 2018;232:430–439. doi: 10.1016/j.envpol.2017.09.058. [DOI] [PubMed] [Google Scholar]
- Ferreira G.V.B., Barletta M., Lima A.R.A., Morley S.A., Costa M.F. Dynamics of marine debris ingestion by profitable fish along the estuarine ecocline. Sci. Rep. 2019;9:13514. doi: 10.1038/s41598-019-49992-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein M.E., Gwiazda R.H., Smith D.R. Lead poisoning of seabirds: Environmental risks from leaded paint at a decommissioned military base. Environ. Sci. Technol. 2003;37:3256–3260. doi: 10.1021/es026272e. [DOI] [PubMed] [Google Scholar]
- Fischer V., Elsner N.O., Brenke N., Schwabe E., Brandt A. Plastic pollution of the Kurl-Kamchatka Trench area (NW pacific) Deep-Sea Research II. 2015;111:399–405. [Google Scholar]
- Flach C.F., Pal C., Svensson C.J., Kristiansson E., Östman M., Bengtsson-Palme J., Tysklind M., Larsson D.G.J. Does antifouling paint select for antibiotic resistance? Sci. Total Environ. 2017;590-591:461–468. doi: 10.1016/j.scitotenv.2017.01.213. [DOI] [PubMed] [Google Scholar]
- Fontana, L., Grassi, C., 2010. Process for coating threaded metallic pieces. European Patent Office EP2468929B1.
- Free C.M., Jensen O.P., Mason S.A., Eriksen M., Williamson N.J., Boldgiv B. High-levels of microplastic pollution in a large, remote, mountain lake. Mar. Pollut. Bull. 2014;85:156–163. doi: 10.1016/j.marpolbul.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Galafassi S., Nizzetto L., Volta P. Plastic sources: A survey across scientific and grey literature for their inventory and relative contribution to microplastics pollution in natural environments, with an emphasis on surface water. Sci. Total Environ. 2019;693 doi: 10.1016/j.scitotenv.2019.07.305. [DOI] [PubMed] [Google Scholar]
- Garcia T.M., Campos C.C., Mota E.M.T., Santos N.M.O., de Santana Campelo R.P., Prado L.C.G., Junior M.M., de Oliveira Soares M. Microplastics in subsurface waters of the western equatorial Atlantic (Brazil) Mar. Pollut. Bull. 2020;150 doi: 10.1016/j.marpolbul.2019.110705. [DOI] [PubMed] [Google Scholar]
- Gauci A., Deidun A., Montebello J., Abela J., Galgani F. Automating the characterisation of beach microplastics through the application of image analyses. Ocean Coastal Management. 2019;182 [Google Scholar]
- Gaylarde C.C., Neto J.A.B., da Fonseca E.M. Paint fragments as polluting microplastics: A brief review. Mar. Pollut. Bull. 2021;162 doi: 10.1016/j.marpolbul.2020.111847. [DOI] [PubMed] [Google Scholar]
- Gigault J., ter Halle A., Baudrimont M., Pascal P.-Y., Gauffre F., Phi T.-L., El Hadri H., Grassl B., Reynauld S. Current opinion: What is a nanoplastics? Environ. Pollut. 2018;235:1030–1034. doi: 10.1016/j.envpol.2018.01.024. [DOI] [PubMed] [Google Scholar]
- Gooch J.W. Plenum Press; New York: 1993. Lead-Based Paint Handbook. [Google Scholar]
- Haave M., Lorenz C., Primpke S., Gerdts G. Different stories told by small and large microplastics in sediment – first report of microplastic concentrations in an urban recipient in Norway. Mar. Pollut. Bull. 2019;141:501–513. doi: 10.1016/j.marpolbul.2019.02.015. [DOI] [PubMed] [Google Scholar]
- Hall N.M., Berry K.L.E., Rintou L., Hoogenboom M.O. Microplastic ingestion by scleractinian corals. Mar. Biol. 2015;162:725–732. [Google Scholar]
- Hann S., Sherrington C., Jamieson O., Hickman M., Kershaw P., Bapasola A., Cole G. ICF; London: 2018. Investigating Options For Reducing Releases In The Aquatic Environment Of Microplastics Emitted by (but not intentionally added in) products. Report for DG Environment of the European Commission. [Google Scholar]
- Henderson L., Green C. Making sense of microplastics? Public understandings of plastic pollution. Mar. Pollut. Bull. 2020;152 doi: 10.1016/j.marpolbul.2020.110908. [DOI] [PubMed] [Google Scholar]
- Herrera A., Ŝtindlová A., Martínez I., Rapp J., Kutzner-Romero V., Samper M.D., Montoto T., Aguiar-González B., Packard T., Gómez M. Microplastic ingestion by Atlantic chub mackerel (Scomber colias) in the Canary Islands coast. Mar. Pollut. Bull. 2019;139:127–135. doi: 10.1016/j.marpolbul.2018.12.022. [DOI] [PubMed] [Google Scholar]
- Horton A.A., Svendsen C., Williams R.J., Spurgeon D.J., Lahive E. Large microplastic particles in sediments of tributaries of the River Thames, UK – Abundance, sources and methods for effective quantification. Mar. Pollut. Bull. 2017;114:218–226. doi: 10.1016/j.marpolbul.2016.09.004. [DOI] [PubMed] [Google Scholar]
- Iliff S.M., Wilczek E.R., Harris R.J., Bouldin R., Stoner E.W. Evidence of microplastics from benthic jellyfish (Cassiopea xamachana) in Florida estuaries. Marine Pollution Beulletin. 2020;159 doi: 10.1016/j.marpolbul.2020.111521. [DOI] [PubMed] [Google Scholar]
- Imhof H.K., Laforsch C., Wiesheu A.C., Schmid J., Anger P.M., Niessner R., Ivleva N.P. Pigments and plastic in limnetic ecosystems: a qualitative and quantitative study on microparticles of different size classes. Water Res. 2016;98:64–74. doi: 10.1016/j.watres.2016.03.015. [DOI] [PubMed] [Google Scholar]
- Ivar do Sul J.A., Costa M.F., Fillmann G. Microplastics in the pelagic environment around oceanic islands of the Western Tropical Atlantic Ocean. Water Air Soil Pollut. 2014;225:2004. [Google Scholar]
- Jambeck J.R., Andrady A., Geyer R., Narayan R., Perryman M., Siegler T., Wilcox C., Lavender Law K. Plastic waste inputs from land into the ocean. Science. 2015;347:768–771. doi: 10.1126/science.1260352. [DOI] [PubMed] [Google Scholar]
- Jartun M., Ottesen R.T., Steinnes E., Volden T. Painted surfaces - important sources of polychlorinated biphenyls (PCBs) contamination to the urban and marine environment. Environ. Pollut. 2009;157:295–302. doi: 10.1016/j.envpol.2008.06.036. [DOI] [PubMed] [Google Scholar]
- Jensen L.H., Motti C.A., Garm A.L., Tonin H., Kroon F.J. Sources, distribution and fate of microfibres on the Great Barrier Reef, Australia. Sci. Rep. 2019;9:9021. doi: 10.1038/s41598-019-45340-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R.J. Chemical contamination of a coral reef by the grounding of a cruise ship in Bermuda. Mar. Pollut. Bull. 2007;54:905–911. doi: 10.1016/j.marpolbul.2007.02.018. [DOI] [PubMed] [Google Scholar]
- Kane I.A., Clare M.A. Dispersion, accumulation, and the ultimate fate of microplastics in deep-marine environments: a review and future directions. Front. Marine Science. 2019;7 doi: 10.3389/feart.2019.00080. [DOI] [Google Scholar]
- Kang J.H., Kwon O.Y., Lee K.W., Song Y.K., Shim W.J. Marine neustonic microplastics around the southeastern coast of Korea. Mar. Pollut. Bull. 2015;96:304–312. doi: 10.1016/j.marpolbul.2015.04.054. [DOI] [PubMed] [Google Scholar]
- Kang J.H., Kwon O.Y., Shim W.J. Potential threat of microplastics to zooplanktivores in the surface waters of the southern Sea of Korea. Arch. Environ. Contam. Toxicol. 2015;69:340–351. doi: 10.1007/s00244-015-0210-3. [DOI] [PubMed] [Google Scholar]
- Koponen I.K., Jensen K.A., Schneider T. Sanding dust from nanoparticle-containing paints: Physical characterisation. J. Phys. Conf. Ser. 2009;151 [Google Scholar]
- Kor K., Mehdinia A. Neustonic microplastic pollution in the Persian Gulf. Mar. Pollut. Bull. 2020;150 doi: 10.1016/j.marpolbul.2019.110665. [DOI] [PubMed] [Google Scholar]
- Lacerda A.L.d.F., Rodrigues L.dos S., van Sebille E., Rodrigues F.L., Ribeiro L., Secchi E.R., Kessler F., Proietti M.C. Plastics in sea surface waters around the Antarctic Peninsula. Sci. Rep. 2019;9:3977. doi: 10.1038/s41598-019-40311-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagerström. M., Norling M., Eklund B. Metal contamination at recreational boatyards linked to the use of antifouling paints—investigation of soil and sediment with a field portable XRF. Environ. Sci. Pollut. Res. 2016;23:10146–10157. doi: 10.1007/s11356-016-6241-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laist D.W. Impacts of marine debris: Entanglement of marine life in marine debris including a comprehensive list of species with entanglement and ingestion records. In: Coe J.M., Rogers D.B., editors. Marine Debris. Springer Series on Environmental Management. Springer; New York, NY: 1997. [Google Scholar]
- Lassen C., Hansen S.F., Magnusson K., Hartmann N.B., Rehne Jensen P., Nielsen T.G., Brinch A. Danish Environmental Protection Agency; Copenhagen: 2015. Microplastics occurrence, effects and sources of releases to the environment in Denmark. [Google Scholar]
- Latuta L. Environmental Science Long Read, Leeds University; 2019. Macro Problem of Microplastic: Assessment of Microplastic Pollution Along the Strandline of Kilnsea Beach.https://discuss.leeds.ac.uk/2019/05/08/macro-problem-of-microplastic-assessment-of-microplastic-pollution-along-the-strandline-of-kilnsea-beach/accessed3/21 [Google Scholar]
- Lee P.K., Chang H.J., Yu S., Chae K.H., Bae J.H., Kang M.J., Chae G. Characterization of Cr(VI)–containing solid phase particles in dry dust deposition in Daejeon, South Korea. Environ. Pollut. 2018;243:1637–1647. doi: 10.1016/j.envpol.2018.09.127. [DOI] [PubMed] [Google Scholar]
- Lima A.R.A., Costa M.F., Barletta M. Distribution patterns of microplastics within the plankton of a tropical estuary. Environ. Res. 2014;132:146–155. doi: 10.1016/j.envres.2014.03.031. [DOI] [PubMed] [Google Scholar]
- Liubartseva S., Coppini G., Lecci R., Clementi E. Tracking plastics in the Mediterranean: 2D Lagrangian model. Mar. Pollut. Bull. 2018;129:151–162. doi: 10.1016/j.marpolbul.2018.02.019. [DOI] [PubMed] [Google Scholar]
- Lusher A.L., Hernandez-Milan G., Berrow S., Rogan E., O'Connor I. Incidence of marine debris in cetaceans stranded and bycaught in Ireland: Recent findings and a review of historical knowledge. Environ. Pollut. 2018;232:467–476. doi: 10.1016/j.envpol.2017.09.070. [DOI] [PubMed] [Google Scholar]
- Macali A., Semenov A., Venuti V., Crupi V., D'Amico F., Rossi B., Corsi I., Bergami E. Episodic records of jellyfish ingestion of plastic items reveal a novel pathway for trophic transference of marine litter. Sci. Rep. 2018;8:6105. doi: 10.1038/s41598-018-24427-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes T., Jon B., Stenton C., Roberts E., Hicks R., Bignell J., Vethaak D.A., Leslie H.A., Sanders M. The world is your oyster: low-dose, long-term microplastic exposure of juvenile oysters. Heliyon. 2020;6:e03103. doi: 10.1016/j.heliyon.2019.e03103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahowald N., Albani S., Kok J.F., Engelstaeder S., Scanza R., Ward D.S., Flanner M.G. The size distribution of desert dust aerosols and its impact on the Earth system. Aeolian Res. 2014;15:53–71. [Google Scholar]
- Markic A., Niemand C., Bridson J.H., Mazouni-Gaertner N., Gaertner J.C., Eriksen M., Bowen M. Double trouble in the South Pacific subtropical gyre: Increased plastic ingestion by fish in the oceanic accumulation zone. Mar. Pollut. Bull. 2018;136:547–564. doi: 10.1016/j.marpolbul.2018.09.031. [DOI] [PubMed] [Google Scholar]
- Massos A., Turner A. Cadmium, lead and bromine in beached microplastics. Environ. Pollut. 2017;227:139–145. doi: 10.1016/j.envpol.2017.04.034. [DOI] [PubMed] [Google Scholar]
- McAdams D., Angelskår T. Paint: the big source of ocean microplastics you didn't know about. World Economic Forum. 2020 https://www.weforum.org/agenda/2020/09/how-to-reduce-microplastics-from-paint/ [Google Scholar]
- Meza-Figueroa D., González-Grijalva B., Romero F., Ruiz J., Pedroza-Montero M., Ibañez-Del Rivero C., Acosta-Elías M., Ochoa-Landin L., Navaro-Espinoza S. Source apportionment and environmental fate of lead chromates in atmospheric dust in arid environments. Sci. Total Environ. 2018;630:1596–1607. doi: 10.1016/j.scitotenv.2018.02.285. [DOI] [PubMed] [Google Scholar]
- Mielke H.W., Gonzales C., Powel E., Mielke P.W. Urban soil-lead (Pb) footprint: retrospective comparison of public and private properties in New Orleans. Environ. Geochem. Health. 2008;30:231–242. doi: 10.1007/s10653-007-9111-3. [DOI] [PubMed] [Google Scholar]
- Miller M.E., Kroon F.J., Motti C.A. Recovering microplastics from marine samples: A review of current practices. Mar. Pollut. Bull. 2017;123:6–18. doi: 10.1016/j.marpolbul.2017.08.058. [DOI] [PubMed] [Google Scholar]
- Molnar J.J. Copper storage in the liver of the wild mute swan (Cygnus olor) Arch. Pathol. Lab. Med. 1983;107:629–632. [PubMed] [Google Scholar]
- Morét-Ferguson S., Lavender K., Proskurowski G., Murphy E.K., Peacock E.E., Reddy C.M. The size, mass, and composition of plastic debris in the western North Atlantic Ocean. Mar. Pollut. Bull. 2010;60:1873–1878. doi: 10.1016/j.marpolbul.2010.07.020. [DOI] [PubMed] [Google Scholar]
- Muller-Karanassos C., Turner A., Arundel W., Vance T., Lindeque P.K., Cole M. Antifouling paint particles in intertidal estuarine sediments from southwest England and their ingestion by the harbour ragworm, Hediste diversicolor. Environ. Pollut. 2019;249:163–170. doi: 10.1016/j.envpol.2019.03.009. [DOI] [PubMed] [Google Scholar]
- Muller-Karanassos C., Arundel W., Vance T., Lindeque P.K., Turner A., Cole M. Environmental concentrations of antifouling paint particles are toxic to sediment-dwelling invertebrates. Environ. Pollut. 2021;268 doi: 10.1016/j.envpol.2020.115754. [DOI] [PubMed] [Google Scholar]
- Negri A., Smith L.D., Webster N.S., Heyward A.J. Understanding ship-grounding impacts on a coral reef: potential effects of anti-foulant paint contamination on coral recruitment. Mar. Pollut. Bull. 2002;44:111–117. doi: 10.1016/s0025-326x(01)00128-x. [DOI] [PubMed] [Google Scholar]
- Negri A., Marshall P. TBT contamination of remote marine environments: ship groundings and ice-breakers as sources of organotins in the Great Barrier Reef and Antarctica. J. Environ. Manage. 2009;90:S31–S40. [Google Scholar]
- Neves D., Sobral P., Ferreira J.L., Pereira T. Ingestion of microplastics by commercial fish off the Portuguese coast. Mar. Pollut. Bull. 2015;101:119–126. doi: 10.1016/j.marpolbul.2015.11.008. [DOI] [PubMed] [Google Scholar]
- Not C., Lui C.Y.I., Cannicci S. Feeding behavior is the main driver for microparticle intake in mangrove crabs. Limnol. Oceanogr. Lett. 2020;5:84–91. [Google Scholar]
- OECD, 2005. OECD Series on Emission Scenario Documents No.13. Emission scenario document on antifouling products. Organisation for economic co-operation and development ENV/JM/MONO (2005)8.
- OECD, 2009. OECD series on emission scenario documents. Number 22. In: Emission scenario documents on coating industry (Paints, Lacquers and Varnishes), JT03267833. Organisation for Economic Co-operation and Development 08-Jul-2009.
- Ory N.C., Sobral P., Ferreira J.L., Thiel M. Amberstripe scad Decapterus muroadsi (Carangidae) fish ingest blue microplastics resembling their copepod prey along the coast of Rapa Nui (Easter Island) in the South Pacific subtropical gyre. Sci. Total Environ. 2018;586:430–437. doi: 10.1016/j.scitotenv.2017.01.175. [DOI] [PubMed] [Google Scholar]
- OSPAR Commission . OSPAR Commission; London: 2010. Guideline for monitoring marine litter on the beaches in the OSPAR maritime area. [Google Scholar]
- Öztekin A., Bat L. Microlitter pollution in sea water: a preliminary study from Sinop Sarikum coast of the southern Black Sea. Turkish J. Fish. Aquatic Sciences. 2017;17:1431–1440. [Google Scholar]
- Parks R., Donnier-Marechal M., Frickers P.E., Turner A., Readman J.W. Antifouling biocides in discarded marine paint particles. Mar. Pollut. Bull. 2010;60:1226–1230. doi: 10.1016/j.marpolbul.2010.03.022. [DOI] [PubMed] [Google Scholar]
- Philipp C., Unger B., Ehlers S.M., Koop J.H.E., Siebert U. First evidence of retrospective findings of microplastics in harbour porpoises (Phocoena phocoean) from German waters. Front. Marine Science. 2021;8 [Google Scholar]
- Pope N.D., O'Hara S.C.M., Imamura M., Hutchinson T.H., Langston W.J. Influence of a collapsed coastal landfill on metal levels in sediments and biota – a portent for the future? J. Environ. Monit. 2011;13:1961–1974. doi: 10.1039/c0em00741b. [DOI] [PubMed] [Google Scholar]
- Powell E.A., Zinn B.T. Combustion and Fuels Branch Naval Research Laboratory; Washington DC: 1983. Smoke Hazards Resulting From The Burning Of Shipboard Paints. [Google Scholar]
- PPG Aerospace, 2014. 4123 Epoxy Primer 7835. https://docplayer.net/52068119-4123-epoxy-primer-7835.html. accessed 3/21.
- Reddy M.S., Basha S., Kumar V.G.S., Joshi H.V., Ghosh P.K. Quantification and classification of ship scraping waste at Alang-Sosiya, India. Mar. Pollut. Bull. 2003;46:1609–1614. doi: 10.1016/S0025-326X(03)00329-1. [DOI] [PubMed] [Google Scholar]
- Rees A.B., Turner A., Comber S. Metal contamination of sediment by paint peeling from abandoned boats, with particular reference to lead. Sci. Total Environ. 2014;494-495:313–319. doi: 10.1016/j.scitotenv.2014.06.064. [DOI] [PubMed] [Google Scholar]
- Rodrigues M.O., Gonçalves A.M.M., Gonçalves F.J.M., Abrantes N. Improving cost-efficiency for MPs density separation by zinc chloride reuse. MethodsX. 2020;7 doi: 10.1016/j.mex.2020.100785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruble D. Weight loss versus coating density as a measure of applied coating cost. Met. Finish. 2002;100:53–58. [Google Scholar]
- Rummel C.D., Löder M.G.J., Fricke N.F., Lang T., Griebeler E.-M., Janke M., Gerdt G. Plastic ingestion by pelagic and demersal fish from the North Sea and Baltic Sea. Mar. Pollut. Bull. 2016;102:134–141. doi: 10.1016/j.marpolbul.2015.11.043. [DOI] [PubMed] [Google Scholar]
- Russell D.J., Hargrove S., Balazs G.H. Marine sponges, other animal food, and nonfood items found in digestive tracts of the herbivorous marine turtle Chelonia mydas in Hawai’i. Pacific Science. 2011;65:375–381. [Google Scholar]
- Sakata K., Sakaguchi A., Yokoyama Y., Terada Y., Takahashi Y. Lead speciation studies on coarse and fine aerosol particles by bulk and micro X-ray absorption fine structure spectroscopy. Geochem. J. 2017;51 doi: 10.2343/geochemj.2.0456. [DOI] [Google Scholar]
- Sarkur I., Moore L.R., Paulsen I.T., Tetu S.G. Assessing the toxicity of leachates from weathered plastics on photosynthetic marine bacteria prochlorococcus. Front. Marine Science. 2020 doi: 10.3389/fmars.2020.571929. [DOI] [Google Scholar]
- Shu Z., Axe L., Jahan K., Ramanujachary K.V. Metal leaching from the bridge paint waste in the presence of steel grit. Chemosphere. 2015;119:1105–1112. doi: 10.1016/j.chemosphere.2014.09.061. [DOI] [PubMed] [Google Scholar]
- Sileo L., Fefer S.I. Paint chip poisoning of laysan albatross at Midway Atoll. J. Wildl. Dis. 1987;23:432–437. doi: 10.7589/0090-3558-23.3.432. [DOI] [PubMed] [Google Scholar]
- Silva P.P.G.E., Nobre C.R., Resaffe P., Pereira C.D.S., Gusmao F. Leachate from microplastics impairs larval development in brown mussels. Water Res. 2016;106:364–370. doi: 10.1016/j.watres.2016.10.016. [DOI] [PubMed] [Google Scholar]
- Singh N., Turner A. Trace metals in antifouling paint particles and their heterogeneous contamination of coastal sediments. Mar. Pollut. Bull. 2009;58:559–564. doi: 10.1016/j.marpolbul.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Smith E.C., Turner A. Mobilisation kinetics of Br, Cd, Cr, Hg, Pb and Sb in microplastics exposed to simulated, dietary-adapted digestive conditions of seabirds. Sci. Total Environ. 2020;733 doi: 10.1016/j.scitotenv.2020.138802. [DOI] [PubMed] [Google Scholar]
- Song Y., Hong S., Jang M., Kang J.H., Kwon O.Y., Han G.M., Shim W.S. Large accumulation of micro-sized synthetic polymer particles in the sea surface microlayer. Environ. Sci. Technol. 2014;48:9014–9021. doi: 10.1021/es501757s. [DOI] [PubMed] [Google Scholar]
- Soon Z.Y., Jung J.H., Yoon C., Kang J.H., Kim M. Characterization of hazards and environmental risks of wastewater effluents from ship hull cleaning by hydroblasting. J. Hazard. Mater. 2021;403 doi: 10.1016/j.jhazmat.2020.123708. [DOI] [PubMed] [Google Scholar]
- Soroldoni S., Abreu F., Castro Í.B., Duarte F.A., Pinho G.L.L. Are antifouling paint particles a continuous source of toxic chemicals to the marine environment? J. Hazard. Mater. 2017;330:76–82. doi: 10.1016/j.jhazmat.2017.02.001. [DOI] [PubMed] [Google Scholar]
- Soroldoni S., Castro Í.B., Abreu F., Duarte F.A., Choueri R.B., Möller O.O., Fillmann G., Pinho G.L.L. Antifouling paint particles: Sources, occurrence, composition and dynamics. Water Res. 2018;137:47–56. doi: 10.1016/j.watres.2018.02.064. [DOI] [PubMed] [Google Scholar]
- Soroldoni S., da Silva S.V., Castro Í.B., Martins C.M.G., Pinho G.L.L. Antifouling paint particles cause toxicity to benthic organisms: effects on two species with different feeding modes. Chemosphere. 2020;238 doi: 10.1016/j.chemosphere.2019.124610. [DOI] [PubMed] [Google Scholar]
- SpecialChem, 2021. Omnexus material selection platform: Plastics and elastomers. https://omnexus.specialchem.com/polymer-properties/properties/density. accessed 3/21.
- Suaria G., Avio C.G., Mineo A., Lattin G.L., Magaldi M.G., Belmonte G., Moore C.J., Regoli F., Aliani S. The Mediterranean plastic soup: Synthetic polymers in Mediterranean surface waters. Sci. Rep. 2016;6:37551. doi: 10.1038/srep37551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suaria G., Perold V., Lee J.R., Lebouard F., Aliani S., Ryan P.G. Floating macro- and microplastics around the Southern Ocean: Results from the Antarctic Circumnavigation Expedition. Environ. Int. 2020;136 doi: 10.1016/j.envint.2020.105494. [DOI] [PubMed] [Google Scholar]
- Sundt P., Schulzew P.E., Syversen F. Report for the Norwegian Environment Agency Miljødirektoaret; 2014. Sources of microplastic-pollution to the marine environment. [Google Scholar]
- Tagg, A.S., Oberbeckmann, S., Fischer, D., Kreikemeyer, B., Labrenz, M., 219. Paint particles are a distinct and variable substrate for marine bacteria. Mar. Pollut. Bull. 146, 117-124. [DOI] [PubMed]
- Takahashi C.K., Turner A., Millward G.E., Glegg G.A. Persistence and metallic composition of paint particles in sediments from a tidal inlet. Mar. Pollut. Bull. 2012;64:133–137. doi: 10.1016/j.marpolbul.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Thomas K.V., McHugh M., Hilton M., Waldock M. Increased persistence of antifouling paint biocides when associated with paint particles. Environ. Pollut. 2003;123:153–161. doi: 10.1016/s0269-7491(02)00343-3. [DOI] [PubMed] [Google Scholar]
- Turner A. Marine pollution from antifouling paint particles. Mar. Pollut. Bull. 2010;60:159–171. doi: 10.1016/j.marpolbul.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Turner A. Metal contamination of soils, sediments and dusts in the vicinity of marine leisure boat maintenance facilities. J. Soils Sediments. 2013;13:1052–1056. [Google Scholar]
- Turner A., Radford A. Bioaccessibility of trace metals in boat paint particles. Ecotoxicol. Environ. Saf. 2010;73:817–824. doi: 10.1016/j.ecoenv.2010.02.022. [DOI] [PubMed] [Google Scholar]
- Turner A., Solman K.R. Analysis of the elemental composition of marine litter by field-portable-XRF. Talanta. 2016;159:262–271. doi: 10.1016/j.talanta.2016.06.026. [DOI] [PubMed] [Google Scholar]
- Turner A., Lewis M. Lead and other heavy metals in soils impacted by exterior legacy paint in residential areas of south west England. Sci. Total Environ. 2018;619-620:1206–1213. doi: 10.1016/j.scitotenv.2017.11.041. [DOI] [PubMed] [Google Scholar]
- Turner A., Singh N., Millard L. Bioaccessibility and bioavailability of Cu and Zn in sediment contaminated by antifouling paint residues. Environ. Sci. Technol. 2008;42:8740–8746. doi: 10.1021/es801923e. [DOI] [PubMed] [Google Scholar]
- Turner A., Barrett M., Brown M.T. Processing of antifouling paint particles by Mytilus edulis. Environ. Pollut. 2009;157:215–220. doi: 10.1016/j.envpol.2008.07.001. [DOI] [PubMed] [Google Scholar]
- van der Schyff V., du Preez M., Blom K., Kylin H., Choong N.S., Yive K., Merve J., Raffin J., Bouwman H. Impacts of a shallow shipwreck on a coral reef: A case study from St. Brandon's Atoll, Mauritius, Indian Ocean. Mar. Environ. Res. 2020;156 doi: 10.1016/j.marenvres.2020.104916. [DOI] [PubMed] [Google Scholar]
- Verschoor A., de Poorter L., Dröge R., Kuenen J., de Valk E. National Institute for Public Health and the Environment; Bilthoven, Netherlands: 2016. Emission of Microplastics and Potential Mitigation Measures. Abrasive Cleaning Agents, Paints and Tyre Wear. RIVM Report 2016-0026. [Google Scholar]
- Wright R.J., Erni-Cassola G., Zadjelovic V., Latva M., Christie-Oleza J.A. Marine plastic debris: A new surface for microbial colonization. Environ. Sci. Technol. 2020;54:11657–11672. doi: 10.1021/acs.est.0c02305. [DOI] [PubMed] [Google Scholar]
- Wurl O., Wurl E., Miller L., Johnson K., Vagle S. Formation and global distribution of sea-surface microlayers. Biogeosciences. 2011;8:121–135. [Google Scholar]
- Yin L.Y., Chen B.J., Xia B., Shi X.T., Qu K.M. Polystyrene microplastics alter the behavior, energy reserve and nutritional composition of marine jacopever (Sebastes schlegelii) J. Hazard. Mater. 2018;360:97–105. doi: 10.1016/j.jhazmat.2018.07.110. [DOI] [PubMed] [Google Scholar]
- Zhejiang Brother Guidepost Paint, 2021. Reflective road marking paint. https://www.globalsources.com/si/AS/Zhejiang-Brother/6008840609500/pdtl/Reflective-Road-Marking-Paint/1053428054.htm. accessed 3/21.