Abstract
A meta-analysis was developed to model performance depression in heat stress (HS), to analyze the influence of HS type (cyclic or constant), and to assess the correlation between dietary electrolyte balance (DEB) and HS in broilers. Two databases (Dat) with performance and temperature were constructed (initial phase – up to 21 d of age – 14 articles, 7,667 animals, average replicate number treatment-ARN/T, 5 and growing phase – over 21 d of age – 74 articles and 25,145 broilers, ARN/T, 7). The criteria for article selection were (1) experiments using at least 2 temperatures (thermoneutral and high temperature); (2) results of ADFI and ADG; (3) feed and water ad libitum during the experiment. Each treatment was classified as cyclic or constant HS and the HS group response was calculated relative to the thermoneutral group. Performance was evaluated as raw data or as relativized information (indicated as “HS effect or ≠”), expressed as a percentage of the difference between results. The models to predict “HS effect” showed that for the initial phase, only ADG was influenced by HS, while for the grower phase, prediction equations were created for ADFI and ADG. Considering the simplest models, there was a reduction of 1.4% in ADFI and 2.1% in ADG for each unit (°C) above the upper critical temperature for broilers older than 21 d. Feed conversion (FC) was not affected by HS in any of the studied phases. Constant HS proved to be more negative than cyclic HS to broiler performance after 21 d of age. The relation between DEB and performance of broilers under HS was analyzed considering broilers over 21 d of age, and very weak correlations were observed. It was concluded that HS affects broilers over 21 d more, although FC is not affected. ADFI is the most important variable affected by HS and the relation between the DEB of the diet and HS is very weak. The empirical models generated in this study accurately predicted ADG and ADFI of broilers exposed to HS and can be used to minimize those effects on poultry production.
Key words: electrolyte balance, hot climate, meta-analysis, poultry
INTRODUCTION
Genetic advances to increase productive potential have resulted in animals with high growing rates in short periods of time. However, these developments are not completely accompanied by cardiorespiratory development (Havenstein et al., 2003). The result is an increase in metabolic heat production and body temperature, due to a higher quantity of body mass and higher metabolic rates (Borges et al., 2003a), mainly during growing and finishing phases, when the heat loss process is reduced (Laganá et al., 2007a).
Most available studies show a negative effect of heat stress on the performance of broilers. Broilers under heat stress decrease feed intake, to maintain the homeothermia; however, this adjustment results in lower body weight gain and higher feed conversion (Ain Baziz et al., 1996). Regarding feed conversion, several authors have demonstrated its improvement when dietary fat is increased under heat stress (Dale and Fuller, 1980; Bonnet et al., 1997) and with the supplementation of vitamins (E and C) and minerals (Zn and Se), (Laganá et al., 2007a). On the other hand, Lana et al. (2000) did not demonstrate differences in broiler feed conversion at 31°C as compared to thermoneutral conditions, which was attributed to the lower intake and body weight gain of these animals. The type of heat stress is also important. Under constant heat stress, broilers are exposed to continuously high temperatures. Under cyclic heat stress, there is a diurnal environmental temperature oscillation (Ribeiro et al., 2001; Borges et al., 2003a; Souza et al., 2016), and broilers have a better ability to deal with this temperature difference (Piestun et al., 2011). Even though the damage caused by heat stress is already proven, there is a lack of studies that seek to model these differences.
Other nutritional measures suggested to maintain homeostasis and reduce the losses caused by increased temperature are related to electrolytic balance (Borges et al., 2003a,b, 2004), using the value of 250 mEq/kg of diet indicated by Mongin (1981) as the ideal for maintenance of the acid base balance of the animal.
Considering the increase in the number of publications, besides the difficulties regarding the ethical issues of using animals for in vivo experiments, the use of a meta-analysis tool, aiming to summarize and quantify studies already performed for the construction of new hypotheses emerges as an alternative (Sauvant et al., 2008). Therefore, the present study aimed to model the performance of broilers submitted to heat stress in the initial and final growing phases, to determine the influence of the type of heat stress on performance, and to assess the correlation between the electrolytic balance of the diet and the heat stress on broiler performance.
MATERIALS AND METHODS
Database Construction
Digital databases (Google Scholar, Science Direct, Scopus, and PubMed) were searched to identify studies published in scientific journals that reported the effect of heat stress on broiler performance. The keywords “temperature” and “heat stress” combined with “broilers/broilers” were used in the search. The main criteria for article selection were (1) experiments using at least 2 temperatures (thermoneutral and high temperature); (2) experiments that reported results of feed intake and body weight gain; and (3) experiments that supplied feed and water ad libitum during the total period. Each treatment in the databases was classified as cyclic or constant heat stress. In constant heat stress, animals were continuously exposed to high temperature, without difference in intensity throughout the experimental period. Cyclic heat stress was defined as the condition in which the ambient temperature fluctuated during the day, for more than 1 d in a row.
After paper selection, information related to the proposed theoretical model and other additional variables was copied from the original publications’ material and methods and results sections, and transferred to electronic spreadsheets. Based on these data, 2 independent databases were constructed. The first database was developed with data for 1 to 21-day-old broilers (Initial phase), and the second for broilers older than 21 d (Growing phase). Results collected from animals subjected to treatments other than a heat stress challenge (e.g., immune stress) were not included in the databases because they could interfere with the results.
Meta-analysis
The methodology applied to database construction and coding followed proposals described in the literature (Lovatto et al., 2007; Sauvant et al., 2008). Codes were used with qualitative grouping criteria in the analytical models. In this item, the main codes were applied for thermoneutral (control temperature, following the recommendations of the manual for Cobb 500, according to the age) and heat stress environments (temperature levels above the thermoneutral zone, according to the growth phase). Codes were used to consider the variability among all compiled experiments (e.g., effect of study or trial) and heat stress type (e.g., constant and cyclic heat stress).
A meta-analysis was performed following 3 sequential analyses: graphical (to control database quality and observe biological coherence of data); correlation (to identify related factors among all variables); and variance–covariance (to compare the treatments and obtain prediction equations). In the latter case, the General Linear Model procedure was utilized. Statistical analyses were performed using Minitab (Minitab for Windows, v. 18, Pine Hall Rd, PA) software.
Performance results were evaluated as raw data (as presented in the original papers) or as relativized information (indicated in the current study as “heat stress effect” or ≠). For the second approach, the responses to heat stress treatments were relativized (≠WG; ≠FI) to the respective control and expressed as a percentage of difference between results. This procedure was adopted as there was a considerable reduction in the effects of difference among experiments in the database (Pastorelli et al., 2012; Andretta et al., 2016).
Comparison among the performance results observed in control and heat stress condition was developed using General Linear Model procedure. Several factors (e.g., genetic traits, dietary characteristics, and housing conditions) were tested as model factors or as covariables, but most were removed due to the lack of significance (P > 0.05) and/or multicollinearity. All tests were performed considering the random effect of study (heterogeneity between experiments) as an arbitrarily chosen factor because each study is conceptually a random outcome from a large population of studies to which inference is to be made (St-Pierre, 2007). The decision for a mixed model was also based on the study objective, which includes the empirical modeling of biological responses. Final models considered the random effect of study, the fixed effect of treatments (heat stress), the stress type, and age (used as a covariable).
Models were created to predict the effect of heat stress (≠) on animal performance. The study was considered in all analytical models as a random effect, and the difference of temperature over controls was considered as a covariate. Linear and quadratic fits were tested individually in the regression analysis for temperature and age. In order to reduce the variability among studies and because the thermoneutral temperatures varied, the equations were created using the difference of the heat stress temperature related to the upper critical temperature (≠UTC, expressed in °C) physiologically required for chickens, obtained from the manual for Cobb 500 according to age (e.g., if birds required 20°C, but were exposed to 25°C, the temperature used in the modeling procedure was 5°C). A similar approach was used by the NRC (2012) to estimate feed intake of pigs in different temperature conditions.
Due to the importance of humidity in the animals’ heat exchanges, in a second step, this variable was also included to determine the prediction equations. However, only in 32 studies, in the grower phase, it was given. This variable had the same treatment as the temperature: it was used the difference between heat stress treatments humidity and the ideal humidity obtained from the manual for Cobb 500 (≠RH), according to the age.
Validation
To validate the prediction models, 2 new databases were utilized. Initial phase models (1–21 d) were compared to 171 observations. The database to validate broilers after 21 d of age contained 169 observations. These complementary databases were totally independent of the first ones and were collected from dissertations and theses published in the literature after July 2018. This criterion was utilized to avoid data already used in the previous databases. The information collected was organized following the same layout as the first ones, where the columns represented descriptive variables related to temperature, age, and performance (feed intake, body weight gain, and feed conversion) and the lines represented the treatments.
The equations were developed using the ≠FI and ≠WG responses, which indicate the effect of the heat stress on the performance responses (variation between heat challenged and control groups). The estimated means for ≠FI and ≠WG (those obtained from the equations) were compared to the observed ≠FI and ≠WG (present in the original studies reported in the dissertations and thesis). Comparisons were performed by variance analysis using the General Linear Model procedure.
The overall lack of accuracy was evaluated by the mean squared prediction error (MSPE). The MSPE was then broken down into error of central tendency (ECT) and error of regression (ER), which together with ECT indicated the lack of trueness, and error due to disturbances (ED) which indicated the lack of precision, following the proposition described by Theil (1966). Afterward, the interpretation was performed according to Perondi et al. (2018).
Dietary Electrolyte Balance
The dietary electrolyte balance (DEB) study was realized considering only data for broilers of 21 d and older, because from this age heat stress impacts performance substantially. The DEB was calculated according to Mongin (1981) and consisted of the sum of Na, K, minus Cl present in the ingredients provided in the diet composition of each study. The levels of Na, K, and Cl in the ingredients were estimated using the tables in Rostagno et al. (2017) an applying the values of the minerals in the Mongin (1981) formula.
Correlations between calculated electrolyte balance and performance results were evaluated in both groups (control and heat stress) considering 2 approaches: (1) raw performance data; or (2) partial correlation with residual values of performance data after adjustment for study and age effects, using variance analysis. The residual values consisted of data that could not be explained by the adjustment for the effect of the study and the age of the animals. In addition, the correlation between calculated electrolyte balance and difference (heat stress over control group) was assessed.
Database Description
Twenty-seven articles published in peer-reviewed journals from 2001 to 2018 (1–21 d), and 99 articles published from 1983 to 2018 (after 21 d) matched the first selection criterion. For the database for 1 to 21 d, 13 articles were removed (one applied feed restriction, 2 showed incomplete information about performance data, and 10 used other effects besides heat stress in the same treatment). From the database for over 21 d, 25 articles were removed (3, 10, and 12 for the same reasons explained above, respectively). Seven articles presented data between rearing phases (e.g., data for 14–28 d of rearing phase). Therefore, 14 articles remained in the database for 1 to 21 d (Supplementary Table 5), totaling 7,667 broilers, distributed in treatments whose average number of replicates were 5. To compose the database of over 21 d of age (Supplementary Table 6), there were 74 articles, 25,145 broilers and the trials presented an average of 7 replicates per treatment.
Considering the initial phase database (up to 21 d), broiler genetics were described in 93% of the studies (47% Ross, 40% Cobb). The Hubbard genetic was used in just one study. One paper did not specify the genetic strain. At this phase, only male chickens were used in the experiments, totaling 67% of the trials, while 27% did not specify the birds’ sex. In the grower phase database, strain was described in 89% of the studies. Most studies used the Ross genetic (31%), followed by Arbor Acre (20%), Cobb (19%), Hubbard (8%), and Isa (6%). Mixed sex was used in 5% of the studies, while 65% of the trials used only males, and 3% only females, and 25% of the trials did not specify the sex. Genetic and sex were tested in the statistical models, but removed due to the lack of significance (P > 0.05) for the studied responses. In addition, the evaluation of these factors is difficult due to the collinearity with the effect of study because most of the studies were developed using only one sex and only one genetic.
Regarding duration of exposure to heat stress, 50% of the studies submitted the animals to constant heat stress during all the initial phase; in the other studies, the average heat stress duration was 7 d. The relative air humidity was reported in only 6 studies in the initial phase database (Supplementary Table 5) and in 32 studies after 21 d of age (Supplementary Table 6). Only 2 articles used cyclic heat stress in the initial phase, and only 3 studies provided DEB values, with differences from 206 mEq/kg to 218 mEq/kg of the diet (Supplementary Table 5). In the grower phase, the DEB values ranged from 40 mEq/kg to 340 mEq/kg of the diet (Supplementary Table 6). After calculating the DEB, a final number of 50 articles was used to assess the analysis.
RESULTS
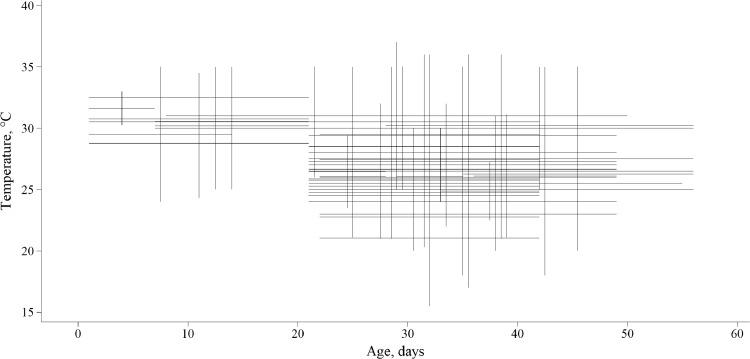
The relationship between environmental temperature and age is shown in Figure 1 and each cross represents a study from both databases. Few studies were conducted in the initial phase, since high temperatures are much more prone to affect old broilers. In this phase, the highest temperature used in the experiments was 37°C in broilers of 8 to 14 d of age, and 36°C in broilers of 15 to 21 d. The majority of the studies involving heat stress temperature were performed from 21 to 42 d of age; few studies were performed after 42 d and the maximum age included was 56 d.
Figure 1.
Meta-design: within-experiment temperature and age in days in broiler. Each experiment is indicated by a cross, of which the horizontal line indicates the age, and the vertical line indicates the ambient temperature.
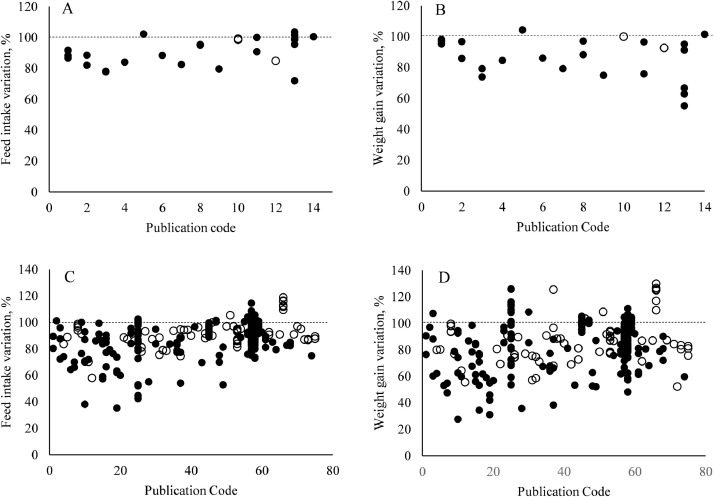
The “heat stress effect” (≠) for 1 to 21 d (A and B) and after 21 d (C and D) is presented in Figure 2. In both databases, feed intake and body weight gain were reduced in heat stress broilers. In the initial phase, a reduction in feed intake was observed in 83% of the comparisons between constant heat stress and control treatments, and in 100% when comparing cyclic heat stress (A). Regarding body weight gain, impaired responses were observed in 92% of the comparisons, but only 33% of the responses had impaired body weight gain when broilers were challenged by cyclic heat stress, in broilers up 21 d of age (B). After 21 d of age, feed intake was lower in 90% of the comparisons for both constant and cyclic heat stress situations (C), and 79% of the responses for constant heat stress and 86% for cyclic heat stress reported impaired body weight gain compared to control treatments (D).
Figure 2.
Performance difference (≠) responses of heat stress treatments relativized to the respective control treatment in the database in 1 to 21 d (A and B) and after 21 d (C and D). Cyclic heat stress is represented by white circles ( ͦ) and constant heat stress is represented by black circles (•) in both database.
The “heat stress effect” (≠) was not significant (P > 0.05) for feed intake and feed conversion of broilers until 21 d of age (Table 1), but body weight gain decreased (−12%; P = 0.001). Type of heat stress could not be evaluated in this phase (few data available). After 21 d, birds exposed to heat stress had a negative difference in feed intake (−12%; P < 0.001) and body weight gain (−15%; P < 0.001). Constant heat stress had the greatest negative influence on feed intake (−29 vs. 20%; P = 0.003) and body weight gain (–21 vs. 16%; P = 0.016), compared to cyclic heat stress. Feed conversion was not influenced (P > 0.05) by heat stress and neither by type of heat stress.
Table 1.
Performance responses of broilers in the control groups (thermoneutral conditions) and the effect (variation, indicated by ≠, %) of heat stress observed in the meta-analysis.
| n | Control1 | S.E. | ≠ (%) | S.E. |
P-values2 |
||
|---|---|---|---|---|---|---|---|
| Heat effect | Stress type3 | ||||||
| 1 to 21 d | |||||||
| Feed intake, g/day | 45 | 55.59 | 4.92 | –9.22 | 1.68 | 0.939 | - |
| Body weight gain, g/day | 48 | 40.24 | 3.25 | –12.33 | 2.45 | 0.001 | - |
| Feed conversion, g/g | 48 | 1.36 | 0.04 | 5.53 | 3.58 | 0.511 | - |
| After 21 d | |||||||
| Feed intake, g/day | 395 | 174.93 | 2.11 | –11.62 | 0.73 | <0.001 | 0.003 |
| Body weight gain, g/day | 415 | 86.51 | 1.04 | –15.01 | 1.06 | 0.001 | 0.016 |
| Feed conversion, g/g | 423 | 2.04 | 0.02 | 6.28 | 0.88 | 0.236 | 0.434 |
- = not specified.
Average performance observed in the control animals (mainteined in thermoneutral conditions).
Study effect was included in all models (P < 0.05).
Means by stress type = feed intake after 21 d, Constant: –29.17 g/day, Cyclic: –19.97 g/day; body weight gain after 21 d, Constant: –20.63 g/day, Cyclic: –15.83 g/day.
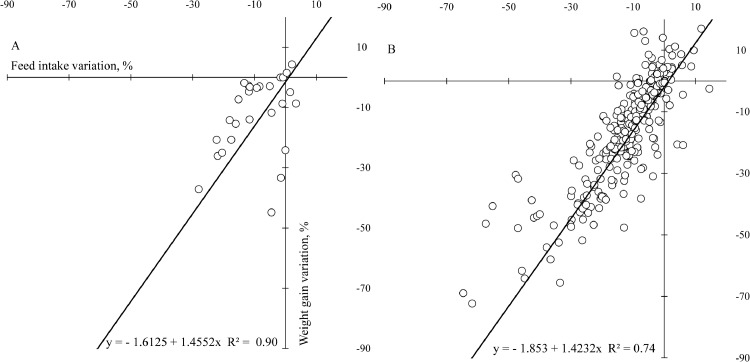
The effect of heat stress in ≠WG and in ≠FI (Figure 3) showed a linear relationship in both databases. Equation for database 1, up to 21 was y = −1.6125 + 1.4552x, R2 = 0.90. Equation for database 2, after 21 d was y = −1.853 + 1.4232x, R2 = 0.74, in which y represents the effect of heat stress on ≠WG and x represents the effect of heat stress on ≠FI. In these equations, intercepts were different from zero and negative in both challenges. According to these results, the ≠WG was −1.6 and −1.8% when ≠FI was null (x = 0), up to 21 and after 21 d, respectively.
Figure 3.
Relationship between body weight gain difference (≠WG) and feed intake difference (≠FI) of broilers in heat stress condition at 1 to 21 d (A) and after 21 d of age (B).
Models to predict the performance difference in HS were developed only for the responses that were significantly affected by heat stress. In addition, only models with at least one significant component are presented in Table 2. The ≠UTC showed a linear fit with ≠WG for broilers (Table 2-Equation 1). A linear effect of ≠UTC was also observed on ≠FI and ≠WG of broilers after 21 d. Considering the simplest models, there was a reduction of 1.397% in feed intake (Table 2-Equation 2) and 2.059% in body weight gain (Table 2-Equation 7) for each unit (°C) above the ≠UTC for broilers older than 21 d. All terms were significant in the models considering the linear effects for both ≠UTC and age on ≠FI (Equation 4) and ≠WG (Equation 9). In general, including the effect of age or quadratic terms did not consistently improve the determination coefficients of the equations.
Table 2.
Empirical models to access the performance difference in broilers under heat stress.
| Models | Terms |
R2 | ||||
|---|---|---|---|---|---|---|
| Intercept | ≠UTC | ≠UTC | A | A2 | ||
| ≠WG (%), 1 to 21 d* | ||||||
| Equation 1 | 22.7** | –5.546*** | 0.90 | |||
| ≠FI (%), after 21 d | ||||||
| Equation 2 | 1.01NS | –1.397*** | 0.70 | |||
| Equation 3 | –1.890NS | –0.102NS | –0.084** | 0.71 | ||
| Equation 4 | 12.1** | –1.327*** | –0.341** | 0.72 | ||
| Equation 5 | 37.1* | –1.308*** | –1.850NS | 0.022NS | 0.72 | |
| Equation 6 | 35.3NS | –0.149NS | –0.075** | –1.930NS | 0.023NS | 0.73 |
| ≠WG (%), after 21 d | ||||||
| Equation 7 | 1.27NS | –2.059*** | 0.74 | |||
| Equation 8 | –2.540NS | –0.345NS | –0.111** | 0.75 | ||
| Equation 9 | 16.0** | –1.976*** | –0.447** | 0.75 | ||
| Equation 10 | 83.6** | –1.923*** | –4.520** | 0.059** | 0.76 | |
| Equation 11 | 81.1** | –0.328NS | –0.104** | –4.640** | 0.062** | 0.77 |
Abbreviations: A, age (days); ≠FI, feed intake difference (%); R2, coefficient of determination; ≠UTC, Difference above the upper critical temperature (°C); ≠WG, body weight gain difference (%).
Probability is expressed as NS = not significant, *P < 0.05, **P < 0.01, ***P < 0.001, NSP > 0.05.
Despite the few studies that provided information on relative humidity, a prediction equation for ≠WG for birds after 21 d of age was determined: ≠WG (%): −53.0 − 8.10≠UTC + 0.182≠UTC2 + 5.39A − 0.0761A2 + 2,049≠RH − 0.1122≠RH2; R2 = 0.95; where: ≠UTC = difference above the upper critical temperature (°C); A = age (days); ≠RH = difference above the upper critical relative humidity. Prediction models for intake and feed conversion were not determined since the relative humidity did not influence these responses.
In Table 3, the agreement between ≠FI and ≠WG difference (in percentage) estimated by the empirical models and the observed values in the new databases are used to evaluate the models in terms of lack of accuracy, trueness, and precision. The criterion determining the best models for ≠FI and ≠WG was choosing the models with the lowest error values (MSPE).
Table 3.
Agreement between observed and estimated difference in feed intake (≠FI) and body weight gain (≠WG) in heat stress broilers.
| Models | Mean | SD1 | Estimated vs. observed |
MSPE4 | MSPE |
|||
|---|---|---|---|---|---|---|---|---|
| P2 | r3 | ECT | ER | ED | ||||
| ≠WG (%), 1 to 21 d | ||||||||
| Observed | –9.05 | 7.62 | ||||||
| Equation 1 | –4.85 | 4.84 | 0.030 | 0.53 | 60.20 | 17.67 | 25.65 | 16.88 |
| ≠FI (%), after 21 d | ||||||||
| Observed | –11.09 | 10.50 | ||||||
| Equation 2 | –13.06 | 3.51 | 0.351 | 0.37 | 99.33 | 3.87 | 84.80 | 10.67 |
| Equation 3 | –11.95 | 4.89 | 0.697 | 0.33 | 101.16 | 0.73 | 79.06 | 21.37 |
| Equation 4 | –11.74 | 4.34 | 0.764 | 0.49 | 85.02 | 0.42 | 70.20 | 14.40 |
| Equation 5 | –11.71 | 5.01 | 0.780 | 0.50 | 82.62 | 0.38 | 63.54 | 18.70 |
| Equation 6 | –10.79 | 6.04 | 0.896 | 0.46 | 88.98 | 0.09 | 59.94 | 28.94 |
| ≠WG (%), after 21 d | ||||||||
| Observed | –18.87 | 10.62 | ||||||
| Equation 7 | –19.15 | 4.62 | 0.916 | 0.62 | 135.90 | 65.00 | 57.87 | 13.03 |
| Equation 8 | –17.43 | 6.29 | 0.615 | 0.59 | 111.56 | 40.23 | 45.74 | 25.59 |
| Equation 9 | –17.74 | 5.60 | 0.683 | 0.63 | 111.27 | 44.16 | 48.28 | 18.83 |
| Equation 10 | –17.88 | 7.26 | 0.739 | 0.62 | 114.20 | 46.10 | 35.80 | 32.30 |
| Equation 11 | –16.64 | 8.44 | 0.478 | 0.63 | 101.35 | 30.78 | 27.22 | 43.35 |
SD, Standard deviation.
P: Probability comparing estimated and observed values (F test).
Correlation between estimated and observed values.
ED, error due to disturbances; composed by ECT, error of central tendency; ER, error of regression; MSPE, mean squared prediction error.
Means estimated by Equation 1 differed (P < 0.05) from the observations of the independent database used for validation. However, all models generated using data for broilers older than 21 d (Equations 2 to 11) estimated similar means (P > 0.05) to the validation database. Correlations between estimated and observed values were slightly higher for ≠WG compared to ≠FI.
Considering only the broilers older than 21 d, the best models in terms of accuracy were Equation 5 for ≠FI and Equation 11 for ≠WG. The accuracy of the models was improved by age inclusion. This improvement was due to an effect on ECT and ER, which together may be interpreted as trueness. However, including age in the predictions impaired the precision (ED) of the models.
Correlations between performance and electrolyte balance (Table 4) were, in general, very low for both control (–0.130 was the strongest correlation) and heat stress performance results (0.194 was the strongest correlation). The partial correlation of feed intake residue (i.e., degree of association between 2 variables, with the effect of a set of controlling random variables removed; in this case, data was adjusted to the effect of article and age) and electrolyte balance was significant and positive in broilers submitted to heat stress. However, this correlation was very low (0.194). In the same way, the correlations of electrolyte balance and the difference between control and heat stress results (≠, %) showed a significant correlation for ≠FI, ≠WG, and ≠FC, but these correlations were also very low (0.163, 0.242, and −0.179, respectively).
Table 4.
Correlations between electrolyte balance and the performance of broilers in heat stress after 21 d of age.
| Approach | FI | WG | FC | |
|---|---|---|---|---|
| Performance | Control treatment, g/d | –0.089 ns | –0.068 ns | –0.032 ns |
| Heat stress treatment, g/d | 0.055 ns | 0.123 ns | –0.144 ns | |
| Residues1 | Control treatment, g/d | 0.028 ns | –0.130 ns | –0.019 ns |
| Heat stress treatment, g/d | 0.194* | –0.035 ns | –0.016 ns | |
| Difference (control vs. heat stress treatments), % | 0.163* | 0.242* | –0.179* | |
| Residues1 of the difference (control vs. heat stress treatments), % | –0.032 ns | 0.013 ns | –0.006 ns | |
Abbreviations: FC, feed conversion; FI, feed intake; WG, body weight gain.
Difference in electrolyte balance was between 40 mEq/kg for 362 mEq/kg.
*P < 0.05.
NSP > 0.05.
Adjusted by study and age effects.
DISCUSSION
Heat stress is one of the main challenges for broiler production in hot climates. Several previous studies had already reported the effect of hot temperatures on the broiler performance. However, the results observed in natural and experimental challenges show great variability and are still inconclusive in some aspects. An alternative to this problem is the meta-analytic approach that allows integrating different variables and establishing systematic responses adjusted to the diversity of available experimental publications. The meta-analysis performed in this study allowed us to address and quantify systematically the association of heat stress with broiler performance. The meta-analysis used the complementarities among previous studies to highlight gaps and to quantify the effects dynamically.
The meta-analysis was divided into 2 databases that included the phases of 1 to 21 d of age and over 21 d. Broilers react in different ways to temperature differences according to their growing phases. With increasing age and weight, heat sensitivity becomes higher, once the heat dissipation area becomes smaller (Laganá, 2008). In our research, most of the studies regarding performance and high temperatures involved broilers of 21 to 42 d of age. The main focus of genetic improvement was rapid growth, aiming at a heavier carcass with higher breast yield (Marchini et al., 2016), although, the respiratory and cardiovascular systems did not develop at the same speed (Havenstein et al., 2003). Besides these physiological changes, the feathers work as thermal insulation, preventing heat dissipation and as per consequence, increasing body temperature (Yahav et al., 1995). The body parts that are not covered by feathers (combs, claws, and wattle) are in a smaller proportion, even though they have an important role in the loss of body heat. Laganá et al. (2007b) used a laser thermometer, without contact, to measure the temperature of the chickens wattles, combs, claws, and head. They showed that 1 h after the beginning of a cyclic heat stress (25–32°C in 3 h), broilers were already presenting high temperatures in these body areas, with difference of more than 5°C compared to the animals in a thermoneutral environment.
On the other hand, it is common to accept that high temperatures are not a problem during the initial phases. The small amount of studies regarding heat stress and performance in this phase is due to young animals having a higher sensitivity to low temperatures (Soren, 2012), as their regulatory system is not totally developed (Gomes et al., 2012), so the heat provided acts to the benefit of the animal, as thermoregulatory maturity is only reached about 10 d after birth (Moraes et al., 2003) and it is completely developed at 21 d, when the feathers start to appear (Laganá, 2012). Even so, this meta-analysis showed that temperatures above the thermoneutrality, negatively affect chicks weight gain.
After 21 d, more studies using constant heat stress were found than using cyclic heat stress. In cyclic heat stress, the heat periods interspersed with normal temperature conditions increase the capacity to deal with high temperatures (Piestun et al., 2011). Even if the broilers decrease feed intake during the periods of heat stress, they are sometimes able to compensate thereafter. That's why the meta-analysis showed biggest losses under constant heat stress, since, in this latter situation, the body heat production is higher and the energy efficiency is lower (Souza et al., 2016). Also, in constant heat stress Bonnet et al. (1997) observed that broilers presented lower digestibility of food, especially proteins and fat, and Mitchell and Carlisle (1992) pointed a decrease in intestinal villi height and jejunum weight, and also in the absorption of nutrients.
According to the equation in which the relationship between ≠WG and ≠FI in heat stress was studied, assuming zero effect on feed intake, there was a small decrease in body weight gain in both phases. Considering this information and the overall reduction in growth, 90 and 74% of the decrease in body weight gain was directly related to the decrease of feed intake in animals up to 21 and after 21 d of age, respectively. Similar interpretation was already used in previous studies to evaluate the effect of health challenge on animal performance (Pastorelli et al., 2012; Andretta et al., 2016). The assessment has an interpretation that could be compared to the experimental protocols with paired feed intake, however, with a larger number of observations and using data collected in more realistic situations. These results reinforce the fact that one of the most effective ways to decrease heat production is to reduce feed intake (Teeter et al., 1985; Geraert et al., 1996; Salabi et al., 2011). Among other factors, the high relative humidity added to high temperature is also responsible for impairing heat transmission from the deeper to the peripheral tissues in older broilers (Lin et al., 2005), making the action of evaporative cooling more difficult (Yahav, 2000). Even though relative humidity is extremely important when exposing broilers to high temperature, most studies do not provide data for it, which limited the possibilities of estimating its effect. Only 38% of the studies that analyzed heat stress in broilers after 21 d of age provided information on relative humidity. The equation generated in this study using relative humidity presented a higher value of R2 (0.95) than the others without this variable. However, it was not possible to validate the equation. Besides the small number of studies, a few strata of humidity within the same study were observed.
In both initial and grower phases, the heat stress effect was not found to influence feed conversion. Whereas up to 21 d of age, only body weight gain was influenced by heat, after that age both body weight gain and feed intake were influenced. If both responses decrease, it is understandable why FCR keeps unchanged, since it is the ratio between these 2 variables. In the grower phase, age was also a significant factor for the generated model. According to Chowdhury et al. (2012), Soren (2012), Gomes et al. (2012), and Moraes et al. (2003), in young broilers, heat production is not enough to increase body temperature to the point where it can be characterized as heat stress.
No models similar to the proposed in this study were found in the literature, preventing any comparison. According to Gonzalez-Esquerra and Leeson 2005, the duration and severity of heat stress may result in different physiological responses. They highlight that comparison between studies is problematic as there is no index that describes accurately the effective degree of suffering of broilers during the heat stress. Furthermore, many studies do not make a distinction between long periods and short periods of heat stress. These authors define acute heat stress as that which persists for less than 7 d, in opposition to the idea of chronic heat stress. Our analyses show that the heat stress should also be divided into constant and cyclic. It is possible that during the mildest hours of the day, the broilers have a compensatory gain (Ribeiro et al., 2001), different from what occurs with broilers in constant heat stress, decreasing the chances of mortality spikes.
The models obtained in the present study were evaluated for their accuracy, trueness, and precision. For FI after 21 d, the accuracy of the model (Equation 5) was limited by the ER, however, this error is systematic and can be corrected by further modeling procedures. However, the accuracy observed for body weight gain estimation after 21 d (Equation 11) was limited by ED, which is a random error, difficult to correct (Perondi et al., 2018). The diversity in the studies that composed the databases may contribute to this higher ED. Despite these limitations, the models created in this study are easily applicable under practical conditions, as they have few terms and can be easily used by nutritionists or even by producers as a strategy to reduce the damage caused by hot temperatures.
The DEB of 250 mEq/kg presented by Mongin (1981), became an ideal goal to many nutritionists. In addition to the fact that it was calculated for animals in thermoneutral environments, the experimental conditions under which this value was generated were very different from current poultry standards: the broilers reach 450 g at 28 d, and the diets had 10 and 20% of Na and 4, 10, and 25% of chlorine. Many authors studying DEB (Borges et al., 2003a; Faria Filho et al., 2005; Faria Filho et al., 2006; Ahmad et al., 2008; Gamba et al., 2015) did not converge to a single number of DEB and no significant effect was observed in the performance of broilers in cyclic heat, even with DEB varying from 0 to 340 mEq/kg (Borges et al., 2003b, 2004). Low or no correlations between DEB and the performance of broilers in high temperatures were found in the current study. Considering these data, it may be said, unlike that suggested by Mongin (1981), that the homeostasis and consequently the better performance of the animal do not depend of the DEB of the diet, assuming the use of diets with normal levels of Na+, K+, and Cl−, in broilers under heat stress or in a thermoneutral temperature. Other factors are responsible for affecting physiological balance and interfere in the performance of broilers exposed to heat stress, such as the animal's age, the ingredients used in the formulation, the protein source – especially animal by-products, the quantity of other elements in the diet and their absorption rate, and the environmental conditions, such as humidity, type, and duration of heat exposure.
CONCLUSIONS
This meta-analytic study allowed the proposition of models to estimate the impairment of body weight gain and feed intake difference of broilers exposed to heat stress. Feed conversion was not affected by heat stress. Constant heat stress was more negative than cyclic heat stress to the performance of broilers after 21 d of age. The correlation between DEB of the diet and heat stress is very weak, showing that physiological balance and animal performance are minimally affected or are not dependent on DEB in the diet when the animal is exposed to heat stress and a thermoneutral environment.
Acknowledgments
ACKNOWLEDGMENTS
The authors thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for funding this study.
Ethics statement: This study was performed with data from published papers; consequently, there was no use of animals. In this situation, it is not necessary for the study to be approved by the Animal Ethics Committee of the Universidade Federal do Rio Grande do Sul.
DISCLOSURES
The authors declare no conflicts of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2021.101338.
Appendix. Supplementary materials
REFERENCES
- Ahmad T., Khalid T., Mushtaq T., Mirza M.A., Nadeem A., Babar M.E., Ahmad G. Effect of potassium chloride supplementation in drinking water on broiler performance under heat stress conditions. Poult. Sci. 2008;87:1276–1280. doi: 10.3382/ps.2007-00299. [DOI] [PubMed] [Google Scholar]
- Ain Baziz H., Geraert P.A., Padilha J.C.F., Guillaumin S. Chronic heat exposure enhances fat deposition and modifies muscle and fat partition in broiler carcasses. Poult. Sci. 1996;75:505–513. doi: 10.3382/ps.0750505. [DOI] [PubMed] [Google Scholar]
- Andretta I., Kipper M., Hauschild L., Lehnen C.R., Remus A., Melchior R. Meta-analysis of individual and combined effects of mycotoxins on growing pigs. Sci. Agric. 2016;73:328–331. [Google Scholar]
- Bonnet S., Geraert P.A., Lessire M., Carre B., Guillaumin S. Effect of high ambient temperature on feed digestibility in broilers. Poult. Sci. 1997;76:857–863. doi: 10.1093/ps/76.6.857. [DOI] [PubMed] [Google Scholar]
- Borges S.A., Fischer da Silva A.V., Ariki J., Hooge D.M., Cummings K.R. Dietary electrolyte balance for broiler chickens under moderately high ambient temperatures and relative humidities. Poult. Sci. 2003;82:301–308. doi: 10.1093/ps/82.2.301. [DOI] [PubMed] [Google Scholar]
- Borges S.A., Fischer da Silva A.V., Ariki J., Hooge D.M., Cummings K.R. Dietary electrolyte balance for broiler chickens exposed to thermoneutral or heat-stress environments. Poult. Sci. 2003;82:428–435. doi: 10.1093/ps/82.3.428. [DOI] [PubMed] [Google Scholar]
- Borges S.A., Fischer da Silva A.V., Majorka A., Hooge D.M., Cummings K.R. Physiological responses of broiler chickens to heat stress and dietary electrolyte balance (sodium plus potassium minus chloride, milliequivalents per kilogram) Poult. Sci. 2004;83:1551–1558. doi: 10.1093/ps/83.9.1551. [DOI] [PubMed] [Google Scholar]
- Chowdhury V.S., Tomonaga S., Nishimura S., Tabata S., Furuse M. Physiological and behavioral responses of young chicks to high ambient temperature. J. Poult. Sci. 2012;49:212–218. [Google Scholar]
- Dale N.M., Fuller H.L. Effect of diet composition on feed intake and growth of chicks under heat stress. II. Constant vs. cycling temperatures. Poult. Sci. 1980;59:1434–1441. doi: 10.3382/ps.0591434. [DOI] [PubMed] [Google Scholar]
- Faria Filho D.E., Rosa P.S., Figueiredo D.F., Dahlke F., Macari M., Furlan R.L. Dietas de baixa proteína no desempenho de frangos criados em diferentes temperaturas. Pesq. Agropec. Bras. 2006;41:101–106. [Google Scholar]
- Faria Filho D.E., Rosa P.S., Vieira B.S., Macari M., Furlan R.L. Protein levels and environmental temperature effects on carcass characteristics, performance, and nitrogen excretion of broiler chickens from 7 to 21 days of age. Rev. Bras. Ci. Av. 2005;7:247–253. [Google Scholar]
- Gamba J.P., Rodrigues M.M., Garcia Neto M., Perri S.H.V., Faria Júnior M.J.A., Pinto M.F. The strategic application of electrolyte balance to minimize heat stress in broilers. Rev. Bras. Cienc. Avic. 2015;17:237–246. [Google Scholar]
- Geraert P.A., Padilha J.C.F., Guillaumin S. Metabolic and endocrine changes induced by chronic heat exposure in broiler chickens: growth performance, body composition and energy retention. Br. J. Nutr. 1996;75:195–204. doi: 10.1079/bjn19960124. [DOI] [PubMed] [Google Scholar]
- Gomes A.R., Litz F.H., Morais H.R., de Oliveira R.P., Nascimento M.R.B.M. Estresse por calor na produção de frangos de corte. Pubvet. 2012;6:1466–1471. [Google Scholar]
- Gonzalez-Esquerra R., Leeson S. Effects of acute versus chronic heat stress on broiler response to dietary protein. Poult. Sci. 2005;84:1562–1569. doi: 10.1093/ps/84.10.1562. [DOI] [PubMed] [Google Scholar]
- Havenstein G.B., Ferket P.R., Qureshi M.A. Carcass composition and yield of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poult. Sci. 2003;82:1509–1518. doi: 10.1093/ps/82.10.1509. [DOI] [PubMed] [Google Scholar]
- Laganá C. Influência de altas temperaturas na alimentação de frangos de corte. Pesquisa Tecnol. 2008;5:1–9. [Google Scholar]
- Laganá C. Avicultura: cuidados com o inverno. Pesquisa Tecnol. 2012;9:1–5. [Google Scholar]
- Laganá C., Ribeiro A.M.L., Kessler A.M., Kratz L.R., Pinheiro C.C. Effect of the supplementation of vitamins and organic minerals on the performance of broilers under heat stress. Rev. Bras. Cienc. Avic. 2007;9:39–43. [Google Scholar]
- Laganá C., Ribeiro A.M.L., Kessler A.M., Kratz L.R., Pinheiro C.C. Effects of the reduction of dietary heat increment on the performance, carcass yield, and diet digestibility of broilers submitted to heat stress. Rev. Bras. Zoot. 2007;9:45–51. [Google Scholar]
- Lana G.R.Q., Rostagno H.S., Albino L.F.T., Lana A.M.Q. Efeito da temperatura ambiente e da restrição alimentar sobre o desempenho e a composição da carcaça de frangos de corte. Rev. Bras. Zootec. 2000;29:1117–1123. [Google Scholar]
- Lin H., Zhang H.F., Du R., Gu X.H., Zhang Z.Y., Buise J., Decuypere E. Thermoregulation responses of broiler chickens to humidity at different ambient temperatures. II. Four weeks of age. Poult. Sci. 2005;84:1173–1178. doi: 10.1093/ps/84.8.1173. [DOI] [PubMed] [Google Scholar]
- Lovatto P.A., Lehnen C.R., Andretta I., Hauschild L., Carvalho A.D. Meta-análise em pesquisas científicas - enfoque em metodologias. Rev. Bras. Zootec. 2007;36:285–294. [Google Scholar]
- Marchini C.F.P., Café M.B., Araújo E.G., Nascimento M.R.B.M. Physiology, cell dynamics of small intestinal mucosa, and performance of broiler chickens under heat stress: a review. Rev. Colom. Cienc. Pecua. 2016;29:159–168. [Google Scholar]
- Mitchell M.A., Carlisle A.J. The effects of chronic exposure to elevated environmental temperature on intestinal morphology and nutrient absorption in the domestic fowl (Gallus domesticus) Comp. Biochem. Physiol. 1992;101A:137–142. doi: 10.1016/0300-9629(92)90641-3. [DOI] [PubMed] [Google Scholar]
- Mongin P. Recent advances in dietary cation-anion balance: applications in poultry. Proc. Nutr. Soc. (Camb.) 1981;40:285–294. doi: 10.1079/pns19810045. [DOI] [PubMed] [Google Scholar]
- Moraes V.M.B., Malheiros R.D., Bruggeman V., Collin A., Tona K., Van As P., Onagbesan O.M., Buyse J., Decuypere E., Macari M. Effect of thermal conditioning during embryonic development on aspects of physiological responses of broilers to heat stress. J. Therm Biol. 2003;28:133–140. [Google Scholar]
- National Research Council (NRC) Natl. Acad. Press; Washington, DC: 2012. Nutrient Requirements of Swine. [Google Scholar]
- Pastorelli H., Milgen J., Lovatto P., Montagne L. Metaanalysis of feed intake and growth responses of growing pigs after a sanitary challenge. Animal. 2012;6:952–961. doi: 10.1017/S175173111100228X. [DOI] [PubMed] [Google Scholar]
- Perondi D., Kipper M., Andretta I., Hauschild L., Lunedo R., Franceschina C.S., Remus A. Empirical models to predict feed intake of growing-finishing pigs reared under high environmental temperatures. Sci. Agric. 2018;75:296–303. [Google Scholar]
- Piestun Y., Halevy O., Shinder D., Ruzal M., Druyan S., Yahav S. Thermal manipulations during broiler embryogenesis improves post-hatch performance under hot conditions. J. Therm. Biol. 2011;36:469–474. [Google Scholar]
- Ribeiro A.M.L., Penz A.M., Jr., Teeter R.G. Effects of 2-hydroxy-4-(methylthio)butanoic acid and DL-methionine on broiler performance and compensatory growth after exposure to two different environmental temperatures. J. Appl. Poult. Res. 2001;10:419–426. [Google Scholar]
- Rostagno H.S., Albino L.F.T, Hannas M.I., Donzele J.L., Sakomura N.K., Perazzo F.G., Saraiva A., Teixeira M.L., Rodrigues P.B., Oliveira R.F., Barreto S.L.T., Brito C.O. 4th ed. Departamento de Zootecnia, Universidade Federal de Viçosa; Viçosa: Brazil: 2017. Tabelas Brasileiras Para Aves e Suínos: Composição de Alimentos e Exigências Nutricionais. [Google Scholar]
- Salabi F., Boujarpor M., Fayazi J., Salari S., Nazari M. Effects of different levels of zinc n the performance and carcass characteristics of broiler reared under heat stress condition. J. Anim. Vet. Adv. 2011;10:1332–1335. [Google Scholar]
- Sauvant D., Schmidely P., Daudin J.J., St-Pierre N.R. Meta-analyses of experimental data in animal nutrition. Animal. 2008;2:1203–1214. doi: 10.1017/S1751731108002280. [DOI] [PubMed] [Google Scholar]
- Soren N.M. Nutritional manipulations to optimize productivity during environmental stresses in livestock. In: Sejian V., Navqi S.M.K., Ezeji T., Lakritz J., Lal R., editors. Pages 181-218 in Environmental Stress and Amelioration in Livestock Production. Springer-Verlag; Berlin Heidelberg, Germany: 2012. [Google Scholar]
- Souza L.F.A.d., Espinha L.P., Almeida E.A., Lunedo R., Furlan R.L., Macari M. How heat stress (continuous or cyclical) interferes with nutrient digestibility, energy and nitrogen balances and performance in broilers. Liv. Sci. 2016;192:39–43. [Google Scholar]
- St-Pierre N.R. Meta-analyses of experimental data in the animal sciences. R. Bras. Zootec. 2007;36:343–358. [Google Scholar]
- Teeter R.G., Smith M.O., Owens F.N., Arp S.C., Sangiah S., Breazile J.E. Chronic heat stress and respiratory alkalosis: occurrence and treatment in broiler chicks. Poult. Sci. 1985;64:1060–1064. doi: 10.3382/ps.0641060. [DOI] [PubMed] [Google Scholar]
- Theil H. North Holland Publishing Co; Amsterdam, The Netherlands: 1966. Applied Economic Forecasting. [Google Scholar]
- Yahav S. Relative humidity at moderate ambient temperatures: its effect on male broiler chickens and turkeys. Br. Poult. Sci. 2000;41:94–100. doi: 10.1080/00071660086475. [DOI] [PubMed] [Google Scholar]
- Yahav S., Goldfeld S., Plavnik I., Hurwitz S. Physiological responses of chickens and turkeys to relative humidity during exposure to high ambient temperature. J. Therm. Biol. 1995;20:245–253. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.