Abstract
This study investigated the effects of dietary supplementation with Bacillus subtilis (B. subtilis) or Bacillus licheniformis (B. licheniformis) on growth performance, immunity, antioxidant capacity, short chain fatty acid (SCFA) production, and the cecal microflora in broiler chickens. In total, 360 male, 1-day-old Cobb 500 birds were randomly divided into 3 groups: the control group was fed a basal diet; the B. subtilis group was fed a basal diet supplemented with 1.5 × 109 CFU/kg B. subtilis; the B. licheniformis group was fed a basal diet supplemented with 1.5 × 109 CFU/kg B. licheniformis. Results showed that chickens supplemented with either B. subtilis or B. licheniformis had comparatively higher (P < 0.05) body weight and average daily gain, whereas no difference (P > 0.05) was observed in feed efficiency. Concentrations of serum IgA, IgY, and IgM, as well as anti-inflammatory IL-10 were significantly increased (P < 0.05), and proinflammatory IL-1β and IL-6 were significantly decreased (P < 0.05) by B. subtilis or B. licheniformis supplementation. Moreover, chickens fed with diets supplemented by either B. subtilis or B. licheniformis had greater antioxidant capacity, indicated by the notable increases (P < 0.05) in glutathione peroxidase, superoxide dismutase, and catalase, along with decrease (P < 0.05) in malondialdehyde. Compared to the control group, levels of SCFA, excluding acetic and propionic acid, in cecal content had improved (P < 0.05) by adding B. licheniformis, and significant increase (P < 0.05) in acetic and butyric acid was observed with B. subtilis supplementation. Microbial analysis showed that both B. subtilis or B. licheniformis supplementation could increase butyrate-producing bacteria such as Alistipes and Butyricicoccus, and decrease pathogenic bacteria such as the Synergistetes and Gammaproteobacteria. In summary, dietary supplemented with B. subtilis or B. licheniformis improved growth performance, immune status, and antioxidant capacity, increased SCFA production, and modulated cecal microbiota in chickens. Moreover, B. licheniformis was more effective than B. subtilis with the same supplemental amount.
Key words: probiotics, broiler, growth performance, immunity, and cecal microflora
INTRODUCTION
Growth-promoting antibiotics are commonly used worldwide to provide protection against disease and ultimately improve growth performance in poultry. However, due to the increasing challenges caused by the extensive use of antibiotics, including environmental pollution and the development of bacterial antibiotic resistance (Mehdi et al., 2018; Roth et al., 2019), growth-promoting antibiotics have been banned for use as feed additives, in Europe since 2006, the United States since 2014, and China since 2020. Therefore, seeking safe and effective antibiotic alternatives is of primary importance in the animal industry.
Probiotics are defined as live microorganisms that can have beneficial physiological effects on their host (Chaucheyras-Durand and Durand, 2010; Santacroce et al., 2019). Use of probiotics as an alternative to antibiotics in poultry diets, has gained considerable attention in the feed industry in recent years (Alagawany et al., 2018; Al-Khalaifah, 2018). Several studies have indicated that probiotics used in the poultry industry can regulate intestinal microbiota structure, enhance immunity to improve disease resistance, promote the digestion and absorption of nutrients, and ultimately improve growth and production performance (Cox and Dalloul, 2015; Khan et al., 2020; Tarradas et al., 2020). Mounting evidence suggests that among the probiotics, Bacillus subtilis (B. subtilis) and Bacillus licheniformis (B. licheniformis) have been recognized as safe for animal dietary use (EFSA, 2007) and can be effectively applied as alternatives to antibiotic growth promoters. Previous studies have shown that B. subtilis can improve the growth performance of heat-stressed broilers and enhance the recovery and restoration processes of damaged intestinal mucosa (Al-Fataftah and Abdelqader, 2014; Abdelqader et al., 2020). Furthermore, B. subtilis can compete with pathogens, balance intestinal microbiota, and enhance immunity in chickens (Elshaghabee et al., 2017; Abudabos et al., 2019; Guo et al., 2020). Similarly, B. licheniformis can enhance the growth performance of chickens (Liu et al., 2012) and maintain intestinal microbiota balance in broilers (Chen and Yu, 2020). Meanwhile, B. licheniformis also helps prevent necrotic enteritis in chickens by normalizing the ileal microbiota (Knap et al., 2010; Xu et al., 2018). Although ample studies have been conducted involving B. subtilis or B. licheniformis in broilers, comparative studies of these 2 probiotics from multiple perspectives, are limited. Therefore, the objective of the present study was to evaluate the effects of continuous dietary supplementation with B. subtilis or B. licheniformis, on growth performance, immunity, antioxidant capacity, short chain fatty acid (SCFA) production and cecal microflora in broilers.
MATERIALS AND METHODS
All animal experiments were conducted in accordance with the principles and specific guidelines presented in Guide for the Care and Use of Agricultural Animals in Research and Teaching (Mcglone et al., 2010), and approved by the Research Center Institutional about Animal Care and Use Committee of Zhejiang Agricultural and Forestry University.
Experimental Design
A total of 360 male, 1-day-old (Cobb 500) broilers were randomly divided into 3 groups fed with different diets treatments. Each group consisted of 8 replicate pens with 15 chickens in per replicate. Dietary treatments included a basal diet (CON), a basal diet supplemented with 1.5 × 109 CFU/kg of B. subtilis (BS), and a basal diet supplemented with 1.5 × 109 CFU/kg of B. licheniformis (BL). The feed and water were available ad libitum. The basal diet was formulated to meet the nutritional requirements of broilers described by the NRC (1994), and the ingredient and chemical compositions of the basal diets used in this study are shown in Table 1. The feeding program consisted of 2 phases, namely starter phase (d 1 to 21) and finisher phase (d 22 to 42). The lighting program provided 23 h of light and 1 h of darkness, up to the end of the experiment. Room temperature was controlled at 32°C to 35°C for the first week and then thereafter reduced by 3°C to 5°C per week, to reach a final temperature of 26°C. The B. Subtilis (HJKC02) and the B. Licheniformis (HJDY01) strains were provided by Vegamax Biotechnology Co., Ltd. (Anji, Zhejiang, China).
Table 1.
Composition and nutrient levels of the basal diet.
| Ages (d) |
||
|---|---|---|
| Items | 1–21 | 22–42 |
| Ingredients (air-dry basis,%) | ||
| Corn | 56.33 | 57.4 |
| Soybean meal | 24.5 | 19 |
| Extruded soybean | 5 | 4 |
| Corn distillers dried grains with solubles | 5 | 8 |
| Corn gluten meal | 2 | 3 |
| Soybean oil | 1.2 | 4.3 |
| Limestone | 1.3 | 1.3 |
| Fermented soybean meal | 1.67 | 0 |
| Premix1,2 | 3 | 3 |
| Total | 100.00 | 100.00 |
| Nutrient levels | ||
| Metabolizable energy (kcal/kg) | 2949 | 3148 |
| Crude protein (%) | 20.6 | 18.6 |
| Crude fat | 4.9 | 8.0 |
| Lysine (%) | 1.17 | 0.99 |
| Methionine + Cysteine (%) | 1.45 | 1.23 |
| Threonine + Tryptophan (%) | 1.13 | 0.95 |
| Calcium (%) | 0.88 | 0.79 |
| Total phosphorus (%) | 0.64 | 0.56 |
Minimal vitamin levels per kg of diets: vitamin A (retinyl acetate), 1,500 IU; cholecalciferol, 200 IU; vitamin E (DL-α-tocopheryl acetate), 10 IU; riboflavin, 3.5 mg; pantothenic acid, 10 mg; niacin, 30 mg; cobalamin, 10μg; choline chloride, 1,000 mg; biotin, 0.15 mg; folic acid, 0.5 mg; thiamine 1.5 mg; pyridoxine 3.0 mg.
Minimal mineral levels per kg of diet: Fe 80.00 mg;Cu 8.00 mg; Mn 60.00 mg; Zn 40.00 mg; I 0.18mg; Se 0.15 mg.
Growth Performance
Body weight (BW) of broiler chickens was measured on d 1, 21, and 42 of the experiment, whereafter the average daily gain (ADG), average daily feed intake (ADFI), and the feed: gain ratio (F: G) for each cage were calculated for each phase. The individual cages were considered as the experimental unit.
Sample Collection
On d 42, 1 bird was chosen from each replicate. After weighing, a blood sample was collected from wing vein of each bird into a 10 mL tube. After centrifuging at 3,000 × g for 15 min at 4°C, serum samples were obtained and stored at –20°C for further study. Broilers were euthanized by cervical dislocation and immediately autopsied, where 2 to 3 g cecal contents were collected from each bird and stored at −80°C until analysis.
Serum Immunoglobulin Content Analyses
The serum IgA (Cat# ANG-E32004C), IgM (Cat# ANG-E32005C), IgY (Cat# ANG-E32209C), IL-1β (Cat# ANG-E32031C), IL-6 (Cat# ANG-E32013C), and IL-10 (Cat# ANG-E32011C) were measured using the chicken-specific ELISA kits obtained from Nanjing Aoqing Biotechnology Co., Ltd (Nanjing, Jiangsu, China).
Serum Biochemical Indexes Content Analyses
The serum biochemical indices, including malondialdehyde (MDA) (Cat# ANG-SH-10112), glutathione peroxidase (GSH-Px) (Cat# ANG-SH-10202), superoxide dismutase (SOD) (Cat# ANG-SH-10012), and catalase (CAT) (Cat# ANG-SH-10122) were assayed separately using specific kits (Nanjing Aoqing Biotechnology Co., Ltd, Nanjing, Jiangsu, China).
SCFAs Measurement and Analysis
Referring to the assay method of Yang et al. (2019), the concentration of SCFAs in cecal content was estimated by gas chromatography using a 7890B Network GC System and 7693 Automatic Liquid Sampler with G4513A injector (Agilent Technologies, Santa Clara, CA) equipped with a 30 m × 0.25 mm × 0.25 μm DB-FFAP Column (Cat# 122-3232, Agilent Technologies,), and a flame ionization detector. Briefly, 1 g of cecal content was dissolved in pure water. Following shock mixing and high-speed centrifugation (12,000 × g for 10 min at 4°C), the supernatant was extracted and mixed with 25% (m/v, 1:3) phosphoric acid. The mixed liquid was stabilized on ice for 35 min and filtered into the special injection bottles for machine detection.
16S rRNA Sequencing and Analysis
Cecal contents were collected for 16S rDNA sequence analysis after 42 days of feeding. The trademarked E.Z.N.A. Soil DNA Kit (Cat# D5625-01, Omega Bio-tek, Norcross, GA) was used to extract total bacterial DNA samples, which were stored at −80°C for further analysis. The V4 regions of 16S rRNA were amplified with primers 515F/806R (515F: 5′-GTGCCAGCMGCCGCGGTAA-3′, 806R: 5′-GGACTACVSGGG TATCTAAT-3′) using the Applied Biosystems GENEAMP 9700 thermocycler PCR system (Cat# 4413750, Thermo Fischer Scientific, Waltham, MA). PCR-amplification products were purified by 2% agarose gel electrophoresis, and the AXYGEN DNA Gel Extraction Kit (Cat# AP-GX-250G, Union City, CA) was used for recovery of DNA from the gels. Purified amplicons were pooled in equimolar and paired-end sequenced (2 × 300) on the Illumina MiSeq platform (PE300, Illumina, San Diego, CA) according to the standard protocols described by Majorbio Bio-Pharm Technology Co. Ltd. (Pudong, Shanghai, China).
Operational taxonomic units (OTUs) were generated at a 97% similarity threshold, and the taxonomy of each 16S rRNA gene sequence was analyzed using the Ribosomal Database Project (RDP) classifier algorithm against the database, at a confidence threshold of 70%. The microbiota diversity analysis included an alpha diversity component—Shannon, Chao, and Simpson, and observed species indices—and a beta diversity component in different samples, Weighted Uniface distances were visualized by principal component analysis (PCA) and principal co-ordinates analysis (PCoA).
Furthermore, linear discriminant analysis coupled with effect size (LEfSe) was performed to identify differential expression at species level between the groups of bacterial taxa and compared that with the relative abundance of the different taxa at species level of the bacterial taxa.
Statistical Analysis
Obtained data were analyzed by one-way ANOVA using IBM SPSS 22.0 software (IBM Corp., Armonk, NY), the results of which were expressed as mean ± SEM. Means were compared using the Tukey-Kramer test and statistical significance was determined at a value of P < 0.05. A histogram was created by GraphPad Prism version 7.0 (GraphPad Software Inc., San Diego, CA). For the analysis of 16S rRNA sequencing results, the relative abundance was calculated for each sample at different taxonomic levels, and the Kruskal-Wallis H test used to analyze the relative abundances at different taxonomic levels, between the groups included in the experiment. Outcomes of the significant difference tests of alpha (two-sided Student's t test) and beta diversity (analysis of similarities) were analyzed using the Quantitative Insights Into Microbial Ecology (QIIME) bioinformatics pipeline.
RESULTS
Growth Performance
The effects of B. subtilis or B. licheniformis supplementation on the growth performance of broilers are shown in Table 2. Results showed that supplementation with either B. subtilis or B. licheniformis from d 1 to 42, significantly improved (P < 0.05) broiler BW, ADG, and ADFI, compared with broilers from the CON group. Notably, the increases in BW and ADG of BL group were higher (P < 0.05) than those of the BS group (P < 0.05) throughout the period from d 1 to 42. No significant differences were observed in F: G ratio (P > 0.05) of the 3 groups during the period from d 1 to 21, but the BL group had a lower F: G ratio than CON group (P < 0.05), during the starter phase.
Table 2.
Effects of dietary supplementation of B. subtilis or B. licheniformis on growth performance in broilers.
| Treatments1,2 |
|||||
|---|---|---|---|---|---|
| Items | NCO | BS | BL | SEM | P-value |
| Body weight, g/bird | |||||
| 1 d | 37.89 | 37.94 | 37.24 | 0.26 | 0.156 |
| 21 d | 758.46 | 802.22 | 803.14 | 9.84 | 0.072 |
| 42 d | 1828.94c | 2069.88b | 2174.37a | 37.63 | 0.001 |
| Average daily gain, g/bird | |||||
| 1-21 d | 34.31 | 36.39 | 36.47 | 0.47 | 0.069 |
| 22–42 d | 50.98c | 60.54b | 65.30a | 1.58 | 0.001 |
| 1–42 d | 42.64c | 48.38b | 50.88a | 0.89 | 0.001 |
| Average daily feed intake, g/bird | |||||
| 1–21 d | 55.93 | 57.30 | 57.07 | 0.66 | 0.689 |
| 22–42 d | 108.54b | 124.26a | 129.02a | 2.50 | 0.001 |
| 1–42 d | 77.58b | 85.00a | 87.97a | 1.20 | 0.001 |
| Feed: Gain ratio, (g:g) | |||||
| 1–21 d | 1.63a | 1.57ab | 1.56b | 0.01 | 0.001 |
| 22–42 d | 2.13 | 2.05 | 1.98 | 0.40 | 0.156 |
| 1–42 d | 1.82 | 1.76 | 1.73 | 0.02 | 0.107 |
Mean with different superscripts in the same row differ significantly (P < 0.05).
NCO = basal diet provided as control; BS = basal diet supplemented with 1.5 × 109CFU/kg B. subtilis; BL = basal diet supplemented with 1.5 × 109CFU/kg B. licheniformis.
The mean represent results from 8 replicate cages per treatment.
Immunoglobulins and Cytokines in Serum
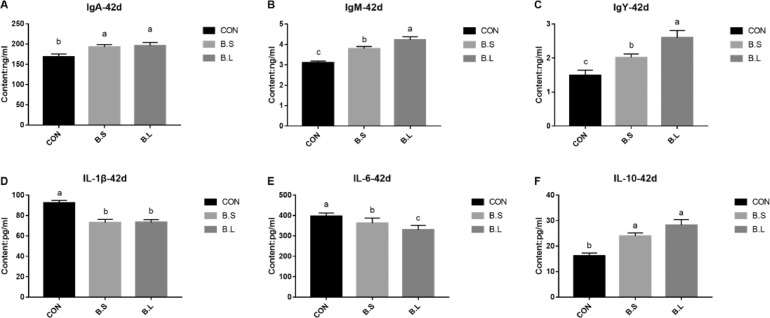
As showed in Figure 1, a significant increase (P < 0.05) in the levels of serum IgA, IgM, and IgY was observed in the BS and BL groups, in comparison to the CON group. Furthermore, levels of IgM and IgY in serum were significantly higher (P < 0.05) in the BL group than in BS group. As for the cytokines in serum, B. subtilis or B. licheniformis supplements significantly increased the concentration of anti-inflammatory factor IL-10 (P < 0.05), but decreased proinflammatory factors IL-1β and IL-6 (P < 0.05). Particularly, the serum IL-6 content of the BL group was significantly lower (P < 0.05) than that of BS group.
Figure 1.
The effects of B. subtilis or B. licheniformis supplement on serum immunoglobulins (A–C) and cytokine (D–F) of broiler chickens at d 42. Levels of immunoglobulins (A–C) and cytokine (D–F) in the serum at d 42. CON = basal diet provided as the control; BS = basal diet supplemented with 1.5 × 109CFU/kg B. subtilis; BL = basal diet supplemented with 1.5 × 109CFU/kg B. licheniformis. Bars represent mean ± SD (n = 8). Different lowercase letters (a, b) above bars represent significantly different means (P < 0.05).
Antioxidant Capacity
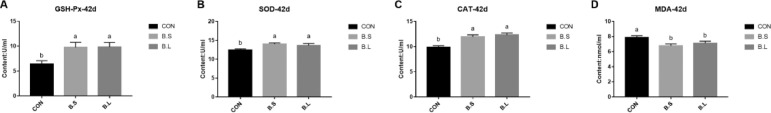
The effects of B. subtilis or B. licheniformis treatment on antioxidant activities are shown in Figure 2. The levels of GSH-Px, SOD, and CAT were notably raised (P < 0.05) in the BS and BL groups compared to the CON group. Furthermore, the levels of MDA were notably lower (P < 0.05) in the BS and BL groups compared to the CON group. No significant difference was observed in antioxidant capacity between the BS and BL groups.
Figure 2.
The effects of B. subtilis and B. licheniformis supplement on serum antioxidant activity of broilers at d 42. CON = basal diet provided as the control; BS = basal diet supplemented with 1.5 × 109CFU/kg B. subtilis; BL = basal diet supplemented with 1.5 × 109CFU/kg B. licheniformis. Bars represent mean ± SD (n = 8). Different lowercase letters (a, b) above bars represent significantly different means (P < 0.05).
SCFA Levels in Cecal contents
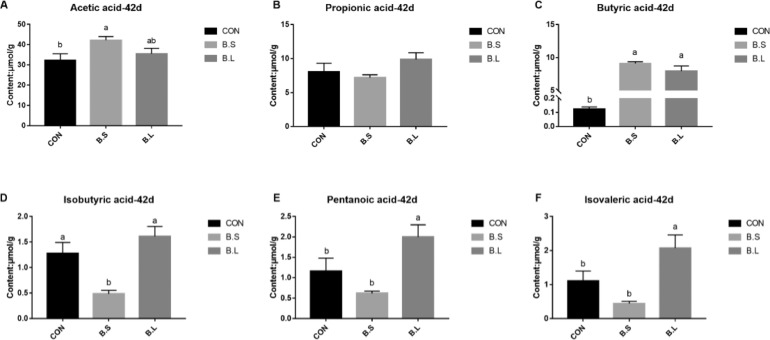
Measured levels of SCFA, including acetic acid, propionic acid, butyric acid, isobutyric acid, valeric acid, and isovaleric acid are presented in Figure 3. Results indicated that B. subtilis supplementation improved (P < 0.05) acetic acid and butyric acid content, compared with the same parameters in the CON group. Furthermore, the levels of butyric acid, valeric acid, and isovaleric acid were upregulated (P < 0.05) after B. licheniformis supplementation. However, no apparent effect was observed in propionic acid levels after administration of B. subtilis or B. licheniformis treatments.
Figure 3.
The effects of B. subtilis or B. licheniformis supplement on the short chain fatty acid in cecum contents of broiler chickens at d 42. CON = basal diet provided as the control; BS = basal diet supplemented with 1.5 × 109CFU/kg B. subtilis; BL = basal diet supplemented with 1.5 × 109CFU/kg B. licheniformis. Bars represent mean ± SD (n = 8). Different lowercase letters (a, b) above bars represent significantly different means (P < 0.05).
Diversity of Cecal Microbiota
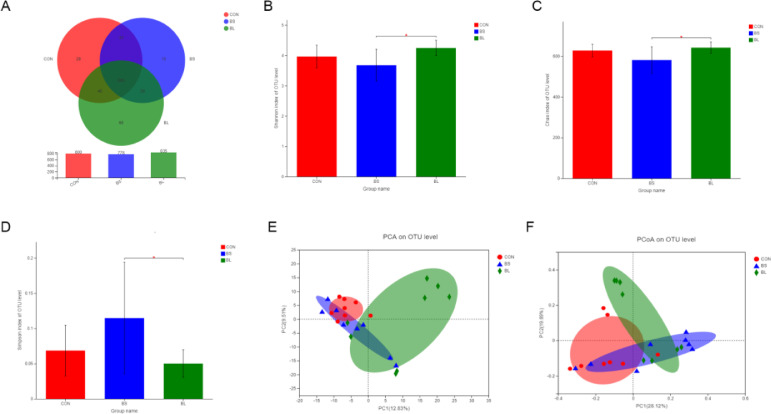
The changes in diversity of cecal microbiota are summarized in Figure 4. Based on the overlapping regions of the Venn diagram, 685 OTUs were shared between the CON, BS, and BL groups, in contrast, nonoverlapping regions showed far more unique OTUs in the BL group (n = 66) were far more than in the CON (n = 29) and BS (n = 15) group (Figure 4A). Alpha diversity analysis included the observed species, as well as Shannon, Chao, and Simpson indices, which were representative of the richness and diversity of the microbial community. Results indicated that diets containing B. licheniformis presented with more significant diversity in cecal microbiota than B. subtilis supplemented diets, whereas neither B. subtilis nor B. licheniformis supplementation appeared to affect community diversity in cecal microbiota, when compared to the CON group diet (Figures 4B–4D). However, as shown in Figures 4E and 4F, PCoA and PCA plots revealed a degree of diversity discrepancy in cecal microbiota communities between all groups, and especially between the BL and CON groups.
Figure 4.
The diversity of microbial community in cecal contents of broilers at d 42. (A) The Venn diagram summarizing the numbers of common and unique OTUs in the microflora community in cecal contents of broilers. (B–D) The shannon index, chao index, simpson reflecting species alpha diversity between groups. (E, F) The principal component analysis (PCA) and principal co-ordinates analysis (PCoA) reflecting species beta diversity within and between groups. CON = basal diet provided as the control; BS = basal diet supplemented with 1.5 × 109CFU/kg B. subtilis; BL = basal diet supplemented with 1.5 × 109CFU/kg B. licheniformis. Bars represent mean ± SD (n = 8). Bars represent mean ± SD (n = 8). *Means different (P < 0.05), **means significant difference (P < 0.01).
Composition of Cecal Microbiota
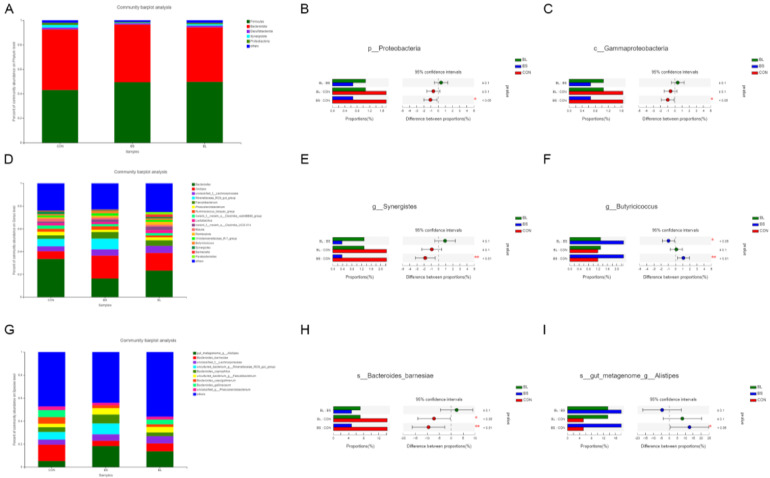
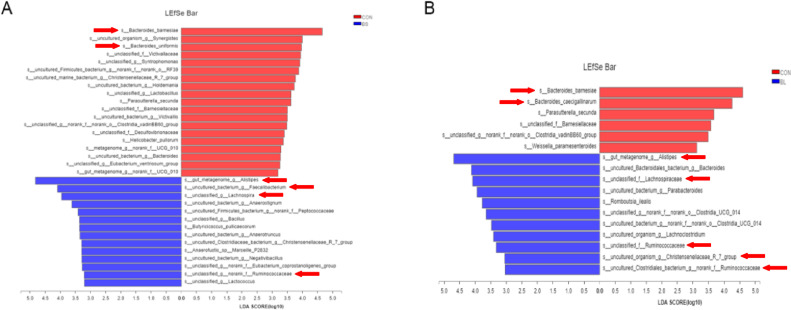
The relative abundance of OTUs of cecal microbiota was analyzed at different ranking levels from phylum to genus, to reflect the change in cecal microbiota community structure. As shown in Figure 5, at phylum level, the relative abundances of Synergistota and Proteobacteria, particularly, c_Gammaproteobacteria in the BS group, were lower than that in the CON group (P < 0.05; Figures 5A–5C, 5E). At genus level, Butyricicoccus that always be considered as a butyric acid producer was increased more significantly (P < 0.05) in the BS group than in CON and BL groups (Figure 5F). At spices level, the s_Bacteroides_barnesiae was increased significantly (P < 0.05) in the CON group, compared with the BS group, and tended to be higher than that in the BL. Conversely, relative abundance of the dominant species s_gut_meta-genome in the BS group was distinctly higher (P < 0.05), than in the CON group (Figures 5G–5I). Furthermore, the outcomes of LEfSe (linear discriminant analysis score = 3), indicated that 19 bacterial taxa were significantly more abundant (P < 0.05) in the CON group, whereas only 14 taxa were overrepresented in the BS group (P < 0.05; Figure 6A). Additionally, 6 bacterial taxa were more abundant in the CON group (P < 0.05), whereas 11 taxa were overrepresented in the BL group (P < 0.05; Figure 6B).
Figure 5.
The abundance of microbial community in cecal contents of broilers at d 42. (A, D, G) The top 10 relative abundance of microflora community between groups (level phylum, level genus, and level species) reveal by the histogram. (D–C, E, F, H, I) The bacteria with significant differences between groups (level phylum, level class, level genus and level species) indicate by histogram. CON = basal diet provided as the control; BS = basal diet supplemented with 1.5 × 109CFU/kg B. subtilis; BL = basal diet supplemented with 1.5 × 109CFU/kg B. licheniformis. Bars represent mean ± SD (n = 8). Bars represent mean ± SD (n = 8). *Means different (P < 0.05), **means significant difference (P < 0.01).
Figure 6.
Taxa that were significantly differentially represented between groups were examined by linear discriminant analysis coupled with effect size (LEfSe) using the default parameters (LDA score = 3). (A) Taxa that were significantly differentially represented between CON and BS. (B) Taxa that were significantly differentially represented between CON and BL. CON = basal diet provided as the control; BS = basal diet supplemented with1.5 × 109CFU/kg B. subtilis; BL = basal diet supplemented with 1.5 × 109CFU/kg B. licheniformis. Red arrow means a significant difference (P < 0.05) between the two groups.
DISCUSSION
With the growing interest in probiotics and their application in animal research, more and mounting supports the concept that dietary B. subtilis or B. licheniformis could promote the growth performance of chickens (Bader and Albin et al., 2012; Liu et al.,2012; Chen and Yu, 2020). Consistent with these studies, our results demonstrated that supplementation of broiler diets with B. subtilis or B. licheniformis improved growth performance by increasing the BW, ADG, ADFI, and reducing the F:G ratio, during the starter feeding phase. Moreover, other studies have shown that dietary B. subtilis supplementation or B. licheniformis can improve the growth parameters even under conditions of heat stress, immunological stress, or necrotic enteritis challenge (Al-Fatafah and Abdelqader, 2014; Gadde et al., 2017; Musa et al., 2019; Sokale et al., 2019; Abdelqader et al., 2020). One hypothesis is that improvements in growth performance may be related to beneficial metabolites produced by B. licheniformis or B. subtilis, such as extracellular digestive enzymes, lysozyme, antifungal proteins, and varieties of antibiotics, amongst others (Kim et al., 2004; Sahu et al., 2008). Another possibility is that administration of B. subtilis or B. licheniformis could enhance the broilers’ immunity (Dong et al., 2020; Guo et al., 2020) and regulate intestinal flora composition and metabolic function (Xu et al., 2018; Chen and Yu, 2020; Rodrigues et al., 2020). Additionally, our results showed that improvements in BW and ADG of the B. licheniformis-supplement group, were significantly greater than those of the B. subtilis-supplemented group, at the same supplemental amount. The reason for this phenomenon requires further research, as few comparative studies are available that demonstrate the differences between B. subtilis or B. licheniformis activity.
Serum immunoglobulins, especially IgA, IgG (its avian counterpart, IgY), and IgM that is produced by B cells, act as important parameters that to reflect the humoral immune status of animals, which relates to their important roles in immune function and providing resistance against various infections (Carlier et al., 2016; Zhang et al., 2017; Balan et al., 2019). In previous studies, levels of IgY and IgA, or IgM in serum were increased in chickens fed with B. subtilis (Bai et al., 2017) or B. licheniformis-supplemented diets (Fazelnia et al., 2021). Our results concurred with previous research in which supplementation with B. subtilis or B. licheniformis significantly increased IgA, IgM and IgY concentrations in serum of broilers after 42 d, reflecting an improvement in immune function.
IL-1β and IL-6 are generally deemed as proinflammatory cytokines produced by classically activated macrophages1. Whereas IL-10, produced by alternatively activated macrophages2, is often considered an anti-inflammatory cytokine. In our research, the levels of IL-1β and IL-6 in serum were reduced and the level of IL-10 increased after B. subtilis or B. licheniformis supplementation. Gadde et al. (2017) and Guo et al. (2020) found that continual administration of B. subtilis following infection with Eimeria maxima or Escherichia coli, down-regulated the expression of IL-1β and IL-6 in the jejunum. Moreover, up-regulated expression of IL-2 and IL-10 in the ileum was observed in an unchallenged experiment (Park et al., 2020). The reason may be that B. subtilis or B. licheniformis could inhibit avian pathogenic growth and enhance functioning of the gut barrier (Wang et al., 2017; Medina et al., 2019; Wang et al., 2020). Interestingly, based on our results, B. licheniformis was more effective than B. subtilis in increasing serum immunoglobulins and decreasing the pro-inflammatory cytokine, IL-6, further research is required to elucidate the exact, mechanism hereof.
Oxidative stress refers to a state of imbalance between antioxidants and free radicals, which could produce varieties of reactive oxygen species (ROS), in the form of hydroxyl free radicals and superoxide anions, in vivo. Excess ROS can damage the proteins, nucleic acids, and other biological macromolecules, and produce large amounts of MDA, which ultimately leads to tissue damage and the development of disease. However, the antioxidant enzymes, including SOD, glutathione (GSH), glutathione reductase (GR), glutathione peroxidase (GPx), and CAT are produced simultaneously, to remove excess ROS and maintain a steady state of health (Maritim et al., 2003; Lauridsen, 2019). Oxidative stress in poultry can occur for many reasons, for example, due to heat stress, poor feed quality or pathogenic infection. Consequently, growth performance is reduced and meat quality could even be affected (Rehman et al, 2018; Zaboli et al, 2019; Wasti et al, 2020). According to previous research, supplementation of broiler diets with B. subtilis can enhance the activity of GSH, GR, GSH-Px, and SOD by increasing the antioxidant gene expression of nuclear factor erythroid 2-related factor 2 (Nrf2), heme oxygenase (HO-1), SOD, and GPx in the liver. (Bai et al., 2017). Furthermore, the findings of Zhao et al. (2020) revealed that MDA in the ileum was lower in a B. licheniformis-supplemented group, in which the activities of antioxidant enzymes, such as SOD, CAT, and total antioxidant capacity (T-AOC), and GSH were improved in the ileum, serum, or liver. In the present study, dietary B. subtilis or B. licheniformis were beneficial in improving antioxidant capacity of broilers by the enhancement of SOD, GPx and CAT, and a decrease in MDA. Nonetheless, research on the antioxidant effects of B. subtilis or B. licheniformis effect on antioxidant is still very limited, and the mechanism of antioxidant regulation remains to be further studied.
SCFAs are a group of saturated fatty acids with fewer than 6 carbon atoms. Apart from being the main energy source of colonocytes, there were an abundance of evidence shows that SCFAs play an important role in the maintenance of health and modulation of immune and inflammatory responses (van der Hee and Wells, 2021). Our results demonstrated that dietary addition of B. subtilis, and especially B. licheniformis, could increase the content of various SCFAs in cecal contents. In particular, the increase in butyric acid level was the most significant. Similar results have been observed in a study by Musa et al. (2019). Moreover, according to research by Parada et al. (2019) and Luu et al. (2020), the observed increase in immunoglobulins and changes in cytokines may be related to an increase in butyric acid production.
Numerous studies have revealed that the gut microbiota play an important role in host health status by absorbing nutrients, improving growth and metabolism, resisting harmful bacteria, and modulating the immune system to such an extent that its function is irreplaceable (Heiss and Olofsson, 2018; Pickard et al., 2017; Cheng et al., 2019). The cecum is the site of most abundant and concentrated intestinal flora in which the biological fermentation processes, especially the production of SCFAs, take place. Moreover, the gut microbiota can utilize or ferment feed in different ways and produce different metabolites (Tungland, 2018; Rychlik, 2020).
Our results obtained from PCA and PCoA conducted on cecal content revealed a degree of diversity discrepancy in cecal microbiota. Similar outcomes were achieved in studies by Ma et al. (2018) and Chen and Yu (2020). Furthermore, previous studies demonstrated that B. subtilis or B. licheniformis can inhibit the growth of pathogenic bacteria in vitro and in vivo, by producing certain antimicrobial peptides or inhibiting biofilm formation (Rivardo et al., 2009; Lin et al., 2017; Du et al., 2019; Guo et al., 2020). The Synergistetes, a group of gram-negative anaerobic organisms that can colonize in chicken cecal, and correlate with the fate of certain antibiotic resistance genes (Kubasova et al., 2019; Zhang et al., 2019), are reported opportunistic pathogens that have been detected frequently in cases of human disease such as cysts, abscesses, and oral cavity and dental diseases (Belibasakis et al., 2016; McCracken and Nathalia, 2020). In our study, the proportion of Synergistetes at phylum level, and even at genera level, trended lower in the BL group, and significantly lower in the BS group, than the CON group. Furthermore, the Gammaproteobacteria, which mainly includes certain pathogenic bacteria, such as Escherichia coli, and Salmonella enterica serovar Typhimurium, among others (Zhao and Houry, 2010), were observed in a downward similar trend to that of Synergistetes. This may also explain changes in immunoglobulin and cytokine levels in BS and BL groups. As one of the dominant intestinal bacteria in broilers, Bacteroides possesses strong ability to utilize amino acids and carbohydrates and could be related to immune system development (Zhou and Zhi, 2016; Singh, 2019). In previous studies, the genera Alistipes and Butyricicoccus, which have been identified as butyrate producers in the gut and demonstrate good anti-inflammatory effects in human and animal experiments (Geirnaert et al., 2014; Eeckhaut et al., 2016; Wu et al., 2020), were observed in higher proportion in the BS group than the CON group. This may account for the increase in butyric acid level and the decrease in inflammatory cytokines, such as IL-1β and IL-6. Moreover, research has indicated that B. subtilis can induce a shift toward butyrate-producing bacteria in the broiler gut microbiome (Jacquier et al., 2019), which was consistent with our results. Per the outcome of LEfSe higher levels of bacteria associated with the production of SCFAs, such as the unclassified_f_Lachnospiraceae, g_Ruminococcus, and g_Faecalibacterium, amongst others (Antonissen et al., 2016; Blasco et al., 2020), were detected after B. subtilis or B. licheniformis supplementation. Considering these combined results, it may explain the significant increase in level of the SCFAs.
In conclusion, supplementary B. subtilis or B. licheniformis significantly improved broiler growth performance, which may have been achieved by enhancing immune functions, modulating gut microbiota, and reducing the number of pathogens.
Moreover, based on our results, B. licheniformis was more effective than B. subtilis in immunomodulating and growth promotion of broilers.
ACKNOWLEDGMENTS
This work was supported by the Zhejiang Provincial Key Research and Development Program (No.2019 C02051; No.2020C02032), Zhejiang Provincial Leading Innovation and Entrepreneurship Team Project (No.2020R01015), and the National Key Research and Development Program Intergovernmental International Innovation Cooperation Project (No.2018YFE0112700). Zhejiang Provincial Key Agricultural Research Institute Project on Green Animal Health Products (No. 2021Y30004)
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- Abdelqader A., Abuajamieh M., Hayajneh F., Al-Fataftah A.R. Probiotic bacteria maintain normal growth mechanisms of heat stressed broiler chickens. J. Therm. Biol. 2020;92 doi: 10.1016/j.jtherbio.2020.102654. [DOI] [PubMed] [Google Scholar]
- Abudabos A.M., Alhouri H.A.A., Alhidary I.A., Nassan M.A., Swelum A.A. Ameliorative effect of Bacillus subtilis, Saccharomyces boulardii, oregano, and calcium montmorillonite on growth, intestinal histology, and blood metabolites on Salmonella-infected broiler chicken. Environ. Sci. Pollut. Res. Int. 2019;26:16274–16278. doi: 10.1007/s11356-019-05105-1. [DOI] [PubMed] [Google Scholar]
- Alagawany M., Abd El-Hack M.E., Farag M.R., Sachan S., Karthik K., Dhama K. The use of probiotics as eco-friendly alternatives for antibiotics in poultry nutrition. Environ. Sci. Pollut. Res. Int. 2018;25:0611–10618. doi: 10.1007/s11356-018-1687-x. [DOI] [PubMed] [Google Scholar]
- Al-Fataftah A.R., Abdelqader A. Effects of dietary Bacillus subtilis on heat-stressed broilers performance, intestinal morphology and microflora composition. Anim. Feed Sci. Technol. 2014;198:279–285. [Google Scholar]
- Al-Khalaifah H.S. Benefits of probiotics and/or prebiotics for antibiotic-reduced poultry. Poult. Sci. 2018;97:3807–3815. doi: 10.3382/ps/pey160. [DOI] [PubMed] [Google Scholar]
- Antonissen G., Eeckhaut V., Van D.K., Onrust L., Haesebrouck F., Ducatelle R., Moore R.J., Van I.F. Microbial shifts associated with necrotic enteritis. Avian. Pathol. 2016;45:308–312. doi: 10.1080/03079457.2016.1152625. [DOI] [PubMed] [Google Scholar]
- Bader J., Albin A., Stahl U. Spore-forming bacteria and their utilisation as probiotics. Benef. Microbes. 2012;3:67–75. doi: 10.3920/BM2011.0039. [DOI] [PubMed] [Google Scholar]
- Balan P., Han K., Moughan P.J. Impact of oral immunoglobulins on animal health–a review. Anim. Sci. J. 2019;90:1099–1110. doi: 10.1111/asj.13258. [DOI] [PubMed] [Google Scholar]
- Bai K., Huang Q., Zhang J.F., He J.T., Zhang L.L., Wang T. Supplemental effects of probiotic Bacillus subtilis fmbJ on growth performance, antioxidant capacity, and meat quality of broiler chickens. Poult. Sci. 2017;96:74–82. doi: 10.3382/ps/pew246. [DOI] [PubMed] [Google Scholar]
- Belibasakis G.N., Mir-Mari J., Sahrmann P., Sanz-Martin I., Schmidlin P.R., Jung R.E. Clinical association of spirochaetes and synergistetes with peri-implantitis. Clin. Oral. Implants. Res. 2016;27:656–661. doi: 10.1111/clr.12690. [DOI] [PubMed] [Google Scholar]
- Blasco L., Kahala M., Tampio E., Vainio M., Ervasti S., Rasi S. Effect of inoculum pretreatment on the composition of microbial communities in anaerobic digesters producing volatile fatty acids. Microorganisms. 2020;8:581. doi: 10.3390/microorganisms8040581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier F.M., Sibille Y., Pilette C. The epithelial barrier and immunoglobulin A system in allergy. Clin. Exp. Allergy. 2016;46:1372–1388. doi: 10.1111/cea.12830. [DOI] [PubMed] [Google Scholar]
- Chaucheyras-Durand F., Durand H. Probiotics in animal nutrition and health. Benef. Microbes. 2010;1:3–9. doi: 10.3920/BM2008.1002. [DOI] [PubMed] [Google Scholar]
- Chen Y.C., Yu Y.H. Bacillus licheniformis-fermented products improve growth performance and the fecal microbiota community in broilers. Poult. Sci. 2020;99:1432–1443. doi: 10.1016/j.psj.2019.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H.Y., Ning M.X., Chen D.K., Ma W.T. Interactions between the gut microbiota and the host innate immune response against pathogens. Front. Immunol. 2019;10:607. doi: 10.3389/fimmu.2019.00607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C.M., Dalloul R.A. Immunomodulatory role of probiotics in poultry and potential in ovo application. Benef. Microbes. 2015;6:45–52. doi: 10.3920/BM2014.0062. [DOI] [PubMed] [Google Scholar]
- Dong Y.X., Li R., Liu Y., Ma L.Y., Zha J.H., Qiao X.B., Chai T.J., Wu B. Benefit of dietary supplementation with Bacillus subtilis BYS2 on growth performance, immune response, and disease resistance of broilers. Probio. Antimicrob. Proteins. 2020;12:1385–1397. doi: 10.1007/s12602-020-09643-w. [DOI] [PubMed] [Google Scholar]
- Du Y.P., Xu Z.C., Yu G.L., Liu W., Zhou Q.F., Yang D.H., Li J., Chen L., Zhang Y., Xue C.Y., Cao Y.C. Bacillus subtilis-A newly isolated strain named WS-1 inhibited diarrhea and death caused by pathogenic in newborn piglets. Front. Microbiol. 2019;10:1248. doi: 10.3389/fmicb.2019.01248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeckhaut V., Wang J., Van P.A., Haesebrouck F., Joossens M., Falony G., Raes J., Ducatelle R., Van I.F. Butyricicoccus pullicaecorum–the probiotic reduces feed conversion and protects from potentially harmful intestinal microorganisms and necrotic enteritis in broilers. Front. Microbiol. 2016;7:1416. doi: 10.3389/fmicb.2016.01416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Introduction of a qualified presumption of safety (QSP) approach for assessment of selected microorganisms referred to EFSA - opinion of the scientific committee. EFSA J. 2007;587:1–16. [Google Scholar]
- Elshaghabee F.M.F., Rokana N., Gulhane R.D., Sharma C., Panwar H. Bacillus as potential probiotics: status, concerns, and future perspectives. Front. Microbiol. 2017;8:1490. doi: 10.3389/fmicb.2017.01490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazelnia K., Fakhraei J., Yarahmadi H.M., Amini K. Dietary supplementation of potential probiotics Bacillus subtilis, Bacillus licheniformis, and saccharomyces cerevisiae and synbiotic improves growth performance and immune responses by modulation in intestinal system in broiler chicks challenged with Salmonella typhimurium. Probio. Antimicrob. Proteins. 2021 doi: 10.1007/s12602-020-09737-5. undefined:undefined. [DOI] [PubMed] [Google Scholar]
- Gadde U.D., Oh S.T., Lee Y.S., Davis E., Zimmerman N., Rehberger T., Lillehoj H.S. Dietary Bacillus subtilis-based direct-fed microbials alleviate LPS-induced intestinal immuno logical stress and improve intestinal barrier gene expression in commercial broiler chickens. Res. Vet. Sci. 2017;114:236–243. doi: 10.1016/j.rvsc.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Geirnaert A., Steyaert A., Eeckhaut V., Debruyne B., Arends J.B.A., Van I.F., Boon N., Van D W.T. Butyricicoccus pullicaecorum, a butyrate producer with probiotic potential, is intrinsically tolerant to stomach and small intestine conditions. Anaerobe. 2014;30:70–74. doi: 10.1016/j.anaerobe.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Guo M.J., Li M.T., Zhang C.C., Zhang X.R., Wu Y.T. Dietary administration of the Bacillus subtilis enhances immune responses and disease resistance in chickens. Front. Microbiol. 2020;11:1768. doi: 10.3389/fmicb.2020.01768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss C.N., Olofsson L.E. Gut microbiota-dependent modulation of energy metabolism. J. Innate. Immun. 2018;10:163–171. doi: 10.1159/000481519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquier V., Nelson A., Jlali M., Rhayat L., Brinch K.S., Devillard E. Bacillus subtilis 29784 induces a shift in broiler gut microbiome toward butyrate-producing bacteria and improves intestinal histomorphology and animal performance. Poult. Sci. 2019;98:2548–2554. doi: 10.3382/ps/pey602. [DOI] [PubMed] [Google Scholar]
- Khan S., Moore R.J., Stanley D., Chousalkar K.K. The gut microbiota of laying hens and its manipulation with prebiotics and probiotics to enhance gut health and food safety. Appl. Environ. Microbiol. 2020;86:e00600–e00620. doi: 10.1128/AEM.00600-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Cho J.Y., Kuk J.H., Moon J.H., Cho J.I., Kim Y.C., Park K.H. Identification and antimicrobial activity of phenylacetic acid produced by Bacillus licheniformis isolated from fermented soybean, Chungkook-Jang. Curr. Microbiol. 2004;48:312–317. doi: 10.1007/s00284-003-4193-3. [DOI] [PubMed] [Google Scholar]
- Knap I., Lund B., Kehlet A.B., Hofacre C., Mathis G. Bacillus licheniformis prevents necrotic enteritis in broiler chickens. Avian. Dis. 2010;54:931–935. doi: 10.1637/9106-101509-ResNote.1. [DOI] [PubMed] [Google Scholar]
- Kubasova T., Kollarcikova M., Crhanova M., Karasova D., Cejkova D., Sebkova A., Matiasovicova J., Faldynova M., Sisak F., Babak V., Pokorna A., Cizek A., Rychlik I. Gut anaerobes capable of chicken caecum colonisation. Microorganisms. 2019;7:597. doi: 10.3390/microorganisms7120597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauridsen C. From oxidative stress to inflammation: redox balance and immune system. Poult. Sci. 2019;98:4240–4246. doi: 10.3382/ps/pey407. [DOI] [PubMed] [Google Scholar]
- Lin Y.C., Xu S., Zeng D., Ni X.Q., Zhou M.J., Zeng Y., Wang H .S., Zhou Y., Zhu H., Pan K.C., Li G.Y. Disruption in the cecal microbiota of chickens challenged with Clostridium perfringens and other factors was alleviated by Bacillus licheniformis supplementation. PLoS One. 2017;12 doi: 10.1371/journal.pone.0182426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.L., Yan H., Lv L., Xu Q.Q., Yin C.H., Zhang K.Y., Wang P., Hu J.Y. Growth performance and meat quality of broiler chickens supplemented with Bacillus licheniformis in drinking water. Asian-Australas. J. Anim. Sci. 2012;25:682–689. doi: 10.5713/ajas.2011.11334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu M., Monning H., Visekruna A. Exploring the molecular mechanisms underlying the protective effects of microbial SCFAs on intestinal tolerance and food allergy. Front. Immunol. 2020;11:1225. doi: 10.3389/fimmu.2020.01225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y.B., Wang W.W., Zhang H.J., Wang J., Zhang W.M., Gao J., Wu S.G., Stahl G.H. Supplemental Bacillus subtilis DSM 32315 manipulates intestinal structure and microbial composition in broiler chickens. Sci. Rep. 2018;8:15358. doi: 10.1038/s41598-018-33762-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maritim A.C., Sanders R.A., Watkins J.B. Diabetes, oxidative stress, and antioxidants: a review. J. Biochem. Mol. Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- Mcglone J., Ford S., Mitloehner F., Grandin T., Ruegg P., Stull C., Lewis G., Swanson J., Underwood W., Mench J., Mader T., Eicher S., Hester P., Salak-Johnson J., Galyean M. 3th Rev ed. Poultry. Federation of Animal Science Societies; Champaign, IL: 2010. Pages 102-120 in Chapter 9 Guide for the Care and Use of Agricultural Animals in Research and Teaching. [Google Scholar]
- McCracken B.A., Nathalia G.M. Phylum Synergistetes in the oral cavity: a possible contributor to periodontal disease. Anaerobe. 2020;2020 doi: 10.1016/j.anaerobe.2020.102250. [DOI] [PubMed] [Google Scholar]
- Mehdi Y., Létourneau-Montminy M.P., Gaucher M.L., Chorfi Y., Suresh G., Rouissi T., Brar S.K., Côté C., Ramirez A.A., Godbout S. Use of antibiotics in broiler production: global impacts and alternatives. Anim. Nutr. 2018;4:170–178. doi: 10.1016/j.aninu.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina F.S., Cretenet M., Bernardeau M. In vitro inhibition of avian pathogenic Enterococcus cecorum isolates by probiotic Bacillus strains. Poult. Sci. 2019;98:2338–2346. doi: 10.3382/ps/pey593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musa B.B., Duan Y., Khawar H., Sun Q.Z., Ren Z.Z., Elsiddig M.M.A., Abbasi I.H.R., Yang X.J. Bacillus subtilis B21 and Bacillus licheniformis B26 improve intestinal health and performance of broiler chickens with Clostridium perfringens-induced necrotic enteritis. J. Anim. Physiol. Anim. Nutr. (Berl). 2019;103:1039–1049. doi: 10.1111/jpn.13082. [DOI] [PubMed] [Google Scholar]
- NRC (National Research Council) 9th Rev ed. Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Parada Venegas D., De L.F.M.K., Landskron G., González M.J., Quera R., Dijkstra G., Harmsen H.J.M., Faber K.N., Hermoso M.A. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019;10:277. doi: 10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park I., Lee Y., Goo D., Zimmerman N.P., Smith A.H., Rehberger T., Lillehoj H.S. The effects of dietary Bacillus subtilis supplementation, as an alternative to antibiotics, on growth performance, intestinal immunity, and epithelial barrier integrity in broiler chickens infected with Eimeria maxima. Poult. Sci. 2020;99:725–733. doi: 10.1016/j.psj.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard J.M., Zeng M.Y., Caruso R., Núñez G. Gut microbiota: role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017;279:70–89. doi: 10.1111/imr.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman Z.U., Meng C.C., Sun Y.J., Safdar A., Pasha R.H., Munir M., Ding C. Oxidative stress in poultry: lessons from the viral infections. Oxid. Med. Cell Longev. 2018;2018 doi: 10.1155/2018/5123147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivardo F., Turner R.J., Allegrone G., Ceri H., Martinotti M.G. Anti-adhesion activity of two biosurfactants produced by Bacillus spp. prevents biofilm formation of human bacterial pathogens. Appl. Microbiol. Biotechnol. 2009;83:541–553. doi: 10.1007/s00253-009-1987-7. [DOI] [PubMed] [Google Scholar]
- Rodrigues D.R., Briggs W., Duff A., Chasser K., Murugesan R., Pender C., Ramirez S., Valenzuela L., Bielke L. Cecal microbiome composition and metabolic function in probiotic treated broilers. PLoS One. 2020;15 doi: 10.1371/journal.pone.0225921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth N., Käsbohrer A., Mayrhofer S., Zitz U., Hofacre C., Domig K.J. The application of antibiotics in broiler production and the resulting antibiotic resistance in Escherichia coli: a global overview. Poult. Sci. 2019;98:1791–1804. doi: 10.3382/ps/pey539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rychlik I. Composition and function of chicken gut microbiota. Animals (Basel) 2020;10:103. doi: 10.3390/ani10010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu M.K., Swarnakumar N.S., Sivakumar K., Thangaradjou T., Kannan L. Probiotics in aquaculture: importance and future perspectives. Indian J. Microbiol. 2008;48:299–308. doi: 10.1007/s12088-008-0024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santacroce L.G., Charitos I.A., Bottalico L. A successful history: probiotics and their potential as antimicrobials. Expert. Rev. Anti. Infect. Ther. 2019;17:635–645. doi: 10.1080/14787210.2019.1645597. [DOI] [PubMed] [Google Scholar]
- Singh R.P. Glycan utilisation system in Bacteroides and Bifidobacteria and their roles in gut stability and health. Appl. Microbiol. Biotechnol. 2019;103:7287–7315. doi: 10.1007/s00253-019-10012-z. [DOI] [PubMed] [Google Scholar]
- Sokale A.O., Menconi A., Mathis G.F., Lumpkins B., Sims M.D., Whelan R.A., Doranalli K. Effect of Bacillus subtilis DSM 32315 on the intestinal structural integrity and growth performance of broiler chickens under necrotic enteritis challenge. Poult. Sci. 2019;98:5392–5400. doi: 10.3382/ps/pez368. [DOI] [PubMed] [Google Scholar]
- Tarradas J., Núria T., Esteve-Garcia E., Brufau J. The control of intestinal inflammation: a major objective in the research of probiotic strains as alternatives to antibiotic growth promoters in poultry. Microorganisms. 2020;8:148. doi: 10.3390/microorganisms8020148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tungland B. Short-chain fatty acid production and functional aspects on host metabolism. Hum. Microbio. Health Dis. 2018;2018:37–106. [Google Scholar]
- van der Hee B., Wells J.M. Microbial regulation of host physiology by short-chain fatty acids. Trends. Microbiol. 2021;29:700–712. doi: 10.1016/j.tim.2021.02.001. [DOI] [PubMed] [Google Scholar]
- Wang Y., Du W., Lei K., Wang B.K., Wang Y.Y., Zhou Y.S., Li W.F. Effects of dietary Bacillus licheniformis on gut physical barrier, immunity, and reproductive hormones of laying hens. Probio. Antimicrob. Proteins. 2017;9:292–299. doi: 10.1007/s12602-017-9252-3. [DOI] [PubMed] [Google Scholar]
- Wang Y.Y., Heng C.N., Zhou X.H., Cao G.T., Jiang L., Wang J.S., Li K.X., Wang D.C., Zhan X.A. Supplemental Bacillus subtilis DSM 29784 and enzymes, alone or in combination, as alternatives for antibiotics to improve growth performance, digestive enzyme activity, anti-oxidative status, immune response and the intestinal barrier of broiler chickens. Br. J. Nutr. 2020;125:494–507. doi: 10.1017/S0007114520002755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasti S., Sah N., Mishra B. Impact of heat Stress on poultry health and performances, and potential mitigation strategies. Animals (Basel) 2020;10:1266. doi: 10.3390/ani10081266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Yang S.J., Zi. Wang S., Cao Y., Zhao R., Li X.Y., Xing Y.W., Liu L.T. Effect of berberine on atherosclerosis and gut microbiota modulation and their correlation in high-fat diet-fed apoE-/- mice. Front. Pharmacol. 2020;11:223. doi: 10.3389/fphar.2020.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S., Lin Y.C., Zeng D., Zhou M.J., Zeng Y., Wang H.S., Zhou Y., Zhu H., Pan K.C., Jing B., Ni X.Q. Bacillus licheniformis normalize the ileum microbiota of chickens infected with necrotic enteritis. Sci. Rep. 2018;8:1744. doi: 10.1038/s41598-018-20059-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.M., Zhang L.L., Cao G.T., Feng J., Yue M., Xu. B. Dai Y.L., Han Q.J., Guo X.Q. Effects of dietary supplementation with essential oils and organic acids on the growth performance, immune system, faecal volatile fatty acids and microflora community in weaned piglets. J. Anim. Sci. 2019;97:133–143. doi: 10.1093/jas/sky426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaboli G., Huang X., Feng X., Ahn D.U. How can heat stress affect chicken meat quality? - a review. Poult. Sci. 2019;98:1551–1556. doi: 10.3382/ps/pey399. [DOI] [PubMed] [Google Scholar]
- Zhang J.Y., Lu T.D., Shen P.H., Sui Q.W., Zhong H., Liu J.B., Tong J., Wei Y.S. The role of substrate types and substrate microbial community on the fate of antibiotic resistance genes during anaerobic digestion. Chemosphere. 2019;229:461–470. doi: 10.1016/j.chemosphere.2019.05.036. [DOI] [PubMed] [Google Scholar]
- Zhang X.Y., Calvert R.A., Sutton B.J., Doré K.A. IgY: a key isotype in antibody evolution. Biol. Rev. Camb. Philos. Soc. 2017;92:2144–2156. doi: 10.1111/brv.12325. [DOI] [PubMed] [Google Scholar]
- Zhao B.Y., Houry W.A. Acid stress response in enteropathogenic gammaproteobacteria: an aptitude for survival. Biochem. Cell Biol. 2010;88:301–314. doi: 10.1139/o09-182. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Zeng D., Wang H.S., Qing X.D., Sun N., Xin J., Luo M., Khalique A., Pan K.C., Shu G., Jing B., Ni X.Q. Dietary probiotic Bacillus licheniformis H2 enhanced growth performance, morphology of small intestine and liver, and antioxidant capacity of broiler chickens against Clostridium perfringens-induced subclinical necrotic enteritis. Probio. Antimicrob. Proteins. 2020;12:883–895. doi: 10.1007/s12602-019-09597-8. [DOI] [PubMed] [Google Scholar]
- Zhou Y.T., Zhi F.C. Bacteroides lower level of in the gut microbiota is associated with inflammatory bowel disease: a meta-analysis. Biomed. Res. Int. 2016;2016 doi: 10.1155/2016/5828959. [DOI] [PMC free article] [PubMed] [Google Scholar]