Abstract
Regardless of whether antimicrobial drugs are administered to laying hens legally or illegally, residues of these drugs may be present in the eggs. Even if the eggs are not intended for human consumption, byproducts/biowaste, such as eggshells, may contain residues of the drugs used, which may pose a risk to human health and the environment. In the presented research, 2 different groups of laying hens received enrofloxacin (10 mg/kg body weight) and lincomycin (20 mg/kg body weight) once daily for 5 d. Eggs were collected daily and the concentration of enrofloxacin, its metabolite ciprofloxacin, and lincomycin residue in the eggshells, whole eggs, egg yolks, and egg whites were determined by ultra-high-performance liquid chromatography-tandem mass spectrometry. This study demonstrates the transfer of enrofloxacin, ciprofloxacin, and lincomycin into the eggshells and provides evidence for the distribution into the eggshells after administration of these drugs to laying hens. The enrofloxacin residues were detected in the eggshell for 10 d after cessation of treatment, ciprofloxacin and lincomycin were rapidly eliminated and 2 d after finish drugs administration they were no longer detected in the eggshell.
Key words: enrofloxacin, lincomycin, ciprofloxacin, eggshell, transfer
INTRODUCTION
It is estimated that global egg production will be 85 million tonnes in 2030 and 91 million tonnes in 2050 (Food and Agriculture Organization of the United Nations, 2018). Approximately 10% of the total egg weight is the eggshell (Schaafsma et al., 2000; Abdulrahman et al., 2014; Ketta and Tůamová, 2016; Laca et al., 2017), which means that egg production generates huge amounts of biowaste every year (Abdulrahman et al., 2014; Laca et al., 2017; Ito et al., 2020; Waheed et al., 2020). The European Commission regulations indicate that eggshell waste may be considered as hazardous waste (Mignardi et al., 2020; Owuamanam and Cree, 2020). Eggshells are also ranked by the US Environmental Protection Agency (EPA) as the 15th leading pollution problem in the food industry (Owuamanam and Cree, 2020; Waheed et al., 2020). Therefore, the need to manage agricultural waste in the form of eggshells is very important from an economic, environmental, human and animal safety point of view. Because eggshells contain as much as 94% calcium carbonate, they can be used in many industrial sectors (Schaafsma et al., 2000; Ketta and Tůamová, 2016). In medicine, eggshells can be utilized to produce hydroxyapatite or nan-calcium citrate, a material used in orthopedics (Abdulrahman et al., 2014; Faridi and Arabhosseini, 2018; Mignardi et al., 2020; Waheed et al., 2020), and to produce calcium supplements in the pharmaceutical industry (Schaafsma et al., 2000; Faridi and Arabhosseini, 2018; Waheed et al., 2019; Waheed et al., 2020). They can also be applied in the food industry as additives (a source of a well-absorbable form of calcium) in foods and beverages (Ray et al., 2017; Bartter et al., 2018). In agriculture, they are used as animal feed additives (Faridi and Arabhosseini, 2018; Waheed et al., 2020) and as a fertilizer for the cultivation of plants (King'ori, 2011; Faridi and Arabhosseini, 2018; Khairnar and Nair, 2019; Radha and Karthikeyan, 2019; Waheed et al., 2020). The possibility of using eggshell waste in this way requires ensuring the highest quality product, as many of these uses are directly related to human and animal health, and environmental safety.
In large-scale animal husbandry, sometimes it is necessary to use veterinary drugs (antimicrobials) to treat bacterial diseases. The use of antimicrobials in laying hens during the laying period is limited because many of them can be absorbed through the digestive tract of laying hens and transferred to the eggs (Kan and Petz, 2000). The EU has established maximum residue limits (MRLs) for only a few antimicrobials in eggs (European Parliament and the Council of the European Union, 2010). Also, the Food and Drug Administration (FDA) has approved a limited list of antimicrobials for laying hens in the United States (Marmulak et al., 2010). Despite regulations and controls, inappropriate use of antimicrobial substances in laying hens still occurs. From the European Food Safety Authority (EFSA) reports for 2010–2018, the number of non-compliant results in egg samples for the determination of antimicrobial substances (group B1) was 93, the most commonly detected compound being enrofloxacin (ENR) n = 34 (Report for 2010 - 2018 on the results from the monitoring of veterinary medicinal product residues and other substances in live animals and animal products, European Food Safety Authority, 2012, European Food Safety Authority, 2013, European Food Safety Authority, 2014, European Food Safety Authority, 2015, European Food Safety Authority, 2016, European Food Safety Authority, 2017, European Food Safety Authority, 2018, European Food Safety Authority, 2019, European Food Safety Authority, 2020). Over the past 5 years, ENR has also been the most frequently reported drug in the Rapid Alert System for Food and Feed (RASFF) system. The antibiotic residues in foods and food products of animal origin may lead to adverse health effects for consumers such as allergic reactions, immunopathological effects, nephropathy, hepatotoxicity, reproductive disorders, and even mutagenicity or carcinogenicity and antimicrobial resistance (Darko et al., 2015; Tadesse and Temesgen, 2017; Bacanlı and Başaran, 2019). In 2013, the Center for Disease Control and Prevention (CDC) reported that in the United States, about 2,000,000 people were infected with antibiotic-resistant bacteria, resulting in 23,000 deaths (CDC, 2013). It is predicted that if we do not change anything, for the same reason, up to 10,000,000 people could die by 2050 (Li et al., 2019).
The aim of this study was to investigate the possibility of transfer of 2 selected antimicrobials (ENR and lincomycin (LIN)) into the eggshell and to determine their residue depletion in various egg components (eggshell, whole egg, white, and yolk). ENR was chosen because it is not approved in the EU and is banned in the United States for laying hens (Marmulak et al., 2010), but is still the most commonly detected antibiotic in eggs in the European Union (EU) (Report for 2010 - 2018 on the results from the monitoring of veterinary medicinal product residues and other substances in live animals and animal products, European Food Safety Authority, 2012, European Food Safety Authority, 2013, European Food Safety Authority, 2014, European Food Safety Authority, 2015, European Food Safety Authority, 2016, European Food Safety Authority, 2017, European Food Safety Authority, 2018, European Food Safety Authority, 2019, European Food Safety Authority, 2020). Moreover, genomic studies suggest that quinolone resistance was a key factor in the evolution of hospital methicillin-resistant Staphylococcus aureus (MRSA) (Laxminarayan et al., 2013). In contrast, LIN is approved in the EU for use in laying hen therapy, and the MRL in eggs is 50 µg/kg (European Parliament and the Council of the European Union, 2010).
The literature contains studies on ENR residues in eggs (Gorla et al., 1997; Lolo et al., 2005; Huang et al., 2006; Bogialli et al., 2009), no such information was found for LIN. In the presented study, we identified that chicken eggshells can be a source of antibiotic residues. This research may have implications for preventing the phenomenon of increasing antibacterials resistance. In addition, continuing to improve our understanding of new sources of human, animal, and environmental exposure to antibiotic residues is of great importance to human safety.
MATERIAL AND METHODS
Animals and Experimental Design
Twenty-two domestic hens (Rosa line), aged 25 to 30 wk were used in this study. Their body weights (BW) ranged from 1.7 to 2.2 kg. They were housed under conventional conditions of ventilation, temperature (20°C) and light. The animals were acclimatized for 1 wk to the new environment: each group was housed in a 20 m2 indoor shelter. The birds were examined to be clinically healthy based on blood analysis (complete blood count) and by daily observation of their behavior and appetite. These observations were prepared by licensed veterinary personnel. The hens were fed twice per day (the first portion of the feed was given 2 h, the second one 10 h after the drug was administered) with a pelleted diet (Feed for laying hens, De Heus) and fresh green forage. Water was given ad libitum. Before the experiment, the birds were not treated with any drugs. The study was registered and approved by the Local Ethical Committee in Lublin (Resolution No. 77/2020).
Before the beginning of the study, the hens were randomly divided into 3 groups: group 1 animals were treated (n = 10) with ENR, group 2 (n = 10) with LIN, and group 3 were control animals (n = 2) not receiving any medication. Each hen received a ring with an assigned ID code that was placed on right leg for easier and more efficient identification.
The animals in experimental group 1 were administrated per os, individually, by crop gavage for 5 d with the veterinary medicinal product Enrofloxan 10% (Biofaktor, Poland) at a dose of 10 mg of ENR/kg BW/day. Experimental group 2 was treated in the same way for 5 d with the veterinary medicinal product Lincofort (Biofaktor, Poland) at a dose of 20 mg of LIN/kg BW/day. The animals in the control group (group 3) receive fresh drinking water via the same method.
Twenty-four hours after the first dose administration (d 1, administration period), egg collection was began and continued for another 19 (group 1) and 11 (group 2) d. Eggs were collected daily, and the number of eggs was noted. Nine to 12 eggs were collected each day in groups 1 and 2. At least 6 eggs from each group were analyzed separately for the determination of drug residues in the eggshell and whole egg. The remaining eggs (n = 3–4) were separated for egg yolk and egg white analysis, and all samples were stored at −18°C until analysis.
Chemicals and Reagents
Analytical standard ENR, ciprofloxacin (CIP) and LIN, and internal standard ciprofloxacin-d8 (CIP-d8) and sulfaphenazole (SFF) were obtained from Sigma-Aldrich (St. Louis, MO). All reagents for liquid chromatography-tandem mass spectrometry: methanol and acetonitrile were obtained from J. T. Baker (the Netherlands), formic acid was from Fluka (Buchs, Switzerland), – were at least HPLC grade. Water was deionized (>18 MΩcm-1) by a Millipore system (Bedford, MA).
LC-MS/MS Analysis
The LC-MS/MS analysis for all matrices was performed using the analytical method previously described by Gbylik-Sikorska et al. (2021) (). The LC-MS/MS quantitation was determined using a Nexera X2 ultra high performance liquid chromatography-tandem mass spectrometer (UHPLC–MS/MS) (Shimadzu, Japan) system connected to a QTRAP 4500 triple-quadrupole mass spectrometer (Sciex, Framingham, MA) with Analyst 1.6.3 software (Sciex) controlling the system and processing the data. The chromatography separation was performed in a Luna Omega 1.6 µm Polar C18 10 column (100 × 2.1 mm, Phenomenex, Torrance, CA) and the mobile phase composition was a mixture of 0.075% formic acid and 0.05% formic acid in acetonitrile.
Sample Preparation
The concentrations of ENR, CIP, and LIN in the eggshells were determined using a previously reported sample preparation procedure (Gbylik-Sikorska et al., 2021). Sample preparation of the whole egg, egg yolk, and egg white was performed using a method described by Błądek et al. 2012 () which was modified in-house. One gram of previously homogenized sample (whole egg/egg yolk/egg white) was placed in a 10 mL polypropylene centrifuge tube, then SFF (IS) was added and vortexed for 15 s and left for 15 min. After incubation, 1 mL of 0.02M of oxalic acid, pH = 4; 0.5 mL of 0.1 M of EDTA disodium salt dihydrate and 8 mL of acetonitrile were added to the centrifuge tubes and the samples were vortexed for the 30 s. The samples were centrifuged at 2930 × rcf for 10 min at 4°C. Next, 6 mL of supernatants were evaporated under nitrogen gas at 45 ± 5°C. The dry residues were reconstituted in 1 mL of ultrapure water and transferred to a 1.5 mL centrifuge tube, centrifuged at 14,500 × rcf for 20 min at room temperature and filtered through 0.22 µm PVDF filters into analytical vials.
Validation Procedure for the Determination of the ENR, CIP and LIN in Whole Egg, Egg Yolk, and Egg White
The linearity, precision (repeatability and within-laboratory reproducibility), recovery, limit of detection (LOD), and quantification (LOQ) of the method were evaluated according to Commission Decision 202/657/EC (European Commission, 2002). Commission Decision 2002/657/EC’) and EUR 28099 EN (Wenzl et al., 2016). The linearity of the method (determination coefficient, r2) was validated by matrix-match calibration curves, which were prepared using blank whole egg/egg yolk/egg white samples spiked with 8 different concentration levels (1, 10, 50, 100, 250, 500, 1,000, and 2,000 µg/kg). The repeatability was calculated after analysis of 6 whole egg/egg yolk/egg white samples spiked at 3 concentration levels: 1, 10, and 50 µg/kg by the same operator on the same day with the same instrument. The reproducibility was determined by another 2 sets of 6 spiked samples prepared in the same way as for the repeatability and analyzed on 2 different days by different operators with the same instrument. The average recovery was carried out by analyzing samples spiked at the same concentration levels as for the precision experiment. The LOD was determined at the signal-to-noise ratio (S/N = 3), and the LOQ was calculated as the lowest validated concentration with S/N > 10.
The ENR, CIP and LIN concentrations in the collected samples were determined using the fully validated analytical method. All matrix-matched calibration curves showed good linearity (r2 >0.996). The coefficients of variation, CVs, ranged between 5.3 and 10.0% for repeatability and between 11.6 and 14.8% for within-laboratory reproducibility. The average percentage recoveries were in the range of 86.0 to 111.0%. The LOQ was 1.0 µg/kg, the LOD was 0.50 µg/kg in each matrix, respectively. Validation results for each analyte are presented in Table 1. The results indicated that the method was suitable to be used to quantify ENR, CIP, and LIN concentration in various egg matrices.
Table 1.
Validation parameters of the method for the determination of the enrofloxacin (ENR), ciprofloxacin (CIP) and lincomycin (LIN) in whole eggs/egg yolks/egg whites.
| Analyte | Matrix | Repeatability*, (CV,%) |
Within-lab Reproducibility*, (CV,%) |
LOQ (µg/kg) |
LOD (µg/kg) |
Recovery* (%) |
|---|---|---|---|---|---|---|
| ENR | Whole egg | 7.3 ± 1.9 | 12.2 ± 4.3 | 1.00 | 0.50 | 102.4 ± 5.6 |
| Egg yolk | 10.0 ± 2.4 | 14.3 ± 5.5 | 1.00 | 0.50 | 89.0 ± 4.1 | |
| Egg white | 6.3 ± 2.3 | 12.9 ± 4.1 | 1.00 | 0.50 | 96.0 ± 4.4 | |
| CIP | Whole egg | 8.8 ± 2.5 | 13.1 ± 4.2 | 1.00 | 0.50 | 91.6 ± 5.2 |
| Egg yolk | 9.7 ± 2.7 | 14.8 ± 6.1 | 1.00 | 0.50 | 111.0 ± 5.8 | |
| Egg white | 7.1 ± 1.8 | 11.9 ± 5.3 | 1.00 | 0.50 | 86.0 ± 3.8 | |
| LIN | Whole egg | 6.4 ± 1.7 | 13.3 ± 3.8 | 1.00 | 0.50 | 90.7 ± 4.1 |
| Egg yolk | 9.4 ± 2.1 | 14.4 ± 5.7 | 1.00 | 0.50 | 87.0 ± 4.4 | |
| Egg white | 5.3 ± 1.9 | 11.6 ± 5.1 | 1.00 | 0.50 | 89.0 ± 5.2 |
Abbreviations: LOD, limit of detection; LOQ, limit of quantification.
Average of 3 validation levels with standard deviation (± SD).
RESULTS
Depletion of ENR, CIP and LIN Residue in Whole Egg, Egg Yolk, EGG White, and Eggshell
The ENR, CIP, and LIN concentrations in the collected samples: whole egg, egg yolk, egg white, and eggshell were calculated using the equation from the regression analysis of the matrix-matched calibration curve, individual for each matrix. The concentration range was adjusted to avoid extrapolation.
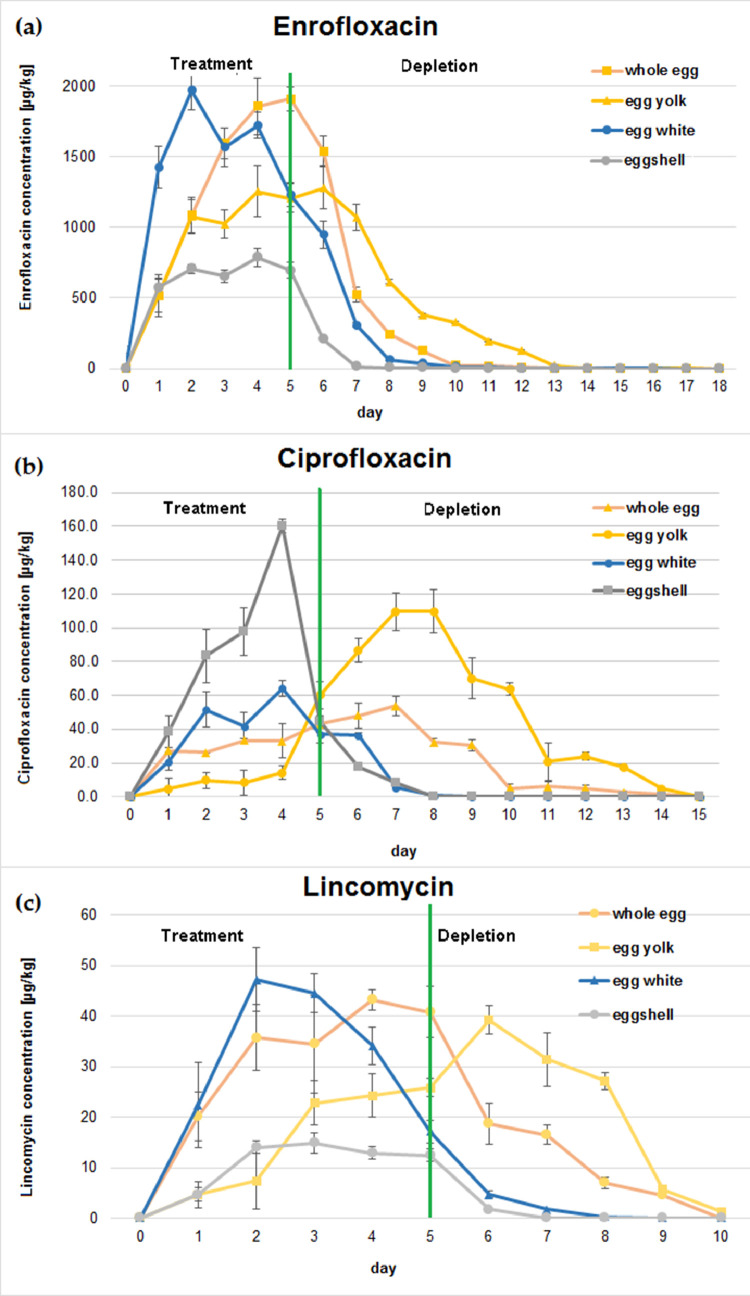
The residues and depletion of ENR, CIP and LIN in the eggshell, whole egg, egg yolk, and egg white after multiple oral doses (ENR – 10 mg/kg BW and LIN – 20 mg/kg BW daily for 5 consecutive days) of veterinary medicines to laying hens were determined. The concentrations (mean ± SD) of each analyte in the 4 matrices at different time points are listed in Table 2. The residue depletion curves are shown in Figure 1. All 3 analytes were determined in eggshell at concentrations ranging from 1.7 to 788 µg/kg for ENR, 8.3 to 160 µg/kg for CIP and 1.8 to 14.9 µg/kg for LIN. These results confirm the possibility of transfer of drug residues (ENR and LIN) administered to laying hens into the eggshell. The maximum concentration of ENR in the whole egg, egg yolk, egg white, and eggshell was detected on the fifth, sixth, second, and fourth day of sample collection, respectively. The highest concentration of ENR was detected in egg white and was 35% and 60% higher in comparison to egg yolk and eggshell, respectively. ENR residues persisted longest in whole egg and egg yolk – for 17 d. It was detected in egg white for 12 d and in the eggshell for 10 d after cessation of treatment. In contrast to ENR, the highest concentration of CIP was determined in the eggshell, it reached the maximum mean concentration (160 ± 14 µg/kg) on the fourth day of drug administration and was 40% higher than the maximum mean concentration detected in egg yolk. CIP was detected in eggshell on the first day after the start of ENR administration, but was rapidly eliminated by the fourth day of the withdrawal period and was no longer detected in the eggshell. It was equally rapidly eliminated in the egg white, where it was detected during the same period as in the eggshell. In whole egg and egg yolk, CIP was present for 10 d after the end of ENR administration, the maximum concentration was achieved on the seventh and eighth day after the beginning of treatment, respectively. LIN was detected in all matrices at concentrations below the egg MRL (50 µg/kg), with a maximum concentration of 47.2 ± 8.5 µg/kg reached on the second day of drug administration in egg white. Its depletion in egg matrices was fastest compared to ENR and CIP, and it persisted longest in egg yolk, where it was detected 6 d after the end of drug administration.
Table 2.
The concentration of enrofloxacin (ENR), ciprofloxacin (CIP) and lincomycin (LIN) in whole egg, egg white, egg yolk, and eggshell after multiple oral administration of ENR and LIN to laying hens.
| Concentration ± SD* µg/kg |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ENR |
CIP |
LIN |
||||||||||||
| Period | Time (d) | Eggshell | Whole egg | Egg yolk | Egg white | Eggshell | Whole egg | Egg yolk | Egg white | Eggshell | Whole egg | Egg yolk | Egg white | |
| Administration | 0 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | |
| 1 | 573 ± 52 | 518 ± 86 | 517 ± 170 | 1423 ± 160 | 38.6 ± 15 | 27.1 ± 1.3 | 4.7 ± 2.0 | 21.0 ± 2.4 | 4.6 ± 0.9 | 20.1 ± 2.5 | 4.7 ± 0.5 | 22.4 ± 5.6 | ||
| 2 | 705 ± 63 | 1087 ± 119 | 1075 ± 150 | 1970 ± 150 | 83.4 ± 9.5 | 26.2 ± 1.6 | 9.7 ± 6.2 | 51.5 ± 5.7 | 14.0 ± 2.5 | 35.7 ± 4.8 | 7.4 ± 1.3 | 47.2 ± 8.5 | ||
| 3 | 655 ± 34 | 1593 ± 129 | 1023 ± 120 | 1566 ± 140 | 97.6 ± 15 | 33.4 ± 0.6 | 8.3 ± 4.6 | 41.7 ± 10 | 14.9 ± 1.3 | 34.5 ± 6.6 | 22.8 ± 5.5 | 44.4 ± 6.3 | ||
| 4 | 788 ± 45 695 ± 63 |
1856 ± 109 | 1253 ± 97 | 1721 ± 90 | 203 ± 14 | 32.9 ± 1.0 | 14.3 ± 7.2 | 64.0 ± 4.7 | 12.9 ± 2.0 | 43.2 ± 9.7 | 24.2 ± 4.4 | 34.1 ± 3.8 | ||
| Depletion | 5 | 1910 ± 203 | 1207 ± 180 | 1230 ± 85 | 24.5 ± 4.1 | 43.1 ± 10 | 60.1 ± 4.2 | 37.1 ± 5.1 | 12.4 ± 1.3 | 40.8 ± 1.9 | 25.8 ± 4.3 | 17.2 ± 3.7 | ||
| 6 | 209 ± 54 | 1540 ± 86 | 1278 ± 100 | 946 ± 96 | 7.9 ± 2.8 | 48.0 ± 5.7 | 86.4 ± 8.2 | 36.4 ± 1.5 | 1.8 ± 1.2 | 18.7 ± 5.1 | 39.2 ± 1.8 | 4.6 ± 2.3 | ||
| 7 | 15.5 ± 3.7 | 523 ± 107 | 1073 ± 150 | 310 ± 15 | <LOQ | 53.8 ± 7.7 | 109 ± 7.0 | 5.5 ± 0.2 | <LOQ | 16.5 ± 3.9 | 31.4 ± 2.8 | 1.8 ± 0.7 | ||
| 8 | 7.5 ± 3.2 | 246 ± 49 | 615 ± 94 | 62.6 ± 11 | <LOQ | 32.3 ± 5.5 | 110 ± 11 | <LOQ | <LOQ | 7.0 ± 1.9 | 27.1 ± 5.3 | <LOQ | ||
| 9 | 5.2 ± 2.1 | 128 ± 19 | 381 ± 17 | 40.2 ± 5.8 | <LOQ | 30.6 ± 2.5 | 69.9 ± 13 | <LOQ | <LOQ | 4.5 ± 1.1 | 5.7 ± 1.7 | <LOQ | ||
| 10 | 2.8 ± 0.7 | 24.8 ± 5.6 | 329 ± 12 | 13.8 ± 4.1 | <LOQ | 4.9 ± 3.5 | 63.6 ± 12 | <LOQ | <LOQ | <LOQ | 1.3 ± 0.6 | <LOQ | ||
| 11 | 2.8 ± 0.7 | 19.1 ± 5.2 | 195 ± 7.4 | 12.2 ± 0.6 | <LOQ | 6.0 ± 2.5 | 20.9 ± 3.6 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | ||
| 12 | 2.9 ± 0.4 | 11.3 ± 6.3 | 126 ± 8.3 | 4.3 ± 1.2 | <LOQ | 5.1 ± 2.8 | 24.0 ± 11 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | ||
| 13 | 1.9 ± 0.5 | 4.5 ± 2.3 | 22.0 ± 3.5 | 3.2 ± 0.6 | <LOQ | 2.7 ± 1.9 | 17.7 ± 2.3 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | ||
| 14 | 1.7 ± 0.5 | 3.0 ± 0.6 | 2.3 ± 0.5 | 3.5 ± 0.4 | <LOQ | 1.4 ± 0.7 | 4.7 ± 1.4 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | ||
| 15 | <LOQ | 2.5 ± 0.1 | 2.5 ± 0.5 | 2.4 ± 1.0 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | ||
| 16 | <LOQ | 1.5 ± 0.1 | 1.8 ± 0.2 | 2.0 ± 0.3 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | ||
| 17 | <LOQ | 1.4 ± 0.1 | 1.4 ± 0.1 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | ||
| 18 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | ||
Abbreviation: LOD, limit of quantification.
Mean concentration ± SD, with n = 6–8 (whole egg, eggshell), n = 3–4 (egg white, egg yolk).
Figure 1.
Residue depletion curves of enrofloxacin (ENR) (A), ciprofloxacin (CIP) (B), and lincomycin (LIN) (C) in whole eggs, egg whites, egg yolks, and eggshells.
DISCUSSION
The use of antimicrobial drugs for the treatment of laying hens for table eggs is very restricted and the number of drugs approved for use is severely limited. In some cases, drugs approved for use in broiler chickens are also used in laying hens that are not intended for human consumption but produce large amounts of waste or by-products (e.g., eggshells) which must be properly managed. Moreover, there are still incidents of the illegal use of drugs whose residues in eggs may pose a risk to the consumer health. Therefore, it is important to investigate the possibility of transferring veterinary medicines, especially antimicrobials, into eggshells, which can be used in various industrial fields and could be another source of antibiotic residues. This study demonstrates the transfer of selected antimicrobials into eggshells. The results provide important evidence for the distribution of ENR, its metabolite CIP, and LIN into the eggshell after administration of the drugs to laying hens. The residue depletion-time of these compounds in whole egg, egg yolk, egg white, and eggshell was also investigated and compared.
The present research indicates that the highest concentration of ENR was obtained in egg white, while the highest concentration of CIP was detected in egg yolk. The finding is similar to earlier results demonstrated by Lolo and Huang (Lolo et al., 2005; Huang et al., 2006). On the other hand, Gorla with co-authors detected trace amounts of CIP only in egg whites (Gorla et al., 1997). This phenomenon can be explained by the fact that CIP is less protein-bound than ENR, because, even though it is more soluble in water than ENR and has lower lipophilicity, it shows a much higher concentration in egg yolk than in white (Gorla et al., 1997; Kan and Petz, 2000; Davis et al., 2007). Lolo et al. (2005) also considered the process of ENR metabolism that occurs in the liver, which produces lipoproteins (a major component of the yolk) as a probable explanation. In our study, both ENR and CIP were present in all matrices 24 h after the first dose of the drug, which was in contrast to the results obtained by Lolo (Lolo et al., 2005), but similar to the results of other authors (Gorla et al., 1997; Huang et al., 2006). However, there is no information in the literature on the transfer of ENR and CIP residues into eggshells and no studies comparing ENR and CIP residues in this matrix have been conducted. Our results indicate that ENR was detected in eggshell 24 h after the start of animal treatment, and its mean concentration was between 573 ± 52 and 788 ± 45 µg/kg for 5 d, then rapidly decreased and again slowed down for another 5 d until d 15 when no residue was detected above the LOQ. The highest concentration of ENR in eggshell was about 60% lower than in egg white. CIP residues were also present in eggshell, but the depletion period was much faster compared to ENR – CIP persisted for only 2 d after the end of treatment. It is interesting to observe that the highest concentration of CIP was observed in the eggshell and was more than 30% higher compared to the maximum concentration in the yolk. Distribution of the drug into the eggshell can take place in several sections of the shell gland. One of them is the first part of the shell gland (uterus), where the process of calcium transfer to the eggshell membranes begins. It can also occur during a process called “plumping,” which stimulates a phase of rapid calcification (300 mg/h) (Roberts, 1995). The transfer of ENR and CIP into eggshell may also be related to their ability to form complexes with metal ions, including calcium (Ca2+, divalent cation) (Uivarosi, 2013). Calcium carbonate, which is the primary component of eggshell, contains about 40% Ca2+ ions (Brennan et al., 1991). Binding to calcium was also one of the physicochemical properties of fluoroquinolones associated with their penetration into human bones (Landersdorfer et al., 2009).
To date, no literature is available on the depletion of LIN residue in eggs after oral multiple administration to laying hens. The results of our experiment indicated that LIN residues in all analyzed matrices were detected 1 d after starting the medication. The mean maximum concentration (47.2 ± 8.5) was observed in egg white on the third day of the drug administration. In egg yolk, the maximum concentration was determined 48 h after the end of LIN administration. The depletion period of LIN residues in egg yolk was the longest and took 6 d; it was 3 d longer than in egg white, in which the elimination of LIN concentration was rapid (3 d) after cessation of the medication. In eggshell, LIN concentration reached a plateau phase on the third day of drug administration and was maintained until the first day after drug cessation. Two days later, it was not detected above the LOQ of the method. Pokrant et al. (2019) reported that the depletion time for LIN residue concentrations in feathers was set at 98 d, but in chicken muscle, its residues could only be detected for 4 d after ceasing treatment (50 mg/kg BW for 7 d). Our results are much closer to those obtained in tissues, which is quite understandable since the process of egg formation takes about 24 h. The ability of LIN transfer into the eggshell may follow the same process as that of ENR and CIP. The LIN residues were also present in human bones after treatment (Nielsen et al., 1976).
This study provides valuable data to add to the current state of knowledge on the residue depletion of veterinary drugs in eggs, especially for LIN. It also provides very important evidence that eggshells, a biowaste or byproduct which can be used in the food and pharmaceutical industries, animal husbandry and plant breeding, can be a source of veterinary drug residues, including antimicrobials that can cause the growth of drug-resistant bacteria. This research suggests that the presence of veterinary drugs in eggshells should be monitored, and further research is needed to investigate the possibility of other drugs transferring into this matrix. Conducting further research and attention to this topic could have important implications for human and animal health, and the environment.
ACKNOWLEDGMENTS
The study was registered and approved by the Local Ethics Commission in Lublin (Ethic Commission Opinion No. 77/2020).
The authors would like to thank Ms. Magdalena Biliecka and Mrs. Aleksandra Kuśmierz for their help in preparing the samples for LC-MS/MS analysis.
DISCLOSURES
The authors declare no financial or personal conflicts of interest.
REFERENCES
- Abdulrahman I., Tijani H.I., Mohammed B.A., Saidu H., Yusuf H., Ndejiko Jibrin M., Mohammed S. From garbage to biomaterials: an overview on egg shell based hydroxyapatite. J. Mater. 2014;2014:1–6. [Google Scholar]
- Bacanlı M., Başaran N. Importance of antibiotic residues in animal food. Food Chem. Toxicol. 2019;125:462–466. doi: 10.1016/j.fct.2019.01.033. [DOI] [PubMed] [Google Scholar]
- Bartter J., Diffey H., Yeung Y.H., O'Leary F., Häsler B., Maulaga W., Alders R. Use of chicken eggshell to improve dietary calcium intake in rural sub-Saharan Africa. Matern. Child Nutr. 2018;14:1–10. doi: 10.1111/mcn.12649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Błądek T., Posyniak A., Gajda A., Gbylik M., Żmudzki J. Multi-class procedure for analysis of antibacterial compounds in eggs by liquid chromatography-tandem mass spectrometry. Bull. Vet. Inst. Pulawy. 2012;56:321–327. [Google Scholar]
- Bogialli S., D'Ascenzo G., Di Corcia A., Laganà A., Tramontana G. Simple assay for monitoring seven quinolone antibacterials in eggs: extraction with hot water and liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. A. 2009;1216:798–800. doi: 10.1016/j.chroma.2008.11.070. [DOI] [PubMed] [Google Scholar]
- Brennan M.J., Duncan W.E., Wartofsky L., Butler V.M., Wray H.L. In vitro dissolution of calcium carbonate preparations. Calcif. Tissue Int. 1991;49:308–312. doi: 10.1007/BF02556251. [DOI] [PubMed] [Google Scholar]
- Darko G., Mensah J.K., Dapaah S.S., Odei J. Estimated dietary exposure to veterinary residues in chicken and eggs. Int. J. Food Contam. 2015;2:16. [Google Scholar]
- Davis J.L., Foster D.M., Papich M.G. Pharmacokinetics and tissue distribution of enrofloxacin and its active metabolite ciprofloxacin in calves. J. Vet. Pharmacol. Ther. 2007;30:564–571. doi: 10.1111/j.1365-2885.2007.00914.x. [DOI] [PubMed] [Google Scholar]
- European Food Safety Authority Report for 2018 on the results from the monitoring of veterinary medicinal product residues and other substances in live animals and animal products. EFSA Support Publ. 2020;17:1775E. [Google Scholar]
- European Food Safety Authority Report for 2012 on the results from the monitoring of veterinary medicinal product residues and other substances in live animals and animal products. 2014. EFSA Support. Publ. 2014;11:EN–540. [Google Scholar]
- European Food Safety Authority Report for 2013 on the results from the monitoring of veterinary medicinal product residues and other substances in live animals and animal products. EFSA Support. EFSA Support. Publ. 2015;12 [Google Scholar]
- European Food Safety Authority Report for 2014 on the results from the monitoring of veterinary medicinal product residues and other substances in live animals and animal product. EFSA Support Publ. 2016;13:EN–923. [Google Scholar]
- European Food Safety Authority Report for 2015 on the results from the monitoring of veterinary medicinal product residues and other substances in live animals and animal products. EFSA Support. Publ. 2017;14:1150E. [Google Scholar]
- European Food Safety Authority Report for 2016 on the results from the monitoring of veterinary medicinal product residues and other substances in live animals and animal products. EFSA Support Publ. 2018;15 [Google Scholar]
- European Food Safety Authority Report for 2017 on the results from the monitoring of veterinary medicinal product residues and other substances in live animals and animal products. EFSA Support Publ. 2019;16:1578E. [Google Scholar]
- European Food Safety Authority Report for 2010 on the results from the monitoring of veterinary medicinal product residues and other substances in live animals and animal products. EFSA Support Publ. 2012;9:212E. [Google Scholar]
- European Food Safety Authority Report for 2011 on the results from the monitoring of veterinary medicinal product residues and other substances in live animals and animal products. EFSA Support Publ. 2013;10:363E. [Google Scholar]
- European Parliament and the Council of the European Union COMMISSION REGULATION (EU) No 37/2010. Off. J. Eur. Union. 2010 Accessed March 2021. https://eur-lex.europa.eu/eli/reg/2010/37(1)/oj. [Google Scholar]
- Faridi H., Arabhosseini A. Application of eggshell wastes as valuable and utilizable products: a review. Res. Agric. Eng. 2018;64:104–114. [Google Scholar]
- Gbylik-Sikorska M., Gajda A., Nowacka-Kozak E., Łebkowska-Wieruszewska B., Posyniak A. Multi-class procedure for analysis of 50 antibacterial compounds in eggshells using ultra-high-performance liquid chromatography–tandem mass spectrometry. Molecules. 2021;26 doi: 10.3390/molecules26051373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorla N., Chiostri E., Ugnia L., Weyers A., Giacomelli N., Davicino R., García Ovando H. HPLC residues of enrofloxacin and ciprofloxacin in eggs of laying hens. Int. J. Antimicrob. Agents. 1997;8:253–256. doi: 10.1016/s0924-8579(97)00018-6. [DOI] [PubMed] [Google Scholar]
- Huang J.-F., Lin B., Yu Q.-W., Feng Y.-Q. Determination of fluoroquinolones in eggs using in-tube solid-phase microextraction coupled to high-performance liquid chromatography. Anal. Bioanal. Chem. 2006;384:1228–1235. doi: 10.1007/s00216-005-0270-8. [DOI] [PubMed] [Google Scholar]
- Ito T., Itokawa H., Miyaki T., Kamimura M., Hamano M., Nakata M., Saikawa Y. Inositol tetrakisphosphate from chicken eggshell. Tetrahedron. 2020;76:1–6. [Google Scholar]
- Kan C.A., Petz M. Residues of veterinary drugs in eggs and their distribution between yolk and white. J. Agric. Food Chem. 2000;48:6397–6403. doi: 10.1021/jf000145p. [DOI] [PubMed] [Google Scholar]
- Ketta M., Tůamová E. Eggshell structure, measurements, and quality-affecting factors in laying hens: a review. Czech J. Anim. Sci. 2016;61:299–309. [Google Scholar]
- Khairnar M.D., Nair S.S. Vol. 17. Proceedings of International Conference on Sustainable Development (ICSD 2019), In Association with Novateur Publications IJIERT-ISSN No: 2394-3696 ISBN. No. 978-93-87901-05-6; 2019. Study on eggshell and fruit peels as a fertilizer; pp. 25–27. [Google Scholar]
- King'ori A.M. A review of the uses of poultry eggshells and shell membranes. Int. J. Poult. Sci. 2011;10:908–912. [Google Scholar]
- Laca A., Laca A., Díaz M. Eggshell waste as catalyst: a review. J. Environ. Manage. 2017;197:351–359. doi: 10.1016/j.jenvman.2017.03.088. [DOI] [PubMed] [Google Scholar]
- Landersdorfer C.B., Bulitta J.B., Kinzig M., Holzgrabe U., Sörgel F. Penetration of antibacterials into bone. Clin. Pharmacokinet. 2009;48:89–124. doi: 10.2165/00003088-200948020-00002. [DOI] [PubMed] [Google Scholar]
- Laxminarayan R., Duse A., Wattal C., Zaidi A.K.M., Wertheim H.F.L., Sumpradit N., Vlieghe E., Hara G.L., Gould I.M., Goossens H., Greko C., So A.D., Bigdeli M., Tomson G., Woodhouse W., Ombaka E., Peralta A.Q., Qamar F.N., Mir F., Kariuki S., Bhutta Z.A., Coates A., Bergstrom R., Wright G.D., Brown E.D., Cars O. Antibiotic resistance-the need for global solutions. Lancet Infect. Dis. 2013;13:1057–1098. doi: 10.1016/S1473-3099(13)70318-9. [DOI] [PubMed] [Google Scholar]
- Li R., Jay J.A., Stenstrom M.K. Fate of antibiotic resistance genes and antibiotic-resistant bacteria in water resource recovery facilities. Water Environ. Res. 2019;91:5–20. doi: 10.1002/wer.1008. [DOI] [PubMed] [Google Scholar]
- Lolo M., Pedreira S., Fente C., Vázquez B.I., Franco C.M., Cepeda A. Study of enrofloxacin depletion in the eggs of laying hens using diphasic dialysis extraction/purification and determinative HPLC-MS analysis. J. Agric. Food Chem. 2005;53:2849–2852. doi: 10.1021/jf048015u. [DOI] [PubMed] [Google Scholar]
- Marmulak T., Tell L.A., Gehring R., Baynes R.E., Vickroy T.W., Riviere J.E. Egg residue considerations during the treatment of backyard poultry. J. Am. Vet. Med. Assoc. 2010;247:1388–1395. doi: 10.2460/javma.247.12.1388. [DOI] [PubMed] [Google Scholar]
- Mignardi S., Archilletti L., Medeghini L., De Vito C. Valorization of eggshell biowaste for sustainable environmental remediation. Sci. Rep. 2020;10:1–10. doi: 10.1038/s41598-020-59324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen M.L., Hansen I., Nielsen J.B. The penetration of lincomycin into normal human bone: determinations of penetration into compact bone, spongy bone and bone marrow. Acta Orthop. Scand. 1976;47:267–270. doi: 10.3109/17453677608991989. [DOI] [PubMed] [Google Scholar]
- Owuamanam S., Cree D. Progress of bio-calcium carbonate waste eggshell and seashell fillers in polymer composites: a review. J. Compos. Sci. 2020;4:70. [Google Scholar]
- Pokrant E., Maddaleno A., Lobos R., Trincado L., Lapierre L., San Martín B., Cornejo J. Assessing the depletion of lincomycin in feathers from treated broiler chickens: a comparison with the concentration of its residues in edible tissues. Food Addit. Contam. Part A. 2019;36:1647–1653. doi: 10.1080/19440049.2019.1662952. [DOI] [PubMed] [Google Scholar]
- Radha T., Karthikeyan G. Hen eggshell waste as fertilizer for the growth of Phaseolus vulgaris (cow pea seeds) Res. J. Life Sci. Bioinform. Pharm. Chem. Sci. 2019;5:398–406. [Google Scholar]
- Ray S., Barman A.K., Roy P.K., Singh B.K. Chicken eggshell powder as dietary calcium source in chocolate cakes. Pharma Innov. J. 2017;6:1–4. [Google Scholar]
- Food and Agriculture Organization of the United Nations. 2018. The future of food and agriculture alternative pathways to 2050 supplementary material. Accessed March 2021. http://www.fao.org/global-perspectives-studies/resources/detail/en/c/1157074/.
- European Commission. 2002. Commission Decision 2002/657/EC. Accessed March 2021. https://op.europa.eu/en/publication-detail/-/publication/ed928116-a955-4a84-b10a-cf7a82bad858/language-en#.
- CDC. 2013. Antibiotic Resistance Threats in the United States, 2013. Accessed March 2021. https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwirrpjJ_ubwAhVqlYsKHRNRD0EQFjABegQIAxAD&url=https%3A%2F%2Fwww.cdc.gov%2Fdrugresistance%2Fpdf%2Far-threats-2013-508.pdf&usg=AOvVaw0QbiCErJYHrlFhCqVmwlnh.
- Roberts J.R..B.C.E. The ultrastructure of avian egg shells. Poult. Sci. Rev. 1995;5:245–272. [Google Scholar]
- Schaafsma A., Pakan I., Hofstede G.J.H., Muskiet F.A.J., Van Der Veer E., De Vries P.J.F. Mineral, amino acid, and hormonal composition of chicken eggshell powder and the evaluation of its use in human nutrition. Poult. Sci. 2000;79:1833–1838. doi: 10.1093/ps/79.12.1833. [DOI] [PubMed] [Google Scholar]
- Tadesse T., Temesgen T. Public health impacts of antibiotic residues in foods of animal origin: a review. Public Policy Adm. Res. 2017;7:6–11. [Google Scholar]
- Uivarosi V. Metal complexes of quinolone antibiotics and their applications: an update. Molecules. 2013;18:11153–11197. doi: 10.3390/molecules180911153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waheed M., Butt M.S., Shehzad A., Adzahan N.M., Shabbir M.A., Rasul Suleria H.A., Aadil R.M. Eggshell calcium: a cheap alternative to expensive supplements. Trends Food Sci. Technol. 2019;91:219–230. [Google Scholar]
- Waheed M., Yousaf M., Shehzad A., Inam-Ur-Raheem M., Khan M.K.I., Khan M.R., Ahmad, Abdullah N., Aadil R.M. Channelling eggshell waste to valuable and utilizable products: a comprehensive review. Trends Food Sci. Technol. 2020;106:78–90. [Google Scholar]
- Wenzl, T., J. Haedrich, A. Schaechtele, P. Robouch, and J. Stroka. 2016. Guidance document on the estimation of LOD and LOQ for measurements in the field of contaminants in feed and food 2016 EUR 28099 EN. Accessed March 2021. https://ec.europa.eu/jrc.