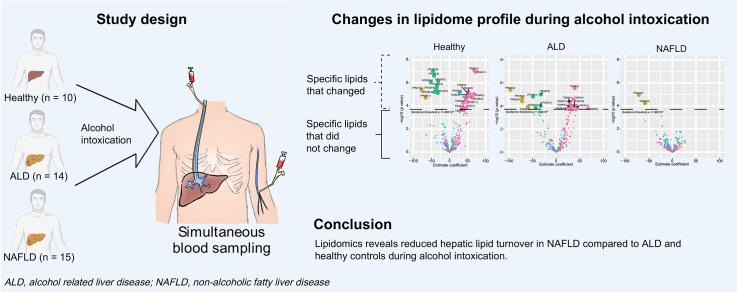
Abstract
Background & Aims
In experimental models, alcohol induces acute changes in lipid metabolism that cause hepatocyte lipoapoptosis and inflammation. Here we study human hepatic lipid turnover during controlled alcohol intoxication.
Methods
We studied 39 participants with 3 distinct hepatic phenotypes: alcohol-related liver disease (ALD), non-alcoholic fatty liver disease (NAFLD), and healthy controls. Alcohol was administrated via nasogastric tube over 30 min. Hepatic and systemic venous blood was sampled simultaneously at 3 time points: baseline, 60, and 180 min after alcohol intervention. Liver biopsies were sampled 240 min after alcohol intervention. We used ultra-high performance liquid chromatography mass spectrometry to measure levels of more than 250 lipid species from the blood and liver samples.
Results
After alcohol intervention, the levels of blood free fatty acid (FFA) and lysophosphatidylcholine (LPC) decreased, while triglyceride (TG) increased. FFA was the only lipid class to decrease in NAFLD after alcohol intervention, whereas LPC and FFA decreased and TG increased after intervention in ALD and healthy controls. Fatty acid chain uptake preference in FFAs and LPCs were oleic acid, linoleic acid, arachidonic acid, and docosahexaenoic acid. Hepatic venous blood FFA and LPC levels were lower when compared with systemic venous blood levels throughout the intervention. After alcohol intoxication, liver lipidome in ALD was similar to that in NAFLD.
Conclusions
Alcohol intoxication induces rapid changes in circulating lipids including hepatic turnaround from FFA and LPC, potentially leading to lipoapoptosis and steatohepatitis. TG clearance was suppressed in NAFLD, possibly explaining why alcohol and NAFLD are synergistic risk factors for disease progression. These effects may be central to the pathogenesis of ALD.
Clinical Trials Registration
The study is registered at Clinicaltrials.gov (NCT03018990).
Lay summary
We report that alcohol induces hepatic extraction of free unsaturated fatty acids and lysophosphatidylcholines, hepatotoxic lipids which have not been previously associated with alcohol-induced liver injury. We also found that individuals with non-alcoholic fatty liver disease have reduced lipid turnover during alcohol intoxication when compared with people with alcohol-related fatty liver disease. This may explain why alcohol is particularly more harmful in people with non-alcoholic fatty liver and why elevated BMI and alcohol have a synergistic effect on the risk of liver-related death.
Keywords: Heavy drinking, Alcohol, Ethanol, Liver disease, Lysophosphatidylcholines, Fatty acids, Triglycerides, Lipidomics
Abbreviations: ALD, alcohol-related liver disease; ALT, alanine aminotransferase; AST, asparagine aminotransferase; Cer, ceramide; CTL, healthy control; DG, diglyceride; FFA, free fatty acid; GGT, gamma-glutamyl transferase; HexCer, hexosylceramide; HOMA-IR, Homeostatic Model Assessment of Insulin Resistance; LacCer, lactosylceramides; LPC, lysophosphatidylcholine; LPE, lysophosphatidylethanolamine; NAFLD, non-alcoholic fatty liver disease; PC, phosphatidylcholine; PE, phosphatidylethanolamine; P-glucose, plasma glucose; PI, phosphatidylinositol; PLA2, phospholipase A2; QC, quality control; SHexCer, sulfatides hexosylceramide; SM, sphingomyelin; TE, transient elastography; TG, triglyceride
Graphical abstract
Highlights
-
•
Alcohol intoxication induces rapid changes in the profile of circulating lipids.
-
•
Alcohol has a profound effect on monosaturated fatty acids.
-
•
Triglyceride clearance is suppressed in NAFLD during alcohol intoxication.
-
•
Hepatic lipid turnover differentiates ALD and NAFLD during alcohol intoxication.
-
•
A suppressed metabolic response may explain why alcohol is particularly harmful in NAFLD.
Introduction
Alcohol is the leading aetiology of cirrhosis and is associated with high morbidity and mortality.1 From 1999 to 2016 the USA experienced a 2–10% annual increase in mortality as a result of alcohol-related liver disease (ALD).2 Worldwide 20% of all people have at least 1 heavy drinking episode per month3 and weekly heavy drinking episodes are seen in 13% of people in the USA.4 Heavy drinking episodes are associated with higher risk of developing chronic liver injury and alcohol-related cirrhosis,5 with a particularly high risk in obese people.[6], [7], [8]
Alcohol-induced lipo-toxicity is an emerging area and it has an important role in the pathogenesis of alcohol-related steatohepatitis. Hepatic alcohol detoxification leads to the formation of toxic metabolites and hepatic steatosis, and it is well-established that alcohol causes hepatic accumulation of free fatty acids (FFAs) by blocking mitochondrial beta-oxidation.9 However, experimental models suggest a more complex interaction between alcohol and hepatic lipid metabolism; alcohol increases the hepatic uptake of FFAs, alcohol has different effects on distinct types of FFA (short vs. long-chain and saturated vs. unsaturated), and alcohol may also affect other lipid classes including lysophosphatidylcholines (LPCs) that can induce lipo-toxicity.9,10 Furthermore, animal models reveal that a high-fat diet increases the susceptibility of alcohol-induced injury and the combination leads to an increased rate of progression of chronic liver injury.11,12
Although experimental studies have revealed mechanistic knowledge, we lack human studies that can aid the clinical understanding of lipid metabolism in relation to alcohol intoxication. To investigate the translation of experimental findings to humans, we developed a study where participants were given a controlled amount of alcohol to replicate alcohol intoxication (Fig. 1A). We recruited participants with 3 different hepatic phenotypes: healthy controls, ALD, and non-alcoholic fatty liver disease (NAFLD). Blood samples were collected from hepatic and systemic veins before and after alcohol intoxication from all 3 hepatic phenotypes. Liver biopsy samples were collected by the end of alcohol intoxication from participants with ALD and NAFLD. Comprehensive lipidomic analyses were performed on these blood and liver tissue samples (Fig. 1B). Our overall aims were to (1) explore the effects of alcohol intoxication on the hepatic venous blood lipid profile, (2) test whether alcohol intoxication causes different reactions in people with ALD or NAFLD and healthy controls, and (3) explore differences between hepatic and systemic blood lipid profiles as well as liver lipid profiles.
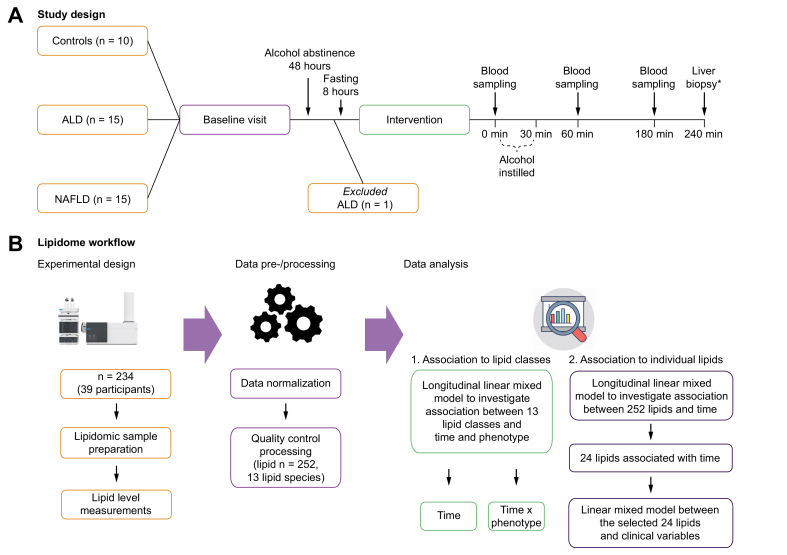
Fig. 1.
Schematic study design and lipidome workflow.
(A) Study design: for the baseline visit, we invited 40 individuals with 3 distinct hepatic phenotypes: 10 healthy controls, 15 with ALD, and 15 with NAFLD. One ALD participant was subsequently excluded owing to protocol violation by consuming alcohol within 48 h before the alcohol intervention. The remaining 39 participants underwent the alcohol intervention: blood was sampled at time 0 min, and alcohol was subsequently instilled over 30 min. Blood was sampled again after 60 and 180 min. ∗Transjugular liver biopsies were collected after 240 min in participants with ALD and NAFLD. (B) Lipidome workflow: lipid levels (n = 252) were measured using ultra-high-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometery (UHPLC-QTOFMS). First, we explored the average level of 13 distinct lipid classes from hepatic venous blood to identify which lipid class changed in its level over time after alcohol intervention. We used longitudinal mixed effect models to investigate 3 different fixed effects: (1) ‘time’, (2) ‘time∗phenotype’, and (3) ‘time∗blood site’. Fixed effect of ‘time’ allowed us to identify lipid classes that changed in their levels after alcohol intervention. Fixed effect of ‘time∗phenotype’ allowed us to see whether levels of 13 lipid classes were different between the hepatic phenotypes before and after alcohol intervention. Fixed effect of ‘time∗blood site’ allowed us to see whether levels of 13 lipid classes were different between 2 blood vein sites (systemic vs. hepatic) before and after alcohol intervention. Finally, we investigated 252 individual lipid species using the same approach where fixed effects were (1) ‘time’, (2) ‘time∗phenotype’, and (3) ‘time∗blood site’. In all models, random effect was the individual participant. ALD, alcohol-related liver disease; NAFLD, non-alcoholic fatty liver disease.
Patients and methods
Participants
Age-matched individuals with 3 hepatic phenotypes were included: (1) healthy controls with no sign of liver disease; (2) individuals with ALD, and (3) individuals with NAFLD. Potential participants received written and oral information followed by a pre-investigation consultation with a physician involved in the study. All investigations were performed at Odense Liver Research Centre. The study was approved by the Ethical Committee of Southern Denmark (S-20160083), registered at ClinicalTrials.gov (NCT03018990), and complied with the Declaration of Helsinki Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects. All participants signed an informed consent.
Participants were recruited through the Odense Liver Research Centre with inclusion criteria of age 18–75 years, body weight >50 kg, capable to be abstinent for 48 h before the investigations, and signed informed consent. Individuals with ALD should be regularly drinking and have a prior or ongoing heavy alcohol intake (males >36 g alcohol/day, females >24 g alcohol/day), biopsy-proven liver fibrosis on a previous liver biopsy, and histological features in keeping with ALD. No participants with ALD were abstinent from alcohol or had a desire to become abstinent from alcohol. Individuals with NAFLD should have biopsy-proven liver fibrosis, histological features in keeping with NAFLD, and no history of heavy alcohol intake. Healthy controls should have normal liver stiffness, BMI <30 kg/m2, normal biochemistry, and no history of heavy alcohol intake. General exclusion criteria were cirrhosis on a previous liver biopsy, competing liver disease of other aetiology, insulin-dependent diabetes mellitus, pregnancy or breastfeeding, antibiotic use within the previous 4 weeks, and all types of cancer.
Investigations
The investigations were initiated in the morning, after 8 h fasting and minimum of 48 h abstinence from alcohol. Under local anaesthesia, a catheter was placed in a hepatic vein via the right jugular vein and inferior vena cava for sampling hepatic venous blood.13 Another catheter was placed in the jugular vein for systemic blood sampling. Radiography (X-ray) was used to ensure correct placement of the catheters before blood sampling.
Alcohol intervention
Ethanol was instilled through a nasogastric tube and was 40% pure ethanol in 9 mg/ml NaCl produced at the hospital pharmacy. Participants received a dose of 2.5 ml of 40% ethanol per kg body weight, instilled over 30 min by infusion pump. To avoid severe intoxication in participants with high body fat percentage, the dose was adjusted by 0.5 ml for each kg body weight encountered from BMI above 25 kg/m2.
Blood sampling
Blood was sampled simultaneously from a hepatic vein and the right external jugular vein at baseline (time 0, just before alcohol intervention), after 60 min, and after 180 min. The Department of Biochemistry and Pharmacology at Odense University Hospital analysed serum alcohol concentrations and routine biochemistry according to standard operating procedures. Blood samples for lipidome analysis were collected in lithium heparin tubes chilled in crushed ice and water. Within 1 h after sampling, we centrifuged and pipetted the samples to secondary tubes and stored them at -80°C. A flowchart of the clinical set-up can be found in Fig. 1.
Liver biopsy sampling
Transjugular liver biopsies were performed using a Tru-Cut® biopsy needle in participants with ALD and NAFLD 240 min after alcohol instillation. The first piece of liver tissue from each participant was immediately stored in formalin 4% and sent for histopathological assessment and scored according to the non-alcoholic steatohepatitis Clinical Research Network system with regards to fibrosis, steatosis, inflammation, and ballooning.14,15 The second piece of tissue was stored immediately in dry tubes at -80°C until lipidome analysis.
Lipidome analysis
Sample preparation for lipidomic analysis has been described elsewhere16,17 and is explained in detail in Supplementary Methods 1. Briefly, a plasma sample (10 μl) was mixed with 10 μl 0.9% w/v NaCl (aq) and internal standards containing 120 μl of chloroform/methanol (2:1) mixture. The lipid containing chloroform was analysed using ultra-high performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometery (UHPLC-QTOFMS). Samples were analysed in a randomised order, with quality control (QC) pooled plasma samples at regular intervals throughout the run (n = 30 for both positive and negative ionisation).
For liver tissue, the needle biopsy sample was first converted into powdered forms by crushing samples using a Covaris CryoPrep impactor CP02. Approximately 2 mg of powdered tissue sample were used for lipid extraction (exact mass was measured and used for the post-processing normalisation step later). The sample was mixed with internal standards containing 480 μl of chloroform/methanol (2:1) mixture. The lipid containing chloroform was analysed in the same manner as the blood samples.
The lipidomics data were pre-processed with MZmine2,18 and lipid features were then normalised to internal standards and log transformed. Liver samples were further normalised to the exact weight used during lipid extraction. We excluded lipid features with >20% missingness across all samples and relative standard deviation values >20% across QC samples. The lipid features were then standardised (scaled) to have a mean of 0 and a standard deviation of 1×104. The data were cross-matched with an in-house library where 252 lipids from 13 different lipid classes were identified in blood samples, whereas 316 lipids from 14 different lipid classes were identified in liver tissue samples at level 1 and 2 lipid identification levels.
Statistical analysis
Participants’ baseline characteristics are reported as mean and SD, counts and proportion, or medians and IQR depending on the distribution. For blood lipidomic analysis, we applied longitudinal mixed effect regression models with participants as a random effect to investigate changes in circulating lipid contents after alcohol intake.
First, we explored the average level of 13 distinct lipid classes from hepatic venous blood to identify which lipid classes changed in level over time after alcohol intervention. For this, we derived average levels from each lipid class and built mixed models between levels of 13 lipid classes and 3 different fixed effects: (1) ‘time’, (2) ‘time∗phenotype’, and (3) ‘time∗blood site’. Fixed effect of ‘time’ allowed us to investigate changes in circulating lipid content after alcohol intervention. From these models we derived estimate coefficient values (representing changes in relative levels per minute) and their corresponding p values. The fixed effect of ‘time∗phenotype’ allowed us to see whether levels of 13 lipid classes were different between phenotypes at baseline (before alcohol intervention) and whether estimate coefficient values of lipid classes differed between the 3 phenotypes. The fixed effect of ‘time∗blood site’ allowed us to see whether baseline levels and estimate coefficient values of the 13 lipids classes differed between the 2 blood vein sites (systemic vs. hepatic).
From the 13 lipid classes, we investigated 252 individual lipid species. We used the same approach as above where fixed effects were (1) ‘time’, (2) ‘time∗phenotype’, and (3) ‘time∗blood site’. In all models, random effect was the individual participant. We report the significant lipids after Bonferroni correction for multiple testing. Mixed models were carried out in R 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria) using the ‘nlme’ package.19
Lipid levels (n = 316, 14 lipid classes) from liver biopsy samples were available from 14 participants with ALD and 15 participants with NAFLD after alcohol intervention. We investigated the effect of alcohol intervention between ALD and NAFLD liver lipids by utilising a logistic regression model where the age of participants at the time of sampling was adjusted. Regression modelling was carried out in R 3.6.0. A schematic workflow of the of the lipidome data analysis is shown in Fig. 1.
Results
Participants
Baseline investigations were performed on 40 individuals from November 2016 to November 2018, however, 1 participant with ALD was subsequently excluded because of protocol violation by consuming alcohol within 48 h of the alcohol intervention. Table 1 shows the participants’ baseline characteristics. The mean age was 54 ± 11 years, 62% were males, and there were no significant differences between the groups. Median alcohol consumption was 60 g/day in those with ALD and below 12 g/day in those with NAFLD and healthy controls (p <0.001). Participants with NAFLD had higher BMI, higher haemoglobin A1c, and lower HDL cholesterol than those with ALD and healthy controls (p <0.01). There was no difference between the ALD and NAFLD groups with regards to liver blood tests and liver histopathology (Table 1). Overall, 55% had significant fibrosis (≥F2), 48% had moderate or severe steatosis (≥S2), and the median NAFLD activity score was 3. All the healthy controls had normal liver stiffness (<6 kPa).
Table 1.
Participant characteristics.
| Characteristics | All | Controls | ALD | NAFLD | p value |
|---|---|---|---|---|---|
| Participants, n | 39 | 10 | 14 | 15 | – |
| Male sex, n | 24 (62%) | 5 (50%) | 12 (86%) | 7 (47%) | 0.066 |
| Age, yr | 54 ± 11 | 53 ± 10 | 57 ± 11 | 53 ± 12 | 0.930 |
| Weight, kg | 87 (74–104) | 75 (67–84) | 85 (74–104) | 99 (86–108)† | 0.006 |
| BMI, kg/m2 | 29 (25–32) | 25 (23–27) | 28 (23–32) | 32 (30–41)∗† | <0.001 |
| Type 2 diabetes, n | 14 (36%) | 0 (0%) | 4 (29%) | 10 (67%)† | 0.002 |
| Metabolic syndrome, n | 18 (46%) | 0 (0%) | 6 (43%)† | 12 (80%)† | <0.001 |
| Daily alcohol consumption, g | 6 (0–48) | 12 (0–12) | 60 (24–120)† | 0 (0–0)∗ | <0.001 |
| Biochemistry | |||||
| Platelet count, 109/L | 221 (190–270) | 210 (169–228) | 216 (105–244) | 252 (196–303) | 0.256 |
| INR | 1 (0.9–1.1) | 1.1 (1–1.1) | 1 (0.9–1.1) | 1 (0.9–1) | 0.192 |
| Albumin, g/L | 45 (42–47) | 46.5 (40–48) | 43 (41–46) | 45 (43–48) | 0.141 |
| ALT, U/L | 36 (23–58) | 26 (17–35) | 44 (27–71) | 44 (26–65) | 0.085 |
| AST, U/L | 29 (25–58) | 23 (22–28) | 49 (29–79)† | 29 (24–61) | 0.007 |
| GGT, U/L | 62 (23–138) | 22 (15–26) | 197 (89–635)† | 65 (45–90)∗† | <0.001 |
| Bilirubin, μmol/L | 10 (7–13) | 10 (9–13) | 13 (7–19) | 10 (6–13)† | 0.490 |
| Metabolic parameters | |||||
| Fasting P-glucose, mmol/L | 6.2 (5.5–7.0) | 5.7 (5.5–6.1) | 6.5 (5.6–7) | 6.7 (6.2–7)† | 0.014 |
| HbA1c, mmol/mol | 38 (34–45) | 35 (31–37) | 37 (31–40) | 46 (40–55)∗† | <0.001 |
| Insulin, pmol/L | 93 (44–183) | 43 (31–47) | 78 (46–217) | 171 (116–188)† | 0.001 |
| HOMA-IR | 5.3 (1.7–8.7) | 1.6 (1.2–1.8) | 3.2 (1.7–10.3) | 7.0 (5.2–8.8)† | <0.001 |
| HDL cholesterol, mmol/L | 1.2 (1.1–1.6) | 1.5 (1.1–2) | 1.5 (1.2–1.6) | 1.1 (0.9–1.3)∗† | 0.005 |
| LDL cholesterol, mmol/L | 2.6 (2.2–3.6) | 3.1 (2.5–4.3) | 2.4 (2.2–3.1) | 2.6 (1.3–3.6) | 0.234 |
| Total cholesterol, mmol/L | 4.8 (4.2–6.0) | 5.3 (4.5–6) | 4.9 (4.4–6.2) | 4.4 (3.8–5.6) | 0.236 |
| Triglycerides, mmol/L | 1.36 (0.78–2.21) | 0.80 (0.72–1.12) | 1.55 (0.85–2.50)† | 1.45 (0.82–3.38)† | 0.033 |
| Liver parameters | – | ||||
| Fibrosis stage (0/1/2/3/4), n | – | – | 2/5/5/1/1 | 1/5/7/1/1 | – |
| Steatosis grade (0/1/2/3), n | – | – | 0/5/2/4 | 0/7/5/3 | – |
| NAFLD activity score | – | – | 3 (1–4) | 3 (2–4) | – |
| Liver stiffness by TE, kPa | 8.9 (4.9–11.0) | 4.6 (3.9–5.0) | 9.0 (5.9–11.0)† | 10.4 (9.5–11.4)† | <0.001 |
Data are presented as means (SD), medians (IQR), or counts (frequencies). Values of p are obtained from comparisons between the 3 groups using Kruskal-Wallis and Chi-square tests. Comparisons between 2 groups are obtained using rank sum and Fisher’s exact test with significance level <0.05 and marked as follows: ∗significant difference between ALD and NAFLD; †significant difference between controls and ALD/NAFLD. Classification of metabolic syndrome was performed according to the International Diabetes Federation criteria.37
ALD, alcohol-related liver disease; ALT, alanine aminotransferase; AST, asparagine aminotransferase; GGT, gamma-glutamyl transferase; HOMA-IR, Homeostatic Model Assessment of Insulin Resistance; NAFLD, non-alcoholic fatty liver disease; P-glucose; plasma glucose; TE, transient elastography.
Alcohol intervention
All participants received the scheduled alcohol dose according to the protocol. The mean serum alcohol concentration was 0 ± 0 mmol/l (0.00 ± 0.0 g/dl) at baseline, peaked at 34 ± 4 mmol/l (0.16 ± 0.02 g/dl) after 60 min, and decreased to 21 ± 3 (0.10 ± 0.1 g/dl) mmol/ after 180 min (p <0.001). Only mild transient symptoms commonly seen in relation to alcohol intake were observed.
Hepatic venous lipid classes before and after alcohol intervention
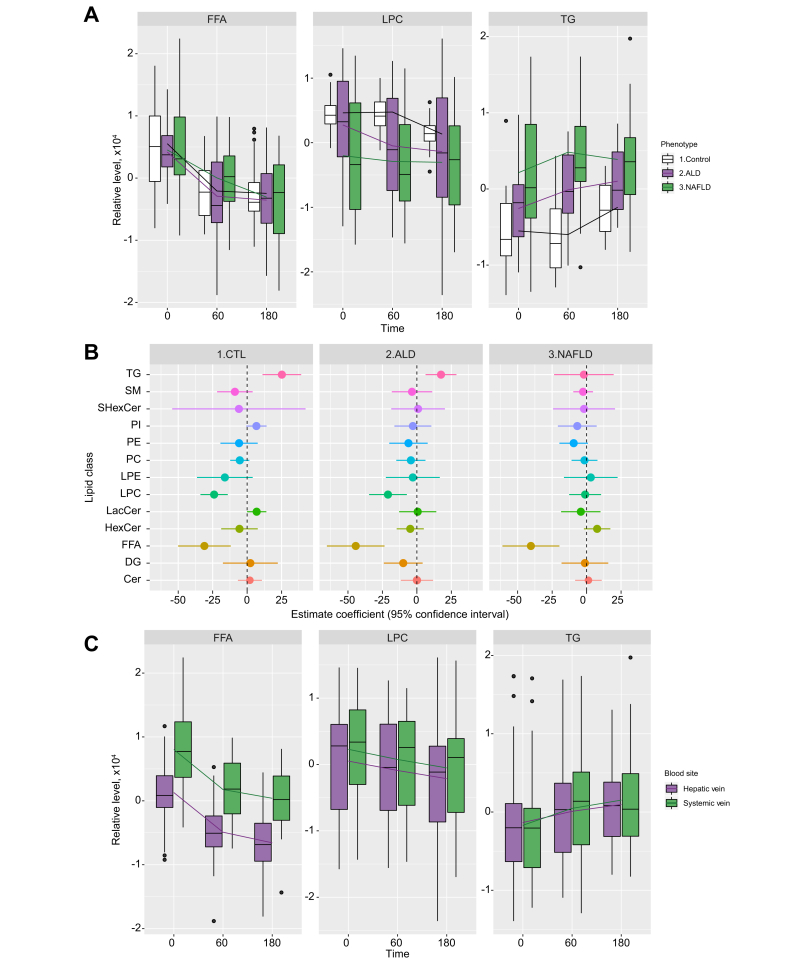
Before the alcohol intervention, healthy controls had lower hepatic venous triglyceride (TG) levels compared with those with ALD and NAFLD (Fig. 2A and Table 2). Alcohol intervention led to significant reductions in levels of FFA (-39.42 units/min, p <0.0001) and LPC (-14.08 units/min, p = 0.0002) (Table 3 and Fig. 2A), but not for other lipid classes. The effect of alcohol intervention on all 13 lipid classes is summarised in Table S1.
Fig. 2.
Lipid profile of 3 hepatic phenotypes.
(A) Boxplots showing FFA, LPC, and TG levels from hepatic vein for the 3 hepatic phenotypes. These 3 lipid classes were selected here because their levels changed after alcohol intervention in at least 1 phenotype (Table 2). FFA showed significant changes over time in Control, ALD, and NAFLD phenotypes, while LPC showed significant changes over time in the Control phenotype. TG showed significant changes over time in ALD and NAFLD phenotypes. Significances were based on p <0.0039. (B) Forrest plot showing magnitude of change after alcohol intervention (estimate coefficients derived from mixed models) for all 13 lipid classes from hepatic vein in each hepatic phenotype. (C) Boxplots comparing FFA, LPC, and TG levels in hepatic and systemic veins. The lines connect the means of boxes. ALD, alcohol-related liver disease; CTL, healthy control; FFA, free fatty acid; LPC, lysophosphatidylcholine; NAFLD, non-alcoholic fatty liver disease; TG, triglyceride.
Table 2.
Comparison of FFA, LPC, and TG at baseline between hepatic and systemic venous blood.
| Lipid class | Average lipid levels in hepatic vein (SD), ×102 |
Average lipid levels in systemic vein (SD), ×102 |
||||||
|---|---|---|---|---|---|---|---|---|
| All | Controls | ALD | NAFLD | All | Controls | ALD | NAFLD | |
| FFA | 12.92 (45.06)† | 5.64 (48.46)† | 21.41 (28.90)† | 9.85 (55.72)† | 80.21 (59.73)† | 104.92 (61.19)† | 62.15 (48.17)∗,† | 80.60 (66.04)∗,† |
| LPC | 5.13 (84.72) | 46.14 (28.05) | 18.49 (79.81) | -34.68 (99.92) | 22.85 (75.97) | 46.26 (34.65) | 36.46 (80.27) | -5.46 (86.45) |
| TG | -13.44 (76.09) | -67.27 (43.10) | -20.68 (47.79)∗ | 29.21 (91.27)∗ | -16.89 (73.27) | -43.01 (63.62) | -30.73 (62.40)∗ | 13.44 (81.79)∗ |
Different to healthy controls at Bonferroni procedure p <0.0039 level according to mixed model.
Difference between hepatic and systemic values at Bonferroni procedure p <0.0039 level according to mixed model. FFA, free fatty acid; LPC, lysophosphatidylcholine; TG, triglyceride.
Table 3.
Summary of mixed models showing relative changes in FFA, LPC, and TG levels from baseline to 180 min after start of alcohol intake.
| Lipid class | Hepatic vein: estimate coefficients (95% CI) |
Systemic vein: estimate coefficients (95% CI) |
||||||
|---|---|---|---|---|---|---|---|---|
| All | Controls | ALD | NAFLD | All | Controls | ALD | NAFLD | |
| FFA | -39.42∗ (-50.95~-27.89) p < 0.0001 |
-31.02∗ (-50.13~-11.90) p = 0.0030 |
-44.36∗ (-65.28~-23.44) p = 0.0002 |
-40.41∗ (-61.08~-19.74) p = 0.0004 |
-37.91∗ (-50.00~-25.82) p <0.0001 |
-45.97 (-76.04~-15.89) p = 0.0047 |
-30.67∗ (-48.95~-12.40) p = 0.0002 |
-39.29∗ (-58.91~-19.67) p = 0.0003 |
| LPC | -14.08∗ (-21.28~-6.87) p = 0.0002 |
-23.96∗ (-33.88~-14.04) p < 0.0001 |
-20.97 (-34.68~-7.26) p = 0.0041 |
-1.05 (-12.64~10.53) p = 0.854 |
-14.84∗ (-21.11~-8.56) p <0.0001 |
-15.50(-30.02~-0.98) p = 0.0377 |
-20.52 (-31.86~-9.18) p = 0.0041 |
-9.09 (-17.96~-0.23) p = 0.0448 |
| TG | 12.00 (2.16~21.84) p = 0.0176 |
25.20∗ (11.19~39.22) p = 0.0013 |
17.52∗ (6.33~28.70) p = 0.0034 |
-1.95 (-23.56~19.65) p = 0.855 |
16.53∗ (8.92~24.14) p <0.0001 |
12.88 (-7.02~32.77) p = 0.1913 |
19.49∗ (6.49~32.50) p = 0.0034 |
16.20∗ (5.77~26.63) p = 0.0035 |
The table shows estimate coefficients (change in relative levels of lipid every minute after alcohol intervention) and their p values are shown.
ALD, alcohol-related liver disease; FFA, free fatty acid; LPC, lysophosphatidylcholine; NAFLD, non-alcoholic steatohepatitis; TG, triglyceride.
Passed Bonferroni procedure p <0.0039.
Effect of alcohol intervention by phenotype on hepatic venous lipid classes
Alcohol caused significant reductions in hepatic venous FFA levels in individuals with all 3 hepatic phenotypes (Fig. 2B): healthy controls (-31.02 units/min, p = 0.0030), ALD (-44.36 units/min, p = 0.0002), and NAFLD (-40.41 units/min, p = 0.0004) (Fig. 2B and Table 3). In contrast, LPC decreased only in healthy controls (-23.96 units/min, p <0.0001) and in ALD (-20.97 units/min, p = 0.0041) (Table 3). Hepatic TG levels increased significantly in healthy controls (+25.20 units/min, p = 0.0013) and in ALD (+17.52 units/min, p = 0.0034) but not in NAFLD (-1.95 units/min, p = 0.855) (Fig. 2B and Table 3).
Estimate coefficient values for each lipid class and between-group comparisons are provided in Tables S1 and S2. Relative levels of each lipid class are provided in Table S3.
Hepatic vs. systemic venous blood
Before alcohol intervention, the level of FFAs in individuals of all 3 hepatic phenotypes was significantly lower in hepatic venous blood than in systemic venous blood (Fig. 2C and Table 2). However, the rates at which lipid classes changed during alcohol intervention (estimate coefficient values) did not differ between hepatic and systemic venous blood for any of the lipid classes (Table S1).
Single lipid levels before and after alcohol intervention
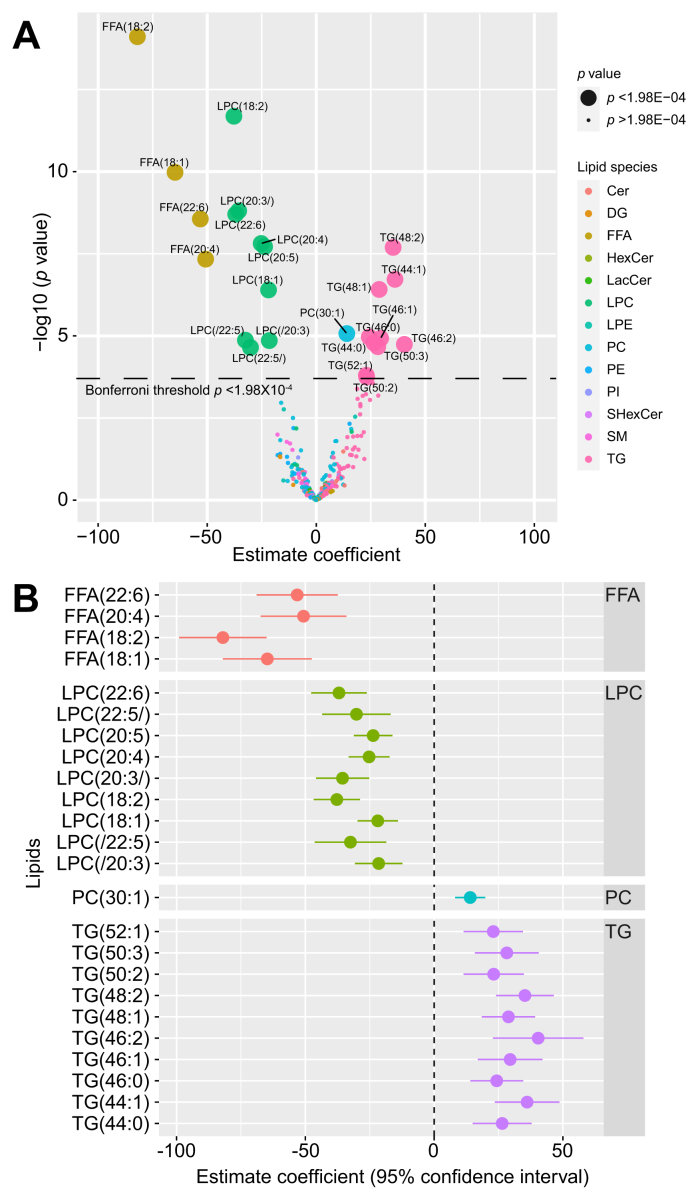
Twenty-four of 252 individual lipids changed significantly in hepatic venous blood after alcohol intervention at Bonferroni level of p <1.98×10-4 (Fig. 3A). Estimated coefficient values for these 24 lipids can be found in Table S4. One monounsaturated fatty acid and 3 polyunsaturated fatty acids decreased in response to alcohol:oleic acid (FFA 18:1), linoleic acid (FFA 18:2), arachidonic acid (FFA 20:4), and docosahexaenoic acid (FFA 22:6). The levels of 9 LPCs decreased, 4 of which contained the same 4 FFAs as above. The 9 LPC molecules contained oleic acid (FFA 18:1), linoleic acid (FFA 18:2), arachidonic acid (FFA 20:4), dihomo-α-linolenic (FFA 20:3), docosapentaenoic acid (FFA 20:5), docosapentaenoic acid (FFA 22:5), and docosahexaenoic acid (FFA 22:6) (Fig. 3B). The alcohol intervention also led to increased levels of 1 phosphatidylcholine and 10 TG molecules.
Fig. 3.
Changes in lipid levels after alcohol intervention.
(A) Volcano plot showing changes in levels (mixed model estimate coefficients) for 252 lipids from hepatic vein after alcohol intervention. Statistical significance (y-axis) is plotted against the magnitude of change in the lipid levels after alcohol intervention (x-axis) to identify the most dynamic lipids that were also statistically significant. Different colours represent different lipid species, and larger dots represent the 24 lipids passing Bonferroni threshold (p <1.98×10-04). (B) Forrest plot showing magnitude of change after alcohol intervention (mixed model estimate coefficients) for the 24 lipids from hepatic vein selected from Fig. 3A. Cer, ceramide; DG, diglyceride; FFA, free fatty acid; HexCer, hexosylceramide; LacCer, lactosylceramides; LPC, lysophosphatidylcholine; LPE, lysophosphatidylethanolamine; NAFLD, non-alcoholic fatty liver disease; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; SHexCer, sulfatides hexosylceramide; SM, sphingomyelin; TG, triglyceride.
Single lipid levels by phenotype before and after alcohol intervention
We focused on these 24 lipids and compared their levels between the different hepatic phenotype groups at baseline. The healthy controls had significantly lower levels of all 10 TG lipids compared to those with ALD while 7 TG lipids were also significantly lower in healthy controls compared with those with NAFLD (Fig. S1 and Table S4). No lipids differed between the groups with ALD and NAFLD before alcohol intervention (Table S5). The 4 FFA levels were similar in healthy controls, and those with ALD and NAFLD before alcohol intervention (Fig. S1).
We then compared changes (estimate coefficient values) between the hepatic phenotypes. Fig. S1 shows TG molecules increased more profoundly in healthy controls, but only TG (44:1) showed a significant difference between healthy controls (+73.08 units/min) and the group with NAFLD (+4.40 units/min). We observed similar patterns for FFA and LPC lipids across all 3 hepatic phenotypes.
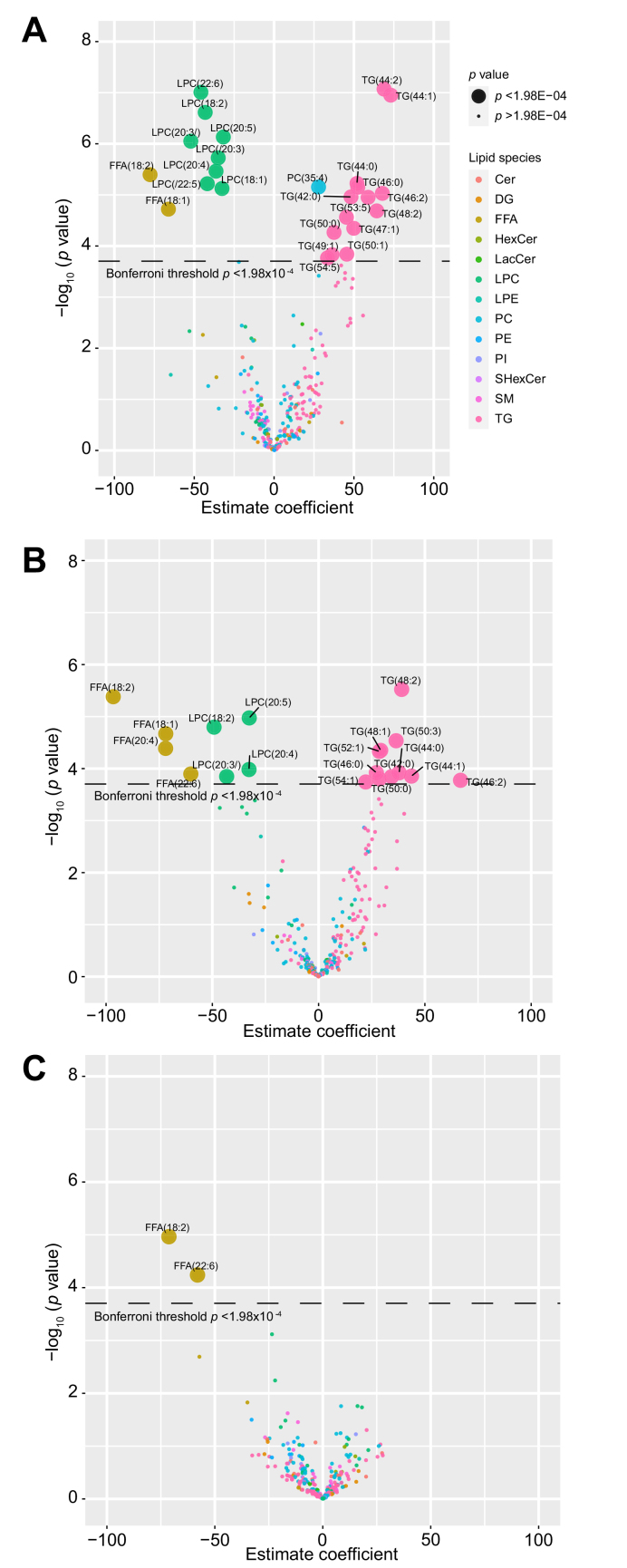
Additionally, we carried out longitudinal models in each phenotype for all individual lipids. The models summarised in volcano plots are shown in Fig. 4. In healthy controls, hepatic venous levels of 25 lipids changed after alcohol intervention (p <1.98×10-4), these included the 24 lipids found from the earlier model. Two FFA and 8 LPC molecules decreased, while 14 TG molecules and 1 PC molecule increased. In those with ALD, 19 hepatic venous lipids had altered levels, 4 FFA and 4 LPC decreased, while 11 TG increased. In those with NAFLD, only 2 FFA hepatic lipids decreased after alcohol intervention.
Fig. 4.
Volcano plot showing changes in levels (mixed model estimate coefficients) for 252 lipids from hepatic vein after alcohol intervention.
(A) Healthy controls, (B) participants with ALD and (C) participants with NAFLD. ALD, alcohol-related liver disease; Cer, ceramide; DG, diglyceride; FFA, free fatty acid; HexCer, hexosylceramide; LacCer, lactosylceramides; LPC, lysophosphatidylcholine; LPE, lysophosphatidylethanolamine; NAFLD, non-alcoholic fatty liver disease; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; SHexCer, sulfatides hexosylceramide; SM, sphingomyelin; TG, triglyceride.
Single lipid levels in hepatic and systemic venous blood before and after alcohol intervention
Baseline levels of the 4 FFAs (oleic acid [18:1], linoleic acid [18:2], arachidonic acid [20:4], and docosahexaenoic acid [22:6]) were lower in the hepatic vein than in the systemic vein (Table S4 and Fig. S2). No single lipid levels changed at a significantly different rate (estimate coefficient) in hepatic and systemic venous blood after alcohol intervention.
ALD vs. NAFLD liver lipidomics
Liver biopsies were collected from participants with ALD and NAFLD after alcohol intervention and liver lipidomic profiles were compared, to identify different lipids levels between the 2 hepatic phenotypes. Logistic regression model showed no lipid being different between the 2 hepatic phenotypes at Bonferroni p <1.58×10-4 level (Fig. S3).
Discussion
In this first investigation in humans of how acute alcohol intoxication affects the circulating hepatic and systemic lipidome, we found that alcohol caused significantly reduced levels of LPCs and FFAs, and increased TG levels. The increase in TGs was restricted to healthy controls and those with ALD, indicating a suppressed or altered TG synthesis in those with NAFLD during acute alcohol intoxication. The TGs that increased were mostly unsaturated or monounsaturated indicating de novo lipogenesis.
Our data show a decrease in circulating LPC after alcohol intake. We hypothesise that alcohol increases hepatic LPC uptake which leads to lipoapoptosis and alcoholic steatohepatitis (Fig. 5). The role of LPC in the pathogenesis of ALD is not yet known, but i.v. LPC injections induced lobular hepatitis in mice,20 and intracellular LPCs were shown to cause caspase activation and generation of oxidative stress resulting in lipoapoptosis of hepatocytes.20,21 Human liver tissue lipidome profiling of simple non-alcoholic fatty liver vs. non-alcoholic steatohepatitis revealed that livers with steatohepatitis contain a higher amount of LPC,22 but lower circulating LPC levels are a marker of poor prognosis in participants with NAFLD.23,24 This indicates an inverse relationship between circulating and intrahepatic LPC levels. In our study, the LPC species that decreased after alcohol intervention contained oleic acid (FFA 18:1), linoleic acid (FFA 18:2), arachidonic acid (FFA 20:4), and docosahexaenoic acid (FFA 22:6). These fatty acids in their free forms were also found to be lower in our study, in line with previous studies which showed alcohol intoxication induced a rapid drop in the systemic circulating FFA in healthy people and people with ALD and NAFLD-related diseases (type 2 diabetes and hypertriglyceridemia).[25], [26], [27], [28], [29] Additionally, we report 2 further novel findings in humans.
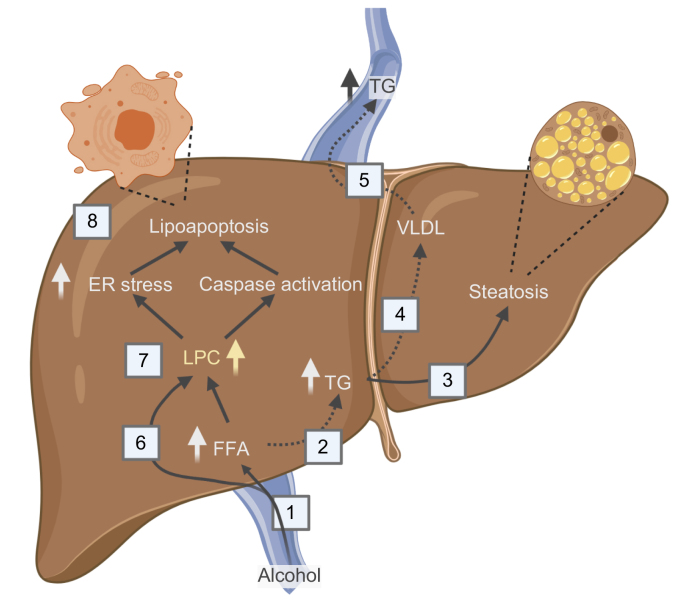
Fig. 5.
A model of hepatic lipid metabolism after alcohol intake.
(1) Alcohol increases hepatic FFA uptake. (2) FFAs can be hydroxylated by CYP2E1 or can be esterified to TGs and either be (3) stored in the hepatocyte leading to steatosis or (4) packaged in VLDL and (5) secreted to the circulation. (6) LPCs can be actively taken up by the liver. Together LPCs and FFAs are the end products of PLA2 metabolism. (7) Increased levels of intracellular LPCs may lead to FFAs via lysophospholipase D (LysoPLD) catalysis or to endoplasmic stress and caspase activation that (8) induce lipoapoptosis.20 Alcohol intoxication does not lead to increased levels of circulating TGs in NAFLD, which might indicate that at least 1 of the dashed arrows is suppressed. This may explain the increased TG storage and alcohol susceptibility reported in individuals with metabolic syndrome. ER, endoplasmic reticulum; FFA, free fatty acid; LPC, lysophosphatidylcholine; PLA2, phospholipase A2; TG, triglycerides.
Firstly, we observed that the drop in circulating FFAs was mainly driven by mono- and polyunsaturated FFAs, that is oleic acid, linoleic acid, arachidonic acid, and docosahexaenoic acid. Previous experimental studies showed alcohol intake resulted in a profound effect on hepatic oleic acid and linoleic acid, arachidonic acid, and docosahexaenoic acid,[30], [31], [32], [33] and now our findings confirm the same effect in humans. However, the role of unsaturated FFAs in fatty liver diseases is unclear and of great importance as the uptake is not observed in the de novo synthesised TG composition. One proposed explanation is that alcohol induces oxidation of unsaturated FFAs and that these oxidised unsaturated FFAs may contribute to the hepatic steatosis and inflammation seen in patients with ALD.34 Our results show that baseline levels and the acute effect of alcohol on unsaturated FFAs are similar in healthy controls, and those with ALD and NAFLD. Therefore, if unsaturated FFAs are involved in development or progression of liver disease, it must be related to either the frequent exposure of alcohol in ALD, or to a change in the subsequent hepatic processing of FFA. Interestingly, the FFA species which changed were same fatty acid chains seen in LPCs, suggesting a profound effect of alcohol on these specific fatty acid chains across 2 lipid species.
Secondly, we observed that the hepatic uptake of FFAs remained constant despite the alcohol-induced drop in circulating FFAs. This is noteworthy because hepatic FFA β-oxidation is inhibited during alcohol metabolism.9 Alcohol-induced suppression of liver FFA β-oxidation in combination with a continued high hepatic uptake of FFAs must lead to an overload of FFAs in the liver. This FFA excess in the liver is metabolised for TG synthesis and released to the circulation. This happened in patients with ALD and healthy controls where circulating TG increased in response to alcohol. In contrast, TG levels remained unaffected after alcohol intake in NAFLD. A suppressed capacity to convert excess hepatic FFA to TG during alcohol intoxication has also been reported in participants with type 2 diabetes and hypercholesterolaemia.26,29 A lack of TG synthesis in response to alcohol could indicate that NAFLD either have suppressed hepatic capacity of FFA esterification to TGs, or impaired hepatocyte secretion of TGs. Such impaired ability to remove FFAs from the liver could explain why binge drinking is particularly harmful in individuals with metabolic syndrome.6 This mechanism may help explain why metabolic risk factors and alcohol have synergistic effect on liver disease.7,12,35
The pathogenesis of NAFLD is closely related to dyslipidaemia.36 In line with that, the NAFLD phenotype was significantly different to the ALD and healthy control phenotypes with respect to fasting levels of TG and LPC. However, the levels of TG and LPC in participants with ALD changed towards a lipid profile more similar to NAFLD after alcohol intake. Therefore, we speculate that during alcohol intoxication, chronic alcohol misusers with ALD have a functional circulating lipid profile similar to those with NAFLD. To support this observation of the circulating lipid levels in ALD changing towards those in NAFLD, we also looked at liver lipid profiles at 240 min in individuals with these 2 hepatic phenotypes who had undergone alcohol intervention and found no difference in lipid profiles after alcohol intoxication.
Limitations of the study warrant consideration. Firstly, all investigations in the present study were performed in the fasting state. Secondly, 38 participants (out of 39) were of European ethnicity. Thirdly, circulating lipid profiles may not directly reflect alcohol-induced lipid changes in hepatic lipid profile. Our hypothesis is derived from measurements of circulating lipid changes after alcohol intoxication without concomitant measurements of liver injury. Thus, future studies should validate if hepatic uptake of LPC leads to liver injury in relation to acute alcohol intoxication.
Despite these limitations, we investigated how alcohol intoxication impacts circulating lipid profiles in humans across 3 distinct hepatic phenotypes. Previous studies have mainly investigated acute alcohol intake in young and healthy individuals. In contrast, our participants were middle-aged with early-stage liver disease and thereby more representative for studying the pathogenesis of chronic fatty liver disease. Furthermore, the comprehensive lipidome analysis enabled us to study lipid classes not previously investigated and indeed revealed that circulating LPCs and specific fatty acid chains are also key in the metabolism of acute alcohol intake.
To conclude, alcohol intoxication induces rapid changes in the profile of circulating lipids. Alcohol has a profound effect on monosaturated fatty acids in their free form or as part of LPC species. Hepatic uptake of LPC may play a central role in the pathogenesis of ALD, whereas the suppressed metabolic response in patients with NAFLD may explain why alcohol is particularly harmful in individuals with metabolic syndrome.
Financial support
Challenge Grant “MicrobLiver”, NNF15OC0016692, Novo Nordisk Foundation.
Authors’ contributions
Conceptualised the study: MI, BSM, TH and AK. Collected data for the study: MI, MK, BSM, CDH, NT and KT. Performed the data analyses: MI, MK, TS, KT and CLQ. Drafted the manuscript: MI, MK, MT, CLQ and AK. All authors contributed to the manuscript with important intellectual content and approved the final version.
Data availability statement
Anonymised data available on request.
Conflicts of interests
All authors declare no conflicts of interests.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
The authors thank Mie Balle Hugger, Annette Fialla, Annette Nielsen, Lotte Kappen, and Jane Brudvig-Lauridsen and the entire staff at Odense Liver Research Centre; Louise Skovborg Just and Lise Ryborg for the managing of GALAXY and MicrobLiver consortia; Mette Andreasen, Anette Tyrsted, and Lea Grip from Open Patient data Explorative Network; Professor Peter Rossing at Steno Diabetes Centre Copenhagen; Associate professor Claire Gudex from University of Southern Denmark; and the MicrobLiver consortium partners.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2021.100325.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Kim D., Li A.A., Gadiparthi C., Khan M.A., Cholankeril G., Glenn J.S. Changing trends in etiology-based annual mortality from chronic liver disease, from 2007 through 2016. Gastroenterology. 2018;155 doi: 10.1053/j.gastro.2018.07.008. 1154–1163.e1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tapper E.B., Parikh N.D. Mortality due to cirrhosis and liver cancer in the United States, 1999–2016: observational study. BMJ. 2018;362 doi: 10.1136/bmj.k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manthey J., Shield K.D., Rylett M., Hasan O.S.M., Probst C., Rehm J. Global alcohol exposure between 1990 and 2017 and forecasts until 2030: a modelling study. Lancet. 2019;393:2493–2502. doi: 10.1016/S0140-6736(18)32744-2. [DOI] [PubMed] [Google Scholar]
- 4.Grant B.F., Chou S.P., Saha T.D., Pickering R.P., Kerridge B.T., Ruan W.J. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001–2002 to 2012–2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74:911–923. doi: 10.1001/jamapsychiatry.2017.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ventura-Cots M., Watts A.E., Bataller R. Binge drinking as a risk factor for advanced alcoholic liver disease. Liver Int. 2017;37:1281–1283. doi: 10.1111/liv.13482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aberg F., Helenius-Hietala J., Puukka P., Jula A. Binge drinking and the risk of liver events: a population-based cohort study. Liver Int. 2017;37:1373–1381. doi: 10.1111/liv.13408. [DOI] [PubMed] [Google Scholar]
- 7.Hart C.L., Morrison D.S., Batty G.D., Mitchell R.J., Davey Smith G. Effect of body mass index and alcohol consumption on liver disease: analysis of data from two prospective cohort studies. BMJ. 2010;340:c1240. doi: 10.1136/bmj.c1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aberg F., Helenius-Hietala J., Puukka P., Farkkila M., Jula A. Interaction between alcohol consumption and metabolic syndrome in predicting severe liver disease in the general population. Hepatology. 2018;67:2141–2149. doi: 10.1002/hep.29631. [DOI] [PubMed] [Google Scholar]
- 9.You M., Arteel G.E. Effect of ethanol on lipid metabolism. J Hepatol. 2019;70:237–248. doi: 10.1016/j.jhep.2018.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ibrahim S.H., Hirsova P., Tomita K., Bronk S.F., Werneburg N.W., Harrison S.A. Mixed lineage kinase 3 mediates release of C-X-C motif ligand 10-bearing chemotactic extracellular vesicles from lipotoxic hepatocytes. Hepatology. 2016;63:731–744. doi: 10.1002/hep.28252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nevzorova Y.A., Boyer-Diaz Z., Cubero F.J., Gracia-Sancho J. Animal models for liver disease – a practical approach for translational research. J Hepatol. 2020;73:423–440. doi: 10.1016/j.jhep.2020.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Xu J., Lai K.K.Y., Verlinsky A., Lugea A., French S.W., Cooper M.P. Synergistic steatohepatitis by moderate obesity and alcohol in mice despite increased adiponectin and p-AMPK. J Hepatol. 2011;55:673–682. doi: 10.1016/j.jhep.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bosch J., Abraldes J.G., Berzigotti A., Garcia-Pagan J.C. The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroenterol Hepatol. 2009;6:573–582. doi: 10.1038/nrgastro.2009.149. [DOI] [PubMed] [Google Scholar]
- 14.Kleiner D.E., Brunt E.M., Van Natta M., Behling C., Contos M.J., Cummings O.W. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology (Baltimore, Md) 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 15.Brunt E.M., Janney C.G., Di Bisceglie A.M., Neuschwander-Tetri B.A., Bacon B.R. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 16.Tofte N., Suvitaival T., Ahonen L., Winther S.A., Theilade S., Frimodt-Moller M. Lipidomic analysis reveals sphingomyelin and phosphatidylcholine species associated with renal impairment and all-cause mortality in type 1 diabetes. Sci Rep. 2019;9:16398. doi: 10.1038/s41598-019-52916-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowden J.A., Heckert A., Ulmer C.Z., Jones C.M., Koelmel J.P., Abdullah L. Harmonizing lipidomics: NIST interlaboratory comparison exercise for lipidomics using SRM 1950-Metabolites in Frozen Human Plasma. J Lipid Res. 2017;58:2275–2288. doi: 10.1194/jlr.M079012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pluskal T., Castillo S., Villar-Briones A., Oresic M. MZmine 2: modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinformatics. 2010;11:395. doi: 10.1186/1471-2105-11-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinheiro J., Bates D., DebRoy S., Sarkar D., Team R.C. 3.1-145. R package version; 2020. https://CRAN.R-project.org/package=nlme (nlme: Linear and Nonlinear Mixed Effects Models). [Google Scholar]
- 20.Han M.S., Park S.Y., Shinzawa K., Kim S., Chung K.W., Lee J.H. Lysophosphatidylcholine as a death effector in the lipoapoptosis of hepatocytes. J Lipid Res. 2008;49:84–97. doi: 10.1194/jlr.M700184-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Mota M., Banini B.A., Cazanave S.C., Sanyal A.J. Molecular mechanisms of lipotoxicity and glucotoxicity in nonalcoholic fatty liver disease. Metabolism. 2016;65:1049–1061. doi: 10.1016/j.metabol.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puri P., Baillie R.A., Wiest M.M., Mirshahi F., Choudhury J., Cheung O. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46:1081–1090. doi: 10.1002/hep.21763. [DOI] [PubMed] [Google Scholar]
- 23.Oresic M., Hyotylainen T., Kotronen A., Gopalacharyulu P., Nygren H., Arola J. Prediction of non-alcoholic fatty-liver disease and liver fat content by serum molecular lipids. Diabetologia. 2013;56:2266–2274. doi: 10.1007/s00125-013-2981-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lehmann R., Franken H., Dammeier S., Rosenbaum L., Kantartzis K., Peter A. Circulating lysophosphatidylcholines are markers of a metabolically benign nonalcoholic fatty liver. Diabetes Care. 2013;36:2331–2338. doi: 10.2337/dc12-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siler S.Q., Neese R.A., Hellerstein M.K. De novo lipogenesis, lipid kinetics, and whole-body lipid balances in humans after acute alcohol consumption. Am J Clin Nutr. 1999;70:928–936. doi: 10.1093/ajcn/70.5.928. [DOI] [PubMed] [Google Scholar]
- 26.Pownall H.J., Ballantyne C.M., Kimball K.T., Simpson S.L., Yeshurun D., Gotto A.M., Jr. Effect of moderate alcohol consumption on hypertriglyceridemia: a study in the fasting state. Arch Intern Med. 1999;159:981–987. doi: 10.1001/archinte.159.9.981. [DOI] [PubMed] [Google Scholar]
- 27.Nishimura M., Hasumura Y., Takeuchi J. Effect of an intravenous infusion of ethanol on serum enzymes and lipids in patients with alcoholic liver disease. Gastroenterology. 1980;78:691–695. [PubMed] [Google Scholar]
- 28.Lieber C.S., Leevy C.M., Stein S.W., George W.S., Cherrick G.R., Abelmann W.H. Effect of ethanol on plasma free fatty acids in man. J Lab Clin Med. 1962;59:826–832. [PubMed] [Google Scholar]
- 29.Christiansen C., Thomsen C., Rasmussen O., Hansen C., Hermansen K. The acute impact of ethanol on glucose, insulin, triacylglycerol,and free fatty acid responses and insulin sensitivity in type 2 diabetes. Br J Nutr. 1996;76:669–675. doi: 10.1079/bjn19960074. [DOI] [PubMed] [Google Scholar]
- 30.Clugston R.D., Jiang H., Lee M.X., Piantedosi R., Yuen J.J., Ramakrishnan R. Altered hepatic lipid metabolism in C57BL/6 mice fed alcohol: a targeted lipidomic and gene expression study. J Lipid Res. 2011;52:2021–2031. doi: 10.1194/jlr.M017368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bradford B.U., O'Connell T.M., Han J., Kosyk O., Shymonyak S., Ross P.K. Metabolomic profiling of a modified alcohol liquid diet model for liver injury in the mouse uncovers new markers of disease. Toxicol Appl Pharmacol. 2008;232:236–243. doi: 10.1016/j.taap.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loftus N., Barnes A., Ashton S., Michopoulos F., Theodoridis G., Wilson I. Metabonomic investigation of liver profiles of nonpolar metabolites obtained from alcohol-dosed rats and mice using high mass accuracy MSn analysis. J Proteome Res. 2011;10:705–713. doi: 10.1021/pr100885w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jang Z.H., Chung H.C., Ahn Y.G., Kwon Y.K., Kim J.S., Ryu J.H. Metabolic profiling of an alcoholic fatty liver in zebrafish (Danio rerio) Mol Biosyst. 2012;8:2001–2009. doi: 10.1039/c2mb25073j. [DOI] [PubMed] [Google Scholar]
- 34.Liu H., Beier J.I., Arteel G.E., Ramsden C.E., Feldstein A.E., McClain C.J. Transient receptor potential vanilloid 1 gene deficiency ameliorates hepatic injury in a mouse model of chronic binge alcohol-induced alcoholic liver disease. Am J Pathol. 2015;185:43–54. doi: 10.1016/j.ajpath.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Israelsen M., Juel H.B., Detlefsen S., Madsen B.S., Rasmussen D.N., Larsen T.R. Metabolic and genetic risk factors are the strongest predictors of severity of alcohol-related liver fibrosis. Clin Gastroenterol Hepatol. 2020 doi: 10.1016/j.cgh.2020.11.038. [DOI] [PubMed] [Google Scholar]
- 36.Buzzetti E., Pinzani M., Tsochatzis E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD) Metabolism. 2016;65:1038–1048. doi: 10.1016/j.metabol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 37.Alberti K.G., Zimmet P., Shaw J. Metabolic syndrome – a new world-wide definition. A consensus statement from the international diabetes federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymised data available on request.